Abstract
Objective
This study assesses the two-year outcomes, costs, and financial sustainability of a medical care management intervention for community mental health settings.
Method
A total of 407 psychiatric outpatients with serious mental illnesses were randomized to usual care or to a medical care manager, who provided care coordination and education. Two-year follow-up chart reviews and interviews assessed quality and outcomes of care, and costs from both the health system and managerial perspective.
Results
Subjects in the intervention group had sustained improvements in quality of primary care preventive services (p<0.001), quality of cardiometabolic care (p<0.001), and mental health-related quality of life (p<0.001). From a health system perspective, by year 2, the program showed a $932 reduction in total costs (95% CI (−1973, 102) with a 92.3% probability that the program was associated with lower costs than usual care. From the community mental health center perspective, the program would break even (i.e., revenues would cover setup costs) if 58% or more of clients had Medicaid or another form of insurance. Given that only 40.5% of clients in the study had Medicaid, the program was not sustainable after grant funding ended.
Conclusions
The positive long-term outcomes and favorable cost profile provided evidence of the potential value of this model. However, the discrepancy between health system and managerial cost perspectives limited the program’s financial sustainability. With anticipated insurance expansions under health reform, there is likely to be a stronger business case for safety net organizations considering implementing these and other evidence-based interventions.
Introduction
An extensive literature has documented that individuals with mental illnesses are at elevated risk for medical morbidity and early mortality.(1) (2) Although this problem was first described nearly a century ago,(3) only recently has this issue become a major focus of mental health advocacy and policy efforts.(4)
For many patients with serious mental disorders, the primary point of contact with the health system is through public-sector mental health programs rather than primary medical care. There has been a growing interest in developing “specialty health homes” for managing medical care for these populations in community mental health settings. (5) Care management, in which staff provide education, advocacy, and linkages to community-based medical services, is a potentially promising approach to delivering care in this population, based on its relative low-cost and flexibility. (5) However, there are currently few evidence-based models for delivering that care.(6, 7)
The Primary Care Access, Referral and Evaluation (PCARE) study is a randomized trial of medical care management for persons with serious mental illnesses treated in community mental health settings. A previous report documented that the intervention improved 1-year quality and outcomes of primary medical care.(8)
This study reports on costs for each year and 2-year outcomes of this intervention. Costs are presented from two vantage points: a health system perspective (which is most applicable to policymakers), and a managerial perspective (which is most relevant to clinic directors considering implementing these programs). The goals of the study are to assess 1) clinical sustainability (i.e. whether one-year improvements were maintained), and 2) financial sustainability (i.e. whether the intervention provided value from a health system perspective and was sustainable under routine funding conditions).
Method
Details of the PCARE study design and intervention are described in an earlier manuscript. (8) These are briefly outlined here to provide context for the 2-year outcome and cost data.
Study Setting
The study was conducted in an urban community mental health center (CMHC) in Atlanta, Georgia. Its target population is individuals age 18 and older from the area who are economically disadvantaged and who experience serious and persistent mental illness with or without comorbid addictive disorders. With the exception of the study intervention, the clinic did not provide any formal medical or mental health care case management or any onsite medical care.
Sample Recruitment
The sample was recruited through a combination of flyers posted at the CMHC, waiting room recruitment, and provider referrals, with approximately 1/3 of potential subjects identified through each of the three approaches. To be eligible, subjects had to be on the active patient roster at the CMHC, have a serious mental illness, (9) and have the capacity to provide informed consent. Inclusion criteria were broad to optimize generalizability to community mental health settings.
Randomization and Follow-Up
Using a computerized algorithm, patients were randomized to the intervention or usual care group. After randomization, interviews were administered every 6 months throughout the course of the study. Interviewers were blinded to subjects’ randomization status. Annual chart reviews were used to calculate quality measures.
Intervention
Two full-time registered nurses provided care management activities combining patient education and activation, and logistical support in obtaining access to ongoing, comprehensive primary care services. Each care manager had a caseload of approximately 75 patients at any given time, each of whom had an initial intake visit followed by monthly follow-up visits.
Care managers enhanced activation using motivational interviewing techniques(10) and action plans, (11) which set and track short-term, achievable goals for medical care or lifestyle change. Coaching was provided to patients to help them interact more effectively with their providers. With the subject’s permission, providers were notified about changes in the patient’s medication regimen and medical status. The care manager worked to helped clients overcome barriers to attending medical appointments
Usual Care
Subjects assigned to usual care were given a list with contact information for local primary care medical clinics that accept uninsured and Medicaid clients. Participants in the usual care condition were not restricted in the medical care or other services that they sought.
Measures
An interview battery administered at baseline and then every 6 months throughout the study was used to identify sites where patients had received medical or mental health services as well as to collect clinical data. Reviews of all medical and mental health charts from these sites at baseline, 12-month, and 24-month follow-up assessed quality of preventive and cardiometabolic care and health service use.
Quality of primary care was assessed at baseline and 12 months using 23 indicators drawn from the U.S. Preventive Services Task Force (USPSTF) guidelines. (12) Among individuals with a cardiometabolic condition (diabetes, hypertension, hypercholesterolemia, or coronary artery disease), quality indicators were drawn from chart reviews using the RAND Community Quality Index (CQI) study.(13) For both of these sets of quality indicators, an aggregate indicator was created representing the proportion of services for which an individual was eligible that were received by the subject.
Health-related quality of life was assessed using the SF-36, developed for the Medical Outcomes Study.(14, 15) Physical Component Summary (PCS) and Mental Component Summary (MCS) scores can be constructed from the survey, ranging from 0 (poor health) to 100 (perfect health). (16) The oblique method, which is the preferred approach when examining persons with comorbid physical and mental conditions, (17, 18) was selected a priori as the approach for calculating the summary scores. Individual subscales were also calculated to provide context for these summary scores.(19)
Among subjects with available fasting laboratory values, the Framingham Cardiovascular Risk Index, which estimates the 10-year risk of developing incident coronary heart disease, was assessed.
Intervention Costs
Staff costs for the nurse care managers were calculated based on median salaries for registered nurses from the Bureau of Labor Statistics. (20) A mean fringe rate of 29% for benefits was included based on national averages for nurses employed in public sector facilities. (20) Training costs were calculated based on daily salaries and fringe rates for the senior staff providing training.
Equipment costs were broken into one-time setup costs (e.g., exam table, sphygmomanometer, scale) and recurring expenses (e.g., gloves, bandages). All prices were drawn from the national medical supply company where the products were purchased.
An indirect rate of 15% was applied to all intervention expenses to account for clinic space and administrative support.(21)
Costs from the Health-System Perspective
A cost analysis was calculated from the health system perspective using standard approaches from the cost-effectiveness literature.(22–24) The quantity of each type of health service (mental and medical outpatient, emergency room, and inpatient) was drawn from chart review data from each site where subjects obtained services. Unit costs were assigned to each service type based on median national expenditures for each type of service from the 2007 Medical Expenditure Panel Survey (MEPS).(25) This survey is well-suited for assessing unit costs from a health system perspective because it uses direct payments, not charges, and captures costs across all insurance groups. All expenditures for each type of service were inflated to 2010 dollars.
Given initial expenditures in setting up a new program, costs were examined separately for each of the two years of the study. Costs for each intervention visit were calculated by applying the hourly intervention cost, based on staff salaries, fringe benefits, equipment, and overhead, to each visit.
Costs from a Managerial Perspective: Budget Impact Analysis
A budget impact analysis involves a careful accounting of the costs of implementing a new program, coupled with the expected returns. (26, 27) The methods followed the approach proposed by the ISPOR Task Force on Good Research Practices.(28) In contrast to cost-effectiveness analyses (CEA), budget impact analyses adopt a managerial rather than societal perspective and have a shorter-term horizon.
For the budget impact analysis, only services provided at the CMHC were considered. Medicaid reimbursement rates were calculated based on 2010 payment rates for clinical nurse visits from Georgia’s Medicaid program. As with the health system-level analysis, intervention expenditures were treated as positive costs. However, individual visit reimbursements were treated as negative expenditures (i.e., revenues).
Typical of most CMHCs nationwide,(29) at the study site, nearly all clients had either Medicaid or were uninsured. Costs for uninsured clients were covered by capitated annual state block grants provided to the clinics. Medicaid services were reimbursed on a fee-for-service basis for each visit. Under varying case-mix scenarios, we calculated a breakeven point, where the total reimbursement offset the costs of funding the care management service during the first year.
Statistical Analysis
All analyses were conducted as intent-to-treat. Two-year clinical outcome analyses were conducted using random regression to calculate the relative difference in change over time. For each outcome measure, the model assessed the outcome as a function of 1) randomization group, 2) time since randomization and 3) group by time interaction. The group by time interaction, which reflects the relative difference in change in the parameters over time, was the primary measure of statistical significance.
To mitigate potential impact of missing interview data on the analysis, we performed multiple imputation by a Monte Carlo Markov Chain approach to impute missing scores for interview data. Covariates used in multiple imputation are age, gender, race, psychiatric diagnosis, medical comorbidity, and values at the 5 time points.
We used bootstrap analysis to generate 95% confidence intervals and to estimate the probability that total healthcare costs were lower in the intervention group than in the usual care group (i.e. a “cost offset”) for each cost category. Bootstrap analysis was conducted by constructing 1000 samples from the study dataset in which the difference in mean costs, defined as the mean among cases minus the mean among controls, was computed. The 95% confidence interval was then derived from the resulting distribution of difference in mean costs across the 1000 bootstrap samples. The probability of a cost offset was estimated by dividing the number of bootstrap samples in which the intervention group had lower healthcare cost than the usual care group by 1000.
Initial analyses indicated that cost data were highly non-normally distributed. Removing extreme outliers (the 3% of the sample beyond 3 standard deviations above or below the mean) substantially improved the normality of the distribution. Confidence intervals and probability of cost-offset were conducted both with and without inclusion of these extreme outliers.
Results
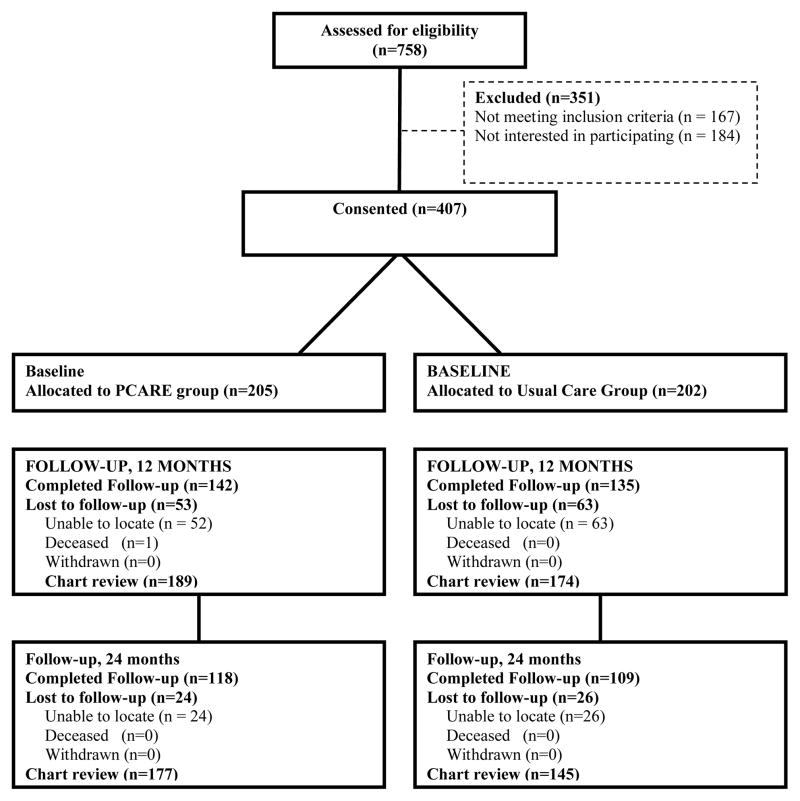
A total of 407 subjects provided informed consent and were randomized. Of those randomized, 68.1% completed 12-month interviews and 55.8% completed 24-month interviews. A total of 89.2% of the sample had complete 12-month chart review data and 79.1% had complete 24-month chart review data. (Figure 1)
Figure 1.
Study Flow Chart
Baseline Characteristics (Table 1)
Table 1.
Demographic and Clinical Characteristics
| Characteristics | PCARE (n=205) | Usual Care (n=202) | p value |
|---|---|---|---|
|
| |||
| Demographics | |||
|
| |||
| Age (years, means ± SD) | 47.0±8.1 | 46.3±8.1 | 0.68 |
| Female, n (%) | 105 (51.2) | 92 (45.5) | 0.18 |
| Race/Ethnicity | 0.78 | ||
| African-American, n (%) | 156 (76.5) | 159 (78.7) | |
| Hispanic or Latino, n (%) | 4 (2.0) | 2 (1.0) | |
| Insurance | |||
| Medicaid | 77(37.9%) | 85(42.1%) | 0.39 |
| Uninsured | 123 (60.3%) | 117 (57.9%) | 0.63 |
| Private | 3 (1.5%) | 0 | 0.08 |
| Monthly income ($, median) | 209.5 (0–603.0) | 374 (80.0–623.0) | 0.20 |
| Single, never married, n (%) | 102 (50.3) | 96 (47.5) | 0.91 |
| Education Completed (years, median) | 12 (11–13) | 12 (11–13) | 0.87 |
| Unemployed | 180 (87.8) | 179 (88,6) | 0.79 |
|
| |||
| Primary Psychiatric Diagnosis | |||
|
| |||
| Schizophrenia/schizoaffective disorder, n (%) | 75 (36.6) | 69 (34.2) | 0.61 |
| Bipolar disorder, n (%) | 22 (10.7) | 30 (14.9) | 0.21 |
| PTSD, n (%) | 11 (5.4) | 9 (4.5) | 0.67 |
| Depression, n (%) | 94 (45.9) | 85 (42.1) | 0.44 |
| Other, n (%) | 0 | 1 (0.5) | 0.31 |
|
| |||
| Co-occurring Substance Use Disorder | 50 (24.4%) | 53 (26.2%) | 0.66 |
Values are means ± SDs, numbers of patients (percentages), or medians (25th to 75th percentiles).
The sample was predominantly African American (77.9%) and poor (median annual income of $3,400). A total of 40% had Medicaid coverage, 59% were uninsured, and 1% had private insurance. The most common psychiatric diagnoses were schizophrenia (42.8%), depression (32.7%), and bipolar disorder (17.2%). A total of 25.3% of the sample had a co-occurring substance use disorder. The most common medical comorbidities were hypertension (45.6%), arthritis (36.6%), tooth/gum disease (25.6%), asthma (20.1%), and diabetes (17.9%). There were no significant differences between the groups in any of the demographic or diagnostic characteristics at baseline.
Two-Year Clinical Outcomes (Table 2)
Table 2.
2-Year Quality and Outcomes of Clinical Care
| PCARE (n=205) | Usual Care (n=202) | p value | p value for change (group*time interaction) | |
|---|---|---|---|---|
|
Quality
| ||||
| Prevention Quality Index | <0.001 | |||
| Baseline (n=391) | 19.9±16.8 | 19.7±17.4 | 0.62 | |
| 1-Year (n=376) | 56.1±23.5 | 20.3±16.2 | <0.0001 | |
| 2-year (n=345) | 56.2±27.7 | 17.4±15.7 | <0.0001 | |
|
| ||||
| Cardiometabolic Quality Index | <0.001 | |||
| Baseline (n=170) | 28.2±33.4 | 31.5±29.3 | 0.30 | |
| 1-Year (n=180) | 34.8±38.5 | 30.3±30.2 | 0.78 | |
| 2-year (n=178) | 43.5±39.5 | 27.8±30.1 | 0.018 | |
|
| ||||
|
Outcomes
| ||||
| SF-36 MCS (n=407) | <0.0001 | |||
| Baseline | 36.4±10.1 | 36.0±10.3 | 0.2981 | |
| 6-month | 37.4±9.9 | 37.0±10.7 | 0.3684 | |
| 1-Year | 39.0±10.2 | 36.5±10.6 | <0.0001 | |
| 18-month | 39.6±10.0 | 37.4±10.2 | <0.0001 | |
| 2-year | 40.5±10.3 | 39.0±10.8 | 0.0012 | |
|
| ||||
| SF-36: PCS(n=407) | 0.47 | |||
| Baseline | 36.4±11.7 | 35.7±11.4 | 0.1744 | |
| 6-month | 37.0±11.6 | 35.9±12.2 | 0.0377 | |
| 1-Year | 36.9±12.0 | 35.7±12.3 | 0.0228 | |
| 18-month | 37.6±12.2 | 36.3±12.2 | 0.0152 | |
| 2-Year | 38.4±12.7 | 37.0±12.9 | 0.0099 | |
|
| ||||
| Framingham Score for 10-year CVD Risk | 0.39 | |||
| Baseline (n=121) | 7.9%±5.4% | 8.5%±6.3% | 0.8329 | |
| 1-Year (n=183) | 7.1%±5.3% | 9.5%±7.4% | 0.0321 | |
| 2-year (n=146) | 7.6%±6.3% | 10.0%±7.8% | 0.0142 | |
Overall, the gains in quality and outcomes of care persisted at 2 years. The total proportion of preventive services for which a client was eligible that were received by the client (primary study outcome) more than doubled between baseline and year 1, and remained highly significant by year 2 (56.2% vs. 17.4%, p<0.001 for group by time interaction). Among the subset of individuals with cardiometabolic diagnoses (diabetes, hypertension, high cholesterol, heart disease), the proportion receiving guideline-concordant cardiometabolic care increased in the PCARE group from 28.2% to 43.5%, while declining slightly in the usual care group (31.5% to 27.8%), resulting in a significant group by time interaction (p<0.001).
Over the two-year follow-up period, there was a significantly greater improvement in the MCS of the SF-36 for the PCARE group than the usual care group (4.1 versus 3 point improvement, p<0.001 for the group by time interaction). The relative improvement on the PCS of the SF-36 was smaller and not statistically significant (2 point versus 1.3 point improvement, p=0.47 for the group by time interaction). Significant improvements, as reflected in significant group by time interactions were seen in the Physical Functioning (p=0.0001), pain (p=0.0006), role-emotional (p<0.0001), social functioning (p<0.0001), general health (p=0.0002), and mental health subscales (p<0.0001) (Appendix).
Among those with fasting blood tests available (n=121), the Framingham Index, which represents the risk of developing cardiovascular disease in 10-years, was significantly lower at two years in the PCARE versus the usual care group (7.6% vs. 10%, p=0.01), however the group by time interaction for the relative change over time was not significant (p=0.39).
Costs: Health System Perspective
Mean annual costs of implementing the intervention, including staff salaries, fringe benefits, supplies and equipment, and overhead, were estimated at $973 per patient. During the second year, mean costs were reduced to $915 per patient, since they did not include the one-time equipment and training costs.
In the health-system perspective analysis at one-year follow-up including all study participants, total costs were $218 higher for PCARE than usual care (95% CI: −$1190, $1585), which reflected a 38.4% probability of a cost offset. Excluding 3% of extreme outliers, total costs at one year were $93 higher for PCARE than usual care (95% CI: −$871, $1012), which reflected a 44.2% probability of a cost offset.
For the second year, subjects in the PCARE program cost $932 less than subjects in usual care, reflecting a 92.3% probability of a cost offset (95% CI: −$1973, $102). Excluding 3% of extreme outliers, subjects in the PCARE program cost $920 less than subjects in usual care, reflecting a 96.1% probability of a cost offset (95% CI: −$1718, −$54).
Costs: Managerial Perspective
Revenues were calculated based on the typical patient flow patterns seen for the nurses in the study (1 new patient and 5 follow-up visit each day). Once caseloads were full, maximal revenues that could be achieved for the program using two nurse care managers working at full capacity, with all clients covered by Medicaid or other insurance, would be $360,840. Assuming minimal or no out-of-pocket payments by uninsured clients (which was the case for this clinic and is typical of CMHCs more generally), (30) the clinic would break even financially –i.e., revenues would exceed expenditures –if more than 58% of clients had Medicaid or some other form of health insurance. Because only 40.5% of clients in the study clinic had Medicaid, it was not financially sustainable under existing conditions. Largely because of challenges in financing, the program closed after the grant was completed.
Discussion
From a clinical perspective, improvements in quality of primary care persisted at two years. From a health-system perspective, the cost profile was highly favorable, and led to a trend towards a cost-offset by the second year, suggesting a good value. However, from a managerial perspective, the program was not financially sustainable under current reimbursement conditions; with a greater proportion of insured clients, it could have been supported. These results, in particular the differences between societal and managerial cost perspective, shed light on barriers to implementing these and other evidence-based practices in routine settings, and how expansion of insurance under health reform might help resolve such obstacles.
The intervention led to sustained improvement in quality and outcomes of primary care. The majority of gains in quality and outcomes were seen during the first year, with continuing, but smaller, improvements during year 2. This asymptotic pattern is similar to that described in other quality improvement interventions, which typically have the greatest relative impact in the first 6 months to one-year, as the greatest deficiencies in care are addressed, with subsequent efforts focused on maintaining those improvements. (31) (32)
For physical health outcomes, even a two-year horizon may be a relatively brief window to reverse the cumulative effects of socioeconomic deprivation, adverse health behaviors, and poor quality of medical care that lead to compromised health in this population. (33) Particularly for patients with high levels of medical morbidity, more aggressive programs including medication management hold potential for substantial improvements in medical outcomes. (34) Nonetheless, the current study found significant improvements in a majority of SF-36 subscales related to physical health (general health, physical functioning, and pain), and the intervention group had a significantly better cardiovascular risk profile at two-year follow up. “Stepped care” models may be able to combine these two approaches, using care management for general mental health clinic populations, with more intensive treatment protocols for patients with preexisiting cardiovascular risk factors or other illnesses.
For health-system perspective, there was a strong trend toward cost savings by the second year, with a 92.3% chance of a cost offset. This result is consistent with research on treating depression in primary care, which has found that these savings may become evident over the long-term, particularly among the costliest and most complex patients.(36, 37) Given the relatively modest costs of establishing care management programs, and their ability to steer patients from inappropriate to more appropriate forms of care, these approaches can represent a particularly good value for society.
However, from a managerial perspective, assessing the “business case” for the intervention was more complex. Because of the high rate (59%) of uninsured clients, revenues would not have covered the costs of running the program in the absence of grant funding. And, despite data supporting its effectiveness and high levels of satisfaction by providers and patients, clinic management was unable to continue the program after the grant was completed. Challenges in achieving financial sustainability are not unique to medical care management programs, but apply to any new clinical programs in public-sector mental health systems with large numbers of uninsured clients. More generally, the mismatch between societal and managerial perspectives, coupled with a lack of a clear locus of accountability for improving quality, may underlie the failure of many cost-effective interventions to be effectively disseminated in routine clinical settings.(26, 38)
Several limitations of the study should be noted. The intervention was tested in a single site; care management approaches might need to differ in other types of settings (e.g., rural areas without nearby medical providers). Similarly, the business case for these programs could differ in sites with lower numbers of eligible patients or where states use different mechanisms of paying for Medicaid patients or for the uninsured. Nonetheless, the characteristics of the site and payment approaches in the current clinic are typical of those used by other urban community health centers nationwide. (39) Also, 2-year follow-up interview rates were relatively low; because cost data, which were the primary outcomes examined in this study, were derived from patient charts, follow up was less of a concern for these analyses.
Expansion of Medicaid under the Patient Protection and Affordable Care Act is likely to disproportionately improve rates of insurance for persons with mental illness who are currently uninsured.(40) This could help improve the business case for implementing evidence-based programs like the one described in the current study in community settings. Other new financing strategies to be tested under new models of care, such as bundled payments that include coverage for care managers, could also help reduce the barriers to implementation of these and other evidence-based quality improvement strategies for persons treated in safety net settings.(41) Finally, for persons with serious and persistent mental conditions, new health home models will include the development of specialty care medical homes that provide primary care services through community mental health providers. (5) These new initiatives hold the potential to begin to better align financial incentives for improving physical healthcare in this vulnerable population, and more broadly, for disseminating evidence-based practices in community mental health settings.
Table 3.
Costs of Care (Health System Perspective)
| Variable | wave | PCARE (n=205) | Usual Care (n=202) | Δ PCARE - Usual Care | Probability of Cost offset (%) |
|---|---|---|---|---|---|
|
| |||||
| Medical outpatient visit, $ | Year 1 | 1855 (1644, 2090) | 1589 (1388, 1801) | 265 (−38, 573) | 7.6 |
| Year 2 | 1338 (1160, 1525) | 1358 (1151, 1577) | −20 (−323, 260) | 53.1 | |
|
| |||||
| Medical ER Visit, $ | Year 1 | 779 (601, 971) | 1038 (838, 1250) | −259 (−564, 23) | 93.3 |
| Year 2 | 580 (435, 743) | 668 (506, 860) | −88 (−328, 141) | 71.1 | |
|
| |||||
| Medical Hospitalization, $ | Year 1 | 1170 (698, 1723) | 1194 (730, 1726) | −23 (−721, 670) | 52.6 |
| Year 2 | 626 (331, 991) | 683 (369, 1064) | −57 (−535, 426) | 58.5 | |
|
| |||||
| Mental Health outpatient visit, $ | Year 1 | 4086 (3721, 4464) | 4346 (3921, 4812) | −260 (−858, 289) | 76.2 |
| Year 2 | 2864 (2495, 3259) | 3451 (3040, 3862) | −587 (−1149, −11) | 95.7 | |
|
| |||||
| Psychiatric ER Visit, $ | Year 1 | 224 (164, 289) | 241 (172, 311) | −17 (−110, 76) | 62.6 |
| Year 2 | 176 (126, 233) | 242 (158, 335) | −66 (−173, 40) | 87.0 | |
|
| |||||
| Psychiatric Hospitalization, $ | Year 1 | 420 (120, 802) | 308 (130, 495) | 112 (−263, 538) | 33.5 |
| Year 2 | 78 (0, 186) | 438 (158, 768) | −360 (−704, −73) | 97.8 | |
|
| |||||
| Total Cost, $ | Year 1 | 8934 (8042, 9868) | 8715 (7784, 9777) | 218 (−1190, 1585) | 38.4 |
| Year 2 | 5908 (5181, 6620) | 6840 (6096, 7629) | −932 (−1973, 102) | 92.3 | |
Acknowledgments
The study was supported by NIMH R01MH070437 and K24MH075867. Dr. Druss had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
None of the authors reports any conflicts of interest.
Clinicaltrials.gov identifier NCT00183313
Contributor Information
Benjamin G. Druss, Rollins School of Public Health, Emory University.
Silke A. von Esenwein, Rollins School of Public Health, Emory University.
Michael T. Compton, The George Washington University School of Medicine and Health Sciences.
Liping Zhao, Rollins School of Public Health, Emory University.
Douglas L. Leslie, Department of Public Health Sciences, Penn State University.
References
- 1.Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. Jama. 2007;298(15):1794–6. doi: 10.1001/jama.298.15.1794. [DOI] [PubMed] [Google Scholar]
- 2.Parks J, Svedsen D. Morbidity and Mortality in People with Serious Mental Illness. Alexandria, VA: 2006. [Google Scholar]
- 3.Malzberg B. Life tables for patients with mental disease. Journal of the American Statistical Association. 1932;27(177A):160–74. [Google Scholar]
- 4.Druss BG, Bornemann TH. Improving health and health care for persons with serious mental illness: the window for US federal policy change. JAMA. 303(19):1972–3. doi: 10.1001/jama.2010.615. [DOI] [PubMed] [Google Scholar]
- 5.Alakeson V, Frank RG, Katz RE. Specialty care medical homes for people with severe, persistent mental disorders. Health Aff (Millwood) 29(5):867–73. doi: 10.1377/hlthaff.2010.0080. [DOI] [PubMed] [Google Scholar]
- 6.Compton MT, Daumit GL, Druss BG. Cigarette smoking and overweight/obesity among individuals with serious mental illnesses: A preventive perspective. Harvard Rev Psychiat. 2006;14(4):212–22. doi: 10.1080/10673220600889256. [DOI] [PubMed] [Google Scholar]
- 7.Butler M, Kane R, McAlpine D, Kathol R, Fu S, Hagedorn H, et al. Integration of Mental Health/Substance Abuse and Primary Care. Minneapolis, Minnesota: Minnesota Evidence-based Practice Center; 2009. [Google Scholar]
- 8.Druss BG, von Esenwein SA, Compton MT, Rask KJ, Zhao L, Parker RM. A randomized trial of medical care management for community mental health settings: the Primary Care Access, Referral, and Evaluation (PCARE) study. Am J Psychiatry. 167(2):151–9. doi: 10.1176/appi.ajp.2009.09050691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Advisory Mental Health Council. Health care reform for Americans with severe mental illness: report of the National Advisory Mental Health Council. Am J Psychiatry. 1993;150:1447–65. doi: 10.1176/ajp.150.10.1447. [DOI] [PubMed] [Google Scholar]
- 10.Rollnick S, Mason P, Butler C. Health Behavior Change: A Guide for Practitioners. Cardiff, UK: Churchill Livingstone; 1999. [Google Scholar]
- 11.Handley M, MacGregor K, Schillinger D, Sharifi C, Wong S, Bodenheimer T. Using action plans to help primary care patients adopt healthy behaviors: a descriptive study. J Am Board Fam Med. 2006;19(3):224–31. doi: 10.3122/jabfm.19.3.224. [DOI] [PubMed] [Google Scholar]
- 12.Thorpe JM, Kalinowski CT, Patterson ME, Sleath BL. Psychological distress as a barrier to preventive care in community-dwelling elderly in the United States. Medical care. 2006;44(2):187–91. doi: 10.1097/01.mlr.0000196965.54871.d5. [DOI] [PubMed] [Google Scholar]
- 13.McGlynn EA, Asch SM, Adams J, Keesey J, Hicks J, DeCristofaro A, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348(26):2635–45. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- 14.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF–36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical care. 1993;31(3):247–63. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 15.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF–36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Medical care. 1994;32(1):40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Ware JE, Jr, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF–36 health profile and summary measures: summary of results from the Medical Outcomes Study. Medical care. 1995;33(4 Suppl):AS264–79. [PubMed] [Google Scholar]
- 17.Simon GE, Revicki DA, Grothaus L, Vonkorff M. SF–36 summary scores: are physical and mental health truly distinct? Med Care. 1998;36(4):567–72. doi: 10.1097/00005650-199804000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Hann M, Reeves D. The SF–36 scales are not accurately summarised by independent physical and mental component scores. Qual Life Res. 2008;17(3):413–23. doi: 10.1007/s11136-008-9310-0. [DOI] [PubMed] [Google Scholar]
- 19.Taft C, Karlsson J, Sullivan M. Do SF–36 summary component scores accurately summarize subscale scores? Qual Life Res. 2001;10(5):395–404. doi: 10.1023/a:1012552211996. [DOI] [PubMed] [Google Scholar]
- 20.Bureau of Labor Statistics. Occupational Employment and Wages. 2009 May; [Google Scholar]
- 21.Wolff N, Helminiak TW, Tebes JK. Getting the cost right in cost-effectiveness analyses. Am J Psychiatry. 1997;154(6):736–43. doi: 10.1176/ajp.154.6.736. [DOI] [PubMed] [Google Scholar]
- 22.Gold MR. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 23.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276(15):1253–8. [PubMed] [Google Scholar]
- 24.Manning WG., Jr Panel on cost-effectiveness in health and medicine recommendations: identifying costs. J Clin Psychiatry. 1999;60 (Suppl 3):54–6. discussion 7–8. [PubMed] [Google Scholar]
- 25.Cohen JW, Cohen SB, Banthin JS. The medical expenditure panel survey: a national information resource to support healthcare cost research and inform policy and practice. Med Care. 2009;47(7 Suppl 1):S44–50. doi: 10.1097/MLR.0b013e3181a23e3a. [DOI] [PubMed] [Google Scholar]
- 26.Leatherman S, Berwick D, Iles D, Lewin LS, Davidoff F, Nolan T, et al. The business case for quality: case studies and an analysis. Health Aff (Millwood) 2003;22(2):17–30. doi: 10.1377/hlthaff.22.2.17. [DOI] [PubMed] [Google Scholar]
- 27.Neumann PJ. Budget impact analyses get some respect. Value Health. 2007;10(5):324–5. doi: 10.1111/j.1524-4733.2007.00237.x. [DOI] [PubMed] [Google Scholar]
- 28.Mauskopf JA, Sullivan SD, Annemans L, Caro J, Mullins CD, Nuijten M, et al. Principles of good practice for budget impact analysis: report of the ISPOR Task Force on good research practices--budget impact analysis. Value Health. 2007;10(5):336–47. doi: 10.1111/j.1524-4733.2007.00187.x. [DOI] [PubMed] [Google Scholar]
- 29.Jarvis D. Healthcare reform and Oament reform and the behaviroal health safety net: what’s on the hoizon for the community behavioral healthcare system. Seattle, WA: 2009. [Google Scholar]
- 30.Mauer BJ, Druss BG. Mind and body reunited: improving care at the behavioral and primary healthcare interface. J Behav Health Serv Res. 2010;37(4):529–42. doi: 10.1007/s11414-009-9176-0. [DOI] [PubMed] [Google Scholar]
- 31.Wells KB, Tang L, Miranda J, Benjamin B, Duan N, Sherbourne CD. The effects of quality improvement for depression in primary care at nine years: results from a randomized, controlled group-level trial. Health Serv Res. 2008;43(6):1952–74. doi: 10.1111/j.1475-6773.2008.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunkeler EM, Katon W, Tang L, Williams JW, Jr, Kroenke K, Lin EH, et al. Long term outcomes from the IMPACT randomised trial for depressed elderly patients in primary care. BMJ. 2006;332(7536):259–63. doi: 10.1136/bmj.38683.710255.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Druss B, Zhao L, Morrato EH, Von Esenwein SA, Marcus SC. Understanding Excess Mortality in Persons with Mental Illness: 17-Year Follow Up of a Nationally Representative US Survey. Med Care. 2011 doi: 10.1097/MLR.0b013e31820bf86e. [DOI] [PubMed] [Google Scholar]
- 34.Katon WJ, Lin EH, Von Korff M, Ciechanowski P, Ludman EJ, Young B, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010;363(27):2611–20. doi: 10.1056/NEJMoa1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Von Korff M, Katon W, Bush T, Lin EHB, Simon GE, Saunders K, et al. Treatment costs, cost offset, and cost-effectiveness of collaborative management of depression. Psychosom Med. 1998;60(2):143–9. doi: 10.1097/00006842-199803000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Unutzer J, Katon WJ, Fan MY, Schoenbaum MC, Lin EH, Della Penna RD, et al. Long-term cost effects of collaborative care for late-life depression. Am J Manag Care. 2008;14(2):95–100. [PMC free article] [PubMed] [Google Scholar]
- 37.Katon WJ, Russo JE, Von Korff M, Lin EH, Ludman E, Ciechanowski PS. Long-term effects on medical costs of improving depression outcomes in patients with depression and diabetes. Diabetes Care. 2008;31(6):1155–9. doi: 10.2337/dc08-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schoenbaum M, Kelleher K, Lave JR, Green S, Keyser D, Pincus H. Exploratory evidence on the market for effective depression care in Pittsburgh. Psychiatr Serv. 2004;55(4):392–5. doi: 10.1176/appi.ps.55.4.392. [DOI] [PubMed] [Google Scholar]
- 39.Druss BG, Bornemann T, Fry-Johnson YW, McCombs HG, Politzer RM, Rust G. Trends in mental health and substance abuse services at the nation’s community health centers: 1998–2003. Am J Public Health. 2008;98(9 Suppl):S126–31. doi: 10.2105/ajph.98.supplement_1.s126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garfield RL, Lave JR, Donohue JM. Health reform and the scope of benefits for mental health and substance use disorder services. Psychiatr Serv. 2010;61(11):1081–6. doi: 10.1176/ps.2010.61.11.1081. [DOI] [PubMed] [Google Scholar]
- 41.Druss BG, Mauer BJ. Health care reform and care at the behavioral health--primary care interface. Psychiatr Serv. 2010;61(11):1087–92. doi: 10.1176/ps.2010.61.11.1087. [DOI] [PubMed] [Google Scholar]