Abstract
Although hydrogels now see widespread use in a host of applications, low fracture toughness and brittleness have limited their more broad use. As a recently described interpenetrating network (IPN) of alginate and polyacrylamide demonstrated a fracture toughness of ∼9000 J/m2, we sought to explore the biocompatibility and maintenance of mechanical properties of these hydrogels in cell culture and in vivo conditions. These hydrogels can sustain a compressive strain of over 90% with minimal loss of Young's Modulus as well as minimal swelling for up to 50 days of soaking in culture conditions. Mouse mesenchymal stem cells exposed to the IPN gel-conditioned media maintain high viability, and although cells exposed to conditioned media demonstrate slight reductions in proliferation and metabolic activity (WST assay), these effects are abrogated in a dose-dependent manner. Implantation of these IPN hydrogels into subcutaneous tissue of rats for 8 weeks led to mild fibrotic encapsulation and minimal inflammatory response. These results suggest the further exploration of extremely tough alginate/PAAM IPN hydrogels as biomaterials.
Introduction
Hydrogels are now ubiquitous in their use in a wide range of fields. The availability of both natural and synthetic variants, the ability to be mechanically and chemically tuned, and the prospect of simultaneously controlling mechanical, chemical, and biological responses all promote hydrogels' use in applications ranging from medical devices, to microfluidics, to household products [1]. However, many hydrogels tend to be brittle, and mechanical limitations have thus precluded their more effective use in applications such as ligament and tendon prostheses, or soft robotic actuators [2]. For example, most hydrogels exhibit fracture toughness of less than 100 J/m2, an order of magnitude lower than that of cartilage [3].
Efforts to surmount these mechanical challenges have included the design of hybrid hydrogels, consisting of interpenetrating networks (IPN] of two different polymers. This approach follows the paradigm that the toughness of a hydrogel can be increased in conjunction with the elastic modulus by adding elements into the system that dissipate energy when strained [4]. Initial attempts at such material designs included IPNs of two covalently crosslinked networks, but these materials were limited as the covalent crosslinks were permanently broken with high strain, precluding repeated loading with the same toughness [4]. More recent approaches have combined covalently and ionically crosslinked polymers, although the highest achievable fracture toughness with those formulations is ∼1000 J/m2 [4-6].
Recently, a new hydrogel consisting of an IPN of alginate and polyacrylamide (PAAM) was reported which displays remarkable mechanical properties [7]. One advantage of this material is its incorporation of two, well-characterized materials, alginate and PAAM. Alginate is a naturally-derived polymer, attractive for, among other properties, its ease of chemical modification, controllable degradation, and self-healing properties when ionically cross-linked [8]. PAAM has long been used in contact lenses, and its hydrophilic and bio-inert nature makes it attractive for biomedical applications, among others [9, 10]. The mechanical properties of these new hydrogels are striking. For example, they provide a fracture toughness of ∼9000 J/m2, an order of magnitude tougher than cartilage and on the order of natural rubber [11]. An optimal formulation of the hydrogel can stretch to over twenty times its original length, and even, when subjected to a notch defect, stretch to over seventeen times its original length [7]. This gel exhibits three mechanisms that likely contribute to its toughness, whereas previous gels have only met two or fewer of these criteria [4, 5, 7]. First, the ionically-crosslinked alginate component provides an energy dissipation mechanism upon straining. Secondly, the long polyacrylamide polymer chain allows for crack bridging and hence maintenance of mechanical integrity once the ionic crosslinks are broken. Thirdly, secondary crosslinks that form between the alginate and polyacrylamide networks allow for force transfer between the two [7]. These properties suggest the potential for the use of these gels in a variety of applications, including orthopedic applications as a prosthetic, non-scaffold material, where their notch-insensitive nature could potentially be exploited to be compatible with suturing. In addition, in orthopedic applications, the failure mode of many materials, both natural and implanted, is fracture, and the high fracture toughness of these gels directly addresses that limitation [12].
Although PAAM is widely used in biomedical applications, toxicity of its monomer, acrylamide, has been documented [13]. Before exploiting these gels for biomedical applications, it is necessary to characterize their biocompatibility, as well as maintenance of mechanical properties when subjected to physiologic conditions. Here, we fabricate alginate/PAAM IPN hydrogels optimized for toughness and provide the first demonstration of the mechanical properties of these materials in compression. The effect of incubation in cell culture medium is then examined. The in vitro viability, metabolic activity, and proliferation of mouse mesenchymal stem cells is explored when cultured in media conditioned by these gels, due to the presence of these cells in orthopedic settings, a potential application area of these gels. Finally, the in vivo immunogenicity of the hydrogel is examined by histology of gels implanted in subcutaneous pockets in male Lewis rats.
Materials and Methods
Cell Culture
Unless otherwise stated, D1 mouse mesenchymal stem cells (ATCC) were cultured in Dulbecco's Modified Eagle Medium (DMEM, Lonza) with 10% Fetal Bovine Serum and 1% Penicilin/Streptomycin in a 37°C, 5% CO2 environment.
Sample Preparation
Alginate/PAAM hybrid gels were prepared as previously described [7]. Alginate (Protanol LF 20/40, FMC Technologies) and acrylamide (Invitrogen) were dissolved in water in a 1:6 ratio in order to achieve a final polymer concentration of 14% in the gel. This solution was rapidly mixed with N,N-methylenebisacrylamide (Invitrogen) as the acrylamide crosslinker, calcium sulfate slurry (Sigma) as the alginate crosslinker, N,N,N′,N′-tetramethylethylenediamine (Invitrogen) as the acrylamide crosslinking accelerator and ammonium persulfate (Sigma) as an acrylamide crosslinking photoinitiator. For the in vitro proliferation and metabolic activity assays, the gels were prepared with two different acrylamide crosslinking densities: 0.06 wt% N,N-methylenebisacrylamide relative to acrylamide, referred to as “optimal” and 0.03 wt%, referred to as “low.” The optimal crosslinking density was used in all other IPNs. This mixture was poured into a glass mold and exposed to a 254 nm light source for 1 hour. The alginate crosslinking was then allowed to complete overnight before gels were removed from the mold.
PAAM and alginate gels were also prepared as controls, using the same weight percentage polymer as in the hybrid gels. Before testing, gels were washed for 30 hours in serum-free DMEM (Lonza).
Two different schemes were to used in the cytotoxicity tests, and will be referred to as 1) cumulative, and 2) snapshot. The general paradigm was to condition cell culture media by soaking gels in the media for various time points and then assaying the media for its impact on cultured cells.
In the first scheme, at time zero, three circular gels (3mm thick, 8mm diameter) per time point were placed in 25 mL complete cell culture media (DMEM, 10% Fetal Bovine Serum, 1% penicillin/streptomycin) and were placed in a 37°C, 5% CO2 environment. At the time points of interest, the gels were removed from the media, which was then frozen. After all time points had been completed, the media was thawed and used as the cell culture media for the assays to follow, described below. Hence, this scheme examines potential cumulative release or degradation products.
In the second scheme, at time zero, fifteen circular gels (3mm thick, 8mm diameter) per time point were placed in 35 mL complete cell culture media (DMEM, 10% Fetal Bovine Serum, 1% penicillin/streptomycin) and were placed in a 37°C, 5% CO2 environment. At the time points of interest, three gels were removed from the media, and were transferred to 35 mL fresh complete media for three days, which was then collected and frozen. After all time points had been completed, the media was thawed and used as the cell culture media for the assays to follow, described below. Hence, this scheme examines snapshots of potential release or degradation.
In Vitro Cytotoxicity Assay
The WST assay is a cytotoxicity assay comparable to the MTT assay. For the conditioned media collected via both schema described above, the WST assay (Millipore) was performed per the manufacturer's instructions after seeding 5000 D1 cells/well in 100 μL complete culture media in 96 well plates for 8 hours before changing to 100μL conditioned media for 72 hours prior to the assay. The plates were read measuring absorbance at 450nm using a BioTek plate reader. Time points were chosen as to span a range during which any release of unincorporated reagents or degradation products would be expected.
In Vitro Proliferation Assay
In 24 well-plates, D1 cells were seeded at 5000 cells/well in 500 μL complete media and allowed to adhere for 6 hours in standard cell culture conditions. Media was changed to conditioned media from both schema and after 72 hours, cells were trypsinized and counted using a Coulter Counter Z2 (Beckman Coulter). Time points were chosen as to span a range during which any release of unincorporated reagents or degradation products would be expected.
Live/Dead Staining
For the 50-day time point in the second scheme, cells were cultured as in the proliferation assay. Instead of counting, however, live/dead staining was performed using the Live/Dead Kit (Life Technologies) per the manufacturer's instructions. Fluorescence images were acquired using 488 nm and 514 nm excitation channels.
Compression Testing
For testing the maintenance of elastic moduli of gels in culture conditions, gels were soaked for various times as described in Scheme 1. Upon removal from the media, gel dimensions were measured with calipers and the gels were subjected to compression tests using a mechanical testing apparatus (Instron Model 3342), with a 50N load cell, a compressive strain rate of 1mm/min, and no preload. Young's Modulus was calculated from the slope of the linear region of the resulting true stress/true strain curve. For testing the compressive properties of the gels outside of culture conditions, cylindrical gels 30mm in diameter and 12mm high were cast and tested as above, but the strain was terminated at a 90% strain and a 1000N load cell was used.
High Performance Liquid Chromatography
IPN gels were fabricated and washed as described above, and placed in sterile PBS in cell culture conditions for 40 days. At this point, the gels were removed and this PBS was saved. A standard curve of endotoxin-free acrylamide monomer (Sigma) in sterile dH20 was prepared and run on an Agilent 1200-series HPLC using an established protocol [14]. Between runs, the machine was flushed and the conditioned PBS was run, comparing the integrated peak area in the total ion count channel of the sample to those of the known concentrations from the standard curve.
In Vivo Biocompatibility
Gel samples were prepared as above using endotoxin-free alginate (FMC Technologies). All other gel components were sourced from the UltraPure line of endotoxin-free reagents (Invitrogen) and all gel solutions were autoclaved prior to casting. Alginate, PAAM, and hybrid gels were prepared in triplicate and implanted in dorsal subcutaneous pockets in Lewis rats in accordance with the Harvard University Standing Committee on the Use of Animals in Research and Teaching. At 8 weeks, animals were sacrificed and the gels and surrounding tissues were removed. Sectioning, paraffin embedding, and Hematoxylin–Eosin (HE) staining was performed by Mass Histology (Worcester, MA). P.A. is a board-certified pathologist and examined all histological sections. The 8-week time point was chosen to in order to gauge the fibrotic response to the hydrogels.
Results
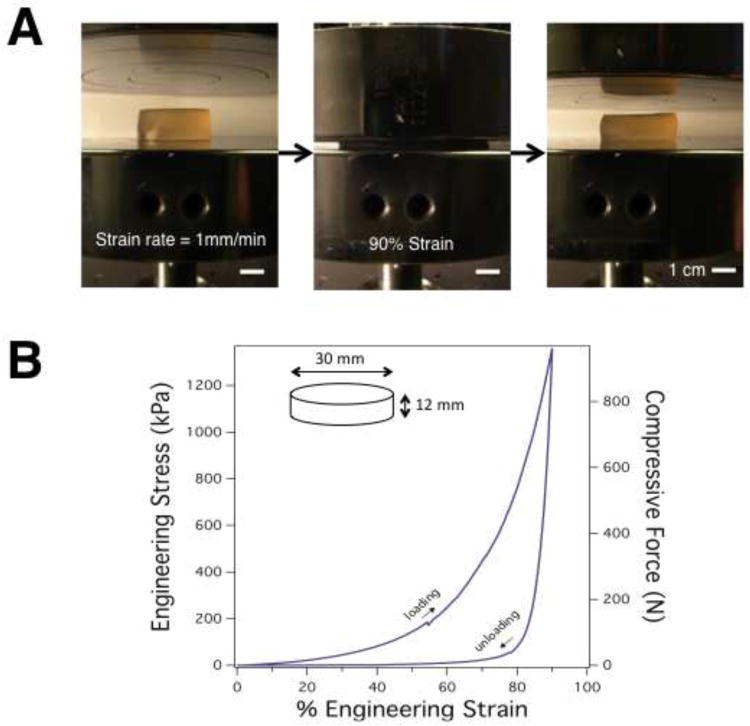
The initial mechanical properties and changes over time in vitro, of the IPNs, were first analyzed. Alginate/PAAM IPN hydrogels were synthesized with a 6:1 PAAM/alginate ratio as previously optimized for fracture toughness [7]. It has been suggested that the emergent properties of these gels, compared to alginate or PAAM alone, result partially from secondary cross-links between amine groups on PAAM and carboxyl groups on alginate, as well as the ability for ionic crosslinks between alginate chains to break under strain and reform after unloading at elevated temperatures. However, the mechanical properties of these gels in compression had not been explored. Strikingly, these gels were able to sustain a compressive strain of over 90% with full strain recovery (Fig. 1A,B). In order to characterize the change in IPN hydrogels' physical and mechanical properties over time, IPN gels were soaked in culture medium and at various time points, their elastic modulus was measured via compression testing. There were no statistically significant deviations in elastic modulus as a function of soaking time (Table 1A). Swelling was also examined, with any changes in gel volume over time potentially attributable to changes in gel porosity or surface effects. However, no appreciable changes in volume were observed as a function of soaking time (Table 1B).
Figure 1.
Compressive mechanical properties of alginate/PAAM IPN gels. A) Images of IPN gel undergoing compression testing to 90% strain. All scale bars 1cm. B) Engineering stress and compressive load as a function of engineering strain from the compressive test shown in A.
Table 1.
Hydrogel properties over time. A) Young's modulus as determined by compression test of optimal-toughness IPN gels as a function of soaking time in cell culture conditions (n=3). No statistically significant (p<0.05) deviation in Young's modulus was detected by one-way ANOVA with a Tukey post-hoc test. B) IPN gel volume as a function of soaking time in cell culture conditions (n=3). No statistically significant (p<0.05) deviation in Young's modulus was detected by one-way ANOVA with a Tukey post-hoc test.
| A | ||||
|---|---|---|---|---|
|
| ||||
| Low Crosslinking Density | High Crosslinking Density | |||
|
| ||||
| Days Soaking | Average Young's Modulus (kPa) | Std. Dev. | Average Young's Modulus (kPa) | Std. Dev. |
|
| ||||
| 1 | 19 | 3 | 25 | 4 |
| 16 | 25 | 6 | 29 | 4 |
| 42 | 18 | 4 | 21 | 1 |
| 50 | 24 | 3 | 30 | 7 |
| B | ||
|---|---|---|
|
| ||
| Days Soaking | Gel Volume (cu. mm) | Std. Dev. (cu. mm) |
|
| ||
| 3 | 352 | 36 |
| 19 | 354 | 25 |
| 45 | 310 | 42 |
| 51 | 328 | 49 |
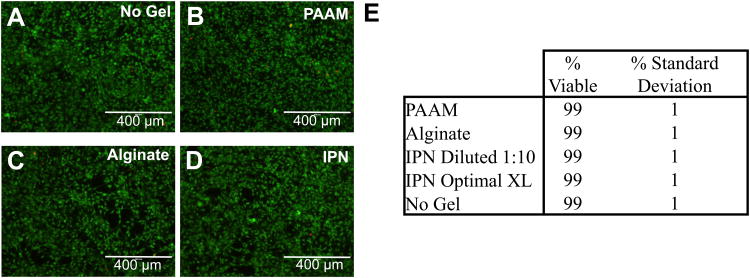
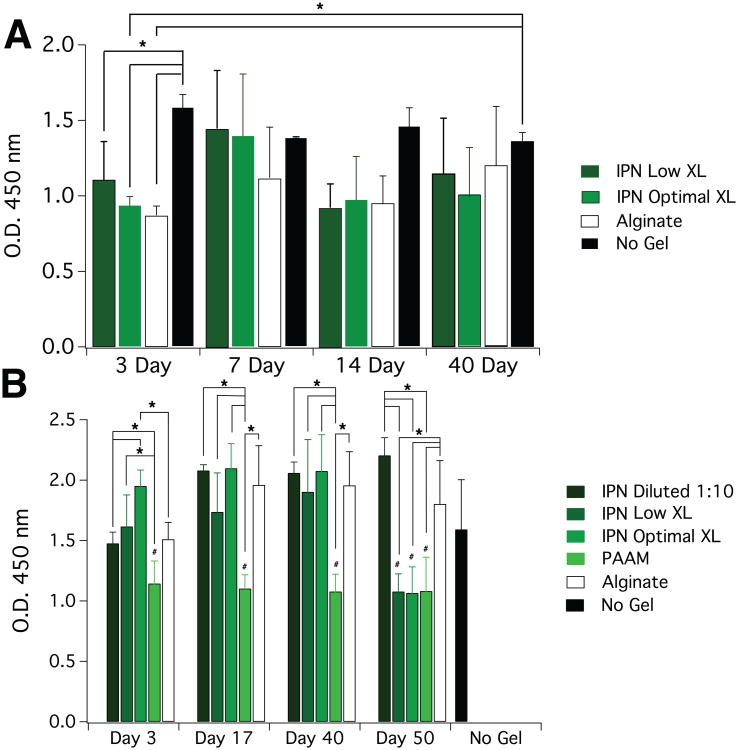
To first examine any potential cytotoxicity of these alginate/polyacrylamide interpenetrating networks, the viability and metabolic activity of mouse mesenchymal stem cells exposed to gel-conditioned media were examined. No significant difference in cell viability was seen between cells exposed to the IPN-conditioned media and controls (Fig. 2). Next, an in vitro cytotoxicity assay (WST assay) was used to profile the metabolic activity of cells exposed to IPN-conditioned media at different time points. In order to asses both the cumulative effects of IPN gel exposure, as well as capture the profile of how any effects may progress over time, cells were exposed to media in which gels continuously could release soluble products (cumulative), or media in which gels could release products for sequential 3-day periods (snapshot). In the cumulative case, activity tended to be lower in all gel cases versus the no gel control, although these differences were only statistically significant at 3 days (Fig. 3A). For all gels in the snapshot scheme, however, no statistically significant reductions in activity were seen for the first three time points, although in both IPN cases, the metabolic activity dropped to the PAAM levels in a statistically significant manner at the 50-day time point (Fig. 3B). This statistically significant drop in metabolic activity was recovered if the IPN-conditioned media was diluted 1:10 with complete media from the no gel condition. Although the dilution results in statistically significantly lower metabolic activity versus the nondiluted condition at 3 days, the activity is still comparable to that of the alginate case. This result suggests a concentration-dependent effect of IPN gel exposure, and that potentially negative effects of these gels on the metabolic activity of cells can be mitigated in a concentration-dependent manner. It should also be noted that there were no statistically significant differences in metabolic activity between cells exposed to media conditioned by the IPNs of different acrylamide crosslinking densities, which was suggestive of a minimal effect of the N,N-methylenebisacrylamide on the cells.
Figure 2.
Viability of mouse mesenchymal stem cells cultured for 3 days in 50-day gel-conditioned media. A-D) Photomicrographs of cell cultures. Live cells stain green and dead cells stain red. A) Complete DMEM control (no gel), B) Pure PAAM gel, C) Pure Alginate Gel, D) IPN Gel. Scale bar represents 400 μm. E) Cell viability percentage as a function of gel type used to condition media.
Figure 3.
Metabolic activity of cells exposed to gel conditioned medium. Plots depict 450 nm absorbance as a function of gel soaking time and gel type used to condition media. A) Cumulative scheme: gels were soaked in media such that they could continuously release soluble products up to the time points. B) Snapshot scheme: gels were soaked in media in culture conditions such that the gels could release products in sequential three day periods. Error bars represent S.D. (n=5) and statistical significance (*,p<0.05) was determined by two-way ANOVA with a Tukey post-hoc test. # marks p<0.05 relative to no gel control in snapshot case
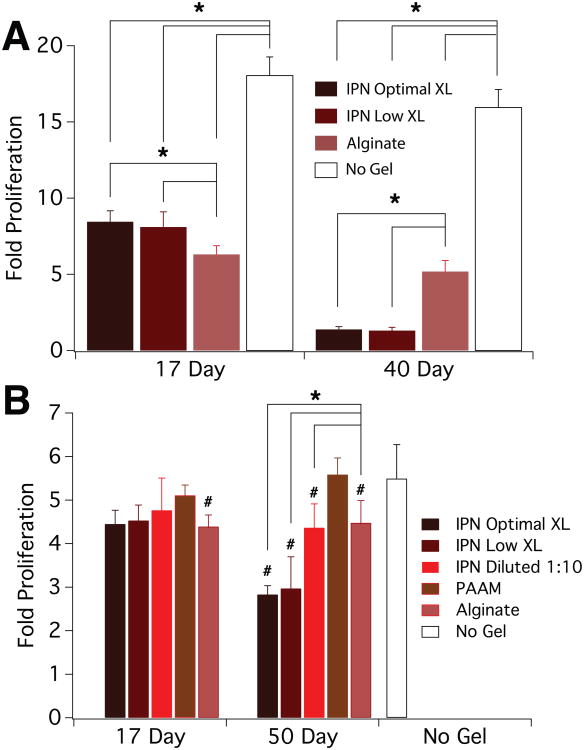
A proliferation assay was then performed in order to test the effects of IPN-conditioned media on this aspect of cell function. Paralleling the cytotoxicity assay, reductions were seen in cell proliferation in all gel conditions containing alginate (Fig. 4A). There also was a reduction in proliferation with conditioned media from longer-incubating IPN gels and this was not seen in the controls. In the snapshot case, a reduction in proliferation with the conditioned medium from alginate-containing gels was observed, but no reduction in proliferation was found with the media conditioned with PAAM gels (Fig. 4B). As in the cumulative scheme proliferation assay, there was a reduction in proliferation with conditioned media from the longer-incubating IPN gels, but this reduction was recovered by diluting 1:10 with control media.
Figure 4.
Proliferation of cells exposed to conditioned media. Plots depict fold increase in number of mouse mesenchymal stem cells normalized to initial seeding density as a function of gel soaking time and gel type used to condition media. A) Cumulative scheme: gels were soaked in media such that they could continuously release soluble products up to the time points. B) Snapshot scheme: gels were soaked in media in culture conditions such that the gels could release products in sequential three day periods. Error bars represent S.D. (n=5) and statistical significance (*,p<0.05) was determined by two-way ANOVA with a Tukey post-hoc test. # marks p<0.05 relative to no gel control in snapshot case
High performance liquid chromatography (HPLC) was used in order to investigate if free acrylamide was released from the IPN gels (Supp. Fig. 1). The IPN gels were soaked in PBS, and HPLC was performed on the conditioned PBS in order to quantify acrylamide release, revealing that 83 ± 3 μg acrylamide was released per gel over 40 days. Assuming that acrylamide release in cell culture media and PBS is comparable, this value also implies that the cells in the 40 day cumulative proliferation study were exposed to an acrylamide concentration of approximately 1 mM. Since the relevant concentration to which cells would be exposed in vivo is unclear due to diffusion of any released monomer and interstitial fluid flow, the experiments to follow serve to examine potentially deleterious effects of these hydrogels in vivo.
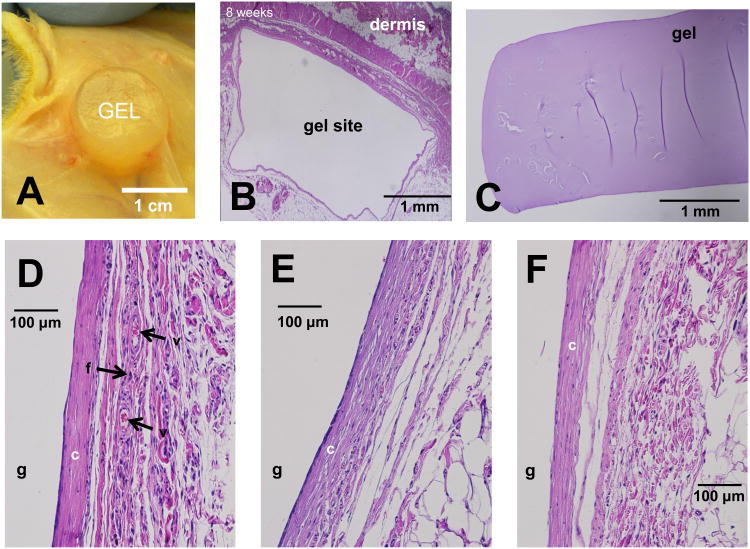
Alginate, PAAM and IPN gels were implanted in dorsal subcutaneous pockets in male Lewis rats (Fig. 5A,B) for 8 weeks in order to assess inflammation, vascularization, and fibrosis of the surrounding tissue. After 8 weeks, the hydrogels had been encapsulated and the resulting tissue pocket displayed new vasculature (Fig. 5B,C). In PAAM (Fig. 5D), alginate (Fig. 5E), and IPN (Fig. 5F) cases, H&E staining of the tissue surrounding the gels showed the absence of macrophages or lymphocytic infiltrations, suggesting a limited inflammatory response, at this 8-week time point. A fibrotic, collagen encapsulation was also visible surrounding the implantation sites in all cases, while the presence of red blood cells suggested the formation of new vasculature in the presence of the implants. The PAAM gels, however, (Fig. 5D, Supp. Fig. 2) led to a higher degree of compaction and vascularity, compared to the alginate and IPN gels. In addition, the PAAM gels displayed more collagen condensation in the fibrotic sheath as well as plump, stellate fibroblasts that could be indicative of a response to the mechanical nature of the gel. It should be noted, however, that the average thickness of the fibrous encapsulation did not vary significantly between gel types (Supp. Fig. 3). Exhaustive examination of the stained hydrogels themselves (Fig. 5E) revealed no cells infiltrating into the gels, as would be predicted by the small pore size of hydrogels at such weight percentages [7].
Figure 5.
Hydrogel implantation in rats. A) View of hydrogel in encapsulated subcutaneous pocket, 8 weeks post-implantation. B-F) Hematoxylin–Eosin-stained paraffin-embedded sections of hydrogel implantation site after 8 week in vivo. B) Representative overview of implantation pocket, with the dermis and gel site. C-F) Representative sections of gel/tissue interface for different gels. Hydrogel location labeled ‘g’, collagen layer labeled ‘c’, active fibroblasts labeled ‘f’, larger blood vessels labeled ‘v’. C) H&E-stained section of implanted IPN gel isolated from surrounding tissue. D) PAAM E) Alginate F) IPN Gel.
Discussion
Characterization of extremely tough IPNs has revealed the ability of these gels to sustain a 90% compressive strain, as well as maintenance of mechanical properties through long-term exposure to cell culture conditions. Minimal degradation of these gels in these conditions is evidenced by the lack of change in the Young's Modulus and swelling behavior as a function of exposure time to culture conditions. It has previously been shown that alginate must be modified via oxidation in order to degrade and that oxidation or a light-induced free radical mechanism is required for PAAM degradation, none of which are present in this study [15-17]. As these IPN hydrogels are particularly attractive for their mechanical properties, the maintenance of such in cell culture and in vivo environments is critical, although more characterization is necessary to ensure that their toughness is maintained after in vivo implantation.
The results from the in vitro cell studies suggest that these IPN gels are generally non-cytotoxic. The high cell viability when exposed to IPN-gel conditioned media is indicative of minimal acute cytotoxicity, while any statistically significant reductions in metabolic activity or cell proliferation are only seen for continuous, long-term concentration of agents released from the gels. These metabolic activity and proliferation results are consistent with literature describing high metabolic activity of cells exposed to alginate gels for fewer than 10 days, as well as proliferation of cells exposed to alginate on the same order of magnitude of the results shown here, although the literature also reports an effect of the relative number of alginate mannuronate and guluronate residues on cell proliferation [18, 19]. In addition, statistically significant reductions in cell metabolic activity with PAAM gels are consistent with literature reporting similar effects that suggests latent acrylamide monomer as a potential source of such reductions [20]. One hypothesis for the reductions in metabolic activity and proliferation with the IPN and alginate gels relative to the no gel controls is that all gels other than PAAM contain alginate, which is known to bind heparin-binding growth factors [21]. The alginate could be sequestering growth factors from uptake by cells and thus slightly inhibiting their metabolic activity. The reductions can be recovered by diluting the cell culture media with media that had not been exposed to a gel, suggesting a concentration-dependent response of cells to these materials, and thus minimal deleterious response of cells to this material if the gel concentration is sufficiently low or the fluid surrounding the gel is periodically replaced. Moreover, the lack of statistically significant differences in cell proliferation and metabolic activity between IPN cases of different acrylamide crosslinking densities suggests that the reductions are not due to the acrylamide crosslinker. Although the in vitro toxicity of acrylamide has not been reported for mesenchymal stem cells, reductions in in vitro proliferation of neural progenitor cells has been documented at acrylamide concentrations in excess of 2.5 mM, and similar reductions in proliferation have been reported in primary rat astrocytes in culture at acrylamide concentrations in excess of 0.5mM [22, 23]. Hence, the projected acrylamide concentration in the cumulative scheme cell studies of approximately 1mM provides a possible cause for these reductions. These in vitro cytotoxicity results are important when considering these IPNs for applications such as microfluidics and soft robotics, and suggest an extensive washing step may be desirable before using in continuous contact with cells.
The implantation study of the IPN gels suggests minimal inflammation in vivo. Histology of the tissue surrounding the gels shows an absence of immune cells and a comparable fibrotic response between alginate and the IPN gels as evidenced by less collagen compaction and vasculature relative to the PAAM gels. These results are consistent with literature exploring the in vivo biocompatibility of alginate and PAAM, which describes alginate as only non-immunogenic and PAAM as eliciting a mild inflammatory response [18, 24]. The favorable histology of the IPN gels relative to the PAAM gels suggests that in vivo exposure to latent acrylamide monomer is minimal [24]. In addition, the similar histological response between the IPN gels and alginate is suggestive of the non-inflammatory nature of the IPNs, as alginate is well characterized and highly used in vivo [18]. This in vivo response to the IPN gels is of critical importance when considering their use in therapeutic applications and medical devices with a high degree of human interaction.
Conclusions
These results demonstrate that alginate/PAAM IPN gels show minimal effects on cells in vitro and in vivo, as compared to pure alginate hydrogels. Combined with results showing minimal mechanical degradation of these gels in cell culture conditions, these studies suggest further exploration into leveraging the striking mechanical properties of this new biomaterial.
Supplementary Material
Supplemental Figure 1. Sample HPLC run of IPN-conditioned PBS after 40 days. Arrows denote peaks representing acrylamide and bis-acrylamide.
Supplemental Figure 2. High magnification (100×) H&E-stained sections of dermal-side tissue surrounding implanted gels after 8 weeks in rats. A) PAAM. B) Alginate. C) IPN
Supplemental Figure 3. Quantification of fibrotic capsule thickness as a function of gel type after 8 weeks subcutaneous implantation (n=3 gels/gel type). No statistically significant (p<0.05) deviation in capsule thickness was detected by oneway ANOVA with a Tukey post-hoc test.
Acknowledgments
The authors would like to thank Dr. Evi Lippens for helpful discussions regarding the histology, and funding support from the National Institutes of Health (R01 DE013349, R37 DEO013033). This research was partially supported by the intramural research program of the NIDCR/NIH (PA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv Mater. 2006;18(11):1345–60. [Google Scholar]
- 2.Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338(6109):917–21. doi: 10.1126/science.1222454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lake GJ, Thomas AG. The strength of highly elastic materials. Proc R Soc London Ser A. 1967;300(1460):108–119. [Google Scholar]
- 4.Gong JP, Katsuyama Y, Kurokawa T, Osada Y. Double-network hydrogels with extremely high mechanical strength. Adv Mater. 2003;15(14):1155–8. [Google Scholar]
- 5.Henderson KJ, Zhou TC, Otim KJ, Shull KR. Ionically cross-linked triblock copolymer hydrogels with high strength. Macromolecules. 2010;43(14):6193–201. [Google Scholar]
- 6.Yu QM, Tanaka Y, Furukawa H, Kurokawa T, Gong JP. Direct observation of damage zone around crack tips in double-network gels. Macromolecules. 2009;42(12):3852–5. [Google Scholar]
- 7.Jeong-Yun S, Xuanhe Z, Widusha RKI, Ovijit C, Kyu Hwan O, David JM, et al. Highly stretchable and tough hydrogels. Nature. 2012;489(7414):133–6. doi: 10.1038/nature11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101(7):1869–80. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 9.Fernández E, López D, López-Cabarcos E, Mijangos C. Viscoelastic and swelling properties of glucose oxidase loaded polyacrylamide hydrogels and the evaluation of their properties as glucose sensors. Polymer. 2005;46(7):2211–7. [Google Scholar]
- 10.Rosiak J, Burozak K, Pȩkala W. Polyacrylamide hydrogels as sustained release drug delivery dressing materials. Radiat Phys Chem (1977) 1983;22(3–5):907–15. [Google Scholar]
- 11.Simha NK, Carlson CS, Lewis JL. Evaluation of fracture toughness of cartilage by micropenetration. J Mater Sci Mater Med. 2004;15(5):631–9. doi: 10.1023/b:jmsm.0000026104.30607.c7. [DOI] [PubMed] [Google Scholar]
- 12.Teoh S. Fatigue of biomaterials: a review. International Journal of Fatigue. 2000;22:825–37. [Google Scholar]
- 13.Exon JH. A review of the toxicology of acrylamide. J Toxicol Environ Health B Crit Rev. 2006;9(5):397–412. doi: 10.1080/10937400600681430. [DOI] [PubMed] [Google Scholar]
- 14.Al-Taher F. Analysis of acrylamide in french fries using Agilent Bond Elut QuEChERS AOAC kit and LC/MS/MS. Agilent Technologies; 2012. [Google Scholar]
- 15.Boontheekul T, K H, Mooney DJ. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials. 2005;26(15):2455–65. doi: 10.1016/j.biomaterials.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 16.Lu M, W X, Wei X. Chemical degradation of polyacrylamide by advanced oxidation processes. Environ Technol. 2012;33:1021–8. doi: 10.1080/09593330.2011.606279. [DOI] [PubMed] [Google Scholar]
- 17.Ver Vers L. Determination of acrylamide monomer in polyacrylamide degradation studies by high-performance liquid chromatography. J Chromatogr Sci. 1999;37(12):486–94. doi: 10.1093/chromsci/37.12.486. [DOI] [PubMed] [Google Scholar]
- 18.Orive G, Carcaboso AM, Hernández RM, Gascón AR, Pedraz JL. Biocompatibility evaluation of different alginates and alginate-based microcapsules. Biomacromolecules. 2005;6(2):927–31. doi: 10.1021/bm049380x. [DOI] [PubMed] [Google Scholar]
- 19.Pariente JL, Kim BS, Atala A. In vitro biocompatibility evaluation of naturally derived and synthetic biomaterials using normal human bladder smooth muscle cells. J Urology. 2002;167(4):1867–71. [PubMed] [Google Scholar]
- 20.Lee K, EA S, Mooney D. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface. 2010;8(55):153–70. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Risbud MV, Bhonde RR. Polyacrylamide-chitosan hydrogels: in vitro biocompatibility and sustained antibiotic release studies. Drug Deliv. 2000;7(2):69–75. doi: 10.1080/107175400266623. [DOI] [PubMed] [Google Scholar]
- 22.Park H. Acrylamide induces cell death in neural progenitor cells and impairs hippocampal neurogenesis. Toxicol Lett. 2010;193(1):86–93. doi: 10.1016/j.toxlet.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi M, T H, Hashimoto K. Effect of acrylamide on cerebellar astrocyte proliferation in vitro. Toxicol in Vitro. 1988;2(2):69–74. doi: 10.1016/0887-2333(88)90016-1. [DOI] [PubMed] [Google Scholar]
- 24.Smetana K, Jr, V J, Souckava D, Krcova Z, Sulc J. The influence of hydrogel functional groups on cell behavior. J Biomed Mater Res. 1990;24(4):463–70. doi: 10.1002/jbm.820240405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Sample HPLC run of IPN-conditioned PBS after 40 days. Arrows denote peaks representing acrylamide and bis-acrylamide.
Supplemental Figure 2. High magnification (100×) H&E-stained sections of dermal-side tissue surrounding implanted gels after 8 weeks in rats. A) PAAM. B) Alginate. C) IPN
Supplemental Figure 3. Quantification of fibrotic capsule thickness as a function of gel type after 8 weeks subcutaneous implantation (n=3 gels/gel type). No statistically significant (p<0.05) deviation in capsule thickness was detected by oneway ANOVA with a Tukey post-hoc test.