Abstract
A literature review was conducted on temporal measures of swallowing in healthy individuals with the purpose of determining the degree of variability present in such measures within the literature. A total of 46 studies that met inclusion criteria were reviewed. The definitions and descriptive statistics for all reported temporal parameters were compiled for meta-analysis. In total, 119 different temporal parameters were found in the literature. The three most-frequently occurring durational measures were: UES opening, laryngeal closure and hyoid movement. The three most-frequently occurring interval measures were: stage transition duration, pharyngeal transit time and duration from laryngeal closure to UES opening. Subtle variations in operational definitions across studies were noted, making the comparison of data challenging. Analysis of forest plots compiling descriptive statistical data (means and 95% confidence intervals) across studies revealed differing degrees of variability across durations and intervals. Two parameters (UES opening duration and the laryngeal-closure-to-UES-opening interval) demonstrated the least variability, reflected by small ranges for mean values and tight confidence intervals. Trends emerged for factors of bolus size and participant age for some variables. Other potential sources of variability are discussed.
Keywords: deglutition, deglutition disorders, temporal, timing, duration, variability
Dysphagia (disordered swallowing) may occur secondary to neurological impairment, structural changes in the head and neck and/or progressive illness. Dysphagia has the potential to impact a person’s nutrition, hydration and quality of life, and may lead to serious sequelae, such as the development of aspiration pneumonia (as a result of material passing below the vocal folds into the respiratory system). Videofluoroscopy (VF) is considered the gold-standard tool for the assessment of dysphagia [1]. In VF, various foods and liquids are mixed with radiographic contrast media such as barium and swallowed under continuous fluoroscopy allowing for direct dynamic visualization of swallowing physiology. Using a standardized protocol, the safety and efficiency of swallowing can be assessed across various textures, volumes and maneuvers [2]. Tn the analysis of VF, several different quantitative parameters can be measured, including kinematic measures of structural displacement and timing measures (durations and intervals). Given that VF involves radiation exposure, which carries a risk of stochastic biohazard, the procedure is necessarily limited to a small number of swallows (usually between three and ten) [3]. Molfenter and Steele [4] previously proposed that the brevity of the exam makes the accuracy of interpretation susceptible to the influence of variability in swallowing performance within a given individual; furthermore, variability across individuals impacts our ability to define a reference context of normative swallowing behavior. It can be difficult to detect and quantify both the extent of impairment and of real change (either improvement or deterioration) in a patient’s swallowing against this backdrop of inherent variability [4]. Consequently, there is a need to better understand the variation that exists in swallowing physiology in order to appreciate the extent to which this constitutes a limitation on clinical decision-making and scientific analysis. In this paper, we report a meta-analysis of temporal variability in swallowing.
In an early and seminal publication, Lof and Robbins [5] studied the test-retest variability of nine temporal measures of swallowing in 16 healthy participants divided equally by sex and age-group (middle-aged versus older adults). To our knowledge, this is the only available publication that explores variability in temporal measures of swallowing. Each participant swallowed three 2 ml boluses of both liquid barium and paste barium. This VF protocol was then repeated an average of 97 days later each middle-aged participants and 45 days later for older participants. The authors reported data for nine durational measures and examined their relative test-retest variability using the coefficient of variation (CV). The CV is a measure that expresses the standard deviation (SD) in reference to the size of the mean, with a higher CV representing greater relative variability in the parameter of interest [6]. Among the nine timing variables that were studied, Lof and Robbins [5] reported that stage transition duration (STD) was the most volatile, with a CV of 1.14 for liquids (mean: −0.22, SD: 0.25) and 17.67 for semisolid (mean: −0.03, SD: 0.053) stimuli.
Unfortunately, there is a statistical limitation in the use of the CV for appreciating relative variability across the parameters studied by Lof and Robbins, because the measure has a tendency to become inflated in the context of small values, especially those near zero [7]. To illustrate this point, imagine a sample in which one subgroup of participants (Group A) has a mean STD of 0.01 seconds but a fairly large standard deviation of 0.25 seconds, thereby yielding a CV of 25 (CV = SD/mean). Consider, then, that a second participant group (Group B) in the same study has a higher mean STD value of 0.5 seconds but the same standard deviation of 0.25 seconds, yielding a comparative CV of 0.5. Given that the standard deviation in both groups is identical in terms of real timing (i.e. 0.25 seconds) it would probably strike most readers as implausible to conclude that the variability seen in Group A is 50 times greater than that seen in Group B, although this is in fact the message implied by the comparison of the two CV statistics. By simply shifting the these hypothetical data away from small values, by adding a fixed value of 0.5 seconds to all proposed group means and SDs, the CVs change to 1.47 for Group A and 0.75 for Group B, dramatically altering the magnitude of relative variability seen between the two groups from 50 to 1.96 and illustrating the tendency for the CV measure to be unduly influenced by actual numeric values. In the Lof and Robbins dataset, STD was the variable that was most volatile (displaying the highest CV values), but also the variable with mean values that fell closest to zero, (i.e., −0.22 sec for liquids and −0.03 sec for semisolids). Clearly, in that particular study, the near-zero values of the STD parameter made the interpretations of its relative variability vulnerable to this weakness in the CV statistic; alternative methods for comparing variability across parameters and studies are needed. Currently, there are no other known studies examining temporal variability in healthy swallowing, although other studies have described substantial variability in event sequencing [8, 9] and in the location of the bolus at swallow onset in healthy swallowing [10, 11].
In a previous meta-analysis of variability conducted from 13 studies reporting data on hyoid and laryngeal kinematics in healthy adult swallowing, Molfenter and Steele [4] inspected relative variability using means and 95% confidence intervals (CI) in order to circumvent the previously-mentioned limitations of the CV statistic [7]. Where variability was found, statistical, methodological, stimulus-related and participant-related sources were proposed. Here, we use a similar approach to describe, synthesize and discuss the variability seen in commonly-reported temporal measures of swallowing from the literature describing healthy deglutition.
Methods
Search Strategy and Inclusion Criteria
A literature search for publications reporting temporal swallowing data was completed using Medline, with the following search terms: (deglutition) and (videofluoroscop*or modified barium) and (timing or duration or temporal). The initial search was limited to studies published in English. This yielded 183 abstracts for further review. The basic inclusion criteria were studies reporting means and SD, or means and standard error of the mean (SEM) for temporal parameters during thin liquid swallowing tasks in healthy adult humans. Studies were excluded from further analysis if they: a) did not employ VF as their instrumental method; b) reported data for pediatric or patient populations (without clear reporting of reference data for healthy adult participants); c) reported timing for solid stimuli only; d) included data limited to measures taken during the performance of compensatory swallow maneuvers;, or e) if the study methods manipulated the natural process of healthy swallowing (e.g., with direct infusion of a bolus to the pharynx). Studies were also excluded if they did not report the quantitative information necessary to calculate confidence intervals for meta-analysis and if they presented statistical results only in graph format.
Studies Included
After the application of these criteria, a subset of 46 publications was retained for in-depth review [5, 8, 9, 12–54]. All temporal variables and statistical data were extracted and compiled in a spreadsheet. Tallies of the frequency of occurrence for each temporal variable were made across all 46 publications. In addition, other factors regarding each study were documented, including participant age and gender distribution, bolus volume, barium density, frame rate and method of analysis used.
Data Extraction
In total, 119 different temporal variables were found in the reviewed literature. Extracted variables were divided into three categories: durations were defined as the time required for a distinct physiological event during swallowing to occur (such as laryngeal closure); intervals were defined as the time or latency elapsing between two gestures in the swallow sequence (such as the time between the onset of laryngeal closure and UES opening); and partial durations were defined as sub-segments of swallow durations (such as the latency between the onset of laryngeal closure and the attainment of maximum laryngeal closure, which is a segment of laryngeal closure duration). Table 1 displays the distribution of the temporal variables by category and their observed frequency within the dataset of 46 articles selected for review. Partial durations were found to be reported with low frequency, representing less than ten percent of all measures, and therefore were not included in the subsequent analyses.
Table 1.
Number of different variables reported and their corresponding frequency of occurrence in the 46 publications reviewed.
| Number of variables | Number of occurrences | % of variable occurrences | |
|---|---|---|---|
| Intervals | 100 | 220 | 0.72 |
| Durations | 8 | 61 | 0.20 |
| Partial Durations | 11 | 23 | 0.08 |
| TOTAL | 119 | 304 |
The three most-frequently occurring duration and interval measures identified in this literature review were selected for further in-depth analysis. The three most-frequently reported durations were: upper esophageal sphincter (UES) opening, laryngeal closure duration (LCD) and hyoid movement duration (HMD). The three most-frequently reported intervals were: stage transition duration (STD), pharyngeal transit time (PTT) and laryngeal-closure-(LC)-to-UES-opening. The distribution of these variables across the reviewed publications is shown in Table 2. It should be noted Table 2 lists studies in alphabetical order, by first author, with a corresponding study key, which is later used to identify these studies in Figures 1–6. Study key numbers in the table and figures do not correspond to citation numbers in the reference list and will therefore be denoted henceforth using italicized numbers, e.g. 7 or 8A, while reference list citations will follow the prescribed convention using square brackets, e.g. [7].
Table 2.
‘Study Key’ by author and variable(s) for the 36 publications that reported data for the 6 temporal variables of interest. The alphanumeric study code for each study/variable corresponds to data presented in Figures 1–6.
| Part 1
| ||||||||
|---|---|---|---|---|---|---|---|---|
| DURATIONS | INTERVALS | |||||||
| Study Key | UES Opening Duration | Laryngeal Closure Duration | Hyoid Movement Duration | Stage Transition Duration | Pharyngeal Transit Time | LC to UES opening duration | ||
| Bisch E et al: Pharyngeal effects of bolus volume, viscosity, and temperature in patients with dysphagia resulting from neurologic impairment and in normal subjects. J Speech Hear Res 37: 1041–1049, 1994. | All boluses pooled | 1A | ✓ | |||||
| 1ml, pooled temp | 1B | ✓ | ||||||
| 5ml, pooled temp | 1C | ✓ | ||||||
| 1ml, cold | 1D | ✓ | ||||||
| 1ml, room temp | 1E | ✓ | ||||||
| 5ml, cold | 1F | ✓ | ||||||
| 5ml, room temp | 1G | ✓ | ||||||
| Chi-Fishman G, Sonies, B: Motor strategy in rapid sequential swallowing: New insights. J Speech Lang Hear Res 43: 1481–92, 2000. | 5cc | 2A | ✓ | ✓ | ✓ | ✓ | ||
| 15cc | 2B | ✓ | ✓ | ✓ | ✓ | |||
| Cook IJ et al: Timing of videofluoroscopic, manometric events, and bolus transit during the oral and pharyngeal phases of swallowing. Dysphagia 4: 8–15,1989. | 2ml | 3A | ✓ | |||||
| 5ml | 3B | ✓ | ||||||
| 10ml | 3C | ✓ | ||||||
| 20ml | 3D | ✓ | ||||||
| Cook IJ et al: Opening mechanisms of the human upper esophageal sphincler. Am J Physiol - Gastro L5: G748–59,1989. | 2ml | 4A | ✓ | |||||
| 5ml | 4B | ✓ | ||||||
| 10ml | 4C | ✓ | ||||||
| 20ml | 4D | ✓ | ||||||
| Daniels SK et al: Dysphagia in stroke: Development of a standard method to examine swallowing recovery, J Rehabil Res Dev 21: 347–355,2006. | 5ml | 5 | ✓ | ✓ | ||||
| Daniels SK et al: Effects of verbal cue on bolus flow during swallowing. Am J Speech Lang Pathol 16: 140–147, 2007. | 5ml, cued | 6A | ✓ | ✓ | ||||
| 5ml, noncued | 6B | ✓ | ✓ | |||||
| Daniels SK et al: Defining and measuring dysphagia following stroke. Am J Speech Lang Pathol 18: 74–81, 2009. | 1ml | 7A | ✓ | |||||
| 5ml | 7B | ✓ | ||||||
| 10ml | 7C | ✓ | ||||||
| 20ml | 7D | ✓ | ||||||
| Dantas RO et al: The effect of high- vs low-density barium preparations on the quantitative features of swallowing. Am J Roentgenol 153: 1191–1195, 1989. | 5ml, low density | 8A | ✓ | ✓ | ✓ | ✓ | ||
| 5ml, high density | 8B | ✓ | ✓ | ✓ | ✓ | |||
| 10ml, low density | 8C | ✓ | ✓ | ✓ | ✓ | |||
| 10ml, high density | 8D | ✓ | ✓ | ✓ | ✓ | |||
| Dantas RO et al: Effect of swallowed bolus variables on oral and pharyngeal phases of swallowing. Am J Physiol - Gastr L258: G675–G681, 1990. | 2ml | 9A | ✓ | ✓ | ||||
| 5ml | 9B | ✓ | ✓ | |||||
| 10ml | 9C | ✓ | ✓ | |||||
| 20ml | 9D | ✓ | ✓ | |||||
| 30ml | 9E | ✓ | ✓ | |||||
| Dantas RO et al: Biomechanics of cricopharyngeal bars. Gastroenterology 99: 1269–1274, 1990. | 2ml | 10A | ✓ | ✓ | ||||
| 5ml | 10B | ✓ | ✓ | |||||
| 10ml | 10C | ✓ | ✓ | |||||
| 20m: | 10D | ✓ | ✓ | |||||
| Dantas RO et al: Effect of Gender on Swallow Event Duration Assessed by Videofluoroscopy. Dysphagia 24: 280–284, 2009. | 5ml, women | 11A | ✓ | ✓ | ||||
| 5ml, men | 11B | ✓ | ✓ | |||||
| 10ml, women | 11C | ✓ | ✓ | |||||
| 10ml, men | 11D | ✓ | ✓ | |||||
| Kahrilas PJ et al: Volitional augmentation of upper esophageal sphincter opening during swallowing. Am Physiol - Gastr L260: G450–456, 1991. | 1ml | 12A | ✓ | |||||
| 10ml | 12B | ✓ | ||||||
| Kang B et al: Influence of Aging on Movement of the Hyoid Bone and Epiglottis during Normal Swallowing: A Motion Analysis. Gerontology 56: 474–482, 2010. | 2ml, <45 yo | 13A | ✓ | ✓ | ✓ | |||
| 2ml, 45–54 yo | 13B | ✓ | ✓ | ✓ | ||||
| 2ml, 55–64yo | 13C | ✓ | ✓ | ✓ | ||||
| 2ml, >65 yo | 13D | ✓ | ✓ | ✓ | ||||
| Kendall KA et al: Timing of events in normal swallowing: A videofluoroscopic study. Dysphagia 15: 74–93, 2000. | 1cc | 14A | ✓ | ✓ | ✓ | |||
| 3cc | 14B | ✓ | ✓ | ✓ | ||||
| 20cc | 14C | ✓ | ✓ | ✓ | ||||
| Kendall KA et al: Accommodation to changes in bolus viscosity in normal deglutition: A videofluoroscopic study. Ann Otol Rhinol Laryngol 110: 1059–1065,2001. | 3cc | 15 | ✓ | ✓ | ✓ | |||
| Kendall KA et al: Hyoid movement during swallowing in older patients with dysphagia. Arch Otolaryngol Head Neck Surg 127: 1224–1229, 2001. | 1cc, young | 16A | ✓ | ✓ | ||||
| 20cc, young | 16B | ✓ | ✓ | |||||
| 1cc, old | 16C | ✓ | ✓ | |||||
| 20cc, old | 16D | ✓ | ✓ | |||||
| Kem M et al: Comparison of upper esophageal sphincter opening in healthy asymptomatic young and elderly volunteers. Ann Olot Rhinol Laryngol 108: 982–989, 1999. | 5ml, young | 17A | ✓ | |||||
| 5ml, older | 17B | ✓ | ||||||
| 10ml, young | 17C | ✓ | ||||||
| 10ml, older | 17D | ✓ | ||||||
| Part 2
| ||||||||
|---|---|---|---|---|---|---|---|---|
| DURATIONS | INTERVALS | |||||||
| Study Key | UES Opening Duration | Laryngeal Closure Duration | Hyoid Movement Duration | Stage Transition Duration | Pharyngeal Transit Time | LC to UES opening duration | ||
| Kim Y et al: Temporal measurements of pharyngeal swallowing in normal populations. Dysphagia 20: 290–296, 2005. | 5ml, young | 18A | ✓ | |||||
| 10ml, young | 18B | ✓ | ||||||
| 5ml, older | 18C | ✓ | ||||||
| 10ml, older | 18D | ✓ | ||||||
| Kim Y et al: Stage transition duration in patients poststroke. Dysphagia 22: 299–315, 2007. | 5ml | 19A | ✓ | |||||
| 10ml | 19B | ✓ | ||||||
| Lazarus CL et al: Effects of bolus volume, viscosity, and repeated swallows in nonstroke subjects and stroke patients. Arch Phys Med Rehabil 74: 1066–1070, 1993. | 1ml | 20A | ✓ | ✓ | ✓ | |||
| 3ml | 20B | ✓ | ✓ | ✓ | ||||
| 5ml | 20C | ✓ | ✓ | ✓ | ||||
| Leonard R. McKenzie S: Hyoid-bolus transit Latencies in normal swallow. Dysphagia 21: 183–190, 2006. | 20cc, young | 21A | ✓ | |||||
| 20cc, older | 21B | ✓ | ||||||
| 20cc, young (H1 B1) | 21C | ✓ | ||||||
| 20cc, old<H1 B1> | 21D | ✓ | ||||||
| Lof GL, Robbins J: Test-retest variability in normal swallowing. Dysphagia 4: 236–242, 1990. | 2ml | 22 | ✓ | ✓ | ✓ | ✓ | ||
| Logemann JA et al: Closure mechanisms of laryngeal vestibule during swallow. Am J Physiol - Gastr L262: G338–G344, 1992. | 1ml | 23A | ✓ | |||||
| 5ml | 23B | ✓ | ||||||
| 10ml | 23C | ✓ | ||||||
| 20ml | 23D | ✓ | ||||||
| Logemann JA et al: Temporal and Biomechanical Characteristics of Oropharyngeal Swallow in Younger and Older Men. J Speech Lang Hear Res 43: 1264–1274, 2000. | 1ml | 24A | ✓ | ✓ | ✓ | |||
| 10ml | 24B | ✓ | ✓ | ✓ | ||||
| young | 24C | ✓ | ✓ | ✓ | ||||
| older | 24D | ✓ | ✓ | ✓ | ||||
| Logemann JA et al: Oropharyngeal swallow in younger and older women: Videofluoroscopic analysis. J Speech Lang Hear Res 45: 434–445, 2002. | 1ml | 25A | ✓ | ✓ | ✓ | |||
| 10ml | 25B | ✓ | ✓ | ✓ | ||||
| young | 25C | ✓ | ✓ | ✓ | ||||
| older | 25D | ✓ | ✓ | ✓ | ||||
| Martin-Harris B et al: Temporal coordination of pharyngeal and laryngeal dynamics with breathing during swallowing: Single liquid swallows. J Appl Physiol 94: 1735–1743, 2003. | 5ml | 26 | ✓ | |||||
| Mendell DA, Logemann JA: A retrospective analysis of the pharyngeal swallow in patients with a clinical diagnosis of GERD compared with normal controls: A pilot study. Dysphagia 17: 220–226, 2002. | All boluses pooled | 27 | ✓ | ✓ | ✓ | ✓ | ||
| Mendell DA, Logemann JA: A retrospective analysis of the pharyngeal swallow in patients with a clinical diagnosis of GERD compared with normal controls: A pilot study. Dysphagia 17: 220–226, 2002. | 20–29 | 28A | ✓ | |||||
| 40–49 | 28B | ✓ | ||||||
| 60–69 | 28C | ✓ | ||||||
| 70–79 | 28D | ✓ | ||||||
| 80–89 | 28E | ✓ | ||||||
| Mokhlesi B et al: Oropharyngeal deglutition in stable COPD. Chest 121: 361–369, 2002. | 3ml | 29A | ✓ | ✓ | ✓ | ✓ | ||
| 5ml | 29B | ✓ | ✓ | ✓ | ✓ | |||
| Ohmae Y et al: Timing of glottic closure during normal swallow. Head Neck 17: 394–402, 1995. | 1ml | 30A | ✓ | ✓ | ✓ | ✓ | ||
| 5ml | 30B | ✓ | ✓ | ✓ | ✓ | |||
| Ohmae Y et al: Effects of two breath-holding maneuvers on oropharyngeal swallow. Ann Otol Rhinol Laryngol 105: 123–131, 1996. | 5ml | 31 | ✓ | ✓ | ✓ | |||
| Palmer JB et al: Coordination of mastication and swallowing. Dysphagia 7: 187–200, 1992. | straw sips | 32 | ✓ | ✓ | ||||
| Park T et al: Initiation and duration of laryngeal closure during the pharyngeal swallow in post-stroke patients. Dysphagia 25: 177–182, 2010. | 5ml | 33A | ✓ | |||||
| 10ml | 33B | ✓ | ||||||
| Pauloski BR et al: Biomechanical analysis of the pharyngeal swallow in postsurgical patients with anterior tongue and floor of mouth resection and distal flap reconstruction. J Speech Hear Res 38: 110–123, 1995. | 1ml | 34 | ✓ | ✓ | ✓ | |||
| Stachler RJ et al: Swallowing of bolus types by postsurgical head and neck cancer patients. Head Neck 16: 413–419, 1994. | 3ml | 35A | ✓ | |||||
| 10ml | 35B | ✓ | ||||||
| Taniguchi H et al: Correspondence between food consistency and suprahyoid muscle activity, tongue pressure, and bolus transit times during the oropharyngeal phase of swallowing. J Appl Physiol 105: 791–799, 2008 | 5ml | 36 | ✓ | |||||
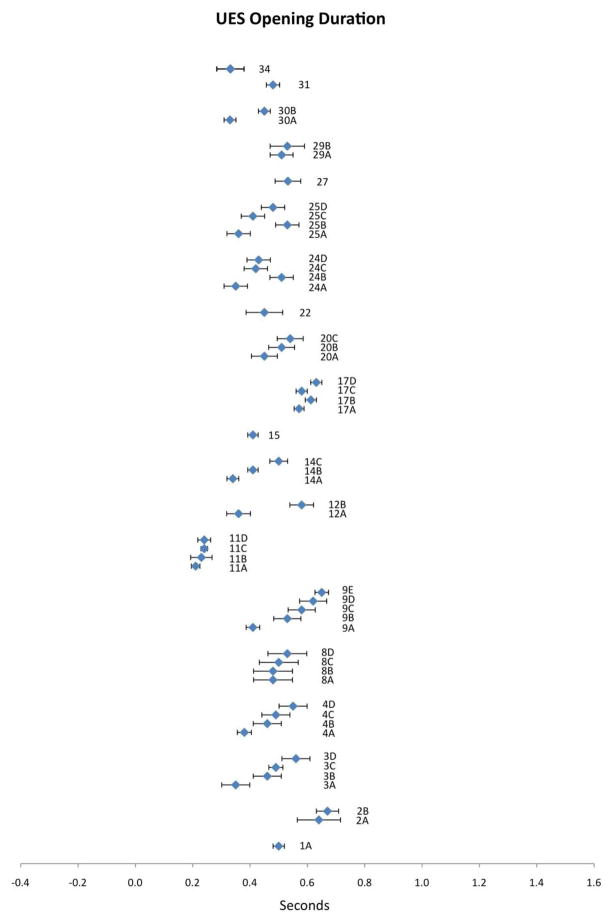
Figure 1.
Means (in sec) and 95% CIs for UES opening duration as reported in the reviewed literature. Alphanumeric codes refer to studies/variables in Table 2.
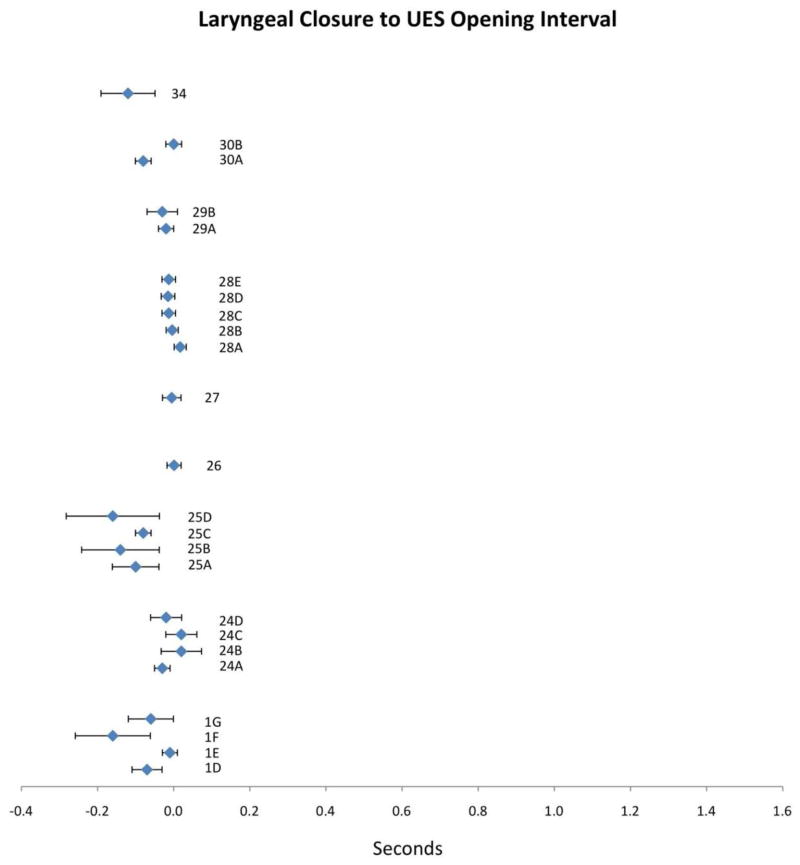
Figure 6.
Means (in sec) and 95% CIs for laryngeal closure to UES opening duration as reported in the reviewed literature. Alphanumeric codes refer to studies/variables in Table 2.
A meta-analysis was performed on the six most-commonly reported temporal parameters (3 durations and 3 interval measures), using descriptive statistics, which were clearly reported in 36 of the 46 publications selected for in-depth review. The remaining 10 publications reported data for variables other than these six highest frequency parameters. All data were converted to units in seconds for uniformity. Means and corresponding measures of dispersion, SD or SEM, were extracted from each publication. One study reported raw data, which allowed us to calculate means and SDs manually [36]. Next, 95% CIs for each study/variable were calculated. This was achieved by multiplying a specific t-value (two-tailed, alpha 0.05, at n−1 df) by the SD/[SQRT(n)] or SEM. By adding and subtracting this product, to/from the mean, one can calculate the 95% CI for that specific mean. These 95% CIs were plotted on modified forest plots for each variable and can be found in Figures 1–6. In all six figures, the diamonds represent the mean (in seconds) for each particular study (or variable, where applicable) and the error bars represent the spread of the 95% CI. The study key appears beside each data point, with corresponding information available in Table 2. The scales for intervals and for durations were held constant across figures to allow for transparent comparison of relative variability across parameters. Inspection of the modified forest plots in Figures 1–6 allows one to appreciate trends in the aggregate data arising from different study factors (for example, factors of bolus volume, age, or gender). It should be noted that the recognition of these trends from visual inspection of the forest plots does not necessarily imply that variation attributable to these apparent factors of importance achieved statistical significance in the original publications.
Results
Durations
UES Opening Duration
Twenty publications reported data for UES opening duration. Compiled results appear in Figure 1 with corresponding study information in Table 2. First, it is apparent that this variable is distributed across a relatively small range of mean values (i.e., 0.21 to 0.67 seconds). Further, the 95% CIs for UES opening duration fall remarkably tight to the means, showing very little spread in the data. Within the UES opening duration data, it is interesting to note that every study in which two or more bolus volumes were studied demonstrated a systematic volume effect: an increase in volume resulted in a corresponding increase in mean UES opening duration (studies 2, 3, 4, 8, 9, 11, 12, 14, 17, 20, 24, 25, 29 and 30). Additionally, a systematic age effect is apparent within the UES opening duration data. Studies 17, 24 and 25 each included a comparison of data between younger and older participant groups. In each of these studies, UES opening durations were longer in the older participants. Study 11 examined a factor of gender, without evidence of any sex effect, while study 8 tested barium density, and reported longer durations with higher density stimuli. These single studies do not allow us to draw any conclusions regarding trends attributable to these factors.
Laryngeal Closure Duration (LCD)
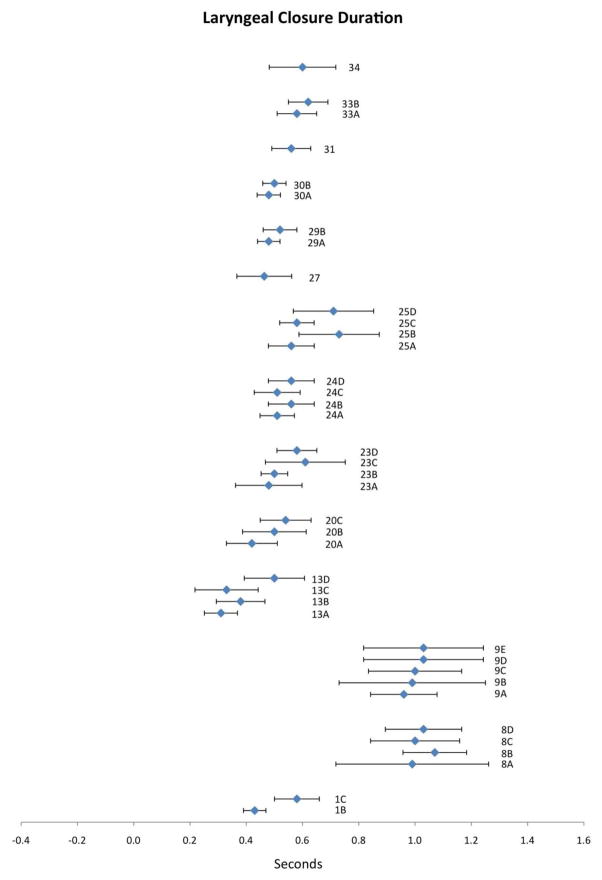
Fourteen publications reported data for LCD. Compiled results appear in Figure 2 with corresponding study information in Table 2. Mean values for LCD ranged from 0.31 to 1.07 seconds. In comparison to the UES opening duration parameter, the 95% CIs for LCD are wider and reflect greater variability around each mean data point. Eight of the ten studies that tested volume effects show apparent support for the notion that LCD increases with increasing bolus volume (1, 9, 20, 24, 25, 29, 30, 33) while two studies contain mixed results in this respect (8 and 23). The contribution of participant age to variation in LCD was tested in three studies and a trend toward increased LCD in older participants was observed in all three (13, 24 and 25). Higher barium density appeared to contribute to longer LCD, but was reported only in study 8, rendering it impossible to draw strong conclusions regarding the pervasiveness of this effect across the literature.
Figure 2.
Means (in sec) and 95% CIs for laryngeal closure duration as reported in the reviewed literature. Alphanumeric codes refer to studies/variables in Table 2.
Hyoid Movement Duration (HMD)
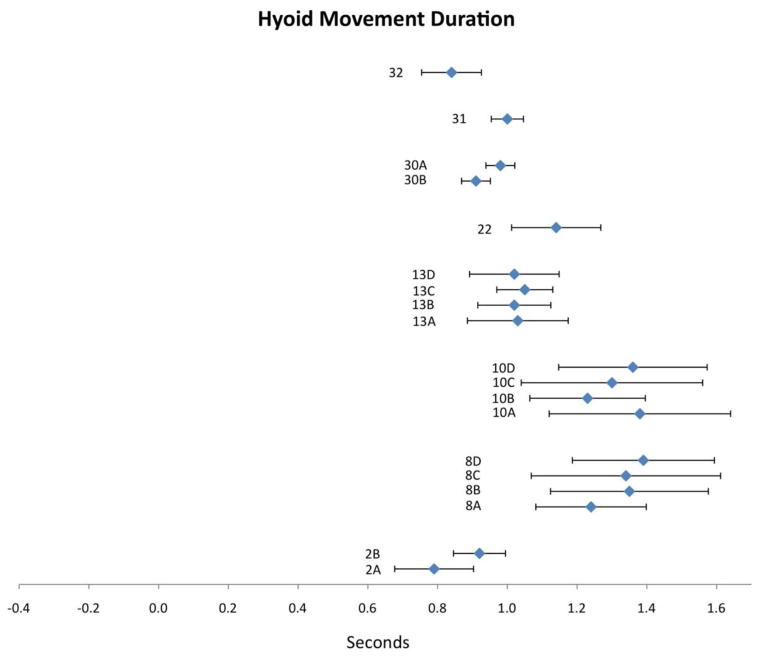
Eight publications reported values for HMD. Compiled results appear in Figure 3 with corresponding study information in Table 2. Mean HMD values were spread across a range from 0.79 to 1.39 seconds. Corresponding CIs appear similar in magnitude to those seen for LCD (and wider than those seen for UES opening duration). Three of four studies that tested the influence of bolus volume on HMD showed that larger volumes were associated with longer durations (2, 8 and 30). Partial support for this trend is also apparent in study 10. Only study 13 tested the contribution of participant age to HMD, with no clear effect emerging for this variable. Higher barium density resulted in longer HMD in the single study examining this factor (study 8).
Figure 3.
Means (in sec) and 95% CIs for hyoid movement duration as reported in the reviewed literature. Alphanumeric codes refer to studies/variables in Table 2.
Intervals
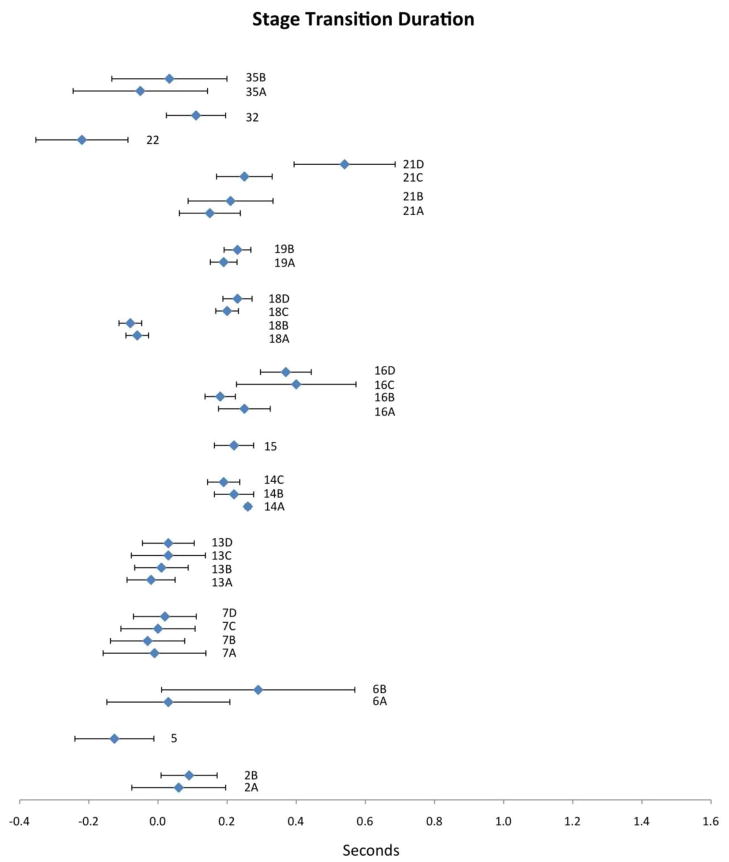
Stage Transition Duration (STD)
STD is defined as the interval between the bolus entering the pharynx (usually demarcated by the bolus passing the shadow of the ramus of the mandible) and the onset of upward and forward hyoid excursion [5]. Fourteen studies reported values for STD. Compiled results appear in Figure 4 with corresponding study information in Table 2. Mean STD values ranged from −0.22 to 0.54 seconds. Not only do the mean values display considerable variability across publications, but the corresponding CIs also appear to be highly variable. Seven of the 14 publications reporting this variable include variations in bolus volume, but there appears to be no clear pattern of this factor across studies. Some publications report longer STD values with larger volumes (2, 19, 35 and 7 partially), while others showed shorter STDs with larger volumes (14, 16 and 18). However, all four studies that included participant age as a factor in their analysis (13, 16, 18 and 21) showed a systematic trend of longer STD values in older participants. In addition, study 6 showed much higher mean values of STD and wider CIs when swallows were uncued (non-command swallow paradigm) versus cued. Finally, of note, a subtle variation in STD definition was noted across studies. The onset of STD reported in 14, 15, 16 and 21C/D was defined as the bolus passing the posterior nasal spine (rather than the shadow of the ramus of the mandible). This variable is designated ‘B1-H1’ by the authors). Thus, it would be logical to expect slightly longer mean values in the STD data for these studies compared to studies referencing STD to bolus movement past the mandible; this expectation is generally consistent with the data shown in Figure 4.
Figure 4.
Means (in sec) and 95% CIs for stage transition duration as reported in the reviewed literature. Alphanumeric codes refer to studies/variables in Table 2.
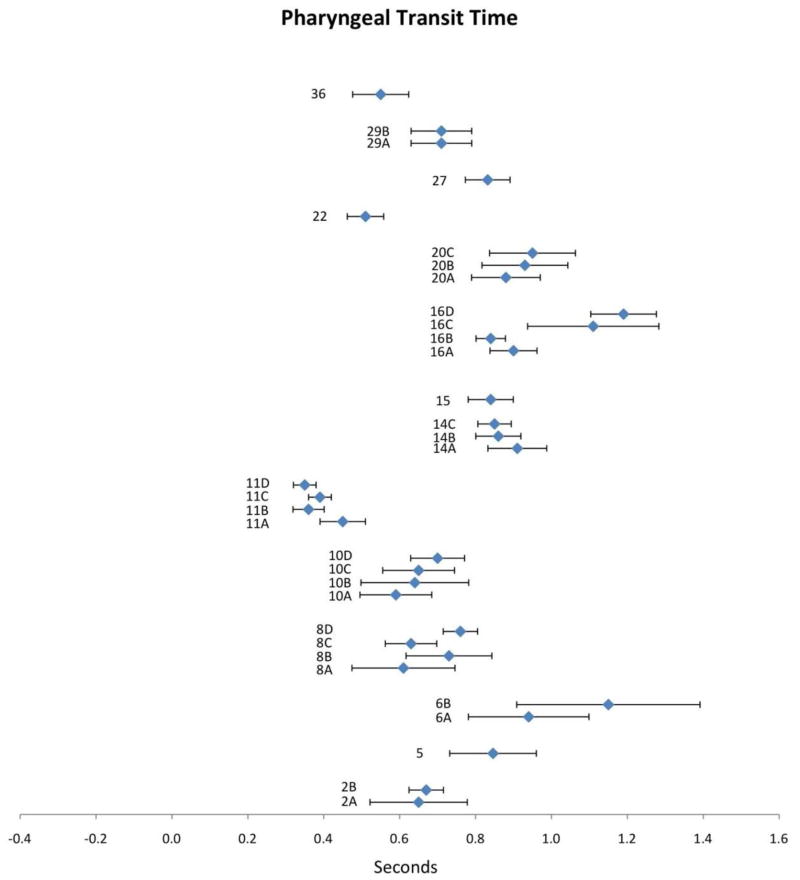
Pharyngeal Transit Time (PTT)
PTT is defined as the interval between the bolus entering the pharynx (usually demarcated by the bolus passing the shadow of the ramus of the mandible) and the bolus tail passing through the UES [5]. Thus, it overlaps with, but extends the interval captured by the STD parameter. Fourteen publications included measures of PTT in their analysis. Compiled results appear in Figure 5 with corresponding study information in Table 2. Mean PTT values displayed a wide range from 0.35 to 1.19s and considerable variability in CIs. As with the STD parameter, there appears to be no clear influence of bolus volume on PTT. Some publications report longer PTT values with larger volumes (2, 8, 10 and 20), others show shorter PTT with larger boluses (11 and 14), while others still show no clear trend (16 and 29). The influence of other factors on PTT was explored in single publications only. Based on these single studies, PTT values appear to be higher in older individuals (study 16), in women (study 11), with higher barium density (study 8) and in non-cued swallow conditions (study 6). However, single studies do not allow for strong conclusions to be drawn regarding the contributions of these factors. Finally, many discrepancies in naming conventions and operational definitions for PTT were noted and will be discussed further below.
Figure 5.
Means (in sec) and 95% CIs for pharyngeal transit time as reported in the reviewed literature. Alphanumeric codes refer to studies/variables in Table 2.
Laryngeal-Closure-(LC)-to-UES-Opening Interval
Nine studies reported the time interval between LC and UES opening. Compiled results appear in Figure 6 with corresponding study information in Table 2. Mean LC-to-UES-opening values display a strikingly tight range from −0.16 to 0.02 seconds with limited variability seen in the 95% CIs. No apparent trends emerge for variation in LC-to-UES-opening-interval based on bolus volume: PTT decreased with increasing bolus size in studies 1, 25 and possibly 29, while it increased with increasing bolus size in study 24. This interval does appear to decrease with increasing age (24, 25 and 28), although it is probably questionable to comment on apparent trends given such a narrow distribution of means. Finally, in study 1, cold temperature appeared to shorten this interval, but was only included in this single study.
Aggregate measures
Given that all six of these frequently-occurring parameters are measured in a common unit (seconds), it is possible to examine the degree of variability present across the measures themselves, and to characterize these parameters in terms of their relative variability. Table 3 compares the aggregate ranges found for mean values (maximum minus minimum mean values reported across studies) and 95% confidence interval widths (maximum upper CI limit minus minimum lower CI limit computed across studies) in our meta-analysis. Table 3 summarizes this comparison exercise and also summarizes the extent to which the metaanalysis of each parameter revealed systematic trends attributable to factors of bolus volume and participant age. Based on this review, it can be seen that the LC-to-UES-opening interval displays, relatively speaking, the smallest mean range and tightest 95% confidence intervals of the six measures reviewed. UES opening duration comes next with a slightly larger mean range and 95% confidence interval range. The values seen for the HMD parameter falls in the middle, displaying what might be interpreted as a typical degree of variability across these timing measures, both for mean range and for 95% confidence interval width. By contrast, the STD, PTT and LCD measures show large mean ranges and widest 95% confidence intervals.
Table 3.
Aggregate mean and 95% CI ranges for all variables with corresponding volume and age effects.
| Parameter | Aggregate Mean Rangea (sec) | Aggregate 95% CI Widthb (sec) | Volume Effect? | Age Effect? |
|---|---|---|---|---|
| UES opening duration | 0.46 | 0.52 | Yes | Yes |
| LCD | 0.76 | 1.04 | Yes | Maybe |
| HMD | 0.60 | 0.96 | Maybe | No |
| STD | 0.76 | 1.04 | No | Yes |
| PTT | 0.84 | 1.07 | No | Maybe |
| LC-to-UES-opening | 0.18 | 0.36 | No | Maybe |
Calculated by subtracting the minimum reported mean value from the maximum reported mean value across the studies reviewed.
Calculated by subtracting the minimum value for the 95% CI lower boundary from the maximum value for the 95% CI upped boundary across the studies reviewed.
Discussion
This meta-analysis compiles data for the three most-frequently reported durations and the three most-frequently reported swallow intervals from the existing literature describing healthy swallowing. Means and CIs were plotted on modified forest plots, with scales held constant to allow for relative inspection of variability across variables. Taken together, it is apparent that not all variables behave similarly with respect to variability, despite being sampled from healthy individuals. Table 3 summarizes the impressions of relative variability gleaned from the inspection of the modified forest plots in Figures 1–6. Of course, it is possible that factors other than those that were directly tested in the original publications might account for some of the variability seen in these data. Here, we discuss several of these potential sources of variability.
Definitional Sources of Variability
Methodological differences across studies may account for a portion of the variability that is seen in these data. One clear opportunity for such variability to occur arises when different operational definitions are used for specific temporal parameters across studies. To illustrate, consider the challenge of defining hyoid movement duration. It has been pointed out that the hyoid is not stable when it is in a resting state [55, 56], making it challenging to define the onset and offset of hyoid movement, and leading to the possibility that even subtle differences in the definitions used for these indices may cause differences in the results of measures made in different studies. In fact, Kendall and colleagues report that the post-swallow hyoid position has such large SDs in their data that they have decided not to routinely include this parameter in their standardized methods for analyzing VF exams [13].
An associated source of variability may be related to challenges in reliable measuring of the durations that are being investigated. Agreement across raters is important not only for calculating durational measures, but in selecting the frames that are used to index such measures. Difficulties in achieving adequate inter-rater and intra-rater reliability for temporal measures on VF have been reported [57, 58]. In this meta-analysis, only 23 of the 46 studies reviewed reported inter-rater reliability, and even fewer reported intra-rater reliability (16 of 46). If acceptable levels of both inter- and intra- rater reliability are not established, the contributions of variable measurement by raters cannot considered or accounted for.
The criteria by which participants are selected for inclusion may also be a methodological source of variability. The definition of ‘healthy’ participants can represent different things for different research groups. For example, in most of the studies reviewed, ‘healthy’ was undefined and could include anyone without a history of structural changes to the head and neck and/or neurological impairment and/or dysphagia. Sometimes, the source of ‘healthy’ control participants may be fundamentally different from control participants in another publication. For example, Stachler and colleagues use a control group of heavy smokers and drinkers to compare to their head and neck surgical patients [45]. It is unclear to what extent these definitional issues may contribute to the variability present in this meta-analysis.
Stimulus Sources of Variability
Bolus volume emerged from this meta-analysis as a factor that influences several timing measures (see Table 3). In particular, it appears that swallow durations are impacted by bolus volume, while swallow intervals are not. UES opening duration was highly influenced by differences in bolus volume, while laryngeal closure duration and hyoid movement duration appeared to be only moderately sensitive to this factor (see Figures 1, 2, 3).
The density of the stimulus may also be a source of variability in timing measures, as suggested in the single study (8) examining this factor. However, while the higher density barium preparation (250% w/v compared with 40% w/v) in that study elicited longer timing measures for all four variables included in that study (UES opening duration, LCD, HMD and PTT), it must also be recognized that we do not have information on intermediate densities that would be necessary to clearly elucidate the effect of density on swallowing timing. Further, it should be noted that only 14 of the 46 studies examined in this review reported the density of the barium used, with values ranging from 35% to 250% w/v. There is a need for clear reporting of methodological decisions like barium density in order to demonstrate the variability attributable to this factor, and to inform the field regarding the potential for manipulations of this factor to reveal clinically important variations in swallowing function.
Participant Sources of Variability
Our meta-analysis suggests that participant age is one source of variability in swallow timing parameters. Among the parameters reviewed, UES duration and STD both showed systematic trends towards longer durations in older participants. By contrast, hyoid movement durations appeared to be robust and invariant across age. Other participant factors, which may be considered as sources of variability, include differences in patient size, such as differences in spine length, which has been explored as a source of kinematic variation but has not, to date, been considered with respect to temporal measures [22, 23, 59, 60]. Variations in pharyngeal size might also be logically considered as a potential source of variability in PTT, given that this measure captures the time for the bolus to travel through the pharynx. Future work should examine whether participant size can account for some of the variability observed in swallowing durations and intervals.
Procedural Sources of Variability
In 2007, Daniels and colleagues published their groundbreaking study on the effect of verbal cueing on temporal measures of swallowing [37]. They demonstrated that uncued swallows were initiated with the bolus head at a more posterior location in the oropharynx, thus resulting in longer temporal measures. In that study, STD and PTT measures for the uncued condition had both longer durations and wider CIs compared to values from other studies and for other variables (see 6B on Figures 5 and 6). Most of the studies included in the current metaanalysis were conducted before Daniels’ work was published and therefore do not report transparent information regarding the use of cueing. Future work should not only describe whether a cueing paradigm was used, but also test its effect on kinematic and other temporal measures of swallowing.
Another potential source of procedural variability is related to the parameters of the fluoroscopy output, namely the frames per second (fps) capture rate. This parameter stipulates how many samples are extracted per second from the continuous fluoroscopy. The majority of studies reviewed reported using 25 fps or greater (n=35), however, the remaining 11 studies do not specify their frame rate. Bonilha and colleagues have recently shown differences in ratings of standardized videofluoroscopic swallowing study measures of residue, overall impression (a physiological composite score) and penetration aspiration scale when a simulated 15 fps condition was compared to the full 30 fps condition [61]. The effect of frame rate on temporal measures of swallowing is unknown; however, one might speculate that higher rates of variability may be observed in lower fps conditions due to the fact that less information is captured during the swallow.
Individual Sources of Variability
Finally, as has been proposed before [4], when all other sources of variability are accounted for, it is highly plausible that each individual participant displays some level of underlying variability in both kinematic and temporal measures of swallowing, across repeated swallows and repeated VF examinations. Within this context, not all variables are likely to fluctuate to the same degree. Rosenbek and colleagues suggest that the challenge of underlying variability for clinicians is “in deciding how many swallows to elicit and how to interpret performance on what is perforce a limited number of swallows” [1]. We feel that underlying individual variability will only be observable in a controlled and standardized assessment paradigm that employs multiple swallows per bolus condition [46, 47]. Martin-Harris and colleagues [2] have shown that 5ml thin liquid and 5ml nectar thick swallows are highly sensitive to physiological measures of swallowing impairment. While this observation needs replication for temporal measures of swallowing, we advocate for the use of multiple trials of thin liquid barium and nectar thick barium (at controlled densities) during standardized VF assessment.
In clinical settings, it is routinely recommended that a VF include the administration of 3 swallows per bolus condition as a minimum for capturing individual variability while balancing radiation exposure [5]. However, 13 of the studies reviewed in this analysis reported data derived from only a single swallow per bolus condition. Under these circumstances, variability may be present, but its influence unrecognized, given that the single observed swallow may not be representative of a typical swallow for that participant. In the case where multiple swallows are administered, the statistical handling of repeated measures can also impact the appreciation of variability. While the majority of studies with more than one swallow per condition averaged the repeated measure, some studies weighted each swallow equally in the analysis [22, 23, 59]. The appropriate handling of repeated measures is well recognized in the literature to influence the impression of variability in a resulting statistic [62, 63].
Limitations
Inconsistent conventions – naming and defining swallow intervals
An important caveat to note regarding this review of the deglutition literature is that we observed a startling number of inconsistencies in the naming conventions and definitions of different temporal variables. For example, the interval between the bolus head entering the pharynx and the bolus tail passing through the UES is most commonly referred to as ‘pharyngeal transit time’ [21, 37, 39, 41], but has also been labeled as ‘pharyngeal transit duration’ [5], ‘pharyngeal clearance duration’ [42] and ‘pharyngeal clearance time’ [53]. Similarly, ‘stage transition duration’ was sometimes referred to as the ‘pharynx-to-swallow interval’ [45, 48].
To make matters more complex, we also observed subtle variations in operational definitions of variables, making the comparison of data across studies challenging. For example, the majority of research describes PTT as the interval between the bolus head passing the shadow of the ramus of the mandible and the tail of the bolus passing through the UES. However, it has also been defined by some as commencing when the bolus head or tail passes the faucial pillars [34, 39, 51, 64] or the posterior nasal spine [13, 15]. This same variable has also been defined as concluding when the bolus head reaches the UES [42], as opposed to the bolus tail passing through the UES. Langmore has also discussed these challenges and points out that the discrepancies for this particular variable appear to stem from changing historical definitions of the boundary between the oral and pharyngeal phase [65]. Similar subtle differences in operational definitions were noted in the literature for measures of oral transit, pharyngeal response time, STD, and onset of hyoid excursion, among others. As has been previously pointed out by Mendell and Logemann [8], some of these disparities can be attributed to different research groups choosing to time-reference swallowing data to different physiological events (such as bolus passing mandible, initial upward/forward movement of the hyoid, or UES opening). We concur with this observation given that very few discrepancies in naming conventions or operational definitions were noted for swallowing durations (which do not require a reference point) compared to swallow intervals. The only exception appeared to be for hyoid movement duration, which was also referred to as ‘pharyngeal response duration’ [42] and ‘swallow duration’ [48]. However, it is reasonable to postulate that wherever such variations in terminology and definition occur, they are likely to contribute to differences in reported mean values, but should not contribute directly to trends in standard deviations and data spread.
This meta-analysis is limited to studies that reported temporal data in healthy adults in a way that allowed us to reconstruct means and confidence intervals. There were many publications that lacked the necessary quantitative information for inclusion. Further, analysis of the influence of specific factors on temporal variability was limited to those variables analyzed and reported in the original publication.
Conclusions
We have compiled descriptive statistical data for the most-frequently occurring temporal variables in the healthy deglutition literature, allowing for an aggregate impression of variability in such measures to be formed. Differences in naming conventions and operational definitions were noted, especially for swallow interval measures. The three most commonly occurring measures of swallowing durations were UES opening, laryngeal closure and hyoid movement. The three most commonly occurring measures of swallow intervals were stage transition duration, pharyngeal transit time and laryngeal closure to UES opening. A meta-analysis of these six variables using modified forest plots has revealed that there is substantial variability in temporal measures of healthy swallowing and that not all variables fluctuate in the same way or to the same degree. Some variables demonstrated tight means and confidence intervals (laryngeal-closure-to-UES-opening interval, UES opening duration). Other variables appeared to be influenced by bolus volume (UES opening duration, laryngeal closure duration) or participant age (UES opening duration, stage transition duration).
We have discussed several factors which may account for a portion of the observed variability in these studies. We also propose that inherent variability in swallowing function may still exist when all these variables are controlled for. Future work should examine within-participant and across-participant variability while controlling methodological, definitional, stimulus, participant, procedural and statistical sources of variability in both healthy individuals and different subgroups of people with dysphagia.
Acknowledgments
The first author has received funding for her doctoral studies from the Natural Sciences and Engineering Research Council (Canada) Create CARE program and the Ontario Student Opportunity Trust Fund. The second author holds a New Investigator award from the Canadian Institutes of Health Research. The authors acknowledge the support of Toronto Rehabilitation Institute who receives funding under the Provincial Rehabilitation Research Program from the Ministry of Health and Long-term Care in Ontario. The views expressed do not necessarily reflect those of the ministry.
Footnotes
Conflicts of Interest
We have no conflicts of interest to declare.
References
- 1.Rosenbek JC, Roecker EB, Wood JL, Robbins J. Thermal application reduces the duration of stage transition in dysphagia after stroke. Dysphagia. 1996;11:225–233. doi: 10.1007/BF00265206. [DOI] [PubMed] [Google Scholar]
- 2.Martin-Harris B, Brodsky MB, Michel Y, Castell DO, Schleicher M, Sandidge J, Maxwell R, Blair J. MBS measurement tool for swallow impairment-MBSimp: Establishing a standard. Dysphagia. 2008;23:392–405. doi: 10.1007/s00455-008-9185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perlman AL, Booth BM, Grayhack JP. Videofluoroscopic predictors of aspiration in patients with oropharyngeal dysphagia. Dysphagia. 1994;9:90–95. doi: 10.1007/BF00714593. [DOI] [PubMed] [Google Scholar]
- 4.Molfenter SM, Steele CM. Physiological variability in the deglutition literature: Hyoid and laryngeal kinematics. Dysphagia. 2011;26:67. doi: 10.1007/s00455-010-9309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lof GL, Robbins J. Test-retest variability in normal swallowing. Dysphagia. 1990;4:236–242. doi: 10.1007/BF02407271. [DOI] [PubMed] [Google Scholar]
- 6.Norman GR, Streiner DL. Biostatistics: The bare essentials. Hamilton, Ontario: B.C. Decker Inc; 2008. [Google Scholar]
- 7.van Geert P, van Dijk M. Focus on variability: New tools to study intra-individual variability in developmental data. Infant Behav Dev. 2002;25:340–374. [Google Scholar]
- 8.Mendell DA, Logemann JA. Temporal sequence of swallow events during the oropharyngeal swallow. J Speech Lang Hear Res. 2007;50:1256–1271. doi: 10.1044/1092-4388(2007/088). [DOI] [PubMed] [Google Scholar]
- 9.Kendall KA, Leonard RJ, McKenzie SW. Sequence variability during hypopharyngeal bolus transit. Dysphagia. 2003;18:85–91. doi: 10.1007/s00455-002-0086-z. [DOI] [PubMed] [Google Scholar]
- 10.Stephen JR, Taves DH, Smith RC, Martin RE. Bolus location at the initiation of the pharyngeal stage of swallowing in healthy older adults. Dysphagia. 2005;20:266–272. doi: 10.1007/s00455-005-0023-z. [DOI] [PubMed] [Google Scholar]
- 11.Martin-Harris B, Brodsky MB, Michel Y, Lee F, Walters B. Delayed initiation of the pharyngeal swallow: Normal variability in adult swallows. J Speech Lang Hear Res. 2007;50:585–594. doi: 10.1044/1092-4388(2007/041). [DOI] [PubMed] [Google Scholar]
- 12.Leonard R, McKenzie S. Hyoid-bolus transit latencies in normal swallow. Dysphagia. 2006;21:183–190. doi: 10.1007/s00455-006-9025-8. [DOI] [PubMed] [Google Scholar]
- 13.Kendall KA, McKenzie S, Leonard RJ, Gonçalves MI, Walker A. Timing of events in normal swallowing: A videofluoroscopic study. Dysphagia. 2000;15:74–83. doi: 10.1007/s004550010004. [DOI] [PubMed] [Google Scholar]
- 14.Kendall KA, Leonard RJ. Bolus transit and airway protection coordination in older dysphagic patients. Laryngoscope. 2001;111:2017–2021. doi: 10.1097/00005537-200111000-00028. [DOI] [PubMed] [Google Scholar]
- 15.Kendall KA, Leonard RJ, McKenzie SW. Accommodation to changes in bolus viscosity in normal deglutition: A videofluoroscopic study. Ann Otol Rhinol Laryngol. 2001;110:1059–1065. doi: 10.1177/000348940111001113. [DOI] [PubMed] [Google Scholar]
- 16.Kendall KA, Leonard RJ, McKenzie S. Common medical conditions in the elderly: Impact on pharyngeal bolus transit. Dysphagia. 2004;19:71–77. doi: 10.1007/s00455-003-0502-z. [DOI] [PubMed] [Google Scholar]
- 17.Kendall KA, Leonard RJ. Videofluoroscopic upper esophageal sphincter function in elderly dysphagic patients. Laryngoscope. 2002;112:332–337. doi: 10.1097/00005537-200202000-00024. [DOI] [PubMed] [Google Scholar]
- 18.Kendall KA, Leonard RJ. Hyoid movement during swallowing in older patients with dysphagia. Arch Otolaryngol Head Neck Surg. 2001;10:1224–1229. doi: 10.1001/archotol.127.10.1224. [DOI] [PubMed] [Google Scholar]
- 19.Kendall KA, Leonard RJ. Pharyngeal constriction in elderly dysphagic patients compared with young and elderly nondysphagic controls. Dysphagia. 2001;16:272–278. doi: 10.1007/s00455-001-0086-4. [DOI] [PubMed] [Google Scholar]
- 20.Kim Y, McCullough GH, Asp CW. Temporal measurements of pharyngeal swallowing in normal populations. Dysphagia. 2005;20:290–296. doi: 10.1007/s00455-005-0029-6. [DOI] [PubMed] [Google Scholar]
- 21.Mendell DA, Logemann JA. A retrospective analysis of the pharyngeal swallow in patients with a clinical diagnosis of GERD compared with normal controls: A pilot study. Dysphagia. 2002;17:220–226. doi: 10.1007/s00455-002-0056-5. [DOI] [PubMed] [Google Scholar]
- 22.Logemann JA, Pauloski BR, Rademaker AW, Kahrilas PJ. Oropharyngeal swallow in younger and older women: Videofluoroscopic analysis. J Speech Lang Hear Res. 2002;45:434–445. doi: 10.1044/1092-4388(2002/034). [DOI] [PubMed] [Google Scholar]
- 23.Logemann JA, Pauloski BR, Rademaker AW, Colangelo LA, Kahrilas PJ, Smith CH. Temporal and Biomechanical Characteristics of Oropharyngeal Swallow in Younger and Older Men. J Speech Lang Hear Res. 2000;43:1264–1274. doi: 10.1044/jslhr.4305.1264. [DOI] [PubMed] [Google Scholar]
- 24.Kang B, Oh B, Kim IS, Chung SG, Kim SJ, Han TR. Influence of Aging on Movement of the Hyoid Bone and Epiglottis during Normal Swallowing: A Motion Analysis. Gerontology. 2010;56:474–482. doi: 10.1159/000274517. [DOI] [PubMed] [Google Scholar]
- 25.Kim SJ, Han TR, Kwon TK. Kinematic analysis of hyolaryngeal complex movement in patients with dysphagia development after pneumonectomy. Thorac Cardiovasc Surg. 2010;58:108–112. doi: 10.1055/s-0029-1186278. [DOI] [PubMed] [Google Scholar]
- 26.Gay T, Rendell JK, Spiro J, Mosier K, Lurie AG. Coordination of oral cavity and laryngeal movements during swallowing. J Appl Physiol. 1994;77:357–365. doi: 10.1152/jappl.1994.77.1.357. [DOI] [PubMed] [Google Scholar]
- 27.Ohmae Y, Logemann JA, Kaiser P, Hanson DG, Kahrilas PJ. Timing of glottic closure during normal swallow. Head and Neck. 1995;17:394–402. doi: 10.1002/hed.2880170506. [DOI] [PubMed] [Google Scholar]
- 28.Ohmae Y, Logemann JA, Kaiser P, Hanson DG, Kahrilas PJ. Effects of two breath-holding maneuvers on oropharyngeal swallow. Ann Otol Rhinol Laryngol. 1996;105:123–131. doi: 10.1177/000348949610500207. [DOI] [PubMed] [Google Scholar]
- 29.Martin-Harris B, Brodsky MB, Price CC, Michel Y, Walters B. Temporal coordination of pharyngeal and laryngeal dynamics with breathing during swallowing: Single liquid swallows. J Appl Physiol. 2003;94:1735–1743. doi: 10.1152/japplphysiol.00806.2002. [DOI] [PubMed] [Google Scholar]
- 30.Power ML, Hamdy S, Singh S, Tyrrell PJ, Turnbull I, Thompson DG. Deglutitive laryngeal closure in stroke patients. J Neurol Neurosur Ps. 2007;78:141–146. doi: 10.1136/jnnp.2006.101857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Logemann JA, Kahrilas PJ, Cheng J, Pauloski BR, Gibbons PJ, Rademaker AW, Lin S. Closure mechanisms of laryngeal vestibule during swallow. Am J Physiol - Gastr L. 1992;262:G338–G344. doi: 10.1152/ajpgi.1992.262.2.G338. [DOI] [PubMed] [Google Scholar]
- 32.Park T, Kim Y, Ko D, McCullough G. Initiation and duration of laryngeal closure during the pharyngeal swallow in post-stroke patients. Dysphagia. 2010;25:177–182. doi: 10.1007/s00455-009-9237-9. [DOI] [PubMed] [Google Scholar]
- 33.Rofes L, Arreola V, Romea M, Palomera E, Almirall J, Cabré M, Serra-Prat M, Clavé P. Pathophysiology of oropharyngeal dysphagia in the frail elderly. Neurogastroent Motil. 2010;22:851–858+e230. doi: 10.1111/j.1365-2982.2010.01521.x. [DOI] [PubMed] [Google Scholar]
- 34.Dantas RO, De Aguiar Cassiani R, Dos Santos CM, Gonzaga GC, Alves LMT, Mazin SC. Effect of Gender on Swallow Event Duration Assessed by Videofluoroscopy. Dysphagia. 2009;24:280–284. doi: 10.1007/s00455-008-9202-z. [DOI] [PubMed] [Google Scholar]
- 35.Daniels SK, Schroeder MF, DeGeorge PC, Corey DM, Foundas AL, Rosenbek JC. Defining and measuring dysphagia following stroke. Am J Speech Lang Pathol. 2009;18:74–81. doi: 10.1044/1058-0360(2008/07-0040). [DOI] [PubMed] [Google Scholar]
- 36.Daniels SK, Schroeder MF, McClain M, Corey DM, Rosenbek JC, Foundas AL. Dysphagia in stroke: Development of a standard method to examine swallowing recovery. J Rehabil Res Dev. 2006;21:347–355. doi: 10.1682/jrrd.2005.01.0024. [DOI] [PubMed] [Google Scholar]
- 37.Daniels SK, Schroeder MF, DeGeorge PC, Corey DM, Rosenbek JC. Effects of verbal cue on bolus flow during swallowing. Am J Speech Lang Pathol. 2007;16:140–147. doi: 10.1044/1058-0360(2007/018). [DOI] [PubMed] [Google Scholar]
- 38.Komori M, Hyodo M, Gyo K. A swallowing evaluation with simultaneous videoendoscopy, ultrasonography and videofluorography in healthy controls. J Otorhinolaryngol Relat Spec. 2008;70:393–398. doi: 10.1159/000163036. [DOI] [PubMed] [Google Scholar]
- 39.Taniguchi H, Tsukada T, Ootaki S, Yamada Y, Inoue M. Correspondence between food consistency and suprahyoid muscle activity, tongue pressure, and bolus transit times during the oropharyngeal phase of swallowing. J Appl Physiol. 2008;105:791–799. doi: 10.1152/japplphysiol.90485.2008. [DOI] [PubMed] [Google Scholar]
- 40.Kim Y, McCullough GH. Stage transition duration in patients poststroke. Dysphagia. 2007;22:299–305. doi: 10.1007/s00455-007-9085-4. [DOI] [PubMed] [Google Scholar]
- 41.Mokhlesi B, Logemann JA, Rademaker AW, Stangl CA, Corbridge TC. Oropharyngeal deglutition in stable COPD. Chest. 2002;121:361–369. doi: 10.1378/chest.121.2.361. [DOI] [PubMed] [Google Scholar]
- 42.Chi-Fishman G, Sonies BC. Motor strategy in rapid sequential swallowing: New insights. J Speech Lang Hear Res. 2000;43:1481–1492. doi: 10.1044/jslhr.4306.1481. [DOI] [PubMed] [Google Scholar]
- 43.Kern M, Bardan E, Arndorfer R, Hofmann C, Ren J, Shaker R. Comparison of upper esophageal sphincter opening in healthy asymptomatic young and elderly volunteers. Ann Otol Rhinol Laryngol. 1999;108:982–989. doi: 10.1177/000348949910801010. [DOI] [PubMed] [Google Scholar]
- 44.Kahrilas PJ, Logemann JA, Krugler C, Flanagan E. Volitional augmentation of upper esophageal sphincter opening during swallowing. Am J Physiol - Gastr L. 1991;260:G450–G456. doi: 10.1152/ajpgi.1991.260.3.G450. [DOI] [PubMed] [Google Scholar]
- 45.Stachler RJ, Hamlet SL, Mathog RH, Jones L, Heilbrun LK, Manov LJ, O’Campo JM. Swallowing of bolus types by postsurgical head and neck cancer patients. Head Neck. 1994;16:413–419. doi: 10.1002/hed.2880160504. [DOI] [PubMed] [Google Scholar]
- 46.Bisch EM, Logemann JA, Rademaker AW, Kahrilas PJ, Lazarus CL. Pharyngeal effects of bolus volume, viscosity, and temperature in patients with dysphagia resulting from neurologic impairment and in normal subjects. J Speech Hear Res. 1994;37:1041–1049. doi: 10.1044/jshr.3705.1041. [DOI] [PubMed] [Google Scholar]
- 47.Lazarus CL, Logemann JA, Rademaker AW, Kahrilas PJ, Pajak T, Lazar R, Halper A. Effects of bolus volume, viscosity, and repeated swallows in nonstroke subjects and stroke patients. Arch Phys Med Rehabil. 1993;74:1066–1070. doi: 10.1016/0003-9993(93)90063-g. [DOI] [PubMed] [Google Scholar]
- 48.Palmer JB, Rudin NJ, Lara G, Crompton AW. Coordination of mastication and swallowing. Dysphagia. 1992;7:187–200. doi: 10.1007/BF02493469. [DOI] [PubMed] [Google Scholar]
- 49.Cook IJ, Dodds WJ, Dantas RO, Massey B, Kern MK, Lang IM, Brausseur JG, Hogan WJ. Opening mechanisms of the human upper esophageal sphincter. Am J Physiol - Gastr L. 1989;257:G748–759. doi: 10.1152/ajpgi.1989.257.5.G748. [DOI] [PubMed] [Google Scholar]
- 50.Cook IJ, Dodds WJ, Dantas RO, Kern MK, Massey BT, Shaker R, Hogan WJ. Timing of videofluoroscopic, manometric events, and bolus transit during the oral and pharyngeal phases of swallowing. Dysphagia. 1989;4:8–15. doi: 10.1007/BF02407397. [DOI] [PubMed] [Google Scholar]
- 51.Dantas RO, Dodds WJ, Massey BT, Kern MK. The effect of high- vs low-density barium preparations on the quantitative features of swallowing. Am J Roentgenol. 1989;153:1191–1195. doi: 10.2214/ajr.153.6.1191. [DOI] [PubMed] [Google Scholar]
- 52.Dantas RO, Cook IJ, Dodds WJ, Kern MK, Lang IM, Brasseur JG. Biomechanics of cricopharyngeal bars. Gastroenterology. 1990;5:1269–1274. doi: 10.1016/0016-5085(90)91149-z. [DOI] [PubMed] [Google Scholar]
- 53.Dantas RO. Effect of swallowed bolus variables on oral and pharyngeal phases of swallowing. Am J Physiol - Gastr L. 1990;258:G675. doi: 10.1152/ajpgi.1990.258.5.G675. [DOI] [PubMed] [Google Scholar]
- 54.Pauloski BR, Logemann JA, Fox JC, Colangelo LA. Biomechanical analysis of the pharyngeal swallow in postsurgical patients with anterior tongue and floor of mouth resection and distal flap reconstruction. J Speech Hear Res. 1995;38:110–123. doi: 10.1044/jshr.3801.110. [DOI] [PubMed] [Google Scholar]
- 55.Zoratto D. Hyolaryngeal excursion as the physiological source of swallowing accelerometry signals. Physiol Meas. 2010;31:843–855. doi: 10.1088/0967-3334/31/6/008. [DOI] [PubMed] [Google Scholar]
- 56.Ishida R, Palmer JB, Hiiemae KM. Hyoid motion during swallowing: Factors affecting forward and upward displacement. Dysphagia. 2002;17:262–272. doi: 10.1007/s00455-002-0064-5. [DOI] [PubMed] [Google Scholar]
- 57.Stoeckli SJ, Huisman TAGM, Seifert B, Martin-Harris BJW. Interrater reliability of videofluoroscopic swallow evaluation. Dysphagia. 2003;18:53–57. doi: 10.1007/s00455-002-0085-0. [DOI] [PubMed] [Google Scholar]
- 58.McCullough GH, Wertz RT, Rosenbek JC, Mills RH, Webb WG, Ross KB. Inter- and intrajudge reliability for videofluoroscopic swallowing evaluation measures. Dysphagia. 2001;16:110–118. doi: 10.1007/PL00021291. [DOI] [PubMed] [Google Scholar]
- 59.Perlman AL, VanDaele DJ, Otterbacher MS. Quantitative assessment of hyoid bone displacement from video images during swallowing. J Speech Hear Res. 1995;38:579–585. doi: 10.1044/jshr.3803.579. [DOI] [PubMed] [Google Scholar]
- 60.Steele CM, Bailey GL, Chau T, Molfenter SM, Oshalla M, Waito AA, Zoratto DCBH. The relationship between hyoid and laryngeal displacement and swallowing impairment. Clin Otolaryngol. 2011;36:30–36. doi: 10.1111/j.1749-4486.2010.02219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bonilha H, Blair J, Carnes BN, Humphries K, McGrattan K, Michele Y, Martin-Harris B. Effect of frame rate on judgement of swallowing function using the MBSImP. Dysphagia. 2011;26:441. [Google Scholar]
- 62.Max L, Onghena P. Some issues in the statistical analysis of completely randomized and repeated measures designs for speech, language, and hearing research. J Speech Lang Hear Res. 1999;42:261–270. doi: 10.1044/jslhr.4202.261. [DOI] [PubMed] [Google Scholar]
- 63.Johnson CJ. More on correct definition of the experimental unit: an extension of Max and Onghena (1999) J Speech Lang Hear Res. 2000;43:1290–1293. doi: 10.1044/jslhr.4305.1290. [DOI] [PubMed] [Google Scholar]
- 64.Dantas RO, Kern MK, Massey BT, Dodds WJ, Kahrilas PJ, Brasseur JG, Cook IJ, Lang IM. Effect of swallowed bolus variables on oral and pharyngeal phases of swallowing. Am Physiol - Gastr L. 1990;258:G675–G681. doi: 10.1152/ajpgi.1990.258.5.G675. [DOI] [PubMed] [Google Scholar]
- 65.Langmore SE. Endoscopic evaluation and treatment of swallowing disorders. New York: Thieme; 2001. [Google Scholar]