Abstract
Activity monitors provide an objective mechanism for evaluating patient function. It is unclear what similarities or unique information may be yielded using different analyses. Fifteen patients scheduled to undergo shoulder arthroplasty and fifteen matched control subjects wore tri-axial accelerometer activity monitors bilaterally at the lower (wrist) and upper (biceps) arm for 3 days. Measures of central tendency, variance, sample entropy, and asymmetry were calculated. A novel technique to evaluate time distribution of activity intensity was also performed. Within both groups there was a difference in central tendency and variance when comparing dominant and non-dominant limbs for both the lower (Controls: Mean Activity, P < 0.001; Max Activity, P < 0.001; Patients: Mean Activity, P = 0.044; Max Activity, P = 0.009) and upper (Controls: Mean Activity, P < 0.001; Max Activity, P = 0.046; Patients: Mean Activity, P = 0.002; Max Activity, P = 0.049) arm. Within group differences were also present for lower arm entropy in both groups (Controls, P < 0.001; Patients P = 0.041), and at the upper arm for patients (P = 0.003). There were differences between groups for the asymmetry index for both the lower (P = 0.033) and upper arm (P = 0.005), and maximum activity level of the lower arm (P = 0.05). Between group differences were present for time distribution of activity intensity, as the involved upper arm of patients was inactive for a greater time than controls (P = 0.013). These results highlight unique information provided by multiple analysis methods, and include a novel approach of evaluating the distribution of time spent across variable intensity activities.
Keywords: Inertial sensor system, Measurement, Evaluation
1. Introduction
A full return to functional activities is a key goal for patients who have sustained an injury. Evaluation of functional recovery may be performed by measuring movement biomechanics in a laboratory setting. These measures, however, are often expensive and do not adequately replicate the patient’s natural living environment. Function may also be captured with self-report questionnaires. This form of assessment is limited as the accuracy of the reporting is dependent on patient recall and perception of activity (Matthews et al., 2002; Ward et al., 2005). Activity monitors provide an alternative mechanism for evaluating patient function. The devices are typically small in size, relatively inexpensive, and record data for multiple days. Tri-axial digital accelerometers embedded within the activity monitor provide objective data regarding movement characteristics. In light of the advantages of activity monitors, it is not surprising there are a growing number of applications for their use in measuring human movement (Acuna et al., 2010; Aminian et al., 1999; Bouten et al., 1997; Crouter et al., 2006; Currie et al., 1992; Frost, 1978).
One of the more recent applications of activity monitors is measurement of limb use after injury to assess the impact of disease on movement, and the effectiveness of intervention in normalizing limb use (Acuna et al., 2010; Aminian et al., 1999; Lang et al., 2007; Uswatte et al., 2005). The non-injured extremity is often used to establish what is normal for an individual with injury. Thus, side-to-side comparisons are an integral component of the evaluation process. Statistical tests including measures of central tendency and variance are easily performed and may reveal asymmetries in limb performance. Unfortunately, these statistical tests do not provide a measurement of the asymmetry magnitude (Kaufman et al., 2012). Alternatively, an asymmetry index provides insight to side-to-side differences, and the magnitude of these differences (Kaufman et al., 1996). A measurement of the percent difference between two limbs, the index ascribes a single value to the level of asymmetry between two sides (Zifchock et al., 2008). Therefore, it quantifies the imbalance between the sides of an individual, or permits comparisons of asymmetry levels between groups.
Sample entropy is an alternative approach for assessing side-to-side differences in limb activity. Frequently utilized to evaluate physiological measures such as blood pressure and heart rate, entropy analysis has also been a component of gait evaluation across a spectrum of pathologies (Kaipust et al., 2012; Tochigi et al., 2012). Based on the concept that normal, unimpaired human movement is more complex than pathological movement, sample entropy measures the probability that two sequences remain similar at the next point of interest in a time series. This measurement of randomness indicates that lower sample entropy values represent a less random signal produced by an injured limb. A more random signal produced by a healthy limb would be associated with a higher sample entropy value. Unlike statistical measures or asymmetry indexes, sample entropy values provide insight to the presence of what is deemed desirable variability.
Identification of activity deficits of an extremity may not be appreciated with bilateral comparisons. For example, the dominant arm may be more active than the non-dominant arm in asymptomatic individuals. Thus, a side-to-side difference in activity levels may be normal. Furthermore, comparing peak or mean values between limbs may mask how much activity the limb is undergoing throughout the course of a day. Previous investigators have developed algorithms for classifying physical activity. Oshima et al. (2010) collected tri-axial accelerometer data from 63 uninjured subjects while they performed discrete activities in a laboratory setting. After analyzing the data to calculate a cutoff value, the investigators were able to correctly distinguish between household and locomotive activities more than 98% of the time (Oshima et al., 2010). While this algorithm calculation technique is useful for correlating activity monitor data with the type of activity being performed, it does not provide insight into the percentage of the day spent performing activities of different intensity. For example, one limb may be inactive or undergoing small magnitude movement accelerations for a greater percentage of the day when compared to the contralateral limb, or when compared to an uninjured individual. This may represent a gradual degradation in function. Thus, evaluating the distribution of activity intensity may be a useful means by which to assess impairment of the limbs.
Multiple analysis techniques are available for the interpretation of human movement captured by tri-axial accelerometer-based activity monitors. It is unclear what similarities or unique information may be yielded based on different analyses. Therefore, the purpose of this study was to report the results of activity monitor data collected from patients with end-stage shoulder osteoarthri-tis, and a matched group of asymptomatic subjects. Analysis methods of upper extremity activity included statistical measures of central tendency and dispersion of scores (variability), sample entropy, and asymmetry analysis. A fourth analysis method utilized in the study was a novel approach developed to evaluate differences in activity intensity during the course of a day.
2. Methods
2.1. Subjects
The study sample consisted of two groups each with 15 subjects, comprised of 15 patients scheduled to undergo shoulder arthroplasty (SA), and a control group of 15 age and gender matched individuals. All subjects were required to be between 50 and 85 years in age, have no neurological or cognitive impairments that would interfere with completion of testing or inability to follow instructions, and be an independent ambulator. All patients were required to have no other upper extremity injury or pain complaints at the time of testing. All control subjects were required to have no upper extremity injury at the time of testing, no symptoms in either upper extremity, and no history of shoulder surgery to either arm. Individuals who did not meet all participation criteria were not eligible for study enrollment. All testing procedures were approved by the Mayo Clinic Institutional Review Board. Informed consent was obtained from study participants prior to initiating data collections.
2.2. Data collection
2.2.1. Activity monitors
Custom built tri-axial accelerometry based activity monitors developed at Mayo Clinic were utilized for all data collections. Each self-contained sensor contained a tri-axial MEMS accelerometer (analog, ±16 g, Analog Devices, part number ADXL326), microcontroller (12 bit ADC, Texas Instruments), power source (Tadiran battery, semiconductor voltage regulator), and onboard data storage (NAND flash memory, 0.5 GB memory chip, Micron). The battery life capacity for each device was 10 continuous days with a sampling frequency of 100 Hz. Precision and accuracy of the devices were determined to be within ±0.09 g.
2.2.2. Procedures
Monitors were worn inside a pocket attached to Velcro straps, which were secured bilaterally to the upper arms at the mid-biceps, and the lower arms at the wrist. Data were collected over three consecutive days in subjects’ natural living environment, including work and non-work activities. Sleep time was not included in the data collection period of interest.
2.3. Data processing
2.3.1. Acceleration signal
All subsequent filtering and analysis methods were applied uniformly to the signal of all data sets and did not vary between body segments nor motor tasks. An anti-aliasing single-pole RC low-pass filter was implemented at the output of the accelerometer and before the input to the analog-to-digital converter (ADC), with the upper 3 dB cutoff frequency set to 50 Hz and a rolloff of 10 dB/decade. The ADC was set to a sampling rate of 100 samples/s with 12 bits/sample. After testing was completed data was off-loaded onto a personal computer via a USB drive for post-processing and analysis. The tri-axial raw signal was reported in the x, y, and z axes, with axis orientations defined in the anatomical position. Additionally, the raw signals were high-pass filtered using a 4th order Butterworth digital design at 0.1 Hz to remove the gravitational component (Bouten et al., 1997). All analyses were independently performed for each body segment using custom Matlab programs (MathWorks, Natick, MA).
2.3.2. Activity
The filtered signals were parsed into 60-s (1 min) epochs. For each epoch, a single value was obtained by summing the vector magnitudes of the three axes (Eq. (1)) (Hagstromer et al., 2007).
| (1) |
where n is the number of data points within a 60-s epoch and ax, ay, az are the tri-axial accelerations, respectively. Activity is in units of acceleration (m/s2) over each 60s epoch. The average activity for each limb segment was determined each day by calculating the arithmetic mean of the epoch activity value within 1 day for each subject. A within-subject ensemble average activity value was calculated for each limb segment over the 3 days of collection. Additionally, the epoch with the maximum activity (MA) was identified for each limb segment for each day analyzed.
2.3.3. Sample entropy
Sample entropy is defined as “the negative natural logarithm of an estimate of the conditional probability that subseries (epochs) of length, m, that match point wise within a tolerance, r, also match at the next point (Richman and Moorman, 2000).” The algorithm determines if subsequent data epochs match within the tolerance, ‘r’, until there is not a match and keeps track of matches in counters A(k) and B(k) for all lengths, ‘k’ up to ‘M’. The sample entropy values are calculated by
| (2) |
Sample entropy values were calculated for each acceleration signal (x, y, and z) separately for each segment on each day analyzed, and combined entropy over the three axes was determined using the vector sum of the individual sample entropy values from each axis. The selections of M and r values are those typically used in the calculation of entropy for physiologic time series (Pincus and Goldberger, 1994). Software available at PhysioNet was used to calculate the sample entropy (Goldberger et al., 2000).
2.3.4. Asymmetry index
Asymmetry between limbs for the lower arm and upper arm was quantified using the daily mean activity counts (counts/min) using the following index:
| (3) |
| (4) |
where I represents the involved limb, U is the uninvolved limb, and Ax is the asymmetry index. For control subjects, I, or involved limb, for use in Eqs. (3) and (4) was the subject’s limb that matched the dominancy of the patient’s involved limb.
The resulting asymmetry index is a unitless value between positive infinity and negative infinity, with positive values indicating greater activity in the uninvolved limb and negative values indicating greater activity in the involved limb. Smaller values represent greater symmetry (perfect symmetry = 0). The asymmetry index results can be multiplied by 100 to represent them as an asymmetry percentage. The asymmetry index was selected because it was designed to allow the data to form a normal or Gaussian distribution (Kaufman et al., 1996).
2.3.5. Activity bins
A novel technique to evaluate activity over the course of the day included the use of activity bins, which were created to calculate the amount of time subjects were inactive, and the time spent performing activities categorized as low and high intensity. To facilitate between-group comparisons, activity levels for the control and patient groups were normalized to the average maximum value of the control group. The epoch activity count data for control subjects and patients for each day was subsequently divided into activity bins based on the percentage of the control group maximum activity value (CMA) as follows:
Inactive: Activity <110m/s2/min epoch (Hagstromer et al., 2007)
Low activity: 110 m/s2/min epoch ≤ Activity ≤33% of CMA
High activity: Activity >33% of CMA
where 33% of CMA is greater than 110 m/s2 in a segment that experiences accelerations above 0.01 m/s2. The epochs in each bin were summed to determine the percentage of each day spent at the different activity levels, and the total percent time spent in each bin was averaged across days for analysis for each segment and each limb.
2.4. Statistical analysis
Measures of central tendency and dispersion were captured using the statistical mean and standard deviation for the minimum, mean, and maximum activity counts for each limb segment and group. Side-to-side within group differences were calculated for the lower and upper arm for minimum, mean and maximum activity counts, and entropy using paired t-tests. Between group differences were evaluated for the lower and upper arm for minimum, mean, and maximum activity counts with a univariate ANOVA. To account for limb dominance, the limb of interest for control subjects was matched to the involved limb of a patient. For example, if a male patient whose involved limb was his non-dominant arm, a male control subject’s non-dominant arm was utilized for comparison. Between group differences were also evaluated for the time spent across activity bins. Hotelling’s T-Square was utilized to detect statistical significance for differences in activity for the lower and upper arm between groups. A t-test was applied to the asymmetry index results to compare between the control and patient groups for the lower and upper arm separately. When statistical significance was achieved, post hoc testing was performed with a univariate ANOVA to determine where differences were occurring. Statistical testing was performed using commercially available software (SAS Institute Inc., Cary, NC). Statistical significance was established at α ≤ 0.05.
3. Results
The sample consisted of 30 subjects, including nine females and six males in each group. All of the control subjects were right-hand dominant. Two patients were left-hand dominant, and 13 right-hand dominant. There were 10 patients whose right arm was the involved limb and six with left arm involvement. There were no differences between groups for age, height, or weight (Table 1). There was also no difference between groups for total time spent wearing the activity monitors (Controls, Mean = 745 min (SD, 105); Patients, Mean = 770 min (SD, 85); P = 0.591).
Table 1.
Subject demographics by group.
| Controls | Patients | P value | |
|---|---|---|---|
| Age (Years) | 72 (6) | 66 (10) | 0.117 |
| Height (cm) | 163 (9) | 163 (7) | 0.9852 |
| Weight (kg) | 73 (14) | 80 (19) | 0.319 |
For measures of central tendency and dispersion of activity level, the control group exhibited side-to-side differences at the lower and upper arm. Both the average and maximum activity was higher in the dominant arm compared to the non-dominant arm (Table 2). Side-to-side differences within the patient group were also evident in the lower and upper arm, with the non-involved arm average and maximum activity greater than the involved arm (Table 2). There was no side-to-side difference in either group for the minimum activity of the lower or upper arm (Table 2).
Table 2.
Bilateral, within group results (Mean (SD)) for minimum, mean, and maximum activity counts (counts/min). Statistical significance * ≤ 0.05.
| Lower arm
|
Upper arm
|
|||||
|---|---|---|---|---|---|---|
| Min | Mean | Max | Min | Mean | Max | |
| Controls | ||||||
| Dominant | 53 (7) | 1096 (256) | 4136 (678) | 56 (8) | 640 (140) | 2688 (439) |
| Non-Dominant | 56 (11) | 954 (209) | 3696 (570) | 52 (8) | 573 (112) | 2518 (386) |
| P value | 0.079 | <0.001* | <0.001* | 0.197 | <0.001* | 0.046* |
| Patients | ||||||
| Involved | 55 (11) | 939 (306) | 3314 (955) | 59 (13) | 572 (183) | 2291 (572) |
| Non-Involved | 55 (12) | 1031 (324) | 3712 (7385) | 54 (10) | 655 (194) | 2723 (809) |
| P value | 0.643 | 0.044* | 0.009* | 0.128 | 0.002* | 0.049* |
Between group comparisons indicated there was a significant difference in the maximum activity of the lower arm (P = 0.05), with the patients exhibiting significantly greater activity in their involved side compared to the matched limb in control subjects (Table 2). There were no differences between groups for the average (P = 0.307) or minimum (P = 0.961) activity at the lower arm, and there were no differences between groups for activity of the upper arm (Minimum, P = 0.336; Mean, P = 0.603; Maximum, P = 0.147) (Table 2).
When evaluating side-to-side differences within groups, both groups demonstrated significant differences in entropy at the lower arm. Control subjects demonstrated significantly greater lower arm entropy on the dominant arm (Mean, 0.5306; SD, 0.16) compared to the non-dominant arm (Mean, 0.4375; SD, 0.12) (P < 0.001). Patients had significantly greater lower arm entropy on the non-involved limb (Mean, 0.4919; SD, 0.20) than the involved limb (Mean, 0.4326; SD, 0.19) (P = 0.041). For the upper arm, there was a significant side-to-side difference in entropy for patients, with the non-involved limb entropy significantly higher in the non-involved arm (Mean, 0.2436; SD, 0.12) than the involved arm (Mean, 0.1932; SD, 0.10) (P = 0.003). There was no side-to-side difference in upper arm entropy for the control group (D: Mean, 0.2352; SD, 0.08; ND: Mean, 0.2112, SD, 0.06; P = 0.370).
There were significant differences between groups based on the asymmetry index for both the lower and upper arm (Table 3). Control subjects presented with almost no asymmetry in the lower arm while the patients exhibited 13% greater mean activity in the uninvolved lower arm when compared to their involved side (Table 3). Similarly, at the upper arm control subjects exhibited low asymmetry while the patients had 17% greater mean activity in the uninvolved upper arm when compared to the involved side (Table 3).
Table 3.
Between group analysis results for Asymmetry Index (Mean (SD)). Positive values denote greater activity in the uninvolved limb while negative values reflect greater activity in the involved limb, and zero is perfect symmetry. Statistical significance * ≤ 0.05.
| Lower arm | Upper arm | |
|---|---|---|
| Controls | −0.01 (0.20) | 0.02 (0.12) |
| Patients | 0.13 (0.20) | 0.17 (0.17) |
| P value | 0.033* | 0.005* |
Between group differences were also present when evaluating the time spent across activity bins (Fig. 1). With inactive, low, and high activity bins combined in a multivariate analysis, there was a significant difference between groups for the upper arm (P = 0.035). Post hoc analysis indicated differences were occurring for the amount of time spent in inactivity, with patients not using their involved upper arm 20% of the time, compared to 14% of inactive time for the matched upper arm of control subjects (P = 0.013) (Fig. 1). Control subjects spent a greater amount of time in the low and high activity bins compared to patients (Fig. 1). Neither of these differences, however, were statistically significant (P = 0.477). There were no differences between groups for time spent across activity bins for the lower arm (P = 0.125) (Fig. 1).
Fig. 1.
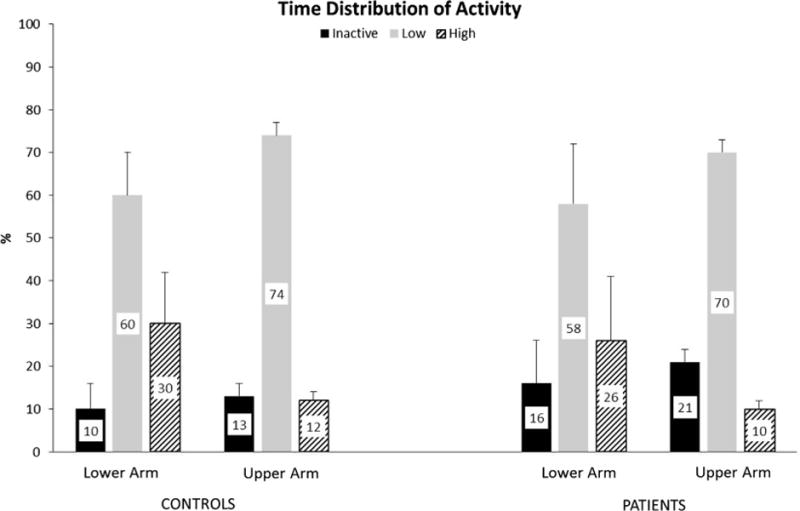
Time distribution (%) of activity for the lower and upper arm in control subjects and patients including time spent inactive, and performing low and high intensity activities. Mean values are displayed within the larger bar, while the thin vertical bars represent the standard deviation.
4. Discussion
To date, there is no consensus regarding which analysis technique to use or what type of information the researcher or clinician may wish to ascertain from data obtained from tri-axial accelerometers worn on the extremities. This study has reported the results of multiple analyses methods for evaluating upper extremity function. Each analysis method provides a different means by which to interpret tri-axial accelerometer data. Ultimately, selection of one or more analysis methods is dependent on the questions of interest. Additionally, data from this study indicate a side-to-side comparison alone is inadequate when evaluating upper extremity function in patients, as an effect of limb dominance was present in control subjects. Finally, these data support distinguishing between the upper and lower arm, as the activity level of each segment was variable in control subjects and patients.
We performed a novel analysis that evaluated the time distribution of activity intensity. These activity bins differentiated the amount of time subjects spent in inactivity, and performing low and high intensity activities. Defining the maximum value, and thus the range for the low and high intensity bins, was based on the control group. Control subjects were matched to patients for gender and age. Thus, interpreting the time spent in activities of low and high intensity for patients was based on a group of asymptomatic peers. Furthermore, the limb of interest for activity bin analysis was chosen based on whether the dominant or non-dominant extremity of the patient was involved. The activity bin data revealed there was a greater amount of inactivity time for the upper arm of patients compared to control subjects. While the amount of time control subjects spent in low and high intensity activities was greater than patients, these values did not reach statistical significance. In contrast, measures of central tendency and variability did not identify any differences between groups for the upper arm, and the only difference between groups for the lower arm was the maximum activity level.
The within subject entropy results highlighted the role of dominancy in the control subjects and the effect of impairments in the patient group. In the control group the dominant limb had greater entropy than the non-dominant limb at the lower arm. There was no difference, however, at the upper arm when comparing limbs. As the main function of the shoulder and elbow (upper arm) are to position the hand in space (Peat, 1986), it may be expected that effects of dominancy would be more readily detected at the lower arm, near the hand. For the patient group, the restrictions in movement as a result of joint pain on the involved side resulted in statistically greater entropy values in the uninvolved lower and upper arm. These results emphasize the utility of the entropy measure to detect pathological movement when performing side-to-side comparisons.
Calculating and interpreting inactivity has previously been described (Hagstromer et al., 2007). Inactivity time is useful in providing insight into movement avoidance. Previous investigators have attempted to distinguish between activities of varying intensity based on classification algorithms from data obtained in controlled environments (de Vries et al., 2011; Oshima et al., 2010; Xia et al., 2011). One of the challenges of extended collections in an individual’s natural living environment is the inability to correlate specific activities with the recorded data. Rather than attempting to identify specific activities that were performed, we suggest differentiating time spent in activities of different intensities may provide meaningful insight of limb function. Sustained avoidance of high intensity activities may represent a shrinking envelope of function associated with more severe clinical impairments. Conversely, an increase in activity intensity after intervention may equate to an expanding range of function associated with clinical improvements in range of motion, strength, or pain. Additionally, if a patient spends less time performing high intensity activities there is the potential to create a vicious circle of disuse, which contributes to limitations in motion and strength. Consequently the patient is unable to perform high intensity activities, and their envelope of function gradually shrinks. Future studies that longitudinally correlate tri-axial accelerometer data with clinical measures will be necessary to confirm these hypotheses and facilitate clinical interventions designed to improve patient function.
There are limitations to this study. It is possible the results of the analyses may be different when evaluating the lower extremities. For example, asymmetries may not be present in asymptomatic individuals. In this case a side-to-side comparison within patients may be appropriate. There may be no differences in activity levels in the upper and lower segments in the legs. This would justify the use of only one monitor per lower extremity. Inclusion of patients with varying diagnoses may have yielded alternative results. The intention of the study, however, was to highlight the findings of different analyses methods. Thus, we do not believe the diagnosis had a meaningful impact on the findings. Finally, precise activities the study participants performed were not documented to validate the classification of low and high intensity activities. Although tri-axial acceleration based activity monitors are a good marker of function, it is important to note these data are limited in nature and do not reflect the range of motion or the plane of motion through which the limb segment is moving.
5. Conclusions
This study highlights the unique information provided by multiple analysis methods when interpreting data collected from tri-axial accelerometers, including a novel approach of evaluating the distribution of time spent across variable intensity activities. Clinicians and researchers evaluating upper extremity function should be mindful of the effect of limb dominance, and include a control group to aide in the interpretation of limb performance in patients. Additionally, activity may not be equivocal in the lower and upper arm. It is therefore recommended activity monitors be worn on both segments to gain greater insight into upper extremity function.
Acknowledgments
Funding for this project was provided by the National Institutes of Health (Grant T-32 HD00447), the Arthritis Foundation, and Mayo Clinic. Tri-axial accelerometers were provided by Dr. Barry K. Gilbert, James E. Bublitz, Kevin J. Buchs, Charles A. Burfield, Christopher L. Felton, Dr. Clifton R. Haider, Michael J. Lorsung, Shaun M. Schreiber, Steven J. Schuster, and Daniel J. Schwab from the Special Purpose Processor Development Group at Mayo Clinic.
Biographies

Hurd is a Research Associate in the Motion Analysis Laboratory, Department of Orthopaedics at the Mayo Clinic in Rochester, MN. She received her undergraduate degree (physical therapy) from the University of Missouri-Columbia, master and doctoral degrees (both in biomechanics and movement science) from the University of Delaware, and completed her post-doctoral (upper extremity biomechanics) training at Mayo Clinic. Dr. Hurd is a physical therapy board certified sports specialist. Her research emphasis is neuromuscular contributions to dynamic joint stability, and clinically she specializes in sports injuries to the shoulder, knee, and elbow.

Melissa M.B. Morrow is a research associate at Mayo Clinic. She received her B.S.E. in Biomedical Engineering at Tulane University in 2003 and doctoral degree in Biomedical Engineering at Mayo Clinic College of Medicine in 2009. Her post-doctoral research is focused using activity and motion monitoring in the field as evidence-based outcome measures following orthopedic surgery in addition to investigating sex differences in knee osteoarthritis.

Kenton Kaufman is the W Hall Wendel Jr Musculoskeletal Research Professor, Director of the Biomechanics-Motion Analysis Laboratory, Professor of Bioengineering, and Consultant in the Departments of Orthopedic Surgery, Physiology and Biomedical Engineering at the Mayo Clinic. He is a registered professional engineer. He received his Ph.D. degree in biomechanical engineering from North Dakota State University in 1988. Dr. Kaufman’s research focuses on musculoskeletal rehabilitation science. He currently holds several grants from NIH and DOD, with projects aimed at improving the mobility of disabled individuals. He has published over 180 scientific papers, 36 book chapters, and holds 6 patents. He is a Fellow in the American Institute for Medical and Biological Engineering and the American Society of Biomechanics.
Footnotes
6. Conflict of Interest Statement
None of the authors of financial or personal relationships to disclose.
References
- Acuna M, Amasay T, Karduna AR. The reliability of side to side measurements of upper extremity activity levels in healthy subjects. BMC Musculoskelet Disord. 2010;11:168. doi: 10.1186/1471-2474-11-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminian K, Rezakhanlou K, De Andres E, Fritsch C, Leyvraz PF, Robert P. Temporal feature estimation during walking using miniature accelerometers: an analysis of gait improvement after hip arthroplasty. Med Biol Eng Comput. 1999;37(6):686–91. doi: 10.1007/BF02513368. [DOI] [PubMed] [Google Scholar]
- Bouten CV, Koekkoek KT, Verduin M, Kodde R, Janssen JD. A triaxial accelerometer and portable data processing unit for the assessment of daily physical activity. IEEE Trans Biomed Eng. 1997;44(3):136–47. doi: 10.1109/10.554760. [DOI] [PubMed] [Google Scholar]
- Crouter SE, Clowers KG, Bassett DR., Jr A novel method for using accelerometer data to predict energy expenditure. J Appl Physiol. 2006;100(4):1324–31. doi: 10.1152/japplphysiol.00818.2005. [DOI] [PubMed] [Google Scholar]
- Currie G, Rafferty D, Duncan G, Bell F, Evans AL. Measurement of gait by accelerometer and walkway: a comparison study. Med Biol Eng Comput. 1992;30(6):669–70. doi: 10.1007/BF02446803. [DOI] [PubMed] [Google Scholar]
- de Vries SI, Engels M, Garre FG. Identification of children’s activity type with accelerometer-based neural networks. Med Sci Sports Exerc. 2011;43(10):1994–9. doi: 10.1249/MSS.0b013e318219d939. [DOI] [PubMed] [Google Scholar]
- Frost JD., Jr Triaxial vector accelerometry: a method for quantifying tremor and ataxia. IEEE Trans Biomed Eng. 1978;25(1):17–27. doi: 10.1109/TBME.1978.326372. [DOI] [PubMed] [Google Scholar]
- Goldberger AL, Amaral LA, Glass L, et al. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation. 2000;101(23):E215–220. doi: 10.1161/01.cir.101.23.e215. [DOI] [PubMed] [Google Scholar]
- Hagstromer M, Oja P, Sjostrom M. Physical activity and inactivity in an adult population assessed by accelerometry. Med Sci Sports Exerc. 2007;39(9):1502–8. doi: 10.1249/mss.0b013e3180a76de5. [DOI] [PubMed] [Google Scholar]
- Kaipust JP, Huisinga JM, Filipi M, Stergiou N. Gait variability measures reveal differences between multiple sclerosis patients and healthy controls. Motor Control. 2012 doi: 10.1123/mcj.16.2.229. [DOI] [PubMed] [Google Scholar]
- Kaufman KR, Miller LS, Sutherland DH. Gait asymmetry in patients with limb-length inequality. J Pediatr Orthop. 1996;16(2):144–50. doi: 10.1097/00004694-199603000-00002. [DOI] [PubMed] [Google Scholar]
- Kaufman KR, Frittoli S, Frigo CA. Gait asymmetry of transfemoral amputees using mechanical and microprocessor-controlled prosthetic knees. Clin Biomech (Bristol, Avon) 2012 doi: 10.1016/j.clinbiomech.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang CE, Wagner JM, Edwards DF, Dromerick AW. Upper extremity use in people with hemiparesis in the first few weeks after stroke. J Neurol Phys Ther. 2007;31(2):56–63. doi: 10.1097/NPT.0b013e31806748bd. [DOI] [PubMed] [Google Scholar]
- Matthews CE, Ainsworth BE, Thompson RW, Bassett DR., Jr Sources of variance in daily physical activity levels as measured by an accelerometer. Med Sci Sports Exerc. 2002;34(8):1376–81. doi: 10.1097/00005768-200208000-00021. [DOI] [PubMed] [Google Scholar]
- Oshima Y, Kawaguchi K, Tanaka S, et al. Classifying household and locomotive activities using a triaxial accelerometer. Gait Posture. 2010;31(3):370–4. doi: 10.1016/j.gaitpost.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Peat M. Functional anatomy of the shoulder complex. Phys Ther. 1986;66(12):1855–65. doi: 10.1093/ptj/66.12.1855. [DOI] [PubMed] [Google Scholar]
- Pincus SM, Goldberger AL. Physiological time-series analysis: what does regularity quantify? Am J Physiol. 1994;266(4 Pt 2):H1643–1656. doi: 10.1152/ajpheart.1994.266.4.H1643. [DOI] [PubMed] [Google Scholar]
- Richman JS, Moorman JR. Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol. 2000;278(6):H2039–2049. doi: 10.1152/ajpheart.2000.278.6.H2039. [DOI] [PubMed] [Google Scholar]
- Tochigi Y, Segal NA, Vaseenon T, Brown TD. Entropy analysis of tri-axial leg acceleration signal waveforms for measurement of decrease of physiological variability in human gait. J Orthop Res. 2012;30(6):897–904. doi: 10.1002/jor.22022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uswatte G, Foo WL, Olmstead H, Lopez K, Holand A, Simms LB. Ambulatory monitoring of arm movement using accelerometry: an objective measure of upper-extremity rehabilitation in persons with chronic stroke. Arch Phys Med Rehabil. 2005;86(7):1498–501. doi: 10.1016/j.apmr.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Ward DS, Evenson KR, Vaughn A, Rodgers AB, Troiano RP. Accelerometer use in physical activity: best practices and research recommendations. Med Sci Sports Exerc. 2005;37(11 Suppl):S582–588. doi: 10.1249/01.mss.0000185292.71933.91. [DOI] [PubMed] [Google Scholar]
- Xia Y, Cheung V, Garcia E, Ding H, Karunaithi M. Development of an automated physical activity classification application for mobile phones. Stud Health Technol Inform. 2011;168:188–94. [PubMed] [Google Scholar]
- Zifchock RA, Davis I, Higginson J, Royer T. The symmetry angle: a novel, robust method of quantifying asymmetry. Gait Posture. 2008;27(4):622–7. doi: 10.1016/j.gaitpost.2007.08.006. [DOI] [PubMed] [Google Scholar]


