Abstract
Background Context
The prevalence of lumbar spinal stenosis (LSS) in the general population and association with low back pain (LBP) remains unclear.
Purpose
1) to evaluate the prevalence of congenital and acquired LSS observed on computed tomography (CT) in a community-based sample; 2) to evaluate the association between LSS and LBP.
Study design/Setting
Cross-sectional observational study. This study was an ancillary project to the Framingham Heart Study.
Sample
3529 participants underwent multi-detector CT. 191 were enrolled in this study.
Outcome Measures
Self-report Measures
LBP in the preceding 12 months was evaluated using a self-report questionnaire.
Physiologic Measures
LSS (congenital and acquired) was characterized using two cut-points: 12 mm for relative LSS, and 10 mm for absolute LSS.
Methods
Using multiple logistic regression we examined the association between LSS and LBP, adjusting for sex, age and BMI.
Results
In the congenital group, relative LSS was found in 4.7% and absolute LSS in 2.6% of patients. Acquired LSS was found in 22.5% and in 7.3%, respectively. Acquired LSS showed increasing prevalence with age: <40 years, the prevalence of relative and absolute LSS was 20.0% and 4.0%, respectively; in those 60–69 years the prevalence was 47.2% and 19.4%, respectively. The presence of absolute LSS was associated with LBP with an odds ratio of 3.16 (95% CI: 1.05–9.53).
Conclusions
The prevalence of congenital and acquired LSS in a community-based sample was characterized. The prevalence of acquired stenosis increased with age. LSS is associated with a three-fold higher risk of experiencing LBP.
Keywords: spinal stenosis, low back pain, computed tomography, spine, prevalence, community-based sample
Introduction
Despite the fact that lumbar spinal stenosis (LSS) is one of the most commonly diagnosed and treated pathologic conditions affecting the spine, very little is known about the epidemiology of stenosis in the general population. The prevalence of acquired, so-called “degenerative” lumbar stenosis has been suggested as ranging from 1.7 to 13.1% [1–3]. However, previously reported studies have utilized asymptomatic populations or selected clinical populations undergoing imaging studies for other reasons. Furthermore, the methods of LSS evaluation were often not described or inappropriate. There have been no prevalence rates reported in unselected populations. Given the increasing use of diagnostic imaging, the unclear association of spinal stenosis radiographic findings with symptoms, and the fact that spinal stenosis is one of the most common indications for spinal surgery a clear understanding of the prevalence of spinal stenosis in the community and its association with symptoms is greatly needed.
One major difficulty in performing any epidemiologic analysis is the absence of universally accepted diagnostic criteria for spinal stenosis [4]. Magnetic resonance imaging (MRI) and computed tomography (CT) are the most frequently utilized diagnostic modalities in clinical practice, but strict measurements defining the presence of clinically significant canal, subarticular, or foraminal narrowing do not exist. The concept that overall rates of spinal stenosis reflect the relative contribution of two distinct types of stenosis, congenital (or developmental) and acquired (or degenerative), is however generally acknowledged [5].
Recognizing these limitations, the aims of the present study were: 1) to evaluate the prevalence of congenital and acquired radiographic spinal stenosis in an unselected community-based population; and 2) to evaluate the association between spinal stenosis observed on CT and the risk of experiencing low back pain (LBP).
Methods
Study design
Cross-sectional study.
Sample
This study was an ancillary project to the Framingham Heart Study. The Framingham Heart Study began in 1948 as a longitudinal population-based cohort study of the causes of heart disease. Initially, 5209 men and women between the ages of 30 and 60 years living in Framingham, Massachusetts were enrolled. Biennial examinations were conducted by trained research staff at the study clinic located in Framingham. In 1971, 5124 offspring (and their spouses) of the original cohort were entered into the Offspring cohort. In 2002, 4095 men and women who were children of the Offspring cohort were enrolled in the Third Generation cohort. A description of the Offspring and Third Generation cohorts has been previously reported [6]. 3590 participants of the Framingham study (participants in both the Offspring and Third Generation cohorts) aged 40–80 years underwent abdominal and chest multi-detector CT scanning to assess coronary and aortic calcification. The recruitment and conduct of CT scanning have also been previously reported [7]. During the latter part of the CT study, 191 participants were consecutively enrolled in this ancillary study to assess the association between radiographic features of the lumbosacral spine and LBP. We were limited to this size sample due to concerns about participant burden from the parent heart study investigators.
LBP evaluation
All study participants who underwent multi-detector CT scan were asked to complete the modified Nordic Low Back Questionnaire [8]. This validated questionnaire defines significant LBP as “low back pain on most days of at least one month in the last 12 months” and is widely used in studies of work-related LBP. In addition, participants were asked if they experienced pain in the buttocks or thighs, pain in a lower leg (below the knee), numbness or tingling in the leg or foot, or weakness in the leg or foot.
Imaging parameters
Study participants were imaged with an eight-slice multi-detector CT scanner (Lightspeed Ultra, GE, Milwaukee, WI, USA). Each subject underwent unenhanced abdominal multi-detector CT performed using a sequential scan protocol with a slice collimation of 8 mm × 2.5 mm (120 KVp, 320/400 mA for .220 lbs body weight, respectively) during a single end-inspiratory breath hold. For the abdominal scan, thirty contiguous 2.5-mm thick slices of the abdomen were acquired covering 150 mm above the level of S1.
Spinal stenosis evaluation
CT scans were evaluated in a blinded fashion with respect to clinical and demographic data. The axial plane was used for measurements of congenital and acquired LSS. Bone windows were used for measurements of congenital LSS. The antero-posterior diameter of the spinal canal was measured at the mid-vertebral body level. This level was selected on the basis of providing the most readily recognizable landmark for measurement of the bony canal and is considered more precise than the mid-sagittal view commonly used in clinical practice due to avoidance of inaccurate measurements resulting from scoliosis or improper patient positioning [9]. Measurements for congenital LSS were obtained for vertebral levels L2 to L5 and for acquired LSS - for L2/L3, L3/L4, L4/L5 and L5/S1 levels. For measurements of acquired LSS, soft tissue windows were used. The antero-posterior diameter of the canal at the level of the intervertebral disc was measured (between intervertebral disc, anteriorly and meeting point of two ligamenta flava, posteriorly (Figure 1)). Measurements were obtained for the L2/L3 to L5/S1 disc levels.
Figure 1.
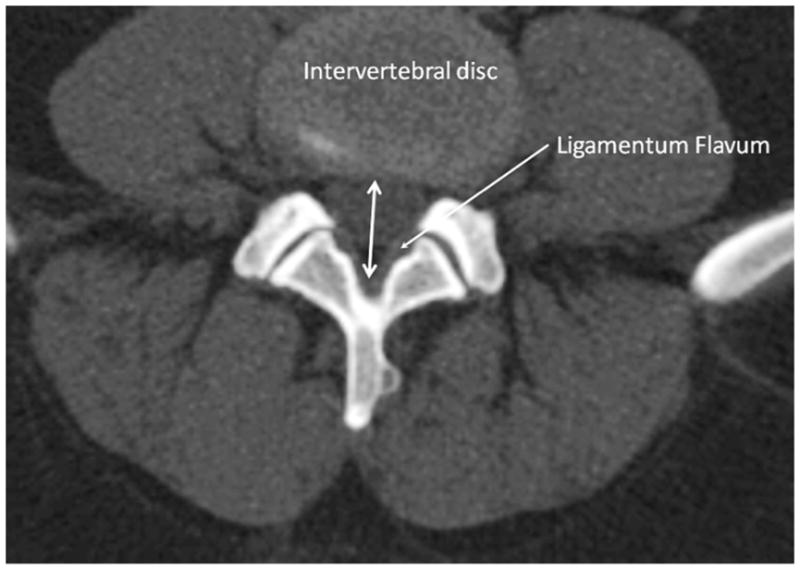
Schema of measurement of spinal canal at the level of intervertebral disc (acquired stenosis).
As previously explained there are no universally accepted definitions of LSS and no generally accepted radiologic diagnostic criteria. For the purposes of this study, two previously proposed threshold measurements were selected to identify the presence of spinal stenosis in the study population: ≤12 mm (“relative” stenosis) and ≤10 mm (“absolute” stenosis) for both congenital [10] and acquired [11, 12] types of stenosis. Although somewhat arbitrary, these cutoffs have been utilized in multiple studies [10–16].
Reliability of CT readings
A reading protocol for evaluation of spinal stenosis based on the previously defined measurements was developed. Using this protocol, intra- and inter-rater reliability was calculated for two readers. To evaluate for reader-drift, intra-rater reliability was periodically reassessed by inserting one repeated “reliability” scan for every 10 new scans. Before analyzing each new set of CT scans, 5 previously analyzed CTs were reevaluated to “recalibrate” the readings to a standard. Intra-observer reliability varied at different spinal levels between 0.95 and 0.98 for congenital LSS and between 0.92 and 0.98 for acquired LSS. The inter-observer reliability ranged from 0.80 to 0.92 and from 0.86 to 0.96, respectively. This range of kappa statistics represents fair to excellent reproducibility that is much higher than was previously reported in studies of non-quantitative measures [15, 17].
Body mass index (BMI)
BMI was computed as the ratio of weight (in kg) divided by height (in square meters).
Statistical analysis
The prevalence of congenital LSS, acquired LSS, and LSS of either type was calculated. The association between spinal stenosis and age, sex and BMI was also evaluated using simple logistic regression analyses.
Stepwise multiple logistic regression analyses were used to examine the association between LBP, as a dependent variable, and spinal stenosis (relative and absolute), age, sex and BMI, as independent variables. All statistical analyses were performed using SAS software, (SAS Institute Inc, Cary, NC, release 9.1).
Results
The study sample included 191 study participants, 104 (55.6%) males and 87 (44.4%) females. The mean age was 52.6±10.8 (age range: 32–79) and the mean BMI was 27.8±5.0. This subsample was representative of the whole group of individuals that underwent multi-detector CT scanning (N=3590). The percent of males in whole sample was 51.8%, mean age 52.7±11.8 and mean BMI 27.8±5.3. The comparison tests showed that there was no difference between the whole sample and the sub-sample studied here in age (p=0.95), BMI (p=0.92) or prevalence of males (p=0.31). In our sub-sample 37 (19.4%) subjects reported experiencing LBP on most days of at least one month in the last 12 months. Among those who complained of LBP, 14 (37.8%) reported pain in a buttocks or thighs, 13 (35.1%) reported pain in lower leg, 8 (21.6%) numbness in the leg or foot, and 5 (13.5%) weakness in the leg or foot. There was an overlap between subjects with these distal symptoms. In total, among 37 subjects that complained of LBP, 28 also complained also of distal symptoms.
The prevalence rates for both congenital and acquired LSS in the study population are shown in Table 1. Congenital LSS was observed in 4.7% of the population in relative terms (12 mm limit) and in 2.6% in absolute terms (10 mm limit). Acquired LSS was found in 22.5% in relative terms and in 7.3% in absolute terms. When subjects with any type of LSS (congenital and/or acquired) were totaled, the prevalence of relative LSS was 23.6% and of absolute LSS 8.4%. Further analysis of the subsample of asymptomatic individuals demonstrated that congenital LSS occurred in 4.0% for relative and 2.7% for absolute LSS; very similar to general sample.
Table 1.
Prevalence of congenital and acquired lumbar spinal stenosis in the population-based sample.
| Type of LSS | Relative (≤12 mm) (N=191) |
Absolute (≤10 mm) (N=191) |
||
|---|---|---|---|---|
| N | % (95% CI) | N | % (95% CI) | |
| Congenital | 9 | 4.71% (2.18–8.76%) | 5 | 2.62% (0.86–6.00%) |
| Acquired | 14 | 22.50% (16.80–29.10%) | 14 | 7.30% (4.07–11.99%) |
| Any type | 45 | 23.60% (17.73–30.23%) | 16 | 8.40% (4.86–13.25%) |
However for acquired LSS the numbers were lower: 20.5% and 4.6%, respectively. For any type of LSS the prevalence was 22.5% and 6.0% respectively.
Prevalence of LSS of any type in subsample of individuals with LBP was 29.7% for relative and 18.9% for absolute LSS.
In the studied population there were 2 subjects with congenital and 10 subjects with acquired LSS that suffered from LBP. 35 subjects without congenital LSS and 27 subjects without acquired LSS had LBP. 7 subjects with congenital LSS and 33 subjects with acquired LSS did not suffer from LBP. 147 subjects without congenital LSS and 121 subjects without acquired LSS did not have LBP.
Analysis of the association between LSS (of any type, congenital or/and acquired) and age, sex, and BMI (Table 2) showed no statistically significant association between sex and LSS (p=0.87 for relative LSS, and p=0.72 for absolute LSS). Significant positive associations were observed between relative LSS and age as well as between relative LSS and BMI; however, these associations were non-significant for absolute LSS.
Table 2.
Association between lumbar spinal stenosis with age, sex and BMI (results of simple logistic regression analyses).
| Association | β | OR | 95%CI for OR | P | |
|---|---|---|---|---|---|
| Relative LSS and Age | 0.036 | 1.037 | 1.004 | 1.071 | 0.026 |
| Relative LSS and Sex* | 0.028 | 1.058 | 0.537 | 2.087 | 0.870 |
| Relative LSS and BMI | 0.077 | 1.080 | 1.012 | 1.152 | 0.021 |
| Absolute LSS and Age | 0.032 | 1.032 | 0.983 | 1.084 | 0.201 |
| Absolute LSS and Sex* | −0.098 | 0.823 | 0.280 | 2.412 | 0.722 |
| Absolute LSS and BMI | 0.064 | 1.066 | 0.977 | 1.162 | 0.149 |
Sex: Female vs. Male (in the dataset males were marked 1 and females 2).
Statistically significant associations at p≤0.05 level are marked bold.
To demonstrate the change of LSS prevalence (congenital and acquired) with age we divided the sample to four age groups: <40, 40–49, 50–59, 60+. The prevalence of congenital LSS did not change with age (p=0.455 for relative LSS, and P=0.601 for absolute LSS). However, as expected, the prevalence of acquired LSS increased with age (p=0.015 for relative LSS, and P=0.034 for absolute LSS) in the study population (Figure 2). The prevalence of relative and absolute LSS increased from 16.0% to 38.8% and from 4.0% to 14.3% between age <40 years and 60+ years, respectively.
Figure 2.
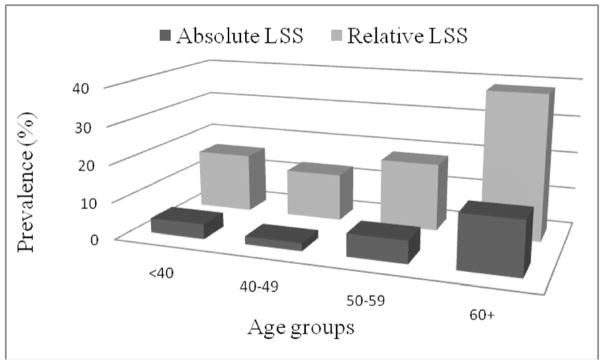
Prevalence of individuals with acquired LSS (%) according the age groups.
The results of multiple logistic regression analyses revealed that age, sex and BMI as well as relative LSS were not significantly associated with LBP; and therefore these variables were removed from the model (p>0.05). However, absolute LSS was found to be significantly associated with LBP in the study population: OR=3.16 (95% CI: 1.05–9.53; p=0.042).
Discussion
Although LSS is one of the most commonly diagnosed spinal disorders, is the major reason for surgery in older adults [18], and is considered a major cause of pain and disability, little is known regarding the epidemiology of this condition. This is the first cross-sectional study to describe the prevalence of radiographic LSS in a community-based population. There are few previously published reports that include any estimate of the population prevalence of LSS. De Villiers and Booysen [1] in a report of 850 myelograms with a water-soluble contrast medium found a 6% prevalence of LSS. However, the authors did not accurately describe the study sample and did not explicitly define their criteria for diagnosing radiographic LSS. Fanuele et al. [3] reported a prevalence of 13.1% among 17,744 patients who presented for evaluation and treatment at multiple spine centers. This study of a selected patient population utilized a multicenter clinical database to identify patients self-reporting their diagnosis on an initial visit questionnaire. No information was provided regarding how LSS diagnosis was established.
There have been multiple studies reporting the occurrence of spinal stenosis in asymptomatic individuals [19, 20]. In an MRI study of 67 individuals who had never had LBP, sciatica, or neurogenic claudication, Boden and colleagues [19] found LSS in one percent of individuals younger than 60, and 21% in individuals over 60 years old. Wiesel and colleagues [20] reported that among 52 asymptomatic individuals over 40 years of age, 50% of CT scans were abnormal with the most common diagnoses being LSS and facet degeneration. Haig and colleagues [16] found 23% prevalence of LSS (11.5 mm cut-point) in 31 asymptomatic individuals. The prevalence of relative LSS in our sample of asymptomatic individuals is very close to those presented by Haig and colleagues [16]. However, even clinically more relevant, absolute LSS was prevalent in 6.0% of asymptomatic individuals vs. 18.9% in individuals with LBP. Our study showed no difference in prevalence of radiographic LSS between men and women. To our knowledge, there are no previous studies regarding sex differences in prevalence of radiographic spinal stenosis in the general population. Jansson and colleagues [21] published statistics of spinal stenosis surgery in Sweden between 1987 and 1999. Among 11,283 cases, no statistically significant differences between sexes were found. No difference was found also among 2751 patients admitted to William Beaumont Hospital, Royal Oak, Michigan with symptomatic LSS [22]. Therefore, we conclude that there is no significant sex difference in the general population prevalence of LSS.
Consistent with previous reports and consensus opinion, our results demonstrate that the prevalence of acquired LSS increases with age.
Verbiest measured the mid-sagittal diameter of the lumbar canal at operation and proposed two major types of stenosis [10]: absolute stenosis, with diameter 10 mm or less; and relative stenosis with diameters ranging from 10 to 12 mm. In a CT study, the same author suggested that midsagittal lumbar canal diameters less than 10 mm represent absolute stenosis and diameters less than 13 mm represent relative stenosis [13]. Ulrich and colleagues suggested that the antero-posterior diameter of the spinal canal (measured on axial plain CT) less than 11.5 mm is small [9]. In another CT study, Lee and colleagues [23] reported that the sagittal diameter of the lumbar spinal canal is never smaller than 10 mm in a normal spine. Schonstrom and associates [24] suggested the cross-sectional area of the dural sac to be a more reliable diagnostic measure and defined cross-sectional areas of greater than 100 mm2 at the narrowest point as normal, 76 to 100 mm2 as moderately stenotic, and less than 76 mm2 as severely stenotic. Taking into account the high correlation (r=0.75) between the sagittal and transverse diameters of the dural sac [24], a sagittal diameter more than 10 mm was defined as normal, 8 to 10 mm as moderately stenotic, and less than 8 mm as severely stenotic. Haig et al. [16] demonstrated that antero-posterior measurements of the spinal canal (using 11.95 mm as a threshold) can distinguish between patients with clinical spinal stenosis and asymptomatic individuals. Jarvik and colleagues [25] also found that severe LSS (gestalt measure) is less common in individuals without LBP and is likely to be diagnostically and clinically relevant. In our community-based study, individuals with sagittal canal diameters ≤10 mm showed a statistically significant association with the occurrence of LBP (OR=3.16 (95% CI: 1.05–9.53)).
One concern of this study was the use of CT images as opposed to MRI images in identifying the presence of spinal stenosis. However, although MRI currently appears to be the imaging modality of choice, for the purposes of the current study, and in regards to previously published literature, CT is a reasonable alternative for the evaluation of lumbar stenosis [26–28]. The purpose of this study was not to argue for a change in standard imaging modality but merely to better define the epidemiology of spinal stenosis. Moreover, the reasonableness of CT use in this context is supported by the excellent intra- and inter-rater reliability of the measurements.
There are several limitations of the present study. First, use of the antero-posterior diameter of the spinal canal can lead to underestimation of the prevalence of spinal stenosis, for example, in patients with trefoil shape of the spinal canal [29]. Second, the LBP index used in this study, although the same measure utilized in work-related LBP studies, is relatively insensitive and does not provide more specific symptomatic data such as pain radiation, pain prolongation and severity, which should be evaluated in future studies.
Conclusions
The prevalence of congenital LSS in the US community based sample is 4.71% and 2.62% for relative and absolute stenosis, respectively. The prevalence of relative and absolute acquired stenosis increases with age to 47.2% and 19.4%, respectively, in the 60–69 year-old age group. The very high prevalence of stenosis in the general population aged over 60 years warns against attributing pain and neurological symptoms in this patient population to LSS based solely on the appearance of radiologic imaging studies. Nevertheless, absolute stenosis (sagittal diameter < 10mm) does appear associated with a three-fold higher risk of experiencing LBP.
Acknowledgments
From the Framingham Heart Study of the National Heart Lung and Blood Institute of the National Institutes of Health and Boston University School of Medicine. This work was supported by the National Heart, Lung and Blood Institute’s Framingham Heart Study contract (No. N01-HC-25195) for the recruitment, enrollment, and examination of the Offspring and Third Generation Cohort and the imaging by computed tomography scan.
L.K. is supported by an Arthritis Foundation Postdoctoral Grant.
Footnotes
Conflict of interest statement: None of the authors have any conflict of interest regarding the contents of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.De Villiers PD, Booysen EL. Fibrous spinal stenosis. A report on 850 myelograms with a water-soluble contrast medium. Clin Orthop Relat Res. 1976:140–144. [PubMed] [Google Scholar]
- 2.Roberson GH, Llewellyn HJ, Taveras JM. The narrow lumbar spinal canal syndrome. Radiology. 1973;107:89–97. doi: 10.1148/107.1.89. [DOI] [PubMed] [Google Scholar]
- 3.Fanuele JC, Birkmeyer NJ, Abdu WA, Tosteson TD, Weinstein JN. The impact of spinal problems on the health status of patients: have we underestimated the effect? Spine. 2000;25:1509–1514. doi: 10.1097/00007632-200006150-00009. [DOI] [PubMed] [Google Scholar]
- 4.Gunzburg R, Keller TS, Szpalski M, Vandeputte K, Spratt KF. A prospective study on CT scan outcomes after conservative decompression surgery for lumbar spinal stenosis. J Spinal Disord Tech. 2003;16:261–267. doi: 10.1097/00024720-200306000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Arnoldi CC, Brodsky AE, Cauchoix J, Crock HV, Dommisse GF, Edgar MA, et al. Lumbar spinal stenosis and nerve root entrapment syndromes. Definition and classification. Clin Orthop Relat Res. 1976:4–5. [PubMed] [Google Scholar]
- 6.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann U, Siebert U, Bull-Stewart A, Achenbach S, Ferencik M, Moselewski F, et al. Evidence for lower variability of coronary artery calcium mineral mass measurements by multi-detector computed tomography in a community-based cohort--consequences for progression studies. Eur J Radiol. 2006;57:396–402. doi: 10.1016/j.ejrad.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 8.Kuorinka I, Jonsson B, Kilbom A, Vinterberg H, Biering-Sorensen F, Andersson G, et al. Standardised Nordic questionnaires for the analysis of musculoskeletal symptoms. Appl Ergon. 1987;18:233–237. doi: 10.1016/0003-6870(87)90010-x. [DOI] [PubMed] [Google Scholar]
- 9.Ullrich CG, Binet EF, Sanecki MG, Kieffer SA. Quantitative assessment of the lumbar spinal canal by computed tomography. Radiology. 1980;134:137–143. doi: 10.1148/radiology.134.1.7350593. [DOI] [PubMed] [Google Scholar]
- 10.Verbiest H. Pathomorphologic aspects of developmental lumbar stenosis. Orthop Clin North Am. 1975;6:177–196. [PubMed] [Google Scholar]
- 11.Bolender NF, Schonstrom NS, Spengler DM. Role of computed tomography and myelography in the diagnosis of central spinal stenosis. J Bone Joint Surg Am. 1985;67:240–246. [PubMed] [Google Scholar]
- 12.Sortland O, Magnaes B, Hauge T. Functional myelography with metrizamide in the diagnosis of lumbar spinal stenosis. Acta Radiol Suppl. 1977;355:42–54. [PubMed] [Google Scholar]
- 13.Verbiest H. The significance and principles of computerized axial tomography in idiopathic developmental stenosis of the bony lumbar vertebral canal. Spine. 1979;4:369–378. doi: 10.1097/00007632-197907000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Eisenstein S. Measurements of the lumbar spinal canal in 2 racial groups. Clin Orthop Relat Res. 1976:42–46. [PubMed] [Google Scholar]
- 15.Drew R, Bhandari M, Kulkarni AV, Louw D, Reddy K, Dunlop B. Reliability in grading the severity of lumbar spinal stenosis. J Spinal Disord. 2000;13:253–258. doi: 10.1097/00002517-200006000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Haig AJ, Geisser ME, Tong HC, Yamakawa KS, Quint DJ, Hoff JT, et al. Electromyographic and magnetic resonance imaging to predict lumbar stenosis, low-back pain, and no back symptoms. J Bone Joint Surg Am. 2007;89:358–366. doi: 10.2106/JBJS.E.00704. [DOI] [PubMed] [Google Scholar]
- 17.Coste J, Judet O, Barre O, Siaud JR, Cohen de Lara A, Paolaggi JB. Inter- and intraobserver variability in the interpretation of computed tomography of the lumbar spine. J Clin Epidemiol. 1994;47:375–381. doi: 10.1016/0895-4356(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 18.Ciol MA, Deyo RA, Howell E, Kreif S. An assessment of surgery for spinal stenosis: time trends, geographic variations, complications, and reoperations. J Am Geriatr Soc. 1996;44:285–290. doi: 10.1111/j.1532-5415.1996.tb00915.x. [DOI] [PubMed] [Google Scholar]
- 19.Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:403–408. [PubMed] [Google Scholar]
- 20.Wiesel SW, Tsourmas N, Feffer HL, Citrin CM, Patronas N. A study of computer-assisted tomography. I. The incidence of positive CAT scans in an asymptomatic group of patients. Spine. 1984;9:549–551. [PubMed] [Google Scholar]
- 21.Jansson KA, Blomqvist P, Granath F, Nemeth G. Spinal stenosis surgery in Sweden 1987–1999. Eur Spine J. 2003;12:535–541. doi: 10.1007/s00586-003-0544-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaBan MM, Imas A. “Young” lumbar spinal stenotic: review of 268 patients younger than 51 years. Am J Phys Med Rehabil. 2003;82:69–71. doi: 10.1097/01.PHM.0000034918.76195.5E. [DOI] [PubMed] [Google Scholar]
- 23.Lee BC, Kazam E, Newman AD. Computed tomography of the spine and spinal cord. Radiology. 1978;128:95–102. doi: 10.1148/128.1.95. [DOI] [PubMed] [Google Scholar]
- 24.Schonstrom NS, Bolender NF, Spengler DM. The pathomorphology of spinal stenosis as seen on CT scans of the lumbar spine. Spine. 1985;10:806–811. doi: 10.1097/00007632-198511000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Jarvik JJ, Hollingworth W, Heagerty P, Haynor DR, Deyo RA. The Longitudinal Assessment of Imaging and Disability of the Back (LAIDBack) Study: baseline data. Spine. 2001;26:1158–1166. doi: 10.1097/00007632-200105150-00014. [DOI] [PubMed] [Google Scholar]
- 26.Kent DL, Haynor DR, Larson EB, Deyo RA. Diagnosis of lumbar spinal stenosis in adults: a metaanalysis of the accuracy of CT, MR, and myelography. AJR Am J Roentgenol. 1992;158:1135–1144. doi: 10.2214/ajr.158.5.1533084. [DOI] [PubMed] [Google Scholar]
- 27.Jarvik JG, Deyo RA. Diagnostic evaluation of low back pain with emphasis on imaging. Ann Intern Med. 2002;137:586–597. doi: 10.7326/0003-4819-137-7-200210010-00010. [DOI] [PubMed] [Google Scholar]
- 28.de Graaf I, Prak A, Bierma-Zeinstra S, Thomas S, Peul W, Koes B. Diagnosis of lumbar spinal stenosis: a systematic review of the accuracy of diagnostic tests. Spine. 2006;31:1168–1176. doi: 10.1097/01.brs.0000216463.32136.7b. [DOI] [PubMed] [Google Scholar]
- 29.Eisenstein S. Lumbar vertebral canal morphometry for computerised tomography in spinal stenosis. Spine. 1983;8:187–191. doi: 10.1097/00007632-198303000-00010. [DOI] [PubMed] [Google Scholar]


