Abstract
Purpose
To evaluate the clinical and immunologic outcomes of DC (dendritic cell) vaccine with interleukin (IL)-2 and IFN-α 2a in metastatic renal cell carcinoma patients.
Experimental Design
Eighteen consented and eligible patients were treated. Peripheral blood monocytes were cultured ex vivo into mature DCs and loaded with autologous tumor lysate. Treatment consisted of five cycles of intranodal vaccination of DCs (1 × 107 cells/1 mL Lactated Ringer’s solution), 5-day continuous i.v. infusion of IL-2 (18MiU/m2), and three s.c. injections of IFN-α 2a (6MiU) every other day. Response Evaluation Criteria in Solid Tumors criteria were used for disease assessment. Correlative immunologic end points included peripheral blood lymphocyte cell phenotype and function as well as peripheral blood anti–renal cell carcinoma antibody and cytokine levels.
Results
All patients received between two and five treatment cycles. Toxicities consisted of known and expected cytokine side effects. Overall objective clinical response rate was 50% with three complete responses. Median time to progression for all patients was 8 months, and median survival has not been reached (median follow up of 37+ months). Treatment-related changes in correlative immunologic end points were noted and the level of circulating CD4+ T regulatory cells had a strong association with outcome. Pre–IP-10 serum levels approached significance for predicting outcome.
Conclusions
The clinical and immunologic responses observed in this trial suggest an interaction between DC vaccination and cytokine therapy. Our data support the hypothesis that modulation of inflammatory, regulatory, and angiogenic pathways are necessary to optimize therapeutic benefit in renal cell carcinoma patients. Further exploration of this approach is warranted.
Renal cell carcinoma (RCC) contributes significantly to cancer-related mortality.14 Improved overall survival (OS) in metastatic RCC (mRCC) patients treated with interleukin (IL)-2, IFN-α, or both have been described (1, 2). Clinical and laboratory data suggest a role for the host immune system and angiogenic pathways in this disease (3–11).
The most successful therapy for mRCC has been single-agent, high-dose aldesleukin (IL-2) with durable complete remissions in a small percent of patients. Prior attempts to improve IL-2 clinical outcome with the addition of other agents or effector cells have failed (5–8, 12, 13). New directed therapies, such as bevacizumab, sorafenib, sunitinib, and temsirolimus, have had significant effect on survival of mRCC patients and are used as first-line therapy in many centers. However, they rarely induce durable complete remissions (8–11).
A major obstacle for cancer immunotherapy is tolerogenic pathways that involve regulatory cells and immunosuppressive cytokines (14–16). IL-2 mobilizes not only immune effector cells capable of destroying cancer but also expands CD4+CD25+FoxP3+ regulatory cells, which modify the immune response. Dendritic cells (DC), the most potent antigen-presenting cells, are able to activate proinflammatory tumor–specific immune pathways as well as disrupt regulatory pathways (17–24). However, clinical responses to DC vaccine (DCV) alone given by different routes have been limited. Intranodal injection seems to be the most effective delivery method for these cells (25, 26). IL-2 and IFN-α can also contribute to overcoming other dysfunctional immune pathways. IL-2 can rectify acquired T-cell receptor signaling defects seen in cancer patients. IFN-α enhances tumor immunogenicity by inducing expression of MHC molecules and tumor-associated antigens, and can enhance DC and T-cell function (27–29). The balance between stimulatory and regulatory immune pathways may explain, in part, the low response rates observed with immunotherapy of mRCC (30–34).
We hypothesized that immunotherapy using intranodal DCV, IL-2, and IFN-α2a could exploit immune pathways to enhance therapeutic benefit. We report the results of a phase II trial using this approach.
Materials and Methods
Patients
The study was approved by Dartmouth Medical School’s Committee for the Protection of Human Subjects and the U.S.A. Food and Drug Administration (IND BB 11162). Eligible patients were ages ≥18 y, had measurable disease and newly progressive metastatic or new metastatic disease, adequate end-organ function, sufficient tumor tissue for vaccine preparation, and signed informed consent. Individuals with a history of brain metastases, HIV disease, hepatitis B/C, autoimmune disease, required the use of corticosteroids or other immunosuppressive agents, or had prior treatment with IFN-α, IL-2, or autologous vaccine, were excluded.
Treatment
Treatment consisted of two induction cycles of IL-2/IFN-α 2a given on days 1 and 14, and three maintenance cycle weeks every 28 d, consistent with the well-established Negrier regimen (12). IL-2 (Chiron, Inc.) was administered by continuous infusion (18 × 106 IU/M2 for 120 h). IFN-α 2a (6 MIU; Hofman La Roche) was given s.c. every other day for three doses (Table 1). Dose reductions followed the National Cancer Institute Common Toxicity Scoring System. IL-2 was stopped for hypotension, atrial fibrillation, renal failure, respiratory distress, mental confusion, and metabolic acidosis. IL-2 was restarted at 75% of the dose at the time of the next cycle. For recurring toxicity, the dose was cut in half (37.5% of original dose). Similar dose modifications were made for IFN-α 2a for liver and renal toxicity as well as atrial fibrillation.
Table 1.
Treatment scheme: Days of therapy and timing of administering each component of treatment during the induction and maintenance phases
| Cycle | DC Vaccinations | IL-2 | IFNα-2a |
|---|---|---|---|
| Induction 1 | Day 0 | Days 1–5 | Days 1, 3, 5 |
| Induction 2 | Day 14 | Days 15–19 | Days 15, 17, 19 |
| Maintenance 1 | Day 33 | Days 43–47 | Days 43, 45, 47 |
| Maintenance 2 | Day 61 | Days 71–75 | Days 71, 73, 75 |
| Maintenance 3 | Day 89 | Days 99–103 | Days 99, 101, 103 |
Leukapheresis for DC preparation was done before treatment and before each maintenance cycle. DCV (1 × 107 DCs in 1 mL Lactated Ringer’s Solution) was given intranodally under ultrasound guidance on the day before starting IL-2/IFN-α 2a. No dose modifications were permitted for DCV. Patients were allowed to continue with IFN-α 2a and vaccine therapy if IL-2 dose limiting toxicity occurred. Duration of vaccine treatment was limited by number of DC available after leukapheresis. Cytokines were given according to protocol up to day 103.
Vaccine preparation
Monocyte precursors were enriched from pheresis product using elutriation (patient 1–15) or an anti-CD14 antibody magnetic bead and CliniMacs platform (patient 16–18; Miltenyi Biotec, Inc.). Elutriated monocytes were cultured in AIM-V serum–free media for 9 d with 500 IU/mL granulocyte macrophage colony-stimulating factor (Berlex, Inc.) and 20 ng/mL IL-4 (R & D Systems; day 0, 3, and 6), tumor lysate (1–3 tumor cell equivalents per DC; day 5), and 50 ng/mL tumor necrosis factor α (R & D System; day 6). CliniMacs enriched monocytes were cultured in X-Vivo 15 media with 1% heat inactivated autologous serum for 8 d with 1,000 IU/mL granulocyte macrophage colony-stimulating factor and 40 ng/mL IL-4 (day 0, 3, and 6), tumor lysate (1–3 tumor cell equivalents per DC; day 5), and 50 ng/mL tumor necrosis factorα and 1 μg/mL PGE2 (Sigma; day 7). The change to CliniMacs purification allowed a closed system for DC preparation and for greater monocyte cell purification. Freeze-fractured and irradiated tumor lysate was obtained from mechanically and enzymatically treated fresh tissue. Frozen DCs from preparation of vaccine #1, stored in 90% autologous serum and 10% DMSO, were thawed and used for vaccine #2. Requirement for release of the final DC preparation included >70% viability, negative sterility test from day 6 to 7 DC culture, endotoxin test, and a negative gram stain.
Clinical assessment
Patients were assessed serially using computed tomography of chest, abdomen, and pelvis, and technetium bone scan. Response was determined by the National Cancer Institute’s Response Evaluation Criteria in Solid Tumors. Follow-up for responding and stable patients occurred quarterly until progression or as clinically indicated.
Correlative immunologic studies
Lymphocyte subpopulations were characterized by standard five-color flow cytometry and analyzed with FloJo software (35). Intracellular staining was done following cell fixation and permeabilization (Il-4, IFNγ) and intranuclear staining for FoxP3 (Biolegend FoxP3 kit). Tumor specific T-cell proliferation was determined using the Dye Dilution Proliferation Assay (36). Culture conditions included lymphocytes alone or combined with tumor lysate–loaded and unloaded DCs, Con A (proliferation control), and Staphylococcus aureus B (IFN-γ control). Pretreatment, midtreatment, and posttreatment time points were evaluated for T-cell receptor function. T-cell receptor function, reported in lytic units, was determined by a standard chromium release–redirected cytotoxicity assay using FcR-positive P815 tumor cell targets, and anti-CD3 antibody (OKT3; ref. 37).
For TREG function, CD4CD25high (TREG) and CD4CD25− T-cells (responder cells) were isolated using the Regulatory T Cell Isolation kit (Miltenyi Biotec). TREG function was determined by [3H]Thymidine uptake in cocultures of responder cells mixed with autologous TREG cells in the presence and absence of T-cell activation beads (anti-CD3, anti-CD28, and anti-CD2: T Cell Activation/Expansion kit; Miltenyi Biotec; ref. 38). All experimental conditions were done in triplicate.
Presence of serum IgG and IgM anti-RCC antibodies was determined by flow cytometry using allogeneic RCC cell lines (CAKI, ACHN, 769-P) and mouse anti-human IgG and IgM. Presence of cell-bound serum antibody was detected using biotinylated goat anti Human IgG or IgM secondary antibody and Streptavidin conjugated DTAF (Jackson Laboratories).
Serum was analyzed for 27 cytokines [IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p70), IL-13, IL-15, IL-17, basic fibroblast growth factor, Eotaxin, granulocyte colony-stimulating factor, granulocyte macrophage colony-stimulating factor, IFN-γ, IP-10, MCP-1 (MCAF), MIP-1α, MIP-1 β, platelet-derived growth factor–BB, RANTES, tumor necrosis factor-α, vascular endothelial growth factor] using the Luminex fluorescent bead technology according to manufacturer’s protocol.
Statistical methods
This phase II trial was planned as a Simon two-stage design to detect a 40% overall response rate (complete plus partial response) compared with a hypothesized response rate of 20%. Institutional Review Board approval had been granted for the original study design, but due to limitation of funds, only the first stage was completed. A 95% confidence interval (CI) for the overall response rate was determined based on the exact binomial method. Progression-free survival (PFS) was defined as the time from study enrollment until disease progression, or death. OS was defined as the time from study enrollment until death. PFS and OS were censored at the date of last follow-up. The product-limit method was used to estimate survival curves corresponding to progression-free survival and OS.
Correlative immunologic studies were assessed using Student’s t test. For precomparisons and postcomparisons, paired t test was used. Mann-Whitney Rank Sum Test was used as a nonparametric test for not normally distributed data.
Results
Patients
Eighteen mRCC patients were enrolled between January 2004 and August 2006. The patient characteristics and outcomes are summarized in Table 2. Fourteen patients received greater than or equal to three DC-vaccinations. One patient was removed from treatment before his 5th cycle due to autoimmune toxicity.
Table 2.
Patient characteristics and response
| Pt # | Gender | Age (y) | Histology | Metastatic disease site | Prognostic criteria MSKCI/UISS | # Vax | Best clinical response | Duration of response in months | Additional treatment | Survival in months |
|---|---|---|---|---|---|---|---|---|---|---|
| 01 | M | 70 | cc | Lung, adrenal | Int/Int | 4 | SD | 17.5 | Soafenib, sunitinib, bevacizumab | 47.0+ |
| 02 | M | 42 | cc | Lung | Int/Int | 5 | PR | 9.2 | Bevacizumab, sunitinib | 46.3+ |
| 03 | M | 76 | cc | LN (mediastinal, RP, pelvic) | L/Int | 2 | SD | 6.1 | Bevacizumab | 12.6 |
| 04 | M | 64 | cc | Lung, LN (mediastinal, para-aortic) | Int/Int | 3 | CR | 43.3+ | None | 43.3+ |
| 05 | M | 71 | s | Lung | L/Int | 1 | PD | 1.4 | Bevacizumab + IFN | 42.4+ |
| 06 | F | 61 | cc | Bone | L/Int | 2 | PD | 1.6 | Bevacizumab, sorafenib | 14.1 |
| 07 | F | 50 | cc | Lung, adrenal, LN | Int/Int | 3 | PR | 9.0 | Sunitinib, sorafenib, bevacizumab | 38.5+ |
| 08 | M | 57 | cc | Lung, bones, LN | Int/Int | 3 | CR | 12.4 | Sorafenib | 37.3+ |
| 09 | M | 69 | cc | Lung, liver, LN | Int/High | 5 | PR | 4.0 | Sorafenib, temsirolimus | 36.4+ |
| 10 | M | 64 | cc | Bone, LN | Int/Int | 5 | SD | 7.0 | Sorafenib | 33.4+ |
| 11 | M | 54 | cc | Lung, LN | L/L | 4 | PR | 2.8 | Sorafenib, sunitinib, bevacizumab | 28.0 |
| 12 | M | 61 | cc | Lung, LN | L/Int | 3 | PR | 9.0 | Not known | 32.3+ |
| 13 | M | 64 | cc | Lung | L/High | 4 | SD | 3.2 | Sorafenib | 7.4 |
| 14 | M | 44 | cc | Lung | Int/Int | 5 | SD | 25.8+ | None | 25.8+ |
| 15 | F | 70 | m | Lung, liver | L/Int | 5 | SD | 4.0 | Sorafenib, sunitinib | 24.0+ |
| 16 | F | 66 | cc | Lung, LN | Int/Int | 4 | PR | 7.0 | Sunitinib | 20.4+ |
| 17 | M | 47 | S | Skin, LN, Lung | Int/Int | 3 | CR | 19.3+ | None | 19.3+ |
| 18 | F | 64 | cc | Bone, LN, Lung, Adrenal | Int/Int | 1 | PD | 1.3 | Sorafenib | 6.4 |
Abbreviations: Pt, patient; MSKCI, Memorial Sloan Kettering Cancer Institute Low, Intermediate, High risk factors; UISS, UCLA Integrated Staging System low, Intermediate, High risk factors; Vax, vaccination; M, male; cc, clear cell; s, sarcomatoid; F, female; SD, stable disease; PR, partial response; PD, progression of disease; +, continuing; LN, lymphnode; m, mixed including clear cell, chromophobe, and papillary.
DCV
Tumor lysate was prepared from primary tumors for 17 patients and nodal metastases for one subject (patient #12). The mature DC phenotype (n = 48 vaccines) was reflected by a mean percent positive value of 5.8 ± 10.0 for monocyte marker CD14, 60.5 ± 23.6 for DC marker CD83, and 91.8 ± 13.0, 83.1 ± 22.8, and 82.7 ± 14.6 for MHC class II, CD80, and CD86, respectively.
Toxicity
Significant clinical toxicities were related to IL-2 and IFN-α2a (Table 3). Two responding patients had autoimmune clinical syndromes (myocarditis, pneumonitis, and nephritis that resolved over the subsequent 3 to 4 months; and parotitis).
Table 3.
Grade III and IV toxicity
| Pt # | Toxicity | Respective toxicity grade |
|---|---|---|
| 01 | Rash, hypotension, confusion | 3,3,3 |
| 02 | Rash | 3 |
| 03 | Rash/itching, INR/PTT, hypoxia, hypotension, hypocalcemia, Creatinine | 3,3,3,3,3,3 |
| 04 | Somnolence, pulmonary embolus, thrombopenia, nausea, right bundle branch block, hypophosphatemia, hyperglycemia, edema, dermatitis, nephritis, cardiomyopathy | 3,3,3,3,3,3,3,3,3,3 |
| 05 | Lymphopenia, hypophosphatemia, hypoalbumenia, dermatitis | 3,3,3 |
| 06 | Pruritus, hyponatremia, c. diff colitis, confusion | 3,3,3,3 |
| 07 | Pruritus, myalgia, metabolic changes, dehydration | 3,3,3 |
| 08 | Pruritus, metabolic changes, anorexia | 3,3,3 |
| 09 | Pruritus, metabolic changes | 3,3 |
| 10 | Hypotension, metabolic changes, hypertension, edema | 3,3,3,3 |
| 11 | Infection, syncope, pruritus, metabolic changes | 3,3,3,3 |
| 12 | Parotitis, edema, confusion | 3,3,3 |
| 13 | Hypotension, confusion, edema | 3,3,3 |
| 14 | Pruritus, pulmonary toxicity, hypoxia, hypotension, metabolic changes, pulmonary edema | 3,3,3,3,3,3,4 |
| 15 | Sinus tachycardia, oliguria, mental status changes, diarrhea | 3,3,3,3 |
| 16 | Leukopenia, hypotension | 3,3 |
| 17 | Uric acid, DVT, fatigue, hyperbilirubinemia | 4, 3, 3, 3 |
| 18 | Increase alkaline phophatase | 3 |
Clinical response results
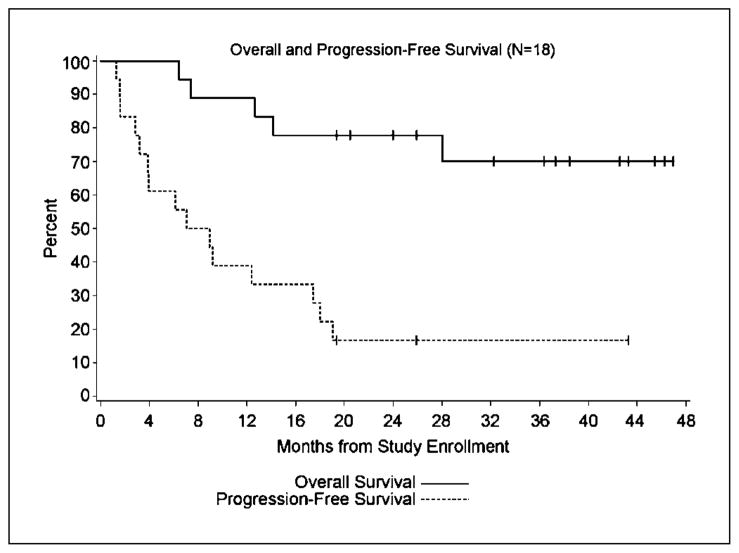
All patients were available for clinical assessment (Table 2). Follow-up ranged from 21 to 48+ months. Median PFS was 8 months (Fig. 1). The median OS has not been reached. A total of 9 objective responses were noted (50%; 95% CI, 22fs66%): three complete responses (CR), two >19.3 and >43.3 months; six partial responses (2.8–9.2 months); and six stable disease (3.2–25.9 months). Responses were seen in lung, liver, mesentery, and adrenal sites as well as a second primary renal tumor determined by biopsy after protocol therapy. Signs of clinical response were observed after the first two cycles as well as after completion of treatment. Histologic subtypes are listed in Table 2. Patients went on to receive targeted therapy as shown in Table 2.
Fig. 1.
Overall and progression free survival: PFS:
 , OS;
, OS;
 , PFS.
, PFS.
Correlative immunologic end points
A treatment-related increase in the precursor frequency of RCC-specific CD8+IFNγ+ T cells was noted in peripheral blood lymphocytes [0.027 ± 0.055 (before) versus 0.082 ± 0.098 (after); P = 0.058]. No relationship with clinical response or PFS was identified.
The percentage of CD4 and CD8 T cells in the peripheral blood lymphocyte population did not change as a result of treatment, but the percentage of CD3−CD56+ natural killer cells increased during treatment [15.1 ± 10.3% (before) versus 23.1 ± 12.0% (after); P = 0.015; Fig. 2A]. No significant changes were seen in the percentage of CD4+IFNγ+ Th1 cells in the T-cell population, but the percentage of CD4+IL-4+ Th2 cells increased with treatment in responding patients [4.7 ± 1.4% (before) versus 13.1 ± 8.0% (after); P = 0.095; Fig. 2C].
Fig. 2.
Treatment effect on lymphocyte cell populations in the peripheral blood: A, CD3, CD4, CD8, and natural killer (CD3-CD56+) cells as percentage of peripheral blood lymphocytes. B, IP-10 serum levels before and after therapy for all patients (n = 8) and prelevels for responding patients (R; n = 4) and nonresponding patients (NR; n = 4). C, CD4+ IL4+ T Cells (TH2) as a percentage of CD3+ lymphocytes for all patients (
 ), nonresponders (■), and responding patients (□).
), nonresponders (■), and responding patients (□).
Cytokine multiplex results revealed a treatment-related increase in antiangiogenic factor and TH1 cytokine IFN γ inducible protein 10 [IP-10: 126 ± 71 pg/mL (before) versus 521 ± 244 pg/mL (after); P = 0.002; Fig. 2B]. Responding patients had higher levels of IP-10 in pretreatment serum than nonresponding patients (169 ± 68 pg/mL versus 83 ± 45 pg/mL; P = 0.07).
CD8+ T cell T-cell receptor function was evaluated in 11 patients who received at least 3 vaccines (4 NR, 7 R). Overall, treatment-related lytic activity increased in CD8+ T cells by 33% but did not reach statistical significance.
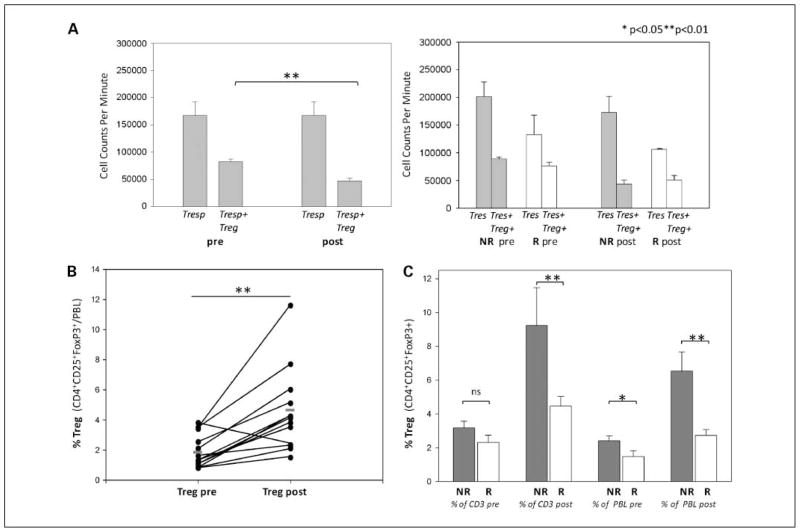
We observed a treatment related increase in the percentage of CD4+CD25+FoxP3+ TREG cells in the lymphocyte population [2.0 ± 1.0% (before) versus 4.4 ± 2.7% (after); P = 0.002; Fig. 3B]. TREG cells from six patients tested showed suppressive function. Surprisingly, TREG cells seem to suppress less effectively in responding versus nonresponding patients, although this may reflect a less brisk proliferative response in the CD4-responding cell population (Fig. 3A).
Fig. 3.
A, Treg cell functional assay (n = 6); left, results of a proliferation assay to quantify suppression of CD4 proliferation by Treg. Right, compares functional suppression in the responding patients (□) and nonresponding patients
 . B, percentage of Treg(CD4+CD25+FoxP3+) in the peripheral blood lymphocyte population before and after treatment. C, comparison of the percentage of circulating Treg cells in the lymphocyte and CD3+ cell population for responding (□) and nonresponding patients (■) before and after treatment.
. B, percentage of Treg(CD4+CD25+FoxP3+) in the peripheral blood lymphocyte population before and after treatment. C, comparison of the percentage of circulating Treg cells in the lymphocyte and CD3+ cell population for responding (□) and nonresponding patients (■) before and after treatment.
After two induction cycles, nonresponders (6.5 ± 2.7%) showed a significantly stronger expansion of Treg cells within the lymphocyte compartment than responding patients (2.7 ± 1.0%; P = 0.004). This difference was also true when Tregs were examined as a percentage of the CD3 population (Fig. 3).
Treatment increased serum IgM anti-RCC antibody levels and led to a significant increase in percent positive IgM staining on allogeneic tumor cell lines [22.1 ± 13.7% (before) versus 38.8 ± 21.7% (after); P = 0.022]. However, no changes in IgG antibody levels were observed.
Discussion
Although the results of small phase II trials have significant limitations, we found a 50% overall objective response rate (95% CI, 27–74%) and a 16% CR (95% CI, 0–34%) that compares favorably to the historical observations of 16% overall objective response rate and 6% CR for high-dose IL-2 or IL-2 plus IFNα therapy (2–7, 12) and an 11% partial response rate recently reported in a small phase I/II single agent autologous tumor lysate-DCV therapy (39). The median time to progression (8 months) seen in this trial also compares favorably with recent reports of targeted therapy (8, 9). Based on historical data from the Memorial Sloan Kettering Cancer Institute and the University of California at Los Angeles Integrated Staging System, we would have predicted a median survival of 13.8 months, and a 2- and 3-year survival of 38.8% and 29%, respectively. At this time, median survival of these 18 patients has not yet been reached and the 2- and 3-year survival is 77% and 70%, respectively. OS data needs to be seen in the context of new directed therapies becoming available during the follow-up time period.
Historically, cancer immune therapies have focused on stimulation of effector cells. Interest in enhancing antitumor immune responses in cancer patients by inhibition of regulatory cells has resurged. Modulation of only one component of the immune equation induces long lasting clinical benefit in only a small minority of patients. We hypothesized that multiple signals, which modulate both effector and regulatory functions, are necessary to improve immune therapy for cancer patients. In this study, we have shown that DCs, IL-2, and IFNα enhance tumor-specific CD8+ effector cells. A statistically significant differential expansion of treatment-induced peripheral blood TREG cells favored a lower percentage of TREG cells in responding patients. This finding of TREG correlation was unexpected. This supports a TREG cell threshold effect on clinical outcome (40). We also found CD4+ IL-4+ tumor-specific precursors increased in responding patients, suggesting a role for Th2 pathway. At present, it is unclear what the significance of the association of this alternative pathway and clinical outcome is and how it may be better exploited in the future.
Interestingly, we observed a treatment-related induction of IP-10, an antiangiogenic cytokine and chemoattractant for activated T cells, natural killer cells, and monocytes and a relationship between outcome and pretreatment IP-10 serum levels. IP-10 expression in RCC tumors has been described as a predictor of outcome, and shown to be induced by IL-2 and implicated as a component of the TH1 response (41, 42). Our observation reinforces the link between immune pathways and tumor angiogenesis and the potential to modulate both these systems for effective therapy.
Until recently, antigen-specific approaches have been limited by the lack of RCC-specific molecules. There are conflicting reports of clinical efficacy using immunodominant peptides derived from carbonic anhydrase-9 (43–45). By using tumor lysate, we incorporated a broader repertoire of potential tumor antigens, which may also include tumor-associated nonprotein molecules. Use of cellular products may also reduce the likelihood of tumor immune escape and expand the eligibility of participating patients.
Development of autoimmunity with immunotherapy has been reported in other studies (46–48). The induction of severe clinical autoimmune-like phenomena in one patient with a complete and durable response suggests autoimmune phenomena may be a potential surrogate marker of benefit.
Clinical responses were observed early and late in the treatment course suggesting there may be a range of predispositions toward immune responsiveness. Our prior studies have also implicated heterogeneity of patients’ immune “readiness” (49). Immune profiling may ultimately define these states more clearly.
In conclusion, this study provides evidence that supports multitargeted immunotherapy for mRCC as a means to regulate effector, regulatory, and angiogenic pathways. Furthermore, elevated levels of circulating vascular endothelial growth factor have been shown to confer poor prognosis in RCC and other solid tumors, and are associated with higher numbers of circulating immature DCs and immunosuppression. Vascular endothelial growth factor blockade can enhance Type-1 cytokine response and could be a reasonable addition to the described DCV/IL-2/IFN regimen (40).
Deeper understanding of immune pathways and the interplay between different targeted therapies provide promising avenues to investigate in future trials.
Translational Relevance.
This article summarizes a phase II trial treating patients with metastatic renal cell carcinoma with a novel autologous dendritic cell (DC) vaccine combined with standard immunotherapy. Although DCs have been hailed as the most potent antigen-presenting cells, DC vaccines in general have been quite unsuccessful. Research over the past decade has shown that these cells play a key role not only in antigen presentation, but also as gatekeepers to immune tolerance. We hypothesized that intranodal DC injection combined with interleukin-2 and IFN-α2a would have enhanced clinical results. In addition, we used autologous tumor lysate to provide an individualized and wide-ranging antigen profile. The clinical results were very impressive with a 50% overall clinical response rate. Immunologic results support these clinical observations. Some of these immunologic insights will have effect on future immunotherapeutic treatment protocols in this disease.
Acknowledgments
We thank Cheryl Carlson and the Norris Cotton Cancer Center’s Office of Clinical Research, Dennis Seguin and the Dartmouth Hitchcock Ultrasound Section, April Peront and the Dartmouth Hitchcock Cell Therapy Center, Peter Seery and the Dartmouth Hitchcock Surgical Pathology Staff, the nurses and staff of the Mary Hitchcock Memorial Hospital Hematology/Oncology Special Care Unit for their assistance in this trial, Mary Robinson for manuscript preparation, and the patients who participated and allowed themselves to be studied as part of this trial.
Grant support: NIH (RO1 CA5648), NE-AUA and Berlex Inc.
Footnotes
Cancer Statistics, American Cancer Society Web Site; http://www.moitherapy.org/
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Schwaab T, Atzpodien J. Renal Cell Carcinoma. In: Kirkwood J, editor. Strategies in Immunoadjuvant Therapies. Dunitz Publishers; 2000. [Google Scholar]
- 2.Motzer RJ, Mazumdar M, Bacik, et al. Effect of cytokine therapy on survival for patients with advanced renal cell carcinoma. J Clin Oncol. 2000;18:1928–35. doi: 10.1200/JCO.2000.18.9.1928. [DOI] [PubMed] [Google Scholar]
- 3.Ernstoff MS, Nair S, Bahnson RR, et al. A phase IA trial of sequential administration recombinant DNA-produced interferons: combination recombinant interferon γ and recombinant interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 1990;8:1637–49. doi: 10.1200/JCO.1990.8.10.1637. [DOI] [PubMed] [Google Scholar]
- 4.Figdor CG, deVries JM, Lesterhuis WJ, Melief CJM. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475–80. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 5.McIntyre CA, Chapman K, Reeder S, et al. Treatment of malignant melanoma and renal cell carcinoma with recombinant human interleukin-2: analysis of cytokine levels in sera and culture supernatants. Eur J Cancer. 1992;26:58–63. doi: 10.1016/0959-8049(92)90385-f. [DOI] [PubMed] [Google Scholar]
- 6.Atkins MB, Sparano J, Fisher RI, et al. Randomized phase II trial of high-dose interleukin-2 either alone or in combination with interferon alfa-2b in advanced renal cell carcinoma. J Clin Oncol. 1993;11:661–70. doi: 10.1200/JCO.1993.11.4.661. [DOI] [PubMed] [Google Scholar]
- 7.Atzpodien J, Kirchner H, Jonas U, et al. Interleukin-2- and interferon alfa-2a-based immunochemotherapy in advanced renal cell carcinoma: a Prospectively Randomized Trial of the German Cooperative Renal Carcinoma Chemoimmunotherapy Group (DGCIN) J Clin Oncol. 2004;22:1188–94. doi: 10.1200/JCO.2004.06.155. [DOI] [PubMed] [Google Scholar]
- 8.Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–34. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad T, Eisen T. Kinase inhibition with BAY 43–9006 in renal cell carcinoma. Clin Cancer Res. 2004;10:6388–92S. doi: 10.1158/1078-0432.CCR-040028. [DOI] [PubMed] [Google Scholar]
- 11.Atkins MB, Hidalgo M, Stadler WM, et al. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol. 2004;22:909–18. doi: 10.1200/JCO.2004.08.185. [DOI] [PubMed] [Google Scholar]
- 12.Negrier S, Escudier B, Lasset C, et al. Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. Groupe Français d’Immunothérapie. N Engl J Med. 1998;338:1272–8. doi: 10.1056/NEJM199804303381805. [DOI] [PubMed] [Google Scholar]
- 13.Goedegebuure PS, Douville LM, Li H, et al. Adoptive immunotherapy with tumor-infiltrating lymphocytes and interleukin-2 in patients with metastatic malignant melanoma and renal cell carcinoma: a pilot study. J Clin Oncol. 1995;13:1939–49. doi: 10.1200/JCO.1995.13.8.1939. [DOI] [PubMed] [Google Scholar]
- 14.Finke JH, Rayman P, Edinger M, et al. Characterization of a human renal cell carcinoma specific cytotoxic CD8+ T cell line. J Immunother. 1992;11:1–11. doi: 10.1097/00002371-199201000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Jantzer P, Schendel DJ. Human renal cell carcinoma antigen-specific CTLs: antigen-driven selection and long-term persistence in vivo. Cancer Res. 1998;58:3078–86. [PubMed] [Google Scholar]
- 16.Wiethe C, Dittmar K, Doan T, et al. Provision of 4-1BB ligand enhances effector and memory CTL responses generated by immunization with dendritic cells expressing a human tumor-associated antigen. J Immunol. 2003;170:2912–22. doi: 10.4049/jimmunol.170.6.2912. [DOI] [PubMed] [Google Scholar]
- 17.Banchereau J, Steinman RM. Dendritic cells and control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 18.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 19.Gabrilovitch DI, Corak J, Ciernik IF, et al. Decreased antigen presentation by dendritic cells in patients with breast cancer. Clin Cancer Res. 1997;3:483–90. [PubMed] [Google Scholar]
- 20.Troy AJ, Summers KL, Davidson PJT, Atkinson CH, Hart DNJ. Minimal recruitment and activation of dendritic cells within renal cell carcinoma. Clin Cancer Res. 1998;4:585–93. [PubMed] [Google Scholar]
- 21.Schwaab T, Schned AR, Heaney JA, et al. In vivo description of dendritic cells in human renal cell carcinoma. J Urol. 1999;162:567–73. [PubMed] [Google Scholar]
- 22.Fields RC, Shimizu K, Mule JJ. Murine dendritic cells pulsed with whole tumor lysates mediate potent antitumor immune responses in vitro and in vivo. Proc Natl Acad Sci U S A. 1998;95:9482–94. doi: 10.1073/pnas.95.16.9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lou Y, Wang G, Lizée G, et al. Dendritic cells strongly boost the antitumor activity of adoptively transferred T cells in vivo. Cancer Res. 2004;64:6783–90. doi: 10.1158/0008-5472.CAN-04-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Javia LR, Rosenberg SA. CD4+CD25+ suppressor lymphocytes in the circulation of patients immunized against melanoma antigens. J Immunother. 2003;26:85–93. doi: 10.1097/00002371-200301000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lambert LA, Gibson GR, Maloney M, et al. Intranodal immunization with tumor lysate-pulsed dendritic cells enhances protective antitumor immunity. Cancer Res. 2001;61:641–6. [PubMed] [Google Scholar]
- 26.Bedrosian I, Mick R, Xu S, et al. Intranodal administration of peptide-pulsed mature dendritic cell vaccines results in superior CD8+ T-cell function in melanoma patients. J Clin Oncol. 2003;21:3826–35. doi: 10.1200/JCO.2003.04.042. [DOI] [PubMed] [Google Scholar]
- 27.Yang JCY. Interleukin-2: Clinical Applications: Renal Cell Carcinoma. In: Rosenberg SA, editor. Principles and Practice of the Biologic Therapy of Cancer. 3. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 73–82. [Google Scholar]
- 28.Kaser A, Nagata S, Tilg H. Interferon α augments activation-induced T cell death by upregulation of Fas (CD95/APO-1) and Fas ligand expression. Cytokine. 1999;11:736–43. doi: 10.1006/cyto.1998.0484. [DOI] [PubMed] [Google Scholar]
- 29.Radvanyi LG, Banerjee A, Weir M, Messner H. Low levels of interferon-α induce CD86 (B7 2) expression and accelerates dendritic cell maturation from human peripheral blood mononuclear cells. Scand J Immunol. 1999;50:499–509. doi: 10.1046/j.1365-3083.1999.00625.x. [DOI] [PubMed] [Google Scholar]
- 30.Anderson PO, Sundstedt A, Yazici Z, et al. IL-2 overcomes the unresponsiveness but fails to reverse the regulatory function of antigen-induced T regulatory cells. J Immunol. 2005;174:310–9. doi: 10.4049/jimmunol.174.1.310. [DOI] [PubMed] [Google Scholar]
- 31.Knoefel B, Nuske K, Steiner T, et al. Renal cell carcinomas produce IL-6, IL-10, IL-11, and TGF-β 1 in primary cultures and modulate T lymphocyte blast transformation. J Interferon Cytokine Res. 1997;17:95–102. doi: 10.1089/jir.1997.17.95. [DOI] [PubMed] [Google Scholar]
- 32.Terabe M, Swann J, Ambrosino E, et al. A non-classical non-Vα14Jα18 CD1d-restricted (type II) NKT cell is sufficient for down regulation of tumor immunosurveillance. J Exp Med. 2005;202:1627–33. doi: 10.1084/jem.20051381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ernstoff MS, Crocenzi TS, Seigne JD, et al. Developing a rational tumor vaccine therapy for renal cell carcinoma: immune yin and yang. Clin Cancer Res. 2007;13:733–40s. doi: 10.1158/1078-0432.CCR-06-2064. [DOI] [PubMed] [Google Scholar]
- 34.Spiotto MT, Fu Y, Schreiber H. Tumor immunity meets autoimmunity: levels and dendritic. Curr Opin Immunol. 2003;15:725–30. doi: 10.1016/j.coi.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 35.Tsunenori K, Hayakazu N. Cancer Sci. 2006;97:780–6. [Google Scholar]
- 36.Givan AL, Fisher JL, Waugh MG, Bercovici N, Wallace PK. Use of cell-tracking dyes to determine proliferation precursor frequencies of antigen-specific T cells. Methods Mol Biol. 2004;263:109–24. doi: 10.1385/1-59259-773-4:109. [DOI] [PubMed] [Google Scholar]
- 37.Crocenzi TS, Tretter CPG, Schwaab T, et al. Impaired cytolytic activity in peripheral blood T cells from renal cell carcinoma patients. Clin Immunol. 2005;117:6–11. doi: 10.1016/j.clim.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 38.Oberg HH, Wesch D, Lenke J, et al. An optimized method for the functional analysis of human regulatory T cells. Scand J Immunol. 2006;64:353–60. doi: 10.1111/j.1365-3083.2006.01825.x. [DOI] [PubMed] [Google Scholar]
- 39.Kim JH, Lee Y, Bae YS, et al. Phase I/II study of immunotherapy using autologous tumor lysate-pulsed dendritic cells in patients with metastatic renal cell carcinoma. Clin Immunol. 2007;125:257–67. doi: 10.1016/j.clim.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 40.Suppiah R. T regulatory cells (Treg) in patients with metastatic renal cell carcinoma (mRCC) decrease during Sunitinib treatment: correlation with clinical responses and T helper 1/T helper 2 (Th1/Th2) bias. Proc ASCO. 2006 [Google Scholar]
- 41.Farber JM. Mig and IP-10: CXC chemokines that target lymphocytes. J Leukoc Biol. 1997;61:246–57. [PubMed] [Google Scholar]
- 42.Panelli MC, White R, Foster M, et al. Forecasting the cytokine storm following systemic interleukin (IL)-2 administration. J Transl Med. 2004;2:17–31. doi: 10.1186/1479-5876-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uemura H, Fujimoto K, Tanaka M, et al. A phase I trial of vaccination of CA9-derived peptides for HLA-A24-positive patients with cytokine-refractory metastatic renal cell carcinoma. Clin Cancer Res. 2006;12:1768–75. doi: 10.1158/1078-0432.CCR-05-2253. [DOI] [PubMed] [Google Scholar]
- 44.Bleumer I, Tiemessen DM, Oosterwijk-Wakka JC, et al. Preliminary analysis of patients with progressive renal cell carcinoma vaccinated with CA9 peptide-pulsed mature dendritic cells. J Immunother. 2007;30:116–22. doi: 10.1097/01.cji.0000211318.22902.ec. [DOI] [PubMed] [Google Scholar]
- 45.Satzger I, Meier A, Schenck F, et al. Autoimmunity as a prognostic factor in melanoma patients treated with adjuvant low-dose interferon α. Int J Cancer. 2007;121:2562–7. doi: 10.1002/ijc.22951. [DOI] [PubMed] [Google Scholar]
- 46.Amato RJ. Vaccine therapy for renal cell carcinoma. Rev Urol. 2003;5:65–71. [PMC free article] [PubMed] [Google Scholar]
- 47.Ernstoff MS. Self-recognition and tumor response to immunotherapy. J Clin Oncol. 2005;23:5875–7. doi: 10.1200/JCO.2005.95.029. [DOI] [PubMed] [Google Scholar]
- 48.Gogas H, Ioannovich J, Dafni U, et al. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med. 2006;354:709–18. doi: 10.1056/NEJMoa053007. [DOI] [PubMed] [Google Scholar]
- 49.Schwaab T, Heaney JA, Schned AR, et al. A randomized phase II trial comparing two different sequence combinations of autologous vaccine and human recombinant interferon γ and human recombinant interferon α 2b therapy in patients with metastatic renal cell carcinoma: clinical outcome and analysis of immunological parameters. J Urol. 2000;163:1322–7. doi: 10.1016/s0022-5347(05)67771-3. [DOI] [PubMed] [Google Scholar]