Abstract
Transient receptor potential (TRP) channels are cellular sensors for a wide spectrum of physical and chemical stimuli. They are involved in the formation of sight, hearing, touch, smell, taste, temperature, and pain sensation. TRP channels also play fundamental roles in cell signaling and allow the host cell to respond to benign or harmful environmental changes. As TRP channel activation is controlled by very diverse processes and, in many cases, exhibits complex polymodal properties, understanding how each TRP channel responds to its unique forms of activation energy is both crucial and challenging. The past two decades witnessed significant advances in understanding the molecular mechanisms that underlie TRP channels activation. This review focuses on our current understanding of the molecular determinants for TRP channel activation.
Introduction
Transient receptor potential (TRP) channels are a group of unique ion channels that serve as cellular sensors for a wide spectrum of physical and chemical stimuli (23,25,142). They respond with exquisite sensitivity to fundamental cell signaling elements such as PIP2, Ca2+, cyclic nucleotides, phosphorylation potential, temperature, and osmotic pressure, as well as environmental inputs that can be either beneficial or harmful. Activation of TRP channels changes the membrane potential, translocates important signaling ions cross the cell membrane, alters enzymatic activity, initiates endocytosis/ exocytosis, and so on. In doing so, TRP channels are known to play crucial roles in many fundamental processes in life such as fertilization, sensory transduction, cell survival, and development. In addition, their activities or malfunctions often signal the presence of harmful conditions to the cell or pathological development. Our understanding of the physiological functions of this group of very diverse and relatively new ion channels is still rather limited; nonetheless, intensive investigations that have been attracted to this area in the last two decades are yielding rich information at a rapid pace. Accurate interpretation of the information requires knowledge on the many TRP channel activation processes, which is the focus of this review.
Another important consideration of TRP channel activation is the polymodality feature. Many TRP channels exhibit exquisite sensitivity to multiple types of stimuli that are distinct in nature, for example, capsaicin, extracellular pH, and heat for TRPV1, menthol and cold for TRPM8. It is believed that evolution has tuned TRP channels to sense multiple stimuli and mediate integrated cellular responses. It should be pointed out, however, that synergistic activation by distinct stimuli is not restricted to TRP channels. Voltage-gated ion channels, for example, can be quite responsive to physiological changes of the intracellular concentration of cyclic nucleotides (e.g., hyperpolarization-activated cyclic nucleotide-gated channels) or Ca2+ [e.g., big potassium (BK) channels]. Ion channels with polymodal activation can act as coincidence detectors that link together otherwise separate cellular events, a role well fit for ion channels whose activity, in general, serves the role of cellular signaling (in both electrical and chemical forms). For TRPV1, polymodal activation by capsaicin and heat may contribute to the common human sensation elicited by these very distinct stimuli (one being chemical, the other physical). While polymodal activation clearly is fundamental to TRP channel functions as cellular sensors, it also poses an additional challenge to the investigation of their activation mechanisms. A TRP channel’s response to a specific stimulus is dependent on the presence and magnitude of many other stimuli that need to be carefully controlled and taken into consideration. Indeed, the sensitivity of TRPV1 to capsaicin is acutely tuned by experimental conditions such as temperature, membrane potential, extracellular pH, and intracellular Ca2+ concentration. Considering that a plethora of potential endogenous TRPV1 agonists and antagonists have also been reported, it is perhaps no wonder that the activation of TRPV1 observed from native cells can vary so dramatically.
Classification
The first TRP channel was discovered in a mutant strain of Drosophila melanogaster in which the lack of a functional copy of the trp gene caused impairment in the fly’s visual system including an abnormal electroretinogram response to light (27). Unlike the wild-type flies, trp mutants showed a unique transient receptor potential (TRP) response to light. The identity of the mutant gene was discovered by Craig Montell and Gerald Rubin in 1989 (111). The predicted trp gene product appeared to resemble an ion channel protein, which was later proved true by Roger Hardie and Baruch Minke who showed that TRP is a Ca2+ channel activated by light in photoreceptor neurons (52). A TRP homolog, TRPL, was also cloned and characterized from the fly (136).
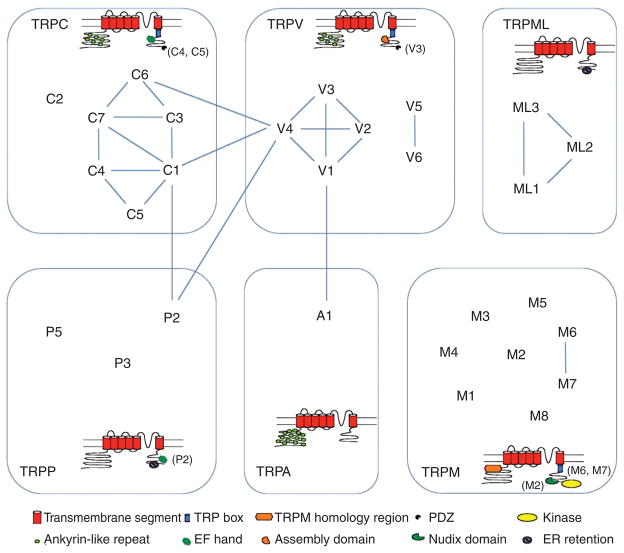
The search for TRP homologs in mammalian species so far has yielded 28 TRP channels (Fig. 1). They are grouped by sequence similarity into several subfamilies: TRPC (canonical), TRPM (melastatin), TRPV (vanilloid), TRPA (ankyrin), TRPML (mucolipin), and TRPP (polycystin) (23, 25). The TRPC subfamily members are most closely related to the Drosophila TRP channels. There are seven TRPC subunits. Human TRPC2 appears to be a pseudogene, though in other mammals TRPC2 is functional. The rest TRPC subunits can be divided into two groups, TRPC1/TRPC4/TRPC5 and TRPC3/TRPC6/TRPC7. TRPC subunits can assemble into homomeric channels, and many of them can also assemble into heteromeric channels (45, 58, 153, 178). Coassembly are reported for TRPC1/TRPC5 (177), TRPC1/TRPC3 (95), TRPC1/TRPC4 (51), TRPC3/TRPC4 (139), TRPC4/TRPC5 (137, 138), and TRPC1/TRPC3/TRPC7 (230). All TRPC subunits contain an N-terminal ankyrin-like repeat domain (ARD), a TRP box after the sixth transmembrane segment, S6, and a Ca2+-binding EF hand domain in the intracellular C terminus. A common feature of the TRPC channels is that they are all activated by the phospholipase C (PLC) signaling pathway.
Figure 1.
Transient receptor potential (TRP) channel subfamilies. The subunit topology is shown with highlights of specific functional domains. Subunits known to coassemble are indicated by lines.
The mammalian TRPM subfamily contains eight members, TRPM1-8. They are divided into three subgroups, TRPM1/TRPM3, TRPM4/TRPM5, TRP6/TRPM7, with TRPM2 and TRPM8 being separate from the rest of the subfamily. Coassembly of TRPM subunits has been reported between TRPM6 and TRPM7 (20, 21, 71, 89). TRPM subunits lack the ARD domain found in the intracellular N terminus of most TRP subunits. Instead, the very long N terminus contains a large TRPM homology region of about 700 amino acids. Most TRPM subunits also contain a well-conserved C-terminal TRP box and a coiled-coil domain (188). TRPM4 and TRPM5 are unique among TRP channels as they are the only two monovalent cation-selective ion channels. In addition, TRPM2, TRPM6, and TRPM7 are special in that they contain an enzymatic domain in the C terminus.
The TRPV subfamily has six members, TRPV1-6, that can be further divided into two groups, TRPV1-4 and TRPV5/TRPV6. TRPV1-4 homomeric channels are weakly Ca2+-selective cation channels. They are also all heat activated. Subunits of the TRPV1-4 group can coassemble to form heteromeric channels (18,53,90,147,166). Likewise, TRPV5 and TRPV6 subunits can coassemble into heteromeric channels (53, 56, 153). TRPV5 and TRPV6 homomeric channels as well as TRPV5/TRPV6 heteromeric channels are highly Ca2+ selective but not heat activated. Like TRPCs, subunits of this subfamily also contain an N-terminal ARD and a TRP box domain. Specific interacting domains in the C terminus are known to promote subunit assembly (42, 195, 233).
The only member of the TRPA subfamily, TRPA1, got its name from its very large ARD domain that contains at least 14 repeats. The TRPML subfamily has three members, TRPML1-3. These are primarily intracellular proteins in cytosolic compartments. An ER retention-signaling domain in the intracellular C terminus is likely a major determinant of their intracellular localization. Coassembly among TRPML subunits has been reported (29, 193). TRPP subfamily has three members, TRPP2 (also known as PKD2), TRPP3 (or PKD2L1), and TRPP5 (or PKD2L2). They are not to be confused with the other polycystins—members of the PKD1 subfamily are 11 transmembrane segments proteins. TRPP subunits also contain a C-terminal ER retention-signaling domain.
The classification of TRP channels is based on sequence similarity instead of common functional features. This is different from most other ion channel families that exhibit not only high sequence similarity but also similar ion selectivity or sensitivity to specific activation stimuli. Because of this classification method, members of the same subfamily may be functionally distinct. On the other hand, members from different subfamilies may share noticeably common features. This can be illustrated by the fact that temperature-activated TRP channels include members of the TRPC, TRPV, TRPM, and TRPA subfamilies, whereas TRPM2, TRPM6, and TRPM7 are the only members of the TRPM subfamily that contain an enzymatic domain. In addition, while subunits of the same subfamily do not always coassemble into heteromeric channels, subunits of different subfamilies are found to interact and possibly form heteromeric channel complexes. Examples of cross-subfamily coassembly are between TRPC1/TRPP2 (4, 82, 187, 234), TRPV4/TRPP2 (83, 172), TRPA1/TRPV1 (152), and TRPC1/TRPC6/TRPV4 (1). Because the TRP channel subfamilies are classified by sequence similarity, their members exhibit great functional diversities. It makes the apparent incoherence in discussing TRP channel as a whole somewhat unavoidable.
Distribution
TRP channels are widely distributed in many tissues and cell types. A number of them appear to be universally expressed, whereas others exhibit more restricted expression patterns. Besides the plasma membrane, TRP channels are also found in intracellular membranes (33). TRPML channels are examples of functional intracellular channels. A detailed account of the distribution of each TRP channel type is beyond the scope of this review; interested readers can refer to excellent reviews for information on comparative distribution (129, 142, 194, 211). It is noted that there exists substantial discrepancies in the literatures on TRP channel distribution. For this reason, it is helpful to pay special attention to the detection method that supports the presence of a TRP channel in specific tissues or cells. The presence of TRP channel mRNA is a good indicator for functional expression. Immunohistochemistry can demonstrate the presence of TRP channel proteins and further reveal their cellular location. Functionally, TRP channel current can be recorded with patch clamping. Furthermore, an increase in intracellular Ca2+ concentration in response to stimuli specific to certain TRP channels serves as a good indicator of functional expression for almost all TRP channels (except TRPM4 and TRPM5) as they are Ca2+ permeable. Radioactive ligand binding also provides direct evidence of TRP protein localization. Genetically modifying a TRP channel locus allows the introduction of reporter genes such as lacZ or Cre into living animals in which the expression of the reporter genes reflects the expression of the channel. Using these techniques, rich information on TRP channel distribution has been gathered.
As the distribution of each TRP channel gives important clues on its functions, a thorough understanding of the expression pattern is of great significance to our appreciation of TRP channel molecular mechanisms. For this reason, tissue-or cell type-specific distribution of particular TRP channels will be mentioned below when their activation mechanisms are discussed. While much has been learned in the past, it is clear that the tissue- and cell-type distribution of TRP channels remains to be an area where additional exploration is needed. For example, it has been thought that TRPV1 is involved in a number of higher functions of the central nervous system including plasticity, learning, and memory. However, a very recent study suggested surprisingly that in mouse, rat, primate, and human the expression of TRPV1 in the brain is limited to only highly restricted areas (14).
A further note of caution can be made on species difference. It is well known that TRP channels from different species exhibit distinct functional properties. Indeed, while human and rodent TRPV1 channels are highly sensitive to capsaicin, TRP channels from birds are insensitive to the plant irritant. Importantly, species difference is also found in the expression pattern of TRP channels. In rodents (both rat and mouse), TRPV3 is predominantly expressed in keratinocytes (131). However, in human and nonhuman primates, expression of TRPV3 in sensory neurons is detected at both mRNA and protein levels (166, 216). Presence of the channel in human sensory neurons might provide a basis for camphor, thymol, eugenol, carvacrol (components from plants such as rosemary, thyme, clove, cinnamon, and oregano), and other chemicals found in food spices to elicit strong and unique warmth human sensation by activating TRPV3 (215). It is further noticed that TRPV3 knockout mice did not seem to exhibit significant defect in heat sensation, which is consistent with a lack of neuronal presence of the channel in rodents (64).
Structure
The structure of ion channels provides invaluable information regarding their functions and activation mechanisms. Unfortunately, structural information is still scarce for TRP channels. Based on sequence similarity and the available low-resolution cryo-electron microscopy (EM) structures, all TRP channels are expected to look somewhat like the voltage-gated potassium channels, with four subunits surrounding a centrally located ion permeation pore. There is no report so far on crystallographic study of a whole TRP channel; structures of isolated channel domains such as the ARD have been solved by crystallography. The following discussion summarizes what we know about the structural features of TRP channels.
Topology and sequence features of TRP subunits
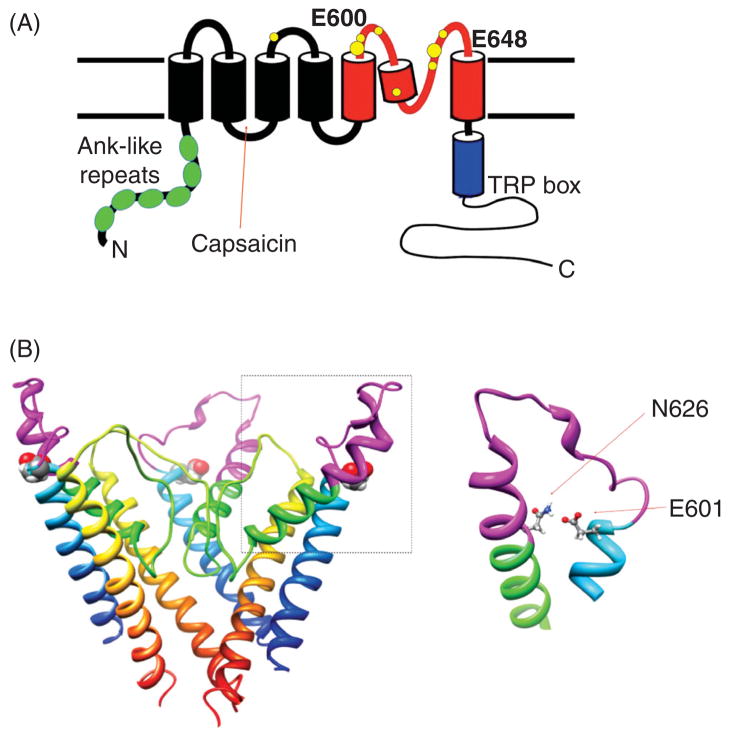
Based mostly on hydropathy analysis, all TRP channels are expected to have six transmembrane segments like the voltage-gated potassium channels (Fig. 1). Both the N and C termini are intracellularly located. Predictions by sequence analysis are largely supported by functional tests such as mutational studies, chemical modification, etc. In many TRP channels, the ion selectivity filter region can be clearly identified from the linear sequence, as they unmistakably resemble the ion selectivity filter of potassium channels. For example, most TRPV channels have an ion selectivity sequence of TIGXGD, with the X being an M or L. Glycosylation sites are suggested in a number of TRP channels in the pore turret after the fifth transmembrane segment, S5. A number of protonation sites are identified in TRP channels. From their sensitivity to pH changes either on the intracellular side or on the extracellular side, the transmembrane location of these protonation sites can be determined (see Table 1).
Table 1.
Summary of pH Effects on TRP Channels
| Channel type | pH effect | Side | Site of action | References |
|---|---|---|---|---|
| TRPV1 | Potentiation (gating) | Extracellular | Pore turret (E600), pre-S6 loop (E648), others | (74) |
| Inhibition (permeation) | Extracellular | Selectivity filter | (6, 207) | |
| TRPV5 | Inhibition (permeation, gating) | Extracellular | Pore turret (E522) | (224) |
| Inhibition (permeation, gating) | Intracellular | Proximal C-terminal (K607) | (223) | |
| TRPM2 | Inhibition (permeation) | Extracellular | Pore turret (H958, R960), pre-S6 loop (E994) | (34) |
| Inhibition (gating) | Intracellular | S4-S5 linker (R933) | (34) | |
| TRPM5 | Inhibition (permeation, gating) | Extracellular | S3-S4 linker (E830), pore turret (H896), pre-S6 loop (H934) | (93) |
| TRPM7 | Potentiation (permeation) | Extracellular | Selectivity filter (E1047, E1052, D1054, E1059) | (70, 88, 121) |
| TRPM6 | Potentiation (permeation) | Extracellular | Selectivity filter (E1024, E1029) | (88) |
| TRPC4 | Potentiation | Extracellular | Unknown | (157) |
| TRPC5 | Potentiation (gating), inhibition (permeation) | Extracellular | Pore turret (E543), pre-S6 loop (E595) | (79, 157) |
| TRPC6 | Inhibition | Extracellular | Unknown | (157) |
| TRPML1 | Potentiation | Luminal (lysosome) | Unknown | (32) |
| TRPML2 | Potentiation | Luminal (lysosome) | Unknown | (32) |
| TRPP3/PKD1L3 | Potentiation (gating, permeation?) | Extracellular | Unknown | (66) |
| TRPA1 | Potentiation (gating) | Extracellular | Unknown | (179) |
Subunit composition
TRP channels are tetrameric assemblies. Evidence of a tetrameric TRPV1 protein complex comes from gel filtration analysis (110), blue native gel electrophoresis (69), electrophoresis in perfluorooctanoic acid (78), and electron micrograph (102, 107, 110, 161). In addition, sucrose gradient centrifugation experiments showed TRPV5 and TRPV6 form tetramers (56). Nearly all the TRP subunits discovered so far can form functional homotetrameric channels [but see reference (173)]. The TRPP2 subunits are shown to form a trimer that then associates with a PKD1 subunit (a 11 putative transmembrane segment-containing protein) to form a channel (227). In addition, there are ample examples of heteromeric channel formation between TRP subunits (16). Figure 1 summarizes what have been reported so far. Some of the heteromeric channels are only observed in expression systems where subunits are coexpressed through transfection. It is yet to be shown that they do exist in native cells.
Detailed mechanisms governing TRP subunit coassembly remain to be determined. The N-terminal ARD domain, which contains a number of highly conserved helix-turn-helix structures and represents one of the most commonly found protein-protein interacting motifs (113,156), was previously reported to affect homomeric and heteromeric TRPV5/TRPV6 channels formation (15, 36). However, no direct interaction between isolated ARDs of any of the TRPV1-4 channels could be detected (233). This result is consistent with crystallography data demonstrating that the ARDs from TRPV1 and TRPV2 remained as monomers in crystal forms (72,92,104), indicating that subunit coassembly of TRPV1-4 does not involve significant interaction between ARDs. It remains possible that ARD interacts with other part(s) of the TRPV protein, through which it affects channel assembly, trafficking, and function (3, 92).
It has been previously proposed by Garcia-Sanz and colleagues (2004) that the TRPV1 C-terminal region reassembles the C-terminus of cyclic nucleotide-gated channels that exists in a fourfold symmetrical arrangement (229). A 38-amino acid segment right after S6 appears to participate in coil-coil interactions between subunits and plays an important role in TRPV1 tetramerization (42). In cyclic nucleotide-gated channels, coil-coil interaction is thought to be a major determinant for subunit assembly as well as subunit stoichiometry of the heteromeric channels in rod photoreceptor neurons (237). Indeed, partial deletions of various TRPV1 C-terminal segments affected channel function, and a complete deletion of the C terminus rendered the truncated channel nonfunctional (195). A short segment of six amino acids in the C-terminal region of the homologous TRPV5 channel was also found to be crucial for subunit assembly (15). In addition, a tetrameric assembly domain in the C terminus proximal to the TRP-like box was recently identified in TRPV1 and possibly other TRPV channels (233).
In addition to the pore-forming subunits, auxiliary subunits, and modulator proteins have been found to be associated with TRP channels. For example, calmodulin (CaM) is a common TRP channel modulator. It is associated with TRPV1 either in the absence of Ca2+ or when the intracellular Ca2+ concentration raises. Interestingly, association of CaM to several TRPV channels is affected by intracellular ATP molecules, through their competition for the N-terminal ARD (135).
EM structure
Besides using the potassium channels as a template for the overall structural features of TRP channels, information from electron microscopy has been very useful in interpreting TRP channel protein architecture. Additionally, attempts have been made to model TRP channel structures (38,42). Clearly, much work is needed to improve the resolution of structural models generated using both methods. In the following discussion, the EM-based TRP channel structures will be examined.
Currently, there are a number of reported EM structures of TRP channels, including TRPV1 (110), TRPV4 (161), TRPM2 (102), TRPA1 (30), and TRPC3 (106,107). All these structures are of limited resolution (>15 Å). Nonetheless, with improvements of the experimental condition and image analysis methods, it is theoretically possible to achieve much higher resolutions that would allow identification of side chains and the generation of atomic resolution models. This promise has been demonstrated by studies of nicotinic acetylcholine receptor (108, 190) and GroEL (97); in both cases, a resolution of about 4 Å has been achieved. Before reaching this level of resolution, the available TRP channel EM structures can provide useful evidence for interpretation of domain arrangement and subunit interactions. The fourfold symmetry seen in the existing structural models of TRP channels mostly originates from the procedure used to refine the model. For this reason, it does not by itself serve as a proof of the assumed tetrameric channel complex.
Two different approaches have been applied to EM studies of TRP channels. In the first approach, negative staining was used to enhance the contrast between channel protein and the background. With this approach the electron density of the staining metal particles forms a “cast” around the protein, which needs to be excluded to yield the protein mass. The staining thus limits the resolution of this EM approach. Additional limitations of the negative staining method on the structural resolution include potential protein structure distortion generated by the staining process and potential inclusion of lipid aggregates to the protein mass. An alternative approach to negative staining is the use of cryo-EM analysis. While the intrinsically low image contrast in the absence of staining currently prohibits the cryo-EM approach to be applied to small proteins, this is the method that has improved dramatically in recent years. Indeed, the high-resolution protein structures mentioned above were solved using the cryo-EM approach.
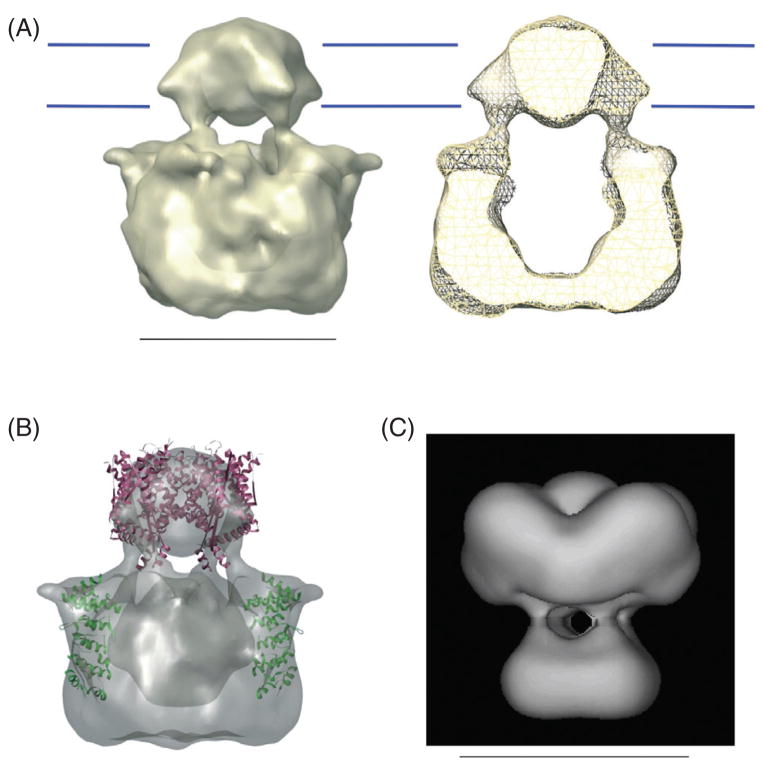
The cryo-EM structure of TRPV1 (110), solved at 19 Å, has two well-defined domains (Fig. 2A). The smaller one of them, having a diameter of 60 Å and a height of 40 Å, is assumed to represent the transmembrane domain. The space occupied by this domain matches roughly with the size of the transmembrane domain of the Kv2.1 crystal structure (Fig. 2B) (96). The larger domain, likely representing the intracellular mass of TRPV1, has a diameter of 100 Å and a height of 75 Å. Its shape resembles the corresponding part of the Shaker potassium channel cryo-EM structure (Fig. 2C) (167) in that both structures look like a “hanging gondola.” The low electron density central space of the TRPV1 structure suggests the existence of a large cavity, which has not been tested experimentally. It is suggested that the N-terminal ARD fits well under the four shoulder-like regions in the cytoplasmic domains near the expected membrane surface (Fig. 2B). If confirmed, this may indicate that the interaction partner for the ARD resides at the intracellular face of the transmembrane region. The EM structure of TRPV4 (161), solved at a lower 35-Å resolution, has similar structural features to TRPV1, including the two identifiable domains and a low-density central cavity.
Figure 2.
Electron microscopy (EM) structures of transient receptor potential (TRP) channels. (A) Cryo-EM structures of TRPV1. Blue lines indicate the position of the cell membrane. (B) The crystal structures of Kv2.1 transmembrane domains (PDB 2A79) and TRPV1 ankyrin-like repeat domains (PDB 2PNN) docked into the TRPV1 cryo-EM structure. (C) Cryo-EM structure of Shaker potassium channel. Adapted, with permission, from references (110,167). Scale bar for A and C, 10 nm.
The structure of TRPM2 (102), solved at 28-Å resolution with the negative staining method, has a distinct bullet-like shape. The dense “bullet head” is interpreted to represent the transmembrane domain. The size of this part is considerably larger than the corresponding part of the TRPV1 and TRPV4 structures, even though the TRPM2 protein complex is larger (690 kDa, compared to about 400 kDa for both TRPV1 and TRPV4) mostly because of its lengthy intracellular termini. Below the dense region is an even larger mass that has many low-density internal cavities. The diameter of this part, presumably representing the intracellular mass of the channel protein including the C-terminal enzymatic domain, is 180 Å. The structure of TRPC3 (107), solved at 15-Å resolution by cryo-EM, has a very different shape. It is very large (240 Å in height and 200 Å wide) given the molecular mass of the TRPC3 channel complex (388 kDa), but contains numerous cavities. These features are consistent with an earlier study of the channel using negative staining (106). Nonetheless, the structural features need to be tested experimentally.
A TRPA1 EM structure at 16-Å resolution was recently published (30). The structure was determined using negative staining and an amphipathic polymer (amphipol) A8-35 in place of detergent. The choice of A8-35, which binds tightly to the transmembrane portion of the protein, is motivated by the idea that it may provide a more native-like environment for membrane proteins (184). For this reason, it has been utilized in a number of nuclear magnetic resonance and EM studies. The overall shape of the TRPA1 structure is reminiscent of the TRPV1 structure. It has a compact transmembrane domain and a basket-like cytoplasmic domain, though the size of the cytoplasmic domain is considerably larger than that of TRPV1, as expected from the long ankyrin-like repeats region of TRPA1.
Crystallographic study of TRP channel structural elements
While at the present time there is no whole TRP channel structure solved by crystallography, high-resolution structures of several intracellular functional domains are available. These studies not only confirmed the expected structural features of these common protein domains, but also they revealed interesting novel features that likely contribute to the function of the host TRP channels.
The ARD is found in the intracellular N terminus of members of the TRPC, TRPV, and TRPA subfamilies. It represents one of the most commonly found protein-protein interaction domain (113, 156). The structural features of ARD in non-TRP channel proteins are found to be largely conserved. Each of the repeats consists of two α-helices folding onto each other followed by a long hairpin loop. ARD is formed by stacking multiple copies of this general structure. In TRPV and TRPC channels, it is believed that up to six repeats exist in ARD, whereas in TRPA1 the copy number is at least 14. TRPN channels, which are found in fruit fly, C. elegans and zebrafish but not mammals, host an N-terminal ARD that contains 29 repeats. So far the ARD structures of rat TRPV1 (92), rat and human TRPV2 (Fig. 3A) (72, 104), and mouse TRPV6 (134) have been solved by crystallography. In general, the structure of ARDs in TRPV channels closely resembles the known ARD structures in other proteins (Fig. 3B). Noted differences include a twist between the fourth and fifth repeat whose functional significance remains to be examined. While ARD is known to serve as a protein-protein interaction domain, its interacting partner is often a much shorter, less-structured peptide segment (113, 156). Consistent with this observation in other proteins, TRPV channel ARDs do not form homooligomers in solution (233). This is confirmed by crystallographic studies. Interacting partners of TRP channel ARDs remain to be identified.
Figure 3.
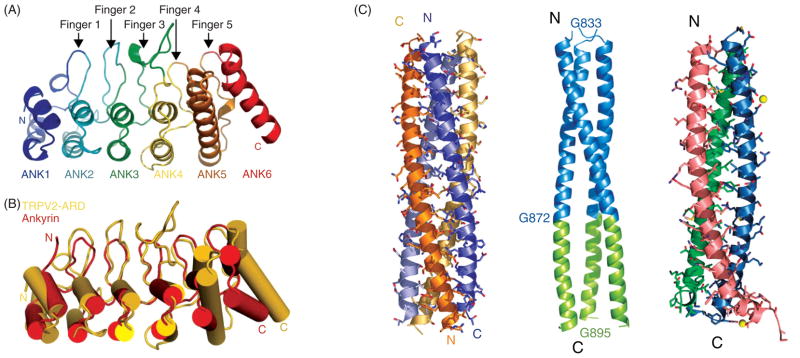
(A) Crystal structure of the TRPV2 ankyrin-like repeat domain. (B) Comparison between TRPV2 ankyrin-like repeat domain and the repeats 16–21 of ankyrin (PDB 1N11). (A) and (B) are adapted, with permission, from reference (72). (C) Crystal structures of the coiled-coil domain of TRPM7 (left), TRPP2 (middle), and CNGA1 (right). Adapted, with permission, from references (40, 163, 227), respectively.
What turned out to be very interesting about the TRPV channel ARD domains is that the ARD of TRPV1, TRPV3, TRPV4 but not TRPV2 also binds ATP (92, 135). Functionally it is shown that ATP sensitizes the response of TRPV1 and TRPV4 to membrane depolarization and agonists such as capsaicin, and desensitizes the response of TRPV3 to repeated agonist (2-APB) stimulation (135). Mutating residues in ARD shown in the crystal structures to be critical for interacting with the ATP molecule indeed disrupted such modulatory effects. These results suggest that changes in the intracellular ATP concentration might be yet another cellular signal that selected TRPV channels can sense. While the molecular mechanism underlying ATP regulation of TRPV channels is still under investigation, one possibility is suggested by the observation that the ARD region that binds ATP also mediates interaction between the channel and Ca2+-CaM (92). Competition between ATP and Ca2+-CaM was observed (135). In TRPV1, repetitive stimulation leads to CaM-mediated channel desensitization. It makes sense then that competitive binding of ATP would remove the inhibitory effect of CaM, leading to channel sensitization.
Coiled-coil is another prevalent protein interaction and assembly domain found in many protein families including transcription factors, membrane fusion proteins, and ion channels (50). It consists of α-helices coming together to form a helical coil bundle of preferred stoichiometry. Interaction between helices is mainly mediated by hydrophobic amino acids that are evenly distributed (every third then fourth position) in the linear sequence. Such characteristic sequences were identified in all TRPM channel proteins (188) that of TRPM7 was crystallized and its structure solved (Fig. 3C, left panel) (40). As indicated by biochemical assays (188), the TRPM7 coiled-coil domain exists as a tetramer, with hydrophobic side chains mediating intermolecular interactions. What is intriguing about the structure, however, is that the orientation of two strands is the opposite of that of the other two strands. The antiparallel arrangement observed in the crystal form was further confirmed by disulfide bond linkage tests (40). This antiparallel arrangement is distinct from the parallel arrangement of the tetrameric coiled-coil domain of the Kv7.4 channel (62) and the trimeric coiled-coil domain of CNG channels (Fig. 3C, right panel) (163). It suggests that in TRPM7 channels this part of the C-terminal region forms a “folded-arms” conformation, placing the C-terminal ends of the coiled-coil domains 77 Å away from each other. About 300 amino acids downstream from this point is the kinase domain, which tends to assemble between subunits to form dimers.
Structure of the TRPM7 kinase domain was solved crystallographically (218). Its sequence suggests that it belongs to the atypical α-kinase family, which is distinct from the classical kinase family. However, unexpectedly its central catalytic core structure resembles the classical protein kinases, despite a lack of sequence similarity between them. Furthermore, in the TRPM7 kinase domain structure, striking conservation of residues known to be important in the catalytic mechanism of classical protein kinases was observed. As mentioned above, the TRPM7 kinase domain forms intersubunit dimers. The N-terminal half of one subunit interacts with the C-terminal half of its partner subunit in a domain swapping arrangement. Hence, the TRPM7 channel complex loses its fourfold symmetry at its intracellular enzymatic domain region. This structural feature resembles that of the tetrameric ionotropic glutamate receptors whose extracellular ligand-binding domains form dimers.
The TRPP2 subunit also contains a C-terminal coiled-coil domain (Fig. 3C, middle panel) (227). Through a combination of biochemical, crystallographic, and fluorescence imaging approaches, it is shown that the TRPP2 coiled-coil domain forms a trimer, like the coiled-coil domain of cyclic nucleotide-gated channels. The trimeric TRPP2 complex then associates with an 11 transmembrane segment-containing PKD1 subunit to form a functional protein complex. In comparison, the cyclic nucleotide-gated channel CNGA1 subunits trimer is associated with a CNGB1 subunit to form a tetrameric channel complex (163, 206, 236, 237). The PKD1 subunit is generally thought to function as a cell surface receptor for extracellular ligands and matrix proteins. The association between PKD1 and TRPP2 would allow coupling of stimuli from the extracellular side to the gating of the TRPP2 channel (109).
Activation Mechanisms
Voltage
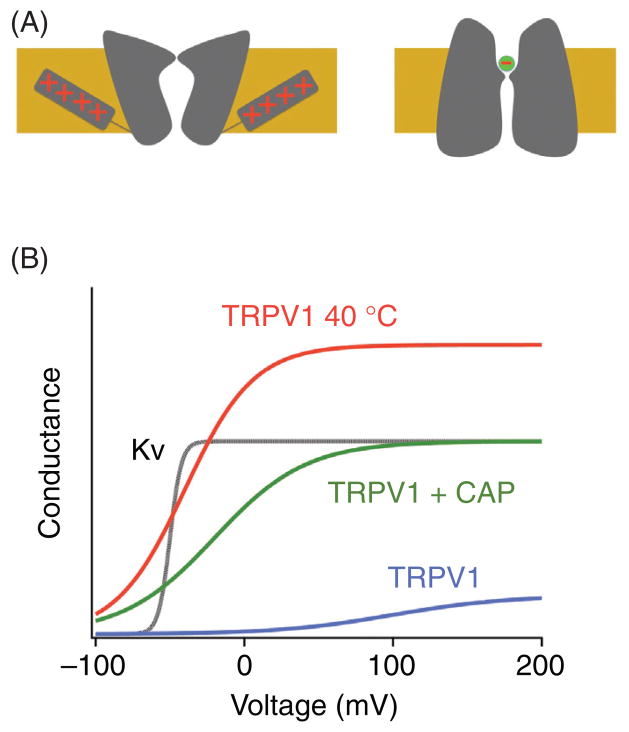
Voltage-dependent activation is one of the best-understood forms of ion channel gating. The predominant form of voltage gating involves a series of positively charged amino acids in the fourth transmembrane segment, S4, of voltage-gated K+, Na+, and Ca2+ channels (Fig. 4A, left panel). The S4 charges sense changes in the electric field across the plasma membrane and move in response to the force exerted by the field (7, 164, 225). In the Shaker potassium channel, movement of S4 and its surrounding structures contributes to the equivalent of about 13 e0 gating charges that move across the transmembrane electric field (154). Recent crystallographic studies start to provide structural underpinning of the voltage sensing process and the subsequent coupling process between the voltage sensor and the activation gate (98). A large number of TRP channels have been reported to be voltage sensitive. However, the voltage-dependent activation process in TRP channels exhibits a couple of noticeable unique features. First, the voltage sensitivity of most TRP channels is quite low in comparison to the classic voltage-gated Na+, K+, and Ca2+ channels (Fig. 4B). TRPV1, for example, has an apparent gating charge of only 0.5 to 0.7 e0 (103, 196). Second, the S4 segment of most TRP channels, with the exception of TRPPs, lacks the series of charged amino acids seen in the classic voltage-gated channels (109). The lack of charged residues in TRP channels provides a simple explanation for their low voltage sensitivity, suggesting that the molecular mechanism underlying the observed voltage dependence in most TRP channels may have a different structural basis. Third, the voltage range in which TRP channels exhibit sensitivity often falls far beyond the physiological range a cell normally experiences. In the case of TRPV1 at room temperature and physiological pH, the threshold voltage for activation is around 0 mV, with a half-activation voltage of about 150 mV.
Figure 4.
(A) schematic drawing of the voltage-dependent gating of the Kv channel (left) and CLC-0 channel (right). The red + and − signs indicate positive charged amino acids and chloride ion, respectively. For the Kv channel, charges carried by arginine and lysine residues in the fourth transmembrane segment, S4, serve as the primary gating charges. Changes in transmembrane voltage drive the movement of S4, which is coupled to the opening of the activation gate. For the CLC-0 channel, the permeant ion Cl− in the pore carries the gating charge. (B) Voltage-dependent gating of TRPV1 and Kv channels.
Despite the low voltage sensitivity that is often in a seemingly nonphysiological range, response to changes in membrane potential by TRP channels is thought to be quite important for their functions. This is because the voltage response can be significantly affected by other channel stimuli, for example, agonists and temperature (196). The coupling between the weakly voltage-dependent activation gating and other gating processes shifts the voltage range at which the channel is responsive, making a voltage change in the physiological range relevant to channel function (Fig. 4B). In addition, voltage-dependent gating also appears to contribute to macroscopic current rectification of many TRP channels, for example, TRPM4 (119).
The importance of voltage-dependent gating is best demonstrated by the heat sensor TRPV1 and cold sensor TRPM8, for which it is thought that the temperature-dependent gating is achieved by shifting the voltage-dependent gating process (196). The work by Voets et al. (2004) demonstrated that, at room temperature, the half-activation voltage of TRPV1 is near +100 mV; raising the temperature to 42°C lowers the half-activation voltage to near −50 mV, a level readily reachable by a sensory neuron (196). A similar negative shift of the activation voltage is also observed in TRPM8 (196, 197). Shift in the voltage dependence is found to be achieved as the opening and closing transitions of TRPV1 and TRPM8 are differentially affected by temperature—for TRPV1, the rate of the opening transition increases rapidly upon heating, leading to a dramatic shift of the equilibrium of the voltage-dependent process toward the open state; for TRPM8, cooling decreases the rate of the closing transition much stronger than the rate of the opening transition, again leading to a shift in equilibrium toward the open state (196). Interestingly, mutations neutralizing charged amino acids in S4 and the S4-S5 linker region are found to reduce the apparent gating charge of TRPM8 (from 0.9 to 0.6 e0) (197), suggesting that a mechanism similar to that of the classic voltage-gated ion channels might exist in this channel as well. A similar mechanism has been proposed for the activation of TRPM4 and TRPM5 channels, whose gating also exhibits voltage dependence and high temperature sensitivity (57, 180).
The molecular basis for the functional coupling between voltage-dependent gating and temperature-dependent gating is currently under debate. While the reports mentioned above indicate that a shared machinery is involved in both gating processes, it is suggested by others that an allosteric mechanism can most economically explain the phenomenon (87). Indeed, ion channels are known to behave as allosteric proteins (155, 228). The large enthalpic and entropic changes associated with the temperature-dependent gating process indicate that large conformational changes, probably affecting many parts of the channel protein, occur during this process (11, 196). In support of the allosteric hypothesis, mutations are found to selectively affect individual activation modality of TRPV1 and TRPV3 (28, 48, 49, 74). Analyses of gating kinetics and equilibrium also suggest that various gating processes may be supported by separate channel structures (103, 219).
Heat
Temperature-dependent activation of TRP channels is a very peculiar gating process that has drawn intensive investigations since its discovery in TRPV1 in1997 by the Julius group (13). Temperature sensing is a basic and indispensable sensory form. Sensitive response of the nervous system to changes in temperature is of paramount importance for homeotherms to maintain a stable body temperature. Temperature cues are used by homeotherms and poikilotherms alike to detect ambient environment, search for favorable conditions, and avoid harm. Indeed, a number of temperature-sensitive TRP channels are studied as nociceptors that respond to extreme temperatures and harmful chemicals. Strong activation of these channels in the nervous system elicits pain. In addition to their roles in neuronal functions, the activity of temperature-sensitive TRP channels are thought to contribute to dynamic local regulations such as dilation or constriction of blood vessels and growth of neurites.
There are a number of TRP channels that are thought to be highly temperature sensitive. Often they are referred to as thermoTRPs. TRPV1 starts to open when the temperature rises to near 40°C under otherwise normal conditions. Its homologs TRPV2, TRPV3, and TRPV4 are also heat activated but with distinct threshold temperatures. TRPV2 activates at more than 50°C, while TRPV3 and TRPV4 activates at lower temperatures around 30°C. (The other two members of the TRPV subfamily, TRPV5 and TRPV6, are not directly activated by heat.) TRPM2, TRPM4, and TRPM5 are also reported to be sensitive to high temperatures. Another heat-sensitive TRP channel is the TRPA1 channel of Drosophila fly and certain snake species. The fly dTRPA1 has several splice variants. The A and D forms, found in multidendritic nociceptor neurons, activate at 24 to 29°C and 34°C, respectively, whereas the B and C forms are not activated by heat (238). The difference in temperature dependence among these variants is found to be associated with regions flanking the N-terminal ARD. The pit organ of viper snakes (e.g., rattlesnakes) expresses a TRPA1 channel that activates at 30°C (47).
The best understood cold-sensitive TRP channel is TRPM8, which activates when temperature drops below about 20°C (105, 130). Another cold-activated TRP channel is TRPC5, which is highly cold sensitive in the temperature range of 37 to 25°C (241). While TRPC5 can coassemble with TRPC1, the heteromeric channel does not show cold-induced channel activity. Mammalian TRPA1 was thought to be a cold sensor with an extremely low threshold temperature below 10°C (174). However, cold activation of TRPA1 remains controversial.
It should be emphasized that thermoTRP channels are not the only highly temperature-sensitive channels. Indeed, the electric ray Torpedo chloride channel CLC-0 has a common gating process that is extremely temperature sensitive (140). (It is, however, unlikely that the heat-induced closure of CLC-0 channel plays a significant physiological role, as the fish may not experience temperatures in the range that affect this gating process. Torpedo california, from which CLC-0 was purified and cloned, lives in northeastern Pacific at a depth of 100–200 m and prefers temperatures of 10–13°C. Temperature-induced common gate closure occurs at temperatures >20°C). The endoplasmic reticulum Ca2+ sensor STIM1 is activated by heat: raising temperature above 35°C causes STIM1 clustering (213). The process may be important for the regulation of intracellular Ca2+ in response to temperature changes. Other highly temperature-sensitive channels include two-pore potassium channels (76) and voltage-gated proton channel (143).
What does high temperature sensitivity of a channel mean? At least two things should be considered. First, for temperature-sensitive TRP channels, their activity can be elicited by the change in temperature alone. TRPV1, for example, opens at resting membrane potential in the absence of capsaicin or other agonists at physiological pH level as long as the temperature reaches near 40°C. The voltage-gated Shaker potassium channel, in comparison, would not open upon raising temperature at resting membrane potential (even though its gating and ion permeation processes would be substantially affected once the channel is activated by membrane depolarization). Second, the activity level of a thermoTRP channel exhibits much higher sensitivity to temperature changes than an “ordinary channel.” A widely used characterization of temperature sensitivity is the Q10 value, which quantifies the fold change in the rate of activation (or deactivation) upon a 10°C increase in temperature. With this standard definition, the Q10 value is exponentially related to the activation energy as defined by the Arrhenius equation (55). For thermoTRPs, the Q10 values are in the range of 10 to 27 (corresponding to an activation energy of 4.2 to 6.0 kcal/mol between 25 and35°C), comparing to a Q10 value of 3 to 7 for an ordinary channel and 40 for CLC-0 common gating. The usage of the Q10 measurement has been relaxed to be applicable to temperature-dependent changes in the current amplitude as well. The current amplitude-based Q10 values cover a wide range and reflect temperature-dependent changes in both permeation and gating. It is a descriptive parameter that is not directly convertible to an energy term.
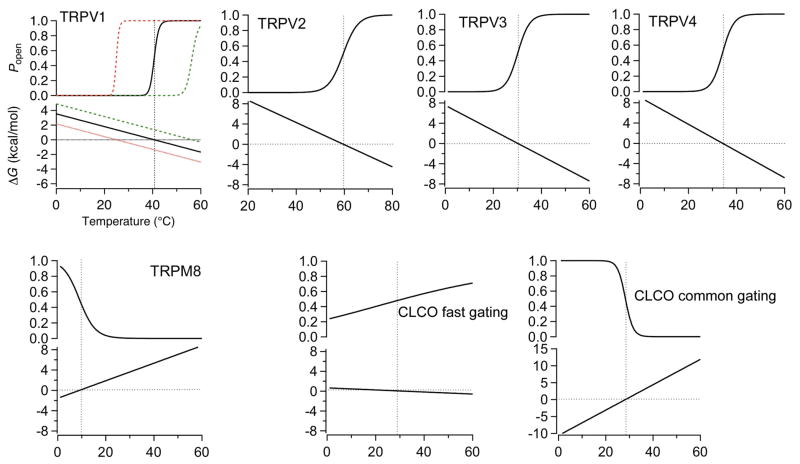
The basic properties of thermoTRP channels heat activation have been extensively characterized from equilibrium measurements of macroscopic and single-channel currents as well as kinetic measurements of current activation (11, 196, 219). These studies suggest that, for thermoTRPs, the heat-induced gating process can be described reasonably well by a single transition between two gating states (C and O). This conclusion is much welcomed as this simple situation can be described by the Gibbs free energy equation ΔG = ΔH − TΔS, in which ΔG is the transition energy between C and O, and ΔH and ΔS are corresponding enthalpic and entropic changes, respectively (Fig. 5). As dictated by thermodynamic principles, ΔS is found to be quite large for thermoTRPs in the range of 100 to 300 cal/mol/K (219). ΔH is also found to be very large, in the range of 30 to 80 kcal/mol. The balance between the two large quantities, ΔS and ΔH, is very important as it allows the transition C→O to occur under physiological conditions. The importance of this fact can be demonstrated by shifting one (say, ΔH) by a small 5% while holding the other (ΔS in this case) unchanged (Fig. 5, top left panel). As a result of this minor deviation from the fine balance point reached through evolution, the system will be stuck indefinitely in either the C state or the O state at the temperature range compatible with life. A lesson from this exercise is that the fine balance between ΔH and ΔS is what determines the functional temperature range (and the threshold temperature) of each thermoTRP channel. It is very likely biology utilizes this property to up- and downregulate the function of these channels under physiological conditions and in response to pathophysiological challenges.
Figure 5.
Temperature-dependent activation of thermo TRP channels and CLC-0 channel. The Popen-T (top panel) and ΔG-T (bottom panel) relationships for each channel are predicted from the measured values for enthalpic and entropic changes. Horizontal and vertical dotted lines indicate the zero ΔG level and the half-activation temperature, respectively. Red and green traces represent the predicted Popen-T and ΔG-T relationships of TRPV1 when the enthalpic change is decreased or increased by 5%, respectively. The ΔG-T relationship of heat-sensitive channels has a negative slope; an increase in temperature causes a decrease in ΔG and a shift of the closed-to-open equilibrium toward the open state. In contrast, the ΔG-T relationship of cold-sensitive channels has a positive slope. CLC-0 has a “normal” fast gating process and a highly temperature-sensitive common gating process. Adapted, with permission, from reference (219).
While the general principles outlined above have been demonstrated to be applicable to thermoTRP channels, the usage of the Gibbs free energy equation likely represents an oversimplification. In particular, the assumption of temperature-independent ΔH and ΔS may not hold, as the molar heat capacity of a channel protein (and its immediate environment including the lipid membrane and the surround water molecules) can be quite large. One can even put forward an argument that the change in molar heat capacity, ΔC, might serve as the driving force for temperature-dependent activation of thermoTRPs (24). Furthermore, it is acknowledged that an equilibrium system between two states, the C and O states, is perhaps an oversimplification of the real channels. Given these limitations, thermodynamic analysis remains a powerful tool for the investigation of the molecular mechanism underlying the high temperature dependence of thermoTRPs, as it has been for the study of heat-dependent protein denaturing process. Indeed, activation of thermoTRPs in many ways resembles the highly temperature-sensitive protein denaturing process, which also exhibits large ΔH and ΔS values.
What happens to thermoTRPs (and their immediate surrounding environment) when temperature is changed? While it is theoretically possible that the lipid and water molecules (either tightly bound to the channel protein or surrounding it) may serve as the heat sensor and transduce thermal energy into protein conformational changes, it is generally recognized that thermoTRP channel proteins themselves are the thermal transducer. Expression of thermoTRP channel proteins introduces high temperature sensitivity into host cells that do not otherwise exhibit high temperature sensitivity; purified channel proteins can bring high temperature sensitivity into artificial lipid membranes they are reconstituted into (231). [The later is not, however, a definitive proof of intrinsic high thermosensitivity, as the channel proteins still function within the lipid/water environment, and as small molecules may be tightly associated with the purified proteins (232)].
Realizing that thermoTRP channel proteins likely behave as a thermal energy transducer, an enthusiastic hunt for “the temperature sensor domain” in the thermoTRP channel proteins has been carried out in many laboratories. Much work has been focused on the capsaicin receptor TRPV1, the canonical heat-sensing channel. So far, most of the investigations rely on mutagenesis (point mutation, deletion, and chimera) to perturb the heat-activation process. While these investigations yielded many informative observations, the intrinsic limitation of the mutation approach for the purpose of identifying the true heat sensor is becoming more and more obvious as we are now facing multiple proposed heat sensor domains spreading across the channel protein. An early study showed that deletion of the last 72 amino acids from the intracellular C terminus substantially affected channel activation by heat (and by other stimuli such as voltage and capsaicin) (195). Noticeably when the whole intracellular C terminus was switched between TRPV1 and the cold-activated TRPM8, their temperature sensitivity properties were also swapped (12). On the N-terminal end, a chimera study on different heat-activated TRPV channels recently suggested that the segment linking the ARD and the first transmembrane domain, S1, determines the steepness of the heat-activation process, while other channel parts including the intracellular C terminus may shift the activation threshold temperature (222). Another noticeable proposal, based on the well-known synergistic gating effects among heat, agonist, and voltage, suggests that heat activation of both TRPV1 and TRPM8 is achieved by mutual effects of all these stimuli on a common activation process (196). Given the location of the binding site for capsaicin and the TRPM8 activator menthol, in the S1-S4 region, and the possibility that this same region may also contribute to the (very weak) voltage activation, one might imagine that a likely location for the shared process would be in the S1-S4 transmembrane core region of the channel. Finally, recordings of temperature-induced changes in fluorescence resonance energy transfer (FRET) signals indicated that the outer pore turret, a structure between the S5 trans-membrane segment and the pore helix, undergoes a significant conformational change during the heat activation of TRPV1 (219). Consistent with real-time recordings of heat-induced structural changes, mutations and chemical modification of the turret substantially changed ΔH and ΔS and the heat-activation process (28,219). Point mutations in the pore helix and the ion selectivity filter-to-S6 linker are also found to selectively affect heat activation, shifting the heat-activation threshold temperature by more than 10°C (49).
One possible interpretation of these observations is that perhaps heat sensing is a widely spread event that involves many protein areas (24). With each part of the protein contributing a little bit to ΔH and ΔS, the well-known cooperativity between conformational changes of different channel structures would pool together the ΔH and ΔS changes to produce a combined highly heat-sensitive process. Indeed, ion channels are known to be allosteric proteins that undergo substantial conformational changes. Cooperativity among subunits has been shown in heteromeric channels formed by TRPV1 and TRPV3 subunits (17). The idea of combining multiple transitions is theoretically feasible as both ΔH and ΔS are extensive parameters and thus are additive. It is also consistent with results from random mutation screenings conducted in TRPV1 and TRPV3 that have identified point mutations affecting heat activation throughout the channel protein (48, 115). This idea, however, does not provide a straightforward explanation for the fact that the activity of many TRP channels of the same subfamilies as thermoTRPs (hence, high sequence similarity and likely high structural similarity) is not highly temperature sensitive, for example, TRPMs and TRPV5-6. Cooperative gating, nonetheless, most likely underlies the synergistic effects of various stimuli on the activation of these polymodal sensors.
The relationship between capsaicin and heat in activating TRPV1 illustrates the cooperative relationship between TRPV1 activators. While the two stimuli have been proposed to converge on the same gating process, substantial evidence are now available in favor of the existence of structurally separate activation pathways for heat and capsaicin that are functionally coupled (Fig. 6). Biophysical recordings showed that capsaicin and heat exhibit additive gating effects even at saturating capsaicin concentrations (103, 219), which is not expected if they work through the same gating machinery. The FRET signal from fluorophores attached to the turret changes only upon heat activation but not capsaicin activation (219). Furthermore, recently published studies (28, 49, 219) and our unpublished observations show that mutations at various outer pore regions selectively affect heat activation but leave the capsaicin activation untouched. Putting all these results together, it is clear that there are two activation pathways, one for heat and another for capsaicin. The two pathways converge onto the same activation gate, yielding the well-known cooperativity between the two stimuli. This dual-allosteric gating mechanism has been previously shown to underlie gating of the Ca2+-sensitive voltage-activated BK channels (61). Mechanistically, the separation of the two gating pathway suggests that heat activation probably does not involve the capsaicin-binding region. In a practical sense, the separation of the capsaicin and heat-activation pathways is good news for pharmaceutical efforts to target TRPV1 for pain management. Indeed, TRPV1 has been actively studied as a potential drug target. While many TRPV1 antagonists have been found or made, it is quickly realized that these molecules lead to severe hyperthermia in animal studies and clinical trials (44). The finding is perhaps well expected from the fact that TRPV1 is involved in body temperature regulation as well as the experience that consuming spicy food can reduce body temperature (despite the flashing and sweating, which are also the consequence of activating the heat sensor). The finding that agonist (and antagonist) pathway is partially separated from the heat pathway suggests that it may be possible to design modality-specific drugs that selectively inhibit certain types of pain-inducing stimuli without changing the channel’s temperature response. A recent report of a study with a number of TRPV1 antagonists is supportive of this idea (128).
Figure 6.
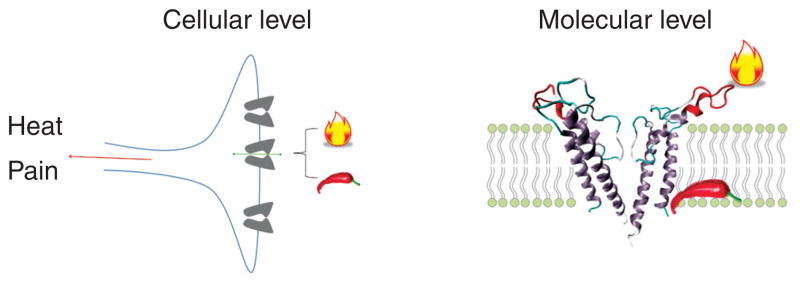
(Left panel) At the cellular level, heat and capsaicin share the same target (TRPV1) for activating sensory neurons, which elicits the sensation of heat or pain. (Right panel) At the molecular level, heat and capsaicin work through distinct activation pathways.
Recent studies of TRPA1 heat activation have yielded a number of important observations. As mentioned above, the viper snake TRPA1 is a heat-activated channel that is thought to function as the snake’s infrared sensor. In contrast, TRPA1 in rat is not heat activated. By making a series of chimeras between the two TRPA1 channels, it was shown that the large ARD is responsible for heat activation of the snake channel (26). Studies of the fruit fly TRPA1 channel further suggest that the regions flanking the ARD can exert regulatory effects on the heat-activation process, making certain dTRPA1 splice variants insensitive to heat (238). Whether a similar mechanism is applicable to TRPV1 remains to be tested, especially as at least two studies seem to have eliminated the TRPV1 ARD as a candidate for the heat-sensing structure (12,222). In comparison, TRPA1 contains at least 14 ankyrin-like repeats and TRPV1-4 channels contain six. Even if it turns out that the ARD is responsible for TRPV heat activation, the search for a cold sensor in TRPM8 would remain because it lacks an N-terminal ARD.
Mechanical force
For ion channels in the plasma membrane, mechanical stimuli come in several forms (150). Transmembrane differences in osmolarity lead to cell swelling or shrinkage, resulting in changes in membrane tension. Tension can also be generated by pressure directly applied to the cell. Alternatively, shear stress due to fluid flow in the blood vessel is an important physiological signal for the regulation of circulation. In a general sense, membrane tension at a sufficiently high level is expected to affect all proteins embedded in the membrane by interfering with their conformational changes; large shear force would directly affect the extracellular part of membrane proteins. While there are ion channels apparently specialized in sensing mechanical stimuli (9, 81, 151, 170), a number of mammalian TRP channels are found to be sensitive to mechanical force either at the physiologically relevant level or with pathological significance. Interestingly, in several cases, TRP channels appear to respond to not the mechanical force per se, but instead to intracellular signaling molecules generated by mechanical stimuli to the cell.
One noticeable osmosensitive TRP channel responsive to hypotonicity-induced cell swelling is TRPV4, which is expressed in kidney, liver, heart, lung, inner-ear hair cells, sensory neurons of the circumventricular organs (responsive to systemic osmotic pressure), and other cell types (91,175,209). It is activated by a decrease in extracellular osmolarity within the physiological range, and is inhibited by an increase in extracellular osmolarity (91, 210). In doing so, TRPV4 may represent a vertebrate osmoreceptor. Unlike volume-regulated anion channels, TRPV4 activation is not sustained at constant extracellular hypotonicity (119). While in the intracellular N-terminus TRPV4 also contains the ARD, which has been proposed to be a spring-like force-sensing protein structure, deletion of this domain did not appear to affect the channel’s mechanosensitivity (91). As TRPV4 is also highly heat sensitive, an increase in temperature potentiates the channel response to osmotic stress (41). Synergistic activation of the channel by heat and hypotonicity, however, does not necessarily imply that the two type of physical stimuli converge to the same channel protein structure that can sense and convert heat and mechanical energy. Indeed, it is thought that activation of TRPV4 by osmotic stress is achieved biochemically, mediated by intracellular signaling molecules produced by membrane-bound mechanosensitive molecules. A number of potential pathways have been proposed. In one potential mechanism, osmotic stress activates the Src family tyrosine kinase that phosphorylates TRPV4 protein (217). Interestingly, the candidate tyrosine residue is identified as Tyr253 in the ARD; mutating this residue eliminated the channel’s stress response (217) (even though, as mentioned above, removing the whole ARD was found to be ineffective). Another tyrosine residue, Tyr110, is also found to be involved (203). In addition, phosphorylation of a residue in the intracellular C terminus, Ser824, by protein kinase C and protein kinase A is found to sensitize TRPV4’s stress response (37,132). Furthermore, PLA2-dependent formation of arachidomic acid and its further conversion to 5′,6′-EET by cytochrome P450 epoxygenase is also involved in TRPV4’s stress response (199).
In addition to TRPV4, extracellular hypotonicity activates TRPM7 (122, 123) and TRPV2 (114) by increasing the channel’s open probability. Membrane tension is also known to affect TRPM4 (112), TRPM7 (123, 204), TRPC1 (101), and TRPC6 (67, 169). In contrast, an N-terminal splice variant of TRPV1 found in arginine-vasopressin neurons of supraoptic nucleus was reported to be activated by hypertonicity-induced cell shrinkage (158). It is thought that activation of TRPV1 in organum vasculosum lamina terminalis neurons serves as the osmosensory transduction mechanism underlying thirst responses (22).
Shear stress is another type of mechanical stimulus that is known to either directly activate TRP channels or potentiate responses of TRP channels to other activators. Examples are found in reports on TRPV4 (41, 203, 210), TRPC6 (67), TRPM7 (122,125,204), and TRPP1/TRPP2 (116). While the significance of this type of TRP channel activation for vascular physiology is obvious, in most cases, it is not clear how mechanical force is transduced to drive channel conformational changes.
Mechanical stimulus not only alters TRP channel functions while the force is present, but also it may induce changes in channel protein distribution. Exposure of TRPM7-expressing cells to physiological levels of laminar fluid flow can trigger rapid (in less than 100 s) translocation of the channel protein to the plasma membrane, which can be monitored by both an increase in TRPM7 current and a near doubling of the fluorescence signal at or near the plasma membrane from channel-attached green fluorescence protein (GFP) (125). Similarly, stretch of myocyte causes TRPV2 channels to translocate to sarcolemma, leading to an increase in Ca2+ influx and the resultant cell damage (68). As might be expected, the translocation response of TRP channels to mechanical force is cell-type specific (68, 77, 125). Its roles in pathological response to vessel wall injury and in myocyte degeneration are being investigated.
Chemicals
Besides activation by energy in physical forms discussed so far, TRP channels are also activated or regulated by chemical-binding energy. Given the great diversity of molecules that interact with TRP channels as well as the physiological and pathological significance many of them present, it is hard to discuss all TRP channel-interacting molecules under one category. Indeed, for TRPV1 alone, the known natural and synthesized chemicals that alter channel functions form a very long list that is still rapidly growing. For the sole purpose of making it manageable, a number of important regulators of multiple TRP channel types are discussed later in separate entries. A couple of example chemicals that interact selectively with specific TRP channels are discussed here.
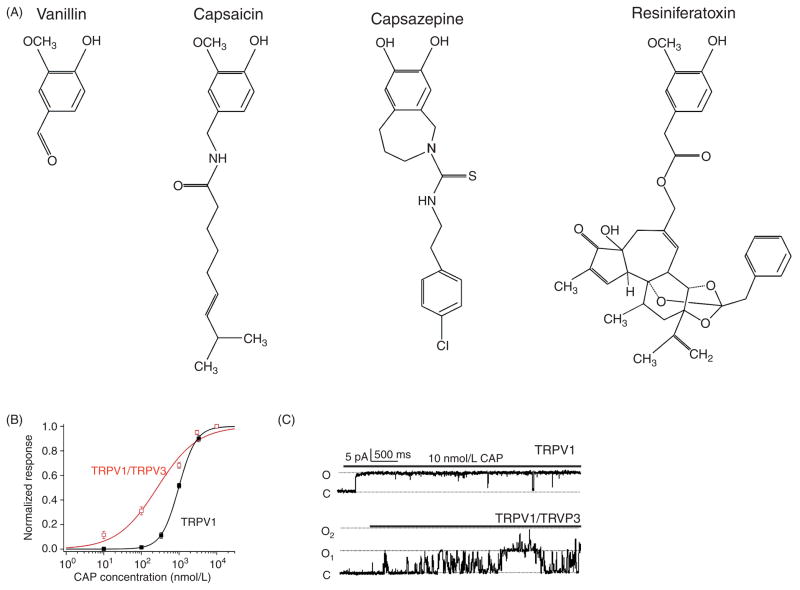
Capsaicin
Capsaicin is the major active vanilloid compound that gives chili peppers its spiciness (Fig. 7A). To mammals including human, it acts as a pungent irritant. Because of the strong burning sensation capsaicin induces in any tissue with which it comes into contact, it has been used in experimental animals to establish pain models. The discovery of TRPV1 was driven by the search for the capsaicin receptor whose existence had been known for years (13). While TRPV1 channels of a mammalian origin are highly sensitive to capsaicin, with EC50 values often far below 1 μmol/L, those from birds are insensitive to capsaicin. In addition, other TRPV channels with substantial sequence homology are also insensitive to capsaicin. These observations suggested that the high affinity binding of capsaicin to mammalian TRPV1 channels occurs at specific binding sites, which was later located to the S2-S4 region by generating chimeric channels between rat TRPV1 and chicken TRPV1 (73).
Figure 7.
(A) The molecular structure of vanillin, TRPV1 activator capsaicin, capsazepine (TRPV1 antagonist), and resiniferatoxin (TRPV1 agonist). (B) Dose-response relationship for TRPV1 homomeric channel and TRPV1/TRPV3 heteromeric channel. (C) Representative single-channel traces from TRPV1 homomeric channel (top) and TRPV1/TRPV3 heteromeric channel (bottom) in response to 10 μmol/L capsaicin. Panels B and C are adapted, with permission, from reference (17).
Binding of capsaicin to the channel’s binding site at the intracellular protein-aqueous solution interface is mediated predominantly by hydrophobic interactions (73). Interestingly, crystallography studies have previously shown that capsaicin binding to the photosynthetic reaction center (PRC) of the plant photosystem II is also mediated mainly by hydrophobic interaction (171). In particular, the vanilloid group of capsaicin and the aromatic ring of a phenylalanine (F216) in PRC stack closely in a nearly parallel orientation. It is possible that the binding of capsaicin to TRPV1 occurs through a similar π- π interaction. Indeed, mutations to aromatic amino acids Y511 and/or F512 (of rat TRPV1) and their adjacent amino acids strongly and specifically attenuate the capsaicin-induced current (73). Additional hydrophobic and hydrophilic interactions mediated by the lipophilic tail of capsaicin and its polar moiety, respectively, may also contribute to the high binding affinity (43, 73). In addition, charged residues, R114 and E761 (also of rat TRPV1), in the intracellular N and C terminals, respectively, may contribute to the capsaicin-induced process as their mutations prevent agonist binding but not heat activation (75). The fact that capsaicin can readily incorporate into the lipid membrane allows rapid diffusion of capsaicin to its binding sites, so that extracellular application of capsaicin can illicit channel activation without a substantial delay.
The dose-dependent activation of TRPV1 by capsaicin can be described by a Hill equation with a slope factor near 2 (Fig. 7B), suggesting the existence of cooperativity between subunits during the capsaicin-dependent activation process. Cooperative gating of the channel by capsaicin is further illustrated by the study of heteromeric channels formed between TRPV1 and TRPV3 subunits (17). The Hill slope factor of the heteromeric channels is significantly shallower; nonetheless, capsaicin can activate heteromeric channels having only two TRPV1 subunits to an open probability of about 60% (Fig. 7C).
It is less understood how binding of capsaicin to TRPV1 induces protein conformational changes that are coupled to the opening of the ion permeation pore. The structural elements that mediate the conformational coupling remain to be determined. In this regard, it is impressive that introduction of capsaicin-binding sites into the capsaicin-insensitive TRPV2 or chicken TRPV1 makes these channels readily activated by capsaicin (73), as if the molecular machinery underlying the agonist activation process is well preserved in these channels and the only thing missing is the affinity binding.
A number of structurally related putative endogenous TRPV1 agonists have been suggested. These include anandamide (242) and 12-HPETE (65). In addition, the strong TRPV1 activator resiniferatoxin (Fig. 7A) also binds to the same binding sites as capsaicin (73). Capsazepine (Fig. 7A) is a capsaicin analog that inhibits TRPV1 activity, apparently by competing for the same binding sites.
Activation of the heat sensor TRPV1 in sensory neurons by capsaicin is thought to form the basis for the “hot” sensation elicited by the consumption of chili peppers (13). At the cellular level, activation of TRPV1 by chemical energy (capsaicin binding) and physical energy (heat) excites the same sensory neurons, leading to the convergence of the two modalities. Indeed, ingestion of spicy food temporary drops body temperature by activating the heat-sensing neurons, causing sweating and enhanced circulation (blushing). In animal and human studies, it has been demonstrated that administration of capsaicin causes hypothermia, while administration of TRPV1 inhibitors such as AMG517 has the opposite effect of inducing hyperthermia (44).
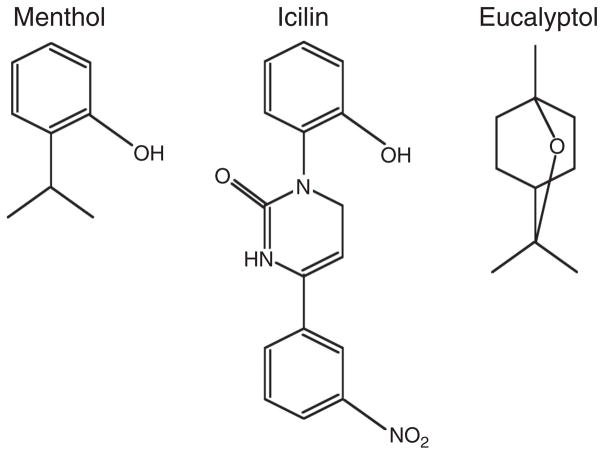
Menthol
Just as activation of the heat-sensor TRPV1 by capsaicin elicits hot sensation, a number of chemical compounds that activate the cold sensor TRPM8 give rise to cooling sensation. Menthol (Fig. 8) is one of the best-studied TRPM8 activators, with an EC50 value below 100 μmol/L (105, 130). Through a screening of random mutations of TRPM8, it was found that an aromatic residue in the S2 segment, Y745 (of mouse TRPM8), strongly affected the efficacy of menthol in activating the channel; mutating this and its adjacent residues impaired menthol binding (5). In contrast, mutations to residues in the intracellular C terminal TRP domain such as Y1005 and L1009 reduced menthol efficacy without a substantial change in apparent binding affinity, suggesting that this region may contribute to the coupling of agonist binding-induced conformational change to the pore (5).
Figure 8.
The molecular structure of the TRPM8 activator menthol, icilin, and eucalyptol.
There are a number of agonists that may interact with TRPM8 in a similar way as menthol (Fig. 8) (105). Eucalyptol (the active compound in the oil of Eucalyptus) has a high EC50 of 3.4 mmol/L and low potency. The synthetic supercooling agent icilin (or AG-3-5) (205), on the other hand, potently activates TRPM8 with a very low EC50 value of less than 1 μmol/L. While menthol and icilin also activate other TRP channels (159, 198), more selective TRPM8-activating menthol derivatives have been reported (160).
Ca2+
Most TRP channels are permeable to Ca2+. Opening of TRP channels initiates Ca2+ translocation into the cytosol, leading to a transient increase in intracellular Ca2+ concentration. (For transient channel activity, the increase in the Ca2+ concentration is most likely only local to the vicinity of the channel site.) Ca2+-triggered signaling events are important for many signal transduction processes, such as the PLC-mediated pathways, the release of intracellular Ca2+ store, and the PIP2-mediated pathways. These very important processes have been examined by numerous reviews [for example, reference (23)]. Only the CaM-mediated feedback regulation of TRP channel activity will be discussed in the following section.
Calmodulin
CaM is a ubiquitous Ca2+-binding protein that plays important regulatory roles in many cellular processes. Each CaM molecule contains four EF-hand motifs that differ in their Ca2+ affinities. The existence of multiple Ca2+-binding sites and cooperativity among them makes CaM a highly Ca2+-sensitive regulator, while difference in Ca2+-binding affinity at each sites allows it to function at a wide dynamic range of Ca2+ concentrations occurring at the site of action. A large number of TRP channels are known to be regulated by CaM. The second TRP channel identified in fruit flies, TRP-like, was discovered through its affinity binding to CaM at two intracellular C-terminal sites (136, 185, 201). The prototypical Drosophila fly TRP channel was later found to bind CaM as well (19). Interaction between CaM and TRP channels represents an important link between intracellular Ca2+ dynamics and channel activity, which interestingly also communicates with other Ca2+ signaling pathways of TRP channel regulation.
CaM regulation of channel activity has been well studied in numerous model systems. Of particular relevance to the TRP channels are those channel types that also permeate Ca2+ ions, such as voltage-gated Ca2+ channels (133), small conductance potassium channels (212), and cyclic nucleotide-gated channels (186). In these cases, channel activation allows Ca2+ ions to cross the cell membrane through the open channel, moving from the high concentration (in the low millimolar range) extracellular side to the low concentration (20–200 nmol/L) intracellular side, and bind to CaM in the cytosol; activated CaM in most cases exerts an inhibitory effect on the channel gating. This negative feedback loop works locally and rapidly to regulate the level of channel activity suitable for cellular physiology. For example, it plays an important role in sensory adaptation and other dynamic physiological processes (94). As most TRP channels are Ca2+ permeable, it is likely that a similar process is involved in their regulation. In addition, activation of CaM by Ca2+ influx following TRP channel activation can affect channel function indirectly through the binding of CaM to other proteins such as CaM-sensitive kinases (182). Furthermore, binding of CaM to a TRP channel can occur in the absence of Ca2+. Similar to the situation in voltage-gated Ca2+ channels (35), apo-CaM may be constitutively associated with certain TRP channels, such as TRPV1 (146). In this case, binding of Ca2+ to CaM serves as a molecular switch to initiate the regulation process.
Given the diversity of TRP channels, it is perhaps not surprising that the sites of CaM binding are also quite diverse (239). Using biochemical tests looking for affinity-mediated association, multiple putative CaM-binding sites can often be identified in the intracellular N terminus and C terminus of a TRP channel. Based on sequence analysis, candidate CaM-binding sites can be predicted in the intracellular C terminus of a number of TRP channels (145). However, one should be cautious in interpreting such information if it is not substantiated by functional tests. Biochemical and functional analyses showed that in TRPC4α there are several segments that bind to CaM. One C-terminal CaM-binding site also binds to an N-terminal region of the IP3 receptor (IP3R) (181, 235). This so-called CIRB (CaM- and IP3R-binding) site, also found in all other TRPC channels, might help mediate functional coupling between TRPC channels and IP3R that contributes to store-operated Ca2+ entry (8, 240). Binding of CaM can also disrupt intracellular interactions that exist at low Ca2+ concentrations, as is the case for cyclic nucleotide-gated channels (192). Additional C-terminal CaM-binding site(s) confer either positive [e.g., for TRPC5 (126)] or inhibitory [e.g., for TRPC1 (165)] effect on channel activity. As the C-terminal CaM-binding sites are often found to overlap with the pro-posed PIP2-binding sites, it is likely that competitive binding of CaM and PIP2 underlies at least some of the observed CaM effects on channel gating (46).
N-terminus-located potential CaM-binding sites are reported in TRPV1, TRPV3, TRPC2, and TRPC4α. The crystal form of the ARD of TRPV1 exists as a complex with CaM (92), suggesting that the N-terminal ARD of TRPV1 and other TRP channels might serve as a CaM-binding site. Interestingly, ATP was also found to bind to the CaM-binding site of TRPV1. Function studies showed competition between ATP and CaM in regulating TRPV1 channel activity (92). As deletion of the ARD disrupts channel function, the significance of CaM binding has not been directly tested. Nonetheless, deletion of the C-terminal CaM-binding sites from TRPV1 did not totally eliminate Ca2+-dependent capsaicin desensitization, supporting a role of the N-terminal CaM-binding site in this slow component of TRPV1 desensitization (124, 146). Interaction between CaM and an N-terminal CaM-binding site is also involved in the potentiation process of TRPV3 (214). In TRPC4α, the site is immediately in front of the first transmembrane segment S1, a region exhibiting substantial sequence conservation among TRPC channels (181). The mouse TRPC2 gene, whose corresponding gene in human is a pseudogene, encodes a functional channel whose intracellular N terminus contains a CaM-binding domain (226).
Just like the diversity exhibited by the CaM-binding sites in TRP channels, the functional consequences of CaM binding also vary dramatically according to the channel type. An inhibitory effect is often observed upon binding of CaM to a TRP channel. In TRPV1, repetitive application of capsaicin leads to desensitization that exhibits a fast component and a slow component (146). The fast component of TRPV1 desensitization is linked to Ca2+-induced activation of apo-CaM that is constitutively bound to the C-terminal CaM-binding site, whereas the slow component might be linked to CaM binding to the N-terminal site in a Ca2+-independent manner (124, 146). Similarly, activation of CaM underlies the inactivation of TRPC1 (165) and TRPV6 (86, 118). Interestingly, activation of CaM may underlie the Ca2+-dependent activation of TRPM4 (120) and the Ca2+-dependent potentiation of TRPV3 (214), TRPV4 (176), and TRPV5 (126). In TRPC1, CaM binding contributes to the delay between Ca2+ release and Ca2+ influx (191). Furthermore, deleting the CaM-binding site reduces surface expression of TRPC3 (202) and TRPV1 (146), suggesting that CaM might also participate in the channel protein trafficking and targeting process.
Similar to CaM, other Ca2+-binding proteins may mediate Ca2+ effects on TRP channel functions. Ca2+-binding protein CaBP1, for example, was shown to inhibit TRPC5 activation by binding to N- and C-terminal sites distinct from the CaM-binding sites (80).
pH
Variation in the pH level occurs in normal physiological processes such as respiratory and metabolic acidosis and alkalosis, and more severely under pathological conditions such as tissue damage, inflammation, and ischemia (10). The acid-sensitive ion channels are known to play important roles in mediating cellular response to environmental pH changes (59). A number of TRP channels are also known to be quite sensitive to environmental and intracellular pH. The effect of pH change on TRP channels can be either potentiation of the channel’s response to other stimuli, direct activation, or suppression of the single-channel current amplitude.
An early report of extracellular pH regulation of TRP channels was on the heat-activated capsaicin receptor, TRPV1, for which proton serves as both a potentiator and an activator (13, 183). It is thought that TRPV1’s sensitivity to acidification may serve as a means to detect painful conditions, especially in deep tissues where the temperature is expected to remain constant (183). Acidification-induced TRPV1 activation under a number of pathological conditions may have important contributions to neuronal pain and cardioprotection. Acidosis below pH 7.0 is perceived as painful in human; acidosis at pH 7.1 to 6.7 initiates cardiac pain (189). At this pH level, TRPV1 activity is affected (6,141,148,183). Tissue damage is a direct cause of proton release from intracellular organelles such as lysosome. Similarly, inflammation-induced pain can be mediated by TRPV1 activation triggered by proton release from affected tissues. Ischemia, the cause of approximately 35% of the total death in the United States, triggers an acute drop of the local pH level and activation of TRPV1, causing the release of calcitonin gene-related peptide (CGRP) and substance P (SP) from these nerve terminals, leading to protective cardiovascular responses in addition to pain (60,63,100,127,144,208). It is believed that Ca2+ permeation through TRPV1 leads to an increase in intracellular Ca2+ concentration and exocytosis, which underlies CGRP and SP release (54). TRPV1 has been shown to mediate changes in heart rate and blood pressure induced by intravenous administration of anandamide (99) and lactic acid-induced bronchoconstriction (117, 221). In human and animal trials, TRPV1 antagonist AMG 517 could induce concentration-dependent hyperthermia and skin vasoconstriction (220). Furthermore, TRPV1 gene knockout has been found to severely impair postischemic recovery in isolated perfused hearts in mice (200).
Within each TRPV1 subunit, there are two major pH-sensing sites, E600 and E648, and a number of potentially pH-sensitive sites, E536, D601, E610, E636, D646, and E651 (amino acid numbers are for rat TRPV1) (Fig. 9) (74,85,149,207). These are highly conserved residues among TRPV1 channels. Consistent with the known pKa values for these acidic residues, the pH-dependent curve for TRPV1 reaches the half-maximal level at around pH 5.5. It is noted that all but one of the pH sites are located in the pore-loop region. Similar observations were made in other pH-sensitive TRP channels. Mutations at a number of pH sites in the TRPV1 pore substantially affect heat activation, raising the possibility that pH regulation of TRPV1 may be mediated by the heat-activation pathway (74). Despite the identification of many acidic residues that may directly or indirectly affect pH activation of TRPV1, how neutralizing acidic residues by protonation produces substantial gating effects remains unclear. It is also unknown whether the sites in the pore-loop and that in the S3-S4 linker region are involved in the same or different gating process. To further complicate the mechanistic understanding of the pH-induced TRPV1 activation process, it is reported that mutations of nontitratable residues in both the outer pore region and the S3–S4 linker region could eliminate pH effects on TRPV1 (2, 149).
Figure 9.
(A) A topology plot of TRPV1 subunit showing the major pH sites E600 and E648, and other pH sites. (B) (Left panel) A structural model of the mouse TRPV1 channel showing the location of E601 (equivalent to E600 of the rat TRPV1 channel; shown in space-filling mode). (Right panel) Potential interaction between E601 and N626.
In addition to TRPV1, a number of TRP channels in the TRPV, TRPM, TRPC, TRPML, TRPA, and TRPP subfamilies were found to be sensitive to pH changes (see Table 1). The list is expected to grow longer as more studies are being attracted to this important form of TRP channel regulation. From the reported cases, interesting schemes already start to emerge. For example, residues critical for pH regulation are often located in the pore region where protonation of the side chain of an acidic residue is expected to exert effects on ion permeation as well as gating transitions. Furthermore, it is often observed that pH affects a TRP channel’s responds to other activation stimuli. It is, at least, theoretically possible that activation of a TRP channel by proton is through its effect on channel activation induced by another stimulus, for example, to shift the channel’s voltage dependence or temperature sensitivity. In this regard, it would be interesting to see whether the distinction between potentiation and activation is supported mechanistically.
One potential complication in the investigation of pH regulation of TRP channel gating is the prominent permeation effect exerted by proton. For TRPV1, it is well documented at both the macroscopic and single-channel levels that extracellular proton can substantially reduce current amplitude (6, 207). The inhibitory effect on permeation partially masks the potentiating and activating effect on channel gating. Recovery from proton inhibition of TRPV1 permeation appears to be rather rapid and faster than recovery of the gating response, yielding a transient increase in the current amplitude upon removal of a low pH solution (207). The “off response” observed in the candidate sour taste receptor formed by PKD1L3 and PKD2L2 (a.k.a. TRPP3) may also come from the relief of conductance inhibition which masks a gating effect (66). While it is expected that proton would interfere with ion permeation along the ion conductance pathway, as is the case for TRPM7 (121), in most cases it remains unknown at what channel site(s) proton exerts the permeation effect. For a number of channels, such as TRPM7 (121) and TRPML1 (168), it was reported that proton acts as a permeant ion as well as a gating regulator.
The suppression of some TRP channels by acidification may originate from the inhibitory effect of proton on channel conductance or/and gating. For example, extracellular acidification (from pH 8.4 to pH 5.4) reduces the single-channel conductance of TRPV5 by near 50% (224). The inhibitory effect is observed also in a TRPV5 channel mutant (E522Q) that lacks a gating response to acidification, confirming a direct interaction between proton and other permeant ions. An intracellular proximal C-terminal titratable residue (K607) was attributed to the sensitivity of TRPV5 to intracellular acidification, which is mediated by a gating conformational change in the pore helix (223).
While most studies have focused on the channel response to acidification, it is known that alkalization also affects TRP channels. Currents from both TRPV1 (31) and TRPA1 (39) can be potentiated by extracellular NH4+, which is thought to be mediated by the resultant intracellular alkalization. It is generally unknown how intracellular alkalization affects TRP channels. For TRPM7, it is attributed to a charge screening effect, though it appears that the PIP2 pathway might be also involved (84). Activity of TRPP3 is potentiated by extracellular alkalization (162). It is unknown whether and to what extent the alkalization effect reflects the removal of proton inhibition of conductance.
Conclusion
Active research in the TRP channel field has yielded rich information on their molecular mechanisms for activation. In many cases, the mechanisms resemble those of other better-understood ion channels but with interest twists that fit the functional role of the TRP channels, whereas in other cases, novel mechanisms appear to underlie unique functions of TRP channels. Much remains to be learned about this important superfamily of ion channels that serve as cellular sensors. Understanding their activation mechanisms will undoubtedly help revealing the multifaceted roles TRP channels play in normal physiology as well as pathophysiological conditions.
Acknowledgments
The author is grateful to Fan Yang and Linlin Ma for comments. Research in his laboratory is supported by funding from NIH (R01NS072377).
References
- 1.Alessandri-Haber N, Dina OA, Chen X, Levine JD. TRPC1 and TRPC6 channels cooperate with TRPV4 to mediate mechanical hyperalgesia and nociceptor sensitization. J Neurosci. 2009;29:6217–6228. doi: 10.1523/JNEUROSCI.0893-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aneiros E, Cao L, Papakosta M, Stevens E, Phillips S, Grimm C. The biophysical and molecular basis of TRPV1 proton gating. EMBO J. 2011;30:994–1002. doi: 10.1038/emboj.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arniges M, Fernandez-Fernandez JM, Albrecht N, Schaefer M, Valverde MA. Human TRPV4 channel splice variants revealed a key role of ankyrin domains in multimerization and trafficking. J Biol Chem. 2006;281:1580–1586. doi: 10.1074/jbc.M511456200. [DOI] [PubMed] [Google Scholar]
- 4.Bai CX, Giamarchi A, Rodat-Despoix L, Padilla F, Downs T, Tsiokas L, Delmas P. Formation of a new receptor-operated channel by heteromeric assembly of TRPP2 and TRPC1 subunits. EMBO Rep. 2008;9:472–479. doi: 10.1038/embor.2008.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bandell M, Dubin AE, Petrus MJ, Orth A, Mathur J, Hwang SW, Patapoutian A. High-throughput random mutagenesis screen reveals TRPM8 residues specifically required for activation by menthol. Nat Neurosci. 2006;9:493–500. doi: 10.1038/nn1665. [DOI] [PubMed] [Google Scholar]
- 6.Baumann TK, Martenson ME. Extracellular protons both increase the activity and reduce the conductance of capsaicin- gated channels. J Neurosci. 2000;20:RC80. doi: 10.1523/JNEUROSCI.20-11-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bezanilla F. How membrane proteins sense voltage. Nat Rev Mol Cell Biol. 2008;9:323–332. doi: 10.1038/nrm2376. [DOI] [PubMed] [Google Scholar]
- 8.Birnbaumer L, Zhu X, Jiang M, Boulay G, Peyton M, Vannier B, Brown D, Platano D, Sadeghi H, Stefani E, Birnbaumer M. On the molecular basis and regulation of cellular capacitative calcium entry: Roles for Trp proteins. Proc Natl Acad Sci U S A. 1996;93:15195–15202. doi: 10.1073/pnas.93.26.15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blount P, Sukharev S, Kung C. A mechanosensitive channel protein and its gene in E. coli. Gravit Space Biol Bull. 1997;10:43–47. [PubMed] [Google Scholar]
- 10.Boron WF. Acid-base physiology. In: Boron WF, Boulpaep EL, editors. Medical Physiology. Philadelphia: Elsevier Saunders; 2005. pp. 633–653. [Google Scholar]
- 11.Brauchi S, Orio P, Latorre R. Clues to understanding cold sensation: Thermodynamics and electrophysiological analysis of the cold receptor TRPM8. Proc Natl Acad Sci U S A. 2004;101:15494–15499. doi: 10.1073/pnas.0406773101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brauchi S, Orta G, Salazar M, Rosenmann E, Latorre R. A hot-sensing cold receptor: C-terminal domain determines thermosensation in transient receptor potential channels. J Neurosci. 2006;26:4835–4840. doi: 10.1523/JNEUROSCI.5080-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 14.Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, O’Donnell D, Nicoll RA, Shah NM, Julius D, Basbaum AI. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci. 2011;31:5067–5077. doi: 10.1523/JNEUROSCI.6451-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang Q, Gyftogianni E, van de Graaf SF, Hoefs S, Weidema FA, Bindels RJ, Hoenderop JG. Molecular determinants in TRPV5 channel assembly. J Biol Chem. 2004;279:54304–54311. doi: 10.1074/jbc.M406222200. [DOI] [PubMed] [Google Scholar]
- 16.Cheng W, Sun C, Zheng J. Heteromerization of TRP channel subunits: Extending functional diversity. Protein Cell. 2010;1:802–810. doi: 10.1007/s13238-010-0108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng W, Yang F, Liu S, Colton CK, Wang C, Cui Y, Cao X, Zhu MX, Sun C, Wang KW, Zheng J. Heteromeric heat-sensitive TRP channels exhibit distinct temperature and chemical response. J Biol Chem. 2012;287:7279–7288. doi: 10.1074/jbc.M111.305045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng W, Yang F, Takanishi CL, Zheng J. Thermosensitive TRPV channel subunits coassemble into heteromeric channels with intermediate conductance and gating properties. J Gen Physiol. 2007;129:191–207. doi: 10.1085/jgp.200709731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chevesich J, Kreuz AJ, Montell C. Requirement for the PDZ domain protein, INAD, for localization of the TRP store-operated channel to a signaling complex. Neuron. 1997;18:95–105. doi: 10.1016/s0896-6273(01)80049-0. [DOI] [PubMed] [Google Scholar]
- 20.Chubanov V, Mederos y Schnitzler M, Waring J, Plank A, Gudermann T. Emerging roles of TRPM6/TRPM7 channel kinase signal transduction complexes. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:334–341. doi: 10.1007/s00210-005-1056-4. [DOI] [PubMed] [Google Scholar]
- 21.Chubanov V, Waldegger S, Mederos y Schnitzler M, Vitzthum H, Sassen MC, Seyberth HW, Konrad M, Gudermann T. Disruption of TRPM6/TRPM7 complex formation by a mutation in the TRPM6 gene causes hypomagnesemia with secondary hypocalcemia. Proc Natl Acad Sci U S A. 2004;101:2894–2899. doi: 10.1073/pnas.0305252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ciura S, Bourque CW. Transient receptor potential vanilloid 1 is required for intrinsic osmoreception in organum vasculosum lamina terminalis neurons and for normal thirst responses to systemic hyperosmolality. J Neurosci. 2006;26:9069–9075. doi: 10.1523/JNEUROSCI.0877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 24.Clapham DE, Miller C. A thermodynamic framework for understanding temperature sensing by transient receptor potential (TRP) channels. Proc Natl Acad Sci U S A. 2011;108:19492–19497. doi: 10.1073/pnas.1117485108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clapham DE, Runnels LW, Strubing C. The TRP ion channel family. Nat Rev Neurosci. 2001;2:387–396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- 26.Cordero-Morales JF, Gracheva EO, Julius D. Cytoplasmic ankyrin repeats of transient receptor potential A1 (TRPA1) dictate sensitivity to thermal and chemical stimuli. Proc Natl Acad Sci U S A. 2011;108:E1184–E1191. doi: 10.1073/pnas.1114124108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cosens DJ, Manning A. Abnormal electroretinogram from a Drosophila mutant. Nature. 1969;224:285–287. doi: 10.1038/224285a0. [DOI] [PubMed] [Google Scholar]
- 28.Cui Y, Yang F, Cao X, Yarov-Yarovoy V, Wang KW, Zheng J. Selective disruption of high-sensitivity heat activation but not capsaicin activation of TRPV1 channels by pore turret mutations. J Gen Physiol. 2012;139:273–283. doi: 10.1085/jgp.201110724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curcio-Morelli C, Zhang P, Venugopal B, Charles FA, Browning MF, Cantiello HF, Slaugenhaupt SA. Functional multimerization of mucolipin channel proteins. J Cell Physiol. 2010;222:328–335. doi: 10.1002/jcp.21956. [DOI] [PubMed] [Google Scholar]
- 30.Cvetkov TL, Huynh KW, Cohen MR, Moiseenkova-Bell VY. Molecular architecture and subunit organization of TRPA1 channel revealed by electron microscopy. J Biol Chem. 2011;286:38168–38176. doi: 10.1074/jbc.M111.288993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhaka A, Uzzell V, Dubin AE, Mathur J, Petrus M, Bandell M, Pat-apoutian A. TRPV1 is activated by both acidic and basic pH. J Neurosci. 2009;29:153–158. doi: 10.1523/JNEUROSCI.4901-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong XP, Cheng X, Mills E, Delling M, Wang F, Kurz T, Xu H. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature. 2008;455:992–996. doi: 10.1038/nature07311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong XP, Wang X, Xu H. TRP channels of intracellular membranes. J Neurochem. 2010;113:313–328. doi: 10.1111/j.1471-4159.2010.06626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Du J, Xie J, Yue L. Modulation of TRPM2 by acidic pH and the underlying mechanisms for pH sensitivity. J Gen Physiol. 2009;134:471–488. doi: 10.1085/jgp.200910254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erickson MG, Alseikhan BA, Peterson BZ, Yue DT. Preassociation of calmodulin with voltage-gated Ca(2+) channels revealed by FRET in single living cells. Neuron. 2001;31:973–985. doi: 10.1016/s0896-6273(01)00438-x. [DOI] [PubMed] [Google Scholar]
- 36.Erler I, Hirnet D, Wissenbach U, Flockerzi V, Niemeyer BA. Ca2+-selective transient receptor potential V channel architecture and function require a specific ankyrin repeat. J Biol Chem. 2004;279:34456–34463. doi: 10.1074/jbc.M404778200. [DOI] [PubMed] [Google Scholar]
- 37.Fan HC, Zhang X, McNaughton PA. Activation of the TRPV4 ion channel is enhanced by phosphorylation. J Biol Chem. 2009;284:27884–27891. doi: 10.1074/jbc.M109.028803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernandez-Ballester G, Ferrer-Montiel A. Molecular modeling of the full-length human TRPV1 channel in closed and desensitized states. J Membr Biol. 2008;223:161–172. doi: 10.1007/s00232-008-9123-7. [DOI] [PubMed] [Google Scholar]
- 39.Fujita F, Uchida K, Moriyama T, Shima A, Shibasaki K, Inada H, Sokabe T, Tominaga M. Intracellular alkalization causes pain sensation through activation of TRPA1 in mice. J Clin Invest. 2008;118:4049–4057. doi: 10.1172/JCI35957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujiwara Y, Minor DL., Jr X-ray crystal structure of a TRPM assembly domain reveals an antiparallel four-stranded coiled-coil. J Mol Biol. 2008;383:854–870. doi: 10.1016/j.jmb.2008.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao X, Wu L, O’Neil RG. Temperature-modulated diversity of TRPV4 channel gating: Activation by physical stresses and phorbol ester derivatives through protein kinase C-dependent and -independent pathways. J Biol Chem. 2003;278:27129–27137. doi: 10.1074/jbc.M302517200. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Sanz N, Fernandez-Carvajal A, Morenilla-Palao C, Planells-Cases R, Fajardo-Sanchez E, Fernandez-Ballester G, Ferrer-Montiel A. Identification of a tetramerization domain in the C terminus of the vanilloid receptor. J Neurosci. 2004;24:5307–5314. doi: 10.1523/JNEUROSCI.0202-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gavva NR, Klionsky L, Qu Y, Shi L, Tamir R, Edenson S, Zhang TJ, Viswanadhan VN, Toth A, Pearce LV, Vanderah TW, Porreca F, Blumberg PM, Lile J, Sun Y, Wild K, Louis JC, Treanor JJ. Molecular determinants of vanilloid sensitivity in TRPV1. J Biol Chem. 2004;279:20283–20295. doi: 10.1074/jbc.M312577200. [DOI] [PubMed] [Google Scholar]
- 44.Gavva NR, Treanor JJ, Garami A, Fang L, Surapaneni S, Akrami A, Alvarez F, Bak A, Darling M, Gore A, Jang GR, Kesslak JP, Ni L, Norman MH, Palluconi G, Rose MJ, Salfi M, Tan E, Romanovsky AA, Banfield C, Davar G. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain. 2008;136:202–210. doi: 10.1016/j.pain.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 45.Goel M, Sinkins WG, Schilling WP. Selective association of TRPC channel subunits in rat brain synaptosomes. J Biol Chem. 2002;277:48303–48310. doi: 10.1074/jbc.M207882200. [DOI] [PubMed] [Google Scholar]
- 46.Gordon-Shaag A, Zagotta WN, Gordon SE. Mechanism of Ca(2+)-dependent desensitization in TRP channels. Channels (Austin) 2008;2:125–129. doi: 10.4161/chan.2.2.6026. [DOI] [PubMed] [Google Scholar]
- 47.Gracheva EO, Ingolia NT, Kelly YM, Cordero-Morales JF, Hollopeter G, Chesler AT, Sanchez EE, Perez JC, Weissman JS, Julius D. Molecular basis of infrared detection by snakes. Nature. 2010;464:1006–1011. doi: 10.1038/nature08943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grandl J, Hu H, Bandell M, Bursulaya B, Schmidt M, Petrus M, Pat-apoutian A. Pore region of TRPV3 ion channel is specifically required for heat activation. Nat Neurosci. 2008;11:1007–1013. doi: 10.1038/nn.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grandl J, Kim SE, Uzzell V, Bursulaya B, Petrus M, Bandell M, Pat-apoutian A. Temperature-induced opening of TRPV1 ion channel is stabilized by the pore domain. Nat Neurosci. 2010;13:708–714. doi: 10.1038/nn.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grigoryan G, Keating AE. Structural specificity in coiled-coil interactions. Curr Opin Struct Biol. 2008;18:477–483. doi: 10.1016/j.sbi.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gudermann T, Hofmann T, Mederos y Schnitzler M, Dietrich A. Activation, subunit composition and physiological relevance of DAG-sensitive TRPC proteins. Novartis Found Symp. 2004;258:103–118. discussion 118–122, 155–109, 263–106. [PubMed] [Google Scholar]
- 52.Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992;8:643–651. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- 53.Hellwig N, Albrecht N, Harteneck C, Schultz G, Schaefer M. Homo-and heteromeric assembly of TRPV channel subunits. J Cell Sci. 2005;118:917–928. doi: 10.1242/jcs.01675. [DOI] [PubMed] [Google Scholar]
- 54.Henrich M, Buckler KJ. Acid-evoked Ca2+ signalling in rat sensory neurones: Effects of anoxia and aglycaemia. Pflugers Arch. 2009;459:159–181. doi: 10.1007/s00424-009-0715-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hille B. Ion Channels of Excitable Membranes. Sunderland, Massachusetts: Sinauer Associates, Inc; 2001. [Google Scholar]
- 56.Hoenderop JG, Voets T, Hoefs S, Weidema F, Prenen J, Nilius B, Bindels RJ. Homo- and heterotetrameric architecture of the epithelial Ca2+ channels TRPV5 and TRPV6. EMBO J. 2003;22:776–785. doi: 10.1093/emboj/cdg080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hofmann T, Chubanov V, Gudermann T, Montell C. TRPM5 is a voltage-modulated and Ca(2+)-activated monovalent selective cation channel. Curr Biol. 2003;13:1153–1158. doi: 10.1016/s0960-9822(03)00431-7. [DOI] [PubMed] [Google Scholar]
- 58.Hofmann T, Schaefer M, Schultz G, Gudermann T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc Natl Acad Sci U S A. 2002;99:7461–7466. doi: 10.1073/pnas.102596199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holzer P. Acid-sensitive ion channels and receptors. Handb Exp Pharmacol. 2009;(Suppl 194):283–332. doi: 10.1007/978-3-540-79090-7_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoover DB. Effects of capsaicin on release of substance P-like immunoreactivity and physiological parameters in isolated perfused guinea-pig heart. Eur J Pharmacol. 1987;141:489–492. doi: 10.1016/0014-2999(87)90571-1. [DOI] [PubMed] [Google Scholar]
- 61.Horrigan FT, Aldrich RW. Allosteric voltage gating of potassium channels II. Mslo channel gating charge movement in the absence of Ca(2+) J Gen Physiol. 1999;114:305–336. doi: 10.1085/jgp.114.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Howard RJ, Clark KA, Holton JM, Minor DL., Jr Structural insight into KCNQ (Kv7) channel assembly and channelopathy. Neuron. 2007;53:663–675. doi: 10.1016/j.neuron.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu CP, Xiao L, Deng HW, Li YJ. The cardioprotection of rutaecarpine is mediated by endogenous calcitonin related-gene peptide through activation of vanilloid receptors in guinea-pig hearts. Planta Med. 2002;68:705–709. doi: 10.1055/s-2002-33794. [DOI] [PubMed] [Google Scholar]
- 64.Huang SM, Li X, Yu Y, Wang J, Caterina MJ. TRPV3 and TRPV4 ion channels are not major contributors to mouse heat sensation. Mol Pain. 2011;7:37. doi: 10.1186/1744-8069-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U. Direct activation of capsaicin receptors by products of lipoxygenases: Endogenous capsaicin-like substances. Proc Natl Acad Sci U S A. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Inada H, Kawabata F, Ishimaru Y, Fushiki T, Matsunami H, Tominaga M. Off-response property of an acid-activated cation channel complex PKD1L3-PKD2L1. EMBO Rep. 2008;9:690–697. doi: 10.1038/embor.2008.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Inoue R, Jensen LJ, Jian Z, Shi J, Hai L, Lurie AI, Henriksen FH, Salomonsson M, Morita H, Kawarabayashi Y, Mori M, Mori Y, Ito Y. Synergistic activation of vascular TRPC6 channel by receptor and mechanical stimulation via phospholipase C/diacylglycerol and phospholipase A2/omega-hydroxylase/20-HETE pathways. Circ Res. 2009;104:1399–1409. doi: 10.1161/CIRCRESAHA.108.193227. [DOI] [PubMed] [Google Scholar]
- 68.Iwata Y, Katanosaka Y, Arai Y, Komamura K, Miyatake K, Shigekawa M. A novel mechanism of myocyte degeneration involving the Ca2+-permeable growth factor-regulated channel. J Cell Biol. 2003;161:957–967. doi: 10.1083/jcb.200301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jahnel R, Dreger M, Gillen C, Bender O, Kurreck J, Hucho F. Biochemical characterization of the vanilloid receptor 1 expressed in a dorsal root ganglia derived cell line. Eur J Biochem. 2001;268:5489–5496. doi: 10.1046/j.1432-1033.2001.02500.x. [DOI] [PubMed] [Google Scholar]
- 70.Jiang J, Li M, Yue L. Potentiation of TRPM7 inward currents by protons. J Gen Physiol. 2005;126:137–150. doi: 10.1085/jgp.200409185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiang LH. Subunit interaction in channel assembly and functional regulation of transient receptor potential melastatin (TRPM) channels. Biochem Soc Trans. 2007;35:86–88. doi: 10.1042/BST0350086. [DOI] [PubMed] [Google Scholar]
- 72.Jin X, Touhey J, Gaudet R. Structure of the N-terminal ankyrin repeat domain of the TRPV2 ion channel. J Biol Chem. 2006;281:25006–25010. doi: 10.1074/jbc.C600153200. [DOI] [PubMed] [Google Scholar]
- 73.Jordt SE, Julius D. Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell. 2002;108:421–430. doi: 10.1016/s0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- 74.Jordt SE, Tominaga M, Julius D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc Natl Acad Sci U S A. 2000;97:8134–8139. doi: 10.1073/pnas.100129497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jung J, Lee SY, Hwang SW, Cho H, Shin J, Kang YS, Kim S, Oh U. Agonist recognition sites in the cytosolic tails of vanilloid receptor 1. J Biol Chem. 2002;277:44448–44454. doi: 10.1074/jbc.M207103200. [DOI] [PubMed] [Google Scholar]
- 76.Kang D, Choe C, Kim D. Thermosensitivity of the two-pore domain K+ channels TREK-2 and TRAAK. J Physiol. 2005;564:103–116. doi: 10.1113/jphysiol.2004.081059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kanzaki M, Zhang YQ, Mashima H, Li L, Shibata H, Kojima I. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat Cell Biol. 1999;1:165–170. doi: 10.1038/11086. [DOI] [PubMed] [Google Scholar]
- 78.Kedei N, Szabo T, Lile JD, Treanor JJ, Olah Z, Iadarola MJ, Blumberg PM. Analysis of the native quaternary structure of vanilloid receptor 1. J Biol Chem. 2001;276:28613–28619. doi: 10.1074/jbc.M103272200. [DOI] [PubMed] [Google Scholar]
- 79.Kim MJ, Jeon JP, Kim HJ, Kim BJ, Lee YM, Choe H, Jeon JH, Kim SJ, So I. Molecular determinant of sensing extracellular pH in classical transient receptor potential channel 5. Biochem Biophys Res Commun. 2008;365:239–245. doi: 10.1016/j.bbrc.2007.10.154. [DOI] [PubMed] [Google Scholar]
- 80.Kinoshita-Kawada M, Tang J, Xiao R, Kaneko S, Foskett JK, Zhu MX. Inhibition of TRPC5 channels by Ca2+-binding protein 1 in Xenopus oocytes. Pflugers Arch. 2005;450:345–354. doi: 10.1007/s00424-005-1419-1. [DOI] [PubMed] [Google Scholar]
- 81.Kloda A, Petrov E, Meyer GR, Nguyen T, Hurst AC, Hool L, Martinac B. Mechanosensitive channel of large conductance. Int J Biochem Cell Biol. 2008;40:164–169. doi: 10.1016/j.biocel.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 82.Kobori T, Smith GD, Sandford R, Edwardson JM. The transient receptor potential channels TRPP2 and TRPC1 form a heterotetramer with a 2:2 stoichiometry and an alternating subunit arrangement. J Biol Chem. 2009;284:35507–35513. doi: 10.1074/jbc.M109.060228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kottgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, Boehlke C, Steffl D, Tauber R, Wegierski T, Nitschke R, Suzuki M, Kramer-Zucker A, Germino GG, Watnick T, Prenen J, Nilius B, Kuehn EW, Walz G. TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol. 2008;182:437–447. doi: 10.1083/jcb.200805124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kozak JA, Matsushita M, Nairn AC, Cahalan MD. Charge screening by internal pH and polyvalent cations as a mechanism for activation, inhibition, and rundown of TRPM7/MIC channels. J Gen Physiol. 2005;126:499–514. doi: 10.1085/jgp.200509324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kuzhikandathil EV, Wang H, Szabo T, Morozova N, Blumberg PM, Oxford GS. Functional analysis of capsaicin receptor (vanilloid receptor subtype 1) multimerization and agonist responsiveness using a dominant negative mutation. J Neurosci. 2001;21:8697–8706. doi: 10.1523/JNEUROSCI.21-22-08697.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lambers TT, Weidema AF, Nilius B, Hoenderop JG, Bindels RJ. Regulation of the mouse epithelial Ca2(+) channel TRPV6 by the Ca(2+)-sensor calmodulin. J Biol Chem. 2004;279:28855–28861. doi: 10.1074/jbc.M313637200. [DOI] [PubMed] [Google Scholar]
- 87.Latorre R, Brauchi S, Orta G, Zaelzer C, Vargas G. ThermoTRP channels as modular proteins with allosteric gating. Cell Calcium. 2007;42:427–438. doi: 10.1016/j.ceca.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 88.Li M, Du J, Jiang J, Ratzan W, Su LT, Runnels LW, Yue L. Molecular determinants of Mg2+ and Ca2+ permeability and pH sensitivity in TRPM6 and TRPM7. J Biol Chem. 2007;282:25817–25830. doi: 10.1074/jbc.M608972200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li M, Jiang J, Yue L. Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J Gen Physiol. 2006;127:525–537. doi: 10.1085/jgp.200609502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liapi A, Wood JN. Extensive co-localization and heteromultimer formation of the vanilloid receptor-like protein TRPV2 and the capsaicin receptor TRPV1 in the adult rat cerebral cortex. Eur J Neurosci. 2005;22:825–834. doi: 10.1111/j.1460-9568.2005.04270.x. [DOI] [PubMed] [Google Scholar]
- 91.Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lishko PV, Procko E, Jin X, Phelps CB, Gaudet R. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron. 2007;54:905–918. doi: 10.1016/j.neuron.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 93.Liu D, Zhang Z, Liman ER. Extracellular acid block and acid-enhanced inactivation of the Ca2+-activated cation channel TRPM5 involve residues in the S3-S4 and S5-S6 extracellular domains. J Biol Chem. 2005;280:20691–20699. doi: 10.1074/jbc.M414072200. [DOI] [PubMed] [Google Scholar]
- 94.Liu M, Chen TY, Ahamed B, Li J, Yau KW. Calcium-calmodulin modulation of the olfactory cyclic nucleotide-gated cation channel. Science. 1994;266:1348–1354. doi: 10.1126/science.266.5189.1348. [DOI] [PubMed] [Google Scholar]
- 95.Liu X, Bandyopadhyay BC, Singh BB, Groschner K, Ambudkar IS. Molecular analysis of a store-operated and 2-acetyl-sn-glycerol-sensitive non-selective cation channel. Heteromeric assembly of TRPC1-TRPC3. J Biol Chem. 2005;280:21600–21606. doi: 10.1074/jbc.C400492200. [DOI] [PubMed] [Google Scholar]
- 96.Long SB, Campbell EB, Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- 97.Ludtke SJ, Baker ML, Chen DH, Song JL, Chuang DT, Chiu W. De novo backbone trace of GroEL from single particle electron cryomicroscopy. Structure. 2008;16:441–448. doi: 10.1016/j.str.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 98.MacKinnon R. Potassium channels. FEBS Lett. 2003;555:62–65. doi: 10.1016/s0014-5793(03)01104-9. [DOI] [PubMed] [Google Scholar]
- 99.Malinowska B, Kwolek G, Gothert M. Anandamide and methanandamide induce both vanilloid VR1- and cannabinoid CB1 receptor-mediated changes in heart rate and blood pressure in anaesthetized rats. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:562–569. doi: 10.1007/s00210-001-0498-6. [DOI] [PubMed] [Google Scholar]
- 100.Manzini S, Perretti F, De Benedetti L, Pradelles P, Maggi CA, Geppetti P. A comparison of bradykinin- and capsaicin-induced myocardial and coronary effects in isolated perfused heart of guinea-pig: Involvement of substance P and calcitonin gene-related peptide release. Br J Pharmacol. 1989;97:303–312. doi: 10.1111/j.1476-5381.1989.tb11955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Maroto R, Raso A, Wood TG, Kurosky A, Martinac B, Hamill OP. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol. 2005;7:179–185. doi: 10.1038/ncb1218. [DOI] [PubMed] [Google Scholar]
- 102.Maruyama Y, Ogura T, Mio K, Kiyonaka S, Kato K, Mori Y, Sato C. Three-dimensional reconstruction using transmission electron microscopy reveals a swollen, bell-shaped structure of transient receptor potential melastatin type 2 cation channel. J Biol Chem. 2007;282:36961–36970. doi: 10.1074/jbc.M705694200. [DOI] [PubMed] [Google Scholar]
- 103.Matta JA, Ahern GP. Voltage is a partial activator of rat thermosensitive TRP channels. J Physiol. 2007;585:469–482. doi: 10.1113/jphysiol.2007.144287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McCleverty CJ, Koesema E, Patapoutian A, Lesley SA, Kreusch A. Crystal structure of the human TRPV2 channel ankyrin repeat domain. Protein Sci. 2006;15:2201–2206. doi: 10.1110/ps.062357206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 106.Mio K, Ogura T, Hara Y, Mori Y, Sato C. The non-selective cation-permeable channel TRPC3 is a tetrahedron with a cap on the large cytoplasmic end. Biochem Biophys Res Commun. 2005;333:768–777. doi: 10.1016/j.bbrc.2005.05.181. [DOI] [PubMed] [Google Scholar]
- 107.Mio K, Ogura T, Kiyonaka S, Hiroaki Y, Tanimura Y, Fujiyoshi Y, Mori Y, Sato C. The TRPC3 channel has a large internal chamber surrounded by signal sensing antennas. J Mol Biol. 2007;367:373–383. doi: 10.1016/j.jmb.2006.12.043. [DOI] [PubMed] [Google Scholar]
- 108.Miyazawa A, Fujiyoshi Y, Unwin N. Structure and gating mechanism of the acetylcholine receptor pore. Nature. 2003;423:949–955. doi: 10.1038/nature01748. [DOI] [PubMed] [Google Scholar]
- 109.Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A, Kimberling WJ, Breuning MH, Deltas CC, Peters DJ, Somlo S. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272:1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- 110.Moiseenkova-Bell VY, Stanciu LA, Serysheva, Tobe BJ, Wensel TG. Structure of TRPV1 channel revealed by electron cryomicroscopy. Proc Natl Acad Sci U S A. 2008;105:7451–7455. doi: 10.1073/pnas.0711835105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: A putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–1323. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- 112.Morita H, Honda A, Inoue R, Ito Y, Abe K, Nelson MT, Brayden JE. Membrane stretch-induced activation of a TRPM4-like nonselective cation channel in cerebral artery myocytes. J Pharmacol Sci. 2007;103:417–426. doi: 10.1254/jphs.fp0061332. [DOI] [PubMed] [Google Scholar]
- 113.Mosavi LK, Cammett TJ, Desrosiers DC, Peng ZY. The ankyrin repeat as molecular architecture for protein recognition. Protein Sci. 2004;13:1435–1448. doi: 10.1110/ps.03554604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, Imaizumi Y. TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ Res. 2003;93:829–838. doi: 10.1161/01.RES.0000097263.10220.0C. [DOI] [PubMed] [Google Scholar]
- 115.Myers BR, Bohlen CJ, Julius D. A yeast genetic screen reveals a critical role for the pore helix domain in TRP channel gating. Neuron. 2008;58:362–373. doi: 10.1016/j.neuron.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 117.Nault MA, Vincent SG, Fisher JT. Mechanisms of capsaicin- and lactic acid-induced bronchoconstriction in the newborn dog. J Physiol. 1999;515(Pt 2):567–578. doi: 10.1111/j.1469-7793.1999.567ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Niemeyer BA, Bergs C, Wissenbach U, Flockerzi V, Trost C. Competitive regulation of CaT-like-mediated Ca2+ entry by protein kinase C and calmodulin. Proc Natl Acad Sci U S A. 2001;98:3600–3605. doi: 10.1073/pnas.051511398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nilius B, Prenen J, Droogmans G, Voets T, Vennekens R, Freichel M, Wissenbach U, Flockerzi V. Voltage dependence of the Ca2+-activated cation channel TRPM4. J Biol Chem. 2003;278:30813–30820. doi: 10.1074/jbc.M305127200. [DOI] [PubMed] [Google Scholar]
- 120.Nilius B, Prenen J, Tang J, Wang C, Owsianik G, Janssens A, Voets T, Zhu MX. Regulation of the Ca2+ sensitivity of the nonselective cation channel TRPM4. J Biol Chem. 2005;280:6423–6433. doi: 10.1074/jbc.M411089200. [DOI] [PubMed] [Google Scholar]
- 121.Numata T, Okada Y. Proton conductivity through the human TRPM7 channel and its molecular determinants. J Biol Chem. 2008;283:15097–15103. doi: 10.1074/jbc.M709261200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Numata T, Shimizu T, Okada Y. Direct mechano-stress sensitivity of TRPM7 channel. Cell Physiol Biochem. 2007;19:1–8. doi: 10.1159/000099187. [DOI] [PubMed] [Google Scholar]
- 123.Numata T, Shimizu T, Okada Y. TRPM7 is a stretch- and swelling-activated cation channel involved in volume regulation in human epithelial cells. Am J Physiol Cell Physiol. 2007;292:C460–C467. doi: 10.1152/ajpcell.00367.2006. [DOI] [PubMed] [Google Scholar]
- 124.Numazaki M, Tominaga T, Takeuchi K, Murayama N, Toyooka H, Tominaga M. Structural determinant of TRPV1 desensitization interacts with calmodulin. Proc Natl Acad Sci U S A. 2003;100:8002–8006. doi: 10.1073/pnas.1337252100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Oancea E, Wolfe JT, Clapham DE. Functional TRPM7 channels accumulate at the plasma membrane in response to fluid flow. Circ Res. 2006;98:245–253. doi: 10.1161/01.RES.0000200179.29375.cc. [DOI] [PubMed] [Google Scholar]
- 126.Ordaz B, Tang J, Xiao R, Salgado A, Sampieri A, Zhu MX, Vaca L. Calmodulin and calcium interplay in the modulation of TRPC5 channel activity. Identification of a novel C-terminal domain for calcium/calmodulin-mediated facilitation. J Biol Chem. 2005;280:30788–30796. doi: 10.1074/jbc.M504745200. [DOI] [PubMed] [Google Scholar]
- 127.Oroszi G, Szilvassy Z, Nemeth J, Ferdinandy P, Szolcsanyi J, Tosaki A. Interaction between capsaicin and nitrate tolerance in isolated guinea-pig heart. Eur J Pharmacol. 1999;368:R1–R3. doi: 10.1016/s0014-2999(99)00056-4. [DOI] [PubMed] [Google Scholar]
- 128.Papakosta M, Dalle C, Haythornthwaite A, Cao L, Stevens EB, Burgess G, Russell R, Cox PJ, Phillips SC, Grimm C. The chimeric approach reveals that differences in the TRPV1 pore domain determine species-specific sensitivity to block of heat activation. J Biol Chem. 2011;286:39663–39672. doi: 10.1074/jbc.M111.273581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pedersen SF, Owsianik G, Nilius B. TRP channels: An overview. Cell Calcium. 2005;38:233–252. doi: 10.1016/j.ceca.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 130.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 131.Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntyre P, Bevan S, Pat-apoutian A. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002;296:2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- 132.Peng H, Lewandrowski U, Muller B, Sickmann A, Walz G, Wegierski T. Identification of a Protein Kinase C-dependent phosphorylation site involved in sensitization of TRPV4 channel. Biochem Biophys Res Commun. 2010;391:1721–1725. doi: 10.1016/j.bbrc.2009.12.140. [DOI] [PubMed] [Google Scholar]
- 133.Peterson BZ, DeMaria CD, Adelman JP, Yue DT. Calmodulin is the Ca2+ sensor for Ca2+-dependent inactivation of L-type calcium channels. Neuron. 1999;22:549–558. doi: 10.1016/s0896-6273(00)80709-6. [DOI] [PubMed] [Google Scholar]
- 134.Phelps CB, Huang RJ, Lishko PV, Wang RR, Gaudet R. Structural analyses of the ankyrin repeat domain of TRPV6 and related TRPV ion channels. Biochemistry. 2008;47:2476–2484. doi: 10.1021/bi702109w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Phelps CB, Wang RR, Choo SS, Gaudet R. Differential regulation of TRPV1, TRPV3, and TRPV4 sensitivity through a conserved binding site on the ankyrin repeat domain. J Biol Chem. 2010;285:731–740. doi: 10.1074/jbc.M109.052548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Phillips AM, Bull A, Kelly LE. Identification of a Drosophila gene encoding a calmodulin-binding protein with homology to the trp pho-totransduction gene. Neuron. 1992;8:631–642. doi: 10.1016/0896-6273(92)90085-r. [DOI] [PubMed] [Google Scholar]
- 137.Plant TD, Schaefer M. TRPC4 and TRPC5: Receptor-operated Ca2+-permeable nonselective cation channels. Cell Calcium. 2003;33:441–450. doi: 10.1016/s0143-4160(03)00055-1. [DOI] [PubMed] [Google Scholar]
- 138.Plant TD, Schaefer M. Receptor-operated cation channels formed by TRPC4 and TRPC5. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:266–276. doi: 10.1007/s00210-005-1055-5. [DOI] [PubMed] [Google Scholar]
- 139.Poteser M, Graziani A, Rosker C, Eder P, Derler I, Kahr H, Zhu MX, Romanin C, Groschner K. TRPC3 and TRPC4 associate to form a redox-sensitive cation channel. Evidence for expression of native TRPC3-TRPC4 heteromeric channels in endothelial cells. J Biol Chem. 2006;281:13588–13595. doi: 10.1074/jbc.M512205200. [DOI] [PubMed] [Google Scholar]
- 140.Pusch M, Ludewig U, Jentsch TJ. Temperature dependence of fast and slow gating relaxations of ClC-0 chloride channels. J Gen Physiol. 1997;109:105–116. doi: 10.1085/jgp.109.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Qin F, Auerbach A, Sachs F. Maximum likelihood estimation of aggregated Markov processes. Proc Biol Sci. 1997;264:375–383. doi: 10.1098/rspb.1997.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- 143.Ramsey IS, Moran MM, Chong JA, Clapham DE. A voltage-gated proton-selective channel lacking the pore domain. Nature. 2006;440:1213–1216. doi: 10.1038/nature04700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rang WQ, Du YH, Hu CP, Ye F, Xu KP, Peng J, Deng HW, Li YJ. Protective effects of evodiamine on myocardial ischemia-reperfusion injury in rats. Planta Med. 2004;70:1140–1143. doi: 10.1055/s-2004-835841. [DOI] [PubMed] [Google Scholar]
- 145.Rhoads AR, Friedberg F. Sequence motifs for calmodulin recognition. FASEB J. 1997;11:331–340. doi: 10.1096/fasebj.11.5.9141499. [DOI] [PubMed] [Google Scholar]
- 146.Rosenbaum T, Gordon-Shaag A, Munari M, Gordon SE. Ca2+/calmodulin modulates TRPV1 activation by capsaicin. J Gen Physiol. 2004;123:53–62. doi: 10.1085/jgp.200308906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rutter AR, Ma QP, Leveridge M, Bonnert TP. Heteromerization and colocalization of TrpV1 and TrpV2 in mammalian cell lines and rat dorsal root ganglia. Neuroreport. 2005;16:1735–1739. doi: 10.1097/01.wnr.0000185958.03841.0f. [DOI] [PubMed] [Google Scholar]
- 148.Ryu S, Liu B, Qin F. Low pH potentiates both capsaicin binding and channel gating of VR1 receptors. J Gen Physiol. 2003;122:45–61. doi: 10.1085/jgp.200308847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ryu S, Liu B, Yao J, Fu Q, Qin F. Uncoupling proton activation of vanilloid receptor TRPV1. J Neurosci. 2007;27:12797–12807. doi: 10.1523/JNEUROSCI.2324-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sachs F. Stretch-activated ion channels: What are they? Physiology (Bethesda) 2010;25:50–56. doi: 10.1152/physiol.00042.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sachs F, Sokabe M. Stretch-activated ion channels and membrane mechanics. Neurosci Res Suppl. 1990;12:S1–S4. doi: 10.1016/0921-8696(90)90003-l. [DOI] [PubMed] [Google Scholar]
- 152.Salas MM, Hargreaves KM, Akopian AN. TRPA1-mediated responses in trigeminal sensory neurons: Interaction between TRPA1 and TRPV1. Eur J Neurosci. 2009;29:1568–1578. doi: 10.1111/j.1460-9568.2009.06702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schaefer M. Homo- and heteromeric assembly of TRP channel subunits. Pflugers Arch. 2005;451:35–42. doi: 10.1007/s00424-005-1467-6. [DOI] [PubMed] [Google Scholar]
- 154.Schoppa NE, McCormack K, Tanouye MA, Sigworth FJ. The size of gating charge in wild-type and mutant Shaker potassium channels. Science. 1992;255:1712–1715. doi: 10.1126/science.1553560. [DOI] [PubMed] [Google Scholar]
- 155.Schoppa NE, Sigworth FJ. Activation of Shaker potassium channels. III. An activation gating model for wild-type and V2 mutant channels. J Gen Physiol. 1998;111:313–342. doi: 10.1085/jgp.111.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sedgwick SG, Smerdon SJ. The ankyrin repeat: A diversity of interactions on a common structural framework. Trends Biochem Sci. 1999;24:311–316. doi: 10.1016/s0968-0004(99)01426-7. [DOI] [PubMed] [Google Scholar]
- 157.Semtner M, Schaefer M, Pinkenburg O, Plant TD. Potentiation of TRPC5 by protons. J Biol Chem. 2007;282:33868–33878. doi: 10.1074/jbc.M702577200. [DOI] [PubMed] [Google Scholar]
- 158.Sharif Naeini R, Witty MF, Seguela P, Bourque CW. An N-terminal variant of Trpv1 channel is required for osmosensory transduction. Nat Neurosci. 2006;9:93–98. doi: 10.1038/nn1614. [DOI] [PubMed] [Google Scholar]
- 159.Sherkheli MA, Benecke H, Doerner JF, Kletke O, Vogt-Eisele AK, Gisselmann G, Hatt H. Monoterpenoids induce agonist-specific desensitization of transient receptor potential vanilloid-3 (TRPV3) ion channels. J Pharm Pharm Sci. 2009;12:116–128. doi: 10.18433/j37c7k. [DOI] [PubMed] [Google Scholar]
- 160.Sherkheli MA, Vogt-Eisele AK, Bura D, Beltran Marques LR, Gisselmann G, Hatt H. Characterization of selective TRPM8 ligands and their structure activity response (S.A.R) relationship. J Pharm Pharm Sci. 2010;13:242–253. doi: 10.18433/j3n88n. [DOI] [PubMed] [Google Scholar]
- 161.Shigematsu H, Sokabe T, Danev R, Tominaga M, Nagayama K. A 3.5-nm structure of rat TRPV4 cation channel revealed by Zernike phase-contrast cryoelectron microscopy. J Biol Chem. 2010;285:11210–11218. doi: 10.1074/jbc.M109.090712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shimizu T, Janssens A, Voets T, Nilius B. Regulation of the murine TRPP3 channel by voltage, pH, and changes in cell volume. Pflugers Arch. 2009;457:795–807. doi: 10.1007/s00424-008-0558-6. [DOI] [PubMed] [Google Scholar]
- 163.Shuart NG, Haitin Y, Camp SS, Black KD, Zagotta WN. Molecular mechanism for 3:1 subunit stoichiometry of rod cyclic nucleotide-gated ion channels. Nat Commun. 2011;2:457. doi: 10.1038/ncomms1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sigworth FJ. Voltage gating of ion channels. Q Rev Biophys. 1994;27:1–40. doi: 10.1017/s0033583500002894. [DOI] [PubMed] [Google Scholar]
- 165.Singh BB, Liu X, Tang J, Zhu MX, Ambudkar IS. Calmodulin regulates Ca(2+)-dependent feedback inhibition of store-operated Ca(2+) influx by interaction with a site in the C terminus of TrpC1. Mol Cell. 2002;9:739–750. doi: 10.1016/s1097-2765(02)00506-3. [DOI] [PubMed] [Google Scholar]
- 166.Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, Egerton J, Charles KJ, Smart D, Randall AD, Anand P, Davis JB. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418:186–190. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- 167.Sokolova O, Kolmakova-Partensky L, Grigorieff N. Three-dimensional structure of a voltage-gated potassium channel at 2.5 nm resolution. Structure. 2001;9:215–220. doi: 10.1016/s0969-2126(01)00578-0. [DOI] [PubMed] [Google Scholar]
- 168.Soyombo AA, Tjon-Kon-Sang S, Rbaibi Y, Bashllari E, Bisceglia J, Muallem S, Kiselyov K. TRP-ML1 regulates lysosomal pH and acidic lysosomal lipid hydrolytic activity. J Biol Chem. 2006;281:7294–7301. doi: 10.1074/jbc.M508211200. [DOI] [PubMed] [Google Scholar]
- 169.Spassova MA, Hewavitharana T, Xu W, Soboloff J, Gill DL. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci U S A. 2006;103:16586–16591. doi: 10.1073/pnas.0606894103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Spencer RH, Chang G, Rees DC. ‘Feeling the pressure’: Structural insights into a gated mechanosensitive channel. Curr Opin Struct Biol. 1999;9:448–454. doi: 10.1016/S0959-440X(99)80063-3. [DOI] [PubMed] [Google Scholar]
- 171.Spyridaki A, Fritzsch G, Kouimtzoglou E, Baciou L, Ghanotakis D. The natural product capsaicin inhibits photosynthetic electron transport at the reducing side of photosystem II and purple bacterial reaction center: Structural details of capsaicin binding. Biochim Biophys Acta. 2000;1459:69–76. doi: 10.1016/s0005-2728(00)00114-6. [DOI] [PubMed] [Google Scholar]
- 172.Stewart AP, Smith GD, Sandford RN, Edwardson JM. Atomic force microscopy reveals the alternating subunit arrangement of the TRPP2-TRPV4 heterotetramer. Biophys J. 2010;99:790–797. doi: 10.1016/j.bpj.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Storch U, Forst AL, Philipp M, Gudermann T, Mederos YSM. TRPC1 reduces the calcium permeability in heteromeric channel complexes. J Biol Chem. 2011;287:3530–3540. doi: 10.1074/jbc.M111.283218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 175.Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol. 2000;2:695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- 176.Strotmann R, Schultz G, Plant TD. Ca2+-dependent potentiation of the nonselective cation channel TRPV4 is mediated by a C-terminal calmodulin binding site. J Biol Chem. 2003;278:26541–26549. doi: 10.1074/jbc.M302590200. [DOI] [PubMed] [Google Scholar]
- 177.Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron. 2001;29:645–655. doi: 10.1016/s0896-6273(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 178.Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J Biol Chem. 2003;278:39014–39019. doi: 10.1074/jbc.M306705200. [DOI] [PubMed] [Google Scholar]
- 179.Takahashi N, Mizuno Y, Kozai D, Yamamoto S, Kiyonaka S, Shibata T, Uchida K, Mori Y. Molecular characterization of TRPA1 channel activation by cysteine-reactive inflammatory mediators. Channels (Austin) 2008;2:287–298. doi: 10.4161/chan.2.4.6745. [DOI] [PubMed] [Google Scholar]
- 180.Talavera K, Yasumatsu K, Voets T, Droogmans G, Shigemura N, Ni-nomiya Y, Margolskee RF, Nilius B. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature. 2005;438:1022–1025. doi: 10.1038/nature04248. [DOI] [PubMed] [Google Scholar]
- 181.Tang J, Lin Y, Zhang Z, Tikunova S, Birnbaumer L, Zhu MX. Identification of common binding sites for calmodulin and inositol 1,4,5-trisphosphate receptors on the carboxyl termini of trp channels. J Biol Chem. 2001;276:21303–21310. doi: 10.1074/jbc.M102316200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tokumitsu H, Muramatsu M, Ikura M, Kobayashi R. Regulatory mechanism of Ca2+/calmodulin-dependent protein kinase kinase. J Biol Chem. 2000;275:20090–20095. doi: 10.1074/jbc.M002193200. [DOI] [PubMed] [Google Scholar]
- 183.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 184.Tribet C, Audebert R, Popot JL. Amphipols: Polymers that keep membrane proteins soluble in aqueous solutions. Proc Natl Acad Sci U S A. 1996;93:15047–15050. doi: 10.1073/pnas.93.26.15047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Trost C, Marquart A, Zimmer S, Philipp S, Cavalie A, Flockerzi V. Ca2+-dependent interaction of the trpl cation channel and calmodulin. FEBS Lett. 1999;451:257–263. doi: 10.1016/s0014-5793(99)00588-8. [DOI] [PubMed] [Google Scholar]
- 186.Trudeau MC, Zagotta WN. Calcium/calmodulin modulation of olfactory and rod cyclic nucleotide-gated ion channels. J Biol Chem. 2003;278:18705–18708. doi: 10.1074/jbc.R300001200. [DOI] [PubMed] [Google Scholar]
- 187.Tsiokas L, Arnould T, Zhu C, Kim E, Walz G, Sukhatme VP. Specific association of the gene product of PKD2 with the TRPC1 channel. Proc Natl Acad Sci U S A. 1999;96:3934–3939. doi: 10.1073/pnas.96.7.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tsuruda PR, Julius D, Minor DL., Jr Coiled coils direct assembly of a cold-activated TRP channel. Neuron. 2006;51:201–212. doi: 10.1016/j.neuron.2006.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ugawa S, Ueda T, Ishida Y, Nishigaki M, Shibata Y, Shimada S. Amiloride-blockable acid-sensing ion channels are leading acid sensors expressed in human nociceptors. J Clin Invest. 2002;110:1185–1190. doi: 10.1172/JCI15709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J Mol Biol. 2005;346:967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 191.Vaca L, Sampieri A. Calmodulin modulates the delay period between release of calcium from internal stores and activation of calcium influx via endogenous TRP1 channels. J Biol Chem. 2002;277:42178–42187. doi: 10.1074/jbc.M204531200. [DOI] [PubMed] [Google Scholar]
- 192.Varnum MD, Zagotta WN. Interdomain interactions underlying activation of cyclic nucleotide-gated channels. Science. 1997;278:110–113. doi: 10.1126/science.278.5335.110. [DOI] [PubMed] [Google Scholar]
- 193.Venkatachalam K, Hofmann T, Montell C. Lysosomal localization of TRPML3 depends on TRPML2 and the mucolipidosis-associated protein TRPML1. J Biol Chem. 2006;281:17517–17527. doi: 10.1074/jbc.M600807200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Vennekens R, Voets T, Bindels RJ, Droogmans G, Nilius B. Current understanding of mammalian TRP homologues. Cell Calcium. 2002;31:253–264. doi: 10.1016/s0143-4160(02)00055-6. [DOI] [PubMed] [Google Scholar]
- 195.Vlachova V, Teisinger J, Susankova K, Lyfenko A, Ettrich R, Vyklicky L. Functional role of C-terminal cytoplasmic tail of rat vanilloid receptor 1. J Neurosci. 2003;23:1340–1350. doi: 10.1523/JNEUROSCI.23-04-01340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004;430:748–754. doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- 197.Voets T, Owsianik G, Janssens A, Talavera K, Nilius B. TRPM8 voltage sensor mutants reveal a mechanism for integrating thermal and chemical stimuli. Nat Chem Biol. 2007;3:174–182. doi: 10.1038/nchembio862. [DOI] [PubMed] [Google Scholar]
- 198.Vogt-Eisele AK, Weber K, Sherkheli MA, Vielhaber G, Panten J, Gisselmann G, Hatt H. Monoterpenoid agonists of TRPV3. Br J Pharmacol. 2007;151:530–540. doi: 10.1038/sj.bjp.0707245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci U S A. 2004;101:396–401. doi: 10.1073/pnas.0303329101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wang L, Wang DH. TRPV1 gene knockout impairs postischemic recovery in isolated perfused heart in mice. Circulation. 2005;112:3617–3623. doi: 10.1161/CIRCULATIONAHA.105.556274. [DOI] [PubMed] [Google Scholar]
- 201.Warr CG, Kelly LE. Identification and characterization of two distinct calmodulin-binding sites in the Trpl ion-channel protein of Drosophila melanogaster. Biochem J. 1996;314(Pt 2):497–503. doi: 10.1042/bj3140497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wedel BJ, Vazquez G, McKay RR, St JBG, Putney JW., Jr A calmodulin/inositol 1,4,5-trisphosphate (IP3) receptor-binding region targets TRPC3 to the plasma membrane in a calmodulin/IP3 receptor-independent process. J Biol Chem. 2003;278:25758–25765. doi: 10.1074/jbc.M303890200. [DOI] [PubMed] [Google Scholar]
- 203.Wegierski T, Lewandrowski U, Muller B, Sickmann A, Walz G. Tyrosine phosphorylation modulates the activity of TRPV4 in response to defined stimuli. J Biol Chem. 2009;284:2923–2933. doi: 10.1074/jbc.M805357200. [DOI] [PubMed] [Google Scholar]
- 204.Wei C, Wang X, Chen M, Ouyang K, Song LS, Cheng H. Calcium flickers steer cell migration. Nature. 2009;457:901–905. doi: 10.1038/nature07577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wei ET, Seid DA. AG-3-5: A chemical producing sensations of cold. J Pharm Pharmacol. 1983;35:110–112. doi: 10.1111/j.2042-7158.1983.tb04279.x. [DOI] [PubMed] [Google Scholar]
- 206.Weitz D, Ficek N, Kremmer E, Bauer PJ, Kaupp UB. Subunit stoichiometry of the CNG channel of rod photoreceptors. Neuron. 2002;36:881–889. doi: 10.1016/s0896-6273(02)01098-x. [DOI] [PubMed] [Google Scholar]
- 207.Welch JM, Simon SA, Reinhart PH. The activation mechanism of rat vanilloid receptor 1 by capsaicin involves the pore domain and differs from the activation by either acid or heat. Proc Natl Acad Sci U S A. 2000;97:13889–13894. doi: 10.1073/pnas.230146497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wharton J, Gulbenkian S, Mulderry PK, Ghatei MA, McGregor GP, Bloom SR, Polak JM. Capsaicin induces a depletion of calcitonin gene-related peptide (CGRP)-immunoreactive nerves in the cardiovascular system of the guinea pig and rat. J Auton Nerv Syst. 1986;16:289–309. doi: 10.1016/0165-1838(86)90035-4. [DOI] [PubMed] [Google Scholar]
- 209.Wissenbach U, Bodding M, Freichel M, Flockerzi V. Trp12, a novel Trp related protein from kidney. FEBS Lett. 2000;485:127–134. doi: 10.1016/s0014-5793(00)02212-2. [DOI] [PubMed] [Google Scholar]
- 210.Wu L, Gao X, Brown RC, Heller S, O’Neil RG. Dual role of the TRPV4 channel as a sensor of flow and osmolality in renal epithelial cells. Am J Physiol Renal Physiol. 2007;293:F1699–F1713. doi: 10.1152/ajprenal.00462.2006. [DOI] [PubMed] [Google Scholar]
- 211.Wu LJ, Sweet TB, Clapham DE International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev. 2010;62:381–404. doi: 10.1124/pr.110.002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Xia XM, Fakler B, Rivard A, Wayman G, Johnson-Pais T, Keen JE, Ishii T, Hirschberg B, Bond CT, Lutsenko S, Maylie J, Adelman JP. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature. 1998;395:503–507. doi: 10.1038/26758. [DOI] [PubMed] [Google Scholar]
- 213.Xiao B, Coste B, Mathur J, Patapoutian A. Temperature-dependent STIM1 activation induces Ca(2)+ influx and modulates gene expression. Nat Chem Biol. 2011;7:351–358. doi: 10.1038/nchembio.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Xiao R, Tang J, Wang C, Colton CK, Tian J, Zhu MX. Calcium plays a central role in the sensitization of TRPV3 channel to repetitive stimulations. J Biol Chem. 2008;283:6162–6174. doi: 10.1074/jbc.M706535200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Xu H, Delling M, Jun JC, Clapham DE. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci. 2006;9:628–635. doi: 10.1038/nn1692. [DOI] [PubMed] [Google Scholar]
- 216.Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, DiStefano PS, Curtis R, Clapham DE. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- 217.Xu H, Zhao H, Tian W, Yoshida K, Roullet JB, Cohen DM. Regulation of a transient receptor potential (TRP) channel by tyrosine phosphorylation. SRC family kinase-dependent tyrosine phosphorylation of TRPV4 on TYR-253 mediates its response to hypotonic stress. J Biol Chem. 2003;278:11520–11527. doi: 10.1074/jbc.M211061200. [DOI] [PubMed] [Google Scholar]
- 218.Yamaguchi H, Matsushita M, Nairn AC, Kuriyan J. Crystal structure of the atypical protein kinase domain of a TRP channel with phosphotransferase activity. Mol Cell. 2001;7:1047–1057. doi: 10.1016/s1097-2765(01)00256-8. [DOI] [PubMed] [Google Scholar]
- 219.Yang F, Cui Y, Wang K, Zheng J. Thermosensitive TRP channel pore turret is part of the temperature activation pathway. Proc Natl Acad Sci U S A. 2010;107:7083–7088. doi: 10.1073/pnas.1000357107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Yao J, Liu B, Qin F. Kinetic and energetic analysis of thermally activated TRPV1 channels. Biophys J. 99:1743–1753. doi: 10.1016/j.bpj.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Yao J, Liu B, Qin F. Rapid temperature jump by infrared diode laser irradiation for patch-clamp studies. Biophys J. 2009;96:3611–3619. doi: 10.1016/j.bpj.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yao J, Liu B, Qin F. Modular thermal sensors in temperature-gated transient receptor potential (TRP) channels. Proc Natl Acad Sci U S A. 2011;108:11109–11114. doi: 10.1073/pnas.1105196108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Yeh BI, Kim YK, Jabbar W, Huang CL. Conformational changes of pore helix coupled to gating of TRPV5 by protons. EMBO J. 2005;24:3224–3234. doi: 10.1038/sj.emboj.7600795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Yeh BI, Sun TJ, Lee JZ, Chen HH, Huang CL. Mechanism and molecular determinant for regulation of rabbit transient receptor potential type 5 (TRPV5) channel by extracellular pH. J Biol Chem. 2003;278:51044–51052. doi: 10.1074/jbc.M306326200. [DOI] [PubMed] [Google Scholar]
- 225.Yellen G. The moving parts of voltage-gated ion channels. Q Rev Bio-phys. 1998;31:239–295. doi: 10.1017/s0033583598003448. [DOI] [PubMed] [Google Scholar]
- 226.Yildirim E, Dietrich A, Birnbaumer L. The mouse C-type transient receptor potential 2 (TRPC2) channel: Alternative splicing and calmodulin binding to its N terminus. Proc Natl Acad Sci U S A. 2003;100:2220–2225. doi: 10.1073/pnas.0438036100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Yu Y, Ulbrich MH, Li MH, Buraei Z, Chen XZ, Ong AC, Tong L, Isacoff EY, Yang J. Structural and molecular basis of the assembly of the TRPP2/PKD1 complex. Proc Natl Acad Sci U S A. 2009;106:11558–11563. doi: 10.1073/pnas.0903684106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zagotta WN, Hoshi T, Dittman J, Aldrich RW. Shaker potassium channel gating. II: Transitions in the activation pathway. J Gen Physiol. 1994;103:279–319. doi: 10.1085/jgp.103.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zagotta WN, Olivier NB, Black KD, Young EC, Olson R, Gouaux E. Structural basis for modulation and agonist specificity of HCN pacemaker channels. Nature. 2003;425:200–205. doi: 10.1038/nature01922. [DOI] [PubMed] [Google Scholar]
- 230.Zagranichnaya TK, Wu X, Villereal ML. Endogenous TRPC1, TRPC3, and TRPC7 proteins combine to form native store-operated channels in HEK-293 cells. J Biol Chem. 2005;280:29559–29569. doi: 10.1074/jbc.M505842200. [DOI] [PubMed] [Google Scholar]
- 231.Zakharian E, Cao C, Rohacs T. Gating of transient receptor potential melastatin 8 (TRPM8) channels activated by cold and chemical agonists in planar lipid bilayers. J Neurosci. 2010;30:12526–12534. doi: 10.1523/JNEUROSCI.3189-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zakharian E, Thyagarajan B, French RJ, Pavlov E, Rohacs T. Inorganic polyphosphate modulates TRPM8 channels. PLoS One. 2009;4:e5404. doi: 10.1371/journal.pone.0005404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zhang F, Liu S, Yang F, Zheng J, Wang K. Identification of a tetrameric assembly domain in the C terminus of heat-activated TRPV1 channels. J Biol Chem. 2011;286:15308–15316. doi: 10.1074/jbc.M111.223941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zhang P, Luo Y, Chasan B, Gonzalez-Perrett S, Montalbetti N, Timpanaro GA, Cantero Mdel R, Ramos AJ, Goldmann WH, Zhou J, Cantiello HF. The multimeric structure of polycystin-2 (TRPP2): Structural-functional correlates of homo- and hetero-multimers with TRPC1. Hum Mol Genet. 2009;18:1238–1251. doi: 10.1093/hmg/ddp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zhang Z, Tang J, Tikunova S, Johnson JD, Chen Z, Qin N, Dietrich A, Stefani E, Birnbaumer L, Zhu MX. Activation of Trp3 by inositol 1,4,5-trisphosphate receptors through displacement of inhibitory calmodulin from a common binding domain. Proc Natl Acad Sci U S A. 2001;98:3168–3173. doi: 10.1073/pnas.051632698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zheng J, Trudeau MC, Zagotta WN. Rod cyclic nucleotide-gated channels have a stoichiometry of three CNGA1 subunits and one CNGB1 subunit. Neuron. 2002;36:891–896. doi: 10.1016/s0896-6273(02)01099-1. [DOI] [PubMed] [Google Scholar]
- 237.Zhong H, Molday LL, Molday RS, Yau KW. The heteromeric cyclic nucleotide-gated channel adopts a 3A:1B stoichiometry. Nature. 2002;420:193–198. doi: 10.1038/nature01201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Zhong L, Bellemer A. Thermosensory and nonthermosensory isoforms of Drosophila melanogaster TRPA1 reveal heat-sensor domains of a thermoTRP channel. Cell Reports. 2012;1:43–55. doi: 10.1016/j.celrep.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zhu MX. Multiple roles of calmodulin and other Ca(2+)-binding proteins in the functional regulation of TRP channels. Pflugers Arch. 2005;451:105–115. doi: 10.1007/s00424-005-1427-1. [DOI] [PubMed] [Google Scholar]
- 240.Zhu X, Jiang M, Peyton M, Boulay G, Hurst R, Stefani E, Birnbaumer L. trp, a novel mammalian gene family essential for agonist-activated capacitative Ca2+ entry. Cell. 1996;85:661–671. doi: 10.1016/s0092-8674(00)81233-7. [DOI] [PubMed] [Google Scholar]
- 241.Zimmermann K, Lennerz JK, Hein A, Link AS, Kaczmarek JS, Delling M, Uysal S, Pfeifer JD, Riccio A, Clapham DE. Transient receptor potential cation channel, subfamily C, member 5 (TRPC5) is a cold-transducer in the peripheral nervous system. Proc Natl Acad Sci U S A. 2011;108:18114–18119. doi: 10.1073/pnas.1115387108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
Further Reading
- 1.Hille Bertil. Ion Channels of Excitable Membranes. 3. Sunderland: Sinauer Associates, Inc; 2001. [Google Scholar]