Abstract
Objective
This exploratory study examines the role of psychosocial–behavioral variables as predictors of elevated corticotropin-releasing hormone (CRH) at 14–20 weeks of gestation.
Method
One hundred and twenty women were enrolled into the study. Blood samples were collected at 14–20 weeks of pregnancy and assayed for CRH. Participants completed questionnaires that included the Perceived Stress Scale, the Center for Epidemiologic Studies (CES) Depression Scale, the Pregnancy-Specific Anxiety (PAS) Scale, the Norbeck Social Support Questionnaire, the Life Orientation Test, the Brief COPE scale, and questions regarding violence/abuse, and work, sleep, and nutritional patterns.
Results
Pregnant women with high CRH levels (15 pcg/ml and above) perceived their income to be inadequate, slept more hours at night, stood more hours during the day, and used the coping styles of disengagement or religion but not humor. Logistic regression identified three predictors for high CRH (accounting for 42.2% of the variance): perceived inadequacy of income and the use of “religion” and “disengagement” to cope with stress.
Conclusions
These results are the first known to identify coping style and perceived income inadequacy as predictors of high CRH. Women with perceived inadequacy of income had almost three times the odds for high CRH. Women who used religion or disengagement to cope with stress had 14 times and 7 times the odds for high CRH levels, respectively. Higher CRH levels are associated with preterm birth (PTB). Thus, it may be important to include maternal coping style and perceptions of income inadequacy in future investigations of CRH levels and PTB.
Keywords: corticotropin-releasing hormone, maternal stress, preterm birth, pregnancy, coping, income, biobehavioral
In the United States, there has been a largely unexplained 31% increase in the occurrence of preterm birth (PTB) since the 1980s (Martin et al., 2007). The current PTB rate stands at 12.7%, and approximately half of these births are idiopathic and spontaneous (Goldenberg, Culhane, Iams, & Romero, 2008; Steer, 2005). Furthermore, these spontaneous PTBs are often associated with factors referred to as psychosocial “stressors,” which may be contributing to a chronic maternal stress response that adversely affects the physiology and timing of birth (Hobel, Goldstein, & Barrett, 2008). In fact, research has consistently documented associations between PTB and chronic maternal stress and/or psychosocial factors (Rich-Edwards & Grizzard, 2005; Steer, 2005).
Several studies have identified relationships between “stress” hormones (i.e., adrenocorticotrophin hormone [ACTH] and cortisol) and either an increased occurrence of PTB and/or increased maternal blood levels of corticotropin-releasing hormone (CRH) in pregnancy (Erickson et al., 2001; Norwitz, Robinson, & Challis, 1999; Sandman et al., 2006). Increased levels of CRH have been found in association with an increased risk of PTB. For example, in a prospective study of 232 pregnant women (Wadhwa et al., 2004), an association was found between elevated maternal plasma CRH levels at 33 weeks of gestation and a 3.3-fold increase in spontaneous PTB (adjusted relative risk = 3.3; 95% confidence interval [1.2, 9.4]).
A plausible explanation for the mechanisms of stress-related PTB is based on links between psychosocial stressors, the physiologic stress response, and the cascade of events that occur in the mother, uterus, placenta, and fetus before birth. In the case of stress-associated PTB, it has been proposed that the neuro-hormonal effectors of the maternal stress response, as well as the fetus, contribute to an early and excessive placental CRH production, ultimately triggering the final cascade of events that culminates in PTB (Challis, Matthews, Gibb, & Lye, 2000). The pathophysiologic mechanisms and pathways involved in the associations between chronic stress and PTB may involve complex interactions between the maternal stress response and the physiology of pregnancy and birth (Hillhouse & Grammatopoulos, 2002; Hobel et al., 2008; Latendresse, 2009; Norwitz et al., 1999; Wadhwa et al., 2004).
A conceptual framework for studying the relationship between stress and PTB includes concepts of acute and chronic stress. Stress is a physiologic response to psychological and physical demands and threats (stressors). Acute stress response is a short-lived activation of the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system (SNS), while a chronic stress condition occurs when the individual stress response is unable to resolve a threat or demand as a result of an ineffective response, an insurmountable challenge, or ongoing repetitive demands. Chronic stress, as opposed to acute stress (with the exception of acute catastrophic stress), has consistently been associated with disease and dysfunction (Forshee, Clayton, & McCance, 2010; McEwen, 2008), including poor pregnancy outcome (Behrman & Stith Butler, 2007; Hobel et al., 2008), likely due to the persistent exposure to cortisol, catecholamines, and other stress mediators. Indeed, the developing theories of allostatic load (the cumulative effects of a lifetime of exposure to stress-response mediators, i.e., ACTH, cortisol, catecholamines, inflammatory cytokines, etc.) implicate chronic stress, rather than acute stress, as the apparent contributor to poor health outcomes, particularly in health disparities research (Lu & Chen, 2004; McEwen, 2008; Shannon, King, & Kennedy, 2007). Based on this understanding of stress, we focused the current study on the impact of chronic stress during pregnancy.
Difficulty in measuring chronic stress due to the complexity and uniqueness of the experience is a major challenge in stress research (Cohen, Kessler, & Gordon, 1997). There is currently no single measure, either psychological or biological, that provides an adequate measure of chronic stress, leading many researchers in the field to include several measures in their investigations in an attempt to more comprehensively evaluate the phenomenon. Based on current literature, Hobel et al. (2008) suggest that an ideal comprehensive approach for evaluating stress during pregnancy would be to use a multidimensional tool to measure variables such as significant life events, mental health issues (e.g., anxiety and depression), racism/discrimination, daily hassles, social support, nutrition, smoking, perceived stress, and work demands, among others. Establishing stress as a composite concept with identifiably separate components has improved the ability of researchers to evaluate stress-related outcomes, including pregnancy outcomes (Lobel, 1994). Recognizing this multidimensional nature of chronic stress, we included a comprehensive collection of measurement tools in the current study.
The physiology of birth includes complex interactions among mother, placenta, and fetus, mediated by prostaglandins, estradiol, progesterone, cortisol (maternal and fetal), and dehydroepiandrosterone-sulfate (DHEA-S produced by the fetal adrenals), in the cascade of events that culminates in birth (Challis et al., 2000; Norwitz et al., 1999; Smith, Mesiano, & McGrath, 2002). Maternal CRH appears to play a prominent role within this cascade. During normal pregnancy, the CRH peptide is produced by the placenta beginning at approximately 14–20 weeks of pregnancy and incrementally increases throughout gestation (Reis, Fadalti, Florio, & Petraglia, 1999; Smith et al., 2002). CRH can be measured peripherally via maternal blood sampling, which is not the case in nonpregnant humans, where CRH is a noncirculating neuropeptide confined to the brain and central to the activation of the HPA stress response (Chrousos, 2000).
McLean et al. (1995) documented that the final trajectory of placental CRH production in PTBs frequently mirrors the final trajectory in term births. However, this CRH trajectory in many PTBs is a precocious one, with elevated CRH levels appearing many weeks earlier than for women with term births. Many other studies support the role of CRH in the occurrence of pre-term as well as term birth (Challis et al., 2000; Majzoub et al., 1999; McLean et al., 1995; Reis et al., 1999; Wadhwa et al., 2004). Due to the well-documented role of CRH in birth physiology, as well as the associations with PTB, in the current study, we focused on factors associated with this specific peptide.
A biobehavioral pathway linking chronic stress to CRH (and thus to PTB) has previously been proposed (Latendresse, 2009) and is based on several studies that document associations among maternal psychosocial stress, neuroendocrine markers (i.e., ACTH, cortisol, and placental CRH), and PTB (Erickson et al., 2001; Herrmann, Siega-Riz, Hobel, Arora, & Dunkel-Schetter, 2001; Hobel, Dunkel-Schetter, Roesch, Castro, & Arora, 1999; Holzman, Jetton, Siler-Khodr, Fisher, & Rip, 2001; Mancuso, Schetter, Rini, Roesch, & Hobel, 2004; Ruiz, Fullerton, Brown, & Dudley, 2002). Notably, in a case–control study, Hobel et al. (1999) found significant differences in measures of ACTH, cortisol, and CRH at all three trimesters of pregnancy between women who delivered term and pre-term. Furthermore, PTB was associated with higher levels of CRH and measures of stress (a combined perceived stress and anxiety score) at 18–20 weeks’ gestation. Indeed, maternal stress level at 18–20 weeks’ gestation accounted for a significant amount of variance in CRH later in pregnancy (28–30 weeks gestation). Mancuso et al. (2004) found that women with high CRH levels and high maternal prenatal anxiety at 28–30 weeks’ gestation delivered earlier than women with lower CRH levels and anxiety. Furthermore, they found that CRH levels at 28–30 weeks’ gestation mediated the relationship between anxiety and gestational age at delivery when controlling for confounding variables (medical risk, income, education, and parity).
Although psychosocial stress appears to contribute to the risk of PTB via CRH mediation (Mancuso et al., 2004), we could only find three studies that specifically examined psychosocial–behavioral variables as predictors of CRH levels during pregnancy. Two of those studies were limited to a narrow selection of predictor variables: fasting ≥13 hr (Herrmann et al., 2001) and a combined maternal perceived stress/state anxiety score (Hobel et al., 1999). In the third study, Kramer et al. (2009) investigated a large number of stress variables in relation to CRH and PTB, finding only that pregnancy-related anxiety was consistently associated with PTB.
As mentioned, CRH appears to play a central role in the timing of birth, and some biological stress mediators, such as ACTH and cortisol, appear to increase its production. However, there are a number of important limitations in previous studies, including (a) the limited use of stress measures and (b) a primary focus on psychological distress (i.e., anxiety and perceived stress). With the designs of these previous studies, investigators failed to acknowledge that chronic stress is a multidimensional phenomenon. In only one of the above-mentioned studies did the authors, Erickson et al. (2001), attempt to assess chronic stress in a comprehensive manner (i.e., as life events, work demands, self-reported psychosocial stressors, health behaviors, and risk taking); however, the report does not adequately identify and describe the instruments used or their reliability and validity.
With the current study, we extend previous research using a multidimensional collection of stress measures (see Figure 1) to evaluate associations with maternal CRH levels and by focusing on an earlier gestational time point (14–20 weeks’ gestation) rather than advanced gestational periods (i.e., 28–30 weeks) or the outcome of PTB, as other studies have. This approach may increase our knowledge of the biobehavioral environment at a time point (early pregnancy) that is potentially more sensitive to interventions aimed at modulating the maternal/fetal/placental neuroendocrine cascades that may lead to PTB.
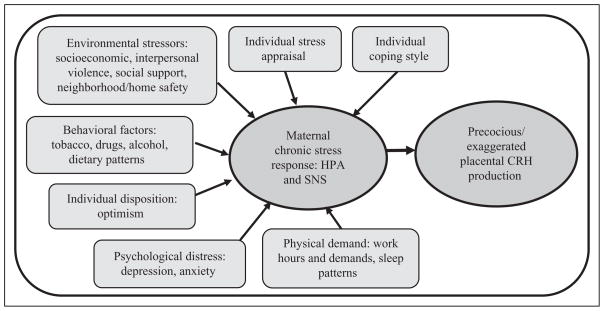
Figure 1.
Conceptual model: proposed pathway from chronic stress contributors to precocious/exaggerated placental CRH production. CRH = corticotropin-releasing hormone; HPA = hypothalamic-pituitary-adrenal axis; SNS = sympathetic nervous system.
The aims of this study, which was part of a larger study of CRH, psychosocial/behavioral factors, and the outcome of PTB, were to (a) describe the relationships between CRH levels at 14–20 weeks’ gestation and the following psychosocial and behavioral variables: psychological distress (depression and anxiety), individual disposition (optimism), stress appraisal (perceived stress), environmental stressors (neighborhood safety, perceived adequacy of income, and social support), physical demand (work hours, standing hours, and sleep patterns), behavioral factors (smoking, alcohol and substance use, and dietary patterns), and coping style (i.e., active, planning, reframing, acceptance, humor, religion/spiritual, support seeking, denial, distraction, venting, self blame, disengagement, and substance use) and (b) determine whether psychosocial and behavioral variables are predictive of high levels of CRH.
Method
Study Design
Data for this study were part of a larger study evaluating the relationships among CRH, psychosocial/behavioral factors, and the outcome of PTB. The study used a cross-sectional correlational design and was approved by the institutional review board of the University of Utah Health Sciences Center. Enrollment into the study began in March 2007 and continued through February 2008. Women were made aware of the study by clinic staff and prenatal care providers at the time of a pre-natal visit and were invited to hear more about the study at the conclusion of the visit. The researcher then met women who indicated interest in the study in the privacy of an exam room. Women were screened, consented, and enrolled into the study in accordance with the protection of human subjects research protocols.
Participants
Participants were recruited prior to 20 weeks of gestation from three community prenatal clinics operated by the University of Utah Health Sciences Center and included a predominately Euro-American population of women consistent with proportions in the wider community population. A target enrollment of 120 women was determined by a priori power analysis (Cohen’s Power and Precision software program) to provide at least 90% power to detect medium effect sizes and with an expected retention rate of approximately 85%.
Pregnant women aged 18 and older, who could speak and read English and who were at less than 20 weeks’ gestation were eligible for participation in the study. Gestation was determined by adequate dating criteria, which included a known date of last menstrual period confirmed by clinical dating prior to 13 weeks’ gestation and ultrasound examination prior to 20 weeks’ gestation. Women were excluded from participation in the study, if any of the following potential confounding variables were present at the time of screening: Previous or current maternal history of hypertension, diabetes, cardiovascular disease, or immune or autoimmune disorders; or any of the following during the current pregnancy: spontaneous abortion, nonviable pregnancy, multiple gestation, vaginal bleeding more than spotting (more than two episodes of 2 days or less in duration), or current use of systemic corticosteroid therapy.
A total of 120 women, aged 18 to 40 years (M = 25.8 years), were enrolled into the study; 100 of these women were retained in the study through collection of the second-trimester maternal blood sample, and 85 women completed study questionnaires. Women who did not complete and return questionnaires were more likely to be single (χ2 = 9.19, p = .01), Medicaid recipients or uninsured (χ2 = 10.89, p = .005), taking prescriptions for mood disorders (χ2 = 3.42, p = .064), and 18–24 years of age (χ2 = 5.34, p = .07). Of the initial group of women enrolled in the study, 20 were excluded from the study: 8 who had spontaneous abortion, 3 who declined to continue their participation, and 9 who transferred to other clinics and were thus lost to follow-up. Of the final study sample, five women had a history of PTB in a previous pregnancy and were included in the data analysis.
Table 1 provides the demographics for the participants who completed questionnaires. The data reflect a population of women of whom over half had an annual family income less than $40,000, approximately 62% had not completed more than high school education, and only one third owned their homes. Notably, half of the participants reported some type of physical, sexual, or emotional abuse at some time during their life but extremely low levels of alcohol, cigarette, and substance use (<5%).
Table 1.
Sample (N = 85) Demographics
| Demographic Variables | % |
|---|---|
| Ethnicity | |
| ”Hispanic” | 24 |
| “Non-Hispanic” | 76 |
| Racial | |
| “White” | 69 |
| Asian | 2.4 |
| American Indian/Alaskan Native | 2.4 |
| Pacific Islander | 4.8 |
| African/African American | 4.8 |
| Hispanic/Mexican American | 14.1 |
| “Multi” | 3.5 |
| Marital status | |
| Married | 64 |
| Living with partner | 13 |
| Single | 21 |
| Divorced/separated | 2.4 |
| Parity | |
| Multiparous | 64 |
| Nulliparous | 36 |
| Insurance coverage | |
| Medicaid | 32 |
| Private insurance | 55 |
| Self-pay/uninsured | 11 |
Measurements
Study questionnaire
Participants received a self-administered study questionnaire to complete at home between 14 and 20 weeks of gestation and return to the researcher by mail (postage paid). The entire questionnaire required approximately 45–60 min to complete.
Psychological distress
We assessed psychological distress with the Center for Epidemiologic Studies–Depression Scale (CES-D) and the Pregnancy-Specific Anxiety (PSA) Scale. The CES-D (Radloff, 1977) is a 20-item instrument designed to measure depression and has been shown to be a reliable and valid measure of depressed feelings and symptoms in a variety of populations, including pregnant women. Although the scale asks participants to indicate how they felt or behaved during the past week, scores are considered reflective of long-standing depressive symptoms, given that test–retest scores separated by 3–6 months are highly correlated. Participants respond on a 0–3 scale (0 = rarely or none of the time, or less than 1 day, and 3 = most or all of the time, or 5–7 days) to questions such as, “I felt that I could not shake off the blues even with help from my family or friends.” The possible range of scores is 0–60, with a score of 16 or more commonly indicating depression. The measure has demonstrated associations with perceived stress, PTB, and physiologic stress response (Christian, Franco, Glaser, & Iams, 2009; Gavin, Chae, Mustillo, & Kiefe, 2009; Yim et al., 2009) and had a correlation coefficient of .93 in the current study.
The PSA (Roesch, Schetter, Woo, & Hobel, 2004) scale was developed to measure levels of context- (pregnancy) specific anxiety and has been associated with length of gestation (Kramer et al., 2009; Mancuso et al., 2004). The reliability (α = .79 in the current study) and validity of the tool has been documented as acceptable. The tool consists of 4 items asking respondents to report how they have felt about being pregnant in the past week, specifically how anxious, concerned, afraid, or panicky they felt in the past week, on a scale of 1 (not at all) to 5 (very much).
Individual disposition
We assessed individual disposition with the Life Orientation Test–Revised (LOT-R; Scheier & Carver, 1985), a 10-item self-report questionnaire that measures a generalized expectancy of positive outcome and is a commonly used measure of optimism. Participants indicate their degree of agreement on a 5-point scale (I disagree a lot to I agree a lot) to questions such as “In uncertain times, I usually expect the best.” The measure has demonstrated divergent and convergent validity and adequate internal consistency (Cronbach’s alpha = .79 in the current study). Optimism has been associated with reduced psychological distress, PTB, and low-birth weight babies (Lobel, 1994).
Stress appraisal
We assessed stress appraisal using the Perceived Stress Scale (PSS; Cohen, Kamarck, & Mermelstein, 1983), a 10-item scale designed to measure the degree to which an individual appraises situations in her life as stressful. It has been validated as a measure of stress in pregnant populations (Glynn, Schetter, Hobel, & Sandman, 2008; Hobel et al., 1999; Ruiz, Fullerton, Brown, & Schoolfield, 2001). The instrument measures participants’ perceptions of the degree to which they feel their lives are unpredictable, uncontrollable, and overwhelming in a global sense over the past month. Participants respond on a 5-point scale, with 0 = never and 4 = almost always, to questions such as “How often have you felt difficulties were piling up so high that you could not overcome them?” The reliability coefficient (Cronbach’s alpha) was .91 using the current study’s data.
Coping style
We assessed coping style using the Brief COPE (Carver, 1997), a 28-item instrument used in a number of studies investigating coping responses. The instrument evaluates how much or how frequently a person engages in particular responses when coping with stress (i.e., “not at all” to “I do this a lot”) on a 1- to 4-point scale to questions such as “I turn to work or other activities to take my mind off things.” Scores are summed for 14, 2-item subscales, with scores of ≥5 identifying the coping style used, as follows (with corresponding Cronbach’s alphas in parentheses): active (.76), planning (.74), reframing (.69), accepting (.5), humor (.68), religion/ spiritual (.80), seeking emotional support (.82), seeking tangible support (.83), distraction (.52), denial (.79), venting (.73), substance use (.93), disengagement (.85), and self-blame (.71). Although some investigators have evaluated coping style in pregnancy (Harville, Savitz, Dole, Herring, & Thorp, 2009; Yali & Lobel, 2002), we could not find evidence for use of the Brief COPE during pregnancy. We selected the instrument due to a desire for instrument brevity.
Environmental stressors
We assessed environmental stressors using the Norbeck Social Support Questionnaire (NSSQ) and a demographic and behavioral questionnaire that included questions on interpersonal violence and abuse, perceived adequacy of income, and safety in the home and neighborhood environment. The NSSQ (Norbeck, Lindsey, & Carrieri, 1981) is a 9-item measure of the number and quality of social support structures. The quality of social support appears to be an important aspect in associations with the occurrence of PTB (Collins, Dunkel-Schetter, Lobel, & Scrimshaw, 1993; Yali & Lobel, 2002). The NSSQ has demonstrated construct and concurrent validity and has been used in diverse populations including pregnant women (Norbeck, Lindsey, & Carrieri, 1983). It asks participants to list members of their personal network and their relationship and to answer questions pertaining to each, such as “How much does this person make you feel liked or loved?” The measure also asks participants to list relationship losses during the past year, including how many, who, and how important the relationships were to the individual. Scoring of the instrument results in a total “functional” social support score comprised of two subscales measuring the constructs of emotional (i.e., how much a person feels loved and valued by those around them) and tangible (i.e., economic assistance) support. Cronbach’s alphas were .91 and .94 for the emotional and tangible support subscales, respectively.
We constructed the DBQ specifically for the current study to collect information on annual income, perceived income adequacy (on a 1–5 scale, never to always, in answer to, “Do you feel that your income is enough to pay for everything you need—food, housing, clothing, power and heat, transportation, child care, etc.?), housing/living arrangements (own/rent and number of people living in the home), education level, interpersonal violence and abuse (10-item measure indicating never, anytime in the past” or during the past year, on questions regarding emotional, physical, or sexual abuse), age, marital status, race/ethnicity, and home/neighborhood safety (“How safe do you feel in your current living situation?” and “How safe do you feel in your current neighborhood?” on a 1–5 scale, very unsafe to very safe). The DBQ also assesses physical demands with questions related to sleep patterns (2 items addressing perceived quality of sleep on a 1–5 scale and total number of hours per night), hours worked per week (for pay and not for pay), and hours of standing per day. Behavioral factors are also included in the DBQ, that is, smoking, alcohol and substance use, and dietary patterns (a 24-item yes/no checklist, indicating frequency and types of food intake during the past month, such as, “I eat at least three vegetables every day,” “I eat proteins—beans, meat, eggs, tofu—every day,” and, “I never eat breakfast.”). We selected the behavioral, psychosocial, and socioeconomic variables for the DBQ based on previous documented or suspected associations with an increased risk of PTB (Behrman & Stith Butler, 2007; Curry, Burton, & Fields, 1998; Dole et al., 2003; Wadhwa et al., 2004).
CRH
The measure of CRH was selected based on the documented associations with a higher incidence of PTB and/or chronic psychological and physiological stress during pregnancy (Hobel et al., 2008; Makrigiannakis et al., 2007; McLean et al., 1995; Rich-Edwards & Grizzard, 2005; Sandman et al., 2006; Steer, 2005; Wadhwa et al., 2004). We collected maternal blood samples for the measurement of placental CRH at 14–20 weeks’ gestation, the earliest possible gestational age at which CRH can be detected. The rationale for measuring CRH early in gestation was to identify any associations early enough in pregnancy to inform the design of future intervention studies. Such interventions may only be effective if initiated earlier, rather than later, in pregnancy, as investigators have suggested that the maternal neuroendocrine milieu may be altered early in pregnancy in association with maternal stress (Glynn, Wadhwa, Dunkel-Schetter, Chicz-Demet, & Sandman, 2001).
Following a previously developed laboratory protocol (Latendresse & Ruiz, 2008), we collected maternal samples by venipuncture at the same time as sample collection for routine maternal alphfetoprotein (AFP) screening. We collected samples into chilled 6-ml lavender top vacuum tubes containing ethylenediaminetetraacetic acid (EDTA) and aprotonin (500 KIU/ml blood), centrifuged them at 4°C and 3,000 rpm for 15 min, placed plasma aliquots into 0.5 ml cryotubes, and stored them at −80°C (±10°C) until time of assay (within 6 months).
We measured CRH with a specific and sensitive radioimmunoassay (RIA) kit (Phoenix Pharmaceuticals, Burlingame, CA). The CRH RIA is 100% specific for human CRH with 0% cross-reactivity to ACTH, luteinizing hormone, vasopressin, and urocortin. The RIA has a sensitivity of 0.00095 ng/ ml to 0.02 ng/ml and intra/inter assay coefficients of variability of 2–6/8.5–10%. Because CRH-binding protein interferes with the measurement of CRH in plasma (Linton et al., 1995), we extracted samples by the addition of 3.5 ml of ice-cold methanol to 0.5 ml of sample. We separated the precipitate by centrifugation and dried the methanol-extracted CRH. We suspended the residue in assay buffer prior to RIA. The mean recovery of exogenous CRH was 90% or greater. We assayed the samples in the Biobehavioral Laboratory, School of Nursing, at the University of Texas Medical Branch—Galveston.
Medical Records Review
We reviewed participants’ prenatal records to verify gestational age of 14–20 weeks.
Statistical Analysis
We primarily used nonparametric statistical analyses, given the bimodal distribution of CRH (approximately 82% had CRH levels below 15 pcg/ml, including 23 with nondetectable levels; 16 women had CRH levels at or above 15 pcg/ml, 14 of whom had levels between 162 and 280 pcg/ml). We entered CRH results below the detection limits of the assay as 0 in the statistical analyses. To rule out the possibility that the lowest and nondetectable CRH levels might be attributable to those women at the lower gestational ages within the 14- to 20-week blood sample collection window, we conducted an independent samples t test. The results indicated no difference in mean gestational age between women with CRH levels above and below 15 pcg/ml (17.20 weeks vs.17.35 weeks, respectively; t = 0.352; df = 98; p = .725).
We used chi-square tests for evaluating associations between nominal-level data and CRH levels (low vs. high). Nominal-level data included marital status, racial background, ethnic membership, housing type, cigarette, alcohol, and drug use, interpersonal violence/abuse, parity, insurance coverage, coping styles, and several continuous-level variables that were collapsed (to facilitate interpretation of the results and/or provide adequate cell sizes for statistical analysis). Collapsed variables included income, sense of safety in the home/ neighborhood (safe vs. not safe), interpersonal/intimate violence/abuse (never, anytime in the past, and during the past year), optimism level (low, medium, and high), depression level (low, medium, and high), social support (low, medium, and high), obstetrical history (0, 1–2, and 3–4 complications or adverse events), medical risk (0, 1–2, and 3–4 risk factors or medical conditions unrelated to pregnancy), current pregnancy complications (0, 1–2, 3, or more), nutritional level (good, fair, and poor), age (18–24 years, 25–33 years, and 34–40 years), and 14 coping styles (active, planning, reframing, acceptance, humor, distraction, denial, venting, substance use, disengagement, self-blame religious/spiritual, emotional support seeking, and tangible support seeking) that were dichotomized (no = 1–4; yes = 5–8).
We also computed Kendall’s rank-order correlation coefficients for independent variables with continuous-level data (collapsed and/or ordinal), given that the dependent variable had a bimodal distribution and thus was dichotomized (CRH—low vs. high). These continuous-level variables included perceived adequacy of income to cover needs, hours of standing per day, quality of sleep, hours of sleep/night, education level, paid work hours/day, perceived stress, and pregnancy-specific anxiety. We used a liberal alpha of .1 for statistical significance for all tests in preparation for entry into logistic regression analysis, given the exploratory nature of the analysis and made no adjustments for number of tests being conducted.
Results
We found that two individual coping styles were associated with higher CRH levels. Women who use religious/spiritual practices (i.e., seek comfort in religion and/or spiritual beliefs, prayer, or meditation) or disengage (i.e., give up attempts to cope or to deal with stress) during stressful times were more likely to have high CRH levels than those who did not use these coping strategies, χ2(1, n = 84) = 7.19, p = .007, and χ2(1, n = 84) = 5.49, p = .019, respectively. Women who used humor (i.e., made fun of the situation or made jokes) were more likely to have lower levels of CRH than those who did not, χ2(1, n = 84) = 5.39, p = .02.
We found a negative correlation between perception of income adequacy and CRH level. The less women perceived their income as adequate to meet their needs, the higher their CRH levels were, τb (n = 85) = −.262, p = .008. We found positive correlations between CRH level and hours of standing per day (< 6 hr vs. ≥ 6 hr) and hours of sleep/night, τb (n = 84) =.230, p = .035 and τb (n = 85) = .203, p = .035.
We found weak associations between reported use of alcohol (collapsed into two categories: “no” vs. “yes” or “quit when I became pregnant”) and higher CRH levels (χ2(1, n = 85) = 3.37, p = .066) and between perceived stress and higher levels of CRH (χ2(2, n = 85) = 4.22, p = .12), though these relationships did not meet a p < .05 significance criterion. A comparison between women with low and high CRH levels appears in Table 2.
Table 2.
Comparison of Women With Low- and High-Corticotropin-Releasing Hormone (CRH) Levels at 14–20 Weeks’ Gestational Age
| Variable | Low CRH (<15 pcg/ml)
|
High CRH (≥ 15 pcg/ml)
|
p Value | ||||
|---|---|---|---|---|---|---|---|
| M (SD) | Median | Range | M (SD) | Median | Range | ||
| CES-D | 18.1 (11.5) | 17.0 | 0–49 | 21 (15.3) | 20.0 | 2–49 | .29 |
| PSA | 10.4 (4.3) | 11 | 2–20 | 9.9 (3.3) | 10 | 4–16 | .35 |
| LOT-R | 17.7 (5.3) | 18 | 4–27 | 17.2 (5.0) | 17.5 | 3–25 | .15 |
| PSS | 18.2 (7.3) | 18 | 2–31 | 21.1 (9.8) | 22 | 5–34 | .12 |
| Neighborhood & home safety | 9.2 (1.2) | 10 | 6–10 | 8.8 (1.9) | 9 | 4–10 | .49 |
| Perceived adequacy of income | 3.6 (1.2) | 4 | 1–5 | 2.6 (1.4) | 2 | 1–5 | .008 |
| Social support sum functional | 197.1 (103.1) | 174 | 13–470 | 176.4 (91.1) | 169 | 89–337 | .67 |
| Emotional subscale | 136.8 (71.5) | 132 | 4–316 | 121.9 (62.7) | 112 | 62–236 | .36 |
| Tangible subscale | 60.3 (34.8) | 54 | 6–154 | 58.1 (27.2) | 54 | 20–101 | .94 |
| Work hr/week | .85 | ||||||
| For pay | 23.6 (21.9) | 32 | 0–77.5 | 25.9 (17.4) | 33 | 0–45 | |
| Not for pay | 25.7 (27.6) | 17.5 | 0–100 | 21.9 (26.3) | 10 | 0–70 | |
| Standing hr/day | 3.5 (2.9) | 2.5 | 0–12 | 5 (4.8) | 3.5 | 0–15 | .035 |
| Sleep quality | 3.4 (.84) | 3 | 2–5 | 3.19 (.91) | 3 | 1–5 | .52 |
| Sleep hr/night | 6.8 (1.7) | 7 | 1–12 | 7.5 (1.3) | 8.0 | 4–9 | .035 |
| Nutritional score | 16.8 (6.0) | 18 | 2–26 | 16.1 (7.1) | 17 | 0–24 | .38 |
| Coping style | |||||||
| Active | 6.0 (1.6) | 6 | 2–8 | 5.9 (1.3) | 6 | 3–8 | .302 |
| Planning | 5.8 (1.7) | 6 | 2–8 | 5.6 (1.3) | 6 | 3–8 | .851 |
| Reframing | 5.4 (1.5) | 5 | 2–8 | 5.4 (1.6) | 5.5 | 2–8 | .872 |
| Acceptance | 5.7 (1.6) | 6 | 2–8 | 6.0 (1.0) | 6 | 5–8 | .707 |
| Humor | 4.4 (1.7) | 4 | 2–8 | 3.6 (1.2) | 4 | 2–5 | .020 |
| Religion | 4.6 (2.2) | 4 | 2–8 | 6.1 (1.7) | 6 | 2–8 | .007 |
| Seek emotional support | 6.0 (1.9) | 6 | 2–8 | 5.6 (1.6) | 6 | 2–8 | .767 |
| Seek tangible support | 5.7 (1.7) | 6 | 2–8 | 5.6 (1.8) | 6 | 2–8 | .894 |
| Self distraction | 5.3 (1.6) | 5 | 2–8 | 5.7 (1.8) | 6 | 2–8 | .200 |
| Denial | 3.0 (1.4) | 2 | 2–7 | 2.9 (1.7) | 2 | 2–8 | .977 |
| Venting | 4.8 (1.8) | 5 | 2–8 | 5.7 (1.5) | 6 | 3–8 | .153 |
| Substance use | 2.2 (.55) | 2 | 2–5 | 2.3 (1.3) | 2 | 2–7 | * |
| Disengagement | 3.3 (1.4) | 3 | 2–8 | 4.1 (2.1) | 4 | 2–8 | .019 |
| Self blame | 4.9 (1.7) | 5 | 2–8 | 4.8 (1.8) | 4.5 | 2–8 | .977 |
Note. CES-D = Center for Epidemiologic Studies–Depression Scale; LOT-R = Life Orientation Test–Revised; PSA = Pregnancy-Specific Anxiety Scale; PSS = Perceived Stress Scale.
There were too few cases for statistical calculation (only one respondent had a coping score indicating use of this coping style).
We computed logistic regression using the forward stepwise likelihood ratios method to assess prediction of membership in the two outcome categories (low CRH or high CRH level). The following eight predictors were entered into the model: perceived adequacy of income, hours of sleep/night, hours of standing/day, the three coping styles of humor, disengagement, and religious/spiritual, perceived stress, and alcohol use. Eighty-four cases were available for the analysis (one participant did not fully complete the questionnaire and thus was not included).
Three predictor variables remained in the final logistic regression model and indicated that women who perceived that their income did not meet their needs were 2.7 times more likely to have high CRH levels than women who perceived that their income did meet their needs. In addition, women who used religious/spiritual or disengagement coping style in response to stress were more likely (nearly 15 times and 7 times, respectively) to have high CRH levels than those who did not. Based on Nagelkerke R2 of .417, the logistic regression results indicate that the predictor variables account for nearly 42% of the variance in membership in low and high CRH groups. The final model did not include hours of sleep/night, hours of standing/day, the use of humor as a coping style, perceived stress, or alcohol use. Table 3 provides the results of the logistic regression.
Table 3.
Final Model Parameter Estimates for Logistic Regression: Outcome Variable of Low Versus High Levels of Corticotropin-Releasing Hormone (CRH)
| Predictorsa | B | SE | WALDb | p Value | Exp(B) | 95% Confidence Interval for Exp(B)
|
|
|---|---|---|---|---|---|---|---|
| Upper Bound | Lower Bound | ||||||
| Perceived inadequacy of income | 0.999 | 0.323 | 9.583 | .002 | 2.717 | 1.443 | 5.115 |
| Disengagement coping | 1.946 | 0.937 | 4.314 | .038 | 7.002 | 1.116 | 43.942 |
| Religious/spiritual coping | 2.705 | 0.842 | 10.330 | .001 | 14.961 | 2.874 | 77.888 |
| Constant | 35.059 | 1.134 | 19.904 | .000 | 0.006 | – | – |
Note. B = beta coefficient; Exp(B) = the odds of having CRH levels in the fourth quartile; SE = standard error; WALD = Logistic Regression Method.
Variables left out of final model: perceived stress, alcohol use, hours of sleep/night, hours of standing/day.
One degree of freedom.
We computed the Hosmer and Lemeshow test criterion, which indicated a good fit between the final model and the actual data, χ2(3, n = 84) = 5.142, p = .071. The overall correct classification rate was not particularly impressive at 88.1%, with rates of 97.1% and 50.0% for low CRH and high CRH, respectively. This computation reflects an overclassification of women into the low CRH group and an underclassification of women into the high CRH group.
We evaluated multicolinearity among the predictor variables and found the highest correlation to be between perceived adequacy of income and perceived stress (r = −.526).
Discussion
Stress has been a notoriously difficult concept to measure, likely due to the subjective nature of the experience, individual stress appraisal, genetic variation, and the enormously wide variety and intensity of stressors that individuals encounter over a lifetime. This difficulty is reflected in the diverse and often conflicting results of studies that have attempted to evaluate associations between stress and physiological events and/or health outcomes. This situation is further complicated by the fact that various investigators tend to use their own collections of measures, which are frequently quite different from one another. The current study is no exception.
We found that perceived adequacy of income and the use of disengagement and religious/spiritual coping were significant predictors of CRH level at 14–20 weeks’ gestation. Perceived adequacy of income was a predictor in spite of the fact that there was no association found between the level of income reported and CRH. This finding is consistent with reports that socioeconomic status, rather than income alone, has more influence on health outcomes, including pregnancy outcomes (Chen, Martin, & Mathews, 2007; Poetz, Eyles, Elliott, Wilson, & Keller-Olaman, 2007; Sherbourne, Hays, & Wells, 1995). Ours is the first study to document the association between perceived adequacy of income and CRH levels during pregnancy.
Our results are also the first to implicate coping style as a predictor of CRH, but the coping measure (Brief COPE) has not previously been used in a pregnant population in relation to physiologic effects. Two specific styles of coping (disengagement and religious/spiritual approach) were found to be predictive of high CRH levels in our study. Disengagement is generally considered an ineffective coping approach, and researchers have documented associations with negative health outcomes in nonpregnant populations (Clements & Sawhney, 2000; Griffing et al., 2006).
In relation to religious/spiritual coping, there are no studies that investigate the interactions between psychosocial–behavioral factors and specific religious practices, approaches, and philosophies or the effects on the neuroendocrine system in pregnant women. With no research in this area, it is difficult to begin to compare studies or to explain the link between CRH levels and religious/spiritual coping style. Theoretically, this link would require involvement of a woman’s stress response and is consistent with the premise that some types of religion-related activities actually increase the levels of psychological distress (Hackney & Sanders, 2003).
The association of coping style with CRH levels may indicate that it is how women process the demands presented to them, as opposed to the demands themselves, which influences physiological stress response. There is a paucity of research in the area of coping style and its impact on pregnancy and pregnancy outcome, thus much further research is needed.
We did not find a significant association between maternal anxiety and CRH levels, as some previous studies have (Hobel et al., 1999; Mancuso et al., 2004). Similar to our study, Kramer et al. (2009) found no association between anxiety and CRH. They did, however, find associations between anxiety and PTB and between CRH and PTB.
The CRH values found in the current study sample are difficult to compare to the values found in previous studies due to the wide variation in sample collection, processing, and storage methodology and in the bioassays selected among the different studies. However, the average and median CRH levels for the current study sample (33.6 pcg/ml and 8.16 pcg/ml), as well as the range (0–280 pcg/ml), are comparable to the CRH results from several other studies (Herrmann et al., 2001; Hobel et al., 1999; Holzman et al., 2001; Sandman et al., 2006). It should also be mentioned that only one of the five women in this study with a history of a prior PTB had a CRH level higher than 15 pcg/ml (actual level = 15.7 at 17.8 weeks’ gestation), placing that individual into the “high” CRH category.
Perceived stress had a weak association with CRH levels in our study, but the variable did not remain as a predictor in logistic regression. An explanation for discordance with other studies is likely due to the early gestational age at which we collected maternal samples (14–20 weeks) for our cross-sectional study. Most studies report stronger and more reliable association between CRH, psychosocial–behavioral variables, and PTB when maternal samples are collected later in pregnancy, that is, at 28–30 weeks of gestation, and within longitudinal studies (Herrmann et al., 2001; Hobel et al., 1999; Kramer et al., 2009; Mancuso et al., 2004). Moreover, CRH levels are commonly below detectable limits prior to 16 weeks of pregnancy (Smith et al., 2002); thus; most studies evaluate CRH levels no earlier than 18–20 weeks of gestation and evaluate changes that occur in CRH levels between the second and third trimesters of pregnancy.
Although CRH appears to play an important role in normal placental development early in pregnancy (Choy, Leung, & Lau, 2004; Kalantaridou, Zoumakis, Makrigiannakis, Godoy, & Chrousos, 2007) and for quiescence of the myometrium during much of the gestational period, CRH also has an instrumental role in labor physiology (Hillhouse & Grammatopoulos, 2002). CRH levels that are either too low or too high may result in adverse pregnancy outcomes, but there are no agreed upon “normal,” acceptable, or cutoff values for CRH levels during pregnancy. Attempts to develop CRH as a screening tool for PTB risk have been met with low sensitivity and low specificity as well as low positive predictive value of the measure (Bisits et al., 1998; Goldenberg, Goepfert, & Ramsey, 2005; Inder et al., 2001). In spite of the early gestational age at which we measured CRH levels in our study, the results still add support to the premise that some psychosocial contributors to stress affect the physiology of pregnancy.
Our findings may be consistent with “life course” and allostatic load concepts, which implicate repetitive and cumulative “wear and tear” as a major contributor to the negative health and pregnancy outcomes occurring for those with perceived inadequacy of income and ineffective coping skills (Johner, 2007; Shannon et al., 2007). For example, in the absence of adequate resources and effective coping skills, a lifetime of problems and challenges may go unresolved, perhaps leading to cumulative stresses or allostatic load (McEwen, 2004). Moreover, allostatic load could also explain the disparities in pregnancy outcomes being reported in African American populations, given the cumulative effects of a lifetime (and transgenerational) exposure to stress mediators (Lu & Chen, 2004).
Limitations
This study had a small sample size (n = 100) and measured CRH quite early in pregnancy, which may explain the lack of significant associations between most of the psychosocial measures and CRH. Moreover, the study included predominantly White women (70%), with the remaining 30% of the sample comprising almost exclusively Latina women. Therefore, the results are not likely applicable to pregnant women of other ethnic populations (e.g., African Americans or Asians) or to women outside the unique Utah population (predominantly of the Mormon religion). Participants took the study questionnaire home for completion; thus, there is no guarantee that the home environment (including other persons) did not influence participant responses. Remaining at the clinic for 45–60 min to complete a lengthy questionnaire, however, would likely have been inconvenient and unacceptable to participants. The study also had a cross-sectional design and was underpowered to evaluate possible associations with the pregnancy outcome of ultimate interest (i.e., PTB). A larger sample size with which it would be possible to evaluate the associations with pregnancy outcomes would be most informative, but this study adds important information to a very small body of knowledge related to bio-psycho-social associations during pregnancy.
Conclusions
The results of this study confirm that there are associations between at least some psychosocial–behavioral variables and CRH levels as early as 14–20 weeks’ gestation and that some of these variables have value in predicting which women will have low CRH levels and which women will have high CRH levels. Furthermore, there are differences in psychosocial–behavioral variables between pregnant women with low and high CRH as early as 14–20 weeks’ gestation.
As previously noted in Figure 1, there are many psychosocial–behavioral factors that may contribute to chronic stress response and subsequent alterations in placental CRH production. In this study, the specific factors that appear to make at least a small contribution to altered production of CRH, possibly via maternal/fetal neurohormonal stress response, are related to maternal coping style and perceived lack of adequate income.
Using biobehavioral approaches to investigate pregnancy complications, such as PTB, and possible neuroendocrine contributors may help researchers to better understand the connections between mind and body and to eventually develop interventions and changes in health policy that will finally begin to reduce the occurrence of PTB. The Scientific Advisory Committee (SAC) on Prematurity (advisors to the National March of Dimes Campaign) specifically calls for investigations that target etiologic mechanisms of PTB, such as psychosocial contributors and identification of biomarkers (Green et al., 2005).
The aims of the study were not to predict PTB but to evaluate the associations between psychosocial/behavioral variables and CRH levels at an early gestational age (14–20 weeks’ gestation). Because precocious and elevated maternal CRH levels have been associated with PTB, it may be important to increase our understanding of the psychosocial and behavioral factors that may be contributing to neuroendocrine changes in pregnancy. Moreover, the ability to detect associations between CRH and psychosocial factors as early as possible has the potential to identify a window of opportunity for conducting risk assessment and introducing interventions. Theoretically, such interventions are too late, if introduced later in pregnancy when the physiological parturition “cascade” has advanced beyond the possibility of successful intervention.
For example, could inclusion of coping style identification during first-trimester (or even preconception) risk assessment, followed by effective coping skills training make a positive impact on maternal stress response, thus PTB risk? Likewise, would evaluation of perceived income adequacy and subsequent provision of needed resources (tailored to the individual) early in pregnancy (or even prior to reproductive age) ameliorate adverse maternal stress responses (behavioral and physiological)? The approach of introducing interventions later in pregnancy has thus far resulted in failure to reduce the PTB rate (Behrman & Stith Butler, 2007), which should call into question the appropriate timing of psychosocial risk assessment and intervention.
Understanding the impact of psychosocial contributors to the maternal–fetal–placental neuroendocrine systems (e.g., to precocious and exaggerated CRH production) during pregnancy could also have important implications for the fetus, the newborn, and even for adult health and disease. For example, aside from the often profound immediate and long-term effects of being born prematurely (Behrman & Stith Butler, 2007), evidence is mounting that some adult conditions (e.g., type II diabetes, hypertension, mental health issues, and cardiovascular disease) may have fetal origins associated with prenatal exposure to maternal stress hormones (Kinsella & Monk, 2009; Mastorci et al., 2009; Schlotz & Phillips, 2009; Weinstock, 2008). Could simple coping skills assessment and subsequent training and/or stress management prior to reproductive age have any impact on adult health later in life or on the health of offspring? Could identification of income insufficiency followed by provision of appropriate resources (economic, housing, food, health care, transportation, etc.) for women and families enhance the adult health of their offspring?
These important questions demand further investigation. The answers might move us along the path to achieving the long-term goals of reducing the risk of poor pregnancy outcome as well as poor health outcomes for both the infants and the adults they will become who are exposed to excess maternal stress hormones.
Acknowledgments
The authors wish to thank the kind and informative support of Dr. Chander Arora and Dr. Calvin Hobel of Cedars Sinai Medical Center.
Funding
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: The National Institute of Health, National Institute of Nursing Research (1 F31 NR010046-01), the March of Dimes Foundation, and the American College of Nurse-Midwives Foundation.
Footnotes
Reprints and permission: sagepub.com/journalsPermissions.nav
Declaration of Conflicting Interests
The author(s) declared no conflicts of interest with respect to the authorship and/or publication of this article.
References
- Behrman RE, Stith Butler A, editors. Preterm birth: Causes, consequences, and prevention. Washington, DC: National Academies Press; 2007. [PubMed] [Google Scholar]
- Bisits A, Madsen G, McLean M, O’Callaghan S, Smith R, Giles W. Corticotropin-releasing hormone: A biochemical predictor of preterm delivery in a pilot randomized trial of the treatment of preterm labor. American Journal of Obstetrics & Gynecology. 1998;178:862–866. doi: 10.1016/s0002-9378(98)60503-2. [DOI] [PubMed] [Google Scholar]
- Carver CS. You want to measure coping but your protocol’s too long: Consider the Brief COPE. International Journal of Behavioral Medicine. 1997;4:92–100. doi: 10.1207/s15327558ijbm0401_6. [DOI] [PubMed] [Google Scholar]
- Challis JR, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocrinology Reviews. 2000;21:514–550. doi: 10.1210/edrv.21.5.0407. [DOI] [PubMed] [Google Scholar]
- Chen E, Martin A, Mathews K. Trajectories of socioeconomic status across children’s lifetime predict health. Pediatrics. 2007;120:e297–e303. doi: 10.1542/peds.2006-3098. [DOI] [PubMed] [Google Scholar]
- Choy MY, Leung TN, Lau TK. Corticotropin-releasing hormone peptide and human first-trimester placental growth. Early Human Development. 2004;79:77–80. doi: 10.1016/j.earlhumdev.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Christian LM, Franco A, Glaser R, Iams JD. Depressive symptoms are associated with elevated serum proinflammatory cytokines among pregnant women. Brain Behavior & Immunity. 2009;23:750–754. doi: 10.1016/j.bbi.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP. The HPA axis and the stress response. Endocrinology Research. 2000;26:513–514. doi: 10.3109/07435800009048562. [DOI] [PubMed] [Google Scholar]
- Clements CM, Sawhney DK. Coping with domestic violence: Control attributions, dysphoria, and hopelessness. Journal of Traumatic Stress. 2000;13:219–240. doi: 10.1023/A:1007702626960. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health & Social Behavior. 1983;24:385. [PubMed] [Google Scholar]
- Cohen S, Kessler RC, Gordon LU. Measuring stress: A guide for health and social scientists. New York, NY: Oxford University Press; 1997. [Google Scholar]
- Collins NL, Dunkel-Schetter C, Lobel M, Scrimshaw SC. Social support in pregnancy: Psychosocial correlates of birth outcomes and postpartum depression. Journal of Personal Sociological Psychology. 1993;65:1243–1258. doi: 10.1037//0022-3514.65.6.1243. [DOI] [PubMed] [Google Scholar]
- Curry MA, Burton D, Fields J. The Prenatal Psychosocial Profile: A research and clinical tool. Research in Nursing & Health. 1998;21:211–219. doi: 10.1002/(sici)1098-240x(199806)21:3<211::aid-nur4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Dole N, Savitz DA, Hertz-Picciotto I, Siega-Riz AM, McMahon MJ, Buekens P. Maternal stress and pre-term birth. American Journal of Epidemiology. 2003;157:14–24. doi: 10.1093/aje/kwf176. [DOI] [PubMed] [Google Scholar]
- Erickson K, Thorsen P, Chrousos G, Grigoriadis DE, Khongsaly O, McGregor J, Schulkin J. Preterm birth: Associated neuroendocrine, medical, and behavioral risk factors. Journal of Clinical & Endocrinological Metabolism. 2001;86:2544–2552. doi: 10.1210/jcem.86.6.7607. [DOI] [PubMed] [Google Scholar]
- Forshee BA, Clayton MF, McCance KL. Stress and disease. In: McCance KL, Huether SE, Brashers VL, Rote NS, editors. Pathophysiology: Biologic basis for disease in adults and children. 6. Maryland Heights, MO: Mosby Elsevier; 2010. [Google Scholar]
- Gavin AR, Chae DH, Mustillo S, Kiefe CI. Prepregnancy depressive mood and preterm birth in Black and White women: Findings from the CARDIA Study. Journal of Women’s Health. 2009;18:803–811. doi: 10.1089/jwh.2008.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn LM, Schetter CD, Hobel C, Sandman CA. Pattern of perceived stress and anxiety in pregnancy predicts pre-term birth. Health Psychology. 2008;27:43–51. doi: 10.1037/0278-6133.27.1.43. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Wadhwa PD, Dunkel-Schetter C, Chicz-Demet A, Sandman CA. When stress happens matters: Effects of earthquake timing on stress responsivity in pregnancy. American Journal of Obstetrics & Gynecology. 2001;184:637–642. doi: 10.1067/mob.2001.111066. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg RL, Goepfert AR, Ramsey PS. Biochemical markers for the prediction of preterm birth. American Journal of Obstetrics & Gynecology. 2005;192:S36–S46. doi: 10.1016/j.ajog.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Green NS, Damus K, Simpson JL, Iams J, Reece EA, Hobel C, Schwarz RH. Research agenda for pre-term birth: Recommendations from the March of Dimes. American Journal of Obstetrics & Gynecology. 2005;193:626–635. doi: 10.1016/j.ajog.2005.02.106. [DOI] [PubMed] [Google Scholar]
- Griffing S, Lewis C, Chu M, Sage R, Jospitre T, Madry L, Primm BJ. The process of coping with domestic violence in adult survivors of childhood sexual abuse. Journal of Child Sexual Abuse. 2006;15:23–41. doi: 10.1300/J070v15n02_02. [DOI] [PubMed] [Google Scholar]
- Hackney CH, Sanders GS. Religiosity and mental health: A meta-analysis of recent studies. Journal for the Scientific Studies of Religion. 2003;42:43–55. [Google Scholar]
- Harville EW, Savitz DA, Dole N, Herring AH, Thorp JM. Stress questionnaires and stress biomarkers during pregnancy. Journal of Women’s Health. 2009;18:1425–1433. doi: 10.1089/jwh.2008.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann TS, Siega-Riz AM, Hobel CJ, Arora C, Dunkel-Schetter C. Prolonged periods without food intake during pregnancy increase risk for elevated maternal corticotropin-releasing hormone concentrations. American Journal of Obstetrics & Gynecology. 2001;185:403–412. doi: 10.1067/mob.2001.115863. [DOI] [PubMed] [Google Scholar]
- Hillhouse EW, Grammatopoulos DK. Role of stress peptides during human pregnancy and labour. Reproduction. 2002;124:323–329. doi: 10.1530/rep.0.1240323. [DOI] [PubMed] [Google Scholar]
- Hobel CJ, Dunkel-Schetter C, Roesch S, Castro L, Arora C. Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks’ gestation in pregnancies ending in preterm delivery. American Journal of Obstetrics & Gynecology. 1999;180:S257–S263. doi: 10.1016/s0002-9378(99)70712-x. [DOI] [PubMed] [Google Scholar]
- Hobel CJ, Goldstein A, Barrett E. Psychosocial stress and pregnancy outcome. Clinical Obstetrics & Gynecology. 2008;51:333–348. doi: 10.1097/GRF.0b013e31816f2709. [DOI] [PubMed] [Google Scholar]
- Holzman C, Jetton J, Siler-Khodr T, Fisher R, Rip T. Second trimester corticotropin-releasing hormone levels in relation to preterm delivery and ethnicity. Obstetrics & Gynecology. 2001;97:657–663. doi: 10.1016/s0029-7844(00)01209-6. [DOI] [PubMed] [Google Scholar]
- Inder WJ, Prickett TC, Ellis MJ, Hull L, Reid R, Benny PS, Donald RA. The utility of plasma CRH as a predictor of preterm delivery. Journal of Clinical Endocrinology and Metabolism. 2001;86:5706–5710. doi: 10.1210/jcem.86.12.8080. [DOI] [PubMed] [Google Scholar]
- Johner RL. Allostatic load: Single parents, stress-related health issues, and social care. Health and Social Work. 2007;32:89–94. doi: 10.1093/hsw/32.2.89. [DOI] [PubMed] [Google Scholar]
- Kalantaridou SN, Zoumakis E, Makrigiannakis A, Godoy H, Chrousos GP. The role of corticotropin-releasing hormone in blastocyst implantation and early fetal immunotolerance. Hormone and Metabolic Research. 2007;39:474–477. doi: 10.1055/s-2007-980190. [DOI] [PubMed] [Google Scholar]
- Kinsella MT, Monk C. Impact of maternal stress, depression and anxiety on fetal neurobehavioral development. Clinical Obstetrics & Gynecology. 2009;52:425–440. doi: 10.1097/GRF.0b013e3181b52df1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MS, Lydon J, Séguin L, Goulet L, Kahn SR, McNamara H, Platt RW. Stress pathways to spontaneous preterm birth: The role of stressors, psychological distress, and stress hormones. American Journal of Epidemiology. 2009;169:1319–1326. doi: 10.1093/aje/kwp061. [DOI] [PubMed] [Google Scholar]
- Latendresse G. The interaction between chronic stress and pregnancy: Preterm birth from a biobehavioral perspective. Journal of Midwifery & Women’s Health. 2009;54:8–17. doi: 10.1016/j.jmwh.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latendresse G, Ruiz RJ. Bioassay research methodology: Measuring CRH in pregnancy. Biological Research for Nursing. 2008;10:54–62. doi: 10.1177/1099800408320970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton EA, Perkins AV, Hagan P, Poole S, Bristow AF, Tilders F, Wolfe CD. Corticotrophin-releasing hormone (CRH)-binding protein interference with CRH antibody binding: Implications for direct CRH immunoassay. Journal of Endocrinology. 1995;146:45–53. doi: 10.1677/joe.0.1460045. [DOI] [PubMed] [Google Scholar]
- Lobel M. Conceptualizations, measurement, and effects of prenatal maternal stress on birth outcomes. Journal of Behavioral Medicine. 1994;17:225–272. doi: 10.1007/BF01857952. [DOI] [PubMed] [Google Scholar]
- Lu MC, Chen B. Racial and ethnic disparities in preterm birth: The role of stressful life events. American Journal of Obstetrics & Gynecology. 2004;191:691–699. doi: 10.1016/j.ajog.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Majzoub JA, McGregor JA, Lockwood CJ, Smith R, Taggart MS, Schulkin J. A central theory of pre-term and term labor: Putative role for corticotropin-releasing hormone. American Journal of Obstetrics & Gynecology. 1999;180:S232–S241. doi: 10.1016/s0002-9378(99)70707-6. [DOI] [PubMed] [Google Scholar]
- Makrigiannakis A, Semmler M, Briese V, Eckerle H, Minas V, Mylonas I, Jeschke U. Maternal serum corticotropin-releasing hormone and ACTH levels as predictive markers of premature labor. International Journal of Gynaecology & Obstetrics. 2007;97:115–119. doi: 10.1016/j.ijgo.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Mancuso RA, Schetter CD, Rini CM, Roesch SC, Hobel CJ. Maternal prenatal anxiety and corticotropin-releasing hormone associated with timing of delivery. Psychosomatic Medicine. 2004;66:762–769. doi: 10.1097/01.psy.0000138284.70670.d5. [DOI] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S, Munson ML. Births: Final data for 2005. National Vital Statistics Reports. 2007;56:1–103. [PubMed] [Google Scholar]
- Mastorci F, Vicentini M, Viltart O, Manghi M, Graiani G, Quaini F, Sgoifo A. Long-term effects of prenatal stress: Changes in adult cardiovascular regulation and sensitivity to stress. Neuroscience & Biobehavioral Reviews. 2009;33:191–203. doi: 10.1016/j.neubiorev.2008.08.001. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protection and damage from acute and chronic stress: Allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Annals of the New York Academy of Science. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean M, Bisits A, Davies J, Woods R, Lowry P, Smith R. A placental clock controlling the length of human pregnancy. Nature Medicine. 1995;1:460–463. doi: 10.1038/nm0595-460. [DOI] [PubMed] [Google Scholar]
- Norbeck JS, Lindsey AM, Carrieri VL. The development of an instrument to measure social support. Nursing Research. 1981;30:264–269. [PubMed] [Google Scholar]
- Norbeck JS, Lindsey AM, Carrieri VL. Further development of the Norbeck Social Support Questionnaire: Normative data and validity testing. Nursing Research. 1983;32:4–9. [PubMed] [Google Scholar]
- Norwitz ER, Robinson JN, Challis JR. The control of labor. New England Journal of Medicine. 1999;341:660–666. doi: 10.1056/NEJM199908263410906. [DOI] [PubMed] [Google Scholar]
- Poetz A, Eyles JD, Elliott S, Wilson K, Keller-Olaman S. Path analysis of income, coping and health at the local level in a Canadian context. Health& Social Care in the Community. 2007;15:542–552. doi: 10.1111/j.1365-2524.2007.00715.x. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Reis F, Fadalti M, Florio P, Petraglia F. Putative role of placental corticotropin-releasing factor in the mechanisms of human parturition. Journal of the Society for Gynecologic Investigations. 1999;6:109–119. doi: 10.1016/s1071-5576(99)00009-x. [DOI] [PubMed] [Google Scholar]
- Rich-Edwards JW, Grizzard TA. Psychosocial stress and neuroendocrine mechanisms in preterm delivery. American Journal of Obstetrics & Gynecology. 2005;192:S30–S35. doi: 10.1016/j.ajog.2005.01.072. [DOI] [PubMed] [Google Scholar]
- Roesch SC, Schetter CD, Woo G, Hobel CJ. Modeling the types and timing of stress in pregnancy. Anxiety, Stress & Coping: An International Journal. 2004;17:87–102. [Google Scholar]
- Ruiz RJ, Fullerton J, Brown CE, Dudley DJ. Predicting risk of preterm birth: The roles of stress, clinical risk factors, and corticotropin-releasing hormone. Biological Research for Nursing. 2002;4:54–64. doi: 10.1177/1099800402004001007. [DOI] [PubMed] [Google Scholar]
- Ruiz RJ, Fullerton J, Brown CE, Schoolfield J. Relationships of cortisol, perceived stress, genitourinary infections, and fetal fibronectin to gestational age at birth. Biological Research for Nursing. 2001;3:39–48. doi: 10.1177/109980040100300106. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Glynn L, Schetter CD, Wadhwa P, Garite T, Chicz-DeMet A, Hobel C. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): Priming the placental clock. Peptides. 2006;27:1457–1463. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Scheier MF, Carver CS. Optimism, coping, and health: Assessment and implications of generalized outcome expectancies. Health Psychology. 1985;4:219–247. doi: 10.1037//0278-6133.4.3.219. [DOI] [PubMed] [Google Scholar]
- Schlotz W, Phillips DI. Fetal origins of mental health: Evidence and mechanisms. Brain, Behavior, and Immunity. 2009;23:905–916. doi: 10.1016/j.bbi.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Shannon M, King TL, Kennedy HP. Allostasis: A theoretical framework for understanding and evaluating perinatal health outcomes. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 2007;36:125–134. doi: 10.1111/j.1552-6909.2007.00126.x. [DOI] [PubMed] [Google Scholar]
- Sherbourne CD, Hays RD, Wells KB. Personal and psychosocial risk factors for physical and mental health outcomes and course of depression among depressed patients. Journal of Consulting and Clinical Psychology. 1995;63:345–355. doi: 10.1037//0022-006x.63.3.345. [DOI] [PubMed] [Google Scholar]
- Smith R, Mesiano S, McGrath S. Hormone trajectories leading to human birth. Regulatory Peptides. 2002;108:159–164. doi: 10.1016/s0167-0115(02)00105-2. [DOI] [PubMed] [Google Scholar]
- Steer P. The epidemiology of preterm labour. British Journal of Obstetrics & Gynecology. 2005;112:1–3. doi: 10.1111/j.1471-0528.2005.00575.x. [DOI] [PubMed] [Google Scholar]
- Wadhwa P, Garite TJ, Porto M, Glynn L, Chicz-DeMet A, Dunkel-Schetter C, Sandman CA. Placental corticotropin-releasing hormone (CRH), spontaneous preterm birth, and fetal growth restriction: A prospective investigation. American Journal of Obstetrics & Gynecology. 2004;191:1063–1069. doi: 10.1016/j.ajog.2004.06.070. [DOI] [PubMed] [Google Scholar]
- Weinstock M. The long-term behavioural consequences of prenatal stress. Neuroscience and Biobehavioral Reviews. 2008;32:1073–1086. doi: 10.1016/j.neubiorev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Yali AM, Lobel M. Stress-resistance resources and coping in pregnancy. Anxiety, Stress & Coping: An International Journal. 2002;15:289–309. [Google Scholar]
- Yim IS, Glynn LM, Dunkel-Schetter C, Hobel CJ, Chicz-DeMet A, Sandman CA. Risk of postpartum depressive symptoms with elevated corticotropin-releasing hormone in human pregnancy. Archives of General Psychiatry. 2009;66:162–169. doi: 10.1001/archgenpsychiatry.2008.533. [DOI] [PMC free article] [PubMed] [Google Scholar]