Abstract
Purpose
To identify the genes, biochemical signaling pathways and biological themes involved in the pathogenesis of retinopathy of prematurity (ROP).
Methods
Next-generation sequencing (NGS) was performed on the RNA transcriptome of rats with the Penn et al. (1994) oxygen-induced retinopathy (OIR) model of ROP at the height of vascular abnormality, postnatal day (P) 19, and normalized to age-matched, room-air-reared littermate controls. Eight custom developed pathways with potential relevance to known ROP sequelae were evaluated for significant regulation in ROP: The three major Wnt signaling pathways, canonical, planar cell polarity (PCP), and Wnt/Ca2+, two signaling pathways mediated by the Rho GTPases RhoA and Cdc42, which are respectively thought to intersect with canonical and noncanonical Wnt signaling, nitric oxide signaling pathways mediated by two nitrox oxide synthase (NOS) enzymes, neuronal (nNOS) and endothelial (eNOS), and the retinoic acid (RA) signaling pathway. Regulation of other biological pathways and themes were detected by gene ontology using the Kyoto Encyclopedia of Genes and Genomes (KEGG) and the NIH's Database for Annotation, Visualization and Integrated Discovery (DAVID)'s GO terms databases.
Results
Canonical Wnt signaling was found to be regulated, but the non-canonical PCP and Wnt/Ca2+ pathways were not. Nitric oxide (NO) signaling, as measured by the activation of nNOS eNOS, was also regulated, as was RA signaling. Biological themes related to protein translation (ribosomes), neural signaling, inflammation and immunity, cell cycle and cell death, were (among others) highly regulated in ROP rats.
Conclusions
These several genes and pathways identified by NGS might provide novel targets for intervention in ROP.
Introduction
Complex interactions occur between the neural retina and its vascular supply in normal retinal development and in retinal disease [1]. Retinopathy of prematurity (ROP) occurs when the prematurely born infant is exposed to supplemental oxygen [2-7] during ages at which the retina [8-16] and its vascular supply [17] are both immature and the eye is growing rapidly [18-21]. Although a critical role for the neural retina in the pathogenesis of ROP has become increasingly recognized [22, 23], the clinical hallmark of active ROP continues to be abnormal retinal vasculature: tortuous arterioles, dilated venules, and vitreoretinal neovascularization (NV) [24]. Nevertheless, lifelong retinal dysfunction is found even if the ROP was so mild that the vascular disease resolved spontaneously [25-29]. Furthermore, the most common clinical sequela of ROP is altered growth of the eye and its refractive components [18-21, 30-36]. The mechanisms underpinning the altered eye growth in ROP remain poorly understood.
It follows that, to date, most of the ROP research, in patients and in animal models, has been focused on the vascular abnormalities. The critical pro-angiogenesis messenger vascular endothelial growth factor (VEGF) has been targeted in severe, active ROP, with promising therapeutic success [37]. Recently, alternative pathways that impact both vascular and neural tissues rather directly, such as the Wnt signaling pathway, have also been implicated in ROP [38]. The continued discovery of new pathways important to ROP pathology suggests that there are still many more. Because it is so complex, a modern approach is needed to consider the biological factors that lead to the distinct pathology of the ROP eye. Next-generation sequencing (NGS) assesses the relative expression of every gene in an organism's genome and can, therefore, identify a large number of potential targets for intervention. NGS is an hypothesis generator.
We used NGS to evaluate the transcriptome (mRNA) of the Penn et al. “50/10” oxygen-induced retinopathy (OIR) rat model of ROP [39] at the height of vasculopathy (postnatal day 19). Thus, we determined all genes that were regulated in the eye afflicted by OIR at this cross-section in time. Our data supplement those already obtained by microarray [40]. In addition to ascertaining individually regulated genes, we conducted gene analysis on pathways annotated by the Kyoto Encyclopedia of Genes and Genomes (KEGG) and the NIH's Database for Annotation, Visualization and Integrated Discovery (DAVID)'s GO terms. We also tested eight systems for which published data and our own preliminary data suggest might be intimately involved in the ROP disease process for likely involvement in ROP pathogenesis (Table 1).
Table 1. Literature-based biochemical signaling pathways.
| Name | P |
|---|---|
| Canonical Wnt | 2.55E-04 |
| Noncanonical PCP Wnt | 0.0563 |
| Noncanonical Wnt/Ca2+ | 0.412 |
| Rhoa | 0.179 |
| Cdc42 | 0.315 |
| nNOS | 0.0337 |
| eNOS | 0.0733 |
| Retinoic Acid | 0.0368 |
First, we looked at canonical Wnt signaling. This pathway is, by definition, activated when a Wnt ligand binds to a frizzled (Frz) receptor on a cell membrane [41]. Disruption in the canonical Wnt pathway results in reduced neovascularization (NV) in the Smith et al. [42] mouse model of ROP [43]. In patients with familial exudative vitreo-retinopathy (FEVR), particular genetic mutations lead to failure of activation of the Frz receptor. Infants with severe FEVR (and Norrie Disease, ND) have tortuous, dilated retinal vessels, an avascular peripheral retina, subretinal exudation, and extraretinal NV with consequent vitreoretinal traction, features common in ROP. Indeed, recent data suggest that as many as 10% of severe ROP cases are complicated by Wnt signaling disorders [44, 45]. Interestingly, as in mild ROP, in mild FEVR/ND, there may be significant retinal and visual dysfunction [46].
Wnt signaling coopts the ubiquitous, versatile, and complex effectors of biochemical activity, the Rho family of GTPases [47]. The Rho GTPases belong to the ‘rat sarcoma’ (Ras) superfamily. They act as molecular switches. The function of Rho GTPases depends upon their various downstream effectors [48]. There is accumulating evidence that misregulated Rho GTPase signaling is involved in wide variety of vascular and neural pathological processes (e.g., cerebral and coronary spasm [49], hypertension [50], arterio-sclerosis [51], nerve injury/regeneration [52-54], neuronal degeneration [55], and a whole host of ocular diseases (e.g., retinal gliosis [56], retinal ischemia and reperfusion injury [57], glaucoma [58-62], diabetic retinopathy[63]), including ROP [64]. RhoA's primary downstream effector is Rho-kinase (ROCK) [48, 65, 66]. The lysophosphatidic acid receptor LPA1 plays a critical role in oxygen-induced retinal ganglion cell degeneration that is likely mediated by ROCK [67], and the ROCK inhibitor Y-27632 reduces the severity of vascular abnormality in OIR [64]. Cell division cycle 42 (Cdc42) Rho GTPase isoforms interact with other downstream effectors such as p21-activated kinase (PAK). Collectively, Rho GTPase signaling is essential for fundamental cellular functions including cell cycle progression, gene expression, cytoskeleton dynamics regulation, cell adhesion/migration, cell proliferation and (of particular importance to ROP) angiogenesis and neurogenesis [68-77]. Wnt signaling primarily interacts with Rho GTPase signaling via two alternative pathways activated upon Frz activation: the non-canonical planar cell polarity (PCP) pathway and the Wnt/Ca2+ pathway. In the PCP pathway, Dsh complexes with Dsh-associated activator of morphogenesis (Daam) which activates Rho. Additionally, Dsh also recruits the Rho GTPase Rac. Furthermore, Cdc42 is recruited by the other non-canonical pathway, Wnt/Ca2+ [78]. Thus, we also looked at the two major non-canonical Wnt pathways (PCP, Wnt/Ca2+), and the downstream RhoA and Cdc42 GTPase signaling pathways.
Next, we considered the nitric oxide (NO) signaling pathway, a signaling system in neurovascular tissues including the retina [79-81]. Regulation of NO was recently shown to be effective at preventing neuropathology in the early stages of a mouse model of diabetic retinopathy [82]. The generation of NO is controlled by a family of enzymes, called NO synthase (NOS), which catalyze its production from L-arginine: neuronal NOS (nNOS), endothelial NOS (eNOS), and inducible NOS (iNOS). nNOS is the isoform most involved in regulation of neural function, while eNOS is associated with vascular changes, and iNOS is inducible only in pathological conditions [83]. All classes of retinal neurons are likely affected by NO, principally via its activation of soluble guanylyl cyclase (sGC) [84]. NO increases the voltage range of exocytosis in photoreceptors [85, 86], reduces light responses in horizontal cells [87, 88], increases the circulating current of bipolar cells [89, 90], uncouples gap junctions between AII amacrine cells and cone bipolars [91], and more. NO also impacts the retinal vasculature. For instance, NO is involved in helping the choroid respond to changes in oxygen tension [92]. Changes in NOS activity are also associated with profound changes to blood vessels in retinal diseases such as diabetic retinopathy and glaucoma [93].
Finally, mindful of the fact that the most common clinical sequela of ROP is refractive error, we also looked at retinoic acid (RA) signaling, a candidate pathway for mediation of myopia; notably, though, expression of the gene encoding the retinoic acid receptor (RARA) is not likely altered in myopia [94], RA levels are higher in the choroid than any other tissue in the body. Increased levels of RA in the choroid are associated with decreased eye growth while, paradoxically, increased RA in the neural retina is associated with increased eye growth [95]. Vitamin A supplementation at preterm ages is beneficial to retinal function in ROP [96]. It is, therefore, plausible that changes in RA production (and possibly its receptors) are important controllers of eye growth and refractive error in ROP. Furthermore, nNOS is associated with rapid swelling of the choroid and changes in the sclera that alter the size and shape of the ametropic chick eye [97-99]. NOS is strongly up-regulated in mammalian form-deprivation myopia, too, although such dramatic changes in the choroid and sclera are not found [100]. NO nevertheless remains another compelling candidate for controlling eye growth and refractive development [95].
In addition to testing these specific hypothesis, we make the gene expression database available (see online supporting materials) for other labs to inspect and test their own hypothesis against.
Methods
Preparation of Genetic Material
The retinae of ten, 19 day old Sprague-Dawley rats were studied. Half had retinopathy induced following the Penn et al. “50/10” paradigm [101], as previously described [102-105]. The other half were room-air-reared (RAR) littermates. Briefly, newborn ROP rat pups were exposed to alternating 24 h periods of 50% and 10% oxygen from the day of birth until P14, when they were returned to room air (∼21% oxygen). On P19, when neovascularization and arteriolar tortuosity are most severe, the ROP rats and RAR controls were sacrificed with an overdose of sodium pentobarbital and their retinae quickly dissected from the eye. These experiments complied with the ARVO Statement for the Use of Animals in Research.
Total RNA was obtained using an RNA Mini Prep Kit (Qiagen, Valencia CA). The cells of each retina were disrupted and homogenized by pulling samples, immersed in β-mercaptoethanol and Buffer RLT, through sequentially higher-gauged needles. The lysate was centrifuged, the supernatant removed and transferred to a clean container, and 70% ethanol added to precipitate the RNA. The sample was then centrifuged on a column to trap the RNA, rinsed with Buffer RW1 and RPE, and then the RNA was eluted using RNAse-free water. RNA purity and concentration were determined using a spectrophotometer (NanoDrop 8000, Thermo Scientific, Wilmington, DE). The contribution of each retina to the final analysis was normalized within group (ROP, RAR); volume was scaled by the inverse of concentration. Thus, each rat contributed the same amount of RNA to the respective group analysis.
Next-Generation Sequencing and Analysis
Whole-genome RNA next-generation sequencing (RNA-seq) was performed at the Harvard Medical School Biopolymers Core Facility (Boston, MA) using a HiSeq2000 Sequencer (Illumina, San Diego CA). Prior to sequencing, 4 μg of RNA was isolated from each sample (ROP, RAR) using a Dynabeads mRNA Purification Kit (Life Technologies, Carlsbad CA) and processed using the NEBNext mRNA Sample Prep Reagent Set 1 (New England Biolabs, Ipswitch, MA). The samples were next run on a Bioanalyzer 2100 High Sensitivity DNA chip (Agilent, Santa Clara, CA) and then in a SYBR-based qPCR assay to verify the presence of adapters. RNA library samples of ROP and RAR were sequenced with 100 base single-end reads. Reads were mapped to the rattus norvegicus reference genome (Baylor 3.4/rn4) using Tophat 2.0.1 software (http://tophat.cbcb.umd.edu/). Quantification for gene expression was performed as reads per kilobase of exon per million mapped reads (RPKM) [106]. A summary of the mapped reads is in Supplementary Table 1.
Derivation of Pathways
To evaluate the eight custom pathways (Table 1), diagrams were produced (Figs. 1 to 8). Qiagen pathway maps were used as an initial template for all pathways except the relatively simple RA synthesis pathway, which is outlined by Cvekl and Wang [107]. Additionally, the PCP pathway map was expanded using information compiled by Katoh [108]. The NCBI gene database was used to confirm the existence and name of each gene in the rat. Protein interactions in all pathways were confirmed to be accurate to current understanding using recent literature [109-143].
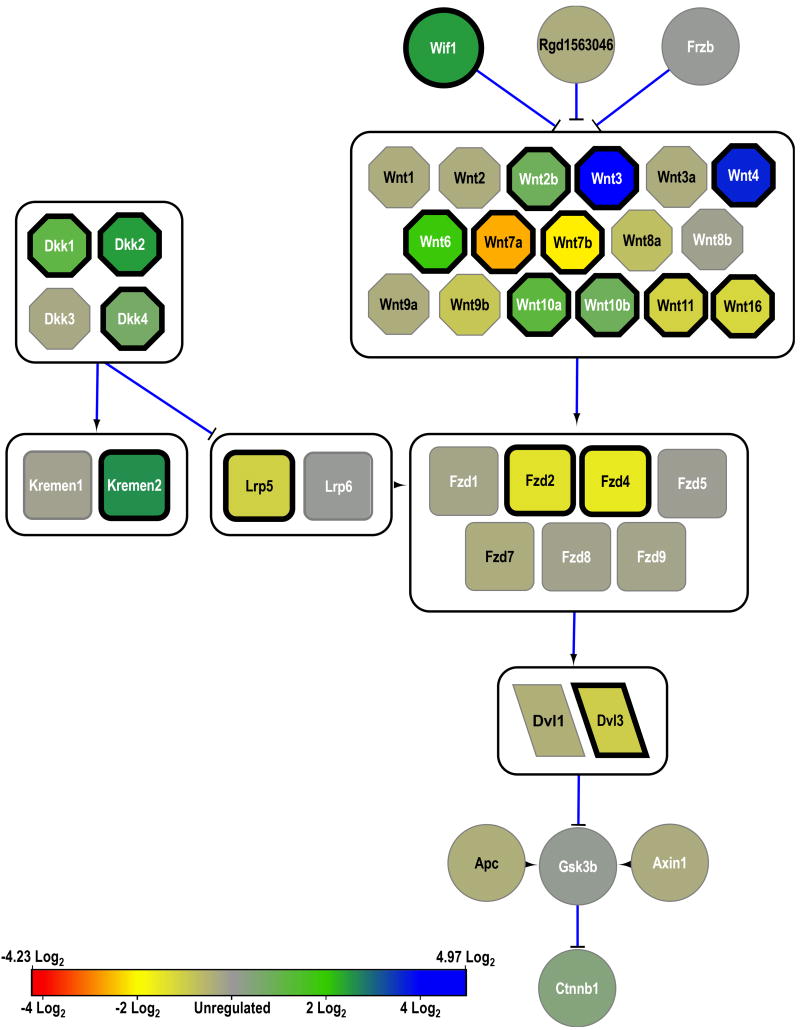
Figure 1.
Major elements of the canonical Wnt signaling pathway. Genes that encode membrane proteins are in squares, ligands in hexagons, and scaffolding proteins in rhomboids. All other genes are represented by circles. Lines ending in flat heads represent inhibition. Lines ending in arrowheads represent excitation. Arrowheads alone represent proteins that act together, such as coenzymes. Large boxes surround genes that act at the same step in the pathway. The expression of each gene in the ROP rats' retinae, relative to the RAR rats' retinae, is color-coded to the bar in the lower left. Bold borders around a gene indicate that it was significantly regulated (±0.77 log2).
Figure 8.
Major elements of retinoic acid signaling. Symbology as in figures 1 and 6.
Isoforms were removed if they were not present in the retina or if their retinal expression was known to be very low. The up- or down-regulation of each gene in each pathway was culled from the NGS database.
Although several papers have employed gene chips to analyze expression in murine OIR models of ROP [144-147], only one (to our knowledge), Recchia et al. [40], did so in the 50/10 ROP rat. Thus, in our study, genes were deemed ‘regulated’ if the fold change (FC) was greater than 1.7, up (+0.77 log2) or down (-0.77 log2), for easy comparison to Recchia et al.'s gene chip results wherein this was the cutoff used for significance. The proportion of regulated genes in the ROP genome, Greg, was calculated. To determine whether the activation of each pathway (Table 1) was significantly altered, the probability of finding the observed number (or more) of regulated genes was calculated using the inverse cumulative binomial probability distribution. We assumed that Greg represented the expected proportion of regulated genes in the pathway. Since eight pathways were tested, marginal significance was defined as P<0.05, but full significance only if P<0.00625.
After testing our hypotheses about the eight custom pathways, gene ontology analysis for ‘significant genes’ (±0.77 log2) was performed using KEGG and NIH DAVID to identify additional regulated biological themes.
Results
Many individual genes were significantly regulated, up and down, in ROP retinae. Table 2 shows those protein-coding genes that were regulated more than ±6.64 log2 (100-fold) in ROP. The complete results are given in Supplementary Table 2. In supplementary figure 1, histograms of the pre-alignment quality of all bases from all reads in the ROP and RAR samples (top) and the qualities of all the reads in ROP and RAR (bottom) are shown; quality of a read is defined as the average of qualities of the bases comprising the read.
Table 2. Protein-expressing ROP genes with >100 fold change (FC) relative to RAR.
| Downregulated Gene | Entrez ID | FC (Log2) | Upregulated Gene | Entrez ID | FC (Log2) |
|---|---|---|---|---|---|
| ‘Similar to ferritin light chain’ | 499244 | -9.28 | Mobp | 25037 | 8.89 |
| RGD1310371 | 308794 | -8.64 | Serpinb5 | 116589 | 8.74 |
| LOC681301 | 681301 | -7.53 | RGD1560723 | 500448 | 8.62 |
| Six6os1 | 500673 | -7.48 | Tpte | 364629 | 8.37 |
| ‘Similar to ribosomal protein S15a’ | 691065 | -7.24 | Tnni2 | 29389 | 8.22 |
| ‘Rho GTPase activating protein 6’ | 100363276 | -7.12 | Tnnt3 | 24838 | 8.17 |
| RGD1560207 | 501568 | -6.83 | Mb | 59108 | 7.95 |
| Myh4 | 360543 | 7.85 | |||
| Pfkfb1 | 24638 | 7.63 | |||
| Myh3 | 24583 | 7.55 | |||
| Krt14 | 287701 | 7.28 | |||
| Ehf | 295965 | 7.27 | |||
| Olr619 | 295843 | 7.26 | |||
| Pkp3 | 293619 | 7.25 | |||
| Kera | 314771 | 7.22 | |||
| Opalin | 361757 | 7.16 | |||
| Olig1 | 60394 | 7.07 | |||
| Iqub | 296936 | 6.89 | |||
| ‘Similar to golgi matrix protein GM130’ | 690485 | 6.88 | |||
| Aspa | 79251 | 6.85 | |||
| Plscr2 | 315883 | 6.82 | |||
| Stc2 | 63878 | 6.78 |
Literature-Based Pathway Analyses (Table 1)
Canonical Wnt signaling (fig.1) was significantly altered in ROP. Six isoforms of Wnt were significantly up-regulated (Wnt2b, Wnt3, Wnt4, Wnt6, Wnt10a, Wnt10b) and four down-regulated (Wnt7a, Wnt7b, Wnt11, Wnt16). However, the lipid-binding Wnt-inhibitory factor, Wif1, was up-regulated, and may have counteracted some of the increased Wnt activity. In addition, three dickkopf-related protein encoding genes (Dkk1, Dkk2, Dkk4) and associated kremen gene (Kremen1), which together inhibit low-density lipoprotein receptors (LRPs), were also up-regulated. Indeed, Lrp5, which is a coreceptor for Fzd binding of Wnt, was down-regulated. Consistent with overall down-regulation of the Wnt pathway, two isoforms of Fzd (Fzd2, Fzd4) were under-expressed; none were up-regulated. The downstream cytoplasmic phosphoprotein encoding dishevelled-like gene (Dvl3) was subsequently down-regulated.
The non-canonical PCP Wnt signaling pathway (fig. 2) was not significantly regulated, although two of the ligands most notably involved in the activation of this pathway (Wnt4, Wnt5b) were markedly up-regulated. This is one pathway by which Wnt and Rho GTPase activity interact (via Daam) [47], and although Daam2 was markedly down-regulated, there was no significant change in Rho expression.
Figure 2.
Major elements of the noncanonical PCP Wnt signaling pathway. Symbology as in figure 1.
Despite the down-regulation of Fzd2, the frizzled isoform most important in the activation of the Wnt/Ca2+ pathway, and despite significant changes in the expression of several G-proteins, both up and down, there was no significant change in the overall expression of the non-canonical Wnt/Ca2+ pathway (fig. 3).
Figure 3.
Major elements of the noncanonical Wnt/Ca2+ signaling pathway. Symbology as in figure 1.
The Rho GTPase pathways initiated by Rho and Cdc42 are respectively shown in figure 4 and figure 5. As mentioned, Rho was not regulated in ROP. Despite marked regulation in several genes in these pathways (as indicated), neither Rho nor Cdc42 signaling were regulated.
Figure 4.
Major elements of the Rho signaling pathway. Symbology as in figure 1.
Figure 5.
Major elements of the Cdc42 signaling pathway, Symbology as in figure 1.
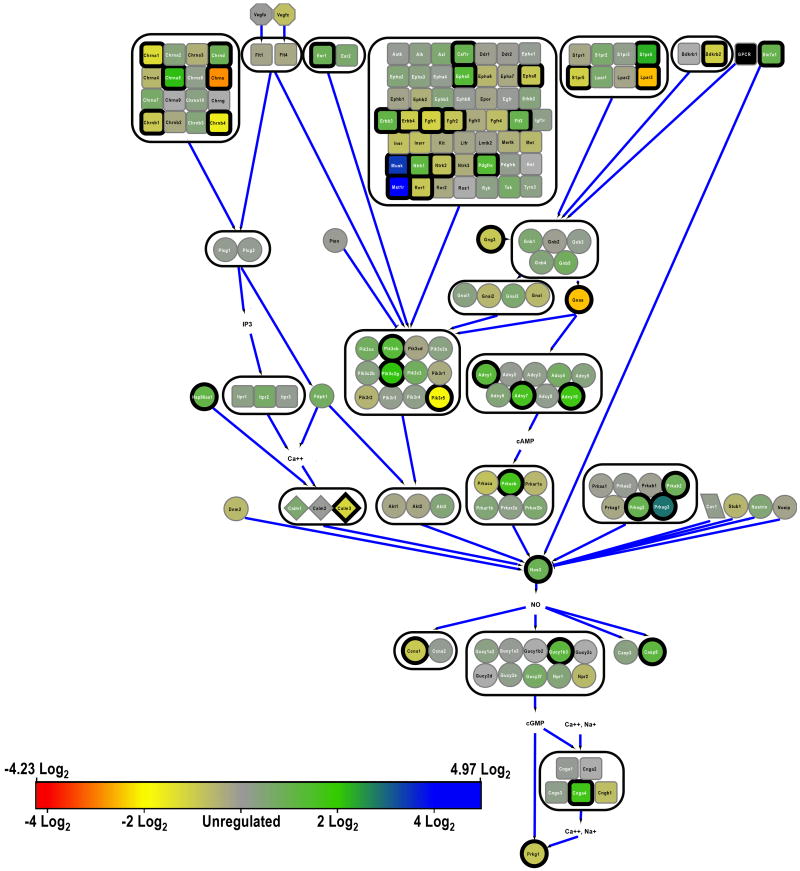
NO activity, indicated by the combined expression of the nNOS (fig. 6) and eNOS (fig. 7) signaling pathways, was marginally significantly regulated (P=0.029). Individually, regulation in the nNOS pathway was marginally significant but in the eNOS pathway not quite so (Table 1). Members of the glutamate (NMDA) receptor superfamily (Grin), which complex to form ligand-gated calcium channels that are instrumental to the activation of nNOS (Nos1), were down-regulated in ROP (Grin2a, Grin2b, Grin2c, Grin2d; fig. 6). Nevertheless, despite the putative decrease in Ca2+ flux in ROP retinal neurons, Nos1 was not significantly regulated. Of note, the downstream Rho GTPase, Rasd1, was significantly down-regulated. In contrast, despite the overall pathway not being significantly regulated, eNOS (Nos3) itself was significantly over-expressed in ROP. Therefore, the total amount of NO catabolized from L-arginine by NOS was likely higher in ROP than RAR retinae.
Figure 6.
Major elements of nitric oxide signaling in neurons (nNOS). Symbology as in figure 1; additionally, genes that encode binding proteins are in diamonds.
Figure 7.
Major elements of nitric oxide signaling in blood vessels (eNOS). Symbology as in figures 1 and 6.
Lastly, RA synthesis (fig. 8) was marginally significantly regulated in ROP. Multiple enzymes in the retinoid cycle were significantly regulated, some down (Adh4, Rdh5, Aldh1a3, Aldh8a1) and one up (Adh1). The gene that encodes the RA receptor (Rara) was not, itself, regulated.
Digital Gene Analyses
The mRNA expression profile was tested against databases of biological themes, including KEGG and DAVID's GO terms. The significance of each theme was calculated following the Benjamini correction for false discovery. Details of the significantly enriched biological themes are given in Tables 3 (KEGG) and 4 (DAVID).
Table 3. Significantly Regulated KEGG Terms.
| Term | rno | P | Benjamini |
|---|---|---|---|
| Ribosome | 03010 | 1.84E-11 | 3.51E-09 |
| Neuroactive ligand-receptor interaction | 04080 | 2.73E-05 | 0.00173 |
| Cytokine-cytokine receptor interaction | 04060 | 2.33E-05 | 0.00222 |
Table 4. A. Significantly Regulated DAVID Terms 1-35 (of 70).
| Term | GO | P | Benjamini |
|---|---|---|---|
|
| |||
| Immune response | 0006955 | 1.08E-09 | 5.93E-06 |
| M phase | 0000279 | 2.33E-08 | 4.26E-05 |
| Translational elongation | 0006414 | 1.93E-08 | 5.28E-05 |
| Response to wounding | 0009611 | 7.80E-07 | 0.00107 |
| Response to organic substance | 0010033 | 2.41E-06 | 0.00264 |
| M phase of mitotic cell cycle | 0000087 | 3.39E-06 | 0.00265 |
| Positive regulation of immune response | 0050778 | 3.91E-06 | 0.00268 |
| Chemical homeostasis | 0048878 | 3.15E-06 | 0.00287 |
| Mitosis | 0007067 | 5.47E-06 | 0.00299 |
| Nuclear division | 0000280 | 5.47E-06 | 0.00299 |
| Regulation of transcription, DNA-dependent | 0006355 | 5.26E-06 | 0.00320 |
| Regulation of RNA metabolic process | 0051252 | 6.84E-06 | 0.00341 |
| Citamin metabolic process | 0006766 | 1.37E-05 | 0.00534 |
| Ion homeostasis | 0050801 | 1.48E-05 | 0.00539 |
| Organelle fission | 0048285 | 1.64E-05 | 0.00561 |
| Cell cycle phase | 0022403 | 1.37E-05 | 0.00575 |
| Positive regulation of immune system process | 0002684 | 2.04E-05 | 0.00588 |
| Regulation of immune effector process | 0002697 | 1.30E-05 | 0.00591 |
| Hemopoietic or lymphoid organ development | 0048534 | 2.03E-05 | 0.00618 |
| Regulation of lymphocyte mediated immunity | 0002706 | 2.00E-05 | 0.00644 |
| Positive regulation of adaptive immune response based on somatic recombination of immune receptors built from immunoglobulin superfamily domains | 0002824 | 3.68E-05 | 0.00957 |
| Positive regulation of adaptive immune response | 0002821 | 3.68E-05 | 0.00957 |
| Regulation of cell proliferation | 0042127 | 3.57E-05 | 0.00974 |
| Cell cycle process | 0022402 | 4.38E-05 | 0.0109 |
| Immune system development | 0002520 | 6.04E-05 | 0.0143 |
| Regulation of adaptive immune response based on somatic recombination of immune receptors built from immunoglobulin superfamily domains | 0002822 | 7.40E-05 | 0.0149 |
| Regulation of adaptive immune response | 0002819 | 7.40E-05 | 0.0149 |
| Regulation of cAMP biosynthetic process | 0030817 | 7.25E-05 | 0.0152 |
| Inflammatory response | 0006954 | 6.70E-05 | 0.0152 |
| Leukocyte differentiation | 0002521 | 8.73E-05 | 0.0153 |
| Homeostatic process | 0042592 | 7.09E-05 | 0.0154 |
| Regulation of programmed cell death | 0043067 | 8.33E-05 | 0.0156 |
| Regulation of cell death | 0010941 | 8.05E-05 | 0.0156 |
| Regulation of leukocyte mediated immunity | 0002703 | 8.66E-05 | 0.0157 |
| Regulation of cAMP metabolic process | 0030814 | 1.00E-04 | 0.0170 |
|
| |||
|---|---|---|---|
| B. Significantly Regulated DAVID Terms 36-70 (of 70) | |||
|
| |||
| Term | GO | P | Benjamini |
|
| |||
| Positive regulation of lymphocyte mediated immunity | 0002708 | 1.08E-04 | 0.0173 |
| Positive regulation of leukocyte mediated immunity | 0002705 | 1.08E-04 | 0.0173 |
| Positive regulation of immune effector process | 0002699 | 1.08E-04 | 0.0177 |
| Regulation of transcription from RNA polymerase II promoter | 0006357 | 1.16E-04 | 0.0180 |
| Regulation of cyclic nucleotide biosynthetic process | 0030802 | 1.23E-04 | 0.0186 |
| Regulation of nucleotide biosynthetic process | 0030808 | 1.23E-04 | 0.0186 |
| Regulation of apoptosis | 0042981 | 1.27E-04 | 0.0187 |
| Ion transport | 0006811 | 1.39E-04 | 0.0188 |
| Cell activation | 0001775 | 1.33E-04 | 0.0189 |
| Regulation of nucleotide metabolic process | 0006140 | 1.38E-04 | 0.0192 |
| Cyclic-nucleotide-mediated signaling | 0019935 | 1.56E-04 | 0.0206 |
| Myeloid cell differentiation | 0030099 | 1.96E-04 | 0.0242 |
| Multicellular organism reproduction | 0032504 | 1.91E-04 | 0.0247 |
| Reproductive process in a multicellular organism | 0048609 | 1.91E-04 | 0.0247 |
| Ppositive regulation of response to stimulus | 0048584 | 1.96E-04 | 0.0247 |
| Cellular ion homeostasis | 0006873 | 2.06E-04 | 0.0248 |
| Regulation of cyclic nucleotide metabolic process | 0030799 | 2.19E-04 | 0.0257 |
| Positive regulation of developmental process | 0051094 | 2.40E-04 | 0.0276 |
| Response to drug | 0042493 | 2.48E-04 | 0.0280 |
| Cellular chemical homeostasis | 0055082 | 2.62E-04 | 0.0283 |
| Regulation of transcription | 0045449 | 2.57E-04 | 0.0284 |
| Cation homeostasis | 0055080 | 2.82E-04 | 0.0287 |
| Monovalent inorganic cation transport | 0015672 | 2.77E-04 | 0.0288 |
| Regulation of lyase activity | 0051339 | 2.77E-04 | 0.0293 |
| Regulation of adenylate cyclase activity | 0045761 | 3.26E-04 | 0.0326 |
| Hemopoiesis | 0030097 | 3.40E-04 | 0.0333 |
| Regulation of cyclase activity | 0031279 | 3.50E-04 | 0.0337 |
| Positive regulation of macromolecule metabolic process | 0010604 | 3.77E-04 | 0.0356 |
| Gamete generation | 0007276 | 3.95E-04 | 0.0366 |
| Sexual reproduction | 0019953 | 4.59E-04 | 0.0418 |
| Elevation of cytosolic calcium ion concentration | 0007204 | 4.86E-04 | 0.0435 |
| Regulation of immunoglobulin mediated immune response | 0002889 | 5.32E-04 | 0.0467 |
| Regulation of T cell mediated immunity | 0002709 | 5.32E-04 | 0.0467 |
| Regulation of B cell mediated immunity | 0002712 | 5.32E-04 | 0.0467 |
| Epidermal cell differentiation | 0009913 | 5.79E-04 | 0.0499 |
|
| |||
Discussion
This data set, obtained at the height of retinal vascular abnormalities (i.e., tortuosity, neovascularization), should provide insights into those genes and pathways activated in the pathogenesis of ROP. It may complement an earlier data set obtained in the 50/10 ROP rat by gene chip [40]. Interestingly, while there is substantial overlap between that study and this NGS study, there is also substantial divergence. In addition to the digital gene expression analysis against standardized databases, we investigated genes in specific pathways of interest to our and other groups.
For instance, the Wnt signaling pathway has been recently implicated in ROP and we found it to be regulated in our sample. However, the KEGG pathway database does not differentiate between the canonical Wnt pathway and the alternative PCP and Wnt/Ca2+ pathways. Thus, we studied these pathways individually, and found that while, unsurprisingly, a significant proportion of the genes in the canonical pathway were regulated, a less marked proportion of the PCP and Wnt/Ca2+ pathways were activated (Table 1, figs. 1-3). Indeed, while the PCP pathway showed some evidence of regulation (=0.056), the Wnt/Ca2+ pathway showed almost no regulation beyond that which would be expected by chance (P=0.41).
It is increasingly clear that Wnt signaling coops Rho GTPase signaling. Therefore, we looked at Rho GTPase activity in the Rho pathway, which has several upstream elements that are regulated by Wnt signaling, and in the Cdc42 pathway, which has equivocal association with Wnt signaling. Neither were regulated.
We have found that, early in the pathogenesis of diabetic retinopathy (DR), NO signaling seems to become activated and be associated with retinal dysfunction [82]. Since both DR and ROP are neovascular diseases, we examined whether genes involved in the activation of NOS, the enzyme that generates NO, were regulated. They were (Table 1): probably those in neurons (P=0.034), and possibly those in blood vessels (P=0.073). Since the retinal blood vessels and neurons are in close physical proximity and mature together, NO may help synchronize the neurovascular congruence in the retina [148]. While the evidence suggested that the nNOS pathway was down-regulated, nNOS itself (Nos1) was not regulated. Conversely, the eNOS pathway was not regulated; however, eNOS itself (Nos3) was up-regulated. Since NO freely diffuses through most cells, neurons may have received increased NO from blood vessels.
In addition, we recently completed a study of the refractive state of the ROP rat's eye [149]. Relative to the control rat, the ROP rat is a myope. Important in the regulation of refractive development is RA, and therefore we looked at RA signaling. We found that it was significantly regulated in ROP (Table 1). Furthermore, we note that the SIX family of sine oculis homeodomain-containing DNA-binding proteins, in particular Six3 and Six6, are critical mediators of vertebrate eye growth and development [150] and that one of the most down-regulated genes in the ROP rat retina is Six6os1 (Table 2). This ‘opposite strand’ natural antisense transcript (NAT) to Six6 is an important mediator of Six6's activity. Six6 and Six6os are expressed almost uniquely in the neural retina. The degree of sequence similarity between murine and human of Six6os is quite high [151]. Also, keratocan (Kera), a gene known for its involvement in corneal development [152], was highly overexpressed in ROP retinae (Table 2). This might contribute to the increased corneal curvature frequently noted in ROP myopia [153].
In our analysis, we defined “marginally significant regulation” of a pathway as having a number of regulated constituent genes that was less than five percent likely to have occurred by chance given the total number of genes that were regulated in ROP. However, we note that statistically significant regulation of a pathway does not imply that the aggregate activity of the pathway is up or down (or even changed at all). For example, the canonical Wnt signaling pathway, the most regulated of our eight literature-based (i.e., hypothesis-driven) pathways, had eleven up-regulated genes and eight down-regulated genes. Further complicating matters, some of the genes that were up-regulated inhibit the activation of other genes in the pathway. Thus, we are tempted to claim that the net effect on Wnt pathway activation is ‘down’, but really, the result is equivocal. Focusing on the ligands, in comparison to the mouse model of ROP in which Wnt3a, 7a, and 10a are all down-regulated [43], in our rat data, expression of 3a was not altered (although expression of 3 was up-regulated), 7a was significantly down-regulated, and 10a was significantly up-regulated.
The most significantly regulated biological theme in the KEGG digital gene expression analysis was ‘Ribosome’ (Table 3). Ribosomes are involved in translation of mRNA into proteins. Ribosomes are involved in so many cellular processes that it is difficult to conclude what their role in ROP might be from this data point alone. Rather, these data suggest that a proteomic approach is likely to yield much new and valuable information.
The KEGG ‘Neuroactive ligand receptor interaction’ theme (Table 3), which contains many G-protein coupled receptors (GPCRs), was also significantly regulated. GPCRs were excluded from our custom pathway analyses because they are so numerous. If these had been included in the custom analyses, the results for the Rho, Cdc42, and eNOS pathways would have been altered. In any event, it is clear that neurotransmitter activity is importantly altered in the ROP rat retina. Certainly, the ERG results in both animal models and patients with ROP indicated that neural retinal dysfunction is a feature of ROP that persists for life.
Cytokines, proteins that act as intercellular messengers, are involved in varied cellular functions including immune processes like inflammation, repair, and maintenance of homeostasis. They are also involved cell growth, differentiation, and death, including in endothelial cells. Several groups of cytokines, distinguished by their structure, exist. ‘Cytokine-cytokine receptor interaction’ was regulated in the KEGG database as shown in Table 3.
Seventy GO terms were regulated in the NIH's DAVID database, following Benjamini correction for multiple tests (Table 4). These terms encompass a variety of biological systems, but two classes seem particularly pronounced due to their repeated appearance: immunity and cell-cycle. There a small but increasing body of evidence that suggests inflammatory and immune system pathways are involved in neovascular retinal diseases. In particular, a role for the complement system has been recently reviewed [154]. Thus, immune system activity may represent an emerging target for ROP therapy. With respect to the cell-cycle, there is little literature to suggest significant changes therein are a feature of ROP. However, changes in mitosis events in endothelial cells, regulated by VEGF and perhaps downstream NO signaling, are associated with the increased arteriolar tortuosity in ROP [155].
There are a few limitations of this kind of study. For instance, while it is fortunate that the output of the HiSeq 2000 sequencing system enables rapid profiling and deep investigation of the transcriptome, we did not perform sequencing on technical replicates. Thus, the degree of certainty in the activation values is somewhat low. Furthermore, while we did have biological replicates in the form of pooled samples, they were sequenced ensemble, which is a less powerful (but much less costly) approach than sequencing individuals' RNA. The declining costs of NGS will make such replication more feasible in needed future studies of this variety. And an additional, final note: The regulation of any given biological theme found to be significant herein may indeed be important to the pathogenesis of ROP, but it may also be compensatory to the pathology. Thus, while it is tempting to imagine interventions that would normalize one or more of the several regulated biological themes discovered herein, caution should be applied since the eye may already be compensating for some other, deleterious biochemical response. In this case, normalizing activation of the signaling pathway could potentially worsen outcomes. Since we assayed only one time point, there is no evidence in our current dataset to distinguish between pathological and compensatory activation of genes.
Supplementary Material
Acknowledgments
This work was supported by NIH EY020308 (JDA) and the Massachusetts Lions Eye Research Fund (RMH).
References
- 1.Feng Y, Busch S, Gretz N, Hoffmann S, Hammes HP. Crosstalk in the retinal neurovascular unit - lessons for the diabetic retina. Exp Clin Endocrinol Diabetes. 2012;120:199–201. doi: 10.1055/s-0032-1304571. [DOI] [PubMed] [Google Scholar]
- 2.Campbell K. Intensive oxygen therapy as a possible cause of retrolental fibroplasia; a clinical approach. Med J Aust. 1951;2:48–50. [PubMed] [Google Scholar]
- 3.Gyllensten LJ, Hellstrom BE. Retrolental fibroplasia; animal experiments: the effect of intermittingly administered oxygen on the postnatal development of the eyes of fullterm mice. Acta Paediatr. 1952;41:577–582. doi: 10.1111/j.1651-2227.1952.tb17854.x. [DOI] [PubMed] [Google Scholar]
- 4.Patz A, Hoeck LE, De La Cruz E. Studies on the effect of high oxygen administration in retrolental fibroplasia. I. Nursery observations. Am J Ophthalmol. 1952;35:1248–1253. doi: 10.1016/0002-9394(52)91140-9. [DOI] [PubMed] [Google Scholar]
- 5.Ashton N, Ward B, Serpell G. Role of oxygen in the genesis of retrolental fibroplasia; a preliminary report. Br J Ophthalmol. 1953;37:513–520. doi: 10.1136/bjo.37.9.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bedrossian R. Retinopathy of prematurity (retrolental fibroplasia) and its relationship to oxygen. Ama Arch Opthalmol. 1953;50:266–267. [PubMed] [Google Scholar]
- 7.Huggert A. The supply of oxygen to prematures and the appearance of retrolental fibroplasia. Acta Paediatr. 1953;42:147–156. doi: 10.1111/j.1651-2227.1953.tb05576.x. [DOI] [PubMed] [Google Scholar]
- 8.Fulton AB. The development of scotopic retinal function in human infants. Doc Ophthalmol. 1988;69:101–109. doi: 10.1007/BF00153690. [DOI] [PubMed] [Google Scholar]
- 9.Fulton AB, Dodge J, Hansen RM, Schremser JL, Williams TP. The quantity of rhodopsin in young human eyes. Curr Eye Res. 1991;10:977–982. doi: 10.3109/02713689109020334. [DOI] [PubMed] [Google Scholar]
- 10.Fulton AB, Hansen RM. The development of scotopic sensitivity. Invest Ophthalmol Vis Sci. 2000;41:1588–1596. [PubMed] [Google Scholar]
- 11.Hansen RM, Fulton AB. Rod-mediated increment threshold functions in infants. Invest Ophthalmol Vis Sci. 2000;41:4347–4352. [PubMed] [Google Scholar]
- 12.Hansen RM, Fulton AB. Development of the cone ERG in infants. Invest Ophthalmol Vis Sci. 2005;46:3458–3462. doi: 10.1167/iovs.05-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hendrickson A. The morphologic development of human and monkey retina. In: Albert DM, Jakobiec FA, editors. Principles and Practice of Ophthalmology: Basic Sciences. Saunders; Philadelphia: 1994. pp. 561–577. [Google Scholar]
- 14.Hendrickson A, Drucker D. The development of parafoveal and mid-peripheral human retina. Behav Brain Res. 1992;49:21–31. doi: 10.1016/s0166-4328(05)80191-3. [DOI] [PubMed] [Google Scholar]
- 15.Mirabella G, Kjaer PK, Norcia AM, Good WV, Madan A. Visual development in very low birth weight infants. Pediatr Res. 2006;60:435–439. doi: 10.1203/01.pdr.0000238249.44088.2c. [DOI] [PubMed] [Google Scholar]
- 16.Wachtmeister L. Oscillatory potentials in the retina: what do they reveal. Prog Retin Eye Res. 1998;17:485–521. doi: 10.1016/s1350-9462(98)00006-8. [DOI] [PubMed] [Google Scholar]
- 17.Provis JM, Leech J, Diaz CM, Penfold PL, Stone J, Keshet E. Development of the human retinal vasculature: cellular relations and VEGF expression. Exp Eye Res. 1997;65:555–568. doi: 10.1006/exer.1997.0365. [DOI] [PubMed] [Google Scholar]
- 18.Cook A, White S, Batterbury M, Clark D. Ocular growth and refractive error development in premature infants without retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2003;44:953–960. doi: 10.1167/iovs.02-0124. [DOI] [PubMed] [Google Scholar]
- 19.Cook A, White S, Batterbury M, Clark D. Ocular growth and refractive error development in premature infants with or without retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2008;49:5199–5207. doi: 10.1167/iovs.06-0114. [DOI] [PubMed] [Google Scholar]
- 20.Fledelius HC. Pre-term delivery and the growth of the eye. An oculometric study of eye size around term-time. Acta Ophthalmol Suppl. 1992:10–15. doi: 10.1111/j.1755-3768.1992.tb04915.x. [DOI] [PubMed] [Google Scholar]
- 21.Fledelius HC. Pre-term delivery and subsequent ocular development. A 7-10 year follow-up of children screened 1982-84 for ROP. 4) Oculometric - and other metric considerations. Acta Ophthalmol Scand. 1996;74:301–305. doi: 10.1111/j.1600-0420.1996.tb00097.x. [DOI] [PubMed] [Google Scholar]
- 22.Fulton AB, Akula JD, Mocko JA, Hansen RM, Benador IY, Beck SC, Fahl E, Seeliger MW, Moskowitz A, Harris ME. Retinal degenerative and hypoxic ischemic disease. Doc Ophthalmol. 2009;118:55–61. doi: 10.1007/s10633-008-9127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fulton AB, Hansen RM, Moskowitz A, Akula JD. The neurovascular retina in retinopathy of prematurity. Prog Retin Eye Res. 2009;28:452–482. doi: 10.1016/j.preteyeres.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ETROP. Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–1694. doi: 10.1001/archopht.121.12.1684. [DOI] [PubMed] [Google Scholar]
- 25.Reisner DS, Hansen RM, Findl O, Petersen RA, Fulton AB. Dark-adapted thresholds in children with histories of mild retinopathy of prematurity. Invest Ophthalmol Vis Sci. 1997;38:1175–1183. [PubMed] [Google Scholar]
- 26.Hansen RM, Fulton AB. Background adaptation in children with a history of mild retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2000;41:320–324. [PubMed] [Google Scholar]
- 27.Fulton AB, Hansen RM, Petersen RA, Vanderveen DK. The rod photoreceptors in retinopathy of prematurity: an electroretinographic study. Arch Ophthalmol. 2001;119:499–505. doi: 10.1001/archopht.119.4.499. [DOI] [PubMed] [Google Scholar]
- 28.Larsson E, Rydberg A, Holmstrom G. Contrast sensitivity in 10 year old preterm and full term children: a population based study. Br J Ophthalmol. 2006;90:87–90. doi: 10.1136/bjo.2005.081653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnaby AM, Hansen RM, Moskowitz A, Fulton AB. Development of scotopic visual thresholds in retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2007;48:4854–4860. doi: 10.1167/iovs.07-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fielder AR, Quinn GE. Myopia of prematurity: nature, nurture, or disease? Br J Ophthalmol. 1997;81:2–3. doi: 10.1136/bjo.81.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fielder AR. Retinopathy of Prematurity. In: Taylor D, Hoyt CS, editors. Pediatric ophthalmology and strabismus. 3rd. Elsevier Saunders; New York: 1997. pp. 537–556. [Google Scholar]
- 32.O'Connor AR, Stephenson T, Johnson A, Tobin MJ, Moseley MJ, Ratib S, Ng Y, Fielder AR. Long-term ophthalmic outcome of low birth weight children with and without retinopathy of prematurity. Pediatrics. 2002;109:12–18. doi: 10.1542/peds.109.1.12. [DOI] [PubMed] [Google Scholar]
- 33.O'Connor AR, Stephenson TJ, Johnson A, Tobin MJ, Ratib S, Fielder AR. Change of refractive state and eye size in children of birth weight less than 1701 g. Br J Ophthalmol. 2006;90:456–460. doi: 10.1136/bjo.2005.083535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snir M, Friling R, Weinberger D, Sherf I, Axer-Siegel R. Refraction and keratometry in 40 week old premature (corrected age) and term infants. Br J Ophthalmol. 2004;88:900–904. doi: 10.1136/bjo.2003.037499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baker PS, Tasman W. Myopia in adults with retinopathy of prematurity. Am J Ophthalmol. 2008;145:1090–1094. doi: 10.1016/j.ajo.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 36.Mactier H, Maroo S, Bradnam M, Hamilton R. Ocular biometry in preterm infants: implications for estimation of retinal illuminance. Invest Ophthalmol Vis Sci. 2008;49:453–457. doi: 10.1167/iovs.07-0540. [DOI] [PubMed] [Google Scholar]
- 37.Quiroz-Mercado H, Martinez-Castellanos MA, Hernandez-Rojas ML, Salazar-Teran N, Chan RV. Antiangiogenic therapy with intravitreal bevacizumab for retinopathy of prematurity. Retina. 2008;28:S19–25. doi: 10.1097/IAE.0b013e318159ec6b. [DOI] [PubMed] [Google Scholar]
- 38.Chen J, Stahl A, Hellstrom A, Smith LE. Current update on retinopathy of prematurity: screening and treatment. Current opinion in pediatrics. 2011;23:173–178. doi: 10.1097/MOP.0b013e3283423f35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barnett JM, Yanni SE, Penn JS. The development of the rat model of retinopathy of prematurity. Documenta ophthalmologica. Advances in ophthalmology. 2010;120:3–12. doi: 10.1007/s10633-009-9180-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Recchia FM, Xu L, Penn JS, Boone B, Dexheimer PJ. Identification of genes and pathways involved in retinal neovascularization by microarray analysis of two animal models of retinal angiogenesis. Investigative ophthalmology & visual science. 2010;51:1098–1105. doi: 10.1167/iovs.09-4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annual review of cell and developmental biology. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 42.Smith LE, Wesolowski E, McLellan A, Kostyk SK, D'Amato R, Sullivan R, D'Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 43.Chen J, Stahl A, Krah NM, Seaward MR, Dennison RJ, Sapieha P, Hua J, Hatton CJ, Juan AM, Aderman CM, Willett KL, Guerin KI, Mammoto A, Campbell M, Smith LE. Wnt signaling mediates pathological vascular growth in proliferative retinopathy. Circulation. 2011;124:1871–1881. doi: 10.1161/CIRCULATIONAHA.111.040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haider MZ, Devarajan LV, Al-Essa M, Kumar H. A C597-->A polymorphism in the Norrie disease gene is associated with advanced retinopathy of prematurity in premature Kuwaiti infants. J Biomed Sci. 2002;9:365–370. doi: 10.1007/BF02256593. [DOI] [PubMed] [Google Scholar]
- 45.Hiraoka M, Takahashi H, Orimo H, Ogata T, Azuma N. Genetic screening of Wnt signaling factors in advanced retinopathy of prematurity. Mol Vis. 2010;16:2572–2577. [PMC free article] [PubMed] [Google Scholar]
- 46.Ohkubo H, Tanino T. Electrophysiological findings in familial exudative vitreoretinopathy. Documenta ophthalmologica. Advances in ophthalmology. 1987;65:461–469. doi: 10.1007/BF00143048. [DOI] [PubMed] [Google Scholar]
- 47.Schlessinger K, Hall A, Tolwinski N. Wnt signaling pathways meet Rho GTPases. Genes & development. 2009;23:265–277. doi: 10.1101/gad.1760809. [DOI] [PubMed] [Google Scholar]
- 48.Luo L. Rho GTPases in neuronal morphogenesis. Nat Rev Neurosci. 2000;1:173–180. doi: 10.1038/35044547. [DOI] [PubMed] [Google Scholar]
- 49.Masumoto A, Mohri M, Shimokawa H, Urakami L, Usui M, Takeshita A. Suppression of coronary artery spasm by the Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation. 2002;105:1545–1547. doi: 10.1161/hc1002.105938. [DOI] [PubMed] [Google Scholar]
- 50.Okamura N, Saito M, Mori A, Sakamoto K, Kametaka S, Nakahara T, Ishii K. Vasodilator effects of fasudil, a Rho-kinase inhibitor, on retinal arterioles in stroke-prone spontaneously hypertensive rats. J Ocul Pharmacol Ther. 2007;23:207–212. doi: 10.1089/jop.2006.128. [DOI] [PubMed] [Google Scholar]
- 51.Miyata K, Shimokawa H, Kandabashi T, Higo T, Morishige K, Eto Y, Egashira K, Kaibuchi K, Takeshita A. Rho-kinase is involved in macrophage-mediated formation of coronary vascular lesions in pigs in vivo. Arterioscler Thromb Vasc Biol. 2000;20:2351–2358. doi: 10.1161/01.atv.20.11.2351. [DOI] [PubMed] [Google Scholar]
- 52.Chan CC, Khodarahmi K, Liu J, Sutherland D, Oschipok LW, Steeves JD, Tetzlaff W. Dose-dependent beneficial and detrimental effects of ROCK inhibitor Y27632 on axonal sprouting and functional recovery after rat spinal cord injury. Exp Neurol. 2005;196:352–364. doi: 10.1016/j.expneurol.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 53.Fournier AE, Takizawa BT, Strittmatter SM. Rho kinase inhibition enhances axonal regeneration in the injured. CNS J Neurosci. 2003;23:1416–1423. doi: 10.1523/JNEUROSCI.23-04-01416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hara M, Takayasu M, Watanabe K, Noda A, Takagi T, Suzuki Y, Yoshida J. Protein kinase inhibition by fasudil hydrochloride promotes neurological recovery after spinal cord injury in rats. J Neurosurg. 2000;93:94–101. doi: 10.3171/spi.2000.93.1.0094. [DOI] [PubMed] [Google Scholar]
- 55.Bauer PO, Nukina N. Enhanced degradation of mutant huntingtin by rho kinase inhibition is mediated through activation of proteasome and macroautophagy. Autophagy. 2009;5:747–748. doi: 10.4161/auto.5.5.8704. [DOI] [PubMed] [Google Scholar]
- 56.Tura A, Schuettauf F, Monnier PP, Bartz-Schmidt KU, Henke-Fahle S. Efficacy of Rho-kinase inhibition in promoting cell survival and reducing reactive gliosis in the rodent retina. Invest Ophthalmol Vis Sci. 2009;50:452–461. doi: 10.1167/iovs.08-1973. [DOI] [PubMed] [Google Scholar]
- 57.Song H, Gao D. Fasudil, a Rho-associated protein kinase inhibitor, attenuates retinal ischemia and reperfusion injury in rats. Int J Mol Med. 2011;28:193–198. doi: 10.3892/ijmm.2011.659. [DOI] [PubMed] [Google Scholar]
- 58.Honjo M, Inatani M, Kido N, Sawamura T, Yue BY, Honda Y, Tanihara H. Effects of protein kinase inhibitor, HA1077, on intraocular pressure and outflow facility in rabbit eyes. Arch Ophthalmol. 2001;119:1171–1178. doi: 10.1001/archopht.119.8.1171. [DOI] [PubMed] [Google Scholar]
- 59.Honjo M, Tanihara H, Inatani M, Kido N, Sawamura T, Yue BY, Narumiya S, Honda Y. Effects of rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facility. Invest Ophthalmol Vis Sci. 2001;42:137–144. [PubMed] [Google Scholar]
- 60.Whitlock NA, Harrison B, Mixon T, Yu XQ, Wilson A, Gerhardt B, Eberhart DE, Abuin A, Rice DS. Decreased intraocular pressure in mice following either pharmacological or genetic inhibition of ROCK. J Ocul Pharmacol Ther. 2009;25:187–194. doi: 10.1089/jop.2008.0142. [DOI] [PubMed] [Google Scholar]
- 61.Waki M, Yoshida Y, Oka T, Azuma M. Reduction of intraocular pressure by topical administration of an inhibitor of the Rho-associated protein kinase. Curr Eye Res. 2001;22:470–474. doi: 10.1076/ceyr.22.6.470.5489. [DOI] [PubMed] [Google Scholar]
- 62.Tian B, Kaufman PL. Effects of the Rho kinase inhibitor Y-27632 and the phosphatase inhibitor calyculin A on outflow facility in monkeys. Exp Eye Res. 2005;80:215–225. doi: 10.1016/j.exer.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 63.Arita R, Hata Y, Nakao S, Kita T, Miura M, Kawahara S, Zandi S, Almulki L, Tayyari F, Shimokawa H, Hafezi-Moghadam A, Ishibashi T. Rho kinase inhibition by fasudil ameliorates diabetes-induced microvascular damage. Diabetes. 2009;58:215–226. doi: 10.2337/db08-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fang X, Ueno M, Yamashita T, Ikuno Y. RhoA activation and effect of Rho-kinase inhibitor in the development of retinal neovascularization in a mouse model of oxygen-induced retinopathy. Current eye research. 2011;36:1028–1036. doi: 10.3109/02713683.2011.593110. [DOI] [PubMed] [Google Scholar]
- 65.Petrie RJ, Doyle AD, Yamada KM. Random versus directionally persistent cell migration. Nat Rev Mol Cell Biol. 2009;10:538–549. doi: 10.1038/nrm2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hahmann C, Schroeter T. Rho-kinase inhibitors as therapeutics: from pan inhibition to isoform selectivity. Cell Mol Life Sci. 2010;67:171–177. doi: 10.1007/s00018-009-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang C, Lafleur J, Mwaikambo BR, Zhu T, Gagnon C, Chemtob S, Di Polo A, Hardy P. The role of lysophosphatidic acid receptor (LPA1) in the oxygen-induced retinal ganglion cell degeneration. Invest Ophthalmol Vis Sci. 2009;50:1290–1298. doi: 10.1167/iovs.08-1920. [DOI] [PubMed] [Google Scholar]
- 68.Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 69.Mueller BK, Mack H, Teusch N. Rho kinase, a promising drug target for neurological disorders. Nat Rev Drug Discov. 2005;4:387–398. doi: 10.1038/nrd1719. [DOI] [PubMed] [Google Scholar]
- 70.Govek EE, Newey SE, Van Aelst L. The role of the Rho GTPases in neuronal development. Genes Dev. 2005;19:1–49. doi: 10.1101/gad.1256405. [DOI] [PubMed] [Google Scholar]
- 71.Sin WC, Haas K, Ruthazer ES, Cline HT. Dendrite growth increased by visual activity requires NMDA receptor and Rho GTPases. Nature. 2002;419:475–480. doi: 10.1038/nature00987. [DOI] [PubMed] [Google Scholar]
- 72.Sebok A, Nusser N, Debreceni B, Guo Z, Santos MF, Szeberenyi J, Tigyi G. Different roles for RhoA during neurite initiation, elongation, and regeneration in PC12 cells. J Neurochem. 1999;73:949–960. doi: 10.1046/j.1471-4159.1999.0730949.x. [DOI] [PubMed] [Google Scholar]
- 73.Albertinazzi C, Gilardelli D, Paris S, Longhi R, de Curtis I. Overexpression of a neural-specific rho family GTPase, cRac1B, selectively induces enhanced neuritogenesis and neurite branching in primary neurons. J Cell Biol. 1998;142:815–825. doi: 10.1083/jcb.142.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leszczynska K, Kaur S, Wilson E, Bicknell R, Heath VL. The role of RhoJ in endothelial cell biology and angiogenesis. Biochem Soc Trans. 2011;39:1606–1611. doi: 10.1042/BST20110702. [DOI] [PubMed] [Google Scholar]
- 75.Bryan BA, D'Amore PA. What tangled webs they weave: Rho-GTPase control of angiogenesis. Cell Mol Life Sci. 2007;64:2053–2065. doi: 10.1007/s00018-007-7008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yuan L, Sacharidou A, Stratman AN, Le Bras A, Zwiers PJ, Spokes K, Bhasin M, Shih SC, Nagy JA, Molema G, Aird WC, Davis GE, Oettgen P. RhoJ is an endothelial cell-restricted Rho GTPase that mediates vascular morphogenesis and is regulated by the transcription factor ERG. Blood. 2011;118:1145–1153. doi: 10.1182/blood-2010-10-315275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mackay DJ, Nobes CD, Hall A. The Rho's progress a potential role during neuritogenesis for the Rho family of GTPases. Trends Neurosci. 1995;18:496–501. doi: 10.1016/0166-2236(95)92773-j. [DOI] [PubMed] [Google Scholar]
- 78.Schlessinger K, McManus EJ, Hall A. Cdc42 and noncanonical Wnt signal transduction pathways cooperate to promote cell polarity. J Cell Biol. 2007;178:355–361. doi: 10.1083/jcb.200701083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Olson KR, Donald JA. Nervous control of circulation--the role of gasotransmitters, NO, CO, and H2S. Acta Histochem. 2009;111:244–256. doi: 10.1016/j.acthis.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 80.Li X, Bazer FW, Gao H, Jobgen W, Johnson GA, Li P, McKnight JR, Satterfield MC, Spencer TE, Wu G. Amino acids and gaseous signaling. Amino Acids. 2009;37:65–78. doi: 10.1007/s00726-009-0264-5. [DOI] [PubMed] [Google Scholar]
- 81.Giove TJ, Deshpande MM, Gagen CS, Eldred WD. Increased neuronal nitric oxide synthase activity in retinal neurons in early diabetic retinopathy. Mol Vis. 2009;15:2249–2258. [PMC free article] [PubMed] [Google Scholar]
- 82.Blom J, Giove T, Favazza T, Akula J, Eldred W. Inhibition of the adrenomedullin/nitric oxide signaling pathway in early diabetic retinopathy. J Ocul Biol Dis Infor. 2011;4:70–82. doi: 10.1007/s12177-011-9072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chiou GC. Review: effects of nitric oxide on eye diseases and their treatment. J Ocul Pharmacol Ther. 2001;17:189–198. doi: 10.1089/10807680151125555. [DOI] [PubMed] [Google Scholar]
- 84.Prast H, Philippu A. Nitric oxide as modulator of neuronal function. Progress in neurobiology. 2001;64:51–68. doi: 10.1016/s0301-0082(00)00044-7. [DOI] [PubMed] [Google Scholar]
- 85.Rieke F, Schwartz EA. A cGMP-gated current can control exocytosis at cone synapses. Neuron. 1994;13:863–873. doi: 10.1016/0896-6273(94)90252-6. [DOI] [PubMed] [Google Scholar]
- 86.Savchenko A, Barnes S, Kramer RH. Cyclic-nucleotide-gated channels mediate synaptic feedback by nitric oxide. Nature. 1997;390:694–698. doi: 10.1038/37803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miyachi E, Murakami M, Nakaki T. Arginine blocks gap junctions between retinal horizontal cells. Neuroreport. 1990;1:107–110. doi: 10.1097/00001756-199010000-00006. [DOI] [PubMed] [Google Scholar]
- 88.McMahon DG, Ponomareva LV. Nitric oxide and cGMP modulate retinal glutamate receptors. J Neurophysiol. 1996;76:2307–2315. doi: 10.1152/jn.1996.76.4.2307. [DOI] [PubMed] [Google Scholar]
- 89.Shiells RA, Falk G. Properties of the cGMP-activated channel of retinal on-bipolar cells. Proc Biol Sci. 1992;247:21–25. doi: 10.1098/rspb.1992.0004. [DOI] [PubMed] [Google Scholar]
- 90.Shiells R, Falk G. Retinal on-bipolar cells contain a nitric oxide-sensitive guanylate cyclase. Neuroreport. 1992;3:845–848. doi: 10.1097/00001756-199210000-00006. [DOI] [PubMed] [Google Scholar]
- 91.Koistinaho J, Swanson RA, de Vente J, Sagar SM. NADPH-diaphorase (nitric oxide synthase)-reactive amacrine cells of rabbit retina: putative target cells and stimulation by light. Neuroscience. 1993;57:587–597. doi: 10.1016/0306-4522(93)90008-4. [DOI] [PubMed] [Google Scholar]
- 92.Hardy P, Peri KG, Lahaie I, Varma DR, Chemtob S. Increased nitric oxide synthesis and action preclude choroidal vasoconstriction to hyperoxia in newborn pigs. Circ Res. 1996;79:504–511. doi: 10.1161/01.res.79.3.504. [DOI] [PubMed] [Google Scholar]
- 93.Tai SC, Robb GB, Marsden PA. Endothelial nitric oxide synthase: a new paradigm for gene regulation in the injured blood vessel. Arterioscler Thromb Vasc Biol. 2004;24:405–412. doi: 10.1161/01.ATV.0000109171.50229.33. [DOI] [PubMed] [Google Scholar]
- 94.Veerappan S, Schache M, Pertile KK, Islam FM, Chen CY, Mitchell P, Dirani M, Baird PN. The retinoic acid receptor alpha (RARA) gene is not associated with myopia, hypermetropia, and ocular biometric measures. Molecular vision. 2009;15:1390–1397. [PMC free article] [PubMed] [Google Scholar]
- 95.Morgan IG. The biological basis of myopic refractive error. Clin Exp Optom. 2003;86:276–288. doi: 10.1111/j.1444-0938.2003.tb03123.x. [DOI] [PubMed] [Google Scholar]
- 96.Mactier H, McCulloch DL, Hamilton R, Galloway P, Bradnam MS, Young D, Lavy T, Farrell L, Weaver LT. Vitamin A supplementation improves retinal function in infants at risk of retinopathy of prematurity. The Journal of pediatrics. 2012;160:954–959. e951. doi: 10.1016/j.jpeds.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 97.Nickla DL, Wilken E, Lytle G, Yom S, Mertz J. Inhibiting the transient choroidal thickening response using the nitric oxide synthase inhibitor l-NAME prevents the ameliorative effects of visual experience on ocular growth in two different visual paradigms. Exp Eye Res. 2006;83:456–464. doi: 10.1016/j.exer.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 98.Nickla DL, Damyanova P, Lytle G. Inhibiting the neuronal isoform of nitric oxide synthase has similar effects on the compensatory choroidal and axial responses to myopic defocus in chicks as does the non-specific inhibitor L-NAME. Exp Eye Res. 2009;88:1092–1099. doi: 10.1016/j.exer.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29:144–168. doi: 10.1016/j.preteyeres.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu J, Liu Q, Yang X, Yang H, Wang XM, Zeng JW. Time-course of changes to nitric oxide signaling pathways in form-deprivation myopia in guinea pigs. Brain Res. 2007;1186:155–163. doi: 10.1016/j.brainres.2007.09.077. [DOI] [PubMed] [Google Scholar]
- 101.Penn JS, Henry MM, Tolman BL. Exposure to alternating hypoxia and hyperoxia causes severe proliferative retinopathy in the newborn rat. Pediatr Res. 1994;36:724–731. doi: 10.1203/00006450-199412000-00007. [DOI] [PubMed] [Google Scholar]
- 102.Liu K, Akula JD, Falk C, Hansen RM, Fulton AB. The retinal vasculature and function of the neural retina in a rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2006;47:2639–2647. doi: 10.1167/iovs.06-0016. [DOI] [PubMed] [Google Scholar]
- 103.Akula JD, Hansen RM, Martinez-Perez ME, Fulton AB. Rod photoreceptor function predicts blood vessel abnormality in retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2007;48:4351–4359. doi: 10.1167/iovs.07-0204. [DOI] [PubMed] [Google Scholar]
- 104.Akula JD, Mocko JA, Benador IY, Hansen RM, Favazza TL, Vyhovsky TC, Fulton AB. The neurovascular relation in oxygen-induced retinopathy. Mol Vis. 2008;14:2499–2508. [PMC free article] [PubMed] [Google Scholar]
- 105.Akula JD, Hansen RM, Tzekov R, Favazza TL, Vyhovsky TC, Benador IY, Mocko JA, McGee D, Kubota R, Fulton AB. Visual cycle modulation in neurovascular retinopathy. Exp Eye Res. 2010;91:153–161. doi: 10.1016/j.exer.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 106.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nature methods. 2008;5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 107.Cvekl A, Wang WL. Retinoic acid signaling in mammalian eye development. Experimental eye research. 2009;89:280–291. doi: 10.1016/j.exer.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Katoh M. WNT/PCP signaling pathway and human cancer (review) Oncology reports. 2005;14:1583–1588. [PubMed] [Google Scholar]
- 109.Bagrodia S, Derijard B, Davis RJ, Cerione RA. Cdc42 and PAK-mediated signaling leads to Jun kinase and p38 mitogen-activated protein kinase activation. The Journal of biological chemistry. 1995;270:27995–27998. doi: 10.1074/jbc.270.47.27995. [DOI] [PubMed] [Google Scholar]
- 110.Aspenstrom P, Lindberg U, Hall A. Two GTPases, Cdc42 and Rac, bind directly to a protein implicated in the immunodeficiency disorder Wiskott-Aldrich syndrome. Current biology : CB. 1996;6:70–75. doi: 10.1016/s0960-9822(02)00423-2. [DOI] [PubMed] [Google Scholar]
- 111.Hotta K, Tanaka K, Mino A, Kohno H, Takai Y. Interaction of the Rho family small G proteins with kinectin, an anchoring protein of kinesin motor. Biochemical and biophysical research communications. 1996;225:69–74. doi: 10.1006/bbrc.1996.1132. [DOI] [PubMed] [Google Scholar]
- 112.Kuroda S, Fukata M, Kobayashi K, Nakafuku M, Nomura N, Iwamatsu A, Kaibuchi K. Identification of IQGAP as a putative target for the small GTPases, Cdc42 and Rac1. The Journal of biological chemistry. 1996;271:23363–23367. doi: 10.1074/jbc.271.38.23363. [DOI] [PubMed] [Google Scholar]
- 113.Ohta Y, Suzuki N, Nakamura S, Hartwig JH, Stossel TP. The small GTPase RalA targets filamin to induce filopodia. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:2122–2128. doi: 10.1073/pnas.96.5.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yamazaki M, Zhang Y, Watanabe H, Yokozeki T, Ohno S, Kaibuchi K, Shibata H, Mukai H, Ono Y, Frohman MA, Kanaho Y. Interaction of the small G protein RhoA with the C terminus of human phospholipase D1. The Journal of biological chemistry. 1999;274:6035–6038. doi: 10.1074/jbc.274.10.6035. [DOI] [PubMed] [Google Scholar]
- 115.Shamah SM, Lin MZ, Goldberg JL, Estrach S, Sahin M, Hu L, Bazalakova M, Neve RL, Corfas G, Debant A, Greenberg ME. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell. 2001;105:233–244. doi: 10.1016/s0092-8674(01)00314-2. [DOI] [PubMed] [Google Scholar]
- 116.Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106:489–498. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- 117.Shyy JY, Chien S. Role of integrins in endothelial mechanosensing of shear stress. Circulation research. 2002;91:769–775. doi: 10.1161/01.res.0000038487.19924.18. [DOI] [PubMed] [Google Scholar]
- 118.Yamashiro S, Totsukawa G, Yamakita Y, Sasaki Y, Madaule P, Ishizaki T, Narumiya S, Matsumura F. Citron kinase, a Rho-dependent kinase, induces di-phosphorylation of regulatory light chain of myosin II. Molecular biology of the cell. 2003;14:1745–1756. doi: 10.1091/mbc.E02-07-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hornstein I, Alcover A, Katzav S. Vav proteins, masters of the world of cytoskeleton organization. Cellular signalling. 2004;16:1–11. doi: 10.1016/s0898-6568(03)00110-4. [DOI] [PubMed] [Google Scholar]
- 120.Wang G, Moniri NH, Ozawa K, Stamler JS, Daaka Y. Nitric oxide regulates endocytosis by S-nitrosylation of dynamin. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1295–1300. doi: 10.1073/pnas.0508354103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schiller MR. Coupling receptor tyrosine kinases to Rho GTPases--GEFs what's the link. Cellular signalling. 2006;18:1834–1843. doi: 10.1016/j.cellsig.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 122.Bustelo XR, Sauzeau V, Berenjeno IM. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. BioEssays : news and reviews in molecular, cellular and developmental biology. 2007;29:356–370. doi: 10.1002/bies.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rossy J, Gutjahr MC, Blaser N, Schlicht D, Niggli V. Ezrin/moesin in motile Walker 256 carcinosarcoma cells: signal-dependent relocalization and role in migration. Experimental cell research. 2007;313:1106–1120. doi: 10.1016/j.yexcr.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 124.Dubash AD, Wennerberg K, Garcia-Mata R, Menold MM, Arthur WT, Burridge K. A novel role for Lsc/p115 RhoGEF and LARG in regulating RhoA activity downstream of adhesion to fibronectin. Journal of cell science. 2007;120:3989–3998. doi: 10.1242/jcs.003806. [DOI] [PubMed] [Google Scholar]
- 125.Munson C, Huisken J, Bit-Avragim N, Kuo T, Dong PD, Ober EA, Verkade H, Abdelilah-Seyfried S, Stainier DY. Regulation of neurocoel morphogenesis by Pard6 gamma b. Developmental biology. 2008;324:41–54. doi: 10.1016/j.ydbio.2008.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hosono Y, Yamaguchi T, Mizutani E, Yanagisawa K, Arima C, Tomida S, Shimada Y, Hiraoka M, Kato S, Yokoi K, Suzuki M, Takahashi T. MYBPH, a transcriptional target of TTF-1, inhibits ROCK1, and reduces cell motility and metastasis. The EMBO journal. 2012;31:481–493. doi: 10.1038/emboj.2011.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen Z, Yuhanna IS, Galcheva-Gargova Z, Karas RH, Mendelsohn ME, Shaul PW. Estrogen receptor α mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. The Journal of clinical investigatio. 1999;103:401–406. doi: 10.1172/JCI5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kroll J, Waltenberger J. VEGF-A induces expression of eNOS and iNOS in endothelial cells via VEGF receptor-2 (KDR) Biochemical and biophysical research communications. 1998;252:743–746. doi: 10.1006/bbrc.1998.9719. [DOI] [PubMed] [Google Scholar]
- 129.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 130.Feron O, Kelly RA. The caveolar paradox: suppressing, inducing, and terminating eNOS signaling. Circulation research. 2001;88:129–131. doi: 10.1161/01.res.88.2.129. [DOI] [PubMed] [Google Scholar]
- 131.Bell RM, Yellon DM. Bradykinin limits infarction when administered as an adjunct to reperfusion in mouse heart: the role of PI3K, Akt and eNOS. Journal of molecular and cellular cardiology. 2003;35:185–193. doi: 10.1016/s0022-2828(02)00310-3. [DOI] [PubMed] [Google Scholar]
- 132.Boo YC, Hwang J, Sykes M, Michell BJ, Kemp BE, Lum H, Jo H. Shear stress stimulates phosphorylation of eNOS at Ser635 by a protein kinase A-dependent mechanism. American journal of physiology. Heart and circulatory physiology. 2002;283:H1819–1828. doi: 10.1152/ajpheart.00214.2002. [DOI] [PubMed] [Google Scholar]
- 133.Stalker TJ, Skvarka CB, Scalia R. A novel role for calpains in the endothelial dysfunction of hyperglycemia. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2003;17:1511–1513. doi: 10.1096/fj.02-1213fje. [DOI] [PubMed] [Google Scholar]
- 134.McCabe TJ, Fulton D, Roman LJ, Sessa WC. Enhanced electron flux and reduced calmodulin dissociation may explain “calcium-independent” eNOS activation by phosphorylation. The Journal of biological chemistry. 2000;275:6123–6128. doi: 10.1074/jbc.275.9.6123. [DOI] [PubMed] [Google Scholar]
- 135.Yao Q, Chen J, Cao H, Orth JD, McCaffery JM, Stan RV, McNiven MA. Caveolin-1 interacts directly with dynamin-2. Journal of molecular biology. 2005;348:491–501. doi: 10.1016/j.jmb.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 136.Dedio J, Konig P, Wohlfart P, Schroeder C, Kummer W, Muller-Esterl W. NOSIP, a novel modulator of endothelial nitric oxide synthase activity. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2001;15:79–89. doi: 10.1096/fj.00-0078com. [DOI] [PubMed] [Google Scholar]
- 137.Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nature reviews. Molecular cell biology. 2010;11:515–528. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- 138.Sessa WC. eNOS at a glance. Journal of cell science. 2004;117:2427–2429. doi: 10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- 139.Weiss SW, Albers DS, Iadarola MJ, Dawson TM, Dawson VL, Standaert DG. NMDAR1 glutamate receptor subunit isoforms in neostriatal, neocortical, and hippocampal nitric oxide synthase neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:1725–1734. doi: 10.1523/JNEUROSCI.18-05-01725.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cooke JP, Dzau VJ. Nitric oxide synthase: role in the genesis of vascular disease. Annual review of medicine. 1997;48:489–509. doi: 10.1146/annurev.med.48.1.489. [DOI] [PubMed] [Google Scholar]
- 141.Cheah JH, Kim SF, Hester LD, Clancy KW, Patterson SE, 3rd, Papadopoulos V, Snyder SH. NMDA receptor-nitric oxide transmission mediates neuronal iron homeostasis via the GTPase Dexras1. Neuron. 2006;51:431–440. doi: 10.1016/j.neuron.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rodriguez-Munoz M, de la Torre-Madrid E, Sanchez-Blazquez P, Wang JB, Garzon J. NMDAR-nNOS generated zinc recruits PKCgamma to the HINT1-RGS17 complex bound to the C terminus of Mu-opioid receptors. Cellular signalling. 2008;20:1855–1864. doi: 10.1016/j.cellsig.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 143.Huelsken J, Behrens J. The Wnt signalling pathway. Journal of cell science. 2002;115:3977–3978. doi: 10.1242/jcs.00089. [DOI] [PubMed] [Google Scholar]
- 144.Lee NE, Park YJ, Chung IY, Seo SW, Park JM, Yoo JM, Song JK. Gene expression changes in a rat model of oxygen-induced retinopathy. Korean J Ophthalmol. 2011;25:42–47. doi: 10.3341/kjo.2011.25.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ishikawa K, Yoshida S, Kadota K, Nakamura T, Niiro H, Arakawa S, Yoshida A, Akashi K, Ishibashi T. Gene expression profile of hyperoxic and hypoxic retinas in a mouse model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2010;51:4307–4319. doi: 10.1167/iovs.09-4605. [DOI] [PubMed] [Google Scholar]
- 146.Tea M, Fogarty R, Brereton HM, Michael MZ, Van der Hoek MB, Tsykin A, Coster DJ, Williams KA. Gene expression microarray analysis of early oxygen-induced retinopathy in the rat. J Ocul Biol Dis Infor. 2009;2:190–201. doi: 10.1007/s12177-009-9041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sato T, Kusaka S, Hashida N, Saishin Y, Fujikado T, Tano Y. Comprehensive gene-expression profile in murine oxygen-induced retinopathy. Br J Ophthalmol. 2009;93:96–103. doi: 10.1136/bjo.2008.142646. [DOI] [PubMed] [Google Scholar]
- 148.Bates D, Taylor GI, Minichiello J, Farlie P, Cichowitz A, Watson N, Klagsbrun M, Mamluk R, Newgreen DF. Neurovascular congruence results from a shared patterning mechanism that utilizes Semaphorin3A and Neuropilin-1. Dev Biol. 2003;255:77–98. doi: 10.1016/s0012-1606(02)00045-3. [DOI] [PubMed] [Google Scholar]
- 149.Chui TY, Bissig D, Berkowitz BA, Akula JD. Refractive Development in the “ROP Rat”. Journal of Ophthalmology. 2012;2012:956705. doi: 10.1155/2012/956705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Anderson AM, Weasner BM, Weasner BP, Kumar JP. Dual transcriptional activities of SIX proteins define their roles in normal and ectopic eye development. Development. 2012;139:991–1000. doi: 10.1242/dev.077255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Alfano G, Vitiello C, Caccioppoli C, Caramico T, Carola A, Szego MJ, McInnes RR, Auricchio A, Banfi S. Natural antisense transcripts associated with genes involved in eye development. Human molecular genetics. 2005;14:913–923. doi: 10.1093/hmg/ddi084. [DOI] [PubMed] [Google Scholar]
- 152.Hassell JR, Birk DE. The molecular basis of corneal transparency. Experimental eye research. 2010;91:326–335. doi: 10.1016/j.exer.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pellegata NS, Dieguez-Lucena JL, Joensuu T, Lau S, Montgomery KT, Krahe R, Kivela T, Kucherlapati R, Forsius H, de la Chapelle A. Mutations in KERA, encoding keratocan, cause cornea plana. Nature genetics. 2000;25:91–95. doi: 10.1038/75664. [DOI] [PubMed] [Google Scholar]
- 154.Yanai R, Thanos A, Connor KM. Complement involvement in neovascular ocular diseases. Advances in experimental medicine and biology. 2012;946:161–183. doi: 10.1007/978-1-4614-0106-3_10. [DOI] [PubMed] [Google Scholar]
- 155.Hartnett ME, Martiniuk D, Byfield G, Geisen P, Zeng G, Bautch VL. Neutralizing VEGF decreases tortuosity and alters endothelial cell division orientation in arterioles and veins in a rat model of ROP: relevance to plus disease. Invest Ophthalmol Vis Sci. 2008;49:3107–3114. doi: 10.1167/iovs.08-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.