Abstract
Prior studies report an association between vitamin D deficiency and hypertension, including the pregnancy-specific disorder, preeclampsia. Circulating vitamin D is almost entirely bound to vitamin D binding protein, which increases 2-fold during pregnancy, but previous studies have not examined vitamin D binding protein or free vitamin D levels. We performed a nested case-control study within the Massachusetts General Hospital Obstetrical Maternal Study (MOMS), measuring first trimester total 25-hydroxyvitamin D and vitamin D binding protein, and calculating free 25-hydroxyvitamin D levels. We compared these levels from pregnancies complicated by subsequent preeclampsia (cases, n=39) with those from normotensive pregnancies (controls, n=131). First trimester total 25-hydroxyvitamin D levels were similar in cases and controls (27.4 ±1.9 ng/ml vs. 28.8±0.80 ng/ml, p=0.435). Despite an association between higher first trimester blood pressures and subsequent preeclampsia, first trimester total 25-hydroxyvitamin D was not associated with first trimester systolic (r=0.11, p=0.16) or diastolic blood pressures (r=0.03, p=0.72). Although there was a trend toward increased risk of preeclampsia with 25-hydroxyvitamin D levels less than 15 ng/ml (OR 2.5, 95% CI 0.89-6.9), this was attenuated after adjustment for body mass index and other covariates (OR 1.35 95% CI 0.40-4.5). First trimester vitamin D binding protein and free 25-hydroxyvitamin D levels were similar in cases and controls and were not associated with first trimester blood pressures. These data suggest that first trimester total and free 25-hydroxyvitamin D levels are not independently associated with first trimester blood pressure or subsequent preeclampsia.
Keywords: vitamin D, vitamin D Binding Protein, preeclampsia, pregnancy
Introduction
In the general population, 25-hydroxyvitamin D (25(OH)D) deficiency has been linked to hypertension.1-3 In preeclampsia, the most severe form of pregnancy-induced hypertension, studies have consistently found alterations in calcium and vitamin D metabolism during clinical disease in late pregnancy; these include hypocalciuria 4-7 and low serum 1,25-dihydroxyvitamin D (1,25(OH)2D).8-11 A recent nested case-control study reported lower early pregnancy levels of 25(OH)D in women who later developed preeclampsia, suggesting that 25(OH)D deficiency antedates disease onset and might contribute to its pathogenesis.12
According to the “free hormone hypothesis,” only hormones liberated from binding proteins are able to enter cells to perform biological actions.13 Vitamin D binding protein (VDBP), abundant in the circulation, serves as the major binding protein for 25(OH)D and 1,25(OH)2D. Circulating vitamin D of both forms is almost entirely bound to albumin (10-15%) and VDBP (85-90%)14-15 which act as vitamin D reservoirs and slow its metabolism.16 VDBP increases in high-estrogen states, such as pregnancy.17-18 Because measurement of total 25(OH)D is necessarily a measurement of the vitamin D/VDBP complex, alterations in VDBP levels in preeclamptic pregnancy could account for lower levels of vitamin D, without necessarily leading to an alteration in the level of free hormone.
We hypothesized that alterations in vitamin D levels previously reported in preeclamptic pregnancy may be mediated by alterations in circulating levels of VDBP. Thus, we aimed to describe and compare levels of total 25(OH)D, VDBP, and calculated free 25(OH)D in the first trimester of normotensive pregnancies and pregnancies subsequently complicated by preeclampsia.
Methods
We conducted a nested case control study of first trimester serum 25(OH)D and VDBP levels within the Massachusetts General Hospital Obstetrical Maternal Study (MOMS). MOMS is a prospective cohort study of pregnant women which has been previously described in detail.19-21 In brief, all women receiving prenatal care at Massachusetts General Hospital or an affiliate between 1998 and 2006 were eligible; 70% of eligible women approached agreed to enroll in the cohort and enrollees had similar characteristics to those who declined. Subjects provided informed consent and were enrolled at their first prenatal visit (n=9930). At that time, subjects provided a serum sample, which was kept on ice for a maximum of three hours, then stored at -80 C for future analysis. Clinical information including the subject's age, estimated date of confinement (from early ultrasound or self-reported last menstrual period), self-reported race, and height was recorded. At each prenatal visit, trained care providers measured subjects' blood pressures and weights. Blood pressure was measured after ∼5 minutes of rest in the sitting position using an appropriate cuff size. The first and fifth Korotkoff phases were used to define systolic and diastolic blood pressures. Blood pressures, weights, and clinical information were transferred directly from the electronic medical record (EMR) into the MOMS study database, along with the results of urine dipstick testing, 24 hour urine protein measurements, the results of glucose loading tests, glucose tolerance tests, and delivery information including date of delivery and infant birth weight. The Partners Human Research Committee approved all study procedures.
All cases of preeclampsia were normotensive at the first prenatal visit (BP<140/90 mmHg) and developed hypertension (systolic blood pressure≥140 or diastolic blood pressure≥90 mmHg) and proteinuria (≥2+ protein on urine dipstick or ≥300mg proteinuria/24 hours) after 20 weeks gestation. A review of clinical records and ICD-9 billing codes was conducted on subjects who met these criteria to verify the diagnosis of preeclampsia. Cases for the present study were subjects whose diagnosis of preeclampsia was confirmed, had serum samples available for analysis, and did not have gestational diabetes mellitus (according to Carpenter-Coustan criteria22). Unmatched controls were chosen randomly from among women who remained normotensive throughout pregnancy, and did not have gestational diabetes mellitus22 or give birth to small for gestational age infants (birth weight less than 5th percentile for gestational age). The sample size selected had 80% power to detect a 5 ng/mL difference in total 25(OH)D levels between cases and controls with a standard deviation of 9.8 ng/mL.
Serum samples were thawed from -80 C for analysis. 25(OH)D was measured by liquid chromatography- tandem mass spectrometry (LC-MS/MS) (laboratory of Michael F. Holick, MD, Boston University School of Medicine), as described previously.23 Inter-assay coefficient of variation (CV) was 8.1% at 37.5 ng/mL. VDBP was measured on samples in duplicate by commercial enzyme linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN, Catalog Number DVDBP0) according to the manufacturer's instructions, after diluting serum samples 1 to 4,000 in Calibrator Diluent RD6-11 (R&D Systems Part Number 895489). For this assay, the manufacturer's reported intra-assay and inter-assay CV's are 6.2 and 7.4%. The assay measures between 89-102% of exogenous VDBP added to human serum and has no significant cross-reactivity with human albumin, vitamin D3, or alfafetoprotein. Albumin was measured using automated clinical chemistry platform at the Massachusetts General Hospital clinical laboratory. Free levels of 25(OH)D were calculated using the following equation:
This equation, based on the binding affinities between albumin and VDBP and 25(OH)D, has been validated in a study of non-pregnant subjects where VDBP was measured by “rocket” immunoelectrophoresis and total 25(OH)D was measured by high performance liquid chromatography.14 The correlation coefficient between calculated free 25(OH)D and free 25(OH)D measured by centrifugal ultrafiltration was 0.925.14
We compared baseline characteristics between cases and controls using t-tests for normally distributed variables (e.g. 25(OH)D), Wilcoxon rank sum test for non-normally distributed variables (e.g. VDBP and free vitamin D), and chi-squared tests for categorical variables. We then conducted univariate and multivariate logistic regression analyses to determine the risk of preeclampsia depending on first trimester levels of 25(OH)D, log-transformed VDBP and log-transformed free 25(OH)D before and after adjustment for body mass index, race, season, nulliparity, and gestational age at blood collection. We further divided subjects into tertiles, quartiles, and quintiles, based on 25(OH)D levels and conducted univariate and multivariate logistic regression analyses to determine whether the risk of preeclampsia differed across these quantiles. We also conducted univariate and multivariate logistic regression analysis to determine the risk of preeclampsia in subjects who were “vitamin D deficient,” (with various definitions of deficiency : 25(OH)D<15 ng/mL, 10 ng/mL, or 20 ng/mL) as compared to those who were not deficient.
Correlations were assessed using Spearman rank order tests. We compared the levels of free and total 25(OH)D and VDBP (after natural log transformation) among subjects with different races, seasons of blood collection, and parity (nulliparous vs. multiparous) using t-tests or one way analysis of variance with Holm-Sidak post-hoc testing, depending on the number of groups. Data are expressed as mean ± standard error or median [interquartile range], unless otherwise noted.
Results
Women who subsequently developed preeclampsia had higher body mass index, systolic and diastolic blood pressures at the first prenatal visit, but were otherwise similar to controls (Table 1).Cases were more likely to be nulliparous and non-white, but these differences did not reach statistical significance.
Table 1. First Trimester Characteristics of Women with and without Pregnancies Subsequently Complicated by Preeclampsia.
| First Trimester Subject Characteristics | Normotensive (controls, n=131) | Preeclampsia (cases, n=39) | p-value |
|---|---|---|---|
| Gestational age at blood collection (weeks) | 11.6 (3.0) | 11.2 (3.6) | 0.481 |
| Age (years) | 30.4 (6.0) | 28.9 (6.4) | 0.193 |
| Body mass index (kg/m2) | 24.4 (4.5) | 30.0 (7.3) | <0.001 |
| SBP (mmHg) | 109 (10.0) | 113 (9.3) | 0.009 |
| DBP (mmHg) | 66.8 (6.6) | 70.4 (6.5) | 0.003 |
| % Nulliparous | 54.2% | 69.2% | 0.095 |
| % White race | 66.4% | 53.8% | 0.152 |
| % Summer blood collection | 29.8% | 28.2% | 0.851 |
Values are expressed as mean(standard deviation) or percent of subjects.
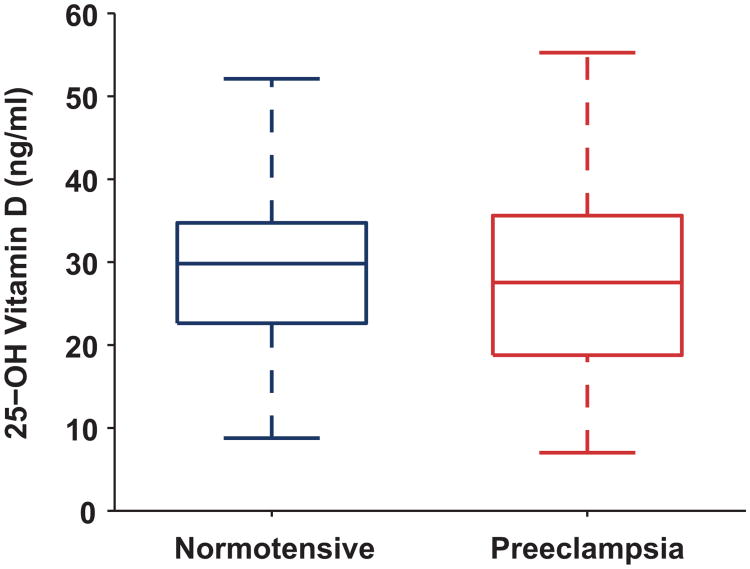
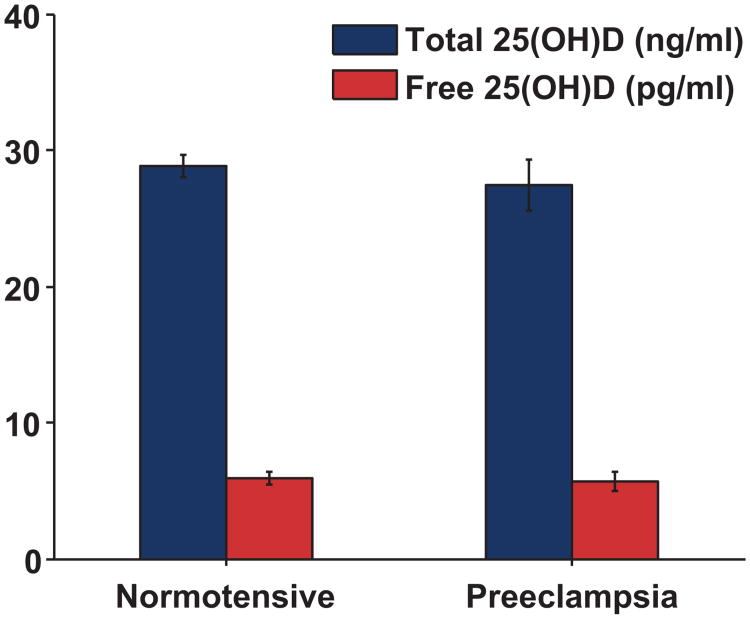
First trimester total 25(OH)D levels were similar in cases and controls (27.4 ±1.9 ng/ml vs. 28.8±0.80 ng/ml, p=0.435, Figure 1). First trimester VDBP levels were also similar between groups (median [IQR]; cases: 469 [217-746] versus controls: 483 [348-650] ug/ml, p=0.802) as were calculated free 25(OH)D levels (Figure 2; cases: 4.13 [2.45-7.66] versus controls: 4.19 [3.14-6.50] pg/ml, p=0.800). These relationships did not change when the analysis was restricted to nulliparous women, among whom first trimester 25(OH)D was 30.0±2.2 ng/mL in cases (n=27) and 28.7±1.1 ng/mL in controls (n=71).
Figure 1.
First trimester serum 25-hydroxyvitamin D concentrations in women who remained normotensive (n=131) and women who developed subsequent preeclampsia (n=39). First trimester 25(OH)D levels were not significantly different in who developed subsequent preeclampsia compared to normotensive women (mean ± standard error=27.4 ± 1.9 vs. 28.8 ± 0.80, p=0.435). To express total 25(OH)D levels (ng/ml) in nmol/L, multiply by conversion factor 2.496.
Figure 2.
First trimester serum levels of Total and Free 25(OH)D in women who remained normotensive (N=131) and women who developed subsequent preeclampsia (N=39). Levels of Free 25(OH)D did not differ significantly between groups (p=0.80). To express total 25(OH)D levels (ng/ml) in nmol/L, multiply by conversion factor 2.496.
Among all subjects, levels of total 25(OH)D were not associated with systolic or diastolic blood pressure at the time of blood collection (r=0.109, p=0.157 and r=0.028, p=0.718). When the analysis was restricted to white women or women without preeclampsia, total 25(OH)D levels remained unassociated with systolic or diastolic blood pressure (r<0.2, p>0.15 for each of these analysis). Similarly, VDBP and free 25(OH)D levels were not associated with first trimester systolic or diastolic blood pressure (r<0.2 p>0.05 for all comparisons).
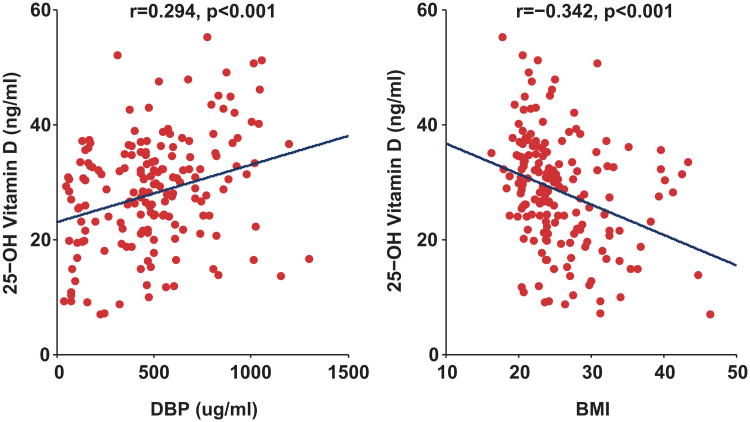
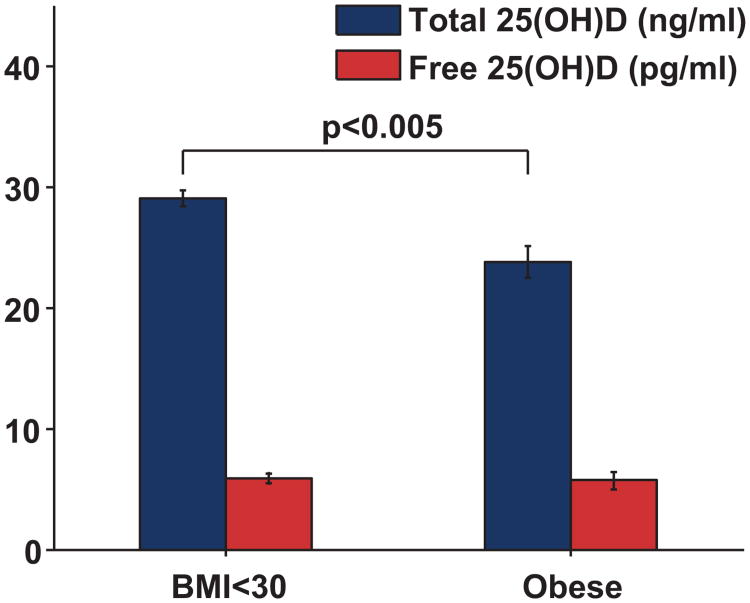
First trimester total 25(OH)D levels were positively correlated with first trimester VDBP levels (r=0.294, p<0.001, Figure 3). VDBP levels (r=0.218, p<0.004) and total 25(OH)D (r=0.178, p<0.021) levels were positively correlated with gestational age at blood collection. Both total 25(OH)D (r=-−0.343, p<0.001) and VDBP (r=−0.190, p=0.013) levels were inversely correlated with body mass index (Figure 3). Total 25(OH)D levels were significantly lower among black non-Hispanic subjects (17.8 ± 2.9 ng/mL, n=8) and Hispanic subjects (23.1 ± 1.2 ng/mL n=44) compared to White non-Hispanic subjects (31.9 ± 0.84 ng/mL, n=108; p<0.001 for both comparisons). VDBP levels were lower in black subjects (204 ± 68 ug/ml) compared to white subjects (519 ± 26 ug/ml, p<0.001). VDBP levels were non-significantly lower in Hispanic subjects (491 ± 44 ug/mL) compared to white subjects (p=0.293). Levels of calculated free 25(OH)D did not differ in black subjects or Hispanic subjects when compared to white subjects (p>0.05 for both comparisons). Levels of free 25(OH)D were not associated with differences in adiposity (Figure 4).
Figure 3.
Relationship between 25-hydroxyvitamin D, Vitamin D Binding Protein, and Body Mass Index (BMI). 25(OH)D levels were positively associated with VDBP levels and inversely associated with body mass index (kg/m2). To express total 25(OH)D levels (ng/ml) in nmol/L, multiply by conversion factor 2.496.
Figure 4.
Levels of Total and Free 25(OH)D by Obesity. Subjects who were obese (body mass index ≥30 kg/m2, n=31) had lower levels of total 25(OH)D compared to non-obese subjects (n=137, p=0.005), but free 25(OH)D levels did not differ (p=0.691). To express total 25(OH)D levels (ng/ml) in nmol/L, multiply by conversion factor 2.496.
Logistic regression analyses revealed no linear association between first trimester total 25(OH)D levels and risk of subsequent preeclampsia in univariate or multivariate analyses (Table 2). There was similarly no significant relationship between 25(OH)D quartile and subsequent preeclampsia (Table 2). Total 25(OH)D level below 15ng/mL was associated with a non-significantly increased risk for preeclampsia, but this relationship was attenuated after adjustment for body mass index alone or in combination with race, and season (Table 2). First trimester VDBP levels or free 25(OH)D levels were not associated with the risk of subsequent preeclampsia (p=0.666 and p=0.734).
Table 2. Odds of Preeclampsia by First Trimester 25-hydroxyvitamin D Concentration.
| Vitamin D Measure/Model | OR for Preeclampsia (95% CI) |
|---|---|
| Per 10 ng/mL Increase in 25(OH)D | |
| Univariate | 0.86 (0.60-1.25) |
| Multivariate 1 | 1.18 (0.78-1.80) |
| Multivariate 2 | 1.24 (0.78-1.98) |
| By Quartiles of 25(OH)D | |
| Quartile 1 | 1.50 (0.57-3.96) |
| Quartile 2 | 1.04 (0.39-2.76) |
| Quartile 3 | 0.67 (0.23-1.91) |
| Quartile 4 | 1.0 (Reference) |
| By 25(OH)D Deficiency (<15ng/mL) | |
| Deficient (Univariate) | 2.49 (0.89-6.9) |
| Deficient (Multivariate 1) | 1.34 (0.42-4.3) |
| Deficient (Multivariate 2) | 1.35 (0.40-4.5) |
| Not Deficient (>15 ng/mL) | 1.0 (Reference) |
Multivariate 1- Adjustment for body mass index, Multivariate 2- Adjustment for body mass index, non-white race, and summer blood collection
Discussion
In this nested case-control study, we found no association between first trimester total 25(OH)D and subsequent preeclampsia. Our data therefore does not support the hypothesis that first trimester 25(OH)D deficiency is a prevalent cause of preeclampsia in this population. Vitamin D binding protein and calculated free levels of 25(OH)D were also not altered in the first trimester among women who later developed preeclampsia. Interestingly, we found that conditions classically associated with lower total levels of total 25(OH)D, including obesity and black race (also observed in the present study), were not associated with alterations in calculated free 25(OH)D levels.
Like previous studies, we found that first trimester blood pressures were higher in women who went on to develop preeclampsia than in women who remained normotensive.24-25 However, unlike Bodnar et al,12 we did not find an independent association between first trimester 25(OH)D levels and subsequent preeclampsia. The discrepancy between our results and those of this prior study may be due to our use of first trimester measured body mass index in multivariate adjustment and differences in the populations studied.
Total 25(OH)D levels are known to be inversely correlated with adiposity 2, 26-29 and this was true in the present study. The difference between our results and those of Bodnar et al.12 following multivariate adjustment may be partly due to differences in the way body mass index was ascertained. We used measured first trimester weights and heights to calculate body mass index rather than self-reported pre-pregnancy weights. When weights and heights are self reported, biased misclassification of body mass index occurs and may lead to incomplete adjustment in multivariate analyses.30 In addition, 25(OH)D levels are more likely to be associated with body mass index at the time of measurement rather than pre-pregnancy body mass index because lower circulating 25(OH)D levels in obese subjects are likely due to the deposition of vitamin D in adipose tissue.28, 31 It is unclear whether the deficit in circulating 25(OH)D levels in the obese has physiologic consequences. However, because women who gain more weight in pregnancy are at greater risk for preeclampsia,32-33 adjustment for pre-pregnancy body mass index may be an inadequate method to account for the potential confounding effect of adiposity, while adjustment for body mass index at the time of 25(OH)D measurement may be more likely to account for this.
In contrast to Bodnar et al., the present study had fewer subjects with very low levels of 25(OH)D.12 Of note, there was a trend toward an increased risk of preeclampsia at very low levels of 25(OH)D in univariate analyses. Accordingly, it is possible that a relationship between preeclampsia and 25(OH)D is present, but only at the low extreme. Furthermore, the MOMS cohort has only a small proportion of black women, when compared to this prior study.12, 29 Vitamin D and calcium metabolism is known to differ by race,34-36 so a relationship between 25(OH)D and preeclampsia risk may be limited to black women, a hypothesis which we were not able to test in our subjects.
Past studies examining vitamin D levels in association with hypertension have not concurrently measured levels of vitamin D binding protein or calculated free vitamin D levels.1-3, 12 While we did not find that VDBP levels were altered in preeclampsia, we found that, like total 25(OH)D, VDBP levels were lower in black subjects and decreased with increasing adiposity. Consequently, calculated free 25(OH)D levels were not lower among black or obese women, who have been previously reported to have vitamin D deficiency.29 To our knowledge these associations with VDBP have not been previously reported in pregnant or non-pregnant populations.18, 37-39 If our results are generalized to the non-pregnant population, reexamination of vitamin D deficiency in the context of VDBP levels is compelling, as such analysis may reveal that some individuals otherwise considered “vitamin D deficient” have normal free-25(OH)D levels due to a concomitant decrease in VDBP. However, the free hormone hypothesis with regards to 25(OH)D has not yet been proven or disproven in a pregnant or non-pregnant population and requires further study.
Sample size may have limited our ability to detect differences in 25(OH)D or VDBP levels between groups. However, in order to detect a difference with an α=0.05 or lower in first trimester total 25(OH)D in women with subsequent preeclampsia of the magnitude we observed in univariate analyses, we would have required approximately 1400 subjects each in the case and control groups. After multivariate analysis, or after restricting the analysis to nulliparous women, higher levels of total 25(OH)D actually appeared to be associated with a non-significantly increased risk of preeclampsia, making a true independent association between low 25(OH)D levels and preeclampsia in this cohort unlikely. We only measured 25(OH)D levels at a single time point, thus we cannot comment on longitudinal changes in 25(OH)D levels and risk of preeclampsia. It is possible that 25(OH)D or VDBP levels measured at time points closer to the clinical onset of preeclampsia may be more closely associated with this disorder. In this regard, 25(OH)D deficiency may be a consequence of preeclampsia, such that levels are not altered until after clinical disease has developed. We also note that the index used to calculate free 25(OH)D has not been validated in pregnancy, therefore we cannot be sure that it accurately reflects the free levels of 25(OH)D in the serum. Finally, sample availability precluded measurement of 1,25(OH)2D in our subjects. While 1,25(OH)2D is thought to be the active form of vitamin D, many tissues, including vascular smooth muscle and endothelial cells, express 1-α hydroxylase, thus may be able to convert circulating 25(OH)D to its active form intracellularly.40-42 Furthermore, the level of free 25(OH)D is highly correlated with the level of free 1,25(OH)2D (Powe, Bhan, Thadhani, unpublished data, 2010), as this more active compound is also bound in the serum by VDBP and albumin in similar proportions.14-15 Future studies should examine levels of total and free 1,25(OH)2D along with VDBP.
Perspectives
This study does not support the hypothesis that first trimester maternal vitamin D deficiency contributes substantially to the risk of preeclampsia in this population. Alterations in VDBP levels were not associated with subsequent preeclampsia, but future research in the general population should continue to explore the relationships between VDBP, free and total vitamin D, and disease risk, given the association between VDBP, race, and body mass index in this pregnant population. Before clinical trials of vitamin D supplementation to prevent or treat preeclampsia are undertaken, ideally an association between early pregnancy or pre-conception vitamin D deficiency and preeclampsia should be demonstrated, after adequate adjustment for adiposity, in large prospective cohort studies.
Acknowledgments
Sources of Funding: C.E.P is a Howard Hughes Medical Institute Medical Research Training Fellow. S. R. is supported by Women's Reproductive Health Research grant (K12HD001255- NIH/NICHD). S.A.K is an investigator of the Howard Hughes Medical Institute.
Footnotes
Disclosure Statement: CEP, ES, SR, JE, IB, and SAK have nothing to declare. RT has a grant from Abbott Laboratories to study vitamin D in kidney disease. RT has filed patents on the use of biomarkers for the prediction and diagnosis of preeclampsia through the Massachusetts General Hospital. R.T. is a consultant to Beckman Coulter and Roche Diagnostics in the area of preeclampsia biomarkers. S.A.K. is a co-inventor on multiple patents related to angiogenic proteins for the diagnosis/therapy of preeclampsia. S.A.K. is a consultant to Abbott, Beckman Coulter , Roche and Johnson & Johnson that are developing angiogenic markers for use in preeclampsia diagnosis/prediction.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, Curhan GC. Plasma 25-hydroxyvitamin d levels and risk of incident hypertension. Hypertension. 2007;49:1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 2.Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, Felsenfeld A, Levine B, Mehrotra R, Norris K. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin d in the united states: Data from the third national health and nutrition examination survey. Arch Intern Med. 2007;167:1159–1165. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 3.Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin d levels and the risk of mortality in the general population. Arch Intern Med. 2008;168:1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taufield PA, Ales KL, Resnick LM, Druzin ML, Gertner JM, Laragh JH. Hypocalciuria in preeclampsia. N Engl J Med. 1987;316:715–718. doi: 10.1056/NEJM198703193161204. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez-Ramos L, Jones DC, Cullen MT. Urinary calcium as an early marker for preeclampsia. Obstet Gynecol. 1991;77:685–688. [PubMed] [Google Scholar]
- 6.Sanchez-Ramos L, Sandroni S, Andres FJ, Kaunitz AM. Calcium excretion in preeclampsia. Obstet Gynecol. 1991;77:510–513. [PubMed] [Google Scholar]
- 7.Frenkel Y, Barkai G, Mashiach S, Dolev E, Zimlichman R, Weiss M. Hypocalciuria of preeclampsia is independent of parathyroid hormone level. Obstet Gynecol. 1991;77:689–691. [PubMed] [Google Scholar]
- 8.Seely EW, Wood RJ, Brown EM, Graves SW. Lower serum ionized calcium and abnormal calciotropic hormone levels in preeclampsia. J Clin Endocrinol Metab. 1992;74:1436–1440. doi: 10.1210/jcem.74.6.1592891. [DOI] [PubMed] [Google Scholar]
- 9.August P, Marcaccio B, Gertner JM, Druzin ML, Resnick LM, Laragh JH. Abnormal 1,25-dihydroxyvitamin d metabolism in preeclampsia. Am J Obstet Gynecol. 1992;166:1295–1299. doi: 10.1016/s0002-9378(11)90625-5. [DOI] [PubMed] [Google Scholar]
- 10.Tolaymat A, Sanchez-Ramos L, Yergey AL, Vieira NE, Abrams SA, Edelstein P. Pathophysiology of hypocalciuria in preeclampsia: Measurement of intestinal calcium absorption. Obstet Gynecol. 1994;83:239–243. [PubMed] [Google Scholar]
- 11.Halhali A, Tovar AR, Torres N, Bourges H, Garabedian M, Larrea F. Preeclampsia is associated with low circulating levels of insulin-like growth factor i and 1,25-dihydroxyvitamin d in maternal and umbilical cord compartments. J Clin Endocrinol Metab. 2000;85:1828–1833. doi: 10.1210/jcem.85.5.6528. [DOI] [PubMed] [Google Scholar]
- 12.Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal vitamin d deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab. 2007;92:3517–3522. doi: 10.1210/jc.2007-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendel CM. The free hormone hypothesis: A physiologically based mathematical model. Endocr Rev. 1989;10:232–274. doi: 10.1210/edrv-10-3-232. [DOI] [PubMed] [Google Scholar]
- 14.Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin d in serum and its regulation by albumin and the vitamin d-binding protein. J Clin Endocrinol Metab. 1986;63:954–959. doi: 10.1210/jcem-63-4-954. [DOI] [PubMed] [Google Scholar]
- 15.Bikle DD, Siiteri PK, Ryzen E, Haddad JG. Serum protein binding of 1,25-dihydroxyvitamin d: A reevaluation by direct measurement of free metabolite levels. J Clin Endocrinol Metab. 1985;61:969–975. doi: 10.1210/jcem-61-5-969. [DOI] [PubMed] [Google Scholar]
- 16.Safadi FF, Thornton P, Magiera H, Hollis BW, Gentile M, Haddad JG, Liebhaber SA, Cooke NE. Osteopathy and resistance to vitamin d toxicity in mice null for vitamin d binding protein. J Clin Invest. 1999;103:239–251. doi: 10.1172/JCI5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bikle DD, Gee E, Halloran B, Haddad JG. Free 1,25-dihydroxyvitamin d levels in serum from normal subjects, pregnant subjects, and subjects with liver disease. J Clin Invest. 1984;74:1966–1971. doi: 10.1172/JCI111617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouillon R, van Baelen H, de Moor P. The measurement of the vitamin d-binding protein in human serum. J Clin Endocrinol Metab. 1977;45:225–231. doi: 10.1210/jcem-45-2-225. [DOI] [PubMed] [Google Scholar]
- 19.Wolf M, Kettyle E, Sandler L, Ecker JL, Roberts J, Thadhani R. Obesity and preeclampsia: The potential role of inflammation. Obstet Gynecol. 2001;98:757–762. doi: 10.1016/s0029-7844(01)01551-4. [DOI] [PubMed] [Google Scholar]
- 20.Wolf M, Sandler L, Jimenez-Kimble R, Shah A, Ecker JL, Thadhani R. Insulin resistance but not inflammation is associated with gestational hypertension. Hypertension. 2002;40:886–891. doi: 10.1161/01.hyp.0000042085.65467.9f. [DOI] [PubMed] [Google Scholar]
- 21.Wolf M, Sandler L, Munoz K, Hsu K, Ecker JL, Thadhani R. First trimester insulin resistance and subsequent preeclampsia: A prospective study. J Clin Endocrinol Metab. 2002;87:1563–1568. doi: 10.1210/jcem.87.4.8405. [DOI] [PubMed] [Google Scholar]
- 22.Metzger BE, Coustan DR. Summary and recommendations of the fourth international workshop-conference on gestational diabetes mellitus. The organizing committee. Diabetes Care. 1998;21(2):B161–167. [PubMed] [Google Scholar]
- 23.Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, Petruschke RA, Chen E, de Papp AE. Prevalence of vitamin d inadequacy among postmenopausal north american women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90:3215–3224. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 24.Moutquin JM, Rainville C, Giroux L, Raynauld P, Amyot G, Bilodeau R, Pelland N. A prospective study of blood pressure in pregnancy: Prediction of preeclampsia. Am J Obstet Gynecol. 1985;151:191–196. doi: 10.1016/0002-9378(85)90010-9. [DOI] [PubMed] [Google Scholar]
- 25.Thadhani R, Ecker JL, Kettyle E, Sandler L, Frigoletto FD. Pulse pressure and risk of preeclampsia: A prospective study. Obstet Gynecol. 2001;97:515–520. doi: 10.1016/s0029-7844(00)01192-3. [DOI] [PubMed] [Google Scholar]
- 26.Snijder MB, van Dam RM, Visser M, Deeg DJ, Dekker JM, Bouter LM, Seidell JC, Lips P. Adiposity in relation to vitamin d status and parathyroid hormone levels: A population-based study in older men and women. J Clin Endocrinol Metab. 2005;90:4119–4123. doi: 10.1210/jc.2005-0216. [DOI] [PubMed] [Google Scholar]
- 27.Hypponen E, Power C. Vitamin d status and glucose homeostasis in the 1958 british birth cohort: The role of obesity. Diabetes Care. 2006;29:2244–2246. doi: 10.2337/dc06-0946. [DOI] [PubMed] [Google Scholar]
- 28.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin d in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 29.Looker AC. Body fat and vitamin d status in black versus white women. J Clin Endocrinol Metab. 2005;90:635–640. doi: 10.1210/jc.2004-1765. [DOI] [PubMed] [Google Scholar]
- 30.Bodnar LM, Siega-Riz AM, Simhan HN, Diesel JC, Abrams B. The impact of exposure misclassification on associations between prepregnancy bmi and adverse pregnancy outcomes. Obesity (Silver Spring) 2010 doi: 10.1038/oby.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris SS, Dawson-Hughes B. Reduced sun exposure does not explain the inverse association of 25-hydroxyvitamin d with percent body fat in older adults. J Clin Endocrinol Metab. 2007;92:3155–3157. doi: 10.1210/jc.2007-0722. [DOI] [PubMed] [Google Scholar]
- 32.DeVader SR, Neeley HL, Myles TD, Leet TL. Evaluation of gestational weight gain guidelines for women with normal prepregnancy body mass index. Obstet Gynecol. 2007;110:745–751. doi: 10.1097/01.AOG.0000284451.37882.85. [DOI] [PubMed] [Google Scholar]
- 33.Kabiru W, Raynor BD. Obstetric outcomes associated with increase in bmi category during pregnancy. Am J Obstet Gynecol. 2004;191:928–932. doi: 10.1016/j.ajog.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 34.Gutierrez OM, Isakova T, Smith K, Epstein M, Patel N, Wolf M. Racial differences in postprandial mineral ion handling in health and in chronic kidney disease. Nephrol Dial Transplant. 2010 doi: 10.1093/ndt/gfq316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weaver CM, McCabe LD, McCabe GP, Braun M, Martin BR, Dimeglio LA, Peacock M. Vitamin d status and calcium metabolism in adolescent black and white girls on a range of controlled calcium intakes. J Clin Endocrinol Metab. 2008;93:3907–3914. doi: 10.1210/jc.2008-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cosman F, Shen V, Morgan D, Gordon S, Parisien M, Nieves J, Lindsay R. Biochemical responses of bone metabolism to 1,25-dihydroxyvitamin d administration in black and white women. Osteoporos Int. 2000;11:271–277. doi: 10.1007/s001980050292. [DOI] [PubMed] [Google Scholar]
- 37.Winters SJ, Chennubhatla R, Wang C, Miller JJ. Influence of obesity on vitamin d-binding protein and 25-hydroxy vitamin d levels in african american and white women. Metabolism. 2009;58:438–442. doi: 10.1016/j.metabol.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 38.Bolland MJ, Grey AB, Ames RW, Horne AM, Mason BH, Wattie DJ, Gamble GD, Bouillon R, Reid IR. Age-, gender-, and weight-related effects on levels of 25-hydroxyvitamin d are not mediated by vitamin d binding protein. Clin Endocrinol (Oxf) 2007;67:259–264. doi: 10.1111/j.1365-2265.2007.02873.x. [DOI] [PubMed] [Google Scholar]
- 39.Haddad JG, Jr, Walgate J. Radioimmunoassay of the binding protein for vitamin d and its metabolites in human serum: Concentrations in normal subjects and patients with disorders of mineral homeostasis. J Clin Invest. 1976;58:1217–1222. doi: 10.1172/JCI108575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merke J, Milde P, Lewicka S, Hugel U, Klaus G, Mangelsdorf DJ, Haussler MR, Rauterberg EW, Ritz E. Identification and regulation of 1,25-dihydroxyvitamin d3 receptor activity and biosynthesis of 1,25-dihydroxyvitamin d3. Studies in cultured bovine aortic endothelial cells and human dermal capillaries. J Clin Invest. 1989;83:1903–1915. doi: 10.1172/JCI114097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zehnder D, Bland R, Chana RS, Wheeler DC, Howie AJ, Williams MC, Stewart PM, Hewison M. Synthesis of 1,25-dihydroxyvitamin d(3) by human endothelial cells is regulated by inflammatory cytokines: A novel autocrine determinant of vascular cell adhesion. J Am Soc Nephrol. 2002;13:621–629. doi: 10.1681/ASN.V133621. [DOI] [PubMed] [Google Scholar]
- 42.Somjen D, Weisman Y, Kohen F, Gayer B, Limor R, Sharon O, Jaccard N, Knoll E, Stern N. 25-hydroxyvitamin d3-1alpha-hydroxylase is expressed in human vascular smooth muscle cells and is upregulated by parathyroid hormone and estrogenic compounds. Circulation. 2005;111:1666–1671. doi: 10.1161/01.CIR.0000160353.27927.70. [DOI] [PubMed] [Google Scholar]