Abstract
The common marmoset (Callithrix jacchus) is poised to become a standard nonhuman primate aging model. With an average lifespan of 5 to 7 years and a maximum lifespan of 16.5 years, marmosets are the shortest-lived anthropoid primates. They display age-related changes in pathologies that mirror those seen in humans, such as cancer, amyloidosis, diabetes, and chronic renal disease. They also display predictable age-related differences in lean mass, calf circumference, circulating albumin, hemoglobin, and hematocrit. Features of spontaneous sensory and neurodegenerative change—for example, reduced neurogenesis, β-amyloid deposition in the cerebral cortex, loss of calbindin D28k binding, and evidence of presbycusis—appear between the ages of 7 and 10 years. Variation among colonies in the age at which neurodegenerative change occurs suggests the interesting possibility that marmosets could be specifically managed to produce earlier versus later occurrence of degenerative conditions associated with differing rates of damage accumulation. In addition to the established value of the marmoset as a model of age-related neurodegenerative change, this primate can serve as a model of the integrated effects of aging and obesity on metabolic dysfunction, as it displays evidence of such dysfunction associated with high body weight as early as 6 to 8 years of age.
Keywords: aging research, hearing loss, marmoset (Callithrix jacchus), neurodegeneration, nonhuman primate (NHP), obesity
Nonhuman Primates in Aging Research
Rationale
Nonhuman primates occupy a special niche as models for health and disease because, with their close phylogenetic relationship to humans, they often closely mirror the physiological processes that take place in humans. They are of particular value in modeling sensory and neurological processes, given the distinct evolutionary trajectory of these traits in primates. Austad (2010) provides an overview of aging in nonhuman primates and their use as models of human aging.
Value of a Short-Lived Nonhuman Primate Model
Primates, as an order, have clearly undergone evolutionary processes resulting in both absolute and relatively long lifespan, with an average longevity quotient of 1.92 (Austad and Fischer 1992; average longevity quotient is the ratio of actual versus expected maximum lifespan of a mammalian species based on its body size). The nonhuman primate (NHP1) species most commonly used for aging research, the rhesus macaque (Macaca mulatta), is a relatively large-bodied species with a maximum lifespan of 40 and a longevity quotient of 2.06.2 The practical implications of working with such a long-lived species often limit the research that can be conducted. Longitudinal studies, for example, are difficult to conduct not only because of the expense but also because of the challenges inherent in controlling environmental variables over decades. Thus one of the drawbacks of nonhuman primates as aging models is their long lifespan.
It is therefore desirable to develop an NHP aging model with the shortest lifespan possible. A reasonable choice for such a model is the common marmoset (Callithrix jacchus; henceforth called simply marmoset), one of the smallest anthropoid primates with one of shortest lifespans of any anthropoid primate. Further, among nonhuman primates, and in comparison with Old World primates like the rhesus monkey, marmosets are easier to handle and maintain. They do not transmit diseases to humans, are more docile than rhesus monkeys, require less space, and are less costly to breed and maintain than Old World primates. In addition, although marmosets are generally maintained in social groups, they can be individually housed with considerably lower risks than seen in macaques for abnormal behavioral outcomes.
Thus, as a relatively short-lived nonhuman primate with small body size and low zoonotic risk, the marmoset has the potential to offer many practical advantages in the study of aging. However, its use in aging research has to this point been limited. Therefore, the aim of this article is to introduce the reader to this animal’s basic biology and life history, present some preliminary information on characteristics associated with aging in marmosets, and provide examples of studies of age-related disease processes in this species.
The Common Marmoset
Basic Biology
Marmosets are part of the primate family Callitrichidae, which includes five to seven genera (depending on the classification scheme used) and approximately 50 species of brightly colored, small New World primates: the marmosets (Callithrix, Callibella, Mico, and Cebuella), the tamarins (Saguinus and Leontopithecus), and Callimico (represented by a single species Callimico goeldii) (Kinzey 1997; Mittermeier 1988; Rylands et al. 1993; Rylands and Mittermeier 2009; Schneider et al. 2001). Callitrichids are distinguished from other primates by a number of characteristics: all digits except the hallux have claws instead of nails, and they have a V-shaped mandible and a nonprehensile tail, are typically diurnal, and show little or no sexual dimorphism (Rylands et al. 1993).
The callitrichids are the smallest of the New World primates, weighing 250 to 600 g. The only callitrichid primate that is frequently used in biomedical (especially neuroscience and infectious disease) research is the marmoset; Mansfield (2003) provides an overview of the common uses of this species in biomedical research. The weight of the adult marmoset averages 350 to 400 g, approximately that of a rat and about 4% of the adult weight of a rhesus macaque. The marmoset’s small size is both one of its greatest advantages (e.g., in terms of ease of handling and housing) and one of its potential drawbacks (e.g., in terms of limitations associated with blood volume).
Callitrichids typically give birth to twin or triplet litters after a 4- to 6-month gestation, and the twins often weigh as much as 20% of the female’s body weight (for an overview of callitrichid reproduction, Tardif et al. 2003). Callitrichids are the only nonhuman primates that do not exhibit lactational anovulation—the female ovulates and can become pregnant with the next litter 7 to 14 days postpartum. In cases of singleton births reports suggest that most are the result of an in utero loss of at least one litter mate (Gengozian et al. 1980; Jaquish et al. 1996; Wislocki 1932, 1939). Littermates are hematopoietic chimeras as a result of chorionic fusion before embryonic gastrulation (Merker et al. 1988). The production of such littermates that share an amazingly integrated in utero environment and then a similar postnatal environment offers many opportunities for studying the importance of pre- and postnatal environmental effects on later health and lifespan.
Wild callitrichid primates typically live in territorial groups of 5 to 15 individuals in which only one female and one male breed. All group members cooperate in infant care activities. Captive housing easily mirrors this normal group structure, with marmosets typically housed as nuclear family groups. Of potential importance in their development as an aging resource, marmosets can be maintained in such social housing in a barrier facility, where it is possible to control exposure to pathogens; such housing is considerably more complex and expensive for the bigger-bodied Old World monkeys that normally live in very large multimale, multifemale groups (for reviews of marmoset housing, husbandry, and nutrition, Layne and Power 2003; Rensing and Oerke 2005; Tardif et al. 2006).
When breeding is not desirable, marmosets are most often housed either individually or in male-female pairs in which the male is vasectomized. Same-sex housing is rare and can result in serious injury due to aggression, particularly among females. Although social housing is preferred, marmosets can be successfully individually housed as long as they have visual, auditory, and olfactory exposure to conspecifics. Serious stereotypies and self-injurious behavior are exceedingly rare, even in individually housed marmosets.
Life History
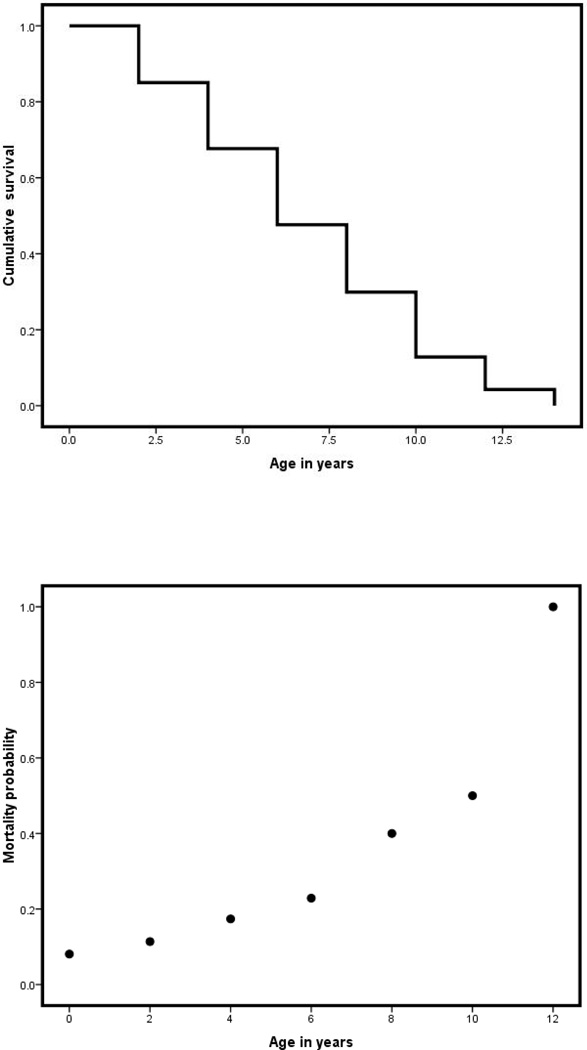
The maximum lifespan for the marmoset is 16.5 years3; reports indicate that the average lifespan of captive marmosets is 5 to 7 years (Abbott et al. 2003; Dyke et al. 1993; Ross et al. 2007; Smucny et al. 2004; Tardif et al. 2003). Figure 1A illustrates postinfancy survival by age for 358 marmosets that were born in the Southwest National Primate Research Center (SNPRC) colony and survived to at least 0.5 years of age, between January 1994 and March 2010 (excluding those whose deaths were related to experiments). For this population, the median lifespan was 5.76 years for all animals surviving to at least 6 months of age and 6.48 for those surviving to 2 years. Age-specific mortality exponentially increased from 0.5 to 12 years of age (Figure 1B).
Figure 1.
Survival analysis of 358 marmosets (Callithrix jacchus) that were born at the Southwest National Primate Research Center (SNPRC) colony and survived at least 6 months, between January 1994 and March 2010, excluding individuals whose deaths were related to experiments. Figure 1A illustrates survival by age; Figure 1B illustrates age-specific mortality.
A survey of mortality patterns in the SNPRC population over time, as well as findings from other colonies, suggests that stable, closed marmoset colonies may experience significantly reduced early adult mortality, producing populations that will be of particular use in aging research. Table 1 shows age-specific mortality for the SNPRC population in three time periods: 1994–1999, the period of colony establishment; 2000–2005, during which the colony was closed and stable; and 2006–2010, during which the colony underwent both significant growth and movement to new animal quarters. The average lifespan of the population extended from 4.82 years during 1994–1999 to 7.07 years during 2000–2005. More importantly, in terms of management for aging research, the colony experienced significantly reduced early adult mortality rates during the stable period—and a reversal in this decline when the colony environment became less stable (with the importation of new animals and the move to new housing). A similar, anecdotal finding is reported in marmoset pairs from the New England NPRC, with less early adult mortality in animals housed in stable, relatively isolated conditions.
Table 1.
Age-specific death rates for SNPRC colony animals during three time periods
| Stage | N | 0–2 y.o. | 2–4 y.o. | 4–6 y.o. | 6–8 y.o. | 8–10 y.o. | 10–12 y.o. |
|---|---|---|---|---|---|---|---|
| 1994–2000: Development | 94 | 0.15 | 0.34 | 0.27 | 0.46 | 0.80 | -- |
| 2000–2005: Stability | 105 | 0.03 | 0.09 | 0.28 | 0.40 | 0.35 | 0.57 |
| 2006–2010: Growth | 273 | 0.14 | 0.15 | 0.21 | 0.23 | 0.49 | 0.55 |
SNPRC, Southwest National Primate Research Center; y.o., years old
Ridley and colleagues (2006) report that in a colony maintained at the University of Cambridge, 16 of 20 (80%) animals in a cohort set aside for breeding were alive at 10 years of age. The specific features that may result in a larger percentage of a marmoset population surviving early adulthood are not defined but may include genetic differences among populations and environmental differences, including minimization of exposure to infectious agents and stressors.
There is evidence of significant differences in survival between the sexes: males have higher age-specific survival and lower age-specific mortality, most notably at later ages (Tardif et al. 2008a), a finding similar to that reported by Allman and colleagues (1998) for other small-bodied New World primates. Given that the majority of the animals in this survival analysis were breeding animals, we propose that the higher mortality in females is caused by an increased risk associated with reproduction.
Characteristics of Aging
Marmosets are often referred to as “aged” at 8 years of age (Abbott et al. 2003). In studies comparing younger (2- to 3-year-old) and older (7- to 8-year-old) marmosets, it is common to find aging effects in the latter group; such effects include fibrous to fibrous cartilaginous changes in intra-articular discs (Berkovitz and Pacy 2000), β-amyloid deposition in the cerebral cortex (Geula et al. 2002), and reduced neurogenesis in the hippocampus (Leuner et al. 2007).
Age-Related Pathologies
The most detailed analyses of age-related pathology in marmosets have been conducted on the population housed at the New England Primate Research Center (NEPRC), established in the late 1960s. For the past 20 years census has averaged 250 to 350 animals, all of them pedigreed and managed for production, with cohorts assigned to experimental (primarily infectious disease) research at 2 to 4 years of age; unassigned animals breed and live out their natural lifespan. The colony consists of 280 individuals with a mean age of 3.8 years and 34 individuals over the age of 8 years (as of March 2010).
Complete gross and histologic pathology on all animals at the time of death revealed the causes of morbidity and mortality in both young and aged animals unassigned to experimental study—and a similar disease profile to that of aging human subjects, in which neoplasia, infectious processes, renal disease, amyloid protein accumulation, and diabetes mellitus are common causes of morbidity and mortality.4
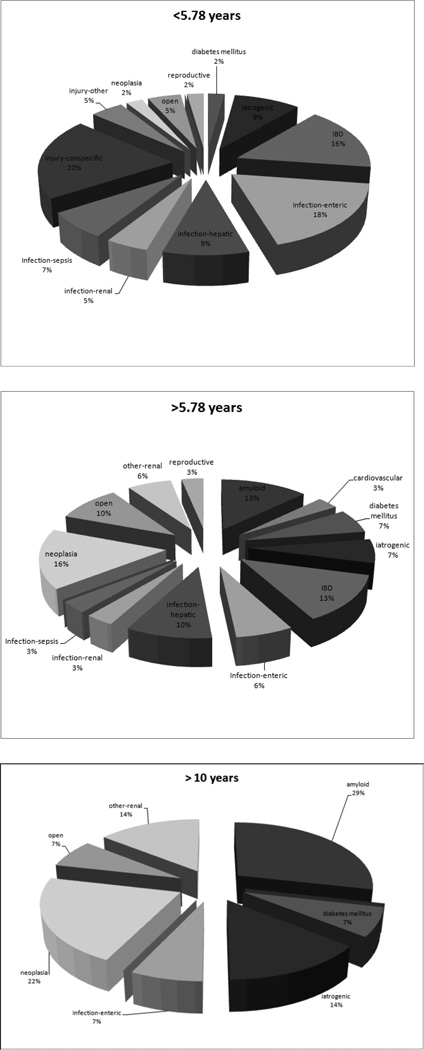
Retrospective analysis from January 2004 to June 2009 (blinded to the animals’ age and the original reports) covered all necropsy findings of animals over 1 year of age: 77 cases were evaluated (36 females, 41 males) with a mean age at death 5.78 years (males 6.08, females 5.44; p = 0.4403). Average weight at death was within the low to normal weight range for healthy adults: 321 g (males 316 g, females 326 g; p = 0.5788). The analysis revealed 30 distinct etiologic or morphologic conditions that had resulted in death; further categorization yielded 15 different groups based on etiology, morphology, and anatomic location.
Comparison of mortality data between these groups reveals significant differences in disease patterns based on age. In juvenile and young adult animals (those less than 5.78 years of age), the predominant causes of mortality were conspecific trauma, inflammatory bowel disease, sepsis, and bacterial infections of the gastrointestinal tract, liver, and kidneys (Figure 2A). In contrast, in aged animals these conditions were absent or greatly reduced and common causes of death included neoplasia, chronic renal disease, amyloidosis, and diabetes mellitus (Figure 2B,C; Ludlage et al. 2005). Adenocarcinoma of the small intestine, infrequently observed before 6 years of age, was the most common malignant neoplasm identified in the aged marmosets (Miller et al. 2010). Lymphoma was the next most common malignant neoplasm and was observed in less than 2% of the cases. Endometriosis, a reproductive proliferative disorder and a common confounder in studies that use rhesus, was not observed in this retrospective analysis and is rarely reported in marmosets.
Figure 2.
Causes of mortality for the New England Primate Research Center population of marmosets (Callithrix jacchus), January 2004 to June 2009. (A) Less than 5.78 years of age; (B) more than 5.78 years of age; (C) more than 10 years of age. n = 77; animals in 2C are included in 2B. IBD, inflammatory bowel disease; open = no cause of death identified; iatrogenic = death from a complication associated with anesthesia or treatment. Unlabeled slivers represent causes of mortality (injury in (B) and injury and iatrogenic in (C)) that occurred in less than 1% of the animals.
Other comorbid conditions increased with age. Myocardial fibrosis, generally an asymptomatic and incidental finding at necropsy, was observed in 60% of the animals older than 10 years; it may correlate with increased risk of anesthetic-associated arrhythmias and, rarely, congestive heart failure. Histologically the condition is characterized by multifocal areas of myocardial fibrosis and, infrequently, inflammatory cell infiltrates. Renal disease was also commonly observed in the aged marmosets, as it is in aged rhesus (KGM, unpublished observation). Multiple etiologies are likely, but these are often unknown and the condition is frequently asymptomatic. The most common clinical sign is weight loss but others are polyuria/polydypsia, azotemia, and anorexia. Morphologic changes include chronic interstitial nephritis, amyloidosis, and glomerulonephropathies. Mixed patterns are common. In animals over 8 years old 75% have some degree of renal pathology at death but renal disease was deemed the primary cause of mortality in only 16.7% of the cases.
Body Composition Changes
Aging and frail humans have been found to show decreases in lean muscle mass, muscular strength and function, and thus quality of life (Walston et al. 2002). Therefore, understanding the relation of age to body composition in an NHP aging model is of value.
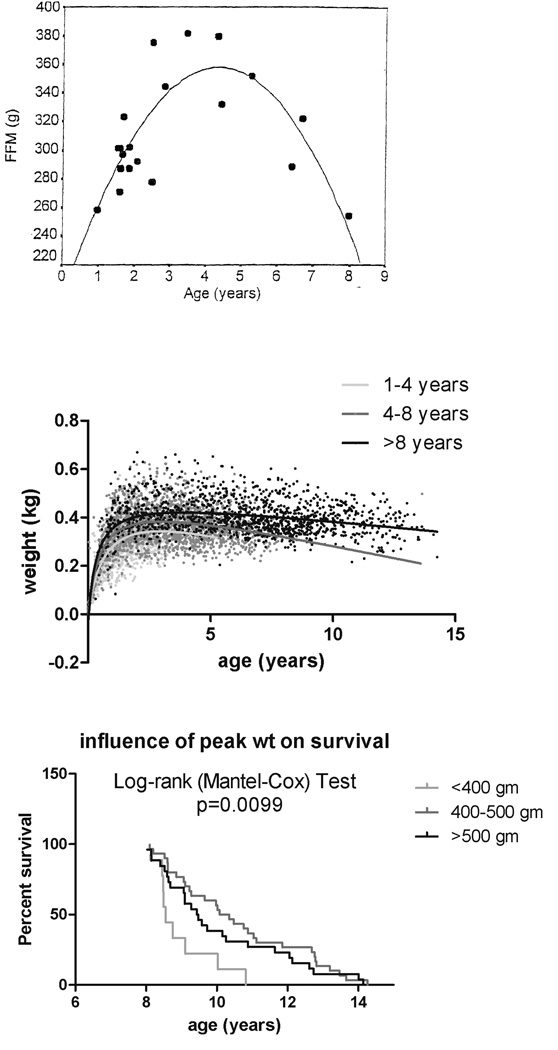
Power and colleagues (2001) reported an inverted U-shaped relationship between age and fat-free mass (as determined by labeled water dilution) in a cross-sectional sample of 20 captive marmosets (10 males and 10 females, ranging in age from 0.96 to 7.97 years), with peak mass from about 2.5 to 5 years of age, at which point mass declined (Figure 3A). Likewise, analyses of weight data from the NEPRC colony showed that body weights plateau between 1.2 and 5 years of age and then decline. The slope for regression of weight by age is not significantly different from zero for ages 1 to 5 years, but it is −7.26 g (p < 0.0001) for ages 5 to 10 years and −14.20 g for ages 10 to 15 years (p < 0.002)—thus weight loss accelerates between late middle age and old age (Figure 3B). The data also revealed that peak weight achieved between 1 and 2 years of age was important in animals surviving over 8 years; those with a peak weight of less than 400 g had decreased survival after 8 years (median survival 8.55 years; p = 0.0099, log-rank [Mantel-Cox] test; Figure 3C). There was no statistically significant difference between animals with peak weights of 400–500 g and those over 500 g.
Figure 3.
(A) Relation of fat-free mass (FFM) to age in a cross-sectional study of 20 marmosets (Callithrix jacchus), as reported by Power and colleagues (2001). (B) Relation of weight to age in years for marmosets surviving less than 4 years (in red), 4 to 8 years (in green), and more than 8 years (in purple). (C) Relation of peak weight to survival.
The data support the contention that weight attained early in life may affect longevity and provide preliminary evidence that the marmoset may be a useful model organism to study complex interactions related to aging phenotype.
The age-related difference in weight reported by Power and colleagues (2001) was most closely associated with fat-free mass in a sample of 20 marmosets. In our preliminary analysis (SDT and CR), we used quantitative magnetic resonance imaging to compare body composition and appendicular girth measures in a group of 40 male marmosets ranging in age from 2 to 13½ years, thus extending the sample size, parameters, and age range from the Power study (Tardif et al. 2008b). Age was significantly negatively correlated with total body mass (−0.336) and lean mass (−0.347) but was not associated with fat mass. Age was also negatively correlated with morphometric measures such as thigh circumference (−0.367) and calf circumference (−0.434) averaged over repeated measures at proximal, mid-, and distal points. The largest age effect was in the proximal calf circumference: that of old animals (8 to 13 years) was 12% lower than that of prime-aged adults (4 to 5 years).
These results suggest that marmosets may display appendicular muscle loss with age similar to that seen in humans and other species. These results await confirmation, through longitudinal analyses, of age-related declines in muscle mass in individual aging animals.
Hematological Changes
Human aging has been associated with significant changes to a number of hematological markers. Hemoglobin, hematocrit, creatinine, and albumin concentrations typically decline with age, while inflammatory cytokine such as c-reactive protein (CRP), interleukin (IL)-6, and tumor necrosis factor (TNF)-α typically increase (Leng et al. 2007; Walston et al. 2002). In our group of 40 marmosets, variation in albumin concentration was negatively associated with age and positively associated with weight. Hemoglobin and hematocrit were both significantly, though weakly, negatively correlated with age (−0.151 and −0.146, respectively). CRP and IL-8 were not associated with age but positively associated with fat mass.
Established and Developing Uses of Marmosets in Research on Age-Related Diseases
Induced Parkinson’s Disease
Parkinson’s disease is the result of the gradual neurodegeneration of dopaminergic cells in the substantia nigra leading to motor rigidity, tremors, slowing of movement (bradykinesia), and instability (Fahn 2003). While Parkinson’s is not considered a fatal disease, patients often have a shorter lifespan than people without the disease and can die from complications of the disease (e.g., pneumonia or fatal falls). Parkinson’s is thought to affect 100 to 200 of every 100,000 Caucasians in the world (Tanner and Goldman 1996). Etiology of the disease is often unknown (idiopathic), but in rare cases it is associated with genetic inheritance, head trauma, or toxins (Fahn 2003). There are several treatments to alleviate the symptoms associated with Parkinson’s disease, but there is no treatment to stop the neurodegeneration.
Animal models have been studied extensively in the exploration of the etiology, pathology, and treatments of Parkinson’s. Marmosets are a very popular model because of their small size, ease of handling, higher brain similarity to humans, standard stereotaxic surgery usage, and diversity of behavioral assessments (Eslamboli 2005). Marmosets also exhibit age-related decreases in neurogenesis before the onset of old age similar to those seen in humans (see Diminished Adult Neurogenesis below) as well as proteosome activity in the brain similar to that of humans (and higher than that of rodents; Zeng et al. 2005).
Through the use of a variety of induction models marmosets have been valuable in studies of the development and treatment of Parkinson’s disease. These models rely on neurotoxin induction of dopaminergic cell death using 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and 6-hydroxydopamine (6-OHDA) (Eslamboli 2005). MPTP subcutaneous injections are effective in both acute and chronic induction models to examine the development of motor deficits and recovery, and stereotaxic injections of 6-OHDA into the striatum are used in studies of end-stage idiopathic disease. These treatments induce the neurodegeneration of dopaminergic cells that leads to the rapid onset of motor deficits and the classical symptomology associated with Parkinson’s disease. Yet, although the symptoms described and quantified mirror those seen in human patients, to date none of the induction models have reproduced the classic human symptom of spontaneous tremors.
A number of behavioral assays are available to examine the decline in motor activity in marmosets after treatment that induces neural damage. These assays involve qualifying spontaneous activity using photocell monitors (Hansard et al. 2002), assessing head posture and balance using video analysis (van Vliet et al. 2008a,b), assessing ability to retrieve food items (hand-eye coordination; van Vliet et al. 2008a,b), and assessing the animals’ vertical movement and ability to right themselves (Verhave et al. 2009).
The need for an NHP model of Parkinson’s disease is most imperative at the stage of testing pharmaceutical treatments. While rodent models may allow for examining initial treatments it is necessary to examine an NHP model before advancing a drug to human clinical trials. The marmoset model has been used to test a number of treatment regimes including modafinil and Δ9-THC, both of which have been found to alleviate symptoms of motor deficits (van Vliet et al. 2008a,b).
Spontaneous Neurodegenerative Change
Beta-amyloid Deposition in the Brain
The deposition of β-amyloid in plaques and β-amyloid angiopathy are diagnostic features of Alzheimer’s disease. The accumulation of β-amyloid in the brains of elderly nonhuman primates has been reported for many species (Gearing et al. 1996, 1997; Lemere et al. 2004; Nakamura et al. 1995; Sawamura et al. 1997; Uno et al. 1996), including marmosets (Geula et al. 2002; Maclean et al. 2000; Ridley et al. 2006). While routine β-amyloid accumulation in Old World monkeys occurs at 22 to 31 years of age, marmosets reliably display such accumulation at 7 to 10 years of age.
Geula and colleagues (2002) and Ridley and colleagues (2006) report similar findings in terms of the form and localization of β-amyloid deposition in the aging marmoset brain, with diffuse Aβ42 positive cortical plaques, accumulation in small cortical vessels, and no apparent neurofibrillary tangles. The reports differ dramatically, however, in the age at which amyloid depositions are commonly found as well as the prevalence of this finding. Geula and colleagues (2002) report all animals over 7 years of age as having at least some deposition whereas Ridley and colleagues (2006) report none in animals under 10 years of age and some deposition in only 17% of animals between 10 and 15 years of age. These differences mirror reported differences in survival rates in these two colonies, suggesting the interesting possibility that marmosets could be specifically managed to produce earlier versus later occurrence of degenerative conditions such as amyloidosis.
Age-Related Calbindin D28k Loss
In addition to documenting the deposition and distribution of β-amyloid in the brain of marmosets, investigators have begun to study other age-related changes such as the loss of calbindin D28k from the basal forebrain cholinergic neurons (BFCNs) (Wu et al. 2003). Loss of BFCNs is a characteristic feature of age-associated human neurodegenerative disorders such as Alzheimer’s disease and may be related to loss of calbindin activity, which normally prevents rises in intracellular calcium (Wu et al. 2003). Localization of calbindin to BFCNs and is observed in the rodent brain and is thought to be primate specific. The brains of young (<4 years), middle-aged (6–8 years), and aged (9–15 years) marmosets were stained immunohistochemically for calbindin and basal forebrain immunoreactivity was determined through stereological techniques. In contrast to other neuronal markers investigators found selective loss of calbindin D28k from BCFNs (Wu et al. 2003). The marmoset may thus represent an appropriate model to study the significance of this age-associated neurodegenerative change.
Diminished Adult Neurogenesis
The marmoset may provide a valuable model of the role of declining neurogenesis in impaired cognitive function. Although new neurons are added to the hippocampus throughout life, many species display declining rates of neurogenesis with age, well before the onset of senescence. Leuner and colleagues (2007) report a significant linear decline with age in BrdU-labeled cells in the dentate gyrus of marmosets from 1.5 to 7 years of age.
Age-Related Hearing Loss
A significant problem among the elderly is difficulty in understanding speech, particularly in noisy social surroundings. It is estimated that nearly 40% of the population at age 65 and 60–80% of those who reach 85 will suffer hearing impairment (Cruickshanks et al. 1998; Gates et al. 1990). As the US population ages, hearing impairment will account for a large percentage of communicative disorders, necessitating novel approaches to understanding, diagnosing, and treating this condition. However, age-related hearing loss (ARHL) has a complex etiology because it can be confounded by noise-induced damage or other insults accumulated over a lifetime. The use of animal models overcomes some of these problems because it is possible to use inbred strains for studying hearing loss in a controlled environment. This has indeed been the case with numerous strains of mice that exhibit some form of inherited hearing disorder (Zheng et al. 1999; for reviews, Frisina and Walton 2001; Frisina 2009).
There are some types of hearing loss for which the development of a model that is more relevant to humans may hold much promise. In elderly humans the most common type of hearing impairment is caused by a progressive decrease in sensory hair cells in the hearing organ (cochlea) (Gates et al. 1990). This sensorineuronal hearing loss, called presbycusis, manifests as a progressive reduction in high-frequency hearing sensitivity over an increasing range of frequencies, eventually affecting the frequency range relevant for speech (Frisina and Frisina 1997). Because presbycusis occurs in the neural periphery (that is, in the peripheral nervous system) it is sometimes informally referred to as peripheral hearing loss.
While mouse models can provide relatively rapid insights into presbycusis in humans (Li and Borg 1991) there are also disadvantages associated with them. Rodents are far removed from the primate taxa and demonstrate markedly different auditory and vocal behaviors. It is important to know, for example, whether presbycusis actually affects behavior in these species. This point is relevant for translational strategies. Viewed as a problem that requires correction, the efficacy of treating presbycusis can be judged only in purely behavioral terms. Assuming that it will at some point be possible to identify specific mechanisms and physiological deficits underlying presbycusis, and to develop strategies for treatment, is there a model to verify the correction of behavioral deficits? Such a model should allow the understanding, diagnosis, and treatment of presbycusis by addressing all aspects of the problem—from cellular to organismal, from neural mechanisms to behavioral response. It is in this light that we propose the development of the marmoset as a model system for presbycusis.
Marmosets, like humans and other nonhuman primates, rely on vocal communication for a variety of social behaviors—they use near and long-distance calls to maintain group contact and territory, identify members of their group and individuals in other groups, and signal distress and alarm (Epple 1968; Ghazanfar and Hauser 2001; Ghazanfar et al. 2002). The importance of vocal communication in this species makes it a promising model for determining the effects of presbycusis on communication behavior, particularly in the noisy and reverberant surroundings of their natural habitat. The auditory challenges that older animals may face are analogous to those faced by elderly humans in noisy social surroundings.
Broadly, we divide the work on NHP hearing into three groups:
investigation of sound production and sound perception in the behavioral context (for reviews, Ghazanfar and Hauser 1999, 2001; Ghazanfar et al. 2002); recent work in New World monkeys has focused on cottontop tamarins (Saguinus oedipus) (Ghazanfar et al. 2002) and the marmoset (Miller and Wang 2006; Miller et al. 2009);
investigation of the neuroethology of audition in NHPs (in the squirrel monkey, Saimiri sciureus, Newman and Wollberg 1973; Winter and Funkenstein 1973; in the marmoset, DiMattina and Wang 2006; Kajikawa et al. 2005; Wang et al. 1995); and
broad neurophysiological (not including neuroanatomical) studies that address fundamental properties of auditory neurons and auditory nuclei (Kajikawa et al. 2005; Lu and Wang 2004; Kadia and Wang 2003).
The literature, which includes studies on Old and New World primates, is vast and therefore beyond our scope; we focus on studies on marmosets. Much of the work has concentrated on the central auditory areas, in particular the auditory cortex (Bendor and Wang 2010; Kajikawa et al. 2005, 2008; Sadagopan and Wang 2009), auditory thalamus (Wang et al. 2008), and midbrain inferior colliculus (Nelson et al. 2009). There are no known reports of neural recordings from the auditory periphery in the marmoset or from deep brain structures such as the cochlear nucleus and superior olivary nucleus, research areas in need of much attention. And to our knowledge, there is only one report on the effects of aging on hearing in nonhuman primates, suggesting moderate hearing loss in older marmosets based on measurements of auditory brainstem responses (ABRs, a noninvasive electrophysiological procedure) (Harada et al. 1999).
We wondered whether the marmoset can be used as a model for presbycusis and, more generally, whether its peripheral auditory system is more closely related to humans than that of other species. Anatomical evidence supporting the similarity of the marmoset cochlea to that of humans comes from the work of Spatz and Lohle (1995), which indicated that the marmoset cochlea has 2½ turns, as in humans. Physiological data supporting this similarity come from a recent study on the measurement of otoacoustic emissions (sounds emitted by the ear in response to stimulation): using a noninvasive technique that measures outer hair cell function Valero and colleagues (2008) showed that the acoustical stimulation parameters required to elicit the strongest emissions are similar to those of humans and Old World primates. Taken together, these studies suggest that cochlear parameters may be largely conserved in humans and nonhuman primates.
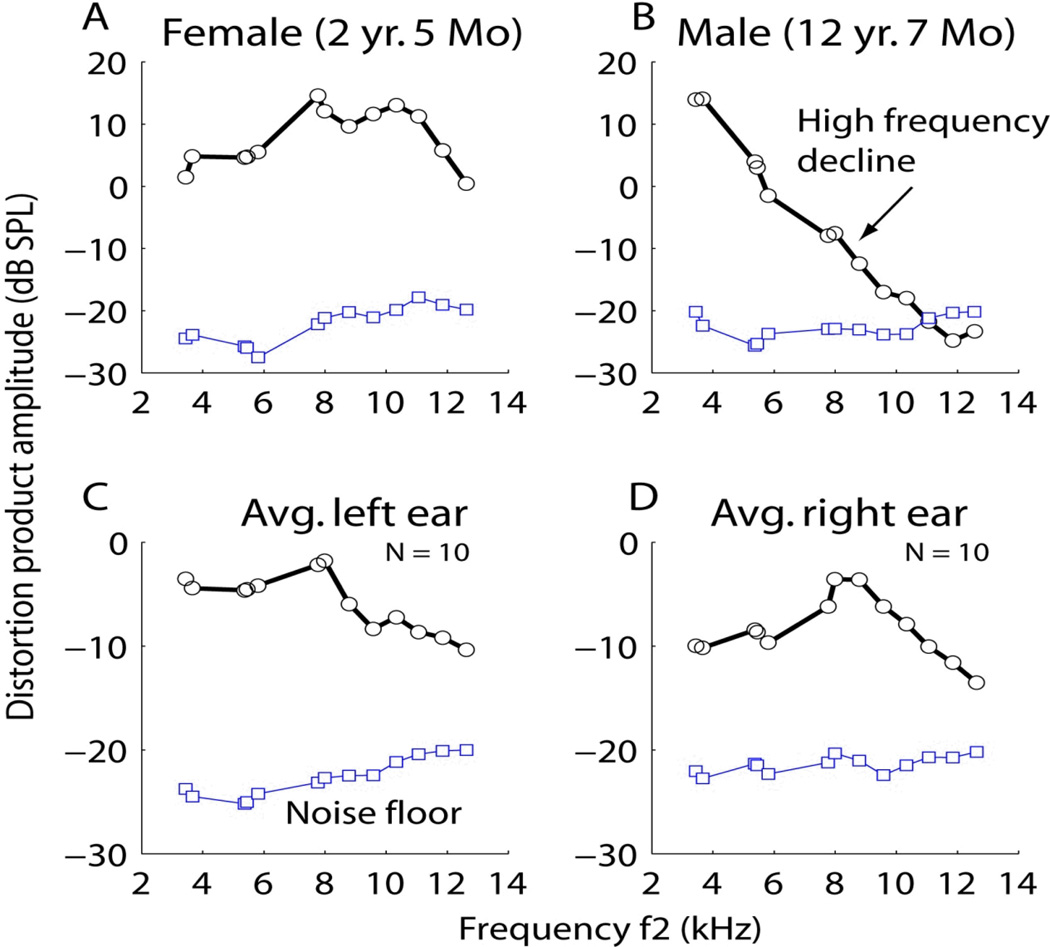
To date, the published evidence for presbycusis in marmosets consists solely of the report by Harada and colleagues (1999). Preliminary data (RR, unpublished) suggest that older marmosets may exhibit presbycusis because their OAEs are diminished in amplitude at higher frequencies (Figure 4), whereas when the cochlea is in good health it responds to stimulation at all frequencies. These data suggest that OAEs could be used to measure the health and status of the marmoset cochlea noninvasively, and thus the marmoset could serve as a useful model for the study of age-related hearing loss.
Figure 4.
Change in strength of distortion product otoacoustic emissions (OAE) with age. For a marmoset with normal hearing the OAE response strength curve, as a function of stimulation frequency, should look similar to the averages over many marmosets (n = 10) for the left and right ears (panels C, D, black lines). The blue line represents noise levels in the system; OAE response is significant if the measured strength exceeds (lies above) the noise curve. In the case of a young female (A) the emission strength was somewhat better than the group average; it remained high for frequencies up to about 12 kHz and declined thereafter. In contrast, for an old male (B) response strength declined as frequency increased, demonstrating a possible sloping hearing loss that is similar to that in humans. At frequencies above 10 kHz it approached the noise level, suggesting that no emissions were present. f2 denotes the high-frequency tone in the two-tone complex used to elicit an OAE; SPL, sound pressure level measured in decibels (dB, 20 µPa).
While evidence for presbycusis may be obtained indirectly from the measurement of ABRs and OAEs (“indirect” because they are not behavioral measures), a major gap in the literature is the lack of data on the extent of hearing loss in older marmosets. Hearing loss can be determined by measuring pure-tone behavioral audiograms, as is done in humans. Audiograms provide a noninvasive and behavioral measure of hearing sensitivity across the frequency range. To establish the presence of presbycusis in older marmosets it is necessary to measure audiograms in young and old animals. Behavioral audiograms of the marmoset have so far been reported in only one study (Sieden 1957); there are no audiograms of older marmosets or of hearing loss in marmosets.
Marmosets offer numerous advantages for hearing research. Because of the importance of vocal communication in this species, researchers can test the effects of aging on hearing at different levels of auditory processing, from behavior to invasive neurophysiology. This aspect deserves special emphasis because hearing performance is fundamentally behavioral performance. The ability to measure behavioral performance related to the hearing deficits that accompany aging makes this model attractive for translational research. However, although in recent years there has been substantial growth in hearing research in the marmoset, much of it has been confined to the higher auditory areas, with behavioral testing only slowly gaining momentum.
Developing the model for presbycusis would open new areas for investigation into lower auditory centers and eventually the entire auditory pathway. With a judicious combination of invasive and noninvasive physiology and with well-designed behavioral tests, the marmoset may very well open the door for understanding and treating human hearing loss.
Obesity and Metabolic Syndrome
In humans, aging and adiposity are both risk factors for metabolic syndrome, which is characterized by impaired lipid trafficking and glucose metabolism. Insulin resistance increases with age and adiposity (Rincon et al. 2006) and, with the US population both aging and becoming fatter, the problems associated with insulin resistance and its sequelae are among the country’s most important public health concerns. Defining the relative roles of age and adiposity in a short-lived primate model of these conditions could provide a valuable tool for testing interventions and therapeutics for this common chronic malady in humans.
Marmosets, in common with most other nonhuman primates (e.g., Bodkin et al. 1993; Comuzzie et al. 2003; Kemnitz 1984; Wagner et al. 2006), display obesity when kept in captivity. Among the phenotypes associated with obesity in marmosets are hypertriglyceridemia and hyperglycemia (Tardif et al. 2009); in a population of 64 animals maintained on a typical low-fat NHP diet and monitored for body composition and lipid and glucose metabolic parameters, 7.8% of subjects had at least three atypical factors, fitting an operational definition of metabolic syndrome.
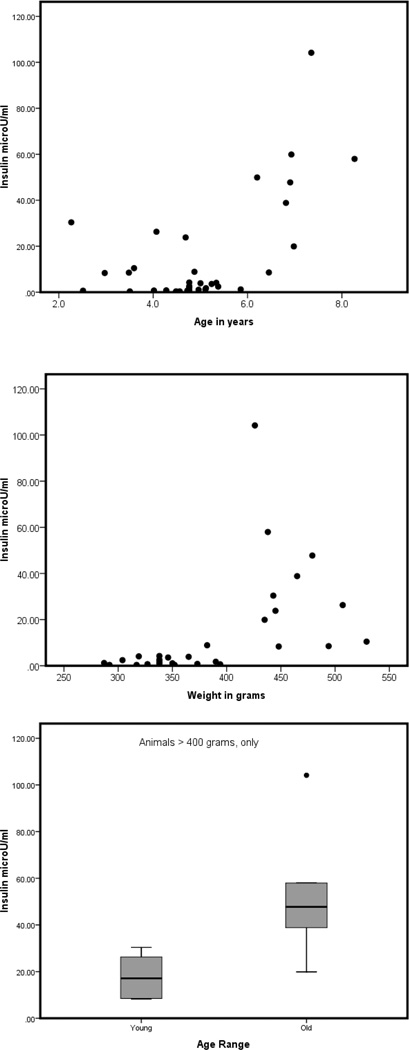
Further examination of this population has raised the possibility of an association between impaired glucose metabolism and aging in marmosets, perhaps separate from its association with obesity. We determined fasting glucose and insulin concentrations in a sample of 31 females ranging from 2.3 to 8.3 years of age and from 287 to 529 g in weight. Weight and age were uncorrelated in this sample population (r = 0.005). Until recently, the lack of a validated insulin assay was a major stumbling block in the development of the marmoset model for obesity and metabolic syndrome; we overcame the technical issues associated with this measurement and can now successfully measure plasma and serum insulin in marmosets using a porcine insulin RIA (Millipore Corp., Billerica, MA). This assay has been fully validated for accuracy (98.89 ± 2.38 SEM) and parallelism (no differences in the slopes of the lines between standards and a serially diluted marmoset pool, p > 0.05).
Age and weight were both independent determinants of fasting insulin in this study population (r2 = 0.578; Figures 5A,B), supporting the contention that marmosets display increasing insulin resistance with age, as is the case in humans. We noted, however, that the weight range in the older population (>5 years of age) was limited to animals over 400 g in weight, and therefore compared fasting insulin in young versus old individuals that weighed more than 400 g (Figure 5C). Older individuals had significantly higher fasting insulin.
Figure 5.
Fasting insulin concentrations in a population of 31 female marmosets (Callithrix jacchus). (A) Relation of insulin concentration to age. (B) Relation of insulin concentration to body weight. (C) Insulin concentration in young (<5 years of age) and old (5 to 8 years of age) marmosets, limited to animals >400 g in weight (n = 11).
We are planning future studies to clarify separate and integrated roles of obesity and aging in the development of metabolic dysfunction in marmosets, as well as possible associated lipid and cardiovascular effects, by including lean aged animals in our study population.
Conclusions
The common marmoset (Callithrix jacchus) offers many opportunities for development of an efficient NHP model of aging and age-related diseases. With an average and maximum lifespan that is 30–40% of that of commonly used Old World monkeys such as macaques, aging studies can be accomplished in a fraction of the time required with larger nonhuman primates. Marmosets exhibit some age-related changes similar to those observed in humans, such as declines in lean mass, calf circumference, circulating albumin, hemoglobin, and hematocrit. In terms of disease, marmosets display higher prevalence of cancer, amyloidosis, diabetes, and chronic renal disease as they age, again similar to humans.
Primate models of neurodegeneration are of particular importance, and, as we have illustrated, marmosets in particular display age-related spontaneous sensory and neurodegenerative changes such as reduced neurogenesis, β-amyloid deposition in the cerebral cortex, loss of calbindin D28k binding, and evidence of presbycusis. The spontaneous occurrence of these phenotypes in marmosets ranging in age from 7 to 10 years suggests that this animal may prove a valuable model for research on selected aspects of age-related neurodegenerative change. Variation among colonies in the age at which neurodegenerative change occurs suggests the interesting possibility that marmosets could be specifically managed to produce earlier versus later occurrence of degenerative conditions associated with differing rates of damage.
In addition to the established value of the marmoset as a model of neurodegenerative change, marmosets are poised to become a model of the integrated effects of aging and obesity on metabolic dysfunction, with evidence that they display metabolic dysfunction associated with high body weight at 6 to 8 years of age. In contrast, the average age at which metabolic dysfunction is reported in rhesus macaques is around 17½ years of age (Colman et al. 2009) and around 20 years of age in baboons (Guardado-Mendoza et al. 2009), ages that exceed the maximum lifespan of marmosets.
The marmoset aging model is poised to reach its full potential in the coming years through the development of colonies specifically managed for aging research combined with the new molecular tools that will stem from the annotated marmoset genome,5 the capacity to produce transgenic marmosets (Sasaki et al. 2009), and the development of marmoset induced pluripotent stem (iPS) cells (Wu et al. 2010).
Acknowledgments
This work was supported by NIH base grants to the New England Primate Research Center (P51-RR000168), the Wisconsin National Primate Research Center (P51-RR000167) and the Southwest National Primate Research Center (P51-RR013986). This work was supported by NIH grants to S. Tardif (R01-DK077639) and R. Ratnam (R03-DC009050).
Footnotes
Abbreviation used in this article: NHP, nonhuman primate
Information from http://genomics.senescence.info/species/entry.php?species=Macaca_mulatta, accessed on November 9, 2010
As reported in the Animal Aging and Longevity Database (AnAge; http://genomics.senescence.info/species/query.php?search=callithrix+jacchus), accessed on November 9, 2010.
The incidence of amyloidosis in humans is lower than that shown for marmosets.
Available online at the Baylor College of Medicine Marmoset Genome Project (www.hgsc.bcm.tmc.edu/project-species-p-Marmoset.hgsc?pageLocation=Marmoset), accessed November 9, 2010.
Contributor Information
Suzette D. Tardif, Barshop Institute for Longevity and Aging Studies at the University of Texas Health Sciences Center in San Antonio, Texas..
Keith G. Mansfield, Resource and Collaborative Affairs and an associate professor of Pathology at Harvard Medical School, and Chair of the Division of Primate Resources at the New England National Primate Research Center in Southborough, Massachusetts..
Rama Ratnam, Systems and Computational Neuroscience in the Department of Biology at the University of Texas at San Antonio..
Corinna N. Ross, Barshop Institute for Longevity and Aging Studies..
Toni E. Ziegler, Wisconsin Primate Research Center, University of Wisconsin in Madison..
References
- Abbott DH, Barnett DK, Colman RJ, Yamamoto ME, Schultz-Darken NJ. Aspects of common marmoset basic biology and life history important for biomedical research. Comp Med. 2003;53:339–350. [PubMed] [Google Scholar]
- Allman J, Rosin A, Kumar R, Hasenstaub A. Parenting and survival in anthropoid primates: Caretakers live longer. Proc Natl Acad Sci U S A. 1998;95:6866–6869. doi: 10.1073/pnas.95.12.6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austad SN. Small nonhuman primates as potential models for aging research. ILAR J. 2010;51 doi: 10.1093/ilar.38.3.142. XXXXXX. [DOI] [PubMed] [Google Scholar]
- Austad SN, Fischer KE. Primate longevity: Its place in the mammalian scheme. Am J Primatol. 1992;28:251–261. doi: 10.1002/ajp.1350280403. [DOI] [PubMed] [Google Scholar]
- Bendor D, Wang X. Neural coding of periodicity in marmoset auditory cortex. J Neurophysiol. 2010;103:1809–1822. doi: 10.1152/jn.00281.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkovitz BKB, Pacy J. Age changes in the cells of the intra-articular disc of the temporomandibular joints of rats and marmosets. Arch Oral Biol. 2000;45:987–995. doi: 10.1016/s0003-9969(00)00067-4. [DOI] [PubMed] [Google Scholar]
- Bodkin N, Hannah J, Ortmeyer H, Hansen B. Central obesity in rhesus monkeys: Association with hyperinsulinemia, insulin resistance and hypertriglyceridemia? Int J Obes Relat Metab Disord. 1993;17:53–61. [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comuzzie A, Cole SA, Martin L, Carey KD, Mahaney MC, Blangero J, VandeBerg JL. The baboon as a nonhuman primate model for the study of the genetics of obesity. Obes Res. 2003;11:75–80. doi: 10.1038/oby.2003.12. [DOI] [PubMed] [Google Scholar]
- Cruickshanks KJ, Wiley TL, Tweed TS, Klein BE, Klein R, Mares-Perlman JA. Prevalence of hearing loss in older adults in Beaver Dam Wisconsin: The epidemiology of hearing loss study. Am J Epidemiol. 1998;148:879–886. doi: 10.1093/oxfordjournals.aje.a009713. [DOI] [PubMed] [Google Scholar]
- DiMattina C, Wang X. Virtual vocalization stimuli for investigating neural representations of species-specific vocalizations. J Neurophysiol. 2006;95:1244–1262. doi: 10.1152/jn.00818.2005. [DOI] [PubMed] [Google Scholar]
- Dyke B, Gage TB, Ballou JD, Petto AJ, Tardif SD, Williams LE. Model life tables for the smaller New World monkeys. Am J Primatol. 1993;29:269–285. doi: 10.1002/ajp.1350290404. [DOI] [PubMed] [Google Scholar]
- Epple G. Comparative studies on vocalization in marmoset monkeys (Hapalidae) Folia Primat. 1968;8:1–40. doi: 10.1159/000155129. [DOI] [PubMed] [Google Scholar]
- Eslamboli A. Marmoset monkey models of Parkinson’s disease: Which model, when and why? Brain Res Bull. 2005;68:140–149. doi: 10.1016/j.brainresbull.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Fahn S. Description of Parkinson’s disease as a clinical syndrome. Ann NY Acad Sci. 2003;991:1–14. doi: 10.1111/j.1749-6632.2003.tb07458.x. [DOI] [PubMed] [Google Scholar]
- Frisina RD. Age-related hearing loss: Ear and brain mechanisms. Ann NY Acad Sci. 2009;1170:708–717. doi: 10.1111/j.1749-6632.2009.03931.x. [DOI] [PubMed] [Google Scholar]
- Frisina DR, Frisina RD. Speech recognition in noise and presbycusis: Relations to possible neural mechanisms. Hear Res. 1997;106:95–104. doi: 10.1016/s0378-5955(97)00006-3. [DOI] [PubMed] [Google Scholar]
- Frisina RD, Walton JP. Aging of the mouse central auditory system. In: Willott JP, editor. Handbook of Mouse Auditory Research: From Behavior to Molecular Biology. New York: CRC Press; 2001. pp. 339–379. [Google Scholar]
- Gates GA, Cooper JC, Jr, Kannel WB, Miller NJ. Hearing in the elderly: The Framingham cohort 1983–1985. Part I Basic audiometric test results. Ear Hear. 1990;11:247–256. [PubMed] [Google Scholar]
- Gearing M, Tigges J, Mori H, Mirra S. Aβ40 is a major form of β-amyloid in nonhuman primates. Neurobiol Aging. 1996;17:903–908. doi: 10.1016/s0197-4580(96)00164-9. [DOI] [PubMed] [Google Scholar]
- Gearing M, Tigges J, Mori H, Mirra S. β-amyloid (Aβ) deposition in the brains of aged orangutans. Neurobiol Aging. 1997;18:139–146. doi: 10.1016/s0197-4580(97)00012-2. [DOI] [PubMed] [Google Scholar]
- Gengozian N, Brewen J, Preston R, Batson J. Presumptive evidence for the absence of functional germ cell chimerism in the marmoset. J Med Primatol. 1980;9:9–27. doi: 10.1159/000460118. [DOI] [PubMed] [Google Scholar]
- Geula C, Nagykery N, Wu C-K. Amyloid-β deposits in the cerebral cortex of the aged common marmoset (Callithrix jacchus): Incidence and chemical composition. Acta Neuropathol. 2002;103:48–58. doi: 10.1007/s004010100429. [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Hauser MD. The neuroethology of primate vocal communication: Substrates for the evolution of speech. Trends Cog Neurosci. 1999;3:377–384. doi: 10.1016/s1364-6613(99)01379-0. [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Hauser MD. The auditory behavior of primates: A neuroethological perspective. Curr Opin Neurobiol. 2001;11:712–720. doi: 10.1016/s0959-4388(01)00274-4. [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Smith-Rohrberg D, Pollen AA, Hauser MD. Temporal cues in the antiphonal longcalling behaviour of cottontop tamarins. Anim Behav. 2002;64:427–438. [Google Scholar]
- Guardo-Mendoza R, Davalli AM, Chavez AO, Hubbard GB, Dick EJ, Majluf-Cruz A, Tene-Perez CE, Goldschmidt L, Hart J, Perego C, Comuzzie AG, Tejera ME, Finzi G, Placidi C, LaRosa S, Capella C, Halff G, Gastaldelli A, DeFronzo RA, Folli F. Pancreatic islet amyloidosis, beta-cell apoptosis, and alpha-cell proliferation are determinants of islet remodling in type-2 diabetic baboons. Proc Natl Acad Sci U S A. 2009;106:13992–13997. doi: 10.1073/pnas.0906471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansard MJ, Smith LA, Jackson MJ, Cheetham P, Jenner P. Dopamine but not norepinephrine or serotonin, reuptake inhibition reverses motor deficits in MPTP treated primates. J Pharmacol Exp Ther. 2002;303:952–958. doi: 10.1124/jpet.102.039743. [DOI] [PubMed] [Google Scholar]
- Harada T, Tokuriki M, Tanioka Y. Age-related changes in the brainstem auditory evoked potentials of the marmoset. Hear Res. 1999;128:119–124. doi: 10.1016/s0378-5955(98)00201-9. [DOI] [PubMed] [Google Scholar]
- Jaquish C, Tardif S, Toal R, Carson R. Patterns of prenatal survival in the common marmoset (Callithrix jacchus) J Med Primatol. 1996;25:57–63. doi: 10.1111/j.1600-0684.1996.tb00194.x. [DOI] [PubMed] [Google Scholar]
- Kadia SC, Wang X. Spectral integration in A1 of awake primates: Neurons with single- and multipeaked tuning characteristics. J Neurophysiol. 2003;89:1603–1622. doi: 10.1152/jn.00271.2001. [DOI] [PubMed] [Google Scholar]
- Kajikawa Y, de La Mothe LA, Blumell S, Hackett TA. A comparison of neuron response properties in areas A1 and CM of the marmoset monkey auditory cortex: Tones and broadband noise. J Neurophysiol. 2005;93:22–34. doi: 10.1152/jn.00248.2004. [DOI] [PubMed] [Google Scholar]
- Kajikawa Y, de la Mothe LA, Blumell S, Sterbing-D'Angelo SJ, D'Angelo W, Camalier CR, Hackett TA. Coding of FM sweep trains and twitter calls in area CM of marmoset auditory cortex. Hear Res. 2008;239:107–125. doi: 10.1016/j.heares.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemnitz J. Obesity in macaques: Spontaneous and induced. Adv Vet Sci Comp Med. 1984;28:81–114. doi: 10.1016/b978-0-12-039228-5.50009-7. [DOI] [PubMed] [Google Scholar]
- Kinzey W. Synopsis of New World primates (16 genera) In: Kinzey WG, editor. New World Primates: Ecology, Evolution and Behavior. New York: Aldine de Gruyter; 1997. [Google Scholar]
- Layne DG, Power RA. Husbandry, handling and nutrition for marmosets. Comp Med. 2003;53:351–359. [PubMed] [Google Scholar]
- Lemere CA, Beierschmitt A, Iglesias M, Spooner ET, Bloom JK, Leverone JF, Zheng JB, Seabrook TJ, Louard D, Li D, Slekoe DJ, Palmour RM, Ervin FR. Alzheimer’s disease Aβ vaccine reduced central nervous system Aβ levels in a nonhuman primate, the Caribbean vervet. Am J Pathol. 2004;165:283–297. doi: 10.1016/s0002-9440(10)63296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng SX, Zue QL, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55:864–871. doi: 10.1111/j.1532-5415.2007.01186.x. [DOI] [PubMed] [Google Scholar]
- Leuner B, Kozorovitskiy Y, Gross CG, Gould E. Diminished adult neurogenesis in the marmoset brain precedes old age. Proc Natl Acad Sci U S A. 2007;104:17169–17173. doi: 10.1073/pnas.0708228104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HS, Borg E. Age-related loss of auditory sensitivity in two mouse genotypes. Acta Otolaryngol. 1991;111:827–834. doi: 10.3109/00016489109138418. [DOI] [PubMed] [Google Scholar]
- Lu T, Wang X. Information content of auditory cortical responses to time-varying acoustic stimuli. J Neurophysiol. 2004;91:301–313. doi: 10.1152/jn.00022.2003. [DOI] [PubMed] [Google Scholar]
- Ludlage E, Murphy CL, Davern SM, Solomon A, Weiss DT, Glenn-Smith D, Dworkin S, Mansfield KM. Systemic AA amyloidosis in the common marmoset. Vet Pathol. 2005;42:117–124. doi: 10.1354/vp.42-2-117. [DOI] [PubMed] [Google Scholar]
- Maclean CJ, Baker HF, Ridley RM, Mori H. Naturally occurring and experimentally induced β-amyloid deposits in the brains of marmosets (Callithrix jacchus) J Neural Transm. 2000;107:799–814. doi: 10.1007/s007020070060. [DOI] [PubMed] [Google Scholar]
- Mansfield K. Marmoset models commonly used in biomedical research. Comp Med. 2003;53:383–392. [PubMed] [Google Scholar]
- Merker H, Sames K, Casto W, Heger W, Neubert D. The embryology of Callithrix jacchus. In: Neubert D, Merker H, Hendrixx A, editors. Nonhuman Primates, Developmental Biology and Toxicology. Berlin: Ueberreuta Wissenscahft-Wein; 1988. [Google Scholar]
- Miller CT, Beck K, Meade B, Wang X. Antiphonal call timing in marmosets is behaviorally significant: Interactive playback experiments. J Comp Physiol A. 2009;195:783–789. doi: 10.1007/s00359-009-0456-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CT, Wang X. Sensory-motor interactions modulate a primate vocal behavior: Antiphonal calling in common marmosets. J Comp Physiol A. 2006;192:27–38. doi: 10.1007/s00359-005-0043-z. [DOI] [PubMed] [Google Scholar]
- Miller AD, Kramer JA, Lin KC, Knight H, Martinot A, Mansfield KG. Small intestinal adenocarcinoma in common marmosets (Callithrix jacchus) Vet Pathol. 2010;47:969–976. doi: 10.1177/0300985810369905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittermeier R. Ecology and behavioral of neotropical primates. Washington: World Wildlife Fund; 1988. [Google Scholar]
- Nakamura S, Tamaoka A, Sawamura N, Shoji S, Nakayama H, Ono F, Sakakibara I, Yoshikawa Y, Mori H, Goto N, Doi K. Carboxyl end-specific monoclonal antibodies to amyloid beta protein (Aβ) subtypes (Aβ40 and Aβ42(43)) differentiate Aβ in senile plaques and amyloid angiopathy in brains of aged cynomolgus monkeys. Neurosci Lett. 1995;201:151–154. doi: 10.1016/0304-3940(95)12160-9. [DOI] [PubMed] [Google Scholar]
- Newman JD, Wollberg Z. Multiple coding of species-specific vocalizations in the auditory cortex of squirrel monkeys. Brain Res. 1973;54:287–304. doi: 10.1016/0006-8993(73)90050-4. [DOI] [PubMed] [Google Scholar]
- Nelson PC, Smith ZM, Young ED. Wide-dynamic-range forward suppression in marmoset inferior colliculus neurons is generated centrally and accounts for perceptual masking. J Neurosci. 2009;29:2553–2562. doi: 10.1523/JNEUROSCI.5359-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power RA, Power ML, Layne DG, Jaquish CE, Oftedal OT, Tardif SD. Relations among measures of body composition, age and sex in the common marmoset monkey (Callithrix jacchus) Comp Med. 2001;51:218–223. [PubMed] [Google Scholar]
- Rensing S, Oerke A-K. Husbandry and management of New World species: Marmosets and tamarins. In: Wolfe-Coote S, editor. The Laboratory Primate. New York: Elsevier; 2005. pp. 145–162. [Google Scholar]
- Ridley RM, Baker HF, Windle CP, Cummings RM. Very long term studies of the seeding of β-amyloidosis in primates. J Neura Transm. 2006;113:1243–1251. doi: 10.1007/s00702-005-0385-2. [DOI] [PubMed] [Google Scholar]
- Rincon M, Muzumdar R, Barzilai N. Aging, body fat and carbohydrate metabolism. In: Masoro EJ, Austad SN, editors. Handbook of the Biology of Aging. 6 th ed. New York: Elsevier; 2006. pp. 498–511. [Google Scholar]
- Ross CN, Fite JE, Jensen H, French JA. Demographic review of a captive colony of callitrichids (Callithrix kuhlii) Amer J Primatol. 2007;69:234–240. doi: 10.1002/ajp.20367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylands A, Coimbra-Filho A, Mittermeier R. Systematics, geographic distribution and some notes on the conservation status of the Callitrichidae. In: Rylands AB, editor. Marmosets and Tamarins: Systematics, Behavior and Ecology. Oxford: Oxford University Press; 1993. [Google Scholar]
- Rylands AB, Mittermeier RA. The Diversity of the New World Primates (Platyrrhini) In: Garber PA, Estrada A, Bicca-Marques JC, Heymann EW, Strier KB, editors. South American Primates: Comparative Perspectives in the Study of Bahavior, Ecology, and Conservation. New York: Springer; 2009. pp. 23–54. [Google Scholar]
- Sadagopan S, Wang X. Nonlinear spectrotemporal interactions underlying selectivity for complex sounds in auditory cortex. J Neurosci. 2009;29:11192–11202. doi: 10.1523/JNEUROSCI.1286-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki E, Suemizu H, Shimada A, Hanazawa K, Oiwa R, Kamioka M, Tomioka I, Sotomaur Y, Hirakawa R, Eto T, Shiozawa S, Maeda T, Ito M, Ito R, Kito C, Yagihashi C, Kawai K, Miyoshi H, Tanioka Y, Tamaoki N, Habu S, Okano H, Nomura T. Generation of transgenic non-human primates with germline transmission. Nature. 2009;459:523–527. doi: 10.1038/nature08090. [DOI] [PubMed] [Google Scholar]
- Sawamura N, Tamaoka A, Shoji S, Koo EH, Walker LC, Mori H. Characterization of amyloid β protein species in cerebral amyloid angiopathy of a squirrel monkey by immunohistochemistry and enzyme-linked immunosorbent assay. Brain Res. 1997;764:225–229. doi: 10.1016/s0006-8993(97)00624-0. [DOI] [PubMed] [Google Scholar]
- Schneider H, Canavez F, Sampaip I, Moreira M, Tagliaro C, Seuanaz H. Can molecular data place each neotropical monkey in its own branch? Chromosoma. 2001;109:515–523. doi: 10.1007/s004120000106. [DOI] [PubMed] [Google Scholar]
- Sieden HR. PhD dissertation. Princeton University; 1957. Auditory acuity of the marmoset monkey (Hapale jacchus) [Google Scholar]
- Smucny DA, Abbott DH, Mansfield KG, Schultz-Darken NJ, Yamamoto ME, Alencar AI, Tardif SD. Reproductive output, maternal age and survivorship in captive common marmoset females (Callithrix jacchus) Am J Primatol. 2004;64:107–121. doi: 10.1002/ajp.20065. [DOI] [PubMed] [Google Scholar]
- Spatz WB, Lohle E. Calcium-binding proteins in the spiral ganglion of the monkey, Callithrix jacchus. Hear Res. 1995;86:89–99. doi: 10.1016/0378-5955(95)00059-d. [DOI] [PubMed] [Google Scholar]
- Tanner CM, Goldman SM. Epidemiology of Parkinson’s disease. Neurolog Clin. 1996;14:317–335. doi: 10.1016/S0733-8619(05)70259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif SD, Smucny DA, Abbott DH, Mansfield K, Schultz-Darken N, Yamamoto ME. Reproduction in captive common marmosets (Callithrix jacchus) Comp Med. 2003;53:364–368. [PubMed] [Google Scholar]
- Tardif S, Bales K, Williams L, Ludlage Moeller E, Abbott D, Schultz-Darken N, Mendoza S, Mason W, Bourgeois S, Ruiz J. Preparing New World monkeys for laboratory research. ILAR J. 2006;47:307–315. doi: 10.1093/ilar.47.4.307. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Araujo A, Arruda MF, French JA, Sousa MBC, Yamamoto ME. Reproduction and aging in marmosets and tamarins. In: Atsalis S, Margulis SW, Hof PR, editors. Primate Reproductive Aging. Interdisciplinary Topics in Gerontology. vol 36. Basel: Karger; 2008a. pp. 29–48. [DOI] [PubMed] [Google Scholar]
- Tardif S, Ross C, Davis K, Dobek G, Brasky K, Espinoza S. Toward a model of frailty. National Harbor MD: Gerontological Society of America; 2008b. Relations among body composition, activity, hematology and age in male marmoset monkeys (Callithrix jacchus): Progr No. 343. [Google Scholar]
- Tardif SD, Power ML, Ross CN, Rutherford JN, Layne-Colon DG, Paulik MA. Characterization of obese phenotypes in a small nonhuman primates, the common marmoset (Callithrix jacchus) Obesity. 2009;17:1499–1505. doi: 10.1038/oby.2009.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno H, Alsum PB, Dong S, Richardson R, Zimbric ML, Thieme CS, Houser WD. Cerebral amyloid angiopathy and plaques and visceral amyloidosis in aged macaques. Neurobiol Aging. 1996;17:275–281. doi: 10.1016/0197-4580(95)02063-2. [DOI] [PubMed] [Google Scholar]
- Valero MD, Pasanen EG, McFadden D, Ratnam R. Distortion-product otoacoustic emissions in the common marmoset (Callithrix jacchus): Parameter optimization. Hear Res. 2008;243:57–68. doi: 10.1016/j.heares.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet AAM, Vanwersch RAP, Jongsma MJ, Olivier B Philippens IHCHM. Therapeutic effects of Δ9-THC and modafinil in a marmoset Parkinson model. Eur Neuropsychopharmacol. 2008a;18:383–389. doi: 10.1016/j.euroneuro.2007.11.003. [DOI] [PubMed] [Google Scholar]
- van Vliet AAM, Blezer ELA, Jongsma MJ, Vanwersch RAP, Olivier B Philippens IHCHM. Exploring the neuroprotective effects of modafinil in a marmoset Parkinson model with immunohistochemistry, magnetic resonance imaging, and spectroscopy. Brain Res. 2008b;1189:219–228. doi: 10.1016/j.brainres.2007.10.059. [DOI] [PubMed] [Google Scholar]
- Verhave PS, Vanwersch RAP, van Helden HPM, Smit AB Philippens IHCHM. Two new test methods to quantify motor deficits in a marmoset model for Parkinson’s disease. Behav Brain Res. 2009;200:214–219. doi: 10.1016/j.bbr.2009.01.022. [DOI] [PubMed] [Google Scholar]
- Wagner JE, Kavanagh K, Ward GM, Auerbach BJ, Harwood HJ, Kaplan JR. Old World primate models of type 2 diabetes mellitus. ILAR J. 2006;47:259–271. doi: 10.1093/ilar.47.3.259. [DOI] [PubMed] [Google Scholar]
- Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, Gottdiener J, Fried LP. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: Results from the Cardiovascular Health Study. Arch Intern Med. 2002;162:2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- Wang X, Lu T, Bendor D, Bartlett E. Neural coding of temporal information in auditory thalamus and cortex. Neuroscience. 2008;157:484–494. doi: 10.1016/j.neuroscience.2008.07.050. [DOI] [PubMed] [Google Scholar]
- Wang X, Merzenich MM, Beitel R, Schreiner CE. Representation of a species-specific vocalization in the primary auditory cortex of the common marmoset: Temporal and spectral characteristics. J Neurophysiol. 1995;74:2685–2706. doi: 10.1152/jn.1995.74.6.2685. [DOI] [PubMed] [Google Scholar]
- Winter P, Funkenstein HH. The effect of species-specific vocalization on the discharge of auditory cortical cells in the awake squirrel monkey (Saimiri sciureus) Exp Brain Res. 1973;18:489–504. doi: 10.1007/BF00234133. [DOI] [PubMed] [Google Scholar]
- Wislocki G. Placentation in the marmoset (Oedipomidas geoffroyi) with remarks on twinning in monkeys. Anat Rec. 1932;52:381–392. [Google Scholar]
- Wislocki G. Observations on twinning in marmosets. Am J Anat. 1939;64:445–483. [Google Scholar]
- Wu C-K, Nagykery N, Hersh LB, Scinto LFM, Geual C. Selective age-related loss of calbindin- D28k from basal forebrain cholinergic neurons in the common marmoset (Callithrix jacchus) Neuroscience. 2003;120:249–259. doi: 10.1016/s0306-4522(03)00248-3. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhang Y, Mishra A, Tardif S, Hornsby P. Generation of induced pluripotent stem cells from newborn marmoset skin fibroblasts. Stem Cell Res. 2010;4:180–188. doi: 10.1016/j.scr.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng B-Y, Medhurst AD, Jackson M, Rose S, Jenner P. Proteasomal activity in brain differs between species and brain regions and changes with age. Mech Aging Dev. 2005;126:760–766. doi: 10.1016/j.mad.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analysis. Hear Res. 1999;130:94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]