Abstract
Objective:
We conducted a clinicopathologic study in a large population with very low levels of education to determine whether very few years of education could contribute to cognitive reserve and modify the relation of neuropathologic indices to dementia.
Methods:
In this cross-sectional study, we included 675 individuals 50 years of age or older from the Brazilian Aging Brain Study Group. Cognitive abilities were evaluated through a structured interview with an informant at the time of autopsy, including the Clinical Dementia Rating (CDR) scale. Neuropathologic examinations were performed using immunohistochemistry and following internationally accepted criteria. Multivariate linear regression models were conducted to determine whether the association between cognitive abilities (measured by CDR sum of boxes) and years of education was independent of sociodemographic variables and neuropathologic indices, including neuritic plaques, neurofibrillary tangles, lacunar infarctions, small-vessel disease, and Lewy bodies. In addition, interaction models were used to examine whether education modified the relation between neuropathologic indices and cognition.
Results:
Mean education was 3.9 ± 3.5 years. Formal education was associated with a lower CDR sum of boxes (β = −0.197; 95% confidence interval −0.343, −0.052; p = 0.008), after adjustment for sociodemographic variables and neuropathologic indices. Furthermore, education modified the relationship of lacunar infarcts with cognitive abilities (p = 0.04).
Conclusions:
Even a few years of formal education contributes to cognitive reserve.
The cognitive reserve theory is increasingly used to explain the clinicopathologic dissociation observed in Alzheimer disease (AD).1 Approximately 30% of cognitively normal subjects have intermediate- to high-likelihood AD pathology at autopsy.2–7 According to this theory, subjects with greater cognitive reserve require a more severe neuropathologic burden to reach the threshold for clinical dementia.8
Previous clinicopathologic studies suggest that although education is not directly related to the development of neuropathologic lesions, it appears to reduce the impact of such lesions on the development of dementia, thereby increasing cognitive reserve. However, the studies supporting this hypothesis have investigated populations with relatively high levels of educational attainment, with mean formal education ranging from 9 to 18 years.8–12 Little information is available regarding the effect of very few years of education on cognitive reserve. Low educational attainment is the reality for a high proportion of the elderly worldwide. According to a report from the United Nations Education, Scientific and Cultural Organization, nearly 800 million adults remained illiterate in 2009, representing about 16% of the global population.13 Most of these individuals live in developing countries, which already are home to approximately 60% of the subjects who have dementia.14
In this study, we examined the cognitive reserve theory in a population with a high prevalence of extremely low levels of formal education. Furthermore, we tested the hypothesis that even a few years of formal education would reduce the deleterious effects of neuropathologic indices (i.e., AD pathologic changes, lacunar infarctions, small-vessel disease, and Lewy body pathology) on the likelihood of having cognitive impairment.
METHODS
Participants.
Subjects were participants in the Brazilian Aging Brain Study Group (BABSG) from the University of São Paulo. Inclusion and exclusion criteria for the BABSG were previously described.15 From February 2004 to February 2009, a total of 1,980 persons aged 50 years or older were included in the BABSG. Of these, the first consecutive 675 persons underwent a complete clinical and neuropathologic diagnosis and were included in this study.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the local ethical committee, and a voluntarily signed informed consent was obtained by the next-of-kin who provided autopsy and brain donation consent and permission to obtain necessary clinical and functional information.
Clinical data.
Upon arrival at the autopsy service, a knowledgeable informant was identified to provide information using a validated semistructured interview. Requirement for being an informant included having close weekly contact with the deceased subject during the last 6 months. Informants were the son or daughter in 69.9%, the spouse in 7.8%, a grandchild in 7.3%, and a sibling in 6.2% of the cases. The remaining 8.8% were other family members. Contact with the informant was categorized as daily or weekly. The interview protocol included demographic data collection of age, sex, ethnicity, and educational attainment. Educational attainment was obtained by inquiring about the number of years in which the deceased attended school. A series of semistructured scales, including the Clinical Dementia Rating (CDR) scale and the Informant Questionnaire of the Cognitive Decline in the Elderly, determined cognitive and functional status.16,17 CDR sum of boxes was obtained for all subjects. The interview was conducted by a skilled team of gerontologists. The final diagnosis was reached by consensus among an experienced gerontologist and a behavioral neurologist.
In a previous study, we prospectively compared the sensitivity and specificity of our protocol when applied to informants of living patients, with the diagnosis established in our memory clinic where the same patients were submitted to a full cognitive assessment, neurologic examination, and neuroimaging. Sensitivity was 86.6% and specificity was 84.4% for the diagnosis of dementia.18
Socioeconomic status was determined through a Brazilian scale that classifies subjects in 5 strata. Stratum A is the highest income and stratum E the lowest. For analysis and inclusion in the multivariate model, individuals were grouped into 3 categories: low socioeconomic status (strata D and E), middle status (stratum C), and high status (strata A and B).19
Neuropathologic assessment.
Autopsy was performed within 20 hours of death. Upon brain procurement, the left hemisphere was fixed in 10% buffered paraformaldehyde for 14 to 21 days, sectioned in 1-cm-thick slabs, and inspected macroscopically. Thirteen samples (middle frontal gyrus, middle and anterior temporal gyri, angular gyrus, superior frontal gyrus and anterior cingulate gyrus, visual cortex, hippocampus and parahippocampal gyrus at the level of lateral geniculate body, amygdala at the level of mammillary bodies including the ambiens gyrus, basal ganglia at the level of the anterior commissure, thalamus, midbrain, pons, medulla oblongata, and cerebellum) were blocked in paraffin for microscopic examination. All extra macroscopic lesions were also sampled for microscopic evaluation.
All sampled regions were stained with hematoxylin & eosin (H&E). Selected sections were immunostained with antibodies against β-amyloid (4G8, dilution 1:10.000; Signet Pathology Systems, Dedham, MA), against phospho-tau (PHF-1, dilution 1:2.000; gift of Prof. Peter Davies, NY), and against α-synuclein (EQV-1, 1:10.000; gift of Kenji Ueda, Tokyo, Japan), as described previously.15 All sections were submitted to antigenic retrieval using a steamer. The reactions were detected using the Vectastain Elite ABC Kit (Vector Laboratories, Burlingame, CA). Whenever necessary for accurate diagnosis, other regions were immunostained and/or additional antibodies including TDP-43 were used. Neuropathologic diagnoses were conducted by 2 experienced neuropathologists blinded to all clinical data.
AD neuropathologic lesions.
The criteria of the Consortium to Establish a Registry for Alzheimer's Disease were used to classify the neuritic plaques into 4 possible groups: absent, sparse, moderate, or frequent.20 The neurofibrillary tangles were staged according to the Braak classification in stages 0 to VI.21
Cerebrovascular assessment.
Vascular changes were analyzed semiquantitatively using H&E staining in all 13 routinely sampled areas plus additional areas with suspected vascular lesions detected at the macroscopic examination.22 Additional staining such as periodic acid-Schiff, Perls, and Klüver-Barrera was used at pathologist discretion. The presence of diffuse small-vessel disease, lacunae, and large infarcts was registered by topography, size, and number. Cerebral amyloid angiopathy was identified using immunohistochemistry for β-amyloid.
Small-vessel disease was assessed in all 13 areas using a 4-point scale (0–3) based on severity. The average of cortical area stages was used. The number and size of lacunar and microinfarcts were recorded in all 14 regions, using H&E-stained sections. The number of lacunar infarcts found in strategic areas to cognition (thalamus, frontocingular cortex, basal forebrain, caudate nucleus, inferotemporal gyri, hippocampus, and angular gyrus) was used for classifying lacunar infarcts on a 6-point scale (0 = none; 1 = up to 2 lacunae; 2 = up to 4 lacunae; 3 = up to 6 lacunae; 4 = up to 8 lacunae; and 5 = more than 8 lacunae).
Lewy body pathology.
The presence of Lewy bodies was classified using the Braak staging for Parkinson disease ranging from stages 0 to VI.23
Statistical analysis.
The main dependent variable was cognitive ability assessed with the CDR sum of boxes (continuous outcome). We initially conducted univariate analysis to examine whether the groups with and without any formal education, and those with and without cognitive impairment were different regarding demographics and neuropathologic features. We used χ2 tests or Fisher exact test when appropriate for categorical variables and unpaired t tests for continuous variables. Multivariate linear regression analysis was conducted to determine whether the association between cognitive ability and education was not confounded by sociodemographic data and neuropathologic features. Education was the main independent variable and was modeled as a continuous measure. The initial model examined the association between education and cognitive ability adjusting for the effects of demographic factors including age at death, sex, socioeconomic status, race, and contact with the informant. Race and socioeconomic status were coded as dummy variables. Further multivariate linear regression models were performed to adjust the association of cognitive ability and education for the presence of neuropathologic indices, measured as ordinal variables.
We conducted additional multivariate linear regression analyses with the addition of interaction terms in order to test the hypothesis that education modifies the relationship of neuropathologic indices and cognitive ability. The p values for interaction and other terms in the model were calculated using likelihood ratio tests.
The level of significance of all tests was set at 5% in 2-tailed tests. The statistical analyses were performed using Stata statistical software version 11.0 (Stata Corp., College Station, TX).
RESULTS
A total of 675 individuals were included in the study; 47.7% were male. The mean age was 74.0 ± 11.7 years and mean educational level was 3.9 ± 3.5 years, 19.3% of the sample had never been to school, and 60.7% had between 1 and 4 years of formal education. Cognitive impairment was present in 50.4% of the subjects divided as CDR 0.5 (30.6%), CDR 1 (22.6%), CDR 2 (15.9%), and CDR 3 (30.9%). Braak stage ≥IV was found in 33.9%, and moderate to severe neuritic plaques were found in 24.3%. Lacunar infarcts and small-vessel disease were present in 31.1% and 42.6% of the subjects, respectively. We found a Braak stage ≥IV (neocortical involvement) for Lewy body pathology in 9.8% of our sample. Univariate analysis was next performed to evaluate the association of the main demographics and neuropathologic variables to groups with and without any formal education (table 1). Those without formal education were older, more likely to be female, had lower socioeconomic status, and had higher frequency of small-vessel disease. We next compared those with and without cognitive impairment (table 2). The group with cognitive impairment was older, more likely to be female, had lower educational attainment, and had a higher frequency of AD-related and vascular pathology.
Table 1.
Demographic, clinical, and neuropathologic data of subjects with and without any formal education (n = 675)
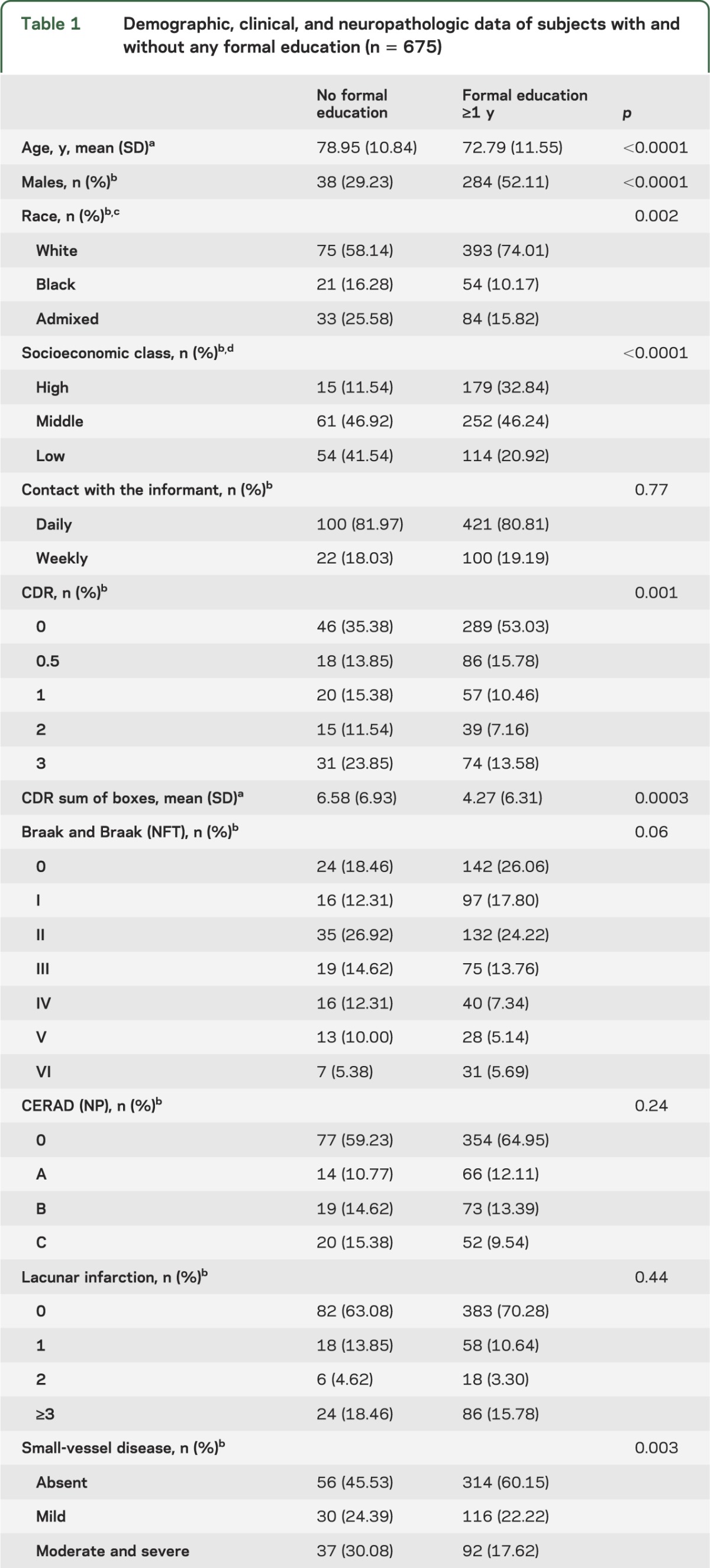
Table 2.
Demographic, clinical, and neuropathologic data from participants with and without cognitive impairment (n = 675)

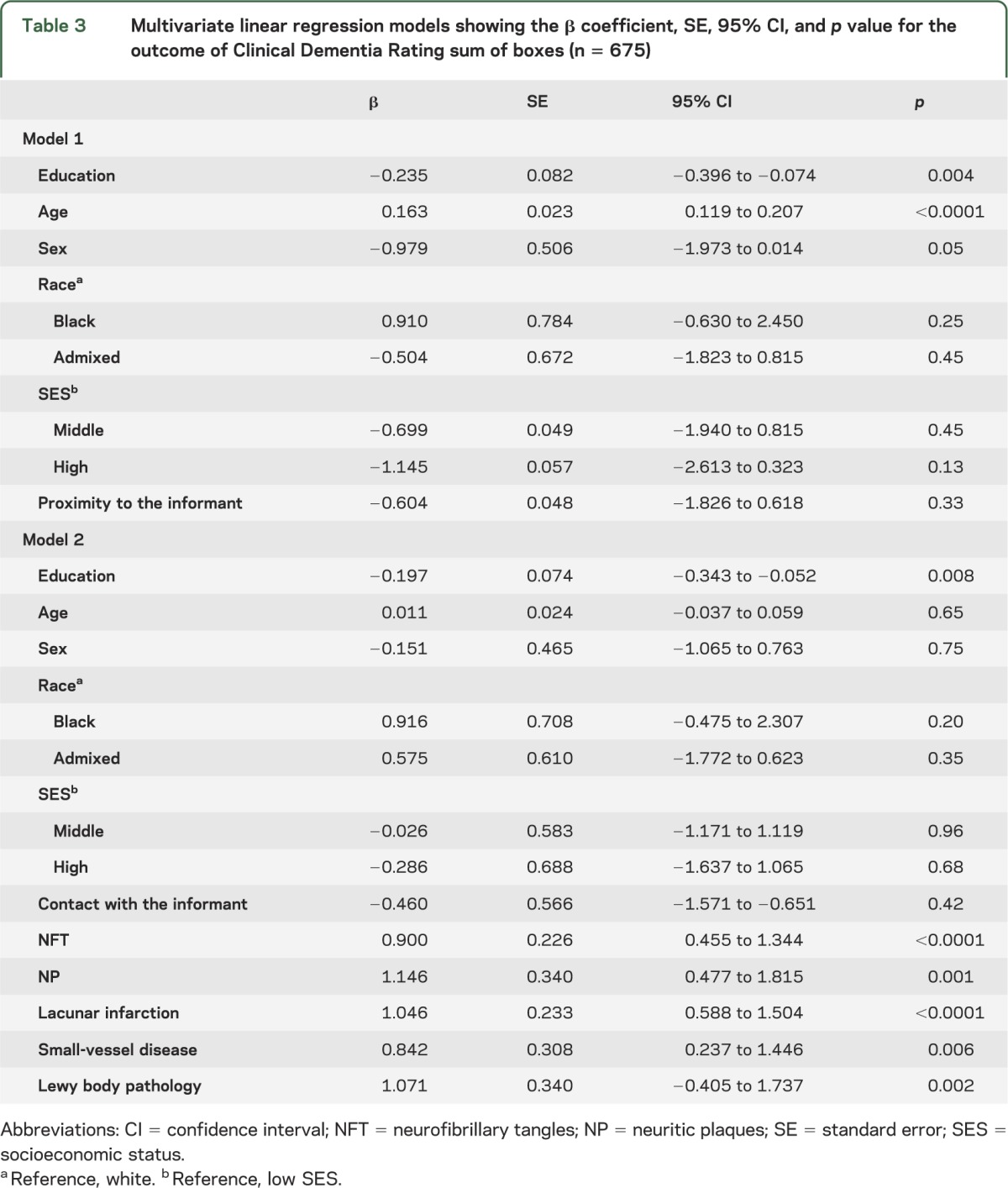
Multivariate linear regression analyses were performed to examine the association between cognitive abilities and education, adjusting for sociodemographics and presence of neuropathologic lesions (table 3). In the full model, each year of education on average was associated with a −0.197 unit lower CDR sum of boxes (β = −0.197; 95% confidence interval −0.343, −0.052; p = 0.008). Therefore, education was associated with better cognitive abilities independent of sociodemographics and neurodegenerative and cerebrovascular lesions.
Table 3.
Multivariate linear regression models showing the β coefficient, SE, 95% CI, and p value for the outcome of Clinical Dementia Rating sum of boxes (n = 675)
Finally, we performed additional linear regression models with CDR sum of boxes as the dependent variable, including terms for interaction between education and each of the neuropathologic lesions, and controlling for sociodemographic characteristics and other lesions. Education modified the relation between lacunar infarcts and cognitive ability (p = 0.04). Significant interactions were not observed between education and AD neuropathologic features, Lewy body pathology, or small-vessel disease (table 4 and the figure).
Table 4.
Multivariate linear regression analysis showing β coefficients (SE), 95% CI, and p values for the outcome of cognitive impairment considering the interaction of education and presence of neuropathologic lesions adjusted for age, sex, race, socioeconomic class, and other neuropathologic lesions (n = 675)
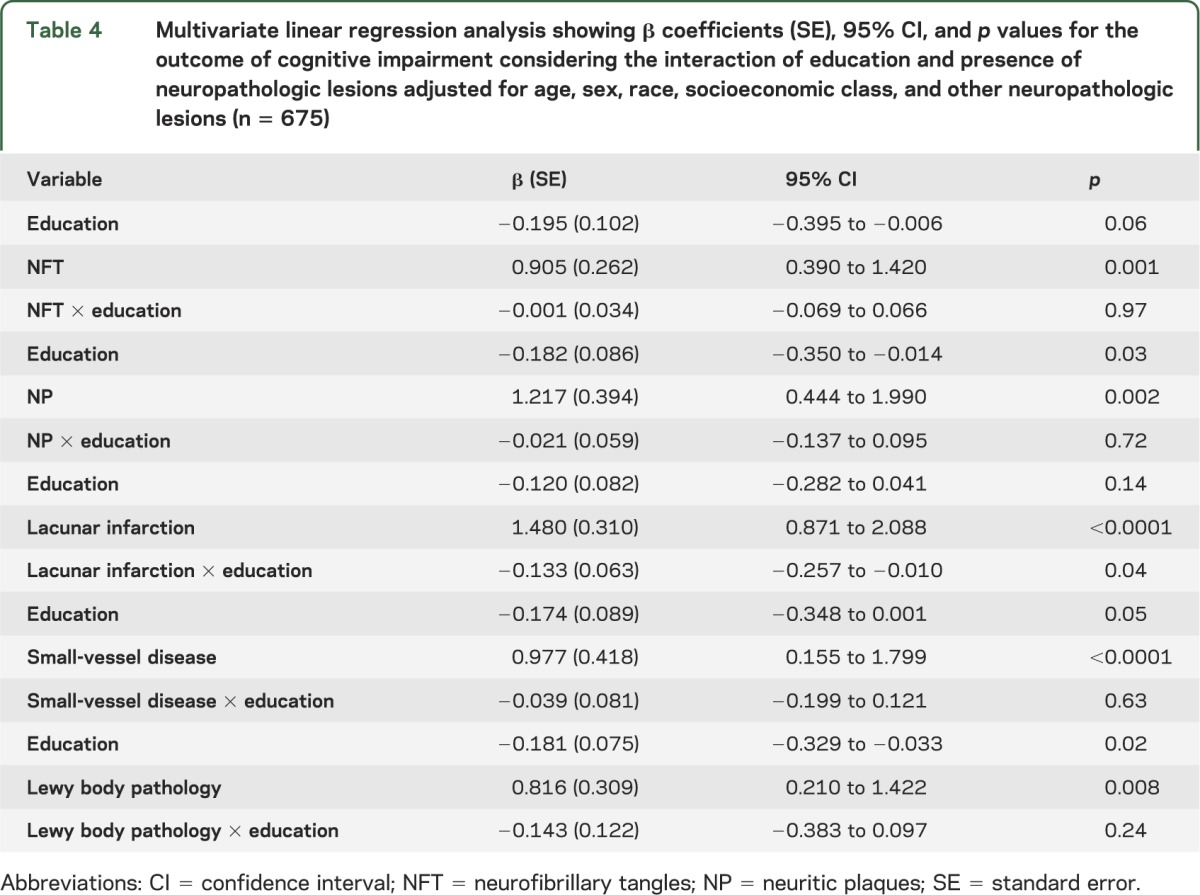
Figure. Predicted values of cognitive impairment considering formal education and neuropathologic lesions.
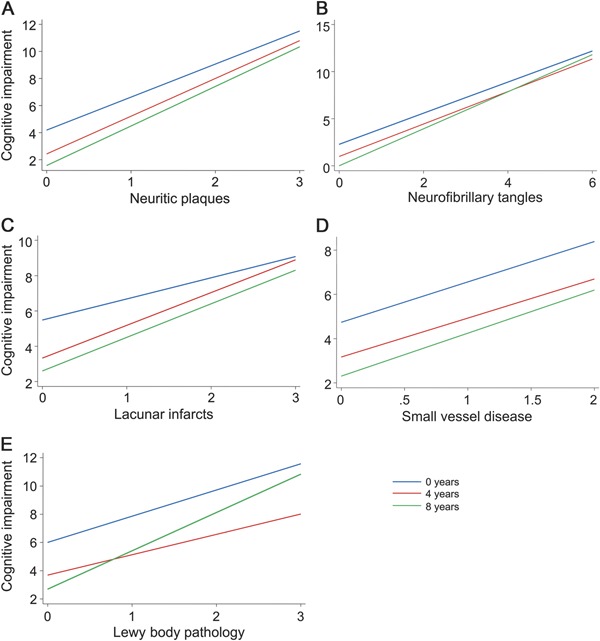
Predicted values of cognitive impairment (Clinical Dementia Rating sum of boxes) considering different neuropathologic lesions (A: neuritic plaques; B: neurofibrillary tangles; C: lacunar infarcts; D: small-vessel disease; and E: Lewy body pathology) for participants with 0 years of education (10th percentile: blue line), 4 years of education (50th percentile: red line), and 8 years of education (90th percentile: green line). Predicted values of cognitive function obtained by multivariate linear models adjusted for age, sex, race, socioeconomic status, closeness of the informant, and all other neuropathologic lesions.
DISCUSSION
Our findings show that very few years of formal education was associated with less cognitive impairment when compared with no formal education and that this relationship does not depend on demographic and socioeconomic characteristics and neuropathologic features related to AD, cerebrovascular lesions, and Lewy body pathology. There was a dose effect of education such that higher levels of schooling were associated with the lowest frequency of cognitive impairment.
Furthermore, our findings agree with previous neuropathologic studies showing that higher education is associated with lower prevalence of dementia through increasing cognitive reserve.8–11 However, other studies included mostly persons with secondary school or more years of education and cannot necessarily be generalized to populations with very low levels of education. The effect of only a few years of education on cognitive reserve has not yet been well established. Thus, the major contribution of this study is to demonstrate that even a few years of education is sufficient to contribute to cognitive reserve and through this mechanism reduce the frequency of cognitive impairment, independent of the neuropathologic burden.
Very high levels of education, as seen in previous studies, may at least partly reflect the intellectual abilities of a person, and it is therefore less clear whether the education itself adds to the cognitive reserve or whether persons who receive more education have more cognitive reserve to begin with.24 Access to only a few years of education will depend less on the intellectual abilities of a child, as opposed to postsecondary education. Therefore, the current study provides additional evidence that education itself actually contributes to the cognitive reserve.
It has been hypothesized that higher prevalence of dementia in low educated individuals with low socioeconomic level is related to increased risk of cerebrovascular lesions, because this population has low access to diagnosis and effective control of hypertension, diabetes, and dyslipidemia.11 Poor control of vascular risk factors may result in cerebrovascular damage, which, in turn, is frequently found in association with AD neuropathologic lesions.25 In fact, in a collaborative neuropathologic study, low education was not associated with increased vascular neuropathology, but it should be noted that in this study the median years of education was 9 years, much higher than the 4 years of our study.10 Our findings supporting the relationship between education and lower frequency of cognitive impairment were also shown and extended to be independent of cerebrovascular lesions, in line with other samples of more educated individuals.
Furthermore, unlike previous studies, we found that the relationship between lacunar infarcts and cognitive ability is modified according to the level of education such that the odds of cognitive impairment associated with infarctions were lower among those with more years of education. These data provide evidence that education not only provides a cognitive advantage such that persons with more education may require a higher degree of cerebrovascular pathology in order to develop cognitive impairment but that education is also associated with mechanisms that reduce the impact of lacunar infarcts on cognition.
We did not find an interaction between education and AD neuropathologic features, as described in other studies.8,9 However, given the truncated range of education, we may not have been able to detect this association in our sample because of limited power. Studies including larger samples and with diverse profiles of education may help to further investigate this hypothesis.
The present study is a postmortem cross-sectional study and therefore has some limitations. We observed a difference of age between the groups with and without formal education. This result is probably related to the secular trend of education in Brazil. Epidemiologic studies demonstrate that older individuals have lower levels of education compared with younger subjects. Older subjects living in urban centers were frequently born and raised in rural areas and had limited access to school.26 However, the cross-sectional nature of our study does not permit excluding the possibility of survival bias. For this reason, all multivariate models were adjusted for age.
The effect attributable to education could be confounded by other variables such as complex mental activities and specific behavior or lifestyle that were not included in our multivariate model. Nevertheless, in a recent study, education was described as the most effective component of cognitive reserve, associated with better performance in diverse cognitive domains, and its effect was independent of reading capacity, socioeconomic status, and cognitive activity throughout life.27 Furthermore, recent evidence demonstrates that literacy and the first few years of education are associated with remarkable changes in cortical network organization and function.28 The advances provided by previous studies together with the results of our study are evidence supporting the hypothesis that elementary education is a simple and powerful strategy to reduce the prevalence of dementia in illiterate populations. Such intervention would have a strong health policy implication because two-thirds of people with dementia live in developing countries where the prevalence of illiteracy is still high.13,14
Additional limitations are acknowledged. The clinical information was obtained through an informant and thus our measurement of cognitive ability may have been less accurate than a direct assessment. Although this is a potential limitation, we used 2 tests validated for informant-based assessment to increase the reliability of this evaluation, as recommended by the literature.29 In addition, high levels of correspondence for the diagnosis of both dementia and normal cognition were obtained between the interviewing protocol used in this study and a gold standard interview performed with the patient and informant, as described previously.18 Furthermore, an immediate postmortem interview may be helpful because the interval of time between the clinical and neuropathologic examination is minimal. This study investigated a convenience population submitted to mandatory autopsy examination due to unknown cause of death. This sample may not represent other subjects not submitted to autopsy. Compared with Brazilian community-dwelling studies, the prevalence of cognitive impairment was higher and the prevalence of illiteracy was slightly lower in this study.30 Further investigations with a broader sample including individuals with known cause of death are required to extend the validity of our results.
ACKNOWLEDGMENT
The authors are grateful to the families of the brain donors and to the São Paulo City Autopsy Service physicians and staff for unconditional support, Brazilian Aging Brain Study Group's participants Ana T. Alho, Katia C. Oliveira, Livia Polichiso, Glaucia A.B. Santos, Mariana Molina, Camila F. Nascimento, Eliza Guccione, and Camila Alves de Souza for outstanding assistance in data collection, and Keila Maria Silva for histologic support. The authors also thank Prof. Kenji Ueda from the University of Tokyo, Japan, and Prof. Peter Davies from Albert Einstein College of Medicine, NY, for generously providing antibodies for immunostaining. Dr. Farfel, Dr. Suemoto, and Dr. Leite are also grateful for the support of the Principles and Practice of Clinical Research Program of the Harvard Medical School.
GLOSSARY
- AD
Alzheimer disease
- BABSG
Brazilian Aging Brain Study Group
- CDR
Clinical Dementia Rating
- H&E
hematoxylin & eosin
AUTHOR CONTRIBUTIONS
Dr. Farfel, Dr. Nitrini, Dr. Suemoto, Dr. Grinberg, and Dr. Jacob Filho were responsible for study design. Dr. Farfel, Dr. Nitrini, Dr. Suemoto, Dr. Grinberg, Dr. Bennett, Dr. Fregni, Dr. Pasqualucci, and Dr. Jacob Filho contributed to manuscript writing. Dr. Farfel, Dr. Nitrini, Dr. Suemoto, Dr. Grinberg, Dr. Ferretti, Dr. Leite, E. Tampellini, L. Lima, D.S. Farias, R.C. Neves, Dr. Rodriguez, Dr. Menezes, and Dr. Jacob Filho were responsible for data analysis or interpretation. Dr. Grinberg and Dr. Rodriguez were responsible for neuropathologic readings. All authors reviewed the manuscript.
STUDY FUNDING
Support for this work was provided by grants from Fundação de Apoio a Pesquisa do Estado de São Paulo—FAPESP (grant 2009/01934-4 and scholarship to R.E.P.L.), LIM22 of the Department of Pathology of University of São Paulo Medical School, Albert Einstein Education and Research Institute, São Paulo (grant 240/07), and Coordenadoria de Apoio ao Pessoal de Nivel Superior—CAPES, Brazil (scholarship to students of BABSG). Dr. Farfel is grateful for the Hospital Israelita Albert Einstein travel fellowship. Dr. Grinberg is supported by grants from the John Douglas French Alzheimer Foundation, Hospital Israelita Albert Einstein and Research Institute, and NIH grants 1R01AG040311-01 and 2P50AG023501. Dr. Bennett is supported by NIH grants P30AG10161 and R01AG17917. This study was not industry-sponsored.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Stern Y. Cognitive reserve. Neuropsychologia 2009;47:2015–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crystal HA, Dickson DW, Sliwinski MJ, et al. Pathological markers associated with normal aging and dementia in the elderly. Ann Neurol 1993;34:566–573 [DOI] [PubMed] [Google Scholar]
- 3.Katzman R, Terry R, De Teresa R, et al. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol 1988;23:138–144 [DOI] [PubMed] [Google Scholar]
- 4.Knopman DS, Parisi JE, Salviati A, et al. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol 2003;62:1087–1095 [DOI] [PubMed] [Google Scholar]
- 5.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol 1999;45:358–368 [DOI] [PubMed] [Google Scholar]
- 6.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 2006;66:1837–1844 [DOI] [PubMed] [Google Scholar]
- 7.Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW, Markesbery WR. “Preclinical” AD revisited: neuropathology of cognitively normal older adults. Neurology 2000;55:370–376 [DOI] [PubMed] [Google Scholar]
- 8.Bennett DA, Wilson RS, Schneider JA, et al. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology 2003;60:1909–1915 [DOI] [PubMed] [Google Scholar]
- 9.Roe CM, Xiong CJ, Miller JP, Morris JC. Education and Alzheimer disease without dementia: support for the cognitive reserve hypothesis. Neurology 2007;68:223–228 [DOI] [PubMed] [Google Scholar]
- 10.EClipSE Collaborative Members, Brayne C, Ince PG, Keage HA, et al. Education, the brain and dementia: neuroprotection or compensation? Brain 2010;133:2210–2216 [DOI] [PubMed] [Google Scholar]
- 11.Del Ser T, Hachinski V, Merskey H, Munoz DG. An autopsy-verified study of the effect of education on degenerative dementia. Brain 1999;122:2309–2319 [DOI] [PubMed] [Google Scholar]
- 12.Koepsell TD, Kurland BF, Harel O, Johnson EA, Zhou XH, Kukull WA. Education, cognitive function, and severity of neuropathology in Alzheimer disease. Neurology 2008;70:1732–1739 [DOI] [PubMed] [Google Scholar]
- 13.United Nations Education, Scientific and Cultural Organization (UNESCO) Institute for Statistics Adult and youth literacy, 1990-2015: analysis of data for 41 selected countries [online]. Available at: http://www.uis.unesco.org/literacy/Documents/UIS-literacy-statistics-1990-2015-en.pdf Accessed December 2012.
- 14.Rodriguez JJ, Ferri CP, Acosta D, et al. Prevalence of dementia in Latin America, India, and China: a population-based cross-sectional survey. Lancet 2008;372:464–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grinberg LT, Ferretti RE, Farfel JM, et al. Brain bank of the Brazilian Aging Brain Study Group: a milestone reached and more than 1,600 collected brains. Cell Tissue Bank 2007;8:151–162 [DOI] [PubMed] [Google Scholar]
- 16.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414 [DOI] [PubMed] [Google Scholar]
- 17.Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med 1989;19:1015–1022 [DOI] [PubMed] [Google Scholar]
- 18.Ferretti RE, Damin AE, Brucki SM, et al. Post-mortem diagnosis of dementia by informant interview. Dement Neuropsychol 2010;4:138–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Almeida P, Wickerhauser H. O critério ABA/ABIPEME—em busca de uma atualização: um estudo e uma proposta submetidos à ABA e à ABIPEME Brazil. São Paulo: Editora da Associação Brasileira dos Institutos de Pesquisa de Mercado; 1991 [Google Scholar]
- 20.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's disease (CERAD). 2. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 1991;41:479–486 [DOI] [PubMed] [Google Scholar]
- 21.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol 1991;82:239–259 [DOI] [PubMed] [Google Scholar]
- 22.Grinberg LT, Thal DR. Vascular pathology in the aged human brain. Acta Neuropathol 2010;119:277–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braak H, Braak E. Pathoanatomy of Parkinson's disease. J Neurol 2000;247:3–10 [DOI] [PubMed] [Google Scholar]
- 24.Ardila A, Bertolucci PH, Braga LW, et al. Illiteracy: the neuropsychology of cognition without reading. Arch Clin Neuropsychol 2010;25:689–712 [DOI] [PubMed] [Google Scholar]
- 25.Fratiglioni L, Grut M, Forsell Y, et al. Prevalence of Alzheimer's disease and other dementias in an elderly urban population: relationship with age, sex, and education. Neurology 1991;41:1886–1892 [DOI] [PubMed] [Google Scholar]
- 26.Scazufca M, Almeida OP, Menezes PR. The role of literacy, occupation and income in dementia prevention: the Sao Paulo Ageing & Health Study (SPAH). Int Psychogeriatr 2010;22:1209–1215 [DOI] [PubMed] [Google Scholar]
- 27.Jefferson AL, Gibbons LE, Rentz DM, et al. A life course model of cognitive activities, socioeconomic status, education, reading ability, and cognition. J Am Geriatr Soc 2011;59:1403–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dehaene S, Pegado F, Braga LW, et al. How learning to read changes the cortical networks for vision and language. Science 2010;330:1359–1364 [DOI] [PubMed] [Google Scholar]
- 29.Bustamante SE, Bottino CM, Lopes MA, et al. Combined instruments on the evaluation of dementia in the elderly: preliminary results. Arq Neuropsiquiatr 2003;61:601–606 [DOI] [PubMed] [Google Scholar]
- 30.Herrera E, Jr, Caramelli P, Silveira AS, Nitrini R. Epidemiologic survey of dementia in a community-dwelling Brazilian population. Alzheimer Dis Assoc Disord 2002;16:103–108 [DOI] [PubMed] [Google Scholar]


