Abstract
Objective:
We explored the relationship between the site of vascular occlusion and the response to endovascular treatment in patients with acute ischemic stroke and also considered the impact of mismatch profile.
Methods:
DEFUSE-2 was a prospective cohort study of patients treated with endovascular therapy. Patients with internal carotid artery (ICA) and middle cerebral artery (MCA) involvement were included in this substudy. Mismatch and reperfusion status was assessed on MRI. Favorable clinical response was defined as an improvement of at least 8 points on the NIH Stroke Scale.
Results:
Reperfusion rates were comparable in both groups (61% for ICA and 59% for MCA). In the setting of reperfusion, percentages of favorable clinical response were similar between patients with stroke due to ICA (65%) and MCA (63%) occlusions. When reperfusion was not achieved, favorable outcomes were less frequent with obstructions of the ICA (9%) than the MCA (52%). Among target mismatch patients, the adjusted odds ratio for favorable clinical response associated with reperfusion was 39.7 (95% confidence interval 1.4–1,132.8) for ICA occlusions vs 5.1 (95% confidence interval 1.4–19.3) for MCA occlusions.
Conclusions:
Endovascular reperfusion is associated with favorable clinical response regardless of the location of the arterial occlusion. This association is strongest for target mismatch patients with ICA occlusions. Target mismatch patients with either ICA or MCA occlusions appear to be good candidates for endovascular reperfusion therapy.
Patients with internal carotid artery (ICA) occlusions have worse outcomes after IV tissue plasminogen activator therapy compared to patients with middle cerebral artery (MCA) occlusions.1–6 Whether patients with ICA occlusions also have worse outcomes than patients with MCA occlusions in the setting of endovascular treatment is less well known. An early study of endovascular therapy reported lower recanalization rates for lesions at the terminus of the ICA.7 In the pooled analysis of the Mechanical Embolus Removal in Cerebral Ischemia (MERCI) and multi-MERCI studies, ICA occlusion was associated with an approximate 2-fold increased chance of mortality after adjusting for reperfusion, baseline NIH Stroke Scale (NIHSS) score, and age.8 These findings have been confirmed in a prospective study of more than 600 patients with acute stroke in the anterior circulation.9 Finally, a systematic review of studies on mechanical therapy suggested that patients with MCA occlusions benefited the most from mechanical endovascular therapy.10 Such reports may lead to reluctance in using endovascular therapy for patients with ICA occlusions. In contrast, a recent systematic review that showed better clinical outcomes with endovascular therapy than with IV thrombolysis for patients with ICA occlusions might encourage the use of endovascular therapy in this population.11
In the Diffusion Weighted Imaging Evaluation for Understanding Stroke Evolution–2 (DEFUSE-2) study, the ability of MRI to identify a patient subgroup (target mismatch) that was likely to show clinical improvement after endovascular reperfusion was assessed.12 This substudy was designed to test whether the site of vascular occlusion (ICA vs MCA) is a predictor of the clinical response after endovascular reperfusion in target mismatch patients.
METHODS
The DEFUSE-2 study comprised a multicenter prospective cohort of ischemic stroke patients who underwent a baseline MRI scan before endovascular therapy. The study was conducted between 2008 and 2011 in 8 centers in the United States and one in Austria. The full methodology of the DEFUSE-2 study has been described previously.12 In brief, patients were eligible if they had an NIHSS score of 5 or more, if a baseline MRI could be obtained within 90 minutes before endovascular therapy, and if the endovascular procedure was anticipated to begin within 12 hours after stroke onset. Patients with wake-up strokes could be included if they were eligible according to these criteria. The endovascular interventions conformed to the practices that were used at the local institutions. No set technique or device choice for intervention was mandated. In patients with proximal ICA occlusions, angioplasty with or without stenting was allowed. Each patient underwent 3 MRI scans: at baseline, within 12 hours after the endovascular procedure, and on day 5. Assessment of the target mismatch profile was based on perfusion-weighted imaging (PWI) and diffusion-weighted imaging (DWI) lesion volumes determined with RAPID image processing software.13 The local investigator's assessment of target mismatch status was used for all analyses. Baseline and early follow-up PWI lesion volumes as well as day 5 infarct volumes based on fluid-attenuated inversion recovery images were determined by investigators at the core imaging laboratory. Reperfusion was defined as a more than 50% reduction in PWI lesion volume between baseline and early follow-up. When the early follow-up PWI was not available or was of insufficient quality, reperfusion was instead assessed on digital subtraction angiography and defined as a more than 50% reduction in tissue with impaired perfusion between the start and the completion of the angiographic procedure (i.e., thrombolysis in cerebral infarction score 2b or 3). Thrombolysis in cerebral infarction scores were assigned by a single reader (M.P.M.) at the core imaging laboratory according to previously described criteria.14
The primary clinical endpoint in the DEFUSE-2 study was favorable clinical response: an improvement of at least 8 points on the NIHSS between baseline and day 30 or a score of 0–1 at day 30. We used the same endpoint in this post hoc analysis of the DEFUSE-2 study to evaluate the association between site of vascular occlusion (proximal and distal ICA vs M1, M2, and M3 occlusions) and response to reperfusion. This association was also analyzed using functional outcomes at day 90 assessed with the modified Rankin scale (mRS) score.
Statistical analyses were performed with SAS/STAT software (version 9.3; SAS Institute, Cary, NC) and SPSS (version 21.0; IBM Corp., Armonk, NY). The Fisher exact test was used to compare categorical variables, the Student t test for comparison of parametric continuous variables, and the Mann-Whitney U test for nonparametric continuous variables. Multivariable logistic regression was used to determine interactions and to adjust odds ratios (ORs) for variables (DWI lesion volume and age) previously reported to be associated with outcome after reperfusion.12
Standard protocol approvals, registrations, and patient consents.
Approval for the study was obtained from local institutional review boards. Written informed consent was obtained from the patients or a relative if the patient was unable.
RESULTS
For this substudy, one patient with anterior cerebral artery occlusion was excluded. The remaining 98 of 99 patients from the DEFUSE-2 cohort with MCA or ICA involvement were included in this study. Twenty-eight patients had an ICA occlusion (17 proximal and 11 distal ICA) and 70 patients had documented MCA involvement (M1: 55, M2: 11, and M3: 4). Baseline characteristics of patients with ICA and MCA lesions are listed in table 1. PWI lesions were larger (p = 0.02) and there were trends toward worse baseline NIHSS scores (p = 0.1) and larger DWI lesions (p = 0.09) in patients with ICA occlusions. Percentages of patients with the target mismatch and reperfusion rates were similar between groups (tables 1 and 2). Clinical outcomes did not differ between patients with MCA vs ICA occlusions, but there was more lesion growth (p = 0.01) and final fluid-attenuated inversion recovery volumes were larger (p = 0.01) when the ICA was involved (table 2). Patients with ICA occlusions had a higher percentage of symptomatic intracerebral hemorrhage than patients with MCA occlusions, but the difference was not significant (p = 0.2).
Table 1.
Baseline characteristics of patients based on site of arterial occlusiona
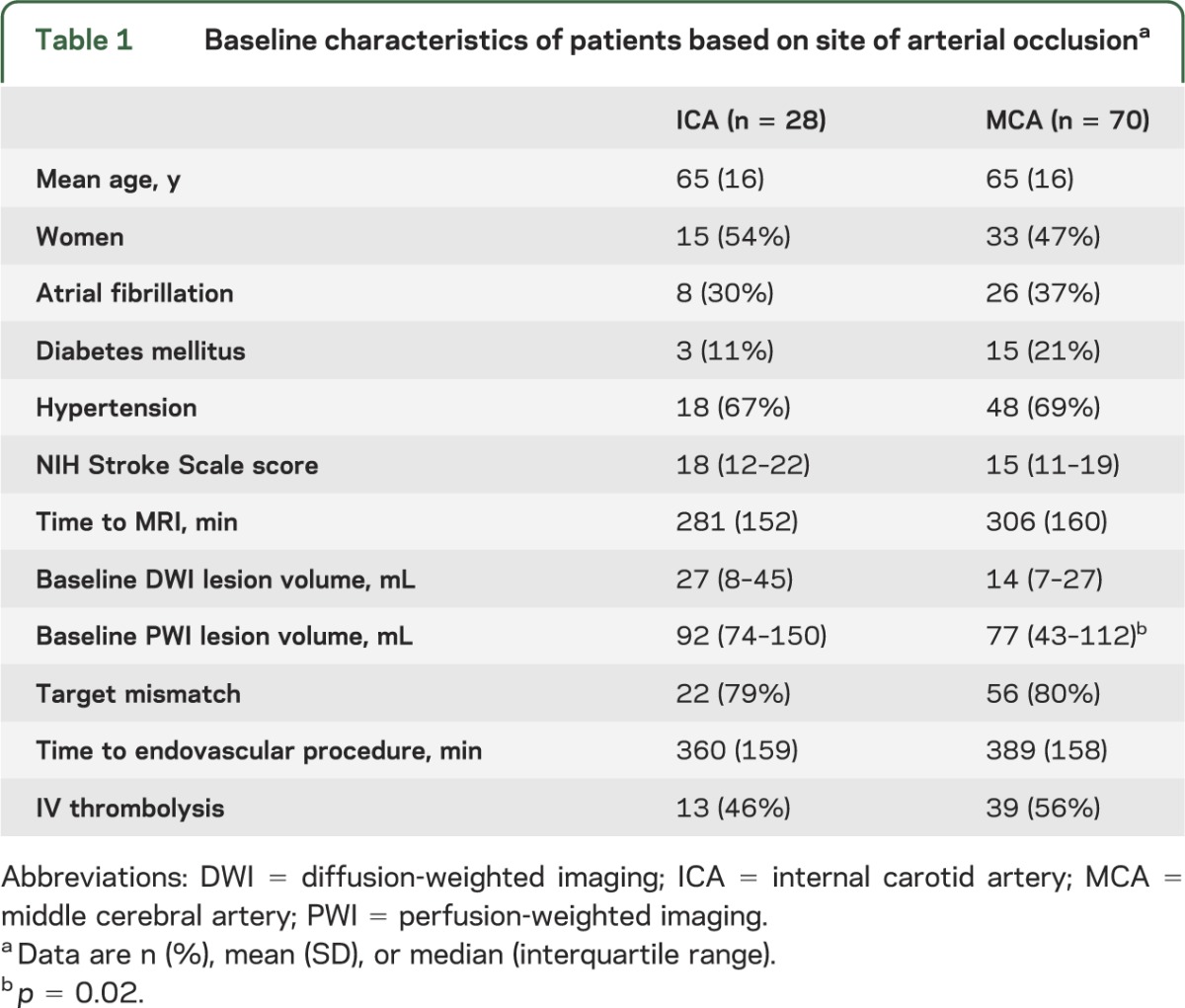
Table 2.
Clinical and radiologic outcomes in patients with ICA vs MCA occlusiona
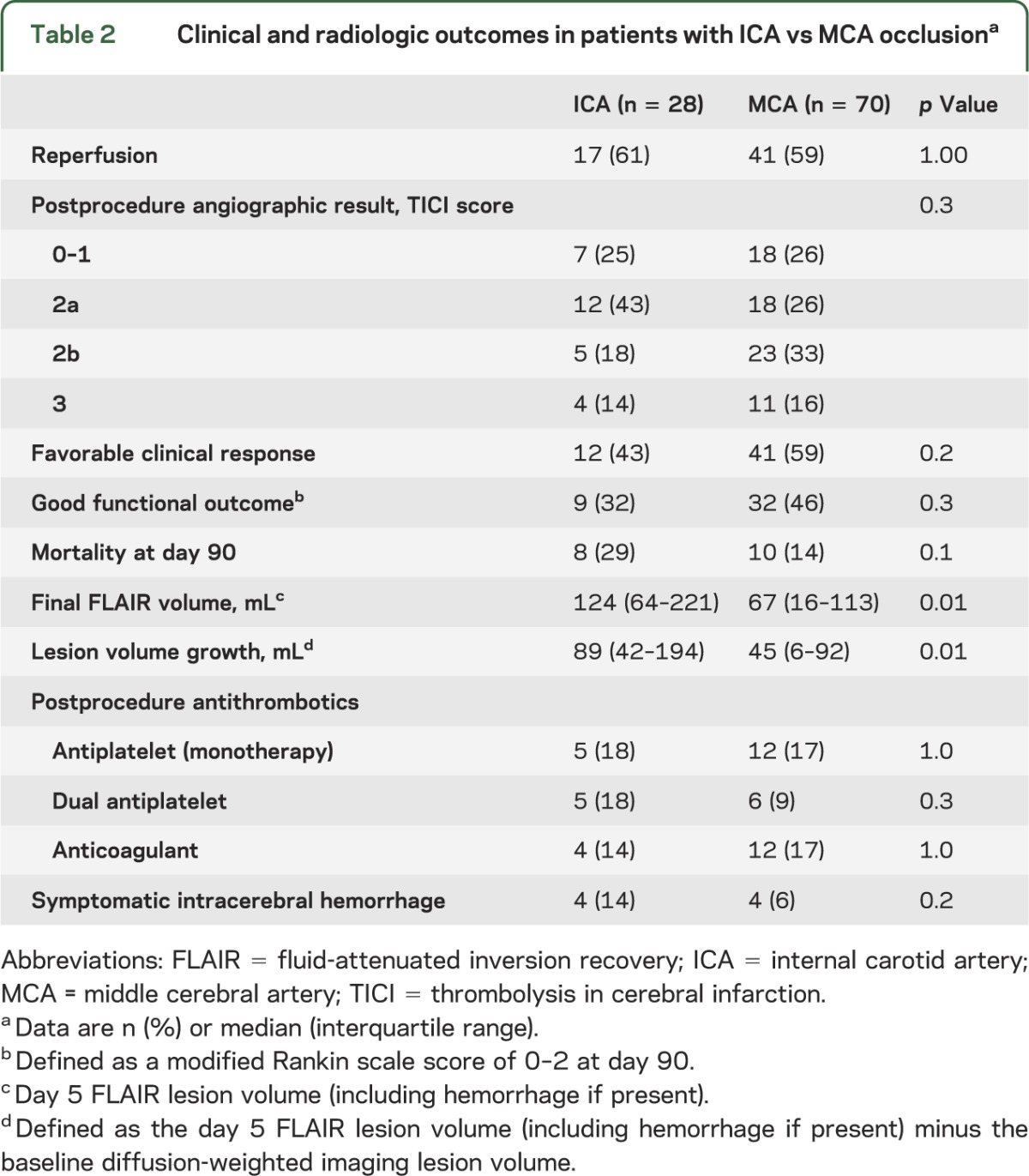
Among patients with MCA occlusions, those who achieved reperfusion had a favorable clinical response rate of 63% compared with 52% in those who did not reperfuse. This corresponds to an OR for favorable clinical response with reperfusion of 1.6 (95% confidence interval [CI] 0.6–4.3). Among patients with ICA occlusions, the percentage of favorable clinical response with reperfusion was 65% compared with 9% without early reperfusion, which corresponds to an OR of 18.3 (95% CI 1.9–179.9) (table 3). The difference between these ORs was significant (p for interaction = 0.04).
Table 3.
Favorable clinical response based on arterial obstruction and mismatch statusa
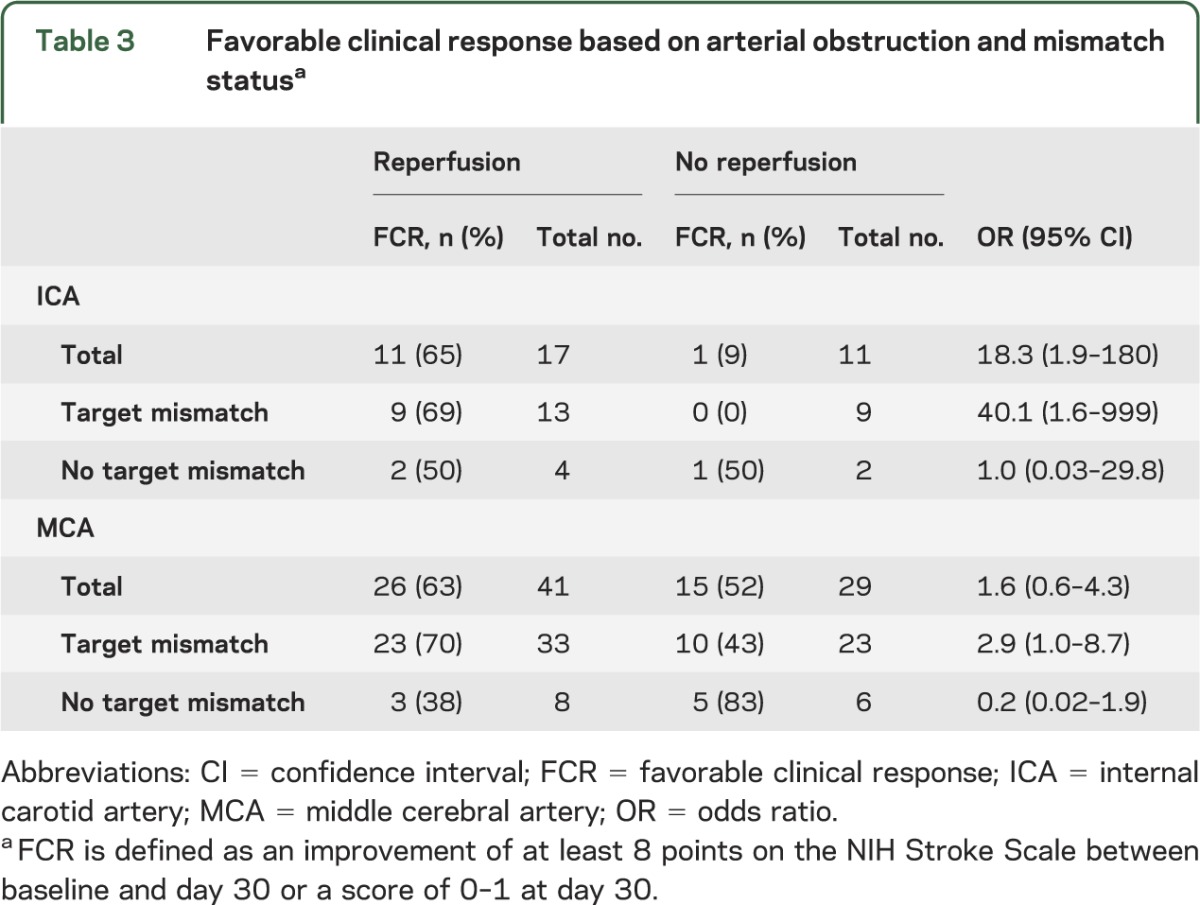
We assessed the same associations between reperfusion and clinical outcomes in the subpopulations with and without the target mismatch profile. Among the 13 target mismatch patients with an ICA occlusion, there was a significant association between reperfusion and favorable clinical response (OR 40.1; 95% CI 1.6–999). This association remained essentially unchanged after adjustment for age and baseline DWI lesion volume (adjusted OR 39.7; 95% CI 1.4–1,133). A relationship between favorable clinical response and reperfusion in target mismatch patients with involvement of the MCA was borderline significant before adjustment (OR 2.9; 95% CI 1.0–8.7). After adjustment for age and initial DWI lesion volume, reperfusion status was associated with favorable clinical response (adjusted OR 5.1; 95% CI 1.4–19.3). In contrast, reperfusion was not associated with clinical outcomes in patients with either MCA or ICA occlusions when target mismatch was not present (table 3).
Stratifying patients by reperfusion status showed a favorable shift on the distribution of mRS scores at day 90 in target mismatch patients with ICA occlusions (p = 0.005), but not a statistically significant difference in target mismatch patients with MCA involvement (p = 0.3) (figure).
Figure. Functional outcome at 3 months in target mismatch patients stratified by site of arterial occlusion and reperfusion status.
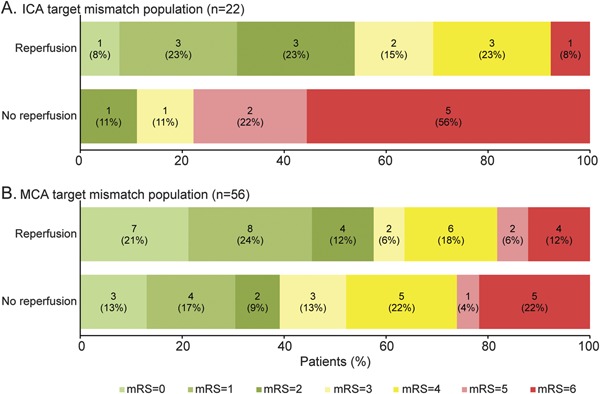
(A) In the DEFUSE-2 cohort, reperfusion was associated with a statistically significant shift in the distribution of outcomes on the mRS score at 3 months in target mismatch patients with ICA occlusions (p = 0.005). (B) There is a favorable trend that does not reach statistical significance in the target mismatch patients with MCA occlusion (p = 0.3). DEFUSE-2 = Diffusion Weighted Imaging Evaluation for Understanding Stroke Evolution–2; ICA = internal carotid artery; MCA = middle cerebral artery; mRS = modified Rankin Scale.
DISCUSSION
This study shows a positive association between reperfusion and favorable clinical responses in target mismatch patients with large-artery occlusions, regardless of the site of the arterial occlusion. The association was stronger for patients with ICA occlusions than for patients with MCA occlusions. This difference was driven by very high rates of poor outcome among target mismatch patients with ICA occlusions who did not reperfuse.
Studies of IV thrombolysis have suggested a less robust treatment effect in patients with ICA occlusions compared with MCA occlusions.1–6 Because recanalization is highly correlated with clinical outcome,15 the low rate of ICA recanalization with IV thrombolysis explains why this treatment does not appear to be very effective for patients with ICA occlusions. In contrast, recanalization rates were similar for patients with MCA (59%) and patients with ICA (61%) occlusions in the DEFUSE-2 study. This is in line with other studies of endovascular therapy that have shown either no difference in reperfusion rates between ICA and MCA occlusions8,10,16 or even higher recanalization rates of the more proximal occlusions.9
Clinical outcomes of patients with ICA occlusions in the DEFUSE-2 study were similar to those reported in a recent meta-analysis of endovascular treatment.11 Thirty-two percent of the DEFUSE-2 patients with ICA occlusions had good functional outcomes (defined as an mRS score of 0–2 at day 90) compared with 33.6% in the meta-analysis.11 The percentage of symptomatic intracerebral hemorrhage in patients with ICA obstructions in the DEFUSE-2 cohort (14%) was slightly higher compared with the percentage reported in that study (11%).11 This may have been attributable in part to the more frequent, albeit nonsignificant, use of dual antiplatelet therapy in the ICA group.
Prior studies that have looked at the site of the arterial obstruction as a predictor of outcome after endovascular therapy have generally shown better outcomes in patients with MCA occlusions. A meta-analysis of endovascular treatment studies has suggested more favorable outcomes with isolated MCA occlusion.10 Another study has shown increased mortality in patients with ICA occlusions even after adjusting for reperfusion status and baseline stroke severity, but no difference in the chance of good outcome.8 Similar to these previous studies, our study also showed a trend toward fewer favorable outcomes and increased mortality in the overall cohort of patients with ICA occlusions compared with patients with MCA occlusions. In our study, this was driven by patients with ICA occlusions having worse outcomes than those with MCA occlusions in the absence of reperfusion. In contrast, in the setting of reperfusion, rates of favorable clinical responses were approximately 65% for both patients with MCA and ICA occlusions. Consequently, the OR for favorable clinical response in the setting of reperfusion is greater for patients with ICA occlusions than for patients with MCA occlusions. These data suggest a differential response to reperfusion with greater clinical efficacy of reperfusion in the subgroup with ICA occlusions.
DEFUSE-2 was designed to study the response to endovascular reperfusion by mismatch status. This substudy shows a positive association between endovascular reperfusion and favorable outcomes in target mismatch patients irrespective of the site of the vascular occlusion, whereas patients without target mismatch did not appear to benefit from reperfusion. The difference in the response to reperfusion between patients with and without the target mismatch explains why the effect of reperfusion was significant in target mismatch patients with MCA occlusions but only showed a trend toward better outcomes when MCA patients with and without target mismatch were considered as a group. These results are in line with the main results of the DEFUSE-2 study, which showed a positive response to reperfusion exclusively in patients with target mismatch, and underscore the importance of stratification by mismatch status.
This study has some limitations. Patients with ICA occlusions who had very large baseline DWI lesions, also referred to as patients with a malignant profile,17 were relatively underrepresented in the DEFUSE-2 cohort. This underrepresentation was the result of investigators canceling the endovascular procedure in some instances after a large baseline DWI lesion was identified. This could partially explain the higher overall good functional outcome (mRS 0–2) rates in this study (42%) compared to the percentages in MERCI18 (28%), TREVO 219 (31%), and SWIFT20 (33%). However, in the endovascular treatment group of IMS III,14 a similar proportion of participants (41%) with good functional outcome was reported. Second, our sample size was not large enough to assess the effect of reperfusion in some specific subgroups. We did not subdivide the ICA and MCA groups in proximal vs distal lesions to clarify whether the location within the ICA or MCA lesion has a differential effect. Additionally, dividing the cohort based on arterial occlusion and mismatch status resulted in small numbers in the subgroups. Because of this, there was insufficient power to analyze the effect of reperfusion in patients without target mismatch. The small sample also likely explains why the effect of reperfusion in target mismatch patients with MCA occlusions was significant for the primary endpoint (favorable clinical response at day 30) but missed statistical significance on the distribution of scores on the mRS at day 90.
CONCLUSION
Reperfusion is associated with high favorable outcome rates irrespective of the site of the vascular occlusion. Location of the occlusion should therefore not solely influence the decision to initiate endovascular treatment. Patients with the target mismatch profile and either an ICA or MCA occlusion appear to be excellent candidates for endovascular reperfusion.
Supplementary Material
GLOSSARY
- CI
confidence interval
- DEFUSE-2
Diffusion Weighted Imaging Evaluation for Understanding Stroke Evolution–2
- DWI
diffusion-weighted imaging
- ICA
internal carotid artery
- MCA
middle cerebral artery
- MERCI
Mechanical Embolus Removal in Cerebral Ischemia
- mRS
modified Rankin Scale
- NIHSS
NIH Stroke Scale
- OR
odds ratio
- PWI
perfusion-weighted imaging
Footnotes
Editorial, page 608
AUTHOR CONTRIBUTIONS
R.L. designed the study, collected, analyzed, and interpreted data, and wrote the manuscript. M.M. analyzed and interpreted the data. M.S., S.K., and R.B. designed the study and collected data. M.P.M. designed the study, and collected and analyzed data. G.W.A. designed the study, collected and interpreted data, and revised the manuscript. M.G.L. designed the study, collected, analyzed, and interpreted data, and wrote the manuscript. All authors reviewed the manuscript.
ACKNOWLEDGMENT
R.L. is Senior Clinical Investigator of FWO Flanders.
STUDY FUNDING
Supported by grants from the National Institute for Neurological Disorders and Stroke (R01 NS03932505 to G.W.A. and K23 NS051372 to M.G.L.).
DISCLOSURE
R. Lemmens, M. Mlynash, M. Straka, S. Kemp, and M. Marks report no disclosures. R. Bammer and G. Albers have equity interest in iSchemaView. M. Lansberg is on the Scientifc Advisory Board for Biogen Idec. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Saqqur M, Uchino K, Demchuk AM, et al. Site of arterial occlusion identified by transcranial Doppler predicts the response to intravenous thrombolysis for stroke. Stroke 2007;38:948–954 [DOI] [PubMed] [Google Scholar]
- 2.De Silva DA, Brekenfeld C, Ebinger M, et al. The benefits of intravenous thrombolysis relate to the site of baseline arterial occlusion in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET). Stroke 2010;41:295–299 [DOI] [PubMed] [Google Scholar]
- 3.Bhatia R, Hill MD, Shobha N, et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke 2010;41:2254–2258 [DOI] [PubMed] [Google Scholar]
- 4.Linfante I, Llinas RH, Selim M, et al. Clinical and vascular outcome in internal carotid artery versus middle cerebral artery occlusions after intravenous tissue plasminogen activator. Stroke 2002;33:2066–2071 [DOI] [PubMed] [Google Scholar]
- 5.Marks MP, Olivot JM, Kemp S, et al. Patients with acute stroke treated with intravenous tPA 3–6 hours after stroke onset: correlations between MR angiography findings and perfusion- and diffusion-weighted imaging in the DEFUSE study. Radiology 2008;249:614–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomalla G, Kruetzelmann A, Siemonsen S, et al. Clinical and tissue response to intravenous thrombolysis in tandem internal carotid artery/middle cerebral artery occlusion: an MRI study. Stroke 2008;39:1616–1618 [DOI] [PubMed] [Google Scholar]
- 7.Gupta R, Vora NA, Horowitz MB, et al. Multimodal reperfusion therapy for acute ischemic stroke: factors predicting vessel recanalization. Stroke 2006;37:986–990 [DOI] [PubMed] [Google Scholar]
- 8.Nogueira RG, Liebeskind DS, Sung G, Duckwiler G, Smith WS. Predictors of good clinical outcomes, mortality, and successful revascularization in patients with acute ischemic stroke undergoing thrombectomy: pooled analysis of the Mechanical Embolus Removal in Cerebral Ischemia (MERCI) and Multi MERCI Trials. Stroke 2009;40:3777–3783 [DOI] [PubMed] [Google Scholar]
- 9.Galimanis A, Jung S, Mono ML, et al. Endovascular therapy of 623 patients with anterior circulation stroke. Stroke 2012;43:1052–1057 [DOI] [PubMed] [Google Scholar]
- 10.Rouchaud A, Mazighi M, Labreuche J, et al. Outcomes of mechanical endovascular therapy for acute ischemic stroke: a clinical registry study and systematic review. Stroke 2011;42:1289–1294 [DOI] [PubMed] [Google Scholar]
- 11.Mokin M, Kass-Hout T, Kass-Hout O, et al. Intravenous thrombolysis and endovascular therapy for acute ischemic stroke with internal carotid artery occlusion: a systematic review of clinical outcomes. Stroke 2012;43:2362–2368 [DOI] [PubMed] [Google Scholar]
- 12.Lansberg MG, Straka M, Kemp S, et al. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol 2012;11:860–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Straka M, Albers GW, Bammer R. Real-time diffusion-perfusion mismatch analysis in acute stroke. J Magn Reson Imaging 2010;32:1024–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broderick JP, Palesch YY, Demchuk AM, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med 2013;368:893–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke 2007;38:967–973 [DOI] [PubMed] [Google Scholar]
- 16.Penumbra Pivotal Stroke Trial Investigators The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke 2009;40:2761–2768 [DOI] [PubMed] [Google Scholar]
- 17.Mlynash M, Lansberg MG, De Silva DA, et al. Refining the definition of the malignant profile: insights from the DEFUSE-EPITHET pooled data set. Stroke 2011;42:1270–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith WS, Sung G, Starkman S, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke 2005;36:1432–1438 [DOI] [PubMed] [Google Scholar]
- 19.Nogueira RG, Lutsep HL, Gupta R, et al. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet 2012;380:1231–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saver JL, Jahan R, Levy EI, et al. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet 2012;380:1241–1249 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


