Abstract
Purpose
Recently, genetic polymorphism (rs3803662C>T) in TOX3 was reported to induce the risk of breast cancer. In this study, we hypothesized that rs3803662 could influence gastric cancer survival outcomes.
Methods
With multiplex SNaPshot method, we genotyped TOX3 rs3803662 in 880 gastric patients with surgical resection. The association between genotype and survival outcomes was performed by the Kaplan-Meier method, Cox regression analysis models and the log-rank test.
Results
There was no association in the analyses of rs3803662 and survival of gastric cancer. However, the stratified analysis by histology showed that rs3803662 CT/TT genotype was associated with a significantly better survival for diffuse-type gastric cancer (log-rank p = 0.030, hazard ratio [HR] = 0.67, 95% confidence interval [CI] = 0.46–0.96), than the CC genotype. In addition, this favorable effect was especially obvious among gastric cancer patients with tumor size >5 cm, T3 and T4 depth of invasion, lymph node metastasis, no drinking, no distant metastasis, no chemotherapy and gastric cardia cancer.
Conclusions
TOX3 rs3803662 might play an important role in the prognostic outcome and treatment of gastric cancer, especially perhaps further help in explaining the reduced risk of death associated with diffuse-type gastric cancer.
Introduction
Gastric cancer is the fourth common type of malignant tumor in the world and the leading cause of cancer deaths in China, Japan and Eastern European countries [1]. A systematic treatment including surgery, chemotherapy, radiotherapy and target therapy may provide a cure for patients with advanced gastric cancer [2]. Despite this, survival in patients with advanced gastric cancer remains poor. Gastric cancer is a complex and multifactor disease that is thought to result from an interaction between genetic background and environmental factors. Some studies have suggested that Helicobacter pylori (H. pylorus) infection, salt-preserved food consumption and tobacco smoking are major exogenous factors and increase the risk of gastric cancer [3], [4]. Extensive epidemiological studies have demonstrated that genetic variants, particularly single nucleotide polymorphisms (SNPs), are likely to modulate the effect of environmental risk factors through modifying functions of various biological pathways involved in gastric carcinogenesis in response to environmental exposure. In recent years, several common low-penetrant genes have been identified as potential gastric cancer susceptibility genetic variants, such as Glutathione S-transferase M1 (GSTM1) polymorphism. It has been found genetic variant may be a useful marker for predicting the prognosis of patients with gastric cancer. Previous studies reported PSCA rs2294008 and APE1 rs1760944 are associated with gastric cancer survival [5], [6].
TOX3 gene, a member of the high mobility group family of non-histone chromatin proteins, also termed trinucleotide repeat containing 9 (TNRC9) gene is located at chromosome 16q12 [7]. This gene regulates Ca2+-dependent neuronal transcription through interaction with the cAMP-response-element-binding protein (CREB) [8]. In normal human tissues, TOX3 is largely expressed in the central nervous system (CNS), in the ileum, and within the brain in the frontal and occipital lobe. TOX3 overexpression induces transcription involving isolated estrogen-responsive elements and estrogen-responsive promoters, and protects neuronal cells from cell death caused by endoplasmic reticulum stress or BAX overexpression through the induction of anti-apoptotic transcripts and repression of pro-apoptotic transcripts [9]. It has been suggested that rs3803662 (a C>T transition) in TOX3 was associated with an increased risk of breast cancer in both BRCA1 and BRCA2-mutation carriers and estrogen receptor (ER) positive patients [10]. Additionally, Fasching et al. reported rs3803662 (TOX3) was associated with OS of breast cancer [11]. This association was seen similarly in other study which showed that when survival was analysed according to molecular subtypes, patients diagnosed with luminal A tumours who carried the risk allele had shorter OS as compared to patients homozygous for the non-risk allele [12].
However, GWAS focused on a loci polymorphism at TOX3 gene that is associated with breast cancer survival, especially in estrogen receptor (ER) positive patients. Some studies suggested that ER expression was associated with survival of gastric cancer [13]. Therefore, the variant identified in breast cancer studies may have the same impact on gastric cancer risk. An association of genetic variation in TOX3 with gastric cancer survival was conducted in our study. We hypothesize that TOX3 rs3803662 is associated with gastric cancer survival in a Chinese population, which can be identified as an independent prognostic marker of gastric cancer survival.
Study population
The study was conducted on a total of 1022 gastric cancer patients with surgical resection of the tumor at Yixing People's hospital, Yixing City, the People's Republic of China (P.R. China), from January 1999 to December 2006 [6]. All patients were neither administrated by adjuvant radiotherapy nor chemotherapy before surgical resection. In our study population, all analyses were restricted to Chinese. Within a maximum of 119.0 months follow-up period, 78 patients were excluded from our study for lacking of enough follow-up information. The present study was approved by the Institutional Ethics Review Board of Nanjing medical university, and all participants gave written informed consent.
Outcomes collection
Overall survival was the main study endpoint. Patients alive on the last follow-up date were considered censored and gastric cancer -related deaths were defined as deaths. The survival time was defined as the date from cancer surgery to gastric cancer -related deaths or the last follow-up. Date of death was obtained from inpatient and outpatient records or the relatives of patients by follow-up telephone calls. Information of pathologic parameters on tumor site, histotype, invasion, lymph node, distant metastasis status, drinking and smoking status were also obtained for gastric cancer patients. Lauren's criterion was used in classifying the tumors into intestinal-type or diffuse-type gastric cancer [14]. TNM stage of the disease was measured in accordance with American Joint Commission for Cancer Staging (AJCCS) [15]. There were 306 patients treated with adjuvant chemotherapy with different regimens after surgery. The therapeutic regimen included FOLFOX4, FOLFOX6, XELOX and so on. Our study was approved by the Institutional Review Board of Nanjing Medical University, Nanjing, P. R. China.
Patients and Methods
Genotyping
Genomic DNA was extracted from tumor specimens by proteinase K digestion, isopropanol extraction and ethanol precipitation [6]. The TOX3 (rs3803662) SNPs were examined by multiplex SNaPshot technology using an ABI fluorescence-based assay allelic discrimination method (Applied Biosystems, Foster city, CA, USA) as described previously [16]. The SNPs were analyzed by using ABI3130 genetic analyzer and the genotypes were determined by using Genemapper4.0 software (Applied Biosystems). In fact, 880 eligible gastric patients were enrolled in the final analysis, 64 cases were excluded from further analyses because of DNA quality failing in genotyping. About 10% of the samples were selected at random for confirmation by repeated genotyping; the results were 100% concordant.
Statistical analysis
The influence of TOX3 variant on gastric cancer survival was estimated by the Kaplan-Meier survival curves and the log–rank test. Three genetic models (codominant, dominant and recessive) were used to assess the association of TOX3 rs3803662 with survival outcome of gastric cancer patients. All P values in this study were 2-sided. P<0.05 was considered statistically significance. The crude hazard ratios (HRs), their 95% confidence intervals (CIs) and adjusted HRs were estimated by Cox regression analysis. Cox stepwise regression analysis was also performed to determine prognostic factor in gastric cancer, with a significance level of P<0.05 for entering and P>0.10 for removing the respective explanatory variables. Mean survival time was provided when the median survival time (MST) could not be calculated. All the statistical analyses were performed with SPSS software.
Results
The characteristics and clinicopathological features of the patients
The characteristics and clinicopathological features of the final 880 gastric cancer patients in this study are summarized in Table 1. The percentage of males was 76.9%. The number of patients died during follow-up was 408. Univariate Cox regression analysis of the pathologic parameters revealed that tumor size, histology, depth of invasion, lymph node metastasis, distant metastasis and TNM stage were significantly associated with survival time (all P<0.05, log-rank test). Specifically, diffuse-type gastric cancer patients had a significantly higher risk of death, compared with intestinal-type gastric cancer patients (HR = 1.46, 95% CI = 1.19–1.79). And patients with tumor size ≤5 cm had a 41% significantly increased survival, compared to those with tumor size>5 cm (HR = 1.41, 95% CI = 1.16–1.71). In addition, as the depth of invasion and TNM stage increased, the lymph node metastasis and distant metastasis expanded, and then the survival time of gastric cancer reduced significantly accordingly (log–rank P<0.001 for depth of invasion, TNM stage and the lymph node metastasis; log–rank P = 0.031 for distant metastasis).
Table 1. Patients' characteristics and clinical features.
| Variable | Patients, n = 880 (%) | Deaths, n = 408 | MST (months) | Log-rank p | HR (95% CI) |
| Age (years) | |||||
| ≤60 | 413 (46.9) | 189 | 97.0 | 0.353 | 1.00 |
| >60 | 467 (53.1) | 219 | 60.0 | 1.10(0.90–1.33) | |
| Sex | |||||
| Male | 677 (76.9) | 310 | 70.0 | 0.331 | 1.00 |
| Female | 203 (23.1) | 98 | 63.0 | 1.12(0.89–1.40) | |
| Tumor size | |||||
| ≤5cm | 546 (62.1) | 231 | 73.53 | 0.001 | 1.00 |
| >5cm | 334 (37.9) | 177 | 48.0 | 1.41(1.16–1.71) | |
| Histological types 1 | |||||
| Intestinal | 371 (42.2) | 143 | 76.93 | <0.001 | 1.00 |
| Diffuse | 505 (57.4) | 262 | 50.0 | 1.46(1.19–1.79) | |
| others | 4 (0.004) | 3 | 11.0 | 2.72(0.87,8.53) | |
| Tumor site | |||||
| Non-cardia | 584 (66.4) | 276 | 67.0 | 0.277 | 1.00 |
| cardia | 296 (33.6) | 132 | 78.0 | 0.89(0.73–1.10) | |
| Depth of invasion2 | |||||
| T1 | 134 (15.2) | 43 | 85.23 | <0.001 | 1.00 |
| T2 | 186 (21.1) | 73 | 73.13 | 1.34(0.92–1.95) | |
| T3 | 520 (59.1) | 271 | 48.0 | 1.98(1.43–2.74) | |
| T4 | 38 (0.04) | 21 | 29.0 | 2.20(1.31–3.71) | |
| Lymph node metastasis | |||||
| N0 | 348 (39.5) | 121 | 81.63 | <0.001 | 1.00 |
| N1/N2/N3 | 532 (60.5) | 287 | 42.0 | 1.84(1.49–2.28) | |
| Distant metastasis | |||||
| M0 | 829 (94.2) | 379 | 74.0 | 0.031 | 1.00 |
| M1 | 51 (5.8) | 29 | 27.0 | 1.51(1.03–2.20) | |
| TNM stage | |||||
| I | 240 (27.3) | 79 | 84.03 | <0.001 | 1.00 |
| II | 174 (19.8) | 70 | 68.73 | 1.32(0.96–1.83) | |
| III | 379 (43.1) | 212 | 38.0 | 2.11(1.63–2.74) | |
| IV | 87 (0.08) | 47 | 47.0 | 1.98(1.38–2.85) | |
| Drinking status | |||||
| NO | 825 (93.8) | 379 | 70.0 | 0.346 | 1.00 |
| YES | 55 (6.2) | 29 | 39.0 | 1.20(0.82–1.75) | |
| Chemotherapy | |||||
| NO | 587(66.7) | 276 | 70.0 | 0.656 | 1.00 |
| YES | 293(33.3) | 132 | 62.0 | 1.05(0.85–1.29) | |
| Smoking status | |||||
| NO | 808 (91.8) | 376 | 67.0 | 0.699 | 1.00 |
| YES | 72 (8.2) | 32 | 97.0 | 0.93(0.65–1.34) | |
Exclude four patients with gastrointestinal stromal tumor and lymphoma. 2Two cases were not available for the information. 3Mean survival time was provided when MST could not be calculated.
Effects of rs3803662 on gastric cancer survival
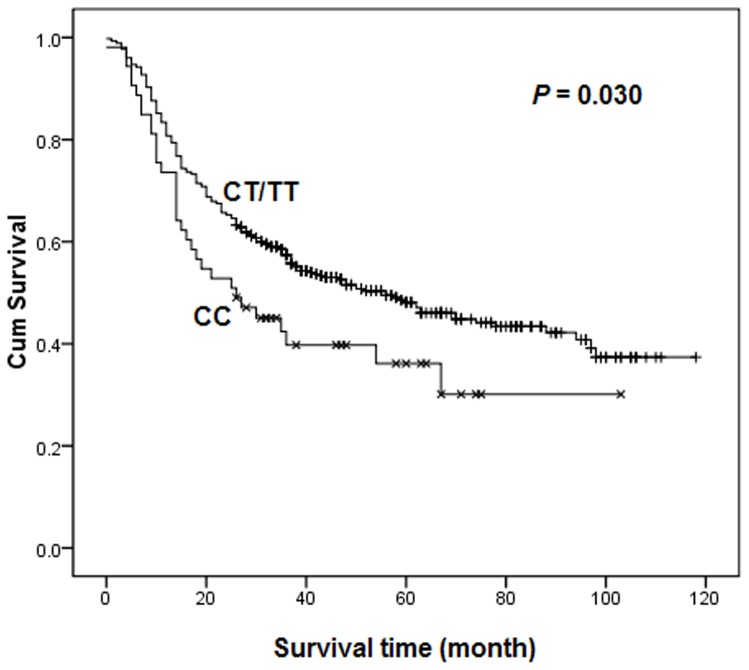
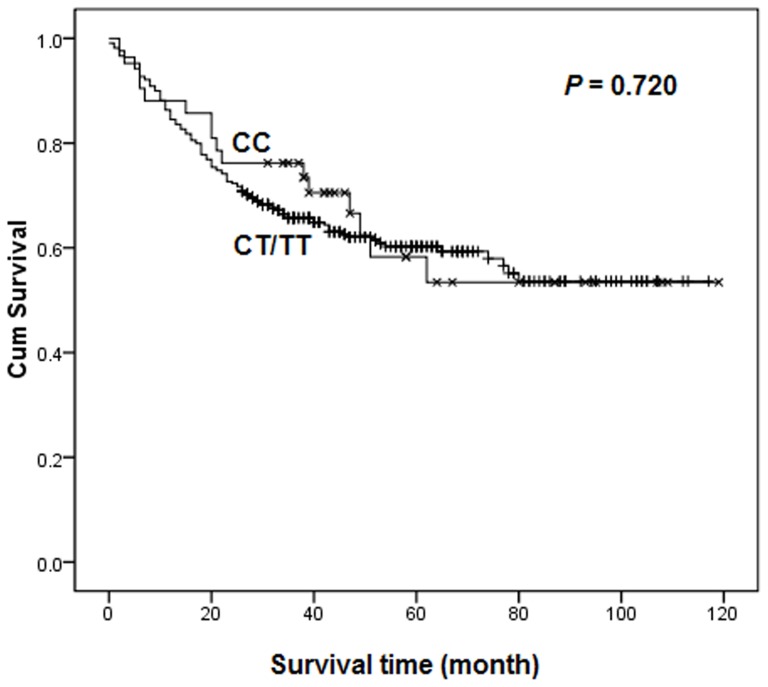
As shown in Table 2, the associations of TOX3 rs3803662 genotypes with gastric cancer survival in different genetic models were assessed by Cox regression analyses. No statistically significant differences were found between the survival of gastric cancer and genotypes in any genetic models. To further evaluate the association, the patients were stratified by histology with intestinal-type and diffuse-type gastric cancer. As a result, rs3803662 was found to be associated with a significantly favorable effect among diffuse-type (log-rank P = 0.030, Fig. 1), but not intestinal-type (log-rank P = 0.720, Fig. 2) gastric cancer patients in dominant model. Cox regression analyses revealed that patients with diffuse-type gastric cancer carrying the CT/TT genotype reduced a 33% risk of death significantly (HR = 0.67, 95% CI = 0.46–0.96), comparing to those who were CC carriers.
Table 2. Association between TOX3 rs3803662 polymorphism and gastric cancer patients' survival.
| Genetic models | Genotypes | All cases | ||||
| Patients, n = 880 | Deaths, n = 408 | MST (months) | Log-rank p | HR (95% CI)1 | ||
| Codominant model | CC | 96 | 50 | 49.0 | 0.194 | 1 |
| CT | 522 | 230 | 80.0 | 0.77(0.57–1.05) | ||
| TT | 262 | 128 | 60.0 | 0.89(0.64–1.23) | ||
| Dominant model | CC | 96 | 50 | 49.0 | 0.200 | 1 |
| CT/TT | 784 | 358 | 74.0 | 0.81(0.60–1.09) | ||
| Recessive model | CC/CT | 618 | 280 | 74.0 | 0.332 | 1 |
| TT | 262 | 128 | 60.0 | 1.10(0.89–1.36) | ||
| Intestinal-type cases | ||||||
| Genetic models | Genotypes | Patients, n = 371 | Deaths, n = 143 | MST (months) | Log-rank p | HR (95% CI) |
| Codominant model | CC | 42 | 16 | 77.72 | 0.308 | 1 |
| CT | 217 | 78 | 78.52 | 0.95(0.55–1.64) | ||
| TT | 112 | 49 | 78.0 | 1.28(0.73–2.27) | ||
| Dominant model | CC | 42 | 16 | 77.72 | 0.720 | 1 |
| CT/TT | 329 | 127 | 75.62 | 1.06(0.63–1.79) | ||
| Recessive model | CC/CT | 259 | 94 | 79.42 | 0.125 | 1 |
| TT | 112 | 49 | 78.0 | 1.34(0.94–1.89) | ||
| Diffuse-type cases | ||||||
| Genetic models | Genotypes | Patients, n = 505 | Deaths, n = 262 | MST (months) | Log-rank p | HR (95% CI) |
| Codominant model | CC | 53 | 33 | 26.0 | 0.078 | 1 |
| CT | 303 | 150 | 56.0 | 0.65(0.44–0.95) | ||
| TT | 149 | 79 | 48.0 | 0.70(0.47–1.06) | ||
| Dominant model | CC | 53 | 33 | 26.0 | 0.030 | 1 |
| CT/TT | 452 | 229 | 56.0 | 0.67(0.46–0.96) | ||
| Recessive model | CC/CT | 356 | 183 | 50.0 | 0.869 | 1 |
| TT | 149 | 79 | 48.0 | 1.02(0.78–1.33) |
Adjusted for age, sex. 2Mean survival time was provided when MST could not be calculated. CI, confidence interval; HR, hazard ratio.
Figure 1. Overall survival of TOX3 rs3803662 dominant genotypes in diffuse-type gastric cancer patients.
Figure 2. Overall survival of TOX3 rs3803662 dominant genotypes in intestinal-type gastric cancer patient.
Stratified analyses among diffuse-type gastric cancer
TOX3 rs3803662 was investigated with survival of diffuse-type gastric cancer patients by stratified analysis of tumor size, histology, depth of invasion, lymph node metastasis, distant metastasis, TNM stage, drinking status, and tumor site. As shown in Table 3, after adjusting for covariates including age and sex, the reduced risk of death was much evident among patients with tumor size >5 cm (HR = 0.35, 95% CI = 0.21–0.56), T3 and T4 depth of invasion (HR = 0.63, 95% CI = .42–0.94 for T3; HR = 0.07, 95% CI = 0.01–0.83 for T4), lymph node metastasis (HR = 0.62, 95% CI = 0.41–0.93), no distant metastasis (HR = 0.68, 95% CI = 0.46–1.00), no drinking (HR = 0.63, 95% CI = 0.43–0.92), no chemotherapy (HR = 0.54, 95% CI = 0.34–0.86) and gastric cardiac cancer (HR = 0.50, 95% CI = 0.25–1.00). Although patients with T1 depth of invasion and stage I showed a similar better outcome, there might have been a suboptimal statistical power to detect a statistical difference in the survival time because of small number of patients in these subgroups. In contrast, there seemed to be a poor survival for patients with tumor size ≤5 cm and drinking although not statistically significant. In addition, a multivariate stepwise Cox regression analysis was also used to confirm an independent role of rs3803662 genotypes in diffuse-type gastric cancer survival. Five variables (drink, lymph node metastasis, tumor site, chemotherapy and rs3803662) were included in the regression model with a significance level of 0.05 for entering and 0.10 for removing a variable (data not shown). When age and sex were included in the final model, the rs3803662 CT/TT genotype was showed to be a significantly favorable predictor for survival of diffuse-type gastric cancer. (CT /TT vs. CC: HR = 0.63, 95% CI = 0.43–0.90) (Table 4).
Table 3. Stratified analysis of TOX3 rs3803662 genotypes associated with diffuse-type gastric cancer patients' survival.
| Variable | Genotypes (death/patients) | ||
| CC | CT/TT | HR (95% CI)1 | |
| Total | 33/53 | 229/452 | 0.67(0.46–0.96) |
| Tumor size | |||
| ≤5cm | 12/30 | 120/252 | 1.19(0.66–2.15) |
| >5cm | 21/23 | 109/200 | 0.35(0.21–0.56) |
| Depth of invasion | |||
| T1 | 0/2 | 9/20 | 0.00 |
| T2 | 3/7 | 31/80 | 0.85(0.26–2.80) |
| T3 | 29/43 | 173/323 | 0.63(0.42–0.94) |
| T4 | 1/1 | 16/29 | 0.07(0.01–0.83) |
| Lymph node metastasis | |||
| N0 | 6/13 | 40/111 | 0.79(0.33–1.87) |
| N1/N2/N3 | 27/40 | 189/341 | 0.62(0.41–0.93) |
| Distant metastasis | |||
| M0 | 29/48 | 214/425 | 0.68(0.46–1.00) |
| M1 | 4/5 | 15/27 | 0.69(0.22–2.20) |
| TNM stage | |||
| I | 0/4 | 19/56 | 0.00 |
| II | 8/13 | 33/84 | 0.52(0.24–1.12) |
| III | 19/29 | 149/259 | 0.69(0.43–1.12) |
| IV | 6/7 | 28/53 | 0.44(0.18–1.13) |
| Drinking status | |||
| NO | 32/51 | 209/426 | 0.63(0.43–0.92) |
| YES | 1/2 | 20/26 | 2.48(0.30–20.70) |
| Chemotherapy | |||
| NO | 21/28 | 153/280 | 0.54(0.34–0.86) |
| YES | 12/25 | 76/172 | 0.84(0.45–1.56) |
| Tumor site | |||
| Non-cardia | 23/36 | 171/323 | 0.74(0.48–1.14) |
| cardia | 10/17 | 58/129 | 0.50(0.25–1.00) |
Adjusted for age, sex.
Table 4. Stepwise Cox regression analysis on diffuse-type gastric cancer patients' survival.
| Final variables | β | SE | HR | 95% CI | P |
| Age | 0.093 | 0.126 | 1.10 | 0.86–1.40 | 0.460 |
| Sex (female vs. male) | −0.023 | 0.147 | 0.98 | 0.73–1.30 | 0.870 |
| Drink (no vs. yes) | 0.606 | 0.238 | 1.83 | 1.15–2.92 | 0.010 |
| Lymph node metastasis (N1/N2/N3 vs. N0) | 0.659 | 0.164 | 1.93 | 1.40–2.67 | <0.001 |
| Tumor site(non-cardia vs. cardia) | −0.374 | 0.146 | 0.69 | 0.52–0.92 | 0.010 |
| Chemotherapy (no vs. yes) | −0.269 | 0.134 | 0.76 | 0.59–0.99 | 0.045 |
| rs3803662 (CT/TT vs. CC) | −0.469 | 0.187 | 0.63 | 0.43–0.90 | 0.012 |
Discussion
In our present study, we investigated the effect of TOX3 rs3803662 on survival of gastric cancer patients. Dominant model was the best-fitting model for TOX3 rs3803662. The rs3803662 CT/TT genotype exhibited a significant association with better survival among diffuse-type gastric cancer patients. Such a difference was not found for intestinal-type gastric cancer patients. The mechanism of gastric cancer survival patterns which were varied by histological subtypes remains to be elucidated. The results differing in the two major histological types of gastric cancer may be due to their diverge in many clinical characteristics and molecular, including their etiology, epidemiology, carcinogenesis and progression, mRNA and/or protein expression profile, gene copy numbers, microsatellite instability, loss of heterozygosity and mutation profile [17]. Furthermore, given the effects of TOX3 on neuronal cells [9], rs3803662 increasing the survival of diffuse-type gastric cancer in a dominant model might be attribute to inhabit a host cell into a tumor cell or cancer cells multiply rapidly by unknown mechanisms. In our study, no drinking is a protective factor for diffuse-type gastric cancer patients' survival, it can be seen that primary prevention popularized into people's daily life will be beneficial to decrease the risk of death among diffuse-type gastric cancer. To the best of our knowledge, this is the first study to examine the association between TOX3 rs3803662 and gastric cancer survival. Our findings provide support for the genome-based studies of gastric cancer survival, especially to Chinese populations.
Gene-gene interaction is a hot topic in genetic epidemiology, including prognosis study. Not a single locus can fully explain their genetic susceptibility and prognosis. Liu et al. reported that the multifactor interactions among polymorphisms in MMP-2, FASL and FAS play more important role in the development of GCA. Similarly, they also found that a gene-dose effect in association with prognosis of NSCLC patients [18], [19]. Winkelmann et al. observed the rs3803662 which is in low LD with rs3104767 showed association to RLS (λ-corrected nominal PGWA = 7.29×10−7). However, logistic regression analysis conditioned on rs3104767 demonstrated that this association is dependent on rs3104767 [20]. Therefore the results above raised the possibility that a combination of SNPs within TOX3 or elsewhere in the gene act cumulatively to increase the risk.
Riaz et al. found that the risk alleles of rs3803662 near the TOX3 gene was associated with a lower expression of TOX3 mRNA in breast cancer and hypothesized a tumor suppressor role of this gene [21]. These findings suggest the critical roles of TOX3 in diseases. It is noted that the specific SNPs and genes related to prognosis may differ from those involved in susceptibility. Nevertheless, the variant identified in breast cancer studies may have the same impact on gastric cancer risk. Thus, whether rs3803662 which changed in the prognosis of gastric cancer may also influence high risk for the disease in patients is needed to warrant. If validated in further research, TOX3 rs3803662 might also be taken as potential genetic marker for gastric cancer susceptibility except for prognosis.
Some limitations should be considered when we interpret the results. First, our study had been implemented in populations of Chinese; whether there is some difference for the same SNP in the characteristics and survival of gastric cancer among different populations needs to be further tested. Second, more research using larger patient populations is needed to confirm our findings due to a relatively small sample size in stratified analysis as well as to exclude the possibility of association occurred by chance. Third, H. pylorus is generally accepted to be the major etiologic agent for peptic ulcer disease as well as for gastric adenocarcinoma [22]. However, we did not investigate on H. pylori infection because there was not enough patient clinical information.
In conclusion, TOX3 rs3803662 plays an important role in the survival of gastric cancer patients, especially can be considered as an independent prognostic marker of diffuse-type gastric cancer. Importantly, our results offer a very promising prospect for exploring and translating our findings into further potential clinical and diagnostic therapeutic applications in gastric cancer.
Funding Statement
The work was supported by grants from National Natural Science Foundation of China to Dr. J Chen (Grant No. 81071641), Dr. Z Xu (Grant No. 81000880); a grant from National Basic Science Research Program to Dr. J Chen (2013CB911300); a grant from Jiangsu Provincial 12th Five-Year Program on Developing Health by Technology and Education Project to Dr. J Chen, Dr. H. Zhu (Grant No. 81001274), Dr. Z Zhang (30972444, 81230068 and BK2010080), Dr. M. Wang (81102089 and BK2011773), the Qin Lan Project of Jiangsu Provincial Department of Education, and by the Priority Academic Program Development of Jiangsu Higher Education Institutions (Public Health and Preventive Medicine). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Wang W, Bu XM, Wang J, Zhang N, Zhao CH (2012) The expression of ARHI in pT2a and pT2b stage gastric cancer and its clinical significance. Oncol Rep 27: 1953–1959. [DOI] [PubMed] [Google Scholar]
- 2. Chen X, Jiang K, Hu J, Zhang B, Gou H, et al. (2008) Cost-effectiveness analysis of chemotherapy for advanced gastric cancer in China. World J Gastroenterol 14: 2715–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meyer H, Wilke H (2011) Treatment strategies in gastric cancer. Dtsch Arztebl Int 108: 698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kushi L, Byers T, Doyle C, Bandera E, McCullough M, et al. (2006) American Cancer Society Guidelines on Nutrition and Physical Activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin 56: 254–281. [DOI] [PubMed] [Google Scholar]
- 5. Zhao Q, Wang W, Zhang Z, Wang S, Wang M, et al. (2011) A genetic variation in APE1 is associated with gastric cancer survival in a Chinese population. Cancer Sci 102: 1293–1297. [DOI] [PubMed] [Google Scholar]
- 6. Wang M, Bai J, Tan Y, Wang S, Tian Y, et al. (2011) Genetic variant in PSCA predicts survival of diffuse-type gastric cancer in a Chinese population. Int J Cancer 129: 1207–1213. [DOI] [PubMed] [Google Scholar]
- 7. Stacey SN, Manolescu A, Sulem P, Rafnar T, Gudmundsson J, et al. (2007) Common variants on chromosomes 2q35 and 16q12 confer susceptibility to estrogen receptor-positive breast cancer. Nat Genet 39: 865–869. [DOI] [PubMed] [Google Scholar]
- 8. Yuan SH, Qiu Z, Ghosh A (2009) TOX3 regulates calcium-dependent transcription in neurons. Proc Natl Acad Sci U S A 106: 2909–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dittmer S, Kovacs Z, Yuan S, Siszler G, Kögl M, et al. (2011) TOX3 is a neuronal survival factor that induces transcription depending on the presence of CITED1 or phosphorylated CREB in the transcriptionally active complex. J Cell Sci 124: 252–260. [DOI] [PubMed] [Google Scholar]
- 10. Antoniou A, Spurdle A, Sinilnikova O, Healey S, Pooley K, et al. (2008) Common breast cancer-predisposition alleles are associated with breast cancer risk in BRCA1 and BRCA2 mutation carriers. Am J Hum Genet 82: 937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gudmundsdottir ET, Barkardottir RB, Arason A, Gunnarsson H, Amundadottir LT, et al. (2012) The risk allele of SNP rs3803662 and the mRNA level of its closest genes TOX3 and LOC643714 predict adverse outcome for breast cancer patients. BMC Cancer 12: 621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fasching PA, Pharoah PD, Cox A, Nevanlinna H, Bojesen SE, et al. (2012) The role of genetic breast cancer susceptibility variants as prognostic factors. Hum Mol Genet 21: 3926–3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xu CY, Guo JL, Jiang ZN, Xie SD, Shen JG, et al. (2010) Prognostic role of estrogen receptor alpha and estrogen receptor beta in gastric cancer. Ann Surg Oncol 17: 2503–2509. [DOI] [PubMed] [Google Scholar]
- 14. Hohenberger P, Gretschel S (2003) Gastric cancer. Lancet 362: 305–315. [DOI] [PubMed] [Google Scholar]
- 15. Sobin L, Fleming I (1997) TNM Classification of Malignant Tumors, fifth edition(1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer 80: 1803–1804. [DOI] [PubMed] [Google Scholar]
- 16. Xiao HW, Lai XY, Luo Y, Shi JM, Tan YM, et al. (2011) Relationship between TNFA, TNFB and TNFRII gene polymorphisms and outcome after unrelated hematopoietic cell transplantation in a Chinese population. Bone Marrow Transplant 46: 400–407. [DOI] [PubMed] [Google Scholar]
- 17. Vauhkonen M, Vauhkonen H, Sipponen P (2006) Pathology and molecular biology of gastric cancer. Best Pract Res Clin Gastroenterol 20: 651–674. [DOI] [PubMed] [Google Scholar]
- 18. Liu L, Wu C, Wang Y, Zhong R, Wang F, et al. (2011) Association of candidate genetic variations with gastric cardia adenocarcinoma in Chinese population: a multiple interaction analysis. Carcinogenesis 32: 336–342. [DOI] [PubMed] [Google Scholar]
- 19. Liu L, Wu C, Wang Y, Zhong R, Duan S, et al. (2011) Combined effect of genetic polymorphisms in P53, P73, and MDM2 on non-small cell lung cancer survival. J Thorac Oncol 6: 1793–1800. [DOI] [PubMed] [Google Scholar]
- 20. Winkelmann J, Czamara D, Schormair B, Knauf F, Schulte EC, et al. (2011) Genome-wide association study identifies novel restless legs syndrome susceptibility loci on 2p14 and 16q12.1. PLoS Genet 7: e1002171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riaz M, Berns EM, Sieuwerts AM, Ruigrok-Ritstier K, de Weerd V, et al.. (2011) Correlation of breast cancer susceptibility loci with patient characteristics, metastasis-free survival, and mRNA expression of the nearest genes. Breast Cancer Res Treat. [DOI] [PubMed]
- 22. Choi IK, Sung HJ, Lee JH, Kim JS, Seo JH (2012) The relationship between Helicobacter pylori infection and the effects of chemotherapy in patients with advanced or metastatic gastric cancer. Cancer Chemother Pharmacol 70: 555–558. [DOI] [PubMed] [Google Scholar]