Abstract
Viruses have developed strategies to counteract signalling through Toll-like receptors (TLRs) that are involved in the detection of viruses and induction of proinflammatory cytokines and IFNs. Vaccinia virus (VACV) encodes A46 protein which disrupts TLR signalling by interfering with TLR: adaptor interactions. Since the innate immune response to viruses is critical to induce protective immunity, we studied whether deletion of A46R gene in a NYVAC vector expressing HIV-1 Env, Gag, Pol and Nef antigens (NYVAC-C) improves immune responses against HIV-1 antigens. This question was examined in human macrophages and in mice infected with a single A46R deletion mutant of the vaccine candidate NYVAC-C (NYVAC-C-ΔA46R). The viral gene A46R is not required for virus replication in primary chicken embryo fibroblast (CEF) cells and its deletion in NYVAC-C markedly increases TNF, IL-6 and IL-8 secretion by human macrophages. Analysis of the immune responses elicited in BALB/c mice after DNA prime/NYVAC boost immunization shows that deletion of A46R improves the magnitude of the HIV-1-specific CD4 and CD8 T cell immune responses during adaptive and memory phases, maintains the functional profile observed with the parental NYVAC-C and enhances anti-gp120 humoral response during the memory phase. These findings establish the immunological role of VACV A46R on innate immune responses of macrophages in vitro and antigen-specific T and B cell immune responses in vivo and suggest that deletion of viral inhibitors of TLR signalling is a useful approach for the improvement of poxvirus-based vaccine candidates.
Introduction
The search for a safe and effective HIV vaccine able to elicit long-lasting protective immunity has encouraged the development of recombinant live vaccine candidates with good safety and immunogenicity profiles. The Thai phase III clinical trial (RV144) using the recombinant poxvirus vector ALVAC and the protein gp120 in a prime-boost strategy and showing a 31.2% protection against HIV infection [1], has raised considerable interest in the use of improved attenuated poxvirus recombinants as HIV vaccine candidates. Among poxviruses, the highly attenuated vaccinia virus (VACV) strain NYVAC is under intense preclinical and clinical evaluation as a vaccine against emergent infectious diseases and cancer [2].
The NYVAC strain was derived from a plaque clone isolate of the Copenhagen vaccinia virus strain (VACV-COP) by the deletion of 18 open reading frames (ORFs) involved in virulence, pathogenesis and host range functions [3]. In spite of its limited replication in human and most mammalian cell types, NYVAC provides a high level of gene expression and induces antigen-specific immune responses when administered to animals and humans [2,4,5,6]. However, the vector still contains other immunomodulatory viral genes that may suppress host immunity, particularly genes encoding proteins that antagonize the innate immune response mediated by Toll-like receptor (TLR) signalling. The deletion of these immunomodulatory genes could be a strategy to further improve NYVAC-based vaccines with the aim to obtain enhanced magnitude, breadth, polyfunctionality and durability of the immune responses.
The sensing of viral pathogens and the subsequent innate immune responses triggered are critical to produce protective immunity. Cells of the innate immune system detect viruses through the recognition of specific pathogen-associated molecular patterns (PAMPs) by pattern recognition receptors (PRRs) [7,8,9,10], among which TLRs are the best characterized [11]. TLR3, TLR7/8 and TLR9 reside predominantly within the endosomes where they recognize viral nucleic acids being involved in the generation of potent antiviral responses [12] while viral glycoprotein products have been shown to interact with TLR2 and TLR4 expressed on the cell surface [13,14]. The implication of TLR2 in the induction of type I IFN in inflammatory monocytes following in vivo infection with VACV has been reported and depletion of these cells leads to elevated levels of VACV in ovaries of mice [15]. TLR2 signalling has also been shown to be important for clonal expansion and memory CD8 T cells formation following VACV infection [16] and in VACV-induced production of proinflammatory cytokines by murine denditic cells (DCs) [17]. The best known role of TLR4 is the detection of lipopolysaccharide (LPS) but this receptor is also involved in the immune response to viruses. For example, TLR4 has been reported to be protective in pulmonary VACV infection since mice deficient for TLR4 signalling showed enhanced viral replication, hypothermia and mortality compared to control animals [18]. Because TLRs are expressed both on specific nonimmune cells, such as epithelial cells at potential sites of entry, and on a variety of immune cells including macrophages, DCs, B cells and certain types of T cells, they play a key role in the defence against pathogens through the induction of proinflammatory cytokines and type I IFNs but also in shaping pathogen-specific humoral and cellular adaptive immune responses.
All TLRs are type I transmembrane glycoprotein receptors comprised of an extracellular N-terminal leucine-rich repeat (LRR) domain involved in ligand binding, a single transmembrane domain and an intracellular C-terminal domain, known as the Toll/IL-1 receptor (TIR) domain, which mediates the interaction and recruitment of various adaptor proteins to activate the downstream signalling pathway [19]. PAMP binding induces receptor homo- or heterodimerization [20,21] and this activated conformation of the receptor triggers the recruitment of TIR domain-containing adaptor proteins that connect downstream signalling molecules leading to the activation of transcription factors such as IFN regulatory factors (IRFs) and NF-κB and the induction of type I IFNs and proinflammatory cytokines, respectively. Ligand recognition by TLRs induces the recruitment of five different adaptor proteins: Myeloid differentiation factor 88 (MyD88), MyD88-adaptor-like (Mal), TIR domain-containing adaptor protein-inducing IFN-β (TRIF), TRIF-related adaptor molecule (TRAM) and sterile α- and armadillo-motif-containing protein (SARM) [22]. Two major pathways can be activated by TLRs: the MyD88-dependent pathway, used by all TLRs except TLR3 [23] and the TRIF-dependent pathway, used by TLR3 and TLR4. TLR4 is the only receptor being able to signal via both pathways due to the differential use of two adaptors, TRAM and Mal. TLR4 uses TRAM to recruit TRIF and induce a type I IFN response via the TRIF-dependent pathway while the use of the coadaptor Mal to recruit MyD88 via the MyD88-dependent pathway induce a proinflammatory response [24]. Crystal structures of the TIR domains of TLR2 [25], TLR10 [26], interleukin-1 receptor accessory protein-like (IL-1RAPL) [27] and Mal [28,29] and NMR structure of the TIR domain of MyD88 [30] have been determined. These studies identified a conserved protruding BB loop between the βB strand and the αB helix, which is essential for functional TLR signalling [31,32,33,34,35].
Viruses have developed strategies to target TLR-mediated signalling to manipulate and evade the host innate immune response [36]. VACV encodes some intracellular negative regulators of TLR signalling including A46 [37], A52 [38], N1 [39], B14 [40], K7 [41] and C6 [42]. A46 was the first virally encoded protein identified to contain a TIR domain [37,43]. Through this domain, A46 binds directly to the TIR domain-containing adaptors MyD88, Mal, TRIF and TRAM, disrupting the formation of Receptor: Adaptor TIR interactions [37] and therefore inhibiting downstream signalling to MAPKs, NF-κB and IRF-3 and interfering with both proinflammatory and type I IFN responses [37]. However, A46 does not interact with SARM, which is a negative regulator of TLR signalling [37]. It has also been shown that A46 protein contributes to virulence since VACV A46R deletion mutant was attenuated in a murine intranasal model [37]. An 11 amino acid peptide derived from A46 (called VIPER) has been reported to specifically inhibit TLR4 responses by directly targeting Mal and TRAM [44] and that A46 binds to Mal via a Bcl-2-like α-helical dimer subdomain [45]. The molecular basis for A46 antagonism of TLR4 has been recently reported [46]. A46 has been shown to impair TLR4 signalling by targeting the conserved BB loop of TIR proteins and thereby disrupting Receptor: Adaptor TIR interactions [46].
Since VACV has been reported to be sensed by TLR2 [15,16,17], TLR4 [18], TLR2-TLR6-MyD88, MDA-5/IPS-1 and NALP3 inflammasome [47] and A46R targets the TIR domain of the adaptors MyD88, Mal, TRIF and TRAM [37], in the present study we have asked to what extent A46R impacts on the immune responses against VACV. This question was addressed with NYCAC-C, an attenuated poxvirus vector expressing HIV-1 Env and Gag-Pol-Nef (GPN) antigens from clade C [48], where A46R was deleted (NYVAC-C-ΔA46R). Specific innate, adaptive and memory immune responses to HIV-1 antigens were evaluated in human macrophages and in a BALB/c mouse model comparing the recombinant virus in the presence or absence of A46R. Our findings provided evidence for an immunomodulatory role of VACV A46 protein.
Results
Generation and in vitro characterization of NYVAC-C-ΔA46R deletion mutant
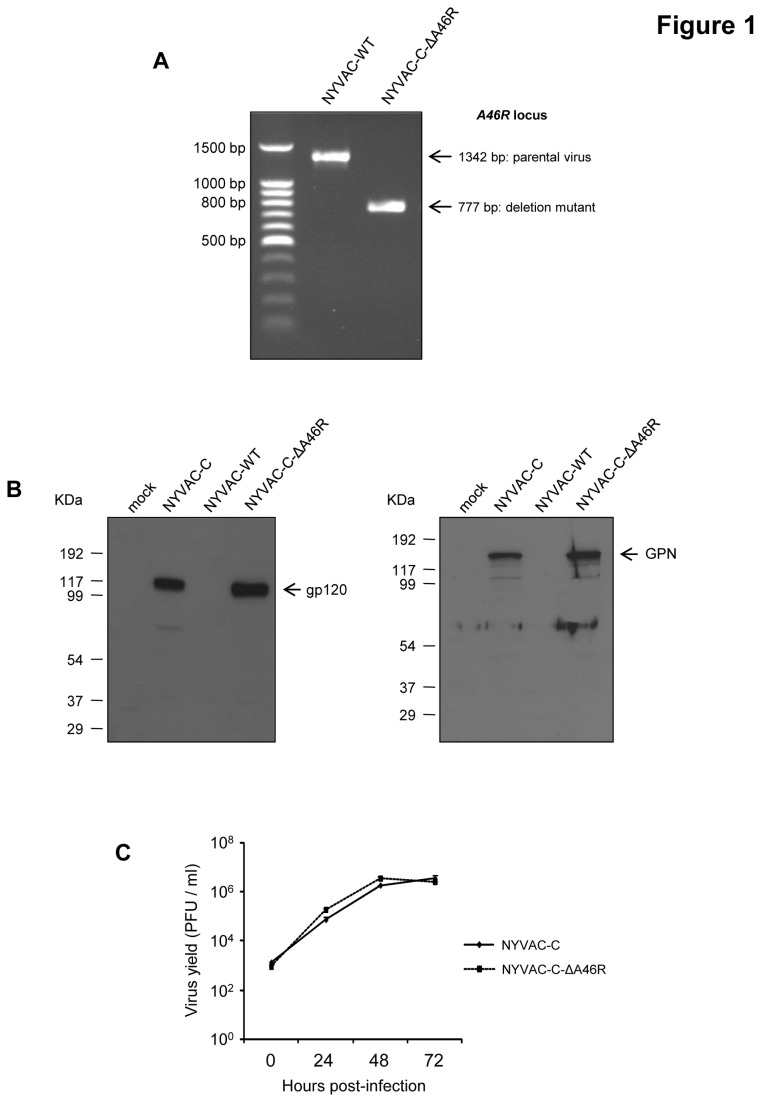
NYVAC-C-ΔA46R deletion mutant was generated as detailed under Materials and Methods using as parental virus the recombinant NYVAC-C that expresses the HIV-1 Env, Gag, Pol and Nef antigens from clade C [48] and following a strategy that allows the deletion of the gene of interest with no fluorescent marker included in the final deletion mutant. The correct deletion of A46R gene was confirmed by PCR using primers annealing in A46R flanking sequences. As shown in Figure 1A, A46R ORF was successfully deleted and no wild-type contamination was present in NYVAC-C-ΔA46R preparation. Analysis by Western-blot confirmed that the A46R deletion mutant expresses the HIV-1 proteins gp120 and GPN at the same level as the parental virus NYVAC-C (Figure 1B). Moreover, analysis by immunostaining showed that all virus plaques have immunoreactivity to anti-WR, anti-gp120 and anti-gag p24 antibodies (data not shown), demonstrating the stability of the antigens expressed by the A46R deletion mutant. To determine if deletion of A46R gene affects virus replication, we compared the growth kinetic of NYVAC-C-ΔA46R deletion mutant with its parental virus NYVAC-C in CEF cells. Figure 1C shows that the growth kinetics were similar between parental and deletion mutant, indicating that A46R gene is not required for virus replication in cultured cells and its deletion does not affect virus growth kinetics.
Figure 1. In vitro characterization of NYVAC-C-ΔA46R deletion mutant.
(A) Confirmation of A46R gene deletion by PCR analysis. Viral DNA was extracted from BSC-40 cells infected with NYVAC-WT or NYVAC-C-ΔA46R at 5 PFU/cell. Primers LFA46R-Aat and RFA46R-Bam spanning A46R flanking sequences were used for PCR analysis of A46R locus. In parental NYVAC, a 1342 bp-product is obtained while in deletion mutant a unique 777 bp-product is observed. (B) Expression of HIV antigens by Western-blot. BSC-40 cells were mock-infected or infected at 5 PFU/cell with NYVAC-WT, NYVAC-C or NYVAC-C-ΔA46R. At 24 hours post-infection, cells were lysed in Laemmli buffer, cells extracts were fractionated by 8% SDS-PAGE and analyzed by Western-blot using a polyclonal anti-gp120 antibody or a polyclonal anti-gag p24 serum to evaluate the expression of gp120 and GPN proteins, respectively. (C) Analysis of virus growth of NYVAC-C-ΔA46R in CEF cells. Monolayers of CEF cells were infected with NYVAC-C or NYVAC-C-ΔA46R at 0.01 PFU/cell. At different times post-infection (0, 24, 48 and 72 hours), cells were collected and infectious viruses were quantified by immunostaining plaque assay in BSC-40 cells.
NYVAC-C-ΔA46R up-regulates TNF, IL-6 and IL-8 production by human macrophages
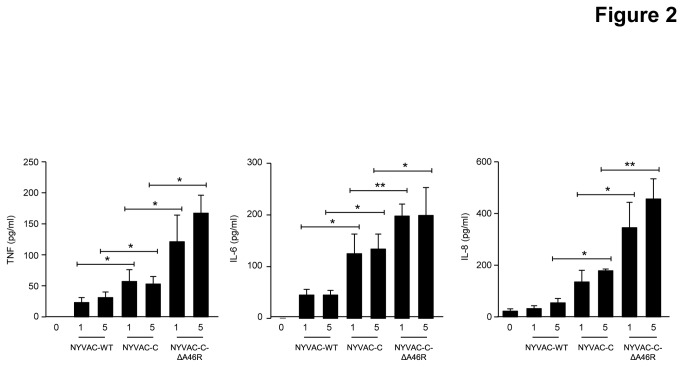
To define whether A46R impairs the response of innate immune cells to NYVAC-C, we measured by ELISA the concentrations of proinflammatory cytokines and chemokines released by human THP-1 macrophages infected for 6 hours with 1 or 5 PFU/cell of NYVAC-WT, NYVAC-C or NYVAC-C-ΔA46R. Compared to NYVAC-WT and to NYVAC-C, the A46R deletion markedly up-regulated the production of TNF, IL-6 and IL-8 by THP-1 cells (Figure 2). Thus, the single deletion of A46R in the NYVAC-C genome triggers a stronger innate immune sensing than NYVAC-C, providing evidence for immune suppression by A46R.
Figure 2. Deletion of A46R gene from NYVAC-C enhances innate immune responses.
Human macrophages were mock-infected (0) or infected with NYVAC-WT, NYVAC-C or NYVAC-C-Δ46R (1 or 5 PFU/cell). 24 hours later, cell-free supernatants were collected to quantify the concentrations of TNF and IL-6 by bioassay and of IL-8 by ELISA. Data are means ± SD of duplicates and are representative of three independent experiments. * p<0.05, ** p<0.005.
Deletion of the viral gene A46R in NYVAC-C induces high, broad and polyfunctional HIV-1-specific T cell adaptive immune responses in BALB/c mice in heterologous prime/boost combination
To assay in vivo the effect of A46R gene deletion on the cellular immunogenicity against HIV-1 antigens, we analyzed the HIV-1-specific T cell adaptive immune responses elicited in mice by using a DNA prime/Poxvirus boost approach since it has been extensively reported that this heterologous immunization protocol is more immunogenic than either component alone to activate T cell responses to HIV-1 antigens [48,49,50].
BALB/c mice, 4 in each group, were immunized as described in Materials and Methods and adaptive T cell immune responses were measured 10 days after the last immunization by polychromatic intracellular cytokine staining (ICS) assay. Splenocytes from immunized animals were stimulated ex vivo for 6 hours with a panel of 464 peptides (15 mers overlapping by 11 amino acids) grouped in three pools: Env (112 peptides), Gag (121 peptides) and GPN (231 peptides) and stained with specific antibodies to identify T cell lineage (CD3, CD4 and CD8), degranulation (CD107a) and responding cells (IL-2, IFN-γ and TNF-α). The percentages of T cells producing IFN-γ and/or IL-2 and/or TNF-α established the overall CD4+ T cell responses whereas the percentages of T cells producing CD107a and/or IFN-γ and/or IL-2 and/or TNF-α determined the overall CD8+ T cell responses.
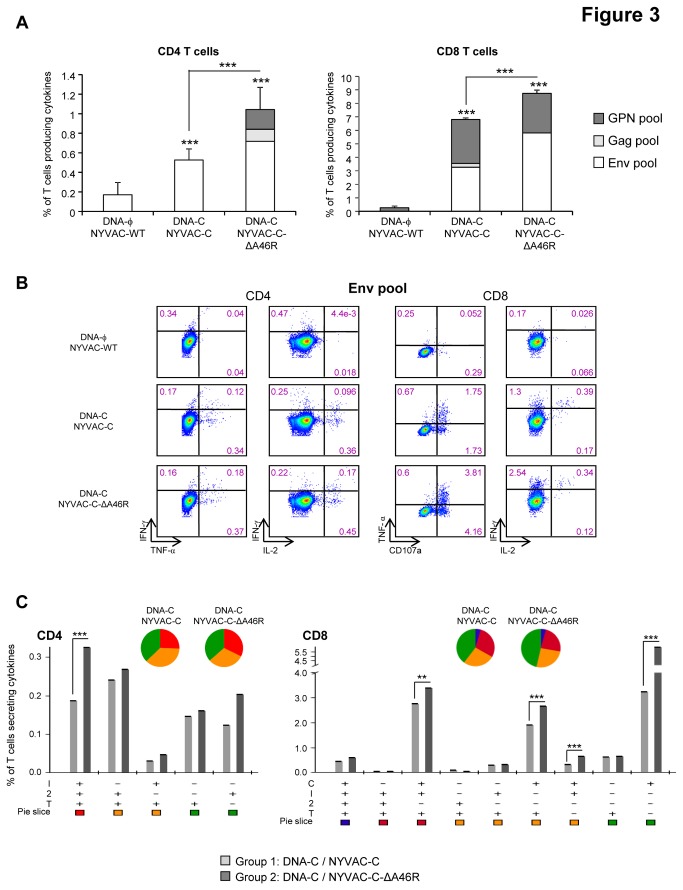
As shown in Figure 3A, in both immunization groups DNA-C/NYVAC-C and DNA-C/NYVAC-C-ΔA46R the magnitudes of the HIV-1-specific CD4 or CD8 T cell responses, determined as the sum of the individual responses obtained for Env, Gag and GPN peptide pools, were significantly higher than those obtained in the control group DNA-ϕ/NYVAC-WT (p<0.001). Furthermore, the magnitudes of the HIV-1-specific CD4 or CD8 T cell responses in the group immunized with NYVAC-C-ΔA46R were significantly higher than those obtained in the group DNA-C/NYVAC-C (p<0.001). In animals immunized with the parental NYVAC-C, the CD4+ T cell response was only directed against the Env pool while in the group boosted with the NYVAC-C-ΔA46R deletion mutant this response was mainly mediated by Env pool but the response against Gag and GPN peptide pools also contributes to the total HIV-1-specific CD4 T cell response. On the other hand, the CD8+ T cell responses were higher in magnitude and A46R gene deletion induced a significant enhancement in the magnitude of the CD8+ T cell responses against the Env pool (p<0.001) whereas the anti-GPN response was maintained. Representative functional profiles of Env-specific CD4 or CD8 T cell responses are shown in Figure 3B.
Figure 3. Adaptive HIV-specific T cell immune responses elicited by A46R deletion mutant in the spleen of BALB/c mice in heterologous prime/boost immunization protocol.
(A) Magnitude of the vaccine-specific CD4 or CD8 T cell response. The HIV-specific CD4 or CD8 T cells were measured 10 days after the last immunization by ICS assay following stimulation of splenocytes derived from immunized animals (n=4) with the different HIV peptide pools. The total value in each group represents the sum of the percentages of CD4+ or CD8+ T cells secreting IFN-γ and/or IL-2 and/or TNF-α (CD4) or CD107a and/or IFN-γ and/or IL-2 and/or TNF-α (CD8) against all HIV peptide pools. All data are background-subtracted. *** p<0.001. p value indicates significantly higher responses compared to parental group or between DNA-C/NYVAC-C-ΔA46R and DNA-C/NYVAC-C immunization groups. (B) Flow cytometry profiles of vaccine-induced CD4 or CD8 T cell responses against Env pool. (C) Functional profile of the adaptive HIV-specific CD4 or CD8 T cell response in the different immunization groups. The possible combinations of the responses are shown on the x axis, whereas the percentages of the functionally distinct cell populations within the total CD4 or CD8 T cell population are shown on the y axis. Combinations that did not contribute significantly to the functional profile are not shown. Responses are grouped and colour-coded on the basis of the number of functions. The non-specific responses obtained in the control group DNA-ϕ/NYVAC-WT were subtracted in all populations. ** p<0.005, *** p<0.001. p values indicate significantly higher responses compared to DNA-C/NYVAC-C immunization group.
The quality of a T cell response can be characterized in part by the pattern of cytokine production and by the cytotoxic potential. On the basis of the analysis of IFN-γ, IL-2 and TNF-α secretion, as well as the study of CD107a expression on the surface of activated T cells as an indirect marker of cytotoxicity, 8 HIV-specific CD4 T cell populations and 16 HIV-specific CD8 T cell populations were identified. Vaccine-induced CD4 T cell responses were highly polyfunctional in both DNA-C/NYVAC-C and DNA-C/NYVAC-C-ΔA46R groups, with more than 60% of CD4 T cells exhibiting two or three functions. CD4 T cells producing IFN-γ+IL-2+TNF-α, IL-2+TNF-α or only TNF-α or IL-2 were the most representative populations induced by the parental NYVAC-C and the A46R deletion mutant, although the percentages of cells producing cytokines were low (Figure 3C). The HIV-1-specific CD8 T cell responses, higher in magnitude, were also polyfunctional in both immunization groups, with more than 50% of CD8+ T cells exhibiting two, three or four functions. CD8+ T cells producing CD107a+ IFN-γ+TNF-α, CD107a+ TNF-α or only CD107a were the most representative populations induced by the parental NYVAC-C and NYVAC-C-ΔA46R deletion mutant (Figure 3C).
Overall, these results indicate that deletion of A46R gene from NYVAC-C genome improved the magnitude of the HIV-1-specific adaptive CD4 and CD8 T cell immune responses and maintained the polyfunctional profile observed with the parental NYVAC-C. Since the contribution of DNA priming is the same for NYVAC-C and NYVAC-C-ΔA46R immunization groups, the differences observed should be attributed to the A46R deletion.
Deletion of the viral gene A46R impacts on the HIV-1-specific CD8 T cell memory phase of the immune response
Phenotypic analysis of memory vaccine-induced T cell immune responses was performed by polychromatic ICS assay 53 days after the last immunization. Splenocytes from immunized mice were stimulated ex vivo for 6 hours with the HIV-1 peptide pools Env, Gag and GPN and stained with specific antibodies to identify T cell lineage (CD3, CD4 and CD8), degranulation (CD107a), responding cells (IL-2, IFN-γ and TNF-α) as well as memory stages (CD127 and CD62L).
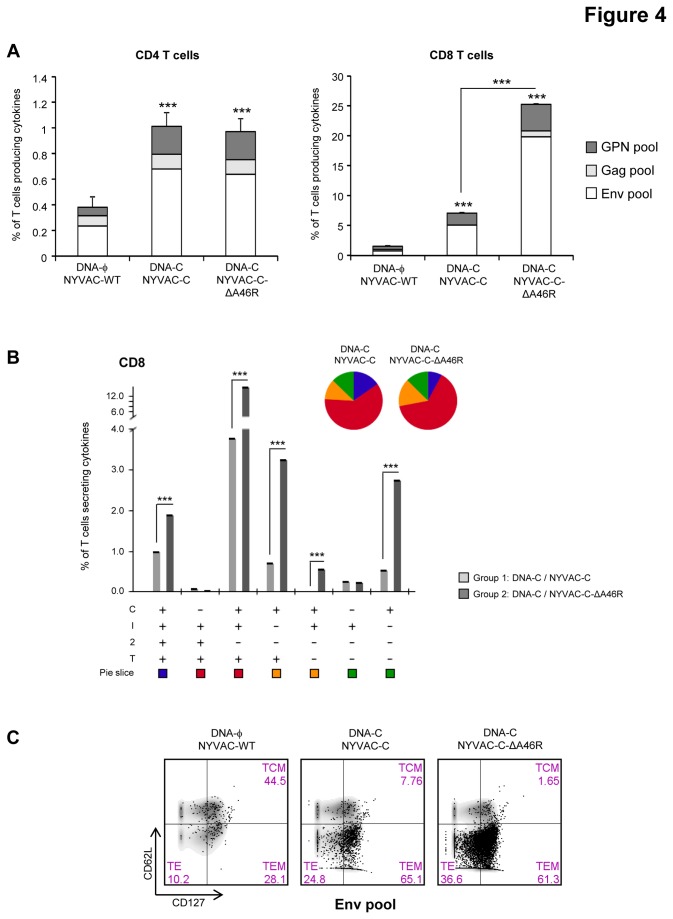
The magnitudes of the memory HIV-1-specific CD4 or CD8 T cell responses, determined as the sum of the individual responses obtained for Env, Gag and GPN peptide pools, were significantly higher in the groups boosted with the parental NYVAC-C or with the NYVAC-C-ΔA46R deletion mutant than in the control group immunized with NYVAC-WT (p<0.001) (Figure 4A).
Figure 4. Memory HIV-specific T cell immune responses elicited by A46R deletion mutant in the spleen of BALB/c mice after prime/boost immunization.
(A) Magnitude of the vaccine-specific CD4 or CD8 T cell responses. The HIV-specific CD4 or CD8 T cells were measured 53 days after the last immunization by ICS assay following stimulation of splenocytes derived from immunized animals (n=4) with the different HIV peptide pools. The total value in each group represents the sum of the percentages of CD4+ or CD8+ T cells secreting IFN-γ and/or IL-2 and/or TNF-α (CD4) or CD107a and/or IFN-γ and/or IL-2 and/or TNF-α (CD8) against all HIV peptide pools. All data are background-subtracted. *** p<0.001. p value indicates significantly higher responses compared to parental group or between DNA-C/NYVAC-C and DNA-C/NYVAC-C-ΔA46R immunization groups. (B) Functional profile of the memory HIV-specific CD8 T cell response in the different immunization groups. The possible combinations of the responses are shown on the x axis, whereas the percentages of the functionally distinct cell populations within the total CD8 T cell population are shown on the y axis. Combinations that did not contribute significantly to the functional profile are not shown. Responses are grouped and colour-coded on the basis of the number of functions. *** p<0.001. p values indicate significantly higher responses compared to DNA-C/NYVAC-C immunization group. (C) Phenotypic profile of memory HIV-specific CD8 T cells. Representative FACS plots showing the percentage of Env-specific CD8 T cells with central memory (TCM; CD127+CD62L+), effector memory (TEM; CD127+CD62L-) or effector (TE; CD127-CD62L-) phenotype.
The magnitude of the HIV-1-specific CD4 T cell response in the group immunized with DNA-C/NYVAC-C-ΔA46R was similar to that obtained in the group DNA-C/NYVAC-C and in both cases it was mainly directed against Env. On the other hand, the CD8+ T cell responses were higher in magnitude and A46R gene deletion clearly induced a significant enhancement in the magnitude of the CD8+ T cell responses against Env and GPN (p<0.001). Representative functional profiles of Env-induced CD8 T cell responses are shown in Figure S1.
HIV-specific CD8 T cell responses were polyfunctional in both immunization groups with 75% of CD8 T cells exhibiting two, three or four functions. CD8 T cells producing CD107a+ IFN-γ+TNF-α, CD107a+ IFN-γ+IL-2+TNF-α, CD107a+ TNF-α or only CD107a were the most representative populations induced (Figure 4B).
Since previous studies have shown that CD127 and CD62L define functionally distinct populations of memory antigen-specific T cells [51], we characterized the differentiation stages of the responding CD8 T cells into central memory (TCM; CD127+CD62L+), effector memory (TEM; CD127+CD62L-) or effector (TE; CD127-CD62L-) populations. As shown in Figure 4C, about 60% of the HIV-specific CD8 T cells were of TEM phenotype in the DNA-C/NYVAC-C and DNA-C/NYVAC-C-ΔA46R groups.
Overall, these results indicate that deletion of A46R gene from NYVAC-C genome improved the magnitude of the HIV-1-specific memory CD8 T cell immune response and maintained the polyfunctional profile and memory differentiation pattern observed with the parental NYVAC-C.
Deletion of the viral gene A46R in NYVAC-C enhances the anti-gp120 humoral response during the memory phase
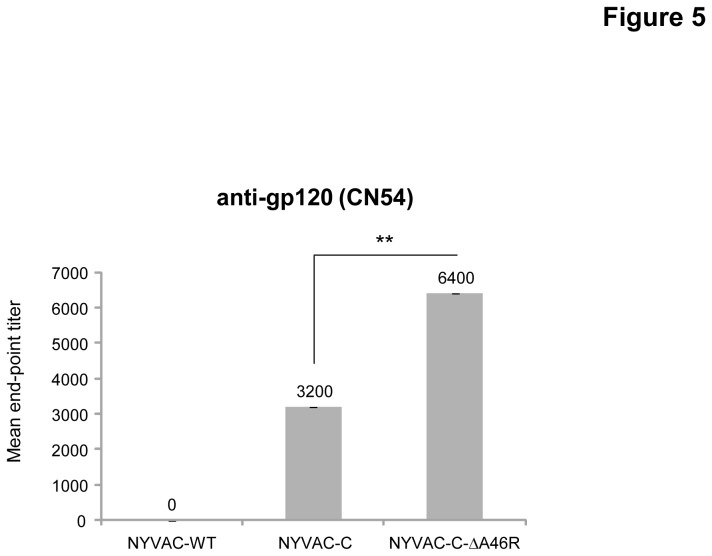
Since cells infected with NYVAC-C release monomeric gp120 [48], we also evaluated the impact of the deletion of viral gene A46R on the humoral response at day 68. We quantified by ELISA the Env-specific IgG titers against the purified gp120 protein from the HIV-1 isolate CN54 (clade C). As shown in Figure 5, the IgG titer obtained in the pool of sera of animals immunized with NYVAC-C-ΔA46R is significantly higher (p<0.005) than the titer obtained in the sera of animals immunized with NYVAC-C indicating that deletion of the viral gene A46R enhances the humoral response induced in mice during the memory phase.
Figure 5. Memory humoral immune response elicited by the A46R deletion mutant against HIV-1 gp120 protein.
Levels of Env-specific IgG binding antibodies were measured in serum from naïve and immunized mice at day 68. The values represent the mean antibodies titer for each group. ** p<0.005.
Deletion of the viral gene A46R impacts on the anti-vector CD8 T cell adaptive and memory phases of the immune response
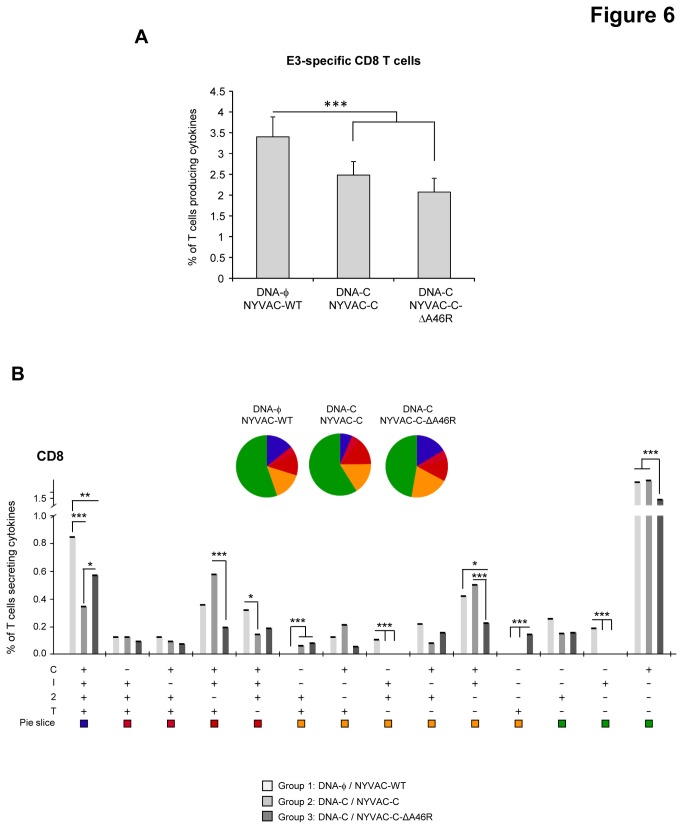
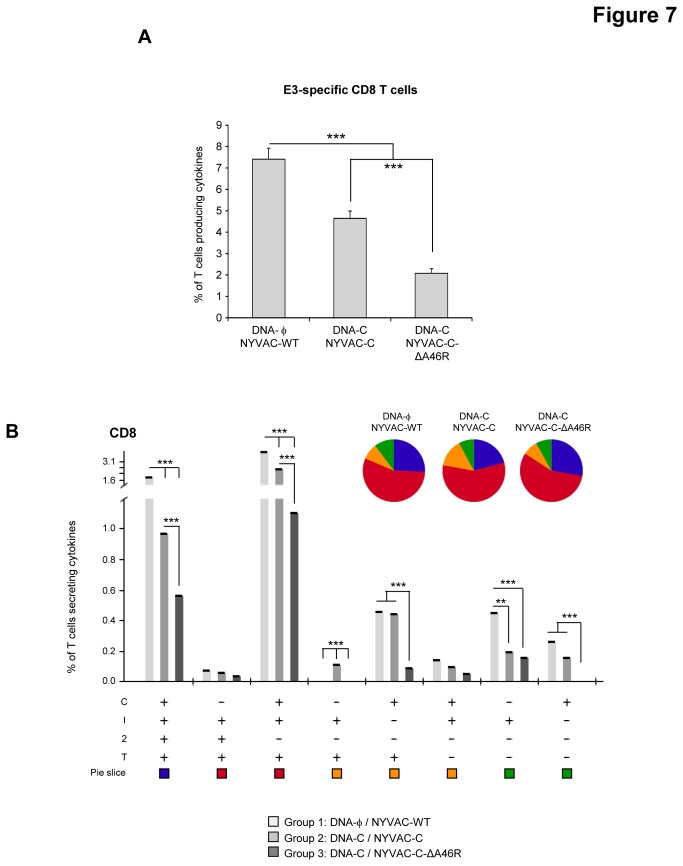
Vaccine-induced anti-vector T cell immune response was measured 10 and 53 days after the last immunization by polychromatic ICS assay. Splenocytes from immunized animals were stimulated ex vivo for 6 hours with VACV E3 peptide, which is specific for CD8 T cells [52]. During the adaptive phase of the immune response, the magnitude of the E3-specific CD8 T cell response was significantly lower in the DNA-C/NYVAC-C and DNA-C/NYVAC-C-ΔA46R immunized groups than in the control group immunized with DNA-ϕ/NYVAC-WT (p<0.001) (Figure 6A). No statistical differences were observed between the DNA-C/NYVAC-C and DNA-C/NYVAC-C-ΔA46R groups. E3-specific CD8 T cell responses were polyfunctional in all the immunization groups with almost 50% of CD8+ T cells exhibiting two, three or four functions. CD8 T cells producing only CD107a were the most representative population induced (Figure 6B). During the memory phase, the magnitude of the E3-specific CD8 T cell response in both immunization groups DNA-C/NYVAC-C and DNA-C/NYVAC-C-ΔA46R was significantly lower than that obtained in the control group DNA-ϕ/NYVAC-WT (p<0.001) and the magnitude of the E3-specific CD8 T cell response observed in the group DNA-C/NYVAC-C-ΔA46R was significantly lower than that obtained in the group DNA-C/NYVAC-C (p<0.001) (Figure 7A). E3-specific CD8 T cell responses were polyfunctional in all the immunization groups with almost 90% of CD8+ T cells exhibiting two, three or four functions. CD8 T cells producing CD107a+ IFN-γ+TNF-α or CD107a+ IFN-γ+IL-2+TNF-α were the most representative populations induced (Figure 7B). Overall, these results indicate that deletion of A46R gene from NYVAC-C genome reduced the magnitude of the VACV E3-specific adaptive and memory CD8 T cell immune response but maintained the polyfunctional profile observed with the parental NYVAC-C. Since adaptive and memory immune responses to HIV antigens were enhanced by the A46R deletion mutant (Figures 3 and 4), the reduced T cell immune response induced by the E3 peptide indicates an immunodominance of HIV antigens.
Figure 6. Adaptive VACV vector-specific T cell immune responses elicited by A46R deletion mutant in the spleen of BALB/c mice after prime/boost immunization.
(A) Magnitude of the VACV-specific CD8 T cell response. The VACV-specific CD8 T cells were measured 10 days after the last immunization by ICS assay following stimulation of splenocytes derived from immunized animals (n=4) with VACV E3 peptide. The total value in each group represents the sum of the percentages of CD8+ T cells secreting CD107a and/or IFN-γ and/or IL-2 and/or TNF-α against E3 peptide. All data are background-subtracted. *** p<0.001. p value indicates significantly higher response compared to DNA-C/NYVAC-C and DNA-C/NYVAC-C-ΔA46R immunization groups. (B) Functional profile of the VACV-specific CD8 T cell response in the different immunization groups. The possible combinations of the responses are shown on the x axis, whereas the percentages of the functionally distinct cell populations within the total CD8 T cell population are shown on the y axis. Combinations that did not contribute significantly to the functional profile are not shown. Responses are grouped and colour-coded on the basis of the number of functions. * p<0.05, ** p<0.005, *** p<0.001.
Figure 7. Memory VACV vector-specific T cell immune responses elicited by A46R deletion mutant in the spleen of BALB/c mice after prime/boost immunization.
(A) Magnitude of the VACV-specific CD8 T cell response. The VACV-specific CD8 T cells were measured 53 days after the last immunization by ICS assay following stimulation of splenocytes derived from immunized animals (n=4) with VACV E3 peptide. The total value in each group represents the sum of the percentages of CD8+ T cells secreting CD107a and/or IFN-γ and/or IL-2 and/or TNF-α against E3 peptide. All data are background-subtracted. *** p<0.001. p values indicate significantly higher response compared to DNA-C/NYVAC-C and DNA-C/NYVAC-C-ΔA46R immunization groups or between DNA-C/NYVAC-C and DNA-C/NYVAC-C-ΔA46R groups. (B) Functional profile of the VACV-specific CD8 T cell response in the different immunization groups. The possible combinations of the responses are shown on the x axis, whereas the percentages of the functionally distinct cell populations within the total CD8 T cell population are shown on the y axis. Combinations that did not contribute significantly to the functional profile are not shown. Responses are grouped and colour-coded on the basis of the number of functions. ** p<0.005, *** p<0.001.
Discussion
Development of non-replicating VACV vectors with enhanced immunogenicity against foreign expressed antigens is a major goal in the poxvirus field, aiming at the application of these vectors as HIV/AIDS vaccines. This is in view of the restricted immunogenicity triggered in clinical trials by the parental vectors expressing HIV antigens, like MVA, NYVAC, canarypox and fowlpox [53]. In fact, the reduced efficacy against HIV infection, 31.2%, of the non-replicating canary poxvirus vector combined with gp120 protein in the RV144 clinical trial [1], highlighted the need of novel poxvirus vectors with improved immunogenicity. With regard to non-replicating poxvirus vectors, different strategies have been pursued to enhance their potency, like the combination of heterologous vectors, use of co-stimulatory molecules and disruption of viral genes encoding immunosuppressive molecules [53]. The latter strategy provides the additional advantage that the immunomodulatory role of a viral gene can be easily quantified in an organism.
A number of MVA deletion mutants in viral immune modulators have been generated to date and tested in mice [54,55,56,57,58] and macaques [59,60]. These studies have shown that MVA recombinant viruses with a single deletion of viral genes encoding inhibitors of type 1 IFN signalling pathway (C6L [55]), apoptosis (F1L [56]), IL-18 binding protein (C12L [57]) or the uracyl-DNA glycosylase gene (UDG [60]), enhanced the overall immune responses to HIV-1 antigens. The HIV-1-specific CD4 and CD8 T cell immune responses were further increased by MVA vectors with deletions of two (A41L/B16R [54]; or C6L/K7R; Garcia-Arriaza, submitted) or four [IL-18 binding protein (MVA008L; C12L), Toll/IL-1 receptor homolog (MVA159R; A46R), CC-chemokine binding protein (MVA153L; B7R) and secreted IL-1β receptor (MVA184R; B16R)] immunomodulatory genes [59], while an additional fifth deletion of the uracyl-DNA glycosylase gene (MVA101R) decreased the responses [59]. Similarly, NYVAC vectors with single or double deletions in VACV genes B19R and B8R encoding type I and type II IFN binding proteins, respectively, increased the immune responses to HIV antigens in the mouse model [61].
In an effort to uncover the role of VACV genes as immune modulators and search for potential applications of these vectors in the development of optimized vaccines, in this investigation we showed that deletion of the viral TLR inhibitor A46R gene in the NYVAC-C genome has no effect on the replication capacity of the virus in CEF cells but triggers expression of immunoregulatory genes in infected macrophages. NYVAC-C also enhances, though to a lesser extent, the production of these proinflammatory cytokines and chemokines compared to NYVAC-WT indicating that the expression of HIV-1 antigens has an effect on innate immune cells. The impact on antigen-presenting cells of the expression of HIV antigens from an attenuated poxvirus vector has been previously reported by using microarray technology in human dendritic cells infected with an MVA-based recombinant virus expressing gp120 and GPN from clade B [62].
Significantly, in mice immunized following a DNA prime/NYVAC boost protocol, the deletion mutant NYVAC-C-ΔA46R enhanced HIV-specific T cell immune responses. Both CD4 and CD8 T cells specific for HIV antigens were activated. In the adaptive phase, the magnitudes of the HIV-1-specific CD4 or CD8 T cell responses in the group immunized with NYVAC-C-ΔA46R were significantly higher than those obtained in the group DNA-C/NYVAC-C (p<0.001), maintaining the polyfunctional profile observed with the parental NYVAC-C. In the memory phase, deletion of A46R gene from NYVAC-C genome improved again the magnitude of the HIV-1-specific memory CD8 T cell immune response, while both the polyfunctional profile and memory differentiation pattern observed were similar as those obtained with the parental NYVAC-C. The main phenotype of the memory response was TEM, which is of immunological relevance as this phenotype has been correlated with protection in the macaque-SIV model [63,64].
This enhanced HIV-specific T cell immune response is in contrast with the lack or reduced effect of A46R deletion on VACV E3-specific T cell responses during adaptive or memory phases of the immune response, respectively (Figures 6 and 7). The absence or decrease of immune stimulatory effect observed when E3 was used to stimulate mouse splenocytes in comparison with the increased responses against HIV antigens is likely to be related to the immune dominance of the HIV antigens versus the viral E3 peptide and such immunodominance may be due to the effect of the priming with a DNA encoding the HIV-1 antigens and also to the fact that NYVAC-C expresses E3 under its natural early promoter while the HIV antigens are expressed at early and late times from a strong synthetic early/late promoter. Since both explanations can be applied to NYVAC-C or NYVAC-C-ΔA46R-induced immune responses, the lower E3-specific CD8 T cell adaptive and memory immune responses elicited by NYVAC-C-ΔA46R deletion mutant compared with that induced by NYVAC-C are inversely correlated with the higher HIV-1-specific CD8 T cell responses triggered by the A46R deletion mutant. A similar trend for E3 response in relation to foreign expressed antigens has been observed for other recombinant VACV vectors [65]. Therefore, a reduction of immune responses to NYVAC-C-ΔA46R vector antigens has the additional vaccine advantage that HIV antigens are favoured over viral antigens, thus enhancing the specific immune responses to HIV.
Since humoral response against HIV antigens has been described to be important for protection against HIV acquisition [1], we also evaluated the presence of anti-gp120 antibodies in the serum of immunized animals. This analysis showed an enhanced anti-gp120 humoral response in the mice immunized with NYVAC-C-ΔA46R deletion mutant suggesting that the deletion of A46R gene is also able to modulate positively the humoral response against gp120.
How deletion of A46R impacts on the immune response of NYVAC-C? As previously described, A46 impairs TLR signalling by targeting the TIR domain of the adaptors MyD88, Mal, TRIF and TRAM disrupting Receptor: Adaptor TIR interactions [37]. Hence, deleting A46R in NYVAC restores TLR signalling upon viral infection, enhancing the expression of proinflammatory molecules, which in turn will enhance T cell activation. According to the intraperitoneal route used in the present study, the effect of A46R gene deletion on immunogenicity against HIV-1 antigens should be explained by the effect of TLR signalling restoration in the cell types present in the peritoneal cavity (mainly B cells, macrophages and granulocytes and, to a lesser extent, T cells [66]). In this context, the increased secretion of proinflammatory cytokines and chemokines by NYVAC-C-ΔA46R-infected macrophages could induce an enhanced recruitment of immature DCs and lymphocytes, generating an appropriate environment for the uptake and presentation of HIV-1 antigens to T cells. Immature NYVAC-C-ΔA46R-infected DCs can also migrate to the lymph nodes, maturing in route, and activate HIV-1-specific T cells enhancing the overall immunogenicity against HIV antigens. According to this, it has been previously reported that the total number of cells in the lungs of mice immunized intranasally with a VACV A46R deletion mutant was increased on day 2 post-infection compared with parental virus whereas on days 5 and 8 was reduced [37]. Since the main innate sensors of VACV vectors are TLR2 [15,16,17], TLR2-TLR6-MyD88, MDA-5/IPS-1 and NALP3 inflammasome [47] and A46R targets the TIR domain of the adaptors MyD88, Mal, TRIF and TRAM [37], our findings of enhanced production of TNF, IL-6 and IL-8 in conjunction with an increase in the magnitude of CD4 and CD8 T cell immune responses to HIV antigens and an enhanced gp120-specific humoral response, reveal that A46R plays an important role as immune modulator. This observation, in combination with the biochemical data on the mode of action of A46, establishes the immunological role of VACV A46R on T and B cell responses.
Materials and Methods
Ethics statement
The animal studies were approved by the Ethical Committee of Animal Experimentation (CEEA-CNB) of Centro Nacional de Biotecnologia (CNB-CSIC, Madrid, Spain) in accordance with national and international guidelines and with the Royal Decree (RD 1201/2005) (Permit numbers: 152/07 and 080030).
Cells and viruses
African green monkey kidney cells (BSC-40; American Type Culture Collection, Manassas, VA) and primary chicken embryo fibroblast cells (CEF; Intervet, s.a, Salamanca, Spain) were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 100 IU/ml of penicillin, 100 µg/ml of streptomycin and 10% newborn calf serum (NCS) for BSC-40 cells or 10% fetal calf serum (FCS) for CEF cells. The human monocytic THP-1 cells (American Type Culture Collection, Manassas, VA) were cultured in RPMI 1640 medium containing 2 mM L-glutamine, 50 µM 2-mercaptoethanol, 100 IU/ml of penicillin, 100 µg/ml of streptomycin and 10% FCS. Cells were maintained in a humidified air 5% CO2 atmosphere at 37°C. The poxvirus strains used in this work included the genetically attenuated vaccinia virus-based vector NYVAC-WT and the recombinant NYVAC-C expressing gp120 as a cell-released product and Gag-Pol-Nef as an intracellular polyprotein from the clade C CN54 HIV-1 isolate [48], used as the parental vector for the generation of the A46R deletion mutant. Virus infections were performed with 2% NCS or FCS. All viruses were grown in primary CEF cells, similarly purified through two 36% (w/v) sucrose cushions and the virus titers were determined by immunostaining plaque assay in BSC-40 cells as previously described [67]. The titer determinations of the different viruses were performed at least three times.
Construction of plasmid transfer vector pGem-RG-A46R wm
The plasmid transfer vector pGem-RG-A46R wm, used for the construction of the recombinant virus NYVAC-C-ΔA46R, with A46R ORF deleted, was obtained by the sequential cloning of A46R recombination flanking sequences into the plasmid pGem-Red-GFP wm, containing dsRed2 and rsGFP genes as fluorescent markers, and previously described [68]. NYVAC genome was used as the template to amplify the left flank of A46R gene (432 bp) with oligonucleotides LFA46R-Aat (5´-CACGATGACGTCAGAGGAGTTAT-3´) (AatII site underlined) and LFA46R-Xba (5´-CGTATGTCTAGATTATTTTGCTGAG-3´) (XbaI site underlined). This left flank was digested with AatII and XbaI and cloned into plasmid pGem-Red-GFP wm previously digested with the same restriction enzymes to generate pGem-RG-LFsA46R wm (4939 bp). The repeated left flank of A46R gene (432 bp) was amplified by PCR from NYVAC genome with oligonucleotides LFA46R’-Eco (5´-CACGATGAATTCAGAGGAGTTAT-3´) (EcoRI site underlined) and LFA46R’-Cla (5´-CGTATGATCGATT TATTTTGCTGAG-3´) (Cla I site underlined), digested with EcoRI and Cla I and inserted into the EcoRI / Cla I-digested pGem-RG-LFsA46R wm to generate pGem-RG-LFdA46R wm (5330 bp). The right flank of A46R gene (360 bp) was amplified by PCR from NYVAC genome with oligonucleotides RFA46R-Cla (5´-CTGAGAATCGATAGGATGAATTTG-3´) (Cla I site underlined) and RFA46R-Bam (5´-ATTTAAGGATCCAGAACGGCAAC-3´) (BamHI site underlined), digested with Cla I and BamHI and inserted into the Cla I / BamHI-digested pGem-RG-LFdA46R wm. The resulting plasmid pGem-RG-A46R wm (5660 bp; Figure S2) was confirmed by DNA sequence analysis and directs the deletion of A46R gene from NYVAC-C genome.
Construction of NYVAC-C-ΔA46R deletion mutant
The deletion mutant NYVAC-C-ΔA46R was constructed using dsRed2 and rsGFP as fluorescent markers. 3 x 106 BSC-40 cells were infected with 0.01 PFU/cell of NYVAC-C and transfected 1 hour later with 6 µg DNA of plasmid pGem-RG-A46R wm using Lipofectamine (Invitrogen) according to the manufacturer’s recommendations. Forty-eight hours post-infection, the cells were harvested, lysed by freeze-thaw cycling, sonicated and used for recombinant virus screening. Deletion mutant was selected from progeny virus by consecutive rounds of plaque purification in BSC-40 cells during which plaques were screened for Red2/GFP fluorescence. In the first three passages, viruses from selected plaques expressed both fluorescent proteins, while in the next two passages viral progeny from selected plaques expressed only one fluorescent marker (Red2). In the last two passages (seven passages in total), viruses from selected plaques do not express any marker due to the loss of the fluorescent marker by homologous recombination within the repeated flanking DNA sequences. The resulting NYVAC-C-ΔA46R virus was expanded in BSC-40 cells and the crude preparation obtained was used for the propagation of the virus in large cultures of primary chicken fibroblasts (CEF) followed by virus purification through two 36% (w/v) sucrose cushions and titrated by immunoplaque assay in BSC-40 cells.
PCR analysis of NYVAC-C-ΔA46R deletion mutant
To test the identity and purity of the recombinant virus NYVAC-C-ΔA46R, viral DNA was extracted from BSC-40 cells infected at 5 PFU/cell with NYVAC-WT or NYVAC-C-ΔA46R. Cell membranes were disrupted using sodium dodecyl sulphate (SDS) followed by proteinase K treatment (0.2 mg/ml proteinase K in 50 mM Tris-HCl pH 8, 100 mM EDTA pH 8, 100 mM NaCl and 1% SDS for 1 hour at 55°C) and phenol extraction of viral DNA. Primers LFA46R-Aat and RFA46R-Bam spanning A46R flanking regions were used for PCR analysis of A46R locus. The amplification reactions were carried out with Platinum Taq DNA polymerase (Invitrogen) according to the manufacturer’s recommendations. The correct sequence of deleted A46R locus was confirmed by DNA sequence analysis.
Expression of HIV-1 proteins gp120 and GPN
To test the correct expression of HIV-1 antigens by the A46R deletion mutant, monolayers of BSC-40 cells were mock-infected or infected at 5 PFU/cell with NYVAC-WT, NYVAC-C or NYVAC-C-ΔA46R. At 24 hours post-infection, cells were lysed in Laemmli buffer, cells extracts fractionated by 8% SDS-PAGE and analyzed by Western-blot using the polyclonal anti-gp120 antibody (Centro Nacional de Biotecnología; diluted 1:3000) or the polyclonal anti-gag p24 serum (ARP 432, NIBSC, Centralised Facility for AIDS reagent, UK; diluted 1:1000) to evaluate the expression of gp120 and GPN proteins, respectively. The anti-rabbit-HRPO (SIGMA; diluted 1:5000) was used as secondary antibody. The immunocomplexes were detected by enhanced chemiluminescence (ECL, GE Healthcare).
Analysis of virus growth
To determine virus growth profiles, monolayers of CEF cells grown in 12-well plates were infected in duplicate at 0.01 PFU/cell with NYCAC-C or NYVAC-C-ΔA46R deletion mutant. Following virus adsorption for 60 min at 37°C, the inoculum was removed. The infected cells were washed once with DMEM without serum and incubated with fresh DMEM containing 2% FCS at 37°C in a 5% CO2 atmosphere. At different times post-infection (0, 24, 48 and 72 hours), cells were harvested by scraping (lysates at 5 x 105 cells/ml), freeze-thawed three times and briefly sonicated. Virus titers in cell lysates were determined by immunostaining plaque assay in BSC-40 cells using rabbit polyclonal anti-vaccinia virus strain WR (Centro Nacional de Biotecnología; diluted 1:1000), followed by anti-rabbit-HRPO (SIGMA; diluted 1:1000).
Measurement of cytokine production by macrophages
THP-1 cells were differentiated into macrophages by treatment with 0.5 mM phorbol 12-myristate 13-acetate (PMA, Sigma-Aldrich) for 24 h. The medium was changed and cells were either mock-infected or infected with 1 or 5 PFU/cell of NYVAC-WT, NYVAC-C or NYVAC-C-Δ46R. Cell-free supernatants were collected after 6 hours to quantify the concentrations of TNF, IL-6 and IL-8. The concentrations of human IL-8 (BD Biosciences) in cell-culture supernatants were measured by ELISA as previously described [47]. TNF and IL-6 concentrations were measured by bioassay as described elsewhere [69].
DNA vectors
The two DNA constructs expressing the HIV-1 CN54gp120 (pcDNA- CN54gp120) and HIV-1 CN54Gag-Pol-Nef (GPN) polyprotein (pcDNA- CN54GPN) have been previously reported [48]. Plasmids were purified using Maxi-prep purification kits (Qiagen) and diluted for injection in endotoxin-free PBS.
Peptides
The HIV-1 peptide pools Gag-1, Gag-2, Env-1, Env-2, GPN-1, GPN-2, GPN-3 and NEF were provided by the EuroVacc Foundation and were previously described [48]. They spanned the HIV-1 Env, Gag, Pol and Nef antigens from clade C included in the immunogens as consecutive 15-mers overlapping by 11 amino acids. For immunological analyses we grouped the pools as follows: Env pool (Env-1+Env-2), Gag pool (Gag-1+Gag-2) and GPN pool (GPN-1+GPN-2+GPN-3+NEF). The VACV E3140-148 peptide (VGPSNSPTF; CNB), previously described as immunodominant epitope in BALB/c mice [52], was used to detect the anti-vector cellular immune response.
Mouse immunization schedule
BALB/c mice (6-8 weeks old) were purchased from Harlan. For the heterologous DNA prime/NYVAC boost immunization protocol performed to assay the immunogenicity of NYVAC-C-ΔA46R deletion mutant, groups of animals (n=8) received 100 µg of DNA-C (50 µg of pcDNA- CN54gp120 + 50 µg of pcDNA- CN54GPN) or 100 µg of DNA-ϕ (100 µg of pcDNA) by intramuscular route (i.m.). Two weeks later, animals were immunized with 1 x 107 PFU of NYVAC-WT, NYVAC-C or NYVAC-C-ΔA46R by intraperitoneal route (i.p.). Mice immunized with sham DNA (DNA-ϕ) followed by NYVAC-WT boost were used as control group. At 10 and 53 days after the last immunization, 4 mice in each group were sacrificed and spleens processed for Intracellular Cytokine Staining (ICS) assay to measure the adaptive and memory cellular immune responses against HIV-1 antigens, respectively. Two independent experiments have been performed for the different groups.
Intracellular Cytokine Staining assay (ICS)
The magnitude, polyfunctionality and phenotype of the HIV-specific T cell responses were analyzed by ICS. After an overnight rest, 4 x 106 splenocytes (depleted of red blood cells) were seeded on 96-well plates and stimulated during 6 hours in complete RPMI 1640 media supplemented with 10% FCS containing 1 µl/ml GolgiPlug (BD Biosciences), anti-CD107a-Alexa 488 (BD Biosciences) and 5 µg/ml of the different HIV peptide pools. At the end of the stimulation period, cells were washed, stained for the surface markers, fixed and permeabilized (Cytofix/Cytoperm Kit; BD Biosciences) and stained intracellularly using the appropriate fluorochromes. Dead cells were excluded using the violet LIVE/DEAD stain kit (Invitrogen). For functional analyses the following fluorochrome-conjugated antibodies were used: CD3-PE-CF594, CD4-APC-Cy7, CD8-V500, IFN-γ-PE-Cy7, IL-2-APC and TNF-α-PE (all from BD Biosciences). In addition, for phenotypic analyses the following antibodies were used: CD62L-Alexa 700 (BD Biosciences) and CD127-PerCP-Cy5.5 (eBioscience). Cells were acquired using a GALLIOS flow cytometer (Beckman Coulter). Analyses of the data were performed using the FlowJo software version 8.5.3 (Tree Star, Ashland, OR). The number of lymphocyte-gated events ranged between 1 x 105 and 1 x 106. After gating, Boolean combinations of single functional gates were then created using FlowJo software to determine the frequency of each response based on all possible combinations of cytokine expression or all possible combinations of differentiation marker expression. For each population, background responses detected in the non-stimulated control samples were subtracted from those detected in stimulated samples for every specific functional combination and the percentages of cells producing cytokines obtained in the DNA-ϕ/NYVAC-WT control populations were also subtracted in all the groups in order to remove the non-specific responses detected as background. Only positive responses are represented.
Antibody measurement by ELISA
Binding antibodies to Env protein in serum were determined by enzyme-linked immunosorbent assay (ELISA) as previously described [48]. Serum samples from naïve and immunized mice were serially 2-fold diluted in duplicate and reacted against 2 µg/ml of the recombinant CN54gp120 purified protein (ARP683, HIV-1 CN54gp120 clade C; EU Programme EVA from the Centre for AIDS Reagents). The antibody titer of Env-specific IgG was defined as the last dilution of serum that resulted in 3 times the mean optical density at 450 nm of the naïve control.
Data analysis and statistics
For the statistical analysis of ICS data, we used a novel approach that corrects measurements for the medium response (RPMI) and allows the calculation of confidence intervals and p values of hypothesis tests [54,70]. Only antigen responses values significantly higher than the corresponding RPMI are represented and the background for the different cytokines in the unstimulated controls never exceeded 0.05%. Analysis and presentation of distributions was performed using SPICE version 5.1, downloaded from http://exon.niaid.nih.gov [71]. Comparison of distributions was performed using a Student’s T test and a partial permutation test as described [71]. All values used for analyzing proportionate representation of responses are background-subtracted. For the statistical analysis of ELISA data, a 1-way ANOVA with Tukey’s honestly significant difference criterion as post-hoc analysis was performed.
Supporting Information
Profile of memory HIV-specific T cell immune responses elicited by A46R deletion mutant in the spleen of BALB/c mice after prime/boost immunization. Flow cytometry profiles of vaccine-induced CD8 T cell responses against Env pool in splenocytes from immunized animals.
(TIF)
Scheme of construction of the plasmid transfer vector pGem-RG-A46R wm. The plasmid transfer vector pGem-RG-A46R wm was obtained by the sequential cloning of A46R recombination flanking sequences into the plasmid pGem-Red-GFP wm, containing dsRed2 and rsGFP genes as fluorescent markers. NYVAC genome was used as the template to amplify the left flank of A46R gene by PCR. This left flank was digested with AatII and XbaI and cloned into plasmid pGem-Red-GFP wm previously digested with the same restriction enzymes to generate pGem-RG-LFsA46R wm (4939 bp). The repeated left flank of A46R gene was amplified by PCR from NYVAC genome, digested with EcoRI and Cla I and inserted into the EcoRI / Cla I-digested pGem-RG-LFsA46R wm to generate pGem-RG-LFdA46R wm (5330 bp). The right flank of A46R gene was amplified by PCR from NYVAC genome, digested with Cla I and BamHI and inserted into the Cla I / BamHI-digested pGem-RG-LFdA46R wm. The resulting plasmid pGem-RG-A46R wm (5660 bp) directs the deletion of A46R gene from NYVAC-C genome.
(TIF)
Acknowledgments
We acknowledge Prof. Ian Jones from Reading University and the Programme EVA Centre for the CN54gp120 purified protein. We thank Victoria Jimenez for expert technical assistance.
Funding Statement
This investigation was supported by grants from the Ministry of Science and Innovation of Spain (SAF2008-02036), Foundation FIPSE and PTVDC/CAVD program with support from the Bill and Melinda Gates Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J et al. (2009) Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 361: 2209-2220. doi:10.1056/NEJMoa0908492. PubMed: 19843557. [DOI] [PubMed] [Google Scholar]
- 2. Gómez CE, Nájera JL, Krupa M, Perdiguero B, Esteban M (2011) MVA and NYVAC as vaccines against emergent infectious diseases and cancer. Curr Gene Ther 11: 189-217. doi:10.2174/156652311795684731. PubMed: 21453284. [DOI] [PubMed] [Google Scholar]
- 3. Tartaglia J, Cox WI, Taylor J, Perkus M, Riviere M et al. (1992) Highly attenuated poxvirus vectors. AIDS Res Hum Retroviruses 8: 1445-1447. PubMed: 1466978. [DOI] [PubMed] [Google Scholar]
- 4. Flynn BJ, Kastenmüller K, Wille-Reece U, Tomaras GD, Alam M et al. (2011) Immunization with HIV Gag targeted to dendritic cells followed by recombinant New York vaccinia virus induces robust T-cell immunity in nonhuman primates. Proc Natl Acad Sci U S A 108: 7131-7136. doi:10.1073/pnas.1103869108. PubMed: 21467219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paoletti E (1996) Applications of pox virus vectors to vaccination: an update. Proc Natl Acad Sci U S A 93: 11349-11353. doi:10.1073/pnas.93.21.11349. PubMed: 8876138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Perkus ME, Tartaglia J, Paoletti E (1995) Poxvirus-based vaccine candidates for cancer, AIDS, and other infectious diseases. J Leukoc Biol 58: 1-13. PubMed: 7616101. [DOI] [PubMed] [Google Scholar]
- 7. Kumar H, Kawai T, Akira S (2009) Pathogen recognition in the innate immune response. Biochem J 420: 1-16. doi:10.1042/BJ20090272. PubMed: 19382893. [DOI] [PubMed] [Google Scholar]
- 8. Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124: 783-801. doi:10.1016/j.cell.2006.02.015. PubMed: 16497588. [DOI] [PubMed] [Google Scholar]
- 9. Perdiguero B, Esteban M (2009) The interferon system and vaccinia virus evasion mechanisms. J Interferon Cytokine Res 29: 581-598. doi:10.1089/jir.2009.0073. PubMed: 19708815. [DOI] [PubMed] [Google Scholar]
- 10. Keating SE, Baran M, Bowie AG (2011) Cytosolic DNA sensors regulating type I interferon induction. Trends Immunol 32: 574-581. doi:10.1016/j.it.2011.08.004. PubMed: 21940216. [DOI] [PubMed] [Google Scholar]
- 11. Kawai T, Akira S (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11: 373-384. doi:10.1038/ni.1863. PubMed: 20404851. [DOI] [PubMed] [Google Scholar]
- 12. Kawai T, Akira S (2008) Toll-like receptor and RIG-I-like receptor signaling. Ann N Y Acad Sci 1143: 1-20. doi:10.1196/annals.1443.020. PubMed: 19076341. [DOI] [PubMed] [Google Scholar]
- 13. Bowie AG, Haga IR (2005) The role of Toll-like receptors in the host response to viruses. Mol Immunol 42: 859-867. doi:10.1016/j.molimm.2004.11.007. PubMed: 15829275. [DOI] [PubMed] [Google Scholar]
- 14. Thompson MR, Kaminski JJ, Kurt-Jones EA, Fitzgerald KA (2011) Pattern recognition receptors and the innate immune response to viral infection. Viruses 3: 920-940. doi:10.3390/v3060920. PubMed: 21994762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barbalat R, Lau L, Locksley RM, Barton GM (2009) Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat Immunol 10: 1200-1207. doi:10.1038/ni.1792. PubMed: 19801985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Quigley M, Martinez J, Huang X, Yang Y (2009) A critical role for direct TLR2-MyD88 signaling in CD8 T-cell clonal expansion and memory formation following vaccinia viral infection. Blood 113: 2256-2264. doi:10.1182/blood-2008-03-148809. PubMed: 18948575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhu J, Martinez J, Huang X, Yang Y (2007) Innate immunity against vaccinia virus is mediated by TLR2 and requires TLR-independent production of IFN-beta. Blood 109: 619-625. doi:10.1182/blood-2006-06-027136. PubMed: 16973959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hutchens MA, Luker KE, Sonstein J, Núñez G, Curtis JL et al. (2008) Protective effect of Toll-like receptor 4 in pulmonary vaccinia infection. PLOS Pathog 4: e1000153 PubMed: 18802464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gay NJ, Gangloff M, O’Neill LA (2011) What the Myddosome structure tells us about the initiation of innate immunity. Trends Immunol 32: 104-109. doi:10.1016/j.it.2010.12.005. PubMed: 21269878. [DOI] [PubMed] [Google Scholar]
- 20. Bryant CE, Spring DR, Gangloff M, Gay NJ (2010) The molecular basis of the host response to lipopolysaccharide. Nat Rev Microbiol 8: 8-14. PubMed: 19946286. [DOI] [PubMed] [Google Scholar]
- 21. Jin MS, Lee JO (2008) Structures of the toll-like receptor family and its ligand complexes. Immunity 29: 182-191. doi:10.1016/j.immuni.2008.07.007. PubMed: 18701082. [DOI] [PubMed] [Google Scholar]
- 22. O’Neill LA, Bowie AG (2007) The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol 7: 353-364. doi:10.1038/nri2079. PubMed: 17457343. [DOI] [PubMed] [Google Scholar]
- 23. Takeuchi O, Akira S (2002) MyD88 as a bottle neck in Toll/IL-1 signaling. Curr Top Microbiol Immunol 270: 155-167. doi:10.1007/978-3-642-59430-4_10. PubMed: 12467250. [DOI] [PubMed] [Google Scholar]
- 24. Thompson AJ, Locarnini SA (2007) Toll-like receptors, RIG-I-like RNA helicases and the antiviral innate immune response. Immunol Cell Biol 85: 435-445. doi:10.1038/sj.icb.7100100. PubMed: 17667934. [DOI] [PubMed] [Google Scholar]
- 25. Xu Y, Tao X, Shen B, Horng T, Medzhitov R et al. (2000) Structural basis for signal transduction by the Toll/interleukin-1 receptor domains. Nature 408: 111-115. doi:10.1038/35040600. PubMed: 11081518. [DOI] [PubMed] [Google Scholar]
- 26. Nyman T, Stenmark P, Flodin S, Johansson I, Hammarström M et al. (2008) The crystal structure of the human toll-like receptor 10 cytoplasmic domain reveals a putative signaling dimer. J Biol Chem 283: 11861-11865. doi:10.1074/jbc.C800001200. PubMed: 18332149. [DOI] [PubMed] [Google Scholar]
- 27. Khan JA, Brint EK, O’Neill LA, Tong L (2004) Crystal structure of the Toll/interleukin-1 receptor domain of human IL-1RAPL. J Biol Chem 279: 31664-31670. doi:10.1074/jbc.M403434200. PubMed: 15123616. [DOI] [PubMed] [Google Scholar]
- 28. Lin Z, Lu J, Zhou W, Shen Y (2012) Structural insights into TIR domain specificity of the bridging adaptor Mal in TLR4 signaling. PLOS ONE 7: e34202. doi:10.1371/journal.pone.0034202. PubMed: 22485159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Valkov E, Stamp A, Dimaio F, Baker D, Verstak B et al. (2011) Crystal structure of Toll-like receptor adaptor MAL/TIRAP reveals the molecular basis for signal transduction and disease protection. Proc Natl Acad Sci U S A 108: 14879-14884. doi:10.1073/pnas.1104780108. PubMed: 21873236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ohnishi H, Tochio H, Kato Z, Orii KE, Li A et al. (2009) Structural basis for the multiple interactions of the MyD88 TIR domain in TLR4 signaling. Proc Natl Acad Sci U S A 106: 10260-10265. doi:10.1073/pnas.0812956106. PubMed: 19506249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dunne A, Ejdeback M, Ludidi PL, O’Neill LA, Gay NJ (2003) Structural complementarity of Toll/interleukin-1 receptor domains in Toll-like receptors and the adaptors Mal and MyD88. J Biol Chem 278: 41443-41451. doi:10.1074/jbc.M301742200. PubMed: 12888566. [DOI] [PubMed] [Google Scholar]
- 32. Núñez Miguel R, Wong J, Westoll JF, Brooks HJ, O’Neill LA et al. (2007) A dimer of the Toll-like receptor 4 cytoplasmic domain provides a specific scaffold for the recruitment of signalling adaptor proteins. PLOS ONE 2: e788. doi:10.1371/journal.pone.0000788. PubMed: 17726518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Loiarro M, Capolunghi F, Fantò N, Gallo G, Campo S et al. (2007) Pivotal Advance: Inhibition of MyD88 dimerization and recruitment of IRAK1 and IRAK4 by a novel peptidomimetic compound. J Leukoc Biol 82: 801-810. doi:10.1189/jlb.1206746. PubMed: 17548806. [DOI] [PubMed] [Google Scholar]
- 34. Loiarro M, Sette C, Gallo G, Ciacci A, Fantò N et al. (2005) Peptide-mediated interference of TIR domain dimerization in MyD88 inhibits interleukin-1-dependent activation of NF-{kappa}B. J Biol Chem 280: 15809-15814. doi:10.1074/jbc.C400613200. PubMed: 15755740. [DOI] [PubMed] [Google Scholar]
- 35. Jiang Z, Georgel P, Li C, Choe J, Crozat K et al. (2006) Details of Toll-like receptor:adapter interaction revealed by germ-line mutagenesis. Proc Natl Acad Sci U S A 103: 10961-10966. doi:10.1073/pnas.0603804103. PubMed: 16832055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bowie AG, Unterholzner L (2008) Viral evasion and subversion of pattern-recognition receptor signalling. Nat Rev Immunol 8: 911-922. doi:10.1038/nri2436. PubMed: 18989317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stack J, Haga IR, Schröder M, Bartlett NW, Maloney G et al. (2005) Vaccinia virus protein A46R targets multiple Toll-like-interleukin-1 receptor adaptors and contributes to virulence. J Exp Med 201: 1007-1018. doi:10.1084/jem.20041442. PubMed: 15767367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harte MT, Haga IR, Maloney G, Gray P, Reading PC et al. (2003) The poxvirus protein A52R targets Toll-like receptor signaling complexes to suppress host defense. J Exp Med 197: 343-351. doi:10.1084/jem.20021652. PubMed: 12566418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. DiPerna G, Stack J, Bowie AG, Boyd A, Kotwal G et al. (2004) Poxvirus protein N1L targets the I-kappaB kinase complex, inhibits signaling to NF-kappaB by the tumor necrosis factor superfamily of receptors, and inhibits NF-kappaB and IRF3 signaling by toll-like receptors. J Biol Chem 279: 36570-36578. doi:10.1074/jbc.M400567200. PubMed: 15215253. [DOI] [PubMed] [Google Scholar]
- 40. Chen RA, Ryzhakov G, Cooray S, Randow F, Smith GL (2008) Inhibition of IkappaB kinase by vaccinia virus virulence factor B14. PLOS Pathog 4: e22. doi:10.1371/journal.ppat.0040022. PubMed: 18266467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schröder M, Baran M, Bowie AG (2008) Viral targeting of DEAD box protein 3 reveals its role in TBK1/IKKepsilon-mediated IRF activation. EMBO J 27: 2147-2157. doi:10.1038/emboj.2008.143. PubMed: 18636090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Unterholzner L, Sumner RP, Baran M, Ren H, Mansur DS et al. (2011) Vaccinia virus protein C6 is a virulence factor that binds TBK-1 adaptor proteins and inhibits activation of IRF3 and IRF7. PLOS Pathog 7: e1002247 PubMed: 21931555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bowie A, Kiss-Toth E, Symons JA, Smith GL, Dower SK et al. (2000) A46R and A52R from vaccinia virus are antagonists of host IL-1 and toll-like receptor signaling. Proc Natl Acad Sci U S A 97: 10162-10167. doi:10.1073/pnas.160027697. PubMed: 10920188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lysakova-Devine T, Keogh B, Harrington B, Nagpal K, Halle A et al. (2010) Viral inhibitory peptide of TLR4, a peptide derived from vaccinia protein A46, specifically inhibits TLR4 by directly targeting MyD88 adaptor-like and TRIF-related adaptor molecule. J Immunol 185: 4261-4271. doi:10.4049/jimmunol.1002013. PubMed: 20802145. [DOI] [PubMed] [Google Scholar]
- 45. Oda S, Franklin E, Khan AR (2011) Poxvirus A46 protein binds to TIR domain-containing Mal/TIRAP via an α-helical sub-domain. Mol Immunol 48: 2144-2150. doi:10.1016/j.molimm.2011.07.014. PubMed: 21831443. [DOI] [PubMed] [Google Scholar]
- 46. Stack J, Bowie AG (2012) Poxviral protein A46 antagonizes Toll-like receptor 4 signaling by targeting BB loop motifs in Toll-IL-1 receptor adaptor proteins to disrupt receptor:adaptor interactions. J Biol Chem 287: 22672-22682. doi:10.1074/jbc.M112.349225. PubMed: 22593572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Delaloye J, Roger T, Steiner-Tardivel QG, Le Roy D, Knaup Reymond M et al. (2009) Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome. PLOS Pathog 5: e1000480 PubMed: 19543380. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48. Gómez CE, Nájera JL, Jiménez V, Bieler K, Wild J et al. (2007) Generation and immunogenicity of novel HIV/AIDS vaccine candidates targeting HIV-1 Env/Gag-Pol-Nef antigens of clade C. Vaccine 25: 1969-1992. doi:10.1016/j.vaccine.2006.11.051. PubMed: 17224219. [DOI] [PubMed] [Google Scholar]
- 49. Gómez CE, Nájera JL, Jiménez EP, Jiménez V, Wagner R et al. (2007) Head-to-head comparison on the immunogenicity of two HIV/AIDS vaccine candidates based on the attenuated poxvirus strains MVA and NYVAC co-expressing in a single locus the HIV-1BX08 gp120 and HIV-1(IIIB) Gag-Pol-Nef proteins of clade B. Vaccine 25: 2863-2885. doi:10.1016/j.vaccine.2006.09.090. PubMed: 17113200. [DOI] [PubMed] [Google Scholar]
- 50. Robinson HL, Sharma S, Zhao J, Kannanganat S, Lai L et al. (2007) Immunogenicity in macaques of the clinical product for a clade B DNA/MVA HIV vaccine: elicitation of IFN-gamma, IL-2, and TNF-alpha coproducing CD4 and CD8 T cells. AIDS Res Hum Retroviruses 23: 1555-1562. doi:10.1089/aid.2007.0165. PubMed: 18160013. [DOI] [PubMed] [Google Scholar]
- 51. Bachmann MF, Wolint P, Schwarz K, Jäger P, Oxenius A (2005) Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor alpha and CD62L. J Immunol 175: 4686-4696. PubMed: 16177116. [DOI] [PubMed] [Google Scholar]
- 52. Tscharke DC, Woo WP, Sakala IG, Sidney J, Sette A et al. (2006) Poxvirus CD8+ T-cell determinants and cross-reactivity in BALB/c mice. J Virol 80: 6318-6323. doi:10.1128/JVI.00427-06. PubMed: 16775319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gomez CE, Perdiguero B, Garcia-Arriaza J, Esteban M (2012) Poxvirus vectors as HIV/AIDS vaccines in humans. Hum Vaccin. J Immunother 8: 1192-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. García-Arriaza J, Nájera JL, Gómez CE, Sorzano CO, Esteban M (2010) Immunogenic profiling in mice of a HIV/AIDS vaccine candidate (MVA-B) expressing four HIV-1 antigens and potentiation by specific gene deletions. PLOS ONE 5: e12395. doi:10.1371/journal.pone.0012395. PubMed: 20811493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. García-Arriaza J, Nájera JL, Gómez CE, Tewabe N, Sorzano CO et al. (2011) A candidate HIV/AIDS vaccine (MVA-B) lacking vaccinia virus gene C6L enhances memory HIV-1-specific T-cell responses. PLOS ONE 6: e24244. doi:10.1371/journal.pone.0024244. PubMed: 21909386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Perdiguero B, Gómez CE, Nájera JL, Sorzano CO, Delaloye J et al. (2012) Deletion of the viral anti-apoptotic gene F1L in the HIV/AIDS vaccine candidate MVA-C enhances immune responses against HIV-1 antigens. PLOS ONE 7: e48524. doi:10.1371/journal.pone.0048524. PubMed: 23119046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Falivene J, Del Médico Zajac MP, Pascutti MF, Rodríguez AM, Maeto C et al. (2012) Improving the MVA vaccine potential by deleting the viral gene coding for the IL-18 binding protein. PLOS ONE 7: e32220. doi:10.1371/journal.pone.0032220. PubMed: 22384183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cottingham MG, Andersen RF, Spencer AJ, Saurya S, Furze J et al. (2008) Recombination-mediated genetic engineering of a bacterial artificial chromosome clone of modified vaccinia virus Ankara (MVA). PLOS ONE 3: e1638. doi:10.1371/journal.pone.0001638. PubMed: 18286194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Garber DA, O’Mara LA, Gangadhara S, McQuoid M, Zhang X et al. (2012) Deletion of specific immune-modulatory genes from modified vaccinia virus Ankara-based HIV vaccines engenders improved immunogenicity in rhesus macaques. J Virol 86: 12605-12615. doi:10.1128/JVI.00246-12. PubMed: 22973033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Garber DA, O’Mara LA, Zhao J, Gangadhara S, An I et al. (2009) Expanding the repertoire of Modified Vaccinia Ankara-based vaccine vectors via genetic complementation strategies. PLOS ONE 4: e5445. doi:10.1371/journal.pone.0005445. PubMed: 19421328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gómez CE, Perdiguero B, Nájera JL, Sorzano CO, Jiménez V et al. (2012) Removal of vaccinia virus genes that block interferon type I and II pathways improves adaptive and memory responses of the HIV/AIDS vaccine candidate NYVAC-C in mice. J Virol 86: 5026-5038. doi:10.1128/JVI.06684-11. PubMed: 22419805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Guerra S, González JM, Climent N, Reyburn H, López-Fernández LA et al. (2010) Selective induction of host genes by MVA-B, a candidate vaccine against HIV/AIDS. J Virol 84: 8141-8152. doi:10.1128/JVI.00749-10. PubMed: 20534857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC et al. (2009) Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med 15: 293-299. doi:10.1038/nm.1935. PubMed: 19219024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM et al. (2011) Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 473: 523-527. doi:10.1038/nature10003. PubMed: 21562493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sánchez-Sampedro L, Gómez CE, Mejías-Pérez E, Sorzano CO, Esteban M (2012) High quality long-term CD4+ and CD8+ effector memory populations stimulated by DNA-LACK/MVA-LACK regimen in Leishmania major BALB/c model of infection. PLOS ONE 7: e38859. doi:10.1371/journal.pone.0038859. PubMed: 22715418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ray A, Dittel BN (2010) Isolation of mouse peritoneal cavity cells. J Vis Exp (35): 1488 PubMed: 20110936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ramírez JC, Gherardi MM, Esteban M (2000) Biology of attenuated modified vaccinia virus Ankara recombinant vector in mice: virus fate and activation of B- and T-cell immune responses in comparison with the Western Reserve strain and advantages as a vaccine. J Virol 74: 923-933. doi:10.1128/JVI.74.2.923-933.2000. PubMed: 10623755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kibler KV, Gomez CE, Perdiguero B, Wong S, Huynh T et al. (2011) Improved NYVAC-based vaccine vectors. PLOS ONE 6: e25674. doi:10.1371/journal.pone.0025674. PubMed: 22096477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Roger T, David J, Glauser MP, Calandra T (2001) MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature 414: 920-924. doi:10.1038/414920a. PubMed: 11780066. [DOI] [PubMed] [Google Scholar]
- 70. Nájera JL, Gómez CE, García-Arriaza J, Sorzano CO, Esteban M (2010) Insertion of vaccinia virus C7L host range gene into NYVAC-B genome potentiates immune responses against HIV-1 antigens. PLOS ONE 5: e11406. doi:10.1371/journal.pone.0011406. PubMed: 20613977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Roederer M, Nozzi JL, Nason MC (2011) SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A 79: 167-174. PubMed: 21265010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Profile of memory HIV-specific T cell immune responses elicited by A46R deletion mutant in the spleen of BALB/c mice after prime/boost immunization. Flow cytometry profiles of vaccine-induced CD8 T cell responses against Env pool in splenocytes from immunized animals.
(TIF)
Scheme of construction of the plasmid transfer vector pGem-RG-A46R wm. The plasmid transfer vector pGem-RG-A46R wm was obtained by the sequential cloning of A46R recombination flanking sequences into the plasmid pGem-Red-GFP wm, containing dsRed2 and rsGFP genes as fluorescent markers. NYVAC genome was used as the template to amplify the left flank of A46R gene by PCR. This left flank was digested with AatII and XbaI and cloned into plasmid pGem-Red-GFP wm previously digested with the same restriction enzymes to generate pGem-RG-LFsA46R wm (4939 bp). The repeated left flank of A46R gene was amplified by PCR from NYVAC genome, digested with EcoRI and Cla I and inserted into the EcoRI / Cla I-digested pGem-RG-LFsA46R wm to generate pGem-RG-LFdA46R wm (5330 bp). The right flank of A46R gene was amplified by PCR from NYVAC genome, digested with Cla I and BamHI and inserted into the Cla I / BamHI-digested pGem-RG-LFdA46R wm. The resulting plasmid pGem-RG-A46R wm (5660 bp) directs the deletion of A46R gene from NYVAC-C genome.
(TIF)