Abstract
Background
Genetic variation in endocannabinoid metabolism is associated with colonic transit in irritable bowel syndrome (IBS) with diarrhea (IBS-D). The nonselective cannabinoid (CB) receptor agonist, dronabinol (DRO), reduced fasting colonic motility in nonconstipated IBS. FAAH and CNR1 variants influenced DRO’s effects on colonic motility. Our aims were: 1) To compare dose-related effects of DRO to placebo (PLA) on gut transit in IBS-D; and 2) To examine influence of genetic variations in CB mechanisms on DRO’s transit effects.
Methods
36 IBS-D volunteers were randomized (double-blind, concealed allocation) to twice daily PLA (n=13), DRO 2.5mg (n=10), or DRO 5mg (n=13) for two days. We assessed gastric, small bowel, and colonic transit by validated radioscintigraphy and genotyped the single nucleotide polymorphisms CNR1 rs806378 and FAAH rs324420. Data analysis utilized a dominant genetic model.
Key Results
Overall treatment effects of DRO on gastric, small bowel, or colonic transit were not detected. CNR1 rs806378 CT/TT was associated with a modest delay in colonic transit at 24h compared to CC (p=0.13 for differential treatment effects on post- minus pre-treatment changes in colonic transit by genotype). No significant interaction of treatment with FAAH rs324420 was detected.
Conclusions/Inferences
Overall, DRO 2.5mg or 5mg twice daily for two days had no effect on gut transit in IBS-D. There appears to be a treatment-by-genotype effect whereby DRO preferentially delays colonic transit in those with the CNR1 rs806378 CT/TT genotypes. Further study of CB pharmacogenetics may help identify a subset of IBS-D patients most likely to benefit from CB agonist therapy.
Keywords: cannabinoid, anandamide, FAAH, motility, nonselective, receptor, gastric, small bowel
Cannabinoid receptors type 1 (CB1) are identified in colonic mucosa and neuromuscular layers1–3; they are also expressed in plasma cells and influence mucosal inflammation4. The endocannabinoid system consists of CB1 and CB2 receptors; the ligands of these receptors are anandamide and 2-arachidonyl glycerol (2-AG), and their respective ligand-inactivating enzymes are fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MGLL)5–8. Activation of CB1 receptors coupled to cholinergic motor neurons inhibits excitatory nerve transmission in human colonic circular muscle in vitro9. In vivo, endocannabinoids acting on myenteric CB1 receptors tonically inhibit colonic propulsion in mice10; they also inhibit gastric and small intestinal transit without altering intraluminal pressure or basal tone in rodents11,12.
We have previously shown that dronabinol (DRO), a nonselective CB receptor agonist, inhibits gastric emptying and colonic motility in healthy humans13,14. The effects on colonic tone and phasic motility were observed with 7.5mg dronabinol, which also induced side effects of drowsiness, lightheadedness, and dizziness13. The 5mg dronabinol dose was more tolerable among healthy participants14. In patients with irritable bowel syndrome (IBS) with diarrhea (IBS-D), genetic variation in endocannabinoid metabolism in the fatty acid amide hydrolase (FAAH) gene was associated with symptoms and colonic transit15. In another prior study of non-constipated IBS patients consisting of both patients with IBS-D and IBS with alternating bowel habit (IBS-A), DRO 5mg reduced fasting colonic motility16.
We hypothesized that dronabinol inhibits colonic transit in IBS, and that these inhibitory effects are modulated by variations in the genes for the CB1 receptor (CNR1) and for FAAH, the rate-limiting enzyme in degradation of the endocannabinoid anandamide. Our specific aims were: 1) to compare effects of two consecutive days of twice daily administration of oral placebo, DRO 2.5mg, and DRO 5mg on gastric, small bowel, and colonic transit in cannabinoid-naive IBS-D patients; and 2) to examine potential influences of genetic variations in CNR1 and FAAH on the transit effects of dronabinol treatment.
MATERIALS AND METHODS
Study Design
This was a double-blind, randomized, placebo-controlled, parallel-group study (registered at ClinicalTrials.gov, identifier NCT01253408) of the pharmacodynamic effects of DRO on gastric, small bowel, and colonic transit of otherwise healthy participants with IBS-D by Rome III criteria. Their ages were between 18 and 69 years, and body mass indices between 21 and 56 kg/m2, with 2 subjects with BMI>40kg/m2. The study was conducted in the Clinical Research Unit at Mayo Clinic in Rochester, MN (NIH CTSA grant RR0024150), where the full trial protocol is kept; the study began on October 2008 and was completed on April 2011. The study was approved by Mayo Clinic Institutional Review Board, and a data safety monitoring plan was established prior to starting the study.
Participants
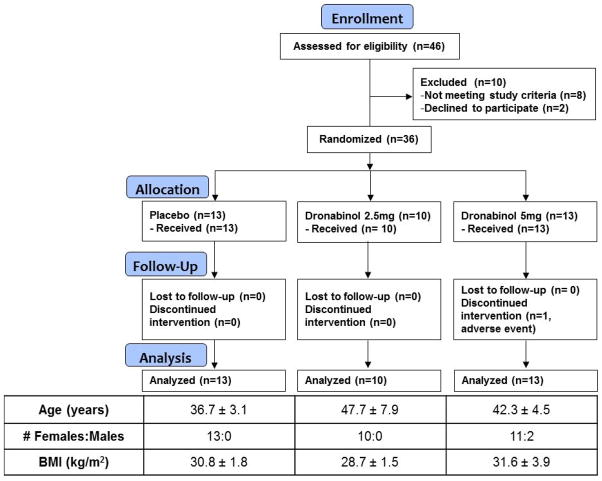
All participants were recruited from a database of patients with IBS who reside within 150 miles of Rochester, MN. Participants completed a validated bowel disease questionnaire (BDQ, including questions that correspond to Rome III criteria)17 and the Hospital Anxiety and Depression Inventory (HAD)18. Potential participants who met the eligibility criteria for the study underwent a complete history and physical examination before enrollment. All candidates were screened by history and by review of their medical records to ensure they were cannabinoid-naïve. All females of childbearing potential had to have a negative pregnancy test within 48 hours of study. The trial flow is summarized in Figure 1.
Figure 1.
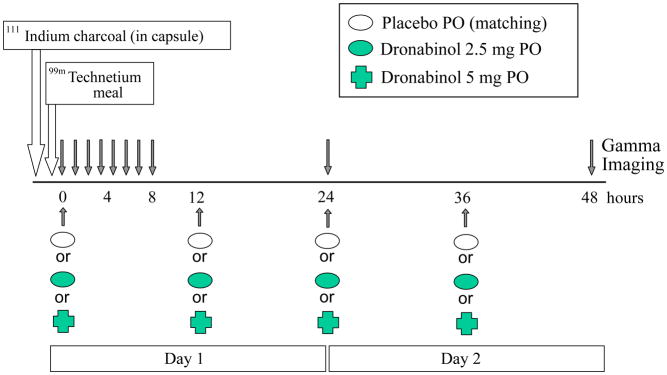
Experimental design showing timing of study medications and scintigraphic transit measurements.
Experimental Design
Thirty-six participants with IBS-D were enrolled and completed most studies as in the protocol with measurements over 48 hours. Participants were randomized to oral administration of matching placebo, dronabinol 2.5mg, or dronabinol 5mg, taken with water twice daily for two days, with the morning dose ingested at the study center under supervision of study staff.
Randomization to treatment group was conducted by computer program. Allocation was concealed, and participants and investigators were blinded to all treatment assignments. The research pharmacist ensured participants were assigned to the appropriate group, in accordance with the random allocation sequence. At study completion, the randomization code was communicated to the study statistician by the research pharmacist.
Pharmacology of Dronabinol
Dronabinol is a synthetic delta-9-tetrahydrocannabinol (Δ9-THC). It is a cannabinoid agonist that is non-selective, with affinity for both CB1 and CB2 receptors. It is highly (~95%) absorbed after a single oral dose19; however, due to high first pass hepatic metabolism (primarily by microsomal hydroxylation) and lipid solubility, only 10–20% of administered oral doses reach the systemic circulation. The onset of action is at 0.5 to 1 hour after oral administration, and the peak effect is at 2 to 4 hours. The elimination phase follows a two-compartment model, with an initial half-life of ~4 hours and a terminal half-life of 25–36 hours. Biliary excretion is the major route of elimination. The rapid onset and peak effect of the medication and the experimental nature of the study with potential for adverse effects (mental, dizziness etc…) led us to choose an experimental design that required only two days of treatment to observe the acute effects of DRO, rather than 7–10 days of treatment which would be required for drug levels to reach steady state. Given the well-published pharmacokinetic results19 and the prior observations of pharmacological effects of DRO using this acute administration design13–14,16, we did not conduct any blood measurements for drug levels.
Experimental Procedure
All participants underwent the following procedures: documentation of eligibility, screening questionnaires, and physical examination within the prior month. The physical examination included standard rectal and pelvic floor examinations20 to exclude rectal evacuation disorder. This was deemed necessary to ensure the diarrhea was not secondary to “retention of stool with overflow”. Participants then underwent baseline colonic transit measurement (GC 24 and GC 48 hours), off treatment. Treatment days corresponded to the scintigraphic transit testing days (days 1 and 2) with participants receiving the medication to which they were randomized. Scintigraphic measurements of gastric, small bowel, and colonic transit were conducted, using a previously validated method (see below) on days 1 and 2, and were completed with a fasting 48-hour scan on day 3 when no medication was administered.
On days 1 and 2, the morning dose of medication was ingested in the research lab, with the participant fasting. On day 1, the morning dose of medication was administered together with the delayed release capsule containing 111In-labeled activated charcoal used to measure colonic transit. On day 2, the morning dose of medication will be given after the 24-hour scan. The evening doses on days 1 and 2 were ingested by participants at bed time in their homes. This timing was selected to reduce the potential for adverse effects such as drowsiness and dizziness which we previously observed in humans who were administered similar doses.
With appropriate consent, a venous blood sample was obtained from all participants, except one from whom a blood sample could not be drawn despite multiple unsuccessful venipuncture attempts, for DNA extraction and pharmacogenomics studies.
Selection of Candidate Endocannabinoid Genetic Polymorphisms
To address the impact of pharmacogenetics on the transit response to dronabinol, we selected two genetic variants, based on the findings in our prior studies15,16 and the minor allele frequencies observed in our database. FAAH and CB1 receptor expressions have been localized to myenteric neurons21.
CNR1 is the gene coding for the CB1 receptor. CNR1 rs806378 (CC vs. CT/TT) showed potential effect on fasting proximal left colonic motility in IBS-D and IBS-A patients16. The T allele of CNR1 polymorphism rs806378 is associated with altered nuclear protein binding in an electrophoretic mobility shift assay, suggesting that rs806378 is a functional polymorphism22. We chose to study rs806378 (whose nearest gene on the chromosome is CNR1), since we previously demonstrated association of this variant with gastric motor functions. In a study by Vazquez-Roque et al.23, rs806378 CC genotype was associated with reduced fasting gastric volume, as well as a modest, non-significant association with gastric emptying of solids compared to the CT/TT group.
FAAH is the rate-limiting enzyme for metabolism of the endocannabinoid, anandamide. The 385C-to-A allelic variant of rs324420 in the human FAAH gene leads to a Pro129Thr amino acid change, which decreases expression of the FAAH protein secondary to reduced protein stability24. The prevalence of the A allele is 16–25% in studies of Caucasians in the NCBI database. Our laboratory previously confirmed the A allele frequency to be 25% in our sample population of healthy controls and IBS patients in southeastern Minnesota15. Reduction in FAAH protein level and activity compromises inactivation of the endocannabinoid anandamide. This leads to higher synaptic concentrations of anandamide and, hypothetically, a greater effect of the exogenously administered cannabinoid, dronabinol, via activation of CB1 and CB2 receptors.
Genotyping
DNA was extracted from whole blood, as previously described24. Genotyping of FAAH rs324420 and CNR1 rs806378 was performed using Taqman™ SNP Genotyping assays (Applied Biosystems, Inc., Foster City, CA) in accordance with manufacturer instructions.
Gastrointestinal and Colonic Transit
A validated scintigraphic method was used to measure gastric, small bowel, and colonic transit. A methacrylate-coated capsule that dissolves in the alkaline pH of the distal ileum was used to release 111In-labeled activated charcoal particles to evaluate colonic transit on sequential scans25. Orally ingested 99mTc-labeled egg meal allows measurement of gastric and small bowel transit. The method has been shown to detect accelerated or delayed transit. These measurements of colonic transit are associated with altered stool frequency and consistency and are predictive of response to therapy for bowel dysfunction 26–29. Three standard meals were ingested during day 1, and patients were instructed to eat their normal diet on day 2.
The primary endpoint for analysis was colonic transit geometric center at 24 hours. Secondary endpoints were colonic transit geometric center at 48 hours, ascending colon emptying T1/2; gastric emptying T1/2; and colonic filling at 6 hours (a surrogate for small bowel transit time).
Sample Size Assessment
Table I (in Supplementary Materials) shows the coefficients of variation (COV) and effect sizes demonstrable with approximately 80% power, assuming an average n=12 per group, using a two-sample t-test with a two-sided α level of 0.05.
Statistical Analysis
The entire research team was blinded to treatment allocation until all studies had been completed. All subjects randomized were included in the analysis under the intention-to-treat (ITT) paradigm. Subjects with missing data had the corresponding values imputed using the overall mean for the remaining subjects. An analysis of covariance (ANCOVA) was used to assess treatment effects on colonic transit incorporating BMI and the corresponding “baseline” value as covariates. An adjustment in the error degrees of freedom was made (subtracting one for each missing value imputed) to adjust the estimates of error variance in the ANCOVA models. In the pharmacogenetic ANCOVA models that explored gene-by-treatment interactions, one patient randomized to DRO 2.5mg was excluded as she did not consent to provide a DNA sample.
RESULTS
Participants and Compliance with Medication
The trial flow is shown in Figure 1. Forty-six patients were assessed for eligibility. Thirty-six IBS volunteers meeting the entry criteria were screened, randomized, and completed the study. A total of 13 volunteers randomly received placebo BID, 10 received DRO 2.5mg BID, and 13 received DRO 5mg BID. One patient in the DRO 5mg group discontinued medication due to an adverse event, and those data were imputed in accordance with the statistical analysis plan (see above). The table within Figure 2 summarizes patient demographics by treatment groups. No clinically important differences in age, sex, and body mass index were observed between treatment groups.
Figure 2.
Trial flow chart and baseline characteristics of study participants (mean ± SEM, unless otherwise noted).
Pharmacodynamic Effects of Dronabinol on Gastric, Small Bowel and Colonic Transit
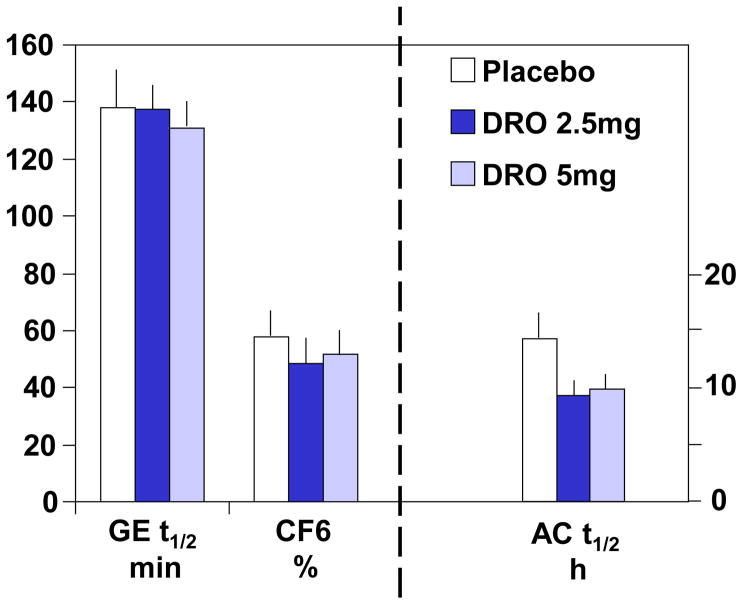
There were no overall or dose–related treatment effects on gastric (p= 0.88), small bowel (p=0.76), and colonic (overall p=0.23; 2.5mg DRO vs placebo, p=0.16; 5mg DRO vs placebo p=0.53) transit (Figures 3A and 3B).
Figure 3.
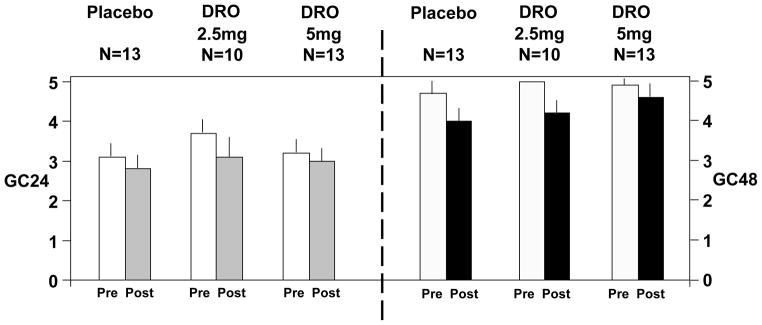
Figure 3A. Effect of dronabinol on gastric emptying, colonic filling, and ascending colon emptying t1/2. Data show mean ± SEM.
Figure 3B. Effect of dronabinol on colonic transit expressed as geometric center at 24 and 48 hours (GC24 and GC48, respectively). Data show mean ± SEM. The pre-drug GC48 for DRO 2.5mg has no SEM bar because all measurements reached a maximum of 5 GC units.
Pharmacogenetics: Treatment by Genotype Interaction Effects for the Entire IBS Group
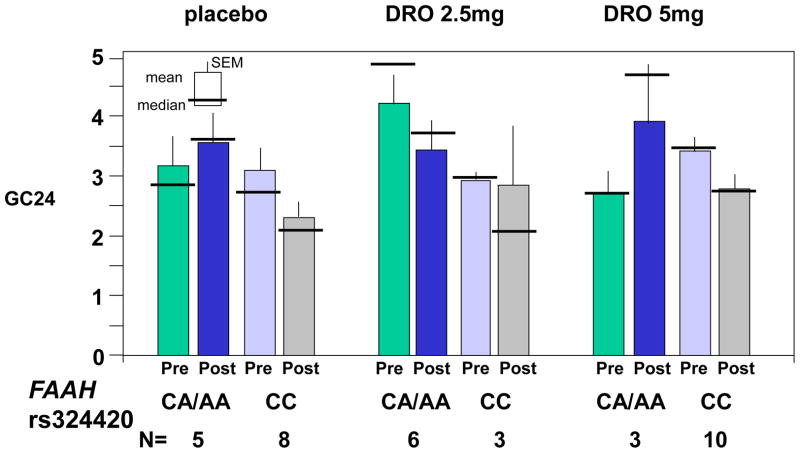
In the CC FAAH rs324420 genotype group the baseline values were fairly similar. There were no qualitative differences for changes (Post-Pre) in colonic transit from pre-treatment baseline values following placebo or DRO treatment (Figure 4A). However, the baseline GC 24 hour values in the CA/AA subgroup were rather dissimilar. In this subgroup, although there appear to be differential treatment effects (e.g. placebo vs. 2.5mg or 5mg doses), a statistically significant treatment-by-gene interaction was not detected (p=0.46).
Figure 4.
Figure 4A. Pharmacogenetics of FAAH rs 324420 and effects of the two doses of dronabinol and placebo on colonic transit at 24 hours (GC24). Data show mean, SEM, and median.
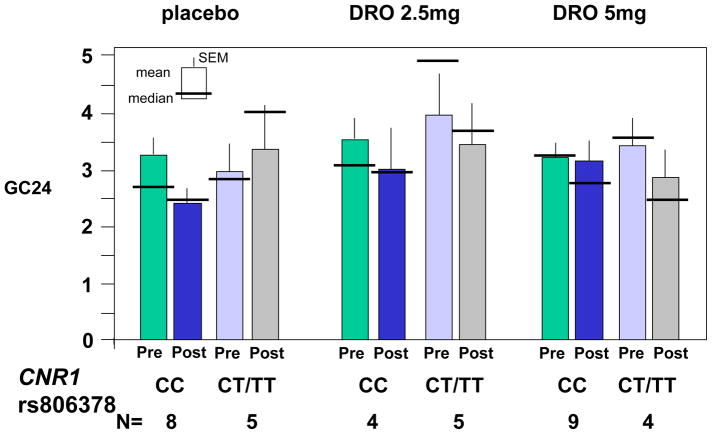
Figure 4B. Pharmacogenetics of CNR1 rs806378 and effects of the two doses of dronabinol and placebo on colonic transit at 24 hours (GC24). Data show mean, SEM, and median.
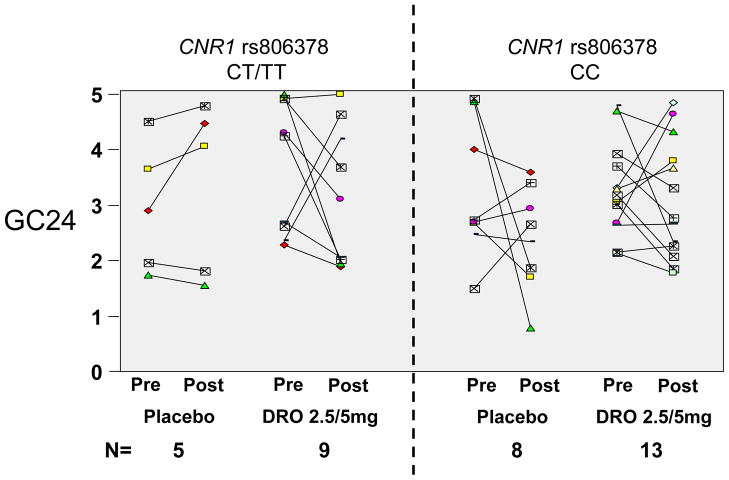
CNR1 rs806378 CC genotype was associated with numerical post-treatment changes in colonic transit GC24 from pre-treatment that were qualitatively similar in the three treatment groups (Figure 4B). In contrast, the CT/TT genotype subgroup showed slowing of colonic transit post-treatment with both 2.5mg and 5mg doses, in contrast to the placebo group which showed a slight acceleration post-treatment. The statistical analysis revealed no significant differences to suggest a gene-by-treatment dose interaction.
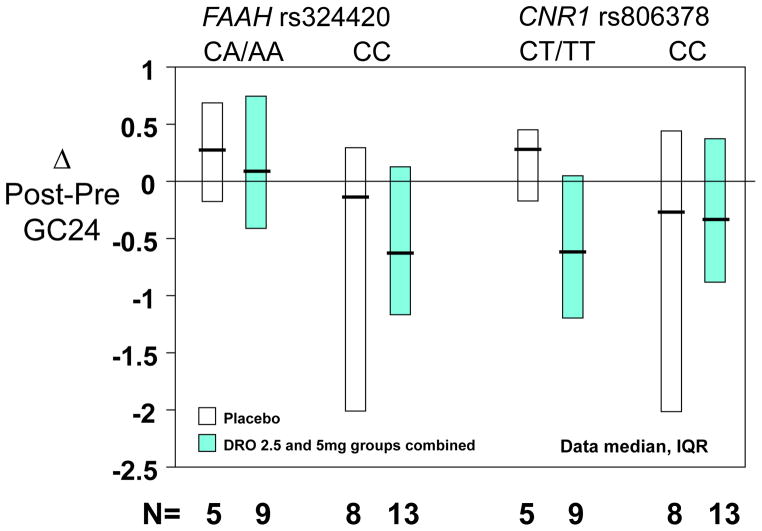
Given the similarity in the slowing effects of the DRO 2.5mg and 5mg doses in the CNR1 rs806378 CT/TT genotype group, we examined potential differential treatment effects associated with genotype comparing placebo with the combined DRO dose groups. As illustrated in Figure 5, the numerical slowing of colonic transit at 24 hours (indicated by individual patient data in Figure 5A, and by the post-pre change in colonic transit at 24 hours in Figure 5B) was confirmed in the CT/TT genotype group, relative to placebo. While this was not statistically significant (p=0.14 for delta GC 24), the unexpected lack of correlation in pre- vs. post-treatment GC 24 values resulted in larger than anticipated variation for changes in colonic transit
Figure 5.
Pharmacogenetics of FAAH rs 324420 and CNR1 rs806378 on pre- and post-treatment colonic transit GC24 for each individual participant (Figure 5A) and the change in colonic transit at 24 hours (Figure 5B) for the combined dronabinol treatment group compared to placebo.
Analysis of FAAH rs324420 genotype for differential treatment effects on post-treatment minus pre-treatment colonic transit at 24 hours was not significant (test for gene–by-treatment interaction, p=0.47).
Adverse Effects
The observed adverse effects (Table II in Supplementary Materials) were not significantly different among the three treatment groups.
DISCUSSION
Cannabinoid receptors are located on cholinergic neurons in the brain stem, stomach and colon. Their activation by the nonselective cannabinoid receptor agonist, dronabinol, inhibits gastrointestinal and colonic muscle excitation by inhibiting cholinergic neurons in the central and enteric nervous systems30. Our overall hypothesis was that cannabinoid receptor modulation is a potential target for therapy in diseases associated with accelerated transit27, as in the increased colonic motor function observed in patients with IBS-D31,32. About 48% of patients with IBS-D have accelerated colonic transit at 24 or 48 hours compared to healthy controls27. Given the effects of CB1 receptor modulation on colonic functions13,16 and the association of FAAH genetic variation with diarrheal symptoms and colonic transit in IBS-D patients24, we conducted a pharmacogenetic analysis exploring the influence of genetic variations in the CB1 receptor and in the rate-limiting catabolic enzyme for anandamide (FAAH) in humans.
This study demonstrates that nonselective cannabinoid CB receptor stimulation with DRO 2.5mg or 5mg b.i.d. does not significantly alter overall gastric, small bowel, or colonic transit in patients with IBS-D, of whom the vast majority (34/36) in the current study was female. In a previous study of gastrointestinal and colonic transit in 30 healthy volunteers (7 males and 8 females randomized in each of two groups to DRO and placebo), we had evaluated the effects of DRO 7.5mg b.i.d and observed significant delay of gastric emptying in females14, but no significant effects on small bowel or colonic transit in either gender13. Therefore, the effect of DRO in IBS-D patients on the primary endpoint of interest (overall colonic transit) is consistent with the effect observed in healthy human subjects. The absence of an effect on gastric emptying in IBS-D patients in the current study may reflect the lower dose of DRO used (5mg b.i.d. instead of 7.5mg b.i.d.).
The current study results do not conform with the observed reduction in intraluminally measured colonic motility and compliance observed with dronabinol treatment of patients with non-constipated IBS16; however, it is worth noting that the 5mg dronabinol inhibited fasting colonic phasic pressure activity and colonic compliance, and there was no demonstrated effect on postprandial colonic tone or phasic pressure activity16. Overall, the two studies question whether a non-selective CB agonist that has potential for central adverse effects can be dosed at sufficient high levels to replicate colonic motor inhibitory effects observed with 7.5mg DRO b.i.d. Clearly peripherally-selective, CB1 modulating agents are required to explore the role of cannabinoid mechanisms in the control or modulation of colonic motor function.
On the other hand, the pharmacogenetic component of the study shows that, even with the small numbers of patients involved in the genotype subgroups, some patients who carry the CNR1 rs806378 CT/TT genotype may show notable delays in colonic transit with DRO treatment.
In the current study, we elected to use the lower maximum dose of 5mg DRO b.i.d. because of the central side effects of drowsiness and dizziness observed in our prior study with a single dose of 7.5mg dronabinol in healthy subjects 13. In contrast, a single 5mg dose in IBS patients did not increase stress or arousal in another previous study in our laboratory14. We perceived that it was important to limit the maximum dose to 5mg DRO b.i.d., as this was associated with tolerable central effects in prior studies14, and it had previously been shown that a single 5mg dose of DRO acutely increased colonic compliance and reduced fasting colonic motility in the subgroups of IBS-D and IBS-A patients16. The same 5mg DRO dose also increased colonic compliance and decreased colonic motility and tone in healthy male and female volunteers13. In fact, the differential effect of DRO (combined 2.5 and 5mg groups) relative to placebo treatment on median colonic transit is about 0.80 geometric center units at 24 hours in the CNR1 rs806378 CT/TT genotype group. This difference in GC24 with DRO relative to placebo is consistent with the magnitude of effects of other agents that significantly affect bowel function, such as linaclotide in patients with IBS-C33 or alosetron in IBS-D patients, in terms of the difference between post- and pre-treatment colonic transit34.
The lack of demonstrable effects of genetic variation in FAAH (rs324420) on transit in response to DRO is not known. However, in contrast to the significant modulation of the effect of DRO by changes in the function of the CB1 receptor due to variation in CNR1, it is conceivable that the FAAH alteration results in only a small additional variation in the level of endocannabinoids at the synapse. In accordance with this hypothesis, the variation in endocannabinoids is not sufficient to modify the overall or combined effects of CB agonist (DRO) and the endocannabinoids reaching the CB1 receptors.
Our study illustrates the potential for cannabinoid agents to modulate colonic function in IBS-D, though this may only pertain to a subgroup of patients, based on a variation in the gene for the CB1 receptor. It is possible that greater peripheral selectivity of the CB1 agonist may have greater effects on transit, and that selection of IBS patients based on CNR1 rs806378 CT/TT genotype may enhance the effects of agents acting on CB1 receptors. The potential for cannabinoids to change gastrointestinal and colonic transit is illustrated by the effects of experimental, more peripherally selective agents. Thus, in vivo studies in mice by Storr et al.35 demonstrated that the inverse CB agonist, AM251, accelerated upper gastrointestinal transit and whole gut transit, while the neutral CB antagonist, AM4113, also increased upper gastrointestinal transit. Regional effects of cannabinoid antagonists also differ in mice, with reduced colonic expulsion with both AM251 and AM4113; whereas, whole gut transit is accelerated by both agents at specific dose levels35. Although there are data suggesting that cannabinoid modulation with the CB1 and CB2 receptor antagonists36 and the FAAH inhibitor, AM350637, may affect visceral sensation in inflammation models36 or gastrointestinal transit and colonic fecal output in mice exposed to endotoxin37, the effects on pain or visceral sensation in human studies have been disappointing38,39.
The strengths of our study include the validated methods measuring gastrointestinal and colonic transit, the clinical significance (number and consistency of bowel movements) of the measurement used as primary endpoint (that is colonic GC24), and inclusion of pharmacogenetic analysis to assess whether DRO’s effects may be influenced by genetic variations in cannabinoid signaling or metabolism.
The weaknesses of this study include assessment of only four doses of DRO over two days and the nonselective nature of dronabinol for CB1 and CB2 receptors. Importantly, our study had insufficient power to detect gene-by-treatment interactions. We conducted a post-hoc analysis of the statistical power of the study based on the variation in the primary response measure (GC at 24 hours), the sample sizes of each group based on specific genotypes, and the pattern of delta GC24 hour data among the four gene-by-drug combinations. Using the dominant genetic model (homozygous major versus combined heterozygous plus homozygous minor groups), treatment group categorized as placebo compared to the combined 2.5 and 5 mg groups, and the pattern of median values in Figure 5B, there would have been approximately 80% power to detect gene-by-treatment interactions of a magnitude illustrated in Table III (in Supplementary Materials), if the variation in delta (post-pre) GC24 values would have been the same as the variation in baseline GC24 values (SD=1.04). Unfortunately the observed variation in delta GC24 values was greater (SD=1.44) and, as a result, there was insufficient power to detect a potential gene-by-treatment interaction. In fact, the usual advantage gained from using baseline GC24 as a covariate did not manifest itself in this study. In summary, the analysis for differential drug effects depending on genotype was limited both by the sample sizes, as well as by the larger than expected observed variation in delta GC24. Thus, there was only 52% power to detect the “interactions” illustrated by the patterns in Table III (in Supplementary Materials), given the observed variation in delta GC24.. To achieve 80% power with the observed SD in GC24 of 1.44, we would require approximately 40 patients per treatment arm assuming a similar distribution of genotypes in each arm (Table III, Supplementary Material). This information is helpful to plan future studies.
Therefore, the pharmacogenetic results in particular are to be viewed as hypothesis- generating. However, the individual responses in the patients who carry the CNR1 rs806378 CT/TT genotype suggest that inclusion of pharmacogenetics in drug appraisal is relevant, at least at the proof-of-concept stage with quantifiable endpoints.
In summary, our study shows that the nonselective cannabinoid receptor agonist, DRO, does not significantly affect colonic transit; however, DRO may inhibit colonic transit in a subset of IBS-D patients, based on a specific genetic variation in CB1. A selective CB1 agonist may have potential as therapy in diarrhea-predominant IBS patients. Further studies to assess the therapeutic role of selective cannabinoid receptor agonists in IBS are warranted. Clinical trials may be enhanced by inclusion of stratification based on CNR1 rs806378 genotype or use of the genotype variation as a covariate in the analysis of results.
Supplementary Material
Acknowledgments
This work was funded by grant RO1-DK079866 from National Institutes of Health (to Dr. Camilleri) and by Mayo Clinic CTSA grant (RR24150). The authors wish to thank Mary Lempke, Pharm.D., research pharmacist, and Mrs. Cindy Stanislav, secretary, for assistance.
Footnotes
ClinicalTrials.gov identifier: NCT01253408
Authors’ contributions: B. Wong: research fellow, patient recruitment and screening, data collection, writing protocol and manuscript; M. Camilleri: study conceptualization, writing of protocol and manuscript; D. Eckert: patient recruitment; P. Carlson: genotyping; M Ryks: data collection; D. Burton: data processing; A.R. Zinsmeister: database management and statistical analysis
Competing Interests: The authors have no conflicts of interest to report.
References
- 1.Howlett AC International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 2.Matsuda LA, Lolait SJ, Brownstein MJ, et al. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 3.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 4.Database of Single Nucleotide Polymorphisms (dbSNP) Bethesda (MD): National Center for Biotechnology Information, National Library of Medicine; (dbSNP Build ID: {132}). Available from: http://www.ncbi.nlm.nih.gov/SNP/ [Google Scholar]
- 5.Devane WA, Hanus L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 6.Hillard CJ. Biochemistry and pharmacology of the endocannabinoids arachidonyl-ethanolamide and 2-arachidonylglycerol. Prostaglandins Other Lipid Mediat. 2000;61:3–18. doi: 10.1016/s0090-6980(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 7.Mechoulam R, Benshabat S, Hanus L, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 8.Sugiura T, Kondo S, Sukagawa A, et al. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 9.Hinds NM, Ullrich K, Smid SD. Cannabinoid 1 (CB(1)) receptors coupled to cholinergic motorneurones inhibit neurogenic circular muscle contractility in the human colon. Br J Pharmacol. 2006;148:191–199. doi: 10.1038/sj.bjp.0706710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinto L, Izzo AA, Cascio MG, et al. Endocannabinoids as physiological regulators of colonic propulsion in mice. Gastroenterology. 2002;123:227–234. doi: 10.1053/gast.2002.34242. [DOI] [PubMed] [Google Scholar]
- 11.Izzo AA, Mascolo N, Capasso R, et al. Inhibitory effect of cannabinoid agonists on gastric emptying in the rat. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:221–223. doi: 10.1007/s002109900054. [DOI] [PubMed] [Google Scholar]
- 12.Shook JE, Burks TF. Psychoactive cannabinoids reduce gastrointestinal propulsion and motility in rodents. J Pharmacol Exp Therapeutics. 1989;249:444–449. [PubMed] [Google Scholar]
- 13.Esfandyari T, Camilleri M, Busciglio I, et al. Effects of a cannabinoid receptor agonist on colonic motor and sensory functions in humans: a randomized, placebo-controlled study. Am J Physiol. 2007;293:G137–G145. doi: 10.1152/ajpgi.00565.2006. [DOI] [PubMed] [Google Scholar]
- 14.Esfandyari T, Camilleri M, Ferber I, et al. Effect of a cannabinoid agonist on gastrointestinal transit and postprandial satiation in healthy human subjects: a randomized, placebo-controlled study. Neurogastroenterol Motil. 2006;18:831–838. doi: 10.1111/j.1365-2982.2006.00834.x. [DOI] [PubMed] [Google Scholar]
- 15.Camilleri M, Carlson P, McKinzie S, et al. Genetic variation in endocannabinoid metabolism, gastrointestinal motility and sensation. Am J Physiol. 2008;294:G13–G19. doi: 10.1152/ajpgi.00371.2007. [DOI] [PubMed] [Google Scholar]
- 16.Wong BS, Camilleri M, Busciglio I, et al. Pharmacogenetic trial of a cannabinoid agonist shows reduced fasting colonic motility in patients with nonconstipated irritable bowel syndrome. Gastroenterology. 2011;141:1638–1647. doi: 10.1053/j.gastro.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talley NJ, Phillips SF, Wiltgen CM, et al. Assessment of functional gastrointestinal disease: the bowel disease questionnaire. Mayo Clin Proc. 1990;65:1456–1479. doi: 10.1016/s0025-6196(12)62169-7. [DOI] [PubMed] [Google Scholar]
- 18.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 19.MICROMEDEX H. Physician’s Desk Reference. Micromedex; 2005. [Google Scholar]
- 20.Lembo T, Camilleri M. Chronic constipation. N Engl J Med. 2003;349:1360–1368. doi: 10.1056/NEJMra020995. [DOI] [PubMed] [Google Scholar]
- 21.Duncan M, Davison JS, Sharkey KA. Review article: endocannabinoids and their receptors in the enteric nervous system. Aliment Pharmacol Ther. 2005;22:667–683. doi: 10.1111/j.1365-2036.2005.02648.x. [DOI] [PubMed] [Google Scholar]
- 22.Tiwari AK, Zai CC, Likhodi O, et al. A common polymorphism in the cannabinoid receptor 1 (CNR1) gene is associated with antipsychotic-induced weight gain in Schizophrenia. Neuropsychopharmacology. 2010;35:1315–1324. doi: 10.1038/npp.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vazquez-Roque MI, Camilleri M, Vella A, et al. Association of genetic variation in cannabinoid mechanisms and gastric motor functions and satiation in overweight and obesity. Neurogastroenterol Motil. 2011;23:637–e257. doi: 10.1111/j.1365-2982.2011.01711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiang KP, Gerber AL, Sipe JC, et al. Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use. Hum Mol Genet. 2004;13:2113–2119. doi: 10.1093/hmg/ddh216. [DOI] [PubMed] [Google Scholar]
- 25.Burton DD, Camilleri M, Mullan BP, Forstrom LA, Hung JC. Colonic transit scintigraphy labeled activated charcoal compared with ion exchange pellets. J Nucl Med. 1997;38:1807–1810. [PubMed] [Google Scholar]
- 26.Charles F, Phillips SF, Camilleri M, Thomforde GM. Rapid gastric emptying in patients with functional diarrhea. Mayo Clin Proc. 1997;72:323–328. doi: 10.4065/72.4.323. [DOI] [PubMed] [Google Scholar]
- 27.Camilleri M, McKinzie S, Busciglio I, et al. Prospective study of motor, sensory, psychologic, and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2008;6:772–781. doi: 10.1016/j.cgh.2008.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deiteren A, Camilleri M, Bharucha AE, et al. Performance characteristics of scintigraphic colon transit measurement in health and irritable bowel syndrome and relationship to bowel functions. Neurogastroenterol Motil. 2010;22:415–423. doi: 10.1111/j.1365-2982.2009.01441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Camilleri M. Scintigraphic biomarkers for colonic dysmotility. Clin Pharmacol Ther. 2010;87:748–753. doi: 10.1038/clpt.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sibaev A, Yüce B, Kemmer M, et al. Cannabinoid-1 (CB1) receptors regulate colonic propulsion by acting at motor neurons within the ascending motor pathways in mouse colon. Am J Physiol. 2009;296:G119–G128. doi: 10.1152/ajpgi.90274.2008. [DOI] [PubMed] [Google Scholar]
- 31.Choi MG, Camilleri M, O’Brien MD, et al. A pilot study of motility and tone of the left colon in patients with diarrhea due to functional disorders and dysautonomia. Am J Gastroenterol. 1997;92:297–302. [PubMed] [Google Scholar]
- 32.Chey WY, Jin HO, Lee MH, et al. Colonic motility abnormality in patients with irritable bowel syndrome exhibiting abdominal pain and diarrhea. Am J Gastroenterol. 2001;96:1499–1506. doi: 10.1111/j.1572-0241.2001.03804.x. [DOI] [PubMed] [Google Scholar]
- 33.Andresen V, Camilleri M, Busciglio I, et al. Effect of 5 days linaclotide on transit and bowel function in females with constipation-predominant irritable bowel syndrome. Gastroenterology. 2007;133:761–768. doi: 10.1053/j.gastro.2007.06.067. [DOI] [PubMed] [Google Scholar]
- 34.Viramontes BE, Camilleri M, McKinzie S, Pardi DS, Burton D, Thomforde GM. Gender related differences in slowing colonic transit by a 5-HT3 antagonist in subjects with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol. 2001;92:2671–2676. doi: 10.1111/j.1572-0241.2001.04138.x. [DOI] [PubMed] [Google Scholar]
- 35.Storr MA, Bashashati M, Hirota C, et al. Differential effects of CB(1) neutral antagonists and inverse agonists on gastrointestinal motility in mice. Neurogastroenterol Motil. 2010;22:787–796. doi: 10.1111/j.1365-2982.2010.01478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanson M, Bueno L, Fioramonti J. Involvement of cannabinoid receptors in inflammatory hypersensitivity to colonic distension in rats. Neurogastroenterol Motil. 2006;18:949–956. doi: 10.1111/j.1365-2982.2006.00819.x. [DOI] [PubMed] [Google Scholar]
- 37.Bashashati M, Storr MA, Nikas SP, et al. Inhibiting fatty acid amide hydrolase normalizes endotoxin-induced enhanced gastrointestinal motility in the mouse. Br J Pharmacol. 2011 Aug 30; doi: 10.1111/j.1476-5381.2011.01644.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buggy DJ, Toogood L, Maric S, et al. Lack of analgesic efficacy of oral delta-9-tetrahydrocannabinol in postoperative pain. Pain. 2003;106:169–172. doi: 10.1016/s0304-3959(03)00331-2. [DOI] [PubMed] [Google Scholar]
- 39.Klooker TK, Leliefeld KE, Van Den Wijngaard RM, et al. The cannabinoid receptor agonist delta-9-tetrahydrocannabinol does not affect visceral sensitivity to rectal distension in healthy volunteers and IBS patients. Neurogastroenterol Motil. 2011;23:30–35. doi: 10.1111/j.1365-2982.2010.01587.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.