Abstract
Background
The incidence of hepatocellular carcinoma (HCC) continues to increase in Japan, but the clinical characteristics of Japanese patients with HCC have not been well described. The aim of this study was to determine the frequencies and utilities of elevated α-fetoprotein (AFP) and des-gamma-carboxy prothrombin (DCP) levels as biomarkers in cryptogenic HCC.
Material/Methods
A total of 2638 patients with HCC diagnosed between 1999 and 2010 in the Nagasaki Association Study of Liver (NASLD) were recruited for this study. The cause of HCC was categorized into 4 groups; HCC-B, HCC-C, HCC-BC, and HCC-nonBC. The significance of factors was examined for HCC-nonBC using logistic regression analysis in all patients.
Results
Multivariate analysis identified age, sex, BMI, alcohol consumption, platelet count, AST, ALT, AFP, DCP, and TNM stage as independent and significant risk factors for HCC-nonBC. According to TNM stage, the median AFP levels in HCC-nonBC with TNM stages I, II, and III were significantly lower than in either HCC-B or HCC-C. In TNM stage IV, the median AFP level in HCC-nonBC was significantly lower than in either HCC-B or HCC-BC. The median DCP levels in HCC-nonBC with TNM stages I and II were significantly higher than those in either HCC-B or HCC-C. In TNM stage III, the median DCP level in HCC-nonBC was significantly higher than that in HCC-C.
Conclusions
DCP was more sensitive than AFP for the diagnosis of early stage cryptogenic HCC. DCP should be used as the main serum test for cryptogenic HCC detection.
Keywords: HCC, DCP, AFP
Background
Primary liver cancer accounts for approximately 6% of all human malignancies. It is estimated that half a million cases occur worldwide annually, making primary liver cancer the fifth most common malignancy in men and the ninth in women [1–6]. Hepatocellular carcinoma (HCC) accounts for 85% to 90% of primary liver cancers [7] and the age-adjusted HCC mortality rate has increased in recent decades in Japan [8]. Similarly, a rising HCC trend has been reported in several developed countries in North America, Europe, and Asia [9,10]. HCC often develops in patients with liver cirrhosis caused by hepatitis B virus (HBV), hepatitis C virus (HCV), excessive alcohol consumption, or nonalcoholic fatty liver disease. Of the hepatitis viruses that cause HCC, HCV is predominant in Japan [11–14]. However, it has been reported that the absolute numbers and proportion of HBsAg and HCVab negative HCC (HCC-nonBC) have both been steadily increasing in Japan [15,16].
The prognosis for patients with HCC is still poor. Surgical resection and liver transplantation are the standard curative treatments available. Recently, radio-frequency ablation (RFA) and percutaneous ethanol injection (PEI) have also been recognized as effective methods of achieving complete tumor necrosis for small HCCs [17]. However, the chance of curative treatment is often limited by several features of HCC itself. HCCs are usually large before they produce symptoms. Bilobar or multifocal tumors are common. The incidence of associated cirrhosis is also high, exceeding 80% in most series [18–20]. Transcatheter intra-arterial chemoembolization (TACE), with which complete necrosis of HCCs is thought to be difficult to achieve, is also impacted by the above factors [21]. To increase opportunities for meaningful intervention and to improve survival, early detection of HCC by measuring alpha-fetoprotein (AFP) and/or imaging screening is implemented in many countries [15,22–25]. However, the poorer prognosis of patients with HCC-nonBC is reportedly attributable to its late detection in an advanced stage, owing to the lack of a surveillance system for early detection of HCC [26].
In this retrospective cohort study, our aim was to characterize consecutive patients who had been diagnosed with HCC-nonBC during an 11-year period (1999–2010) at the centers comprising the Nagasaki Association Study of Liver Disease (NASLD) group. We evaluated the clinical characteristics of patients with HCC-nonBC, their tumor stages, treatment, AFP and DPC as potential biomarkers, and survival.
Material and Methods
Patients
In total, 2638 patients with HCC diagnosed between 1999 and 2010 in the NASLD were recruited for this study. The diagnosis of HCC was based on AFP and/or DCP levels, as well as the results of imaging techniques such as ultrasonography (USG), computerized tomography (CT), magnetic resonance imaging (MRI), and hepatic angiography (HAG), and/or liver biopsy. The diagnostic criteria included characteristic liver biopsy findings, elevated AFP (≥20 ng/mL) and/or DCP (≥40 ng/mL), and neovascularization on HAG, CT, and/or MRI.
The diagnosis of chronic HCV infection was positive for both anti-HCV, by a third-generation enzyme-linked immunosorbent assay (ELISA), and for HCV RNA by polymerase chain reaction (PCR). The diagnosis of chronic HBV infection was based on the presence of HBsAg (enzyme-linked immunosorbent assay; Abbott Laboratories); serum AFP was measured by radioimmunoassay (Abbott Laboratories). The history of alcohol intake was obtained from medical records; habitual drinking was defined as an average daily consumption of an amount equivalent to 80 g of pure ethanol for a period of more than 10 years.
HCC etiologies were categorized into 4 groups: (1) HCC-B, HBsAg positive, and HCVAb negative; (2) HCC-C, HCVAb positive, and HBsAg negative; (3) HCC-BC, both HBsAg and HCVAb positive; and (4) HCC-nonBC, both HbsAg, and HCVAb negative. The significance of age, sex, body mass index (BMI), alcohol intake, diabetes mellitus, underlying liver disease Child-Pugh score, platelet count, prothrombin time (PT), albumin (ALB), total bilirubin (Bil), aspartate aminotransferase (AST), alanine aminotransferase (ALT), AFP, DCP, and Tumor-Node-Metastasis (TNM) stage were examined to identify possible relationships with HCC-nonBC using logistic regression analysis.
Treatment modalities
Patients diagnosed with HCC were assessed for surgery based on the extent of lobar involvement and liver function status. The extent of lobar involvement was evaluated based on a combination of USG, CT, MRI, and HAG findings. Patients were considered to be poor candidates for resection if they met any of the following criteria: (1) bilobar involvement, (2) evidence of tumor infiltration into the main portal vein or thrombosis of the vein, (3) evidence of extrahepatic metastases, (4) Child’s grade C cirrhosis, or (5) poor cardiac and/or respiratory status. If surgery was contraindicated or the patient refused to undergo an operation, RFA or PEI therapy was the second treatment choice, offered to those with HCCs less than 3 cm in diameter. The remaining patients without main portal vein thrombosis or extrahepatic metastasis were advised to undergo TACE, regardless of tumor size or number.
After initial treatment, the AFP levels and liver functions of the patients were assessed every 1 to 3 months, and USG imaging was performed every 3 to 6 months during the follow-up period. Patients suspected to have HCC recurrence were further evaluated by CT and/or MRI. The assessment of treatment for recurrent HCC was based on lobar involvement and liver function status as described for the initial treatment. RFA or liver transplantation to treat HCC was started at our institution in 2002. Furthermore, none of the subjects in our study received either of these treatments for recurrent HCC during the follow-up period.
Statistical analysis
The survival duration was the time from the diagnosis of HCC until the time of death or the time of preparation of this manuscript. The survival rate was analyzed using the Kaplan-Meier method, and differences between the survival probability curves were tested using the log-rank test. Descriptive summaries of study groups are reported as the median value (SD: standard deviation) and number (%). Data were analyzed using the Mann-Whitney U test for continuous ordinal data, and the chi-square test with Yates’ correction and Fisher’s exact test were used for intergroup comparisons to determine the association between 2 qualitative variables. P values <0.05 were considered to indicate a statistically significant difference. Variables achieving statistical significance according to univariate analysis were subsequently included in the multivariate analysis using a logistic regression model and were described as hazard ratios (HR) with 95% confidence intervals (CI). Coefficients were calculated from the linear discriminating function of the variables. Data analysis was performed using SPSS version 16.0 for Windows.
Results
Patient characteristics at enrollment
We diagnosed 2638 patients with HCC during the study period. Patient characteristics at the time of HCC diagnosis are presented in Table 1. The underlying causes of HCC were as follows: 474 (18%) patients were positive for HBsAg, 1533 (58%) were positive for HCVAb, 40 (2%) were positive for both HBsAg and HCVAb, and 591 (22%) were negative for HBsAg and anti-HCV.
Table 1.
Characteristics of 2,638 HCC patients.
| (SD) | (%) | |
|---|---|---|
| All | 2,638 | |
| Age (years) | 70.0 | 10.3 |
| Sex | ||
| Male | 1,786 | 68 |
| Female | 852 | 32 |
| Alcohol consumption (unknown 454) | ||
| Excessive | 236 | 9 |
| Not excessive | 511 | 13 |
| None | 1,437 | 54 |
| DM (unknown 326) | ||
| (+) | 651 | 25 |
| (−) | 1,661 | 63 |
| BMI (unknown 594) | 22.7 | 0.5 |
| Etiology of liver disease | ||
| HBV | 474 | 18 |
| HCV | 1,533 | 58 |
| HBV+HCV | 40 | 2 |
| NBNC | 591 | 22 |
| Underlying liver disease (unknown, 110) | ||
| Normal | 42 | 2 |
| Chronic hepatitis | 686 | 26 |
| Cirrhosis | 1,800 | 68 |
| Child-Pugh Grade (unknown, 88) | ||
| A | 1796 | 68 |
| B | 606 | 23 |
| C | 148 | 6 |
| TNM stage | ||
| I | 682 | 26 |
| II | 1,039 | 39 |
| III | 579 | 22 |
| IV | 338 | 12 |
| Treatment | ||
| Surgical resection | 391 | 15 |
| PEIT or RFA | 740 | 28 |
| TACE or TAI | 1,221 | 46 |
| Chemotherapy | 51 | 2 |
| Transplantation | 10 | 1 |
| Only palliative care | 225 | 9 |
| Platelets (104/mL) | 11.6 | 8.0 |
| AST (IU/L) | 56 | 101 |
| ALT (IU/L) | 45 | 402 |
| Bil (mg/dL) | 0.9 | 0.5 |
| Alb (g/dL) | 3.7 | 0.6 |
| PT (%) | 83 | 18 |
| AFP (ng/mL) | 25 | 89,580 |
| <20 | 1,209 | 46 |
| 20–199 | 761 | 29 |
| ≥200 | 668 | 25 |
| DCP (mAU/mL) | 89 | 75,076 |
| <40 | 991 | 38 |
| 40–199 | 583 | 22 |
| ≥200 | 1,064 | 40 |
| Observation period (years) | 1.8 | 2.3 |
Alcohol consumption: excessive; average daily consumption of an amount equivalent to 80 g of pure ethanol for a period of more than 10 years. Not excessive; Alcohol consumption: excessive; average daily consumption of an amount equivalent to 1–79 g of pure ethanol for a period of more than 10 years. Data are presented as median value (SD: standard deviation) or frequency (%).
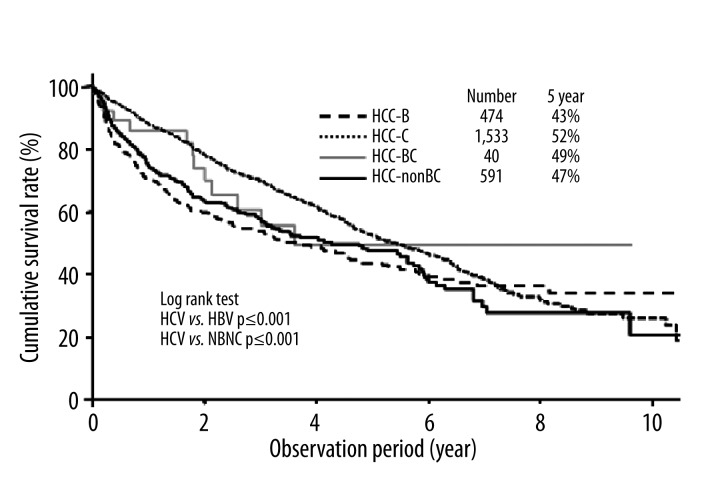
Overall, the median survival of all 2638 patients was 1.8 years. The cumulative 5-year survival rates of the patients with HCC-B, HCC-C, HCC-BC, and HCC-nonBC were 43%, 52%, 49%, and 47%, respectively (Figure 1). Patients in the HCC-C group had a higher cumulative survival rate than those in the HCC-B and HCC-nonBC groups.
Figure 1.
Cumulative survival rate of HCC patients according to chronic viral hepatitis infection.
Univariate and multivariate analyses of the factors associated with HCC-nonBC
Univariate and multivariate analyses were performed to identify factors independently related to HCC-nonBC. In the univariate analysis, the following 13 factors significantly influenced HCC-nonBC: age, sex, BMI, alcohol consumption, diabetes mellitus, underlying liver disease, platelet count, Bil, AST, ALT, AFP, DCP, and TNM stage (Table 2). Multivariate analysis identified age (≥70 years, HR 1.63), sex (female, HR 1.73), BMI (≥25, HR 2.12), alcohol consumption (not excessive, HR 3.41; excessive, HR 14.73), diabetes mellitus (HR 2.42), underlying liver disease (chronic hepatitis, HR 0.46; cirrhosis, HR 0.52), platelet count (<116,000/μL, HR 1.88), AST (<56 IU/L, HR 1.47), ALT (<46 IU/L, HR 2.48), AFP (20–199 ng/mL, HR 0.60; ≥200 ng/mL, HR 0.63), DCP (20–199 mAU/mL, HR 1.64; ≥200 mAU/mL, HR 2.08), and TNM stage (II, HR1.67; III, HR1.88; IV, HR 2.40), as independent and significant factors associated with HCC-nonBC (Table 3).
Table 2.
Univariate analysis of factors associated with HCC-nonBC.
| Parameters | Hazard ratio | P value |
|---|---|---|
| Age (years) ≥70 | 1.59 | <0.001 |
| Sex Female | 0.67 | <0.001 |
| BMI (kg/m2) ≥25 | 1.85 | <0.001 |
| Alcohol consumption | ||
| None | 1 | – |
| Not excessive | 2.57 | <0.001 |
| Excessive | 12.41 | <0.001 |
| Diabetes mellitus (%) | ||
| + | 2.96 | <0.001 |
| Underlying liver disease | ||
| Normal | 1 | – |
| Chronic hepatitis | 0.20 | <0.001 |
| Cirrhosis | 0.20 | <0.001 |
| Child-Pugh grade | ||
| A | 1 | – |
| B | 0.92 | 0.446 |
| C | 1.34 | 0.131 |
| Platelets (103/μL) ≥116 | 2.22 | <0.001 |
| AST (IU/L) <56 | 2.15 | <0.001 |
| ALT (IU/L) <46 | 2.64 | <0.001 |
| PT (%) ≥83 | 1.26 | 0.016 |
| Bil (mg/dL) <0.9 | 0.89 | 0.229 |
| Alb (mg/dL) ≥3.7 | 1.06 | 0.547 |
| AFP (ng/mL) | ||
| <20 | 1 | – |
| 20–199 | 0.48 | <0.001 |
| ≥200 | 0.82 | 0.079 |
| DCP (mAU/mL) | ||
| <40 | 1 | – |
| 40–199 | 1.66 | <0.001 |
| ≥200 | 2.73 | <0.001 |
| TNM stage | ||
| I | 1 | – |
| II | 1.66 | <0.001 |
| III | 2.73 | <0.001 |
| IV | 2.22 | <0.001 |
Table 3.
Multivariate analysis of factors associated with HCC-nonBC.
| Parameters | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Age (years) ≥70 | 1.63 | 1.21–2.20 | 0.001 |
| Sex Female | 1.73 | 1.33–2.85 | <0.001 |
| BMI (kg/m2) ≥25 | 2.12 | 1.58–2.83 | <0.001 |
| Alcohol consumption | |||
| None | 1 | – | – |
| Not excessive | 3.41 | 2.43–4.79 | <0.001 |
| Excessive | 14.73 | 9.48–22.9 | <0.001 |
| Diabetes mellitus (%) | |||
| (+) | 2.42 | 1.82–3.22 | <0.001 |
| Underlying liver disease | |||
| Normal | 1 | – | – |
| Chronic hepatitis | 0.46 | 0.01–0.16 | <0.001 |
| Cirrhosis | 0.52 | 0.02–0.19 | <0.001 |
| Platelets (103/μL) ≥116 | 1.88 | 1.35–2.60 | <0.001 |
| AST (IU/L) <56 | 1.47 | 1.01–2.10 | 0.411 |
| ALT (IU/L) <46 | 2.08 | 1.47–2.94 | <0.001 |
| PT (%) ≥83 | 0.97 | 0.71–1.32 | 0.826 |
| AFP (ng/mL) | |||
| <20 | 1 | – | – |
| 20–199 | 0.60 | 0.42–0.85 | 0.005 |
| ≥200 | 0.63 | 0.43–0.92 | 0.079 |
| DCP (mAU/mL) | |||
| <40 | 1 | – | – |
| 40–199 | 1.64 | 1.13–2.39 | 0.010 |
| ≥200 | 1.88 | 1.35–2.60 | 0.018 |
| TNM stage | |||
| I | 1 | – | – |
| II | 1.67 | 1.13–2.48 | 0.011 |
| III | 1.88 | 1.19–2.96 | 0.007 |
| IV | 2.40 | 1.32–4.35 | <0.001 |
CI – confidence interval.
Comparison of biomarkers according to liver disease cause
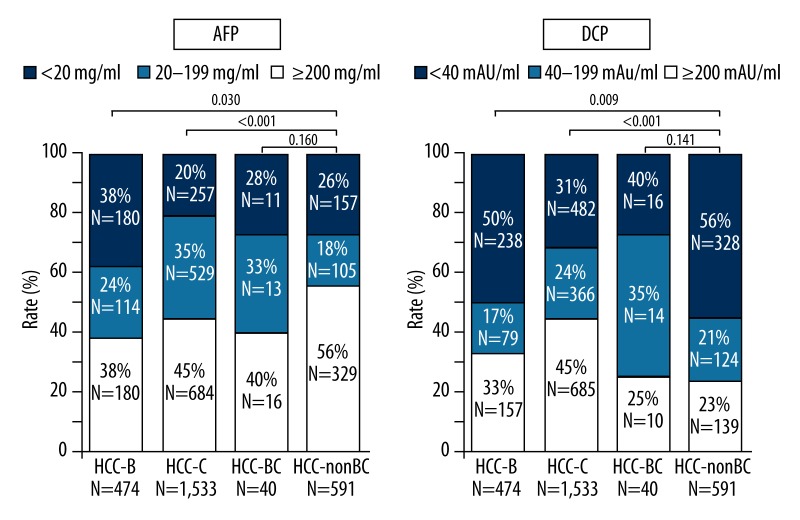
The positive rate of AFP (≥20 ng/ml) in HCC-B, HCC-C, HCC-BC, and HCC-nonBC were 62%, 55%, 61%, and 44%, respectively; whereas the positive rate of DCP (≥40 mAU/ml) were 67%, 55%, 75% and 77%, respectively (Figure 2). The positive rate of AFP in HCC-nonBC was significantly lower than those in the HCC-B and HCC-C groups, whereas the positive rate of DCP was significantly higher than that in the HCC-B and HCC-C groups.
Figure 2.
The positive rate of AFP (≥20 ng/ml) and DCP (≥40 mAU/ml) in HCC-B, HCC-C, HCC-BC and HCC-nonBC.
The median AFP and DCP levels in HCC-B, HCC-C, HCC-BC, and HCC-nonBC according to TNM stage are presented in Table 4. The median AFP levels in HCC-B, HCC-C, HCC-BC, and HCC-nonBC were 60 ng/mL, 25 ng/mL, 29 ng/mL, and 13 ng/mL, respectively; whereas the DCP levels were 4990 mAU/mL, 418 mAU/mL, 612 mAU/mL, and 3077 mAU/mL, respectively. The median AFP level in HCC-nonBC was significantly lower than those in the other groups; whereas the median DCP level was significantly higher than that in the HCC-C group.
Table 4.
Median AFP and DCP levels in HCC-B, HCC-C, HCC-BC and HCC-nonBC according to TNM stage.
| HCC-B | HCC-C | HCC-BC | HCC-nonBC | |
|---|---|---|---|---|
| All patients | ||||
| Number | 474 | 1,533 | 40 | 591 |
| AFP (ng/mL) (range) | 60 (1–2,920,000)* | 25 (1–1,438,472)* | 29 (3–189,850)*** | 13 (1–8,145,000) |
| DCP (mAU/mL) (range) | 4,990 (4–1,497,560) | 418 (1–871,700)* | 612 (10–266,260) | 3,077 (5–265,000,000) |
| TNM stage I | ||||
| Number | 103 | 480 | 13 | 77 |
| AFP (ng/mL) (range) | 15 (1–6,300)** | 16 (1–3,188)* | 28 (3–2,120)* | 6 (1–9,820) |
| DCP (mAU/mL) (range) | 26 (3–1,038)** | 25 (1–20,448)* | 39 (14–353) | 44 (12–12,224) |
| TNM stage II | ||||
| Number | 150 | 624 | 11 | 254 |
| AFP (ng/mL) (range) | 14 (1–181,150)** | 24 (1–200,000)* | 17 (5–952) | 8 (1–114,907) |
| DCP (mAU/mL) (range) | 72 (4–233,780)* | 53 (2–74,493)* | 173 (10–831) | 255 (5–369,000) |
| TNM stage III | ||||
| Number | 92 | 319 | 12 | 157 |
| AFP (ng/mL) (range) | 77 (2–453,000)*** | 48 (1–196,000)** | 47 (4–72,727) | 25 (1–246,940) |
| DCP (mAU/mL) (range) | 535 (10–172,000) | 305 (4–14,410) *** | 206 (16–4,039) | 460 (10–109,350) |
| TNM stage IV | ||||
| Number | 129 | 102 | 4 | 103 |
| AFP (ng/mL) (range) | 4,450 (1.5–2,920,000)** | 2,379 (2–1,438,472) | 64,838 (2882–189,850)*** | 914 (1–8,145,000) |
| DCP (mAU/mL) (range) | 9,573 (10–1,497,560) | 8,954 (19–871,700) | 104,254 (755–266,260) | 3,340 (12–265,000,000) |
p≤0.001 vs. HCC-nonBC;
p≤0.01 vs. HCC-nonBC;
p≤0.05 vs. HCC-nonBC.
According to TNM stage, the median AFP levels in HCC-nonBC with TNM stages I, II, and III were significantly lower than that in either HCC-B or HCC-C. In TNM stage IV, the median AFP level in HCC-nonBC was significantly lower than that in either HCC-B or HCC-BC. The median DCP levels in HCC-nonBC with TNM stages I and II were significantly higher than that in either HCC-B or HCC-C. In TNM stage III, the median DCP level in HCC-nonBC was significantly higher than that in HCC-C. However, there were no significant differences in the median DCP level among TNM stage IV cases.
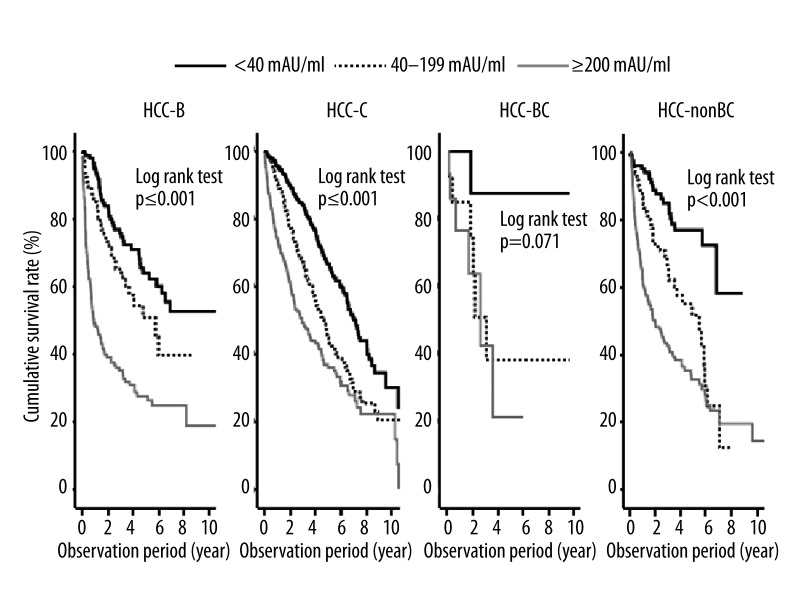
The survival rate of patients in the high DCP group (≥200 mAU/mL) was significantly lower than that of patients classified into the low DCP (40–199 mAU/mL) and DCP negative (<40 mAU/mL) groups among those with HCC-B, HCC-C, and HCC-nonBC (p≤0.001; log-rank test) (Figure 3).
Figure 3.
Cumulative survival rate of HCC patients according to DCP levels stratified by chronic viral hepatitis infection.
Discussion
The age-adjusted mortality rate for HCC has increased over the past few decades in Japan [27]. However, most patients are still diagnosed at an advanced stage and their survival time is therefore short. Patients with chronic HBV and/or HCV infection in addition to cirrhosis should be monitored with USG and/or CT and/or MRI of the liver to detect tumors at an early stage. HCC surveillance using imaging modalities is usually performed at 6-month intervals [28,29]. There is a large population of individuals infected with HCV, HBV, or both in whom cancer is in the latency period. For those who harbor chronic HCV and/or HBV infections, attention must be focused on the detection of HCC at an early stage. In this study, more than 75% of patients with HCC were positive for HBV and/or HCV. Additionally, our data showed patients with HCC-nonBC to generally be diagnosed at an advanced stage. Thus, the target population for HCC surveillance must be easily identifiable. However, it will not be easy to select appropriate subjects for screening of HCC among those negative for both HBsAg and HCVAb.
AFP has long been considered the ideal serological marker for detecting HCC. Persistently elevated AFP is well known to be related to the presence of HCC and its determination can facilitate better identification of patients at risk. However, in our present dataset, the median serum AFP level in HCC-nonBC was not abnormal, whereas those in HCC-B and HCC-C cases were abnormal. Few early-stage HCC-nonBC cases present with abnormal AFP serum levels. Several reports have shown elevated AFP to be a risk factor for HCC development in HCV and/or HBV patients [24,30–36]. However, our results suggest AFP alone to be insufficient for HCC-nonBC surveillance.
Since Liebman et al. demonstrated DCP to be a useful marker for HCC diagnosis, many studies have compared DCP and AFP. Several investigations have made comparisons of the usefulness of DCP and AFP for HCC diagnosis [37–40]. However, whether AFP is superior to DCP in all cases is still controversial. Even the sensitivities and specificities reported by these studies were quite different. One reason for these differences involves the use of different cut-off values in the various studies (e.g., 40, 60, and 100 mAU/mL for DCP; and 20, 100, and 200 ng/mL for AFP). Other possible reasons include differences in the causes of the underlying liver diseases, and patients with cirrhosis tending to have higher AFP levels than those with chronic hepatitis [36,41]. Another possible reason for these differences might be etiological differences in liver diseases among the patients examined in prior studies. In this study, the median AFP level in HCC-nonBC was significantly lower than that in either HCC-B or HCC-C, whereas the median DCP level was significantly higher. Our data suggest that DCP levels differ among liver diseases with different etiologies. The high value identified in our study may be related to the higher DCP values in patients without hepatitis virus infection.
The biological function of AFP is still not well identified. Since AFP is similar to albumin, it is possible that AFP function as a carrier for several ligands such as bilirubin, fatty acids, steroids, heavy metals, flavonoids, phytoestrogens, dioxin, and various drugs [42]. The increase of AFP levels to 500 ng/ml is correlated with the tumor size; 80% of small HCC show no increase of AFP concentration. Furthermore, sensitivity of AFP decreases from 52% to 25% when tumor diameter is >3 and <3 cm, respectively [42].
There are various differences between DCP and total AFP. Firstly, DCP is a more specific HCC marker than AFP because other liver diseases do not cause an increase of DCP serum levels. DCP measurement for HCC has a sensitivity of 48–62% and a specificity of 81–98% [43]. However, we often encounter patients with liver disease who have slightly elevated DCP levels, but undetectable HCC as assessed by imaging studies. It has been reported that aberrant elevation of DCP is occasionally observed in patients with alcoholic cirrhosis, obstructive jaundice, or vitamin K deficiency [44]. Recently, Toyoda et al. measured a novel DCP (NX-DCP) in serum using a newly developed sandwich ECLIA with new anti-DCP monoclonal antibodies p11 and p16, and reported preliminary data from only 20 HCC patients. They showed that the DCP/NX-DCP ratio may be useful for the diagnosis of HCC among warfarin users [45].
Neither DCP alone nor AFP alone was optimal for the detection of HCC, but the combination of both markers enhanced sensitivity, indicating that these 2 markers are complementary. Several other studies have shown DCP and AFP to be complementary, which is consistent with the production of DCP and AFP in HCC occurring through different pathways, possibly explaining why sex, race, underlying liver disease, and hepatic disease etiologies had opposite effects on these 2 markers [46–48].
Conclusions
In conclusion, this retrospective cohort study demonstrated DCP to be more sensitive than AFP for the diagnosis of early-stage cryptogenic HCC. We advocate that DCP be used as the main serum test for detecting cryptogenic HCC.
Footnotes
Conflict of interest
The following people have nothing to disclose: Naota Taura, Tatsuki Ichikawa, Hisamitsu Miyaaki, Eisuke Ozawa, Takuya Tsutsumi, Shotaro Tsuruta, Yuji Kato, Takashi Goto, Noboru Kinoshita, Masanori Fukushima, Hiroyuki Kato, Kazuyuki Ohata, Kazuo Ohba, Junichi Masuda, Keisuke Hamasaki, Hiroshi Yatsuhashi, and Kazuhiko Nakao.
Source of support: Self financing
References
- 1.El-Serag HB, Mason AC. Risk factors for the rising rates of primary liver cancer in the United States. Arch Intern Med. 2000;160:3227–30. doi: 10.1001/archinte.160.21.3227. [DOI] [PubMed] [Google Scholar]
- 2.el-Serag HB. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2001;5:87–107. doi: 10.1016/s1089-3261(05)70155-0. , vi. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Hampel H, Yeh C, et al. Extrahepatic manifestations of hepatitis C among United States male veterans. Hepatology. 2002;36:1439–45. doi: 10.1053/jhep.2002.37191. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB. Hepatocellular carcinoma and hepatitis C in the United States. Hepatology. 2002;36:S74–83. doi: 10.1053/jhep.2002.36807. [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol. 2002;35:S72–78. doi: 10.1097/00004836-200211002-00002. [DOI] [PubMed] [Google Scholar]
- 6.Hassan MM, Frome A, Patt YZ, et al. Rising prevalence of hepatitis C virus infection among patients recently diagnosed with hepatocellular carcinoma in the United States. J Clin Gastroenterol. 2002;35:266–69. doi: 10.1097/00004836-200209000-00013. [DOI] [PubMed] [Google Scholar]
- 7.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 8.Kiyosawa K, Tanaka E. Characteristics of hepatocellular carcinoma in Japan. Oncology. 2002;62:5–7. doi: 10.1159/000048269. [DOI] [PubMed] [Google Scholar]
- 9.McGlynn KA, Tsao L, Hsing AW, et al. International trends and patterns of primary liver cancer. Int J Cancer. 2001;94:290–96. doi: 10.1002/ijc.1456. [DOI] [PubMed] [Google Scholar]
- 10.Bosch FX, Ribes J, Diaz M, et al. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Hamasaki K, Nakata K, Tsutsumi T, et al. Changes in the prevalence of hepatitis B and C infection in patients with hepatocellular carcinoma in the Nagasaki Prefecture, Japan. J Med Virol. 1993;40:146–49. doi: 10.1002/jmv.1890400212. [DOI] [PubMed] [Google Scholar]
- 12.Kato Y, Nakata K, Omagari K, et al. Risk of hepatocellular carcinoma in patients with cirrhosis in Japan. Analysis of infectious hepatitis viruses. Cancer. 1994;74:2234–38. doi: 10.1002/1097-0142(19941015)74:8<2234::aid-cncr2820740805>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 13.Shiratori Y, Shiina S, Imamura M, et al. Characteristic difference of hepatocellular carcinoma between hepatitis B- and C-viral infection in Japan. Hepatology. 1995;22:1027–33. doi: 10.1016/0270-9139(95)90605-3. [DOI] [PubMed] [Google Scholar]
- 14.Shiratori Y, Shiina S, Zhang PY, et al. Does dual infection by hepatitis B and C viruses play an important role in the pathogenesis of hepatocellular carcinoma in Japan? Cancer. 1997;80:2060–67. [PubMed] [Google Scholar]
- 15.Taura N, Yatsuhashi H, Nakao K, et al. Long-term trends of the incidence of hepatocellular carcinoma in the Nagasaki prefecture, Japan. Oncol Rep. 2009;21:223–27. [PubMed] [Google Scholar]
- 16.Taura N, Fukushima N, Yastuhashi H, et al. The incidence of hepatocellular carcinoma associated with hepatitis C infection decreased in Kyushu area. Med Sci Monit. 2011;17(2):PH7–11. doi: 10.12659/MSM.881375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Omata M, Tateishi R, Yoshida H, et al. Treatment of hepatocellular carcinoma by percutaneous tumor ablation methods: Ethanol injection therapy and radiofrequency ablation. Gastroenterology. 2004;127:S159–66. doi: 10.1053/j.gastro.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 18.Calvet X, Bruix J, Gines P, et al. Prognostic factors of hepatocellular carcinoma in the west: a multivariate analysis in 206 patients. Hepatology. 1990;12:753–60. doi: 10.1002/hep.1840120422. [DOI] [PubMed] [Google Scholar]
- 19.Schafer DF, Sorrell MF. Hepatocellular carcinoma. Lancet. 1999;353:1253–57. doi: 10.1016/S0140-6736(98)09148-X. [DOI] [PubMed] [Google Scholar]
- 20.Akriviadis EA, Llovet JM, Efremidis SC, et al. Hepatocellular carcinoma. Br J Surg. 1998;85:1319–31. doi: 10.1046/j.1365-2168.1998.00865.x. [DOI] [PubMed] [Google Scholar]
- 21.Ngan H, Lai CL, Fan ST, et al. Transcatheter arterial chemoembolization in inoperable hepatocellular carcinoma: four-year follow-up. J Vasc Interv Radiol. 1996;7:419–25. doi: 10.1016/s1051-0443(96)72881-6. [DOI] [PubMed] [Google Scholar]
- 22.Oka H, Kurioka N, Kim K, et al. Prospective study of early detection of hepatocellular carcinoma in patients with cirrhosis. Hepatology. 1990;12:680–87. doi: 10.1002/hep.1840120411. [DOI] [PubMed] [Google Scholar]
- 23.Lai CL, Lau JY, Wu PC, et al. Subclinical hepatocellular carcinoma in Hong Kong Chinese. Oncology. 1992;49:347–53. doi: 10.1159/000227071. [DOI] [PubMed] [Google Scholar]
- 24.Colombo M, de Franchis R, Del Ninno E, et al. Hepatocellular carcinoma in Italian patients with cirrhosis. N Engl J Med. 1991;325:675–80. doi: 10.1056/NEJM199109053251002. [DOI] [PubMed] [Google Scholar]
- 25.Pateron D, Ganne N, Trinchet JC, et al. Prospective study of screening for hepatocellular carcinoma in Caucasian patients with cirrhosis. J Hepatol. 1994;20:65–71. doi: 10.1016/s0168-8278(05)80468-4. [DOI] [PubMed] [Google Scholar]
- 26.Akahoshi H, Taura N, Ichikawa T, et al. Differences in prognostic factors according to viral status in patients with hepatocellular carcinoma. Oncol Rep. 2010;23:1317–23. doi: 10.3892/or_00000766. [DOI] [PubMed] [Google Scholar]
- 27.Wada I, Hara T, Kajihara S, et al. Population-based study of hepatitis C virus infection and hepatocellular carcinoma in western Japan. Hepatol Res. 2002;23:18–24. doi: 10.1016/s1386-6346(01)00161-9. [DOI] [PubMed] [Google Scholar]
- 28.Dohmen K, Shirahama M, Onohara S, et al. Differences in survival based on the type of follow-up for the detection of hepatocellular carcinoma: an analysis of 547 patients. Hepatol Res. 2000;18:110–21. doi: 10.1016/s1386-6346(99)00094-7. [DOI] [PubMed] [Google Scholar]
- 29.Okuda H, Nakanishi T, Takatsu K, et al. Comparison of clinicopathological features of patients with hepatocellular carcinoma seropositive for alpha-fetoprotein alone and those seropositive for des-gamma-carboxy prothrombin alone. J Gastroenterol Hepatol. 2001;16:1290–96. doi: 10.1046/j.1440-1746.2001.02610.x. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez-Diaz JL, Rosas-Camargo V, Vega-Vega O, et al. Clinical and pathological factors associated with the development of hepatocellular carcinoma in patients with hepatitis virus-related cirrhosis: a long-term follow-up study. Clin Oncol (R Coll Radiol) 2007;19:197–203. doi: 10.1016/j.clon.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 31.Bruix J, Sherman M Practice Guidelines Committee AAftSoLD. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–36. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 32.Tsukuma H, Hiyama T, Tanaka S, et al. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med. 1993;328:1797–801. doi: 10.1056/NEJM199306243282501. [DOI] [PubMed] [Google Scholar]
- 33.Oka H, Tamori A, Kuroki T, et al. Prospective study of alpha-fetoprotein in cirrhotic patients monitored for development of hepatocellular carcinoma. Hepatology. 1994;19:61–66. [PubMed] [Google Scholar]
- 34.Ganne-Carrie N, Chastang C, Chapel F, et al. Predictive score for the development of hepatocellular carcinoma and additional value of liver large cell dysplasia in Western patients with cirrhosis. Hepatology. 1996;23:1112–18. doi: 10.1002/hep.510230527. [DOI] [PubMed] [Google Scholar]
- 35.Sangiovanni A, Colombo E, Radaelli F, et al. Hepatocyte proliferation and risk of hepatocellular carcinoma in cirrhotic patients. Am J Gastroenterol. 2001;96:1575–80. doi: 10.1111/j.1572-0241.2001.03780.x. [DOI] [PubMed] [Google Scholar]
- 36.Tateyama M, Yatsuhashi H, Taura N, et al. Alpha-fetoprotein above normal levels as a risk factor for the development of hepatocellular carcinoma in patients infected with hepatitis C virus. J Gastroenterol. 2011;46:92–100. doi: 10.1007/s00535-010-0293-6. [DOI] [PubMed] [Google Scholar]
- 37.Weitz IC, Liebman HA. Des-gamma-carboxy (abnormal) prothrombin and hepatocellular carcinoma: a critical review. Hepatology. 1993;18:990–97. doi: 10.1002/hep.1840180434. [DOI] [PubMed] [Google Scholar]
- 38.Kasahara A, Hayashi N, Fusamoto H, et al. Clinical evaluation of plasma des-gamma-carboxy prothrombin as a marker protein of hepatocellular carcinoma in patients with tumors of various sizes. Dig Dis Sci. 1993;38:2170–76. doi: 10.1007/BF01299891. [DOI] [PubMed] [Google Scholar]
- 39.Takikawa Y, Suzuki K, Yamazaki K, et al. Plasma abnormal prothrombin (PIVKA-II): a new and reliable marker for the detection of hepatocellular carcinoma. J Gastroenterol Hepatol. 1992;7:1–6. doi: 10.1111/j.1440-1746.1992.tb00925.x. [DOI] [PubMed] [Google Scholar]
- 40.Grazi GL, Mazziotti A, Legnani C, et al. The role of tumor markers in the diagnosis of hepatocellular carcinoma, with special reference to the des-gamma-carboxy prothrombin. Liver Transpl Surg. 1995;1:249–55. doi: 10.1002/lt.500010410. [DOI] [PubMed] [Google Scholar]
- 41.Marrero JA, Su GL, Wei W, et al. Des-gamma carboxyprothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in american patients. Hepatology. 2003;37:1114–21. doi: 10.1053/jhep.2003.50195. [DOI] [PubMed] [Google Scholar]
- 42.Malaguarnera G, Giordano M, Paladina I, et al. Serum markers of hepatocellular carcinoma. Dig Dis Sci. 2010;55:2744–55. doi: 10.1007/s10620-010-1184-7. [DOI] [PubMed] [Google Scholar]
- 43.Nakagawa T, Seki T, Shiro T, et al. Clinicopathologic significance of protein induced vitamin K absence or antagonist II and alpha-fetoprotein in hepatocellular carcinoma. Int J Oncol. 1999;14:281–86. doi: 10.3892/ijo.14.2.281. [DOI] [PubMed] [Google Scholar]
- 44.Tameda M, Shiraki K, Sugimoto K, et al. Des-gamma-carboxy prothrombin ratio measured by P-11 and P-16 antibodies is a novel biomarker for hepatocellular carcinoma. Cancer Sci. 2013;104:725–31. doi: 10.1111/cas.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toyoda H, Kumada T, Osaki Y, et al. Novel method to measure serum levels of des-gamma-carboxy prothrombin for hepatocellular carcinoma in patients taking warfarin: a preliminary report. Cancer Sci. 2012;103:921–25. doi: 10.1111/j.1349-7006.2012.02232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lok AS, Sterling RK, Everhart JE, et al. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology. 2010;138:493–502. doi: 10.1053/j.gastro.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okuda H, Obata H, Nakanishi T, et al. Production of abnormal prothrombin (des-gamma-carboxy prothrombin) by hepatocellular carcinoma. A clinical and experimental study. J Hepatol. 1987;4:357–63. doi: 10.1016/s0168-8278(87)80546-9. [DOI] [PubMed] [Google Scholar]
- 48.Beale G, Chattopadhyay D, Gray J, et al. AFP, PIVKAII, GP3, SCCA-1 and follisatin as surveillance biomarkers for hepatocellular cancer in non-alcoholic and alcoholic fatty liver disease. BMC Cancer. 2008;8:200. doi: 10.1186/1471-2407-8-200. [DOI] [PMC free article] [PubMed] [Google Scholar]