Abstract
Purpose
We conducted a prospective, open-label study in 54 adult subjects with sickle cell disease to determine the relationship between morphine concentrations, cytochrome P450 (CYP) 2D6 genotype, and clinical outcomes.
Methods
A blood sample was obtained for genotyping and serial blood samples were drawn to measure codeine and its metabolites in the plasma before and after oral codeine sulfate 30mg. Codeine and its metabolites were measured by liquid chromatographytandem mass spec-trometry (LC-MS). CYP2D6 genetic testing included four single nucleotide polymorphisms (SNP) indicative of three variant alleles: *17 (1023T); *29 (1659A, 3183A); and *41 (2988A) alleles.
Results
Thirty subjects (group I) had a mean (standard deviation) maximal morphine concentration of 2.0 (1.0) ng/ ml. Morphine was not measurable in the remaining 24 subjects (group II). Nine (30%) subjects in group I and 11 (46%) subjects in group II carried a variant *17, *29, or *41 allele (p=0.23); one (3%) subject in group I and 5 (21%) subjects in group II were homozygous for *17 or *29 allele (p=0.07). Emergency room visits (group I 1.5± 1.8 vs. group II 2.1±4.3, p=NS) did not differ based on metabolic status, but more hospital admissions (0.9±1.4 vs. 2.2±4.1, p=0.05) were documented in patients with no measurable morphine concentrations.
Conclusions
We conclude that Blacks with sickle cell disease without measurable plasma morphine levels after a single dose of codeine were not more likely to be a carrier of a single variant allele commonly associated with reduced CYP2D6 metabolic capacity; however, homozygosity for a variant CYP2D6 allele may result in reduced metabolic capacity. Furthermore, it appears that subjects without measurable morphine concentrations were more likely to be admitted to the hospital for an acute pain crisis.
Keywords: Sickle cell disease, Codeine, Morphine, Cytochrome P450 enzymes, Drug metabolism, Genotype
Introduction
Sickle cell disease is a group of inherited hemoglobin disorders commonly associated with frequent episodes of severe, debilitating pain. Codeine containing medications (CCM) are frequently prescribed to manage pain crises. However, some patients experience minimal relief with these drugs and require other opioids to manage their pain. Interpatient variability in responses to CCM is not well-understood.
Codeine undergoes O-demethylation by the polymorphic enzyme cytochrome P450 (CYP) 2D6 to the active metabolite morphine (5% to 10% of parent drug) [36]. (Fig. 1) Morphine strongly contributes to the analgesic, antitussive, and antidiarrheal effects commonly associated with CCM [8, 23, 32]. Morphine undergoes further metabolism to a 3- and 6-glucuronide; the 6-glucuronide demonstrates substantially greater analgesic effects compared to morphine. The 3-glucuronide contributes to the neuroexcitatory effects associated with morphine [8, 18, 23, 25, 26, 28–30, 32].
Fig. 1.
The metabolism of codeine. Each of the opioids in the pathway may undergo further metabolism to form one or more glucuroindes. The structures are courtesy of ChemSpider: a database of chemical structures and property predictions (www.chemspider.com)
About 70 CYP2D6 variant alleles along with additional subvariants are identified to date. The most common variant alleles associated with reduced activity in Blacks (Americans and Africans) following standard probe drugs are CYP2D6*17 and *29, with reported frequencies ranging from 0.14 to 0.26 for CYP2D6*17 and 0.08 for CYP2D6*29 [9, 11, 16, 33, 34]. Two other reduced function alleles, CYP2D6*10 and CYP2D6*41 can be found in Blacks [4, 10, 11]. Their frequencies are approximately 0.03 and 0.02, respectively.
We propose that Blacks with sickle cell disease carrying CYP2D6*17 or *29 alleles will report minimal or no analgesia with CCM and that these individuals may be more likely to seek medical care with the need for other opioids for acute pain management. Therefore, we conducted a prospective, open-label study to examine the relationship between morphine concentrations, CYP2D6 genotype, and clinical outcomes in sickle cell disease. Positive associations between morphine concentrations, outcomes and genotype could lead to the ability to predict response to CCMs based on genotype and individualize analgesic therapy for an acute pain crisis in sickle cell disease.
Patients and methods
Study design and population
This was a prospective, open-label study to examine the relationship between morphine concentrations, CYP2D6 genotype, and clinical outcomes. The study population included people with sickle cell disease with the hemoglobin genotype SS who were prescribed CCM and received care at the Sickle Cell Center at the University of Illinois Medical Center (UIMC). Advertisements were posted within the medial center to identify interested individuals. The medical records of potential participants were reviewed to establish eligibility. The exclusion criteria were: contraindications to codeine; serum creatinine > 2.0 mg/dl; serum asparteine or alanine transaminases or direct bilirubin greater than three times the upper limit of normal; pregnancy, and a history of an acute pain crisis within two weeks of the study. Smoking, alcohol consumption, opioid analgesics, or medications known to inhibit CYP2D6 were prohibited for at least 48 hours before the study visit. Study visits were rescheduled for all subjects with an acute pain crisis or the ingestion of CCM within 2 weeks or 48 hours, respectively, of the visit. The study was performed in the Sickle Cell Center (subjects 1 to 34) or the Clinical Research Center (CRC, subjects 35 to 58) at the UIMC, and was approved by the Scientific Advisory Committee for the CRC and the Institutional Review Board for the University of Illinois at Chicago.
Study procedures
Subjects who provided written informed consent were admitted to the Sickle Cell Center or the CRC for an 8-hour outpatient visit. No direct analgesic response was assessed. A venous blood sample was collected to determine CYP2D6 genotype (EDTA-containing collection tube). Codeine sulfate 30 mg was administered with 200 ml of water after at least an 8 hour fast. Serial blood samples were collected 30 min before and 1, 2, 3, 4, and 6 hours after oral codeine intake (heparin containing collection tube). Blood for genotyping was stored at −80°C until analysis. Plasma for pharmacokinetic analysis was separated by centrifugation at 3,000 g for 15 min at 4°C, and samples were stored at −20°C. The number of emergency room (ER) visits and hospital admissions in the 12 months preceding the outpatient visit was determined by interviewing the subject and reviewing the medical record.
Measuring codeine, morphine, morphine-3-glucuronide and morphine-6-glucuronide
Codeine, morphine, M3G and M6G in plasma were measured by modifying a previously published liquid chromatography-tandem mass spectrometry method [14]. Plasma (200µl) samples were spiked with internal standard (50µl; 200ng/ml of morphine-d3, 50ng/ml of M3G-d3, 50ng/ml codeine-d3 in water) and prepared using a solid phase extraction method with Oasis HLB cartridges (Waters, Milford, MA). The analytes were eluted with 1 ml of methanol, evaporated to dryness by nitrogen at 40°C, and then reconstituted with 200µl of 95% water and 5% methanol with 0.1% formic acid. Ten µl of the reconstituted material was injected onto a 5µm Zorbax Eclipse XDB-C18 150×4.6 mm column (Agilent Technologies, Santa Clara, CA). The gradient was started with 95% mobile phase A (water with 0.1% formic acid) and 5% mobile phase B (methanol with 0.1% formic acid) pumped at a flow rate of 0.6 ml/min. From 0.5 to 5 min, a linear gradient was initiated to decrease mobile phase A from 95% to 10%, with 10% mobile phase A maintained for one min. From 6 to 6.1 min, a linear gradient was initiated to increase mobile phase A from 10% to 95%, with 95% mobile phase A maintained until the end of the run (10 min). Retention times for codeine, morphine, M3G and M6G, were 6.3, 5.7, 5.3 and 5.7 min, respectively.
MS analyses were performed on a 3200 Q TRAP triple quadrupole MS with a TurboIonSpray® source (Applied Biosystems, Foster City, CA). The positive ion mode was chosen and quantitation was performed in the multiple reaction mode (MRM) using nitrogen as the collision gas. Precursor-to-product ion transitions of m/z 286152 for morphine, m/z 462286 for M3G and M6G, and m/z 300165 for codeine were used for the MRM with dwell times of 120ms for analytes and 80ms for internal standards.
The analytes were quantified using a calibration curve (r2>0.99) of peak area ratios of the opioid to the internal standard versus the analyte concentration. The lower limit of quantitation was 1 ng/ml for codeine and its metabolites. Samples with concentrations of opioids above the calibration curves were sufficiently diluted and reassayed. Interassay coefficients of variation were below 10% for all quality controls. The accuracy was between 96% and 107% for all opioids. Codeine, morphine and morphine-d3 were purchased from Alltech (State College, PA) and codeine-d3, M3G, M6G, and M3G-d3 were purchased from Cerilliant (Round Rock, TX).
Determining CYP2D6*17, CYP2D6*29 and CYP2D6*41 genotype
The DNA was extracted from whole blood using a Puregene® kit (Gentra Systems, Inc., Minneapolis, MS) and stored at −20°C. The CYP2D6*17, *29 and *41 alleles were identified using polymerase chain reactions (PCR) followed by RFLP, bi-alleleic discrimination or capillary sequencing. If none of these polymorphisms were observed, the allele assignment was defaulted to ‘other’.
To detect the CYP2D6*17 allele (1023C>T), a 199bp fragment of the CYP2D6 gene spanning the polymorphic site of interest was amplified by PCR using the forward primer 5′-CTTCGGGGACGTGTTCAG -3′ and reverse primer 5′-CCCACGGAAATCTGTCTC-3′. The 25µl reaction mixture contained HotStarTaq™ Master Mix (Qiagen, Valencia, CA), 10µM of each forward and reverse primer, 20 to 100 ng/ml of genomic DNA and RNase free water. Thermocycling consisted of an initial denaturation for 15 min at 95°C, followed by 35 cycles (94°C for 30s, 60°C for 30s and 72°C for 1 min) and a final extension for 10 min at 72°C. These products were digested overnight with HphI restriction enzyme (New England Biolabs, Beverly, MA), and the resulting fragments were separated on an 8% polyacrylamide gel. Fragment sizes of 104 and 94 bp indicated the T allele and fragment sizes of 94 and 80 bp indicated the C allele. To verify assay specificity, samples from 6 subjects (2 non-carriers, 2 heterozygous carriers, and 2 homozygous carriers) were analyzed by capillary sequencing using the ABI PRISM 3100 Genetic Analyzer and BigDye Terminator™ technology (v 3.1, Applied Biosystems).
To detect the CYP2D6*29 allele (1659G>A and 3183G>A), long range (XL) PCR was performed as previously described [9]. Subsequently, the 3183G>A SNP was detected by allele-specific PCR using the forward primer 5′-ATTGTGGGGACGCATGTCT-3′ and reverse primer 5′-GGGACGATGTCCCCAAAG-3′. The PCR product was purified using a Qiaquick PCR Purification Kit (Qiagen, Inc., Valencia, CA, USA) and 3183G>A was determined by capillary sequencing. All subjects positive for 3183A (i.e. the CYP2D6*29 allele) were confirmed by testing for 1659G>A also by sequence analysis of purified PCR product.
To detect the CYP2D6*41 allele (2988G>A), bi-allelic discrimination was completed using an inventoried Taqman SNP Genoypting Assays (Applied Biosystems). Reactions were carried out in a final volume of 5µl following the protocol provided. The assay was performed in duplicate for each sample and non-template control used for each assay. Endpoint bi-allelic discrimination was performed using the ABI Prism 7900HT SDS.
Pharmacokinetic analysis
Non-compartmental analysis was used to estimate the pharmacokinetic parameters for codeine, morphine, M3G and M6G using the measured concentrations and actual times with the aide of the program WinNonlin® Professional (v. 4.0, Pharsight Corp., Mountain View, CA). Log-linear regression of the terminal slope was used to estimate the elimination rate constant (k) and the elimination half-life (t1/2) was calculated by the equation t1/2 = 0.693/k. The apparent peak plasma concentration (Cmax) for codeine and its metabolites was visually identified from the plasma concentration time curve. The area under the concentration-time curve from 0 to t=6 h (AUC0→6) was calculated using the log-linear trapezoidal rule. The AUC0→∞ was calculated by adding the AUC0→6 to the terminal area determined by dividing the last measurable plasma concentration by k. The apparent oral clearance (Clo) was calculated from the equation Clo = dose/AUC0→∞
Statistical analysis
The primary outcome measure was CYP2D6 genotype and the secondary outcome measures were ER visits and hospital admissions and the concentrations of the glucuronide metabolites. The mean and standard deviation is reported for all continuous data and the number and percentage is reported for all categorical data. The outcome measures were compared between metabolic status by two sample Wilcoxon test and Fisher Exact test. P-value < 0.05 was considered statistical significant. All of the data were analyzed with the aide of the statistical program SAS (version 9.1.3, SAS Institute, Inc., Cary, NC).
Results
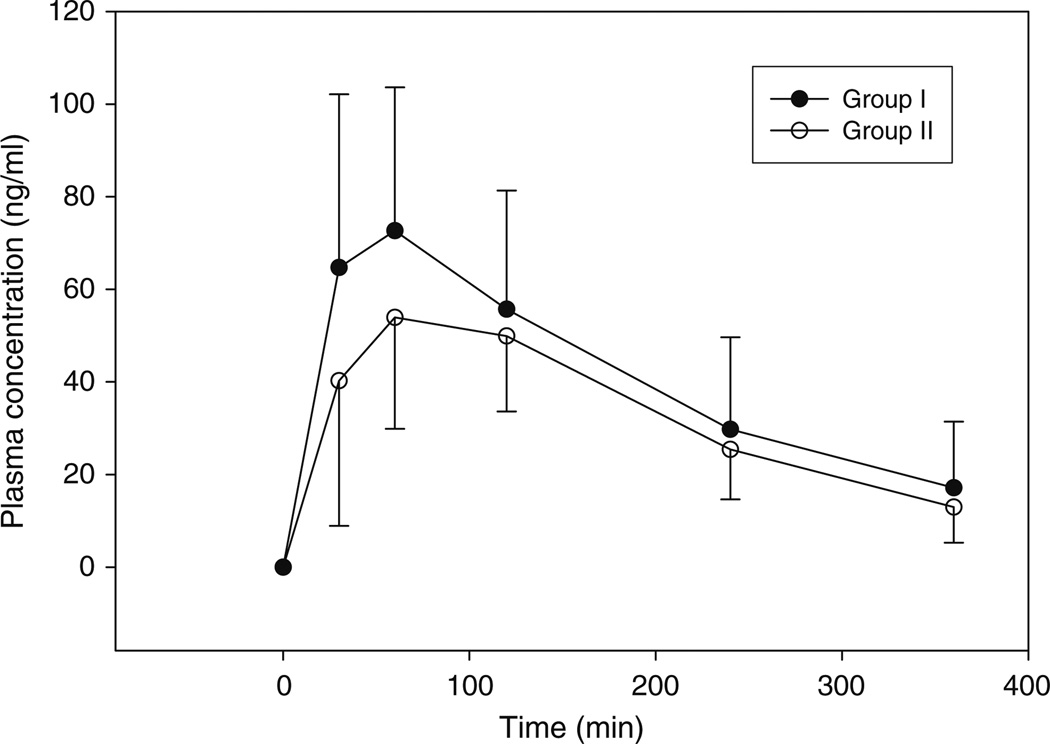
Fifty-four subjects completed the study (Table 1). Morphine was not measurable in the plasma of 24 subjects (group II), whereas morphine was measurable in the remaining 30 subjects (group I). The maximal codeine, morphine, M3G, and M6G concentrations are provided in Table 2. The time to the maximal concentration was delayed, and the maximal codeine concentrations (Cmax) were lower in subjects in group II (P<0.05) (Fig. 2). Not surprisingly, the concentrations of M3G and M6G were lower in subjects in group II based on the absence of morphine concentrations (P< 0.001). The time to the maximal concentrations of either glucuronide was similar between the groups.
Table 1.
Study population
| Characteristic | All (n=54) | Group I (n=30) | Group II (n=24) |
|---|---|---|---|
| Age, years (mean (stdev)) | 32 (12) | 32 (12) | 31 (14) |
| Gender (n (%)) | |||
| Men | 18 (33) | 9 (30) | 9 (38) |
| Women | 36 (67) | 21 (70) | 15 (62) |
| Medications (n (%)) | |||
| Folate | 44 (81) | 26 (87) | 18 (75) |
| Hydroxyurea | 32 (60) | 15 (50) | 17 (70) |
| Co-existing illness (n (%)) | |||
| Acute chest syndrome | 7 (13) | 3 (10) | 4 (17) |
| Avascular necrosis | 6 (11) | 2 (7) | 4 (17) |
| Cerebrovascular accident | 8 (15) | 4 (13) | 4 (17) |
| Cholycystectomy | 15 (28) | 9 (30) | 6 (25) |
| Chronic renal insufficiency | 4 (7) | 2 (7) | 2 (8) |
| Priaprism (men only) | 3 (16) | 2 (22) | 1 (14) |
| Pulmonary hypertension | 4 (7) | 3 (10) | 1 (4) |
| Skin ulcers | 6 (11) | 5 (17) | 1 (4) |
| Visits or admissions documented (mean (stdev)) | |||
| Emergency room | 1.7 (3.1) | 1.5 (1.8) | 2.1 (4.3) |
| Hospital admissiona | 1.5 (2.9) | 0.9 (1.4) | 2.2 (4.1) |
P<0.05
Table 2.
Mean (stdev) pharmacokinetic parameters for codeine and its metabolites based on metabolic status
| Group I (n=30) | Group II (n=24) | |
|---|---|---|
| Codeine | ||
| t1/2 (min) | 125 (48) | 120 (27) |
| Tmax (min)a | 54 (27) | 77 (36) |
| Cmax (ng/ml)a | 81 (32) | 62 (24) |
| AUC(0–∞) (µg*min/ml) | 22 (23) | 14 (6.4) |
| Morphine | ||
| Tmax (min) | 46 (25) | – |
| Cmax (ng/ml) | 2.0 (1.0) | 0.0 |
| AUC(0–∞) (ng*min/ml) | 222 (246) | 0.0 |
| Morphine-3-glucuronide | ||
| Tmax (min) | 104 (69) | 110 (52) |
| Cmax (ng/ml)a | 88 (38) | 38 (21) |
| AUC(0–6) (µg*min/ml)a | 22 (10) | 9.2 (5.3) |
| Morphine-6-glucuronide | ||
| Tmax (min) | 122 (80) | 126 (40) |
| Cmax (ng/ml)a | 9.5 (3.8) | 8.3 (4.5) |
| AUC(0–6) (ng*min/ml)a | 2185 (911) | 1017 (504) |
P<0.05
Fig. 2.
The mean (and standard deviation) plasma concentration time profile for the 30 subjects in group I (•) and the 24 subjects in group II (ο) are illustrated. The whiskers represent the standard deviation
The number of carriers of a variant CYP2D6 allele did not significantly differ between group I and group II subjects. (Table 3). Nine carriers of a variant allele were identified in group I and 11 carriers of a variant allele were identified in group II (p=0.23). More CYP2D6 variant allele homozygotes were identified in group II compared to group I (group I 1 vs. group II 5, p=0.07).
Table 3.
Allele and genotype frequencies in Blacks with sickle cell disease
| Frequencies All |
Group I | Group II | |
|---|---|---|---|
| Allele | n=108 | n=60 | n=48 |
| *17 | 17 (0.16) | 7 (0.12) | 10 (0.21) |
| *29 | 8 (0.07) | 2 (0.03) | 6 (0.12) |
| *41 | 1 (0.01) | 1 (0.02) | 0 (0.0) |
| *other | 82 (0.76) | 50 (0.83) | 32 (0.67) |
| Genotype | n=54 | n=30 | n=24 |
| *other/*other | 34 (0.63) | 21 (0.70) | 13 (0.54) |
| *other/*17 | 7 (0.13) | 5 (0.17) | 2 (0.08) |
| *other/*29 | 6 (0.11) | 2 (0.07) | 4 (0.17) |
| *other/*41 | 1 (0.02) | 1 (0.03) | 0 (0.0) |
| *17/*17 | 5 (0.09) | 1 (0.03) | 4 (0.17) |
| *29/*29 | 1 (0.02) | 0 (0.0) | 1 (0.04) |
Values are numbers with proportion in parenthesis
The mean pharmacokinetic parameters estimated for codeine and its metabolites were compared between the 12 carriers of the *17 allele and the 34 non-carriers of a *17 allele, with non-carriers designated as *other/*other. The mean Cmax and AUC0→6 estimated for morphine was 40% and 30% lower among variant allele carriers, respectively, but the differences did not reach statistical significance. However, we observed significantly lower mean Cmax and AUC0→6 for M3G and M6G in *17 carriers versus noncarriers (p< 0.05) (Fig. 3). Similarly, the Cmax estimated for codeine and its metabolites tended to be lower in *29 allele carriers (n=7) compared to non-carriers (p=0.07).
Fig. 3.
The mean maximal plasma concentrations for codeine, morphine, morphine-3-glucuronide and morphine-6-glucuronide are illustrated in non-carriers and carriers of the three variant alleles identified in this study population. The whiskers represent the standard deviation
The mean number of ER visits and hospital admissions in the 12 months preceding the study visit was higher for subjects in group II compared to group I (Table 1). Emergency room visits (group I 1.5±1.8 vs. group II 2.1±4.3, p = NS) did not differ based on metabolic status, but more hospital admissions (0.9±1.4 vs. 2.2±4.1, p=0.05) were documented in patients with no measurable morphine concentrations.
Discussion
We examined the relationship between morphine concentrations, CYP2D6 genotype, and clinical outcomes in 54 Blacks with sickle cell disease. We found that the variant CYP2D6 allele frequency was significantly higher in patients without measurable morphine concentrations following a dose of codeine. In addition, carriers of two variant CYP2D6 alleles tended to be overrepresented in the group without measurable morphine. Furthermore, the concentrations of morphine or its metabolites tended to be lower in carriers of a variant *17 or *29 allele. These differences did not extend to significant differences in ER visits, but more hospital admissions were documented in subjects without measurable morphine concentrations.
We opted not to label our subjects as poor metabolizers (PM) or extensive metabolizers (EM), since codeine is not a standard probe drug in which phenotype has been predicted based on a well-defined plasma or urinary metabolic ratio. Early systems used to predict phenotype distinguished between PM and EM using a urinary metabolic ratio of a standard probe drug, such as dextromethorphan or debrisoquine [13]. Two studies indicate that less morphine, M3G, and M6G are measurable in Whites identified as PM based on genotype or phenotype [5, 35]. However, these data should be extrapolated to Black populations with caution. Black population samples demonstrate slower CYP2D6 activity compared to Whites that is evidenced as a ‘right-shift’ in the activity distribution and higher mean metabolic ratios. More over, substantial differences have also been observed among Black American and African populations [2, 9, 17, 20, 21].
We examined the *17 and *29 alleles, because these are the most common variant CYP2D6 alleles reported in Blacks, and these alleles are associated with a reduced metabolic capacity using standard probe drugs [3, 11, 27, 31, 34]. Furthermore, two reports indicate that *17 allele is associated with reduced metabolic capacity for codeine [27, 31]. Oscarson et al. [27] reported that the *17 allele is associated with 5- to 10-lower affinity for codeine compared to the wild-type allele and Shen et al. [31] reported that the intrinsic clearance of codeine was reduced by ~20%. Our data also suggests that *17 allele is associated with reduced metabolic capacity, since the Cmax and AUC0→6 of morphine, M3G and M6G were substantially lower for *17 carriers. However, this SNP is also part of different haplotypes which have been found in a number of other allelic variants including CYP2D6*40. This *40 allele is associated with a loss of function and occurs at a frequency 0.006 in Blacks [9]. Therefore, it is conceivable that a fraction of our twelve CYP2D6*17 alleles have been misclassified. Although the metabolic capacity of the *17 and *40 alleles is substantially different, a misclassification is not likely to affect the outcomes of our study with likelihood only one subject in our study may be a carrier of the *40 allele. The affinity and intrinsic clearance for codeine has not been described for *29 allele, but our data appears to indicate that codeine O-demethylation is reduced in *29 carriers.
Of note, the sensitivity of our assay is in agreement with other LC-MS assays using similar equipment [19, 24]. A more sensitive assay would likely lead to more subjects in our study with measurable morphine concentrations and reclassified into group I; the number of subjects that could be reclassified cannot be predicted. However, our mean serum molar ratio of M3G to morphine, M6G to morphine and M3G to M6G for subjects in group I and the M3G to M6G in subjects in group II agrees with previous published reports in which subjects received oral morphine [1, 12, 15].
Interestingly, the mean Cmax and AUC for M3G and M6G were substantially lower for *17 carriers. CYP2D6 catalyzes the O-demethylation of codeine to morphine, and the polymorphic enzyme uridine diphospate-glucuronosyltransferase (UGT) 2B7 catalyzes the metabolism of morphine to M6G and possibly to M3G [6, 7]. Therefore, it can be argued that the rate-limiting step in the metabolism of codeine to morphine to its glucuronides is the metabolism of codeine to morphine; this would support the relationship between CYP2D6 genotype and the concentrations of the glucuronide metabolites. We also noted that mean maximal codeine concentrations were lower in subjects in group II compared to subjects in group I which is contrary to our expectations. It is not apparent why the mean maximal codeine concentrations were lower in this group; we would anticipate that maximal codeine concentrations would be higher in the subjects in group II secondary to reduced metabolism to morphine. However, only about 10% of codeine undergoes O-demethylation by the polymorphic enzyme CYP2D6. Codeine predominantly undergoes metabolism by CYP3A4 to norcodeine. Both norcodeine and the remaining metabolites undergoes further metabolism by the phase II enzyme UGT. It is possible inhibition or reduced function alleles of CYP3A4 or UGT may be more common in the subjects in group I compared to group II and led to a statistically significant increase in the mean maximal codeine concentration in the subjects assigned to group I. Alternatively, it appears that the orocecal transit time following codeine is prolonged in extensive metabolizers of CYP2D6 [22]. The prolonged transit time would allow for increased absorption and could account for the higher mean maximal plasma concentration of the parent drug codeine in our subjects assigned to group I.
In this study population, we identified a relationship between metabolic status and hospital admissions, but not ER visits. ER visits and hospital admissions for an acute pain crisis were quantified as a clinical surrogate marker of response to codeine. A more direct measure of response, such as heat intolerance, cold intolerance and pinprick response, was not used in this study, because these techniques could elicit a pain crisis. An indirect measure of a response to codeine was therefore the best estimation of treatment response and provided a non-invasive opportunity to explore the relationship between genotype, phenotype and outcomes. Our failure to demonstrate a relationship between the ER visits and phenotype may be due to the use of a poor surrogate marker for clinical response to codeine; this marker is likely influenced by a large number of factors that could wash out any possible influence of phenotype.
In summary, the data suggest that subjects with sickle cell disease with no measurable plasma morphine levels after a single dose of codeine were not more likely to possess a variant allele commonly associated with reduced CYP2D6 metabolic capacity in Blacks. However, we did observe differences in the frequency of heterozygotes and homozygotes for some variants between subjects with and without measurable morphine concentrations and carriers of these alleles demonstrated reduced concentrations of morphine or its metabolites. It is possible the observed relationship may be clinically relevant, although we did not identify a statistically significant relationship with the both surrogate markers. Additional prospective studies are warranted to determine if clinical response to codeine or hydrocodone may be predicted based on phenotype.
Acknowledgements
We would like to extend our gratitude to Dr. Andrea Gaedigk who provided her expertise during the completion of this study.
This study was supported in part by the Illinois Department of Public Health awarded to the Sickle Cell Center, Janssen Medical Affairs, LLC (FEN-EMR4007) and a Clinical Translational Science Award from the Center for Clinical Translational Science at the University of Illinois awarded to Dr. Stacy S. Shord and the General Clinical Research Center at the University of Illinois Medical Center at Chicago (NIH grant M01-RR-13987).
Contributor Information
Stacy S. Shord, Email: sshord@uic.edu, Department of Pharmacy Practice, University of Illinois, College of Pharmacy (MC 886), 833 S Wood Street, Room 164, Chicago, IL 60612, USA.
Larisa H. Cavallari, Department of Pharmacy Practice, University of Illinois, College of Pharmacy (MC 886), 833 S Wood Street, Room 164, Chicago, IL 60612, USA
Weihua Gao, University of Illinois School of Public Health, Chicago, IL, USA.
Hyun-Young Jeong, Department of Pharmacy Practice, University of Illinois, College of Pharmacy (MC 886), 833 S Wood Street, Room 164, Chicago, IL 60612, USA.
Kelly Deyo, Department of Pharmacy Practice, University of Illinois, College of Pharmacy (MC 886), 833 S Wood Street, Room 164, Chicago, IL 60612, USA.
Shitalben R. Patel, Department of Pharmacy Practice, University of Illinois, College of Pharmacy (MC 886), 833 S Wood Street, Room 164, Chicago, IL 60612, USA
Joseph R. Camp, Department of Pharmacy Practice, University of Illinois, College of Pharmacy (MC 886), 833 S Wood Street, Room 164, Chicago, IL 60612, USA
Susan M. Labott, Department of Psychiatry, University of Illinois, College of Medicine (MC 913), 912 S Wood Street, Chicago, IL, USA Molokie University of Illinois Sickle Cell Center, Chicago, IL, USA.
Robert E. Molokie, Department of Medicine, Section of Hematology/Oncology, University of Illinois, College of Medicine (MC 713), 840 S Wood Street, Chicago, IL, USA Molokie University of Illinois Sickle Cell Center, Chicago, IL, USA.
References
- 1.Aasmundstad TA, Storset P. Influence of ranitidine on the morphine-3-glucuronide to morphine-6-glucuronide ratio after oral administration of morphine in humans. Hum Exp Toxicol. 1998;17:347–352. doi: 10.1177/096032719801700611. [DOI] [PubMed] [Google Scholar]
- 2.Aklillu E, Herrlin K, Gustafsson LL, Bertilsson L, Ingelman-Sundberg M. Evidence for environmental influence on CYP2D6-catalysed debrisoquine hydroxylation as demonstrated by phenotyping and genotyping of Ethiopians living in Ethiopia or in Sweden. Pharmacogenetics. 2002;12:375–383. doi: 10.1097/00008571-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Bapiro TE, Hasler JA, Ridderstrom M, Masimirembwa CM. The molecular and enzyme kinetic basis for the diminished activity of the cytochrome P450 2D6.17 (CYP2D6.17) variant. Potential implications for CYP2D6 phenotyping studies and the clinical use of CYP2D6 substrate drugs in some African populations. Biochem Pharmacol. 2002;64:1387–1398. doi: 10.1016/s0006-2952(02)01351-5. [DOI] [PubMed] [Google Scholar]
- 4.Cai WM, Nikoloff DM, Pan RM, de Leon J, Fanti P, Fairchild M, Koch WH, Wedlund PJ. CYP2D6 genetic variation in healthy adults and psychiatric African-American subjects: implications for clinical practice and genetic testing. Pharmacogenomics J. 2006;6:343–350. doi: 10.1038/sj.tpj.6500378. [DOI] [PubMed] [Google Scholar]
- 5.Caraco Y, Tateishi T, Guengerich FP, Wood AJ. Micro-somal codeine N-demethylation: cosegregation with cytochrome P4503A4 activity. Drug Metab Dispos. 1996;24:761–764. [PubMed] [Google Scholar]
- 6.Coffman BL, King CD, Rios GR, Tephly TR. The glucuronidation of opioids, other xenobiotics, and androgens by human UGT2B7Y(268) and UGT2B7H(268) Drug Metab Dispos. 1998;26:73–77. [PubMed] [Google Scholar]
- 7.Coffman BL, Rios GR, King CD, Tephly TR. Human UGT2B7 catalyzes morphine glucuronidation. Drug Metab Dispos. 1997;25:1–4. [PubMed] [Google Scholar]
- 8.Desmeules J, Gascon MP, Dayer P, Magistris M. Impact of environmental and genetic factors on codeine analgesia. Eur J Clin Pharmacol. 1991;41:23–26. doi: 10.1007/BF00280101. [DOI] [PubMed] [Google Scholar]
- 9.Gaedigk A, Bradford LD, Marcucci KA, Leeder JS. Unique CYP2D6 activity distribution and genotype-phenotype discordance in black Americans. Clin Pharmacol Ther. 2002;72:76–89. doi: 10.1067/mcp.2002.125783. [DOI] [PubMed] [Google Scholar]
- 10.Gaedigk A, Ryder DL, Bradford LD, Leeder JS. CYP2D6 poor metabolizer status can be ruled out by a single genotyping assay for the −1584G promoter polymorphism. Clin Chem. 2003;49:1008–1011. doi: 10.1373/49.6.1008. [DOI] [PubMed] [Google Scholar]
- 11.Gaedigk A, Simon SD, Pearce RE, Bradford LD, Kennedy MJ, Leeder JS. The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin Pharmacol Ther. 2008;83:234–242. doi: 10.1038/sj.clpt.6100406. [DOI] [PubMed] [Google Scholar]
- 12.Goucke CR, Hackett LP, Ilett KF. Concentrations of morphine, morphine-6-glucuronide and morphine-3-glucuronide in serum and cerebrospinal fluid following morphine administration to patients with morphine-resistant pain. Pain. 1994;56:145–149. doi: 10.1016/0304-3959(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 13.Kirchheiner J. CYP2D6 phenotype prediction from genotype: which system is the best? Clin Pharmacol Ther. 2008;83:225–227. doi: 10.1038/sj.clpt.6100455. [DOI] [PubMed] [Google Scholar]
- 14.Kirchheiner J, Schmidt H, Tzvetkov M, Keulen JT, Lotsch J, Roots I, Brockmoller J. Pharmacokinetics of codeine and its metabolite morphine in ultra-rapid metabolizers due to CYP2D6 duplication. Pharmacogenomics J. 2007;7:257–265. doi: 10.1038/sj.tpj.6500406. [DOI] [PubMed] [Google Scholar]
- 15.Klepstad P, Borchgrevink PC, Dale O, Zahlsen K, Aamo T, Fayers P, Fougner B, Kaasa S. Routine drug monitoring of serum concentrations of morphine, morphine-3-glucuronide and morphine-6-glucuronide do not predict clinical observations in cancer patients. Palliat Med. 2003;17:679–687. doi: 10.1191/0269216303pm835oa. [DOI] [PubMed] [Google Scholar]
- 16.Leathart JB, London SJ, Steward A, Adams JD, Idle JR, Daly AK. CYP2D6 phenotype-genotype relationships in African-Americans and Caucasians in Los Angeles. Pharmacogenetics. 1998;8:529–541. doi: 10.1097/00008571-199812000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Lennard MS, Iyun AO, Jackson PR, Tucker GT, Woods HF. Evidence for a dissociation in the control of sparteine, debrisoquine and metoprolol metabolism in Nigerians. Pharmaco-genetics. 1992;2:89–92. doi: 10.1097/00008571-199204000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Lotsch J. Opioid metabolites. J Pain Symptom Manage. 2005;29:S10–S24. doi: 10.1016/j.jpainsymman.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Maralikova B, Weinmann W. Confirmatory analysis for drugs of abuse in plasma and urine by high-performance liquid chromatography-tandem mass spectrometry with respect to criteria for compound identification. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;811:21–30. doi: 10.1016/j.jchromb.2004.04.039. [DOI] [PubMed] [Google Scholar]
- 20.Masimirembwa C, Persson I, Bertilsson L, Hasler J, Ingelman-Sundberg M. A novel mutant variant of the CYP2D6 gene (CYP2D6*17) common in a black African population: association with diminished debrisoquine hydroxylase activity. Br J Clin Pharmacol. 1996;42:713–719. doi: 10.1046/j.1365-2125.1996.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masimirembwa CM, Hasler JA. Genetic polymorphism of drug metabolising enzymes in African populations: implications for the use of neuroleptics and antidepressants. Brain Res Bull. 1997;44:561–571. doi: 10.1016/s0361-9230(97)00307-9. [DOI] [PubMed] [Google Scholar]
- 22.Mikus G, Trausch B, Rodewald C, Hofmann U, Richter K, Gramatte T, Eichelbaum M. Effect of codeine on gastrointestinal motility in relation to CYP2D6 phenotype. Clin Pharmacol Ther. 1997;61:459–466. doi: 10.1016/S0009-9236(97)90196-X. [DOI] [PubMed] [Google Scholar]
- 23.Mortimer O, Persson K, Ladona MG, Spalding D, Zanger UM, Meyer UA, Rane A. Polymorphic formation of morphine from codeine in poor and extensive metabolizers of dextro-methorphan: relationship to the presence of immunoidentified cytochrome P-450IID1. Clin Pharmacol Ther. 1990;47:27–35. doi: 10.1038/clpt.1990.4. [DOI] [PubMed] [Google Scholar]
- 24.Musshoff F, Trafkowski J, Kuepper U, Madea B. An automated and fully validated LC-MS/MS procedure for the simultaneous determination of 11 opioids used in palliative care, with 5 of their metabolites. J Mass Spectrom. 2006;41:633–640. doi: 10.1002/jms.1021. [DOI] [PubMed] [Google Scholar]
- 25.Oguri K, Yamada-Mori I, Shigezane J, Hirano T, Yoshimura H. Enhanced binding of morphine and nalorphine to opioid delta receptor by glucuronate and sulfate conjugations at the 6-position. Life Sci. 1987;41:1457–1464. doi: 10.1016/0024-3205(87)90710-7. [DOI] [PubMed] [Google Scholar]
- 26.Osborne R, Thompson P, Joel S, Trew D, Patel N, Slevin M. The analgesic activity of morphine-6-glucuronide. Br J Clin Pharmacol. 1992;34:130–138. doi: 10.1111/j.1365-2125.1992.tb04121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oscarson M, Hidestrand M, Johansson I, Ingelman-Sundberg M. A combination of mutations in the CYP2D6*17 (CYP2D6Z) allele causes alterations in enzyme function. Mol Pharmacol. 1997;52:1034–1040. doi: 10.1124/mol.52.6.1034. [DOI] [PubMed] [Google Scholar]
- 28.Pasternak GW, Bodnar RJ, Clark JA, Inturrisi CE. Morphine-6-glucuronide, a potent mu agonist. Life Sci. 1987;41:2845–2849. doi: 10.1016/0024-3205(87)90431-0. [DOI] [PubMed] [Google Scholar]
- 29.Penson RT, Joel SP, Bakhshi K, Clark SJ, Langford RM, Slevin ML. Randomized placebo-controlled trial of the activity of the morphine glucuronides. Clin Pharmacol Ther. 2000;68:667–676. doi: 10.1067/mcp.2000.111934. [DOI] [PubMed] [Google Scholar]
- 30.Penson RT, Joel SP, Clark S, Gloyne A, Slevin ML. Limited phase I study of morphine-3-glucuronide. J Pharm Sci. 2001;90:1810–1816. doi: 10.1002/jps.1131. [DOI] [PubMed] [Google Scholar]
- 31.Shen H, He MM, Liu H, Wrighton SA, Wang L, Guo B, Li C. Comparative Metabolic Capabilities and Inhibitory Profiles of CYP2D6.1, CYP2D6.10, and CYP2D6.17. Drug Metab Dispos. 2007;35:1292–1300. doi: 10.1124/dmd.107.015354. [DOI] [PubMed] [Google Scholar]
- 32.Sindrup SH, Arendt-Nielsen L, Brosen K, Bjerring P, Angelo HR, Eriksen B, Gram LF. The effect of quinidine on the analgesic effect of codeine. Eur J Clin Pharmacol. 1992;42:587–591. doi: 10.1007/BF00265920. [DOI] [PubMed] [Google Scholar]
- 33.Wan YJ, Poland RE, Han G, Konishi T, Zheng YP, Berman N, Lin KM. Analysis of the CYP2D6 gene polymorphism and enzyme activity in African-Americans in southern California. Pharmacogenetics. 2001;11:489–499. doi: 10.1097/00008571-200108000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Wennerholm A, Johansson I, Hidestrand M, Bertilsson L, Gustafsson LL, Ingelman-Sundberg M. Characterization of the CYP2D6*29 allele commonly present in a black Tanzanian population causing reduced catalytic activity. Pharmacogenetics. 2001;11:417–427. doi: 10.1097/00008571-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Williams DG, Patel A, Howard RF. Pharmacogenetics of codeine metabolism in an urban population of children and its implications for analgesic reliability. Br J Anaesth. 2002;89:839–845. doi: 10.1093/bja/aef284. [DOI] [PubMed] [Google Scholar]
- 36.Yue QY, Svensson JO, Alm C, Sjoqvist F, Sawe J. Interindividual and interethnic differences in the demethylation and glucuronidation of codeine. Br J Clin Pharmacol. 1989;28:629–637. doi: 10.1111/j.1365-2125.1989.tb03555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]