Introduction
With the advent of potent antiretroviral therapy (ART), the lifespan of individuals infected with HIV has significantly increased [1]. However, while mortality from end-stage AIDS has declined, chronic diseases, including type 2 diabetes mellitus (DM2), have emerged as important causes of morbidity and mortality in the HIV-infected population [2].
While the exact mechanisms leading to DM2 in HIV-infected patients have yet to be fully characterized, the pathophysiology is multifactorial, and includes adverse metabolic effects of antiretroviral agents [3–5], associated body morphology changes [6, 7], immune activation and chronic inflammation [8, 9], and an increase in the prevalence of usual risk factors for DM2, such as obesity [10, 11].
Given that HIV-infected individuals are at increased risk of comorbid conditions such as cardiovascular disease [12], effective medical management of hyperglycemia may be particularly important in this population. However, there are only limited data on the response to diabetic medications in HIV-infected patients with DM2, with currently published studies focused on patients with body morphology disorders and associated insulin resistance [13–19]. It is unknown whether patients with HIV infection respond to diabetic medical therapy similarly to the HIV-uninfected population. However, given the persistent inflammation and adverse metabolic effects characteristic of HIV infection, we evaluated the hypothesis that HIV-infected individuals achieve a smaller reduction in glycemia with medical therapy compared to patients without HIV. To do so, we compared the effectiveness of initial medical therapy for DM2 in patients with and without HIV infection.
METHODS
Study Setting and Population
This study was conducted in the Centers for AIDS Research (CFAR) Network of Integrated Clinical Systems (CNICS) cohort, a longitudinal observational study of HIV-infected patients receiving primary care at 8 sites. The CNICS data repository integrates comprehensive clinical data from all outpatient and inpatient encounters, including demographic, clinical, laboratory, and medication data using electronic health records (EHRs) and other institutional data sources [20, 21].
In addition, patients without HIV infection were included from the Pennsylvania Integrated Clinical and Research Database (PICARD) at the University of Pennsylvania, Philadelphia. PICARD integrates information from multiple health systems including the EPIC EHR and includes information on patient-related clinic notes, orders, laboratory data, diagnoses, and medication prescriptions. PICARD serves as a comprehensive resource for obtaining a large population of HIV-uninfected subjects with DM2 and has been used successfully in prior pharmacoepidemiologic studies [22, 23].
Patients were included in the study if they were adults ≥18 years or older with DM2. Prevalent DM2 was identified based on meeting at least one of the following criteria: 1) DM2 diagnoses (e.g., ICD-9-CM code 250.02) or 2) prescription(s) for a diabetes-specific medication such as a sulfonylurea. This algorithm was previously found to have a positive predictive value of 91% for identifying HIV-infected diabetic patients [24]. The use of the same algorithm in PICARD had a positive predictive value of 90% for identifying HIV-uninfected adult patients with diabetes (Han J, et al., unpublished data).
The date of the first prescription for a diabetic medication was denoted as the index date (Figure 1, Supplemental Digital Content). Subjects who initiated a new diabetic medication between 1 January 1999 and 1 February 2010 were eligible for inclusion. In order to further ensure “new-user” status and to exclude subjects who may have switched medications, subjects were required to 1) have been enrolled in the cohort ≥ 6 months pre-index date; 2) not have had a prescription for a diabetic medication in the 6 months preceding the index date; and 3) have at least one re-order of the new therapy during the year after the index date.
Study Design
This was a retrospective cohort study using a “new-user” design[25] of subjects with DM2 who initiated diabetic medical therapy, with the primary exposure of interest being HIV infection. To increase the uniformity of comparison groups, subjects without HIV infection were matched in a 3:1 ratio to HIV-infected subjects based on age (± 5 years) and sex.
The primary outcome of interest was absolute change in hemoglobin A1c (HbA1c) during the first year of therapy. Subanalyses were planned a priori with the goal of in-depth exploration of mechanisms that may play an important role in determining glycemic control in HIV, namely protease inhibitor (PI) use and HIV disease stage, which correlates with levels of chronic inflammation. For the latter, CD4+ T-cell count and viral load were used as approximate surrogate measures, given established associations between lower CD4+ T-cell counts and higher viral loads with greater levels of inflammation [9, 26–28]. Treatment responses in patients without HIV infection were compared separately to HIV-infected patients 1) with CD4+ T-cell counts <200 cells/μL; 2) with detectable viremia; and 3) receiving PI-based ART. The secondary outcome of interest was the proportion of patients in each group that achieved the American Diabetes Association (ADA) goal of a HbA1c <7% at any time in the post-index period.
In evaluating the effects of diabetic medications on glycemic response, no adjustment was made for medication dose changes, as the majority of subjects receiving an oral medication (>90%) were titrated to maximum doses. Medication intensification was considered as the addition of another diabetic medication to the initial regimen at any time during the 12-month post-index period. The study was reviewed and approved by the institutional review board of the University of Pennsylvania. All CNICS sites also have local institutional review board approval.
Demographic and clinical variables
The following baseline data were collected for all subjects at the time of diabetic medication initiation: age, sex, height, weight, and race and ethnicity. The presence of the following comorbid conditions at baseline that could potentially affect glycemic response and/or adherence were collected (Table 1, Supplemental Digital Content): major psychiatric comorbid conditions (≥1 diagnosis code for a major psychiatric disorder ≤12 months pre-index date); alcohol abuse (≥1 diagnosis code for current alcohol abuse ≤12 months pre-index date); substance abuse (≥1 diagnosis code for current illicit substance abuse ≤12 months pre-index date); and hepatitis C infection [29, 30] (≥1 diagnosis code for hepatitis C and/or positive hepatitis C antibody status).
HbA1c results from 6 months pre-index date up to 12 months post-index date were included. Baseline HbA1c, HIV RNA levels, and CD4+ T-cell counts were defined as those measured in the 6-month pre-index date period closest to but not after the start of diabetic medical therapy.
Diabetic medications were categorized by class (i.e., sulfonylureas, metformin, thiazolidinediones [TZDs], insulin, or combination oral therapy) and included for up to 12 months after the index date. For patients with HIV infection, ART regimens were classified into PI-based ART (i.e., inclusion of a PI) or non-PI based ART at baseline (i.e., absence of a PI).
Statistical Analysis
Baseline characteristics among the groups were compared using the Student’s t-test or Wilcoxon rank-sum test for continuous variables and the χ2 or Fisher’s exact test for categorical variables. A generalized estimating equation (GEE) model was used for the primary outcome analyses. The GEE model accounts for correlation within a subject, including for longitudinal, repeated measurements, and allowed inclusion of all available HbA1c measurements during the first year of treatment [31]. Follow-up time intervals during which HbA1c values were obtained were categorized as follows for analysis: 1–3 months, 4–6 months, 7–9 months, and 10–12 months. If more than one follow-up HbA1c value was available for a specified time interval, the most recent value was entered into the model. Variables that could act as potential confounders of the association between HIV infection and change in HbA1c, including comorbid conditions, diabetic medication class, and therapy intensification, were considered for inclusion and maintained in the final model if their inclusion resulted in a ≥10% change in the effect measure for the primary association of interest and/or were considered to be clinically important [32]. Baseline HbA1c was retained in the model regardless of change in point estimate given its known influence on initial response to therapy [33, 34]. Given its reported association with insulin resistance [29, 30], hepatitis C was considered a priori as a potential effect modifier. Subanalyses were then performed in a similar fashion using a GEE model, with treatment responses in subjects without HIV infection compared to responses in HIV-infected subjects 1) on a PI-based versus a non-PI-based ART regimen; 2) with and without a baseline undetectable viral load; and 3) with a baseline CD4+ T-cell count ≥200 or <200 cells/μL. An undetectable viral load was categorized as one that was below the lower limit of detection for the specific assay in use at the time of specimen collection (at least ≤400 copies/mL).
Secondary outcome analysis was performed using multiple logistic regression [35, 36], with adjustment for potential confounders in the final model as noted in the primary outcome analyses [32]. For all calculations, a 2-tailed P value of <0.05 was considered significant. All statistical calculations were performed using commercially available software (STATA v11.0; College Station, Texas).
RESULTS
Baseline Characteristics
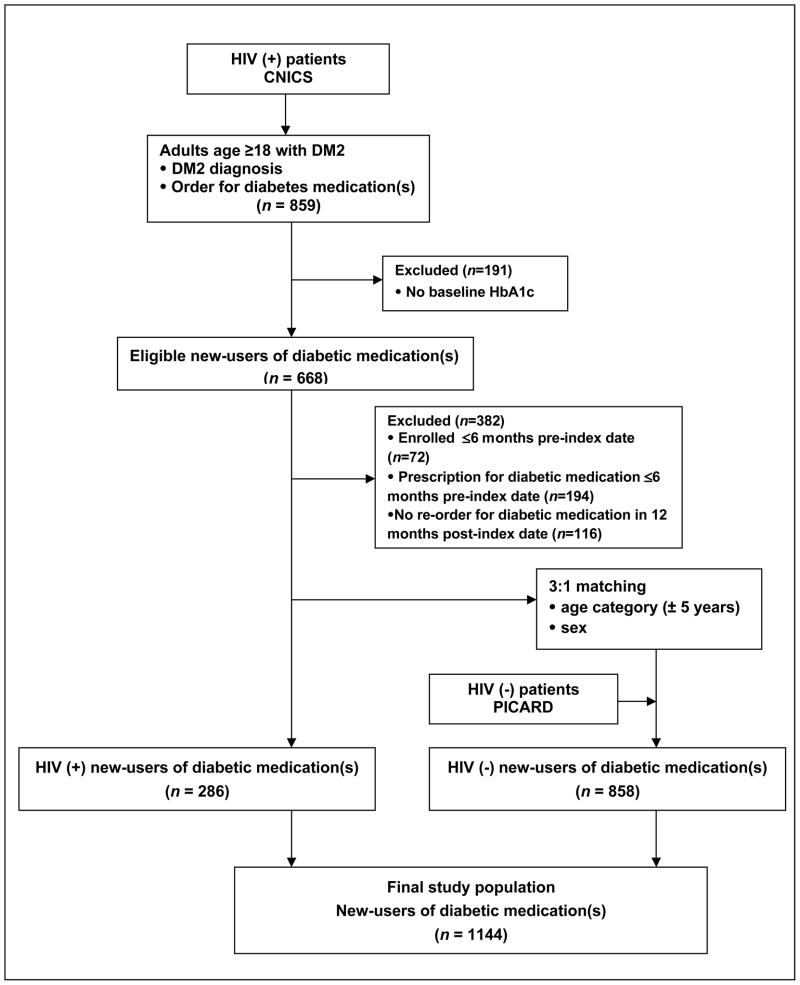
A total of 286 patients with HIV infection and 858 patients without HIV infection qualified for the study (Figure 1). Baseline clinical and demographic characteristics of new-users of diabetic medications with and without HIV infection are shown in Table 1. Compared to patients without HIV infection, patients with HIV infection had lower mean baseline HbA1c values (7.82% [standard deviation (SD), 2.3] versus 8.62% [SD, 2.4], respectively; P<0.001). Both groups had a median of two follow-up HbA1c tests (interquartile range 1, 3) performed in the year after initiating pharmacologic therapy, and at least one follow-up HbA1c value for each of the four follow-up time intervals was available for approximately half of subjects in both groups (data not shown).
Figure 1.
Study flow diagram based on inclusion and exclusion criteria.
HIV (+) = HIV-infected; HIV (−) = HIV-uninfected.
Table 1.
Baseline Characteristics of New-Users of Diabetic Medical Therapy
| Characteristica | HIV-Infected Patients (n = 286) | HIV-Uninfected Patients (n = 858) | P Value |
|---|---|---|---|
| Age category | |||
| <35 | 9 (3.2) | 27 (3.2) | N/Ab |
| ≥35 to <45 | 100 (35.0) | 300 (35.0) | |
| ≥45 to <55 | 119 (41.6) | 357 (41.6) | |
| ≥55 to <65 | 47 (16.4) | 141 (16.4) | |
| ≥65 | 11 (3.8) | 33 (3.8) | |
|
| |||
| Female sex | 48 (16.8) | 144 (16.8) | N/Ab |
|
| |||
| Race/ethnicity | |||
| White | 127 (44.4) | 405 (47.2) | <0.001 |
| Black | 93 (32.5) | 362 (42.2) | |
| Hispanic | 50 (17.5) | 56 (6.5) | |
| Other c | 16 (5.6) | 35 (4.1) | |
|
| |||
| Mean BMI (SD), kg/m2 | 29.7 (6.5) | 33.2 (7.5) | <0.001 |
|
| |||
| Obesityd | 118 (41.5) | 511 (62.2) | <0.001 |
|
| |||
| Hepatitis C infection | 35 (12.2) | 30 (3.5) | <0.001 |
|
| |||
| Major psychiatric disordere | 44 (15.4) | 116 (13.5) | 0.43 |
|
| |||
| Alcohol abusee | 38 (14.2) | 48 (5.6) | <0.001 |
|
| |||
| Substance abusee | 40 (14.9) | 91 (10.6) | 0.05 |
|
| |||
| Mean baseline HbA1c (SD), % | 7.8 (2.3) | 8.6 (2.4) | <0.001 |
|
| |||
| Initial medication class | |||
| Sulfonylurea | 72 (25.2) | 193 (22.5) | 0.11 |
| Metformin | 144 (50.4) | 495 (57.6) | |
| Thiazolidinedione | 38 (13.3) | 89 (10.4) | |
| Combination oral medications | 19 (6.6) | 60 (7.0) | |
| Insulin | 13 (4.5) | 21 (2.5) | |
|
| |||
| Initial combination oral therapy | 19 (6.6) | 60 (7.0) | 0.84 |
|
| |||
| Metformin as initial therapy | 144 (50.4) | 495 (57.6) | 0.03 |
|
| |||
| Median medication start year (IQR) | 2006 (2004, 2008) | 2005 (2003, 2007) | 0.001 |
|
| |||
| Medication intensification | 17 (5.9) | 46 (5.4) | 0.71 |
|
| |||
| HIV-transmission risk factorf | |||
| MSM | 147 (51.4) | N/A | |
| IDU | 24 (8.4) | ||
| MSM and IDU | 20 (7.0) | ||
| Heterosexual | 68 (23.8) | ||
| Other | 8 (2.8) | ||
| Unknown | 19 (6.6) | ||
|
| |||
| Mean CD4+ T-cell count (SD), cells/μL | 467 (305) | N/A | |
|
| |||
| CD4+ T-cell count <200 cells/μL | 55 (19.2) | N/A | |
|
| |||
| Undetectable HIV RNA level | 151 (52.8) | N/A | |
|
| |||
| Antiretroviral therapy regimeng | |||
|
| |||
| PI-based | 151 (52.9) | N/A | |
| Non-PI-based | 123 (42.9) | ||
Abbreviations: N/A, not applicable; BMI, body mass index; SD, standard deviation; HbA1c, hemoglobin A1c; IQR, interquartile range; MSM, men who have sex with men; IDU, injection drug use; PI, protease inhibitor.
Data are presented as numbers (percentages), except where otherwise noted.
Subjects without HIV infection were matched to those with HIV infection on age and sex in a 3:1 ratio.
Self-identified as “other” race/ethnicity.
Defined as a BMI ≥30 kg/m2.
Data were not available from the Case Western Reserve University CNICS site (n=18 subjects).
As per the Centers for Disease Control and Prevention (CDC) HIV surveillance system.
12 (4.2%) of HIV-infected patients were not on antiretroviral therapy at the time of diabetic medication initiation.
Initial Diabetic Medical Therapy and Treatment Responses Over the First Year
The majority of new-users initiated one oral diabetic medication, with the most commonly prescribed therapy for patients with and without HIV infection being metformin (50.4% and 57.6%, respectively). Only a small proportion of patients with and without HIV infection started combination therapy (6.6% and 7.0%, respectively; P=0.84) or underwent a medication switch (5.2% and 5.9%, respectively; P=0.76) during the 12-month post-index period. Unadjusted mean HbA1c values during the first year of therapy by HIV infection status are shown in Figure 2, Supplemental Digital Content.
Overall, patients had an adjusted absolute mean reduction in HbA1c of 1.04% (95% CI, −0.87, −1.22) during the first year of diabetic medical therapy (Table 2). HIV-infected patients achieved significantly smaller reductions in HbA1c compared to patients without HIV infection, with an absolute mean difference in reduction in HbA1c of −0.17% during the first year of treatment (95% CI, −0.28, −0.06; P=0.003), after adjustment for baseline HbA1c (Table 2). In the final GEE model including age, race/ethnicity, use of initial combination oral therapy, baseline HbA1c, HIV infection status, and time (duration of therapy), there was no evidence of effect modification by hepatitis C status (Wald test for interaction P=0.39). Furthermore, the group-by-time interaction, which was formally tested during model building, was not significant (Wald test for interaction P=0.080). Changes in HbA1c in adjusted GEE analyses are shown in Table 3.
Table 2.
Adjusted Changes in HbA1c Values Compared with Baseline by HIV Infection Status and Duration of Therapy.
| Duration of Therapy (Post-Index Date) | Change From Baseline, % (95% CI)a | Difference Between HIV-Infected and HIV-Uninfected Patients (95% CI)b |
|---|---|---|
| 1–3 months | −1.11 (−0.96, −1.26) | −0.17 (−0.28, −0.06) |
| 4–6 months | −1.20 (−1.05, −1.36) | |
| 7–9 months | −1.15 (−1.00, −1.31) | |
| 10–12 months | −1.04 (−0.87, −1.22) |
Abbreviations: HbA1c, hemoglobin A1c; CI, confidence interval.
Absolute reduction in HbA1c (%).
Of absolute reduction in HbA1c (%), with reference group patients without HIV infection.
Table 3.
Adjusted Changes in HbA1c in New-Users of Diabetic Medical Therapy Based on a Multivariable Regression Model of HIV Infection Using a Generalized Estimating Equation.
| Variable | Regression Coefficient (95% CI) | P Value |
|---|---|---|
| Age | −0.007 (−0.01, −0.001) | 0.02 |
|
| ||
| Race/ethnicitya | ||
| Black | 0.09 (−0.03, 0.21) | |
| Hispanic | −0.13 (−0.33, 0.06) | 0.046b |
| Other | −0.17 (−0.41, 0.06) | |
|
| ||
| Initial combination therapy | 0.37 (0.08, 0.66) | 0.01 |
|
| ||
| Baseline HbA1c | 0.56 (0.53, 0.59) | <0.001 |
|
| ||
| HIV infection | −0.17 (−0.28, −0.06) | 0.003 |
|
| ||
| Timec | ||
| 1–3 months | −1.11 (−1.26, −0.96) | |
| 4–6 months | −1.20 (−1.36, −1.05) | <0.001d |
| 7–9 months | −1.15 (−1.31, −1.00) | |
| 10–12 months | −1.04 (−1.22, −0.87) | |
Abbreviations: HbA1c, hemoglobin A1c; CI, confidence interval.
Reference category, “White.”
Wald test of race/ethnicity terms in the final model.
Reference category, “baseline,” prior to initiation of diabetic medical therapy.
Wald test for individual and all time terms in the final model.
On posthoc analyses, HIV-infected patients with either low (≤8%) or high (>8%) baseline HbA1c values achieved statistically significantly smaller reductions in HbA1c compared to patients without HIV infection. Furthermore, this less robust response appeared more pronounced for those beginning with high compared to low baseline HbA1c values, as follows: a difference of −0.25% (95% CI, −0.47, −0.04; P=0.02) versus a difference of −0.12% (95% CI, −0.22, −0.01; P=0.03), respectively. The results of this posthoc analysis indicate that the difference in response is likely not due primarily to lower baseline HbA1c values in patients with HIV infection.
Subanalyses
After adjustment for age, baseline HbA1c, BMI, year of medication initiation, medication intensification, and time (duration of therapy), HIV-infected patients on a PI-based regimen, but not those on non-PI-based regimens, achieved significantly smaller reductions in HbA1c during the first year of treatment compared to patients without HIV infection (difference −0.21%, 95% CI, −0.35, −0.08; P=0.002 in those on PIs, versus difference −0.10%, 95% CI, −0.25, 0.06; P=0.21 in those not on PIs) (Table 2, Supplemental Digital Content).
There was no significant difference in reduction in HbA1c after initiation of therapy in HIV-infected patients with a) either CD4+ T-cell counts <200 cells/μL or CD4+ T-cell counts ≥200 cells/μL (P=0.162 and 0.50, respectively) or b) either detectable or undetectable viral loads (P=0.35 and 0.27, respectively) in comparison to HIV-uninfected patients in multivariable analyses.
HIV Infection and Achievement of the American Diabetes Association Goal for HbA1c
In unadjusted analysis, 59.1% of patients without HIV infection and 72.7% of patients with HIV infection achieved a HbA1c of <7% at any time in the 12-month post-index date period. In multivariable analyses, HIV infection was associated with the achievement of a HbA1c <7%, with an adjusted OR of 1.69 (95% CI, 1.21, 2.36; P=0.002), even after adjusting for baseline HbA1c (Table 4).
Table 4.
Multivariable Model of Variables Associated with Achievement of a HbA1c <7% in New-Users of Diabetic Medical Therapy.
| Variable | OR (95%CI) | P Value |
|---|---|---|
| Baseline HbA1c | 0.74 (0.70, 0.79) | <0.001 |
|
| ||
| Initial medication classa | ||
| Metformin | 1.54 (1.12, 2.12) | 0.008 |
| TZD | 1.38 (0.85, 2.24) | 0.19 |
| Insulin | 0.88 (0.40, 1.94) | 0.75 |
| Combination oral therapy | 0.59 (0.33, 1.05) | 0.07 |
|
| ||
| Medication intensification | 0.52 (0.30, 0.91) | 0.02 |
|
| ||
| HIV infection | 1.69 (1.21, 2.36) | 0.002 |
Abbreviations: HbA1c, hemoglobin A1c; OR, odds ratio; CI, confidence interval; TZD, thiazolidinedione.
Reference category, sulfonylureas.
DISCUSSION
In this longitudinal cohort study, patients with DM2 and HIV infection who initiated diabetic medical therapy achieved smaller absolute reductions in HbA1c than patients with DM2 and no HIV infection in the course of routine clinical care. This less robust response was more pronounced in HIV-infected patients on a PI-based regimen compared to those on a non-PI-based regimen, and was independent of several important potentially confounding factors including the baseline HbA1c level.
Overall, the entire cohort of new-users, who were almost exclusively treated with monotherapy and predominantly with metformin, achieved an absolute reduction in HbA1c of approximately 1% after initiation of pharmacologic therapy. This response is consistent with that reported in studies in the HIV-uninfected population after one year of oral monotherapy [37–39]. Notably, HIV-infected patients in our study had lower HbA1c values compared to HIV-uninfected patients at baseline and all time points through the year after initiation of therapy. Several studies suggest that HbA1c may underestimate glycemic control in HIV-infected patients [40–42], and this HbA1c-glucose discordance may in part be explained by hemolysis and use of specific ART agents. Nevertheless, in the present study, HIV-infected patients, even after adjustment for baseline HbA1c, achieved significantly smaller reductions in HbA1c compared to patients without HIV infection. While the mechanisms behind this association are unclear, our subanalyses, based on a limited number of patients, suggest that decreased responses in those with HIV are more pronounced in users of PIs. This finding is consistent with data indicating increased insulin resistance in recipients of PIs [3–5] and use of these medications, particularly in individuals with uncontrolled DM2 and HIV, should be carefully considered when weighing treatment options. The observed differences in glycemic response may also relate in part to persistent inflammation or immune activation, which have been associated with both HIV infection and insulin resistance [26, 28, 43–45]. Although our subanalyses did not support this mechanism, CD4+ T-cell count and viral load, while associated with inflammation and immune activation, are only approximate measures of these underlying processes [26, 28, 46] and future investigations of this issue should characterize these parameters more directly. In particular, insulin resistance itself has been strongly associated with inflammation [44, 45] and future studies should aim to measure levels of inflammatory biomarkers (e.g., C-reactive protein) as well as markers of immune activation.
The rate of achievement of a HbA1c <7% in our study was 72.7% for HIV-infected patients. To our knowledge, there are currently only three studies in the literature evaluating achievement of a HbA1c <7% in HIV-infected patients, all of which reported rates of approximately 50% [47–49]. However, these studies were cross-sectional reports of patients with prevalent DM2 and therefore are not comparable to our cohort of new-users. Furthermore, HbA1c-glucose discordance [40, 41] may in part explain why HIV-infected patients, despite smaller absolute reductions in HbA1c, were more likely to achieve the ADA goal. Nonetheless, although the absolute difference in HbA1c was modest and HIV-infected patients were more likely to achieve goal HbA1c values, studies in the HIV-uninfected literature have demonstrated that even a small reduction in HbA1c of ~0.8% significantly reduces the risk of microvascular and macrovascular complications [50]. Given this less robust glycemic response to standard medical therapy for DM2, it will be important for HIV providers to focus on aggressive, timely management of glycemia as well as achievement of recommended lipid and blood pressure goals to reduce associated morbidity and mortality. The finding that HIV-infected patients who started with higher baseline HbA1c values (>8%) in our study had an even more pronounced poorer response to initial therapy provides further justification for early and prompt initiation of treatment in patients with recently diagnosed DM2.
To our knowledge, the present study is the first in the literature to evaluate the response to initial diabetic medical therapy in patients with HIV infection compared to those without HIV infection. The results are strengthened by the large size of our cohort, capture of comprehensive clinical data for assessment of confounding, and evaluation of HIV-specific, mechanistic factors such as ART regimen and virologic control that could arguably affect glycemic response. Finally, the present study evaluated patients receiving routine medical care, thereby measuring the impact of medications in a “real-world” clinical setting as opposed to the more artificial setting of a clinical trial where patients are highly selected for inclusion.
There are also several potential limitations of the present study. As with any observational study, our results may be affected by confounding. However, we attempted to account for a wide variety of relevant factors in our multivariable analysis, employed a new-user design which minimizes prevalent-user bias, and used a longitudinal model which permitted incorporation of all follow-up HbA1c results in the analyses. Furthermore, we were unable to ascertain data on adherence to diabetic medical therapy; however, we evaluated comorbid conditions that could potentially affect adherence in our analyses, and there were minimal therapy switches in both patient groups, thereby suggesting low rates of adverse medication effects that could lead to poor adherence. The use of separate cohorts for patients with and without HIV infection is another potential limitation; however, both patient cohorts were representative of routine medical care delivered in academic, tertiary care settings, patients were matched by age and sex, and patients with prevalent DM2 were ascertained in both databases using similar, validated algorithms. Furthermore, while the findings of our study demonstrated that HIV-infected patients on PI-based regimens had a less robust glycemic response, increasing evidence suggests that newer agents in the PI class may not have the same adverse metabolic effects compared to older agents [51]. While the majority of HIV-infected patients on PI-based regimens were on atazanavir/ritonavir and lopinavir/ritonavir (42.4% and 31.8%, respectively), given limitations in sample size, we were unable to evaluate the effect of individual PIs on glycemic control. Finally, the present study evaluated clinical care delivered at academic institutions and may not be generalizable to other settings.
In conclusion, HIV-infected patients in routine clinical care achieved smaller absolute reductions in HbA1c in response to initial diabetic medical therapy. Current guidelines on the management of DM2 in HIV-infected patients are based on established recommendations in the HIV-uninfected population [52]. However, given the less robust glycemic response to initial diabetic therapy, optimizing medical therapy and achievement of target lipid and blood pressure goals may be even more critical in HIV-infected patients with DM2. Further research is needed to elucidate the mechanisms leading to this less robust glycemic response, including the contribution of specific PIs and chronic inflammation. Future studies should also evaluate optimal choice of pharmacologic therapy and the most accurate measurement of glycemia in this medically complex population, with the goal of decreasing the risk of clinical complications and mortality.
Supplementary Material
Acknowledgments
The authors thank the following individuals for their role in contributing data from each CNICS site: Michael Saag, MD (University of Alabama); Steven Deeks, MD (University of California, San Francisco); WC Mathews, MD (University of California, San Diego); Joseph Eron, MD (University of North Carolina); Stephen Boswell, MD (Fenway Community Health Center of Harvard University); Mari Kitahata, MD, MPH (University of Washington); and Benigno Rodriguez, MD (Case Western Reserve University). The authors also thank Marie Synnvestedt, PhD and Mark Weiner, MD for their assistance with obtaining data from PICARD.
Funding/Support: This study was supported by Institutional National Research Service Award T32 AI055435 (Han), CNICS grant R24AI067039, Agency for Healthcare Research and Quality grant R21HS019516 (Crane), and the University of Pennsylvania CFAR grant (P30 AI 045008).
Footnotes
Prior presentation: The results of this study were previously presented as a poster presentation at the 19th Conference on Retroviruses and Opportunistic Infections (CROI), Seattle, WA, on March 7, 2012.
Financial Disclosure: Dr. Frank is an advisor to Gilead Sciences. Dr. Bisson is a consultant to Pfizer.
Author Contributions: Study concept and design: Han, Crane, Bellamy, Frank, Cardillo, Bisson. Acquisition of data: Han, Crane, Bisson. Analysis and interpretation of data: Han, Crane, Bellamy, Frank, Cardillo, Bisson. Drafting of the manuscript: Han and Bisson. Critical revision of the manuscript for important intellectual content: Han, Crane, Bellamy, Frank, Cardillo, Bisson. Statistical analysis: Han, Bisson, Bellamy. Obtained funding: Han, Crane, Bisson, Frank. Administrative, technical, and material support: Crane. Study supervision: Bisson.
References
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ. 2009;338:a3172. doi: 10.1136/bmj.a3172. [DOI] [PubMed] [Google Scholar]
- 3.Behrens G, Dejam A, Schmidt H, Balks HJ, Brabant G, Korner T, et al. Impaired glucose tolerance, beta cell function and lipid metabolism in HIV patients under treatment with protease inhibitors. AIDS. 1999;13:F63–70. doi: 10.1097/00002030-199907090-00001. [DOI] [PubMed] [Google Scholar]
- 4.Woerle HJ, Mariuz PR, Meyer C, Reichman RC, Popa EM, Dostou JM, et al. Mechanisms for the deterioration in glucose tolerance associated with HIV protease inhibitor regimens. Diabetes. 2003;52:918–925. doi: 10.2337/diabetes.52.4.918. [DOI] [PubMed] [Google Scholar]
- 5.Noor MA, Seneviratne T, Aweeka FT, Lo JC, Schwarz JM, Mulligan K, et al. Indinavir acutely inhibits insulin-stimulated glucose disposal in humans: a randomized, placebo-controlled study. AIDS. 2002;16:F1–8. doi: 10.1097/00002030-200203290-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hadigan C, Miller K, Corcoran C, Anderson E, Basgoz N, Grinspoon S. Fasting hyperinsulinemia and changes in regional body composition in human immunodeficiency virus-infected women. J Clin Endocrinol Metab. 1999;84:1932–1937. doi: 10.1210/jcem.84.6.5738. [DOI] [PubMed] [Google Scholar]
- 7.Hadigan C, Corcoran C, Stanley T, Piecuch S, Klibanski A, Grinspoon S. Fasting hyperinsulinemia in human immunodeficiency virus-infected men: relationship to body composition, gonadal function, and protease inhibitor use. J Clin Endocrinol Metab. 2000;85:35–41. doi: 10.1210/jcem.85.1.6264. [DOI] [PubMed] [Google Scholar]
- 8.Brown TT, Tassiopoulos K, Bosch RJ, Shikuma C, McComsey GA. Association between systemic inflammation and incident diabetes in HIV-infected patients after initiation of antiretroviral therapy. Diabetes Care. 2010;33:2244–2249. doi: 10.2337/dc10-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deeks SG. Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Top HIV Med. 2009;17:118–123. [PubMed] [Google Scholar]
- 10.Amorosa V, Synnestvedt M, Gross R, Friedman H, MacGregor RR, Gudonis D, et al. A tale of 2 epidemics: the intersection between obesity and HIV infection in Philadelphia. J Acquir Immune Defic Syndr. 2005;39:557–561. [PubMed] [Google Scholar]
- 11.Butt AA, McGinnis K, Rodriguez-Barradas MC, Crystal S, Simberkoff M, Goetz MB, et al. HIV infection and the risk of diabetes mellitus. AIDS. 2009;23:1227–1234. doi: 10.1097/QAD.0b013e32832bd7af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Wijk JP, de Koning EJ, Cabezas MC, op’t Roodt J, Joven J, Rabelink TJ, Hoepelman AI. Comparison of rosiglitazone and metformin for treating HIV lipodystrophy: a randomized trial. Ann Intern Med. 2005;143:337–346. doi: 10.7326/0003-4819-143-5-200509060-00009. [DOI] [PubMed] [Google Scholar]
- 14.Sutinen J, Hakkinen AM, Westerbacka J, Seppala-Lindroos A, Vehkavaara S, Halavaara J, et al. Rosiglitazone in the treatment of HAART-associated lipodystrophy--a randomized double-blind placebo-controlled study. Antivir Ther. 2003;8:199–207. [PubMed] [Google Scholar]
- 15.Mulligan K, Yang Y, Wininger DA, Koletar SL, Parker RA, Alston-Smith BL, et al. Effects of metformin and rosiglitazone in HIV-infected patients with hyperinsulinemia and elevated waist/hip ratio. AIDS. 2007;21:47–57. doi: 10.1097/QAD.0b013e328011220e. [DOI] [PubMed] [Google Scholar]
- 16.Hadigan C, Corcoran C, Basgoz N, Davis B, Sax P, Grinspoon S. Metformin in the treatment of HIV lipodystrophy syndrome: A randomized controlled trial. JAMA. 2000;284:472–477. doi: 10.1001/jama.284.4.472. [DOI] [PubMed] [Google Scholar]
- 17.Yarasheski KE, Cade WT, Overton ET, Mondy KE, Hubert S, Laciny E, et al. Exercise training augments the peripheral insulin-sensitizing effects of pioglitazone in HIV- infected adults with insulin resistance and central adiposity. Am J Physiol Endocrinol Metab. 2011;300:E243–251. doi: 10.1152/ajpendo.00468.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hadigan C, Yawetz S, Thomas A, Havers F, Sax PE, Grinspoon S. Metabolic effects of rosiglitazone in HIV lipodystrophy: a randomized, controlled trial. Ann Intern Med. 2004;140:786–794. doi: 10.7326/0003-4819-140-10-200405180-00008. [DOI] [PubMed] [Google Scholar]
- 19.Carr A, Workman C, Carey D, Rogers G, Martin A, Baker D, et al. No effect of rosiglitazone for treatment of HIV-1 lipoatrophy: randomised, double-blind, placebo- controlled trial. Lancet. 2004;363:429–438. doi: 10.1016/S0140-6736(04)15489-5. [DOI] [PubMed] [Google Scholar]
- 20.Kitahata MM, Rodriguez B, Haubrich R, Boswell S, Mathews WC, Lederman MM, et al. Cohort profile: the Centers for AIDS Research Network of Integrated Clinical Systems. Int J Epidemiol. 2008;37:948–955. doi: 10.1093/ije/dym231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CNICS. [Accessed 5/1/2012];CFAR Network of Integrated Clinical Systems. Available at http://www.uab.edu/cnics/
- 22.Turner BJ, Hollenbeak CS, Weiner M, Ten Have T, Tang SS. Effect of unrelated comorbid conditions on hypertension management. Ann Intern Med. 2008;148:578–586. doi: 10.7326/0003-4819-148-8-200804150-00002. [DOI] [PubMed] [Google Scholar]
- 23.Umscheid CA, Gross R, Weiner MG, Hollenbeak CS, Tang SS, Turner BJ. Racial disparities in hypertension control, but not treatment intensification. Am J Hypertens. 2010;23:54–61. doi: 10.1038/ajh.2009.201. [DOI] [PubMed] [Google Scholar]
- 24.Crane HM, Kadane JB, Crane PK, Kitahata MM. Diabetes case identification methods applied to electronic medical record systems: their use in HIV-infected patients. Curr HIV Res. 2006;4:97–106. doi: 10.2174/157016206775197637. [DOI] [PubMed] [Google Scholar]
- 25.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158:915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 26.Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 27.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. J Pathol. 2008;214:231–241. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]
- 29.Bahtiyar G, Shin JJ, Aytaman A, Sowers JR, McFarlane SI. Association of diabetes and hepatitis C infection: epidemiologic evidence and pathophysiologic insights. Curr Diab Rep. 2004;4:194–198. doi: 10.1007/s11892-004-0023-7. [DOI] [PubMed] [Google Scholar]
- 30.Mehta SH, Brancati FL, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Hepatology. 2001;33:1554. doi: 10.1053/jhep.2001.0103306le01. [DOI] [PubMed] [Google Scholar]
- 31.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 32.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 33.Bloomgarden ZT, Dodis R, Viscoli CM, Holmboe ES, Inzucchi SE. Lower baseline glycemia reduces apparent oral agent glucose-lowering efficacy: a meta-regression analysis. Diabetes Care. 2006;29:2137–2139. doi: 10.2337/dc06-1120. [DOI] [PubMed] [Google Scholar]
- 34.DeFronzo RA, Stonehouse AH, Han J, Wintle ME. Relationship of baseline HbA1c and efficacy of current glucose-lowering therapies: a meta-analysis of randomized clinical trials. Diabet Med. 2010;27:309–317. doi: 10.1111/j.1464-5491.2010.02941.x. [DOI] [PubMed] [Google Scholar]
- 35.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 36.Hosmer DOLS. Applied logistic regression. New York: Wiley and Sons; 1989. [Google Scholar]
- 37.Huizinga MM, Roumie CL, Greevy RA, Liu X, Murff HJ, Hung AM, et al. Glycemic and weight changes after persistent use of incident oral diabetes therapy: a Veterans Administration retrospective cohort study. Pharmacoepidemiol Drug Saf. 2010;19:1108–1112. doi: 10.1002/pds.2035. [DOI] [PubMed] [Google Scholar]
- 38.Karter AJ, Moffet HH, Liu J, Parker MM, Ahmed AT, Go AS, Selby JV. Glycemic response to newly initiated diabetes therapies. Am J Manag Care. 2007;13:598–606. [PMC free article] [PubMed] [Google Scholar]
- 39.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 40.Kim PS, Woods C, Georgoff P, Crum D, Rosenberg A, Smith M, Hadigan C. A1C underestimates glycemia in HIV infection. Diabetes Care. 2009;32:1591–1593. doi: 10.2337/dc09-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diop ME, Bastard JP, Meunier N, Thevenet S, Maachi M, Capeau J, et al. Inappropriately low glycated hemoglobin values and hemolysis in HIV-infected patients. AIDS Res Hum Retroviruses. 2006;22:1242–1247. doi: 10.1089/aid.2006.22.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Polgreen PM, Putz D, Stapleton JT. Inaccurate glycosylated hemoglobin A1C measurements in human immunodeficiency virus-positive patients with diabetes mellitus. Clin Infect Dis. 2003;37:e53–56. doi: 10.1086/376633. [DOI] [PubMed] [Google Scholar]
- 43.Hsue PY, Hunt PW, Schnell A, Kalapus SC, Hoh R, Ganz P, et al. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS. 2009;23:1059–1067. doi: 10.1097/QAD.0b013e32832b514b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 45.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27:813–823. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- 46.Giorgi JV, Lyles RH, Matud JL, Yamashita TE, Mellors JW, Hultin LE, et al. Predictive value of immunologic and virologic markers after long or short duration of HIV-1 infection. J Acquir Immune Defic Syndr. 2002;29:346–355. doi: 10.1097/00126334-200204010-00004. [DOI] [PubMed] [Google Scholar]
- 47.Satlin MJ, Hoover DR, Glesby MJ. Glycemic control in HIV-infected patients with diabetes mellitus and rates of meeting American Diabetes Association management guidelines. AIDS Patient Care STDS. 2011;25:5–12. doi: 10.1089/apc.2010.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bury JE, Stroup JS, Stephens JR, Baker DL. Achieving American Diabetes Association goals in HIV-seropositive patients with diabetes mellitus. Proc (Bayl Univ Med Cent) 2007;20:118–123. doi: 10.1080/08998280.2007.11928265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adeyemi O, Vibhakar S, Max B. Are we meeting the American Diabetes Association goals for HIV-infected patients with diabetes mellitus? Clin Infect Dis. 2009;49:799–802. doi: 10.1086/605286. [DOI] [PubMed] [Google Scholar]
- 50.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 51.Tomaka F, Lefebvre E, Sekar V, Van Baelen B, Vangeneugden T, Vandevoorde A, Diego Miralles G. Effects of ritonavir-boosted darunavir vs. ritonavir-boosted atazanavir on lipid and glucose parameters in HIV-negative, healthy volunteers. HIV Med. 2009;10:318–327. doi: 10.1111/j.1468-1293.2008.00690.x. [DOI] [PubMed] [Google Scholar]
- 52.Aberg JA, Kaplan JE, Libman H, Emmanuel P, Anderson JR, Stone VE, et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 update by the HIV medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:651–681. doi: 10.1086/605292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.