Abstract
The host pathogen interaction is strikingly complex during HIV infection. While several immune effector mechanisms (i.e., cytotoxic T cells, neutralizing antibodies, NK cells, etc) can play a strong antiviral role in vivo, the virus is remarkably able to evade these responses. In addition, the virus preferentially infects and kills activated memory CD4+ T cells, thus exploiting the host antiviral immune response as a source of new cellular targets for infection. Recent advances in understanding (i) how HIV perturbs the host immune system, (ii) how the immune system fights HIV; and (iii) how HIV disease persists when virus replication is suppressed by antiretroviral drugs may hopefully lead to better prevention and treatment strategies for this deadly viral infection.
Introduction
Human Immunodeficiency Virus (HIV) infection, when left untreated, almost invariably results in a progressive and irreversible state of immunodeficiency (i.e., acquired immune deficiency syndrome or AIDS), whose pathogenesis is a complex phenomenon that involves numerous factors related to both the virus and the host immune system. Our understanding of HIV pathogenesis has significantly improved over the past few years, and it is hoped that these advances will soon translate in more effective interventions to prevent, treat, and hopefully cure HIV infection and AIDS. In this article, we will briefly review the most recent advances concerning how HIV infection perturbs the host immune system, how the immune system fights the virus, and how HIV disease persists when virus replication is suppressed by antiretroviral drugs.
How HIV perturbs host immune function
Many aspects of HIV/AIDS pathogenesis have been elucidated by in vivo studies of non-human primates infected with the Simian Immunodeficiency Virus (SIV), including both “experimental” pathogenic hosts, such as Asian macaques, and “natural” non-pathogenic hosts such as African sooty mangabeys (SMs) (1–2). HIV and SIV primarily infect activated CD4+ memory T cells expressing the main virus co-receptor CCR5, and CD4+ memory T cells are progressively depleted in both blood and mucosal tissues during pathogenic HIV/SIV infections. Of note, a recent study showed that, in naïve CD4+ T cell-deficient macaques, in which thymectomy abrogated naïve CD4+ T cell recovery after Ab-mediated CD4+ T cell depletion, SIV replication and CD4+ T cell dynamics post-infection are similar to control animals, thus confirming that memory CD4+ T cells are the key pathogenic players in SIV-infected macaques (3**). However, within the memory CD4+ T cell pool several subsets exist, both in terms of maturation along the axis of central, transitional, and effector memory cells, and in terms of lineage differentiation (Th1, Th2, Th17, regulatory T cells, follicular T helper cells, and possibly others) (4). A series of studies have led to the formulation of a pathogenic model (see Figure 1) in which the pattern of infected CD4+ T cells is the key determinant of HIV/SIV pathogenesis (5–11). In this view, infection of central memory CD4+ T cells (TCM) is a strong correlate of pathogenesis, while infections in which TCM are relatively spared (i.e., SIV-infected SMs or HIV-infected “non-progressors”) are typically non-pathogenic (5–11).
Figure 1.
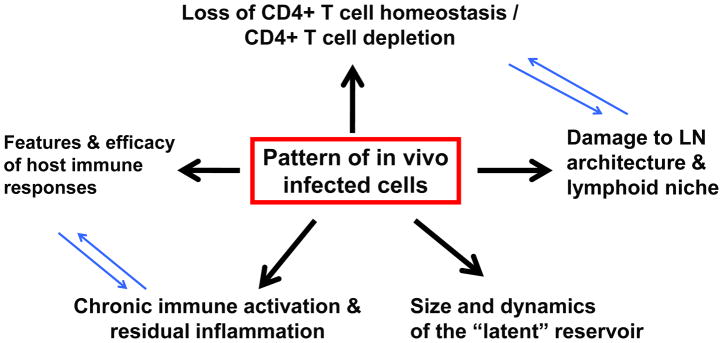
Te pattern of virus-infected cells in vivo influences many aspects of HIV/AIDS pathogenesis.
The molecular mechanisms determining which CD4+ T cell subsets are predominantly infected in vivo remain incompletely understood. In the non-pathogenic model of SIV infection of SMs a factor protecting CD4+ TCM from virus infection is the low expression of CCR5 upon activation (7). However, numerous additional host restriction factors may affect the pattern of infected cells in vivo (12). The family of HIV and SIV host restriction factors (HRFs) include molecules such as TRIM-5a, APOBEC-3G, BST-2/Tetherin, and various others (12). More recently it was discovered that the SAM domain HD domain-containing protein 1 (SAMHD1), a deoxynucleoside triphosphate triphosphohydrolase that restricts HIV and SIV replication by inhibiting viral DNA synthesis through depletion the intracellular dNTP pool, is the host factor counteracted by the viral protein Vpx (13–16). Intriguingly, several recent studies have shown that HRFs, including TRIM-5a (17), Tetherin (18–19), and APOBEC-3G (20), also act as innate immune sensors. Since the expression of HRFs is up-regulated in response to type I interferons, these studies define a novel and potentially important link between host restriction and innate antiviral immunity that may play a major role in determining the pattern of virus-infected cells in vivo during HIV and SIV infections.
While the direct role of HIV in infecting and killing CD4+ T cells is clearly central to HIV/AIDS pathogenesis, several “indirect” mechanisms of immune deficiency have been described, including chronic immune activation and inflammation, bystander death of uninfected cells, and ineffective T cell regeneration (reviewed in 21). The pathogenic role of immune activation is confirmed by the observations that HIV-infected individuals with undetectable viremia but significant immune activation can progress to AIDS (22) and that individuals with preserved CD4+ T cell counts despite persistently high viremia (i.e., viremic non-progressors) show limited immune activation (23–24). Interestingly, systemic immune activation is also a possible determinant of viral acquisition if present prior to infection (25–28). An additional important feature of pathogenic SIV/HIV infections is the functional and structural impairment of lymph nodes due to (i) virus trapping in follicular dendritic cells and virus replication in CD4+ follicular T helper cells (*8, 29, *30, *31), (ii) disruption of the fibroblastic reticular network, with increased collagen deposition (32), and (iii) failure of CD4+ T cell homeostasis (32). In SIV-infected macaques, the structural impairment of lymph nodes can be partially restored by the administration of TNF antagonists during the acute phase of infection (33). An additional hallmark of HIV/AIDS pathogenesis is the loss of mucosal immune integrity with consequent translocation of microbial products from the intestinal lumen to the portal and systemic circulation, where they cause persistent innate immune activation (34). Recent advances in this area include the observations that pathogenic SIV infection is associated with major changes in the intestinal virome (35) and that probiotic/prebiotic supplementation of antiretroviral treatment improves the gastrointestinal immunity in SIV-infected macaques (**36).
How the immune system fights HIV
Several components of the host immune system can suppress virus replication in vivo, including CD8+ T cells, CD4+ T cells, NK cells, neutralizing antibodies, and possibly other mechanisms as well. In the case of CD4+ T cells, the recent characterization of potential direct antiviral effects (37,38) underscores the complex pathophysiologic role of these cells that, when activated, also serve as key targets for the virus (39). As for the CD8+ T cells, their role is well characterized in terms of both benefit, as mediators of the “elite controller” phenotype, and limitations as shown by virus CTL epitope escape and insufficient efficacy when induced by candidate AIDS vaccines (reviewed in 40). The main recent advances in this area are the observations that (i) the protection conferred by live-attenuated SIV vaccines is mediated by activated effector memory CD8+ T cells in lymph nodes that can suppress or even completely contain early SIV replication at these sites (*41); (ii) rhesus cytomegalovirus (rCMV)-based vectors expressing SIV antigens confer strong protection from highly pathogenic challenge through the persistent induction of effector CD8+ T cells in mucosal tissues (**42); and (iii) macaques expressing “protective” MHC class I alleles (a model for HIV elite controllers) that were immunized against the appropriately MHC-restricted Nef and Vif epitopes potently suppressed virus replication after pathogenic SIV challenge (*43). Collectively, these elegant studies in non-human primates directly demonstrated the potential of antiviral CD8+ T cell responses and how this antiviral activity takes different flavors based on the vector used to elicit these responses as well as the host genetic background. Whether similar responses can be elicited in humans by specific immunogens used as candidate AIDS vaccines remains to be determined.
Perhaps the most striking recent advances in our understanding of HIV-specific immune responses are in the area of HIV-specific neutralizing antibodies. A series of breakthrough studies conducted independently in several laboratories have shown that a substantial portion of HIV-infected individuals produce very potent and broadly reactive neutralizing antibodies (bnAbs) (44–50). In addition, these studies revealed the key targets for neutralization in the HIV Envelope protein (i.e., CD4 binding sites, the so-called membrane-proximal external region or MPER, and the N-linked glycans in positions N160 and N332), and some previously unrecognized structural and genetic features of these bnAbs (i.e., long complementary determining region-H3 with ~30 aminoacids, high rate of somatic mutation, and the presence of genetic insertions or deletions) (44–50). Intriguingly, the “germline” versions of a subset of these bnABs (i.e., those directed against the CD4 binding site) do not seem to recognize HIV Envelope proteins (**51), therefore suggesting that a complex, multi-step process of sequential antigenic stimulation of the relevant B cell clones is required. Following viral and antibody evolution from early stages of infection up to the point of bnAb development may provide valuable insight into the interplay between antibody lineage development and viral evolution. A recent study by Liao et al showed that the CH103 CD4-binding site bnAb develops relatively early in infection and that the “unmutated” common ancestor of CH103 avidly binds the transmitted/founder virus (52). However, increased neutralizing breadth occurs after significant diversification of viral variants and the induction of further mutations in CH103 lineage antibodies. In another study, Moore et al. elucidate the case of two HIV-infected individuals wherein the transmitted/founder virus develops an escape mutation resulting in a glycan-shift from Asn 334 to Asn 332 on the viral envelope, thus inducing PGT128-like glycan-dependent bnAbs in these individuals (53). These reports provides evidence that viral evolution within the infected host facilitates development of bnAbs. Of note, Moore et al. also found that while the conserved viral epitope for glycan-dependent bnAB arises quite frequently in HIV-infected individuals, this does not guarantee the development of the bnAb in that individual.
The naturally occurring bnAbs, in most cases, are produced several years after the initial infection and in the context of chronic virus replication, which they are incapable of suppressing likely due to escape mechanisms. However, passive transfer experiments in macaques have convincingly demonstrated that HIV-specific bnAbs can provide complete protection against transmission of chimeric SHIVs expressing the HIV-1 envelope glycoprotein (54,55). These experiments strongly suggest that a vaccine designed to elicit and to maintain such antibodies would protect against HIV-1. Most recently, Moldt et al. demonstrated that PGT121, one of the most potent bnAbs identified so far, provides sterilizing immunity to macaques against high-dose mucosal SHIV challenge after passive administration of only 1 mg/kg of the Ab, thus at lower dose than previously observed (56). Further elucidation of the genetic, structural, and functional properties of these naturally occurring bnAbs provides essential information to be used for the rational design of novel and more effective immunogens. In an attempt to design envelope-based immunogens capable of specifically and robustly inducing potent bnAbs, Jardine et al. have used computation-guided in vitro screening to engineer a gp120 outer domain immunogen that can activate both germline and mature VRC01-class B cells thus providing a promising immunogen to be tested in preclinical macaque studies (57).
In parallel to this progress, there has been significant recent advance in understanding the role of follicular T helper cells (TFH) as both helpers for HIV-specific antibody responses and targets for virus infection. A series of important studies in both HIV-infected humans and SIV-infected macaques have independently shown that while TFH are a preferential target for direct virus infection they are not depleted as compared to other memory CD4+ T cell subsets (8, 29 *30, *31, *58). Recently, Cubas et al. showed that ligation of PD1 on TFH cells reduces IL-21 production and ICOS expression, suggesting an impaired ability of TFH cells to provide “help” to germinal center B cells in chronic stages of HIV infection (59). The full extent to which TFH contribute to the generation of HIV-specific bnABs-- in the context of either natural infection of immunization of uninfected individuals-- is the object of intense investigation.
Pathogenic HIV/SIV infections are associated with vigorous innate immune responses that result in high production of type I interferons (IFN-I) and persistent upregulation of interferon stimulated genes (ISGs) (60–63). These studies have emphasized the spectacular dichotomy of IFN-I signaling in AIDS pathogenesis, in which IFN-dependent HRFs (i.e., TRIM5a, Tetherin, APOBECs, SAMHD1, TREX etc) act as potent antiviral effectors and, at the same time, chronic IFN-I signaling has been associated with both HIV/SIV-disease progression and poor immunological response to ART (60–64). In a recent study, IFN was administered to SIV-infected sooty mangabeys, in which low immune activation is associated with transient ISG up-regulation, resulting in decreased virus replication and no signs of accelerated disease progression (65). This study emphasized the in vivo antiviral effect of IFNs and supported the possibility that this effect may be exploited in the clinical setting of ART-treated HIV-infected individuals (66–68).
Host-pathogen interaction in the age of ART
An increasingly high percentage of HIV-infected individuals receive antiretroviral therapy (ART) that is capable of fully suppressing virus replication for prolonged periods of time. While ART results in a dramatic reduction of HIV-associated morbidity and mortality, the currently available treatments do not eradicate the infection nor achieve a full recovery from the immune dysfunction induced by the virus (68). In fact, many studies have shown that residual immune activation and/or incomplete immune reconstitution occur, at various levels of severity, in virtually all ART-treated HIV-infected individuals, and are involved in the development of the so-called “end-organ disease” which includes cardiovascular disease, HIV-associated neurocognitive dysfunction, metabolic and kidney abnormalities, bone disease, and others (reviewed in 69). Elucidating the exact cellular and molecular mechanisms responsible for this end organ disease will be essential to design intervention to use, in addition to standard ART, in the clinical management of HIV-infected individuals. The potential mechanisms that have been proposed include low level virus replication; disruption of lymphoid tissue architecture; persistent mucosal immune dysfunction and microbial translocation; residual innate immune activation via the TLR7/9-type I IFN pathway; ineffective homeostatic signals; activation of atherogenic pathways; and persistent replication of CMV (69). Importantly, initial studies aimed at reverting these mechanisms with specific pharmacological interventions are showing some promise (**36, 70–71). Whether and to what extent some residual virus replication contributes to the persistent HIV-related morbidity under ART is unclear. On the other hand, a full suppression of virus replication would be necessary to achieve a “functional cure” for HIV infection, which is defined as undetectable viremia in absence of ART, lack of measurable immunological abnormalities, and projected lifespan comparable to age-matched HIV-uninfected individuals. A key obstacle in this regard is the presence of persistent reservoirs of latently infected cells that are able, in most cases, to reinstate high-level virus replication soon after ART interruption (68). Several studies suggested that resting memory CD4+ T cells (and, in particular, central memory CD4+ T cells) are the main contributors to this reservoir (reviewed in 72), consistent with the key role of these cells in the natural history of HIV pathogenesis (5–11). Potential approaches targeting this CD4+ TCM reservoir include inducing the reactivation of latent virus, permanent silencing of latent integrated provirus, promoting the differentiation of TCM into shorter lived cells, boosting of cellular antiviral immune responses, treating the residual immune activation with immunomodulatory agents, and eliminating the reservoirs with replacement by uninfected cells through a combination of lymphoablation and gene and/or stem cell therapy (68, 72). However, any such approach appears unlikely to succeed unless it takes into full account the complex biology of CD4+ T cell subsets, and how this biology may influence the mechanisms by which the persistent reservoirs are established and maintained under ART.
Conclusions
The host-pathogen interaction during HIV infection is peculiarly complex due to the fact that the virus infects immune cells, and that antiviral immune responses end up paradoxically favoring virus replication through the creation of cellular targets for infection. Several aspects of this complex interaction have been clarified, and with further progress it is reasonable to hope that a vaccine and a cure for this deadly infection will emerge in the not so distant future.
Highlights.
Pattern of virus-infected CD4+ T cells as a key factor in HIV/AIDS pathogenesis.
Novel properties of antiviral CTL responses described in macaques.
Immunogens to elicit HIV-specific bnAbs as key to developing an AIDS vaccine.
Viral reservoir clearance under ART as the way to “cure” HIV infection..
Acknowledgments
The authors wish to thank Dr. Ann Chahroudi for critical review of this manuscript. This work was supported in part by the Office of Research Infrastructure Programs/OD P51OD011132 to the Yerkes National Primate Research Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hatziioannou T, Evans DT. Animal models for HIV/AIDS research. Nat Rev Microbiology. 2012;10:852–867. doi: 10.1038/nrmicro2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chahroudi A, Bosinger SE, Vanderford TH, Paiardini M, Silvestri M. Natural SIV Hosts: Showing AIDS the Door. Science. 2012;335(6073):1188–1193. doi: 10.1126/science.1217550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **3.Okoye AA, Rohankhedkar M, Abana C, Pattenn A, Reyes M, Pexton C, Lum R, Sylwester A, Planer SL, Legasse A, et al. Naive T cells are dispensable for memory CD4+ T cell homeostasis in progressive simian immunodeficiency virus infection. J Exp Med. 2012;209(4):641–651. doi: 10.1084/jem.20112071. In this interesting study, the authors used thymectomy followed by CD4+ lymphocyte depletion to develop a model of “CD4+ naïve T cell deficient” macaques. When infected with SIV, these animals show dynamics of virus replication and CD4+ T cell depletion similar to controls, thus confirming that memory CD4+ T cells (and not naive CD4+ T cells) are the primary pathogenic players during lentivirus-induced immunodeficiency. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–89. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okoye A, Meier-Schellersheim M, Brenchley JM, Hagen SI, Walker JM, Rohankhedkar M, Lum R, Edgar JB, Planer SL, Legasse A, Sylwester AW, et al. Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection. J Exp Med. 2007;204(9):2171–85. doi: 10.1084/jem.20070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenchley JM, Silvestri G, Douek DC. Nonprogressive and progressive primate immunodeficiency lentivirus infections. Immunity. 2010;32(6):737–42. doi: 10.1016/j.immuni.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paiardini M, Cervasi B, Reyes-Aviles E, Micci L, Ortiz AM, Chahroudi A, Vinton C, Gordon SN, Bonsinger SE, Francella N, Hallberg PL, et al. Low levels of SIV infections in sooty mangabey central memory CD4+ T cells are associated with limited CCR5 expression. Nat Med. 2011;17:830–836. doi: 10.1038/nm.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *8.Brenchley JM, Vinton C, Tabb B, Hao XP, Connick E, Paiardini M, Lifson JD, Silvestri G, Estes JD. Differential infection patterns of CD4+ T cells and lymphoid tissue viral burden distinguish progressive and nonprogressive lentiviral infections. Blood. 2012;120(20):4172–81. doi: 10.1182/blood-2012-06-437608. This study provides the first demonstration that pathogenic HIV and SIV infections are characterized by higher virus replication in lymph nodes as compared to non-pathogenic SIV infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmitz JE, Ma ZM, Hagan EA, Wilks AB, Furr KL, Linde CH, Zahn RC, Brenchley JM, Miller CJ, Permar SR. Memory CD4(+) T lymphocytes in the gastrointestinal tract are a major source of cell-associated simian immunodeficiency virus in chronic nonpathogenic infection of African green monkeys. J Virol. 2012;86 (20):11380–5. doi: 10.1128/JVI.01556-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Descours B, Avettand-Fenoel V, Blanc C, Samri A, Mélard A, Supervie V, Theodorou I, Carcelain G, Rouzioux C, Autran B ALT ANRS CO15 Study Group. Immune responses driven by protective human leukocyte antigen alleles from long-term nonprogressors are associated with low HIV reservoir in central memory CD4 T cells. Clin Infect Dis. 2012;54(10):1495–503. doi: 10.1093/cid/cis188. [DOI] [PubMed] [Google Scholar]
- 11.Sáez-Cirión A, Bacchus C, Hocqueloux L, Avettand-Fenoel V, Girault I, Lecuroux C, Potard V, Versmisse P, Melard A, Prazuck T, Descours B, Guergnon J, Viard JP, Boufassa F, Lambotte O, Goujard C, Meyer L, Costagliola D, Venet A, Pancino G, Autran B, Rouzioux C S VISCONTI Study Group. Post treatment HIV-1 controllers with a long-term virological remission after interruption of early initiated antiretroviral therapy ANRS VISCONTI study. PLOS Path. 2013 doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bieniasz PD. An overview of intracellular interactions between immunodeficiency viruses and their hosts. AIDS. 2012 Jun 19;26(10):1243–54. doi: 10.1097/QAD.0b013e328353bd04. [DOI] [PubMed] [Google Scholar]
- 13.Laguette N, Sobhian B, Casartelli N, Ringeard M, Chable-Bessia C, Ségéral E, Yatim A, Emiliani S, Schwartz O, Benkirane M. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature. 2011;474(7353):654–7. doi: 10.1038/nature10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hrecka K, Hao C, Gierszewska M, Swanson SK, Kesik-Brodacka M, Srivastava S, Florens L, Washburn MP, Skowronski J. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature. 2011;474(7353):658–61. doi: 10.1038/nature10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstone DC, Ennis-Adeniran V, Hedden JJ, Groom HC, Rice GI, Christodoulou E, Walker PA, Kelly G, Haire LF, Yap MW, de Carvalho LP, Stoye JP, Crow YJ, Taylor IA, Webb M. HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature. 2011;480(7377):379–82. doi: 10.1038/nature10623. [DOI] [PubMed] [Google Scholar]
- 16.Lahouassa H, Daddacha W, Hofmann H, Ayinde D, Logue EC, Dragin L, Bloch N, Maudet C, Bertrand M, Gramberg T. SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat Immunol. 2012;13(3):223–8. doi: 10.1038/ni.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pertel T, Hausmann S, Morger D, Züger S, Guerra J, Lascano J, Reinhard C, Santoni FA, Uchil PD, Chatel L, et al. TRIM5 is an innate immune sensor for the retrovirus capsid lattice. Nature. 2011;472(7343):361–5. doi: 10.1038/nature09976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galão RP, Le Tortorec A, Pickering S, Kueck T, Neil SJ. Innate sensing of HIV-1 assembly by Tetherin induces NFκB-dependent proinflammatory responses. Cell Host Microbe. 2012;12(5):633–44. doi: 10.1016/j.chom.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tokarev Andrey, Suarez Marissa, Kwan Wilson, Fitzpatrick Kathleen, Singh Rajendra, Guatelli John. Stimulation of NF-κB Activity by the HIV Restriction Factor BST2. J Virol. 2013;87:2046–2057. doi: 10.1128/JVI.02272-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norman JM, Mashiba M, McNamara LA, Onafuwa-Nuga A, Chiari-Fort E, Shen W, Collins KL. The antiviral factor APOBEC3G enhances the recognition of HIV-infected primary T cells by natural killer cells. Nat Immunol. 2(10):975–83. doi: 10.1038/ni.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sodora DL, Silvestri G. Immune activation and AIDS pathogenesis. AIDS. 2008;22 (4):439–46. doi: 10.1097/QAD.0b013e3282f2dbe7. [DOI] [PubMed] [Google Scholar]
- 22.Hunt PW, Brenchley J, Sinclair E, McCune JM, Roland M, Page-Shafer K, Hsue P, Emu B, Krone M, Lampiris H, Douek D, Martin JN, Deeks SG. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197(1):126–33. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choudhary SK, Vrisekoop N, Jansen CA, Otto SA, Schuitemaker H, Miedema F, Camerini D. Low immune activation despite high levels of pathogenic human immunodeficiency virus type 1 results in long-term asymptomatic disease. J Virol. 2007;81(16):8838–42. doi: 10.1128/JVI.02663-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rotger M, Dalmau J, Rauch A, McLaren P, Bosinger SE, Martinez R, Sandler NG, Roque A, Liebner J, Battegay M, et al. Comparative transcriptomics of extreme phenotypes of human HIV-1 infection and SIV infection in sooty mangabey and rhesus macaque. J Clin Invest. 2011;121(6):2391–400. doi: 10.1172/JCI45235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bégaud E, Chartier L, Marechal V, Ipero J, Léal J, Versmisse P, Breton G, Fontanet A, Capoulade-Metay C, Fleury H, et al. Reduced CD4 T cell activation and in vitro susceptibility to HIV-1 infection in exposed uninfected Central Africans. Retrovirology. 2006;3:35. doi: 10.1186/1742-4690-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Songok EM, Luo M, Liang B, Mclaren P, Kaefer N, Apidi W, Boucher G, Kimani J, Wachihi C, Sekaly R, et al. Microarray Analysis of HIV Resistant Female Sex Workers Reveal a Gene Expression Signature Pattern Reminiscent of a Lowered Immune Activation State. PLoS One. 2012;7(1):e30048. doi: 10.1371/journal.pone.0030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suy A, Castro P, Nomdedeu M, García F, López A, Fumero E, Gallart T, Lopalco L, Coll O, Gatell JM, et al. Immunological profile of heterosexual highly HIV-exposed uninfected individuals: predominant role of CD4 and CD8 T-cell activation. J Infect Dis. 2007;196(8):1191–201. doi: 10.1086/521193. [DOI] [PubMed] [Google Scholar]
- 28.Naranbhai V, Abdool Karim SS, Altfeld M, Samsunder N, Durgiah R, Sibeko S, Abdool Karim Q, Carr WH CAPRISA004 TRAPS team. Innate Immune Activation Enhances HIV Acquisition in Women, Diminishing the Effectiveness of Tenofovir Microbicide Gel. J Infect Dis. 2012;206(7):993–1001. doi: 10.1093/infdis/jis465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong JJ, Amancha PK, Rogers K, Ansari AA, Villinger F. Spatial alterations between CD4(+) T follicular helper, B, and CD8(+) T cells during simian immunodeficiency virus infection: T/B cell homeostasis, activation, and potential mechanism for viral escape. J Immunol. 2012;188(7):3247–56. doi: 10.4049/jimmunol.1103138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *30.Petrovas C, Yamamoto T, Gerner MY, Boswell KL, Wloka K, Smith EC, Ambrozak DR, Sandler NG, Timmer KJ, Sun X, et al. CD4 T follicular helper cell dynamics during SIV infection. J Clin Invest. 2012;122(9):3281–94. doi: 10.1172/JCI63039. One of three studies showing that while follicular helper T cells are important targets for HIV/SIV infection, their number are relatively expanded compared to other CD4+ T cell subsets. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *31.Perreau M, Savoye AL, De Crignis E, Corpataux JM, Cubas R, Haddad EK, De Leval L, Graziosi C, Pantaleo G. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med. 2013;210(1):143–56. doi: 10.1084/jem.20121932. One of three studies showing that while follicular helper T cells are important targets for HIV/SIV infection, their number are relatively expanded compared to other CD4+ T cell subsets. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng M, Smith AJ, Wietgrefe SW, Southern PJ, Schacker TW, Reilly CS, Estes JD, Burton GF, Silvestri G, Lifson JD, et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest. 2011;121(3):998–1008. doi: 10.1172/JCI45157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tabb B, Morcock DR, Trubey CM, Quiñones OA, Hao XP, Smedley J, Macallister R, Piatak M, Jr, Harris LD, Paiardini M, Silvestri G, Brenchley JM, Alvord WG, Lifson JD, Estes JD. Reduced inflammation and lymphoid tissue immunopathology in rhesus macaques receiving anti-tumor necrosis factor treatment during primary simian immunodeficiency virus infection. J Infect Dis. 2013;207(6):880–92. doi: 10.1093/infdis/jis643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brenchley JM, Douek DC. Microbial translocation across the GI tract. Annu Rev Immunol. 2012;30:149–73. doi: 10.1146/annurev-immunol-020711-075001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Handley SA, Thackray LB, Zhao G, Presti R, Miller AD, Droit L, Abbink P, Maxfield LF, Kambal A, Duan E, et al. Pathogenic simian immunodeficiency virus infection is associated with expansion of the enteric virome. Cell. 2012;151(2):253–66. doi: 10.1016/j.cell.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **36.Klatt NR, Canary LA, Sun X, Vinton CL, Funderburg NT, Morcock DR, Quiñones M, Deming CB, Perkins M, Hazuda DJ, et al. Probiotic/prebiotic supplementation of antiretrovirals improves gastrointestinal immunity in SIV-infected macaques. J Clin Invest. 2013 doi: 10.1172/JCI66227. pii: 66227. This study provides strong experimental evidence suggesting that the HIV/SIV-associated mucosal immune dysfunction and chronic immune activation can be reverse by inducing changes in the intestinal microbiome by treating SIV-infected macaques with prebiotic/probiotic microorganisms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ortiz AM, Klatt NR, Li B, Yi Y, Tabb B, Hao XP, Sternberg L, Lawson B, Carnathan PM, Cramer EM, et al. Depletion of CD4+ T cells abrogates post-peak decline of viremia in SIV-infected rhesus macaques. J Clin Invest. 2011;121:4433–45. doi: 10.1172/JCI46023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soghoian DZ, Jessen H, Flanders M, Sierra-Davidson K, Cutler S, Pertel T, Ranasinghe S, Lindqvist M, Davis I, Lane K, et al. HIV-specific cytolytic CD4 T cell responses during acute HIV infection predict disease outcome. Sci Transi Med. 2012;4(123):123ra25. doi: 10.1126/scitranslmed.3003165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klatt NR, Silvestri G. CD4+ T cells and HIV: A paradoxical Pas de Deux. 4(123):123ps4. doi: 10.1126/scitranslmed.3003862. [DOI] [PubMed] [Google Scholar]
- 40.Makedonas G, Betts MR. Living in a house of cards: re-evaluating CD8+ T-cell immune correlates against HIV. Immunol Rev. 2011;239(1):109–24. doi: 10.1111/j.1600-065X.2010.00968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *41.Fukazawa Y, Park H, Cameron MJ, Lefebvre F, Lum R, Coombes N, Mahyari E, Hagen SI, Bae JY, Reyes MD, 3rd, et al. Lymph node T cell responses predict the efficacy of live attenuated SIV vaccines. Nat Med. 2012;18(11):1673–81. doi: 10.1038/nm.2934. This study revealed an association between virus-specific effector-differentiated CD8+ T cells in lymph nodes and protection from pathogenic SIV challenge conferred by live-attenuated SIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **42.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–7. doi: 10.1038/nature10003. This study demonstrated the potential of persistent vaccine vectors that elicit strong, mucosal effector CD8+ T cell responses to protect against pathogenic SIV challenge. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *43.Mudd PA, Martins MA, Ericsen AJ, Tully DC, Power KA, Bean AT, Piaskowski SM, Duan L, Seese A, Gladden AD, et al. Vaccine-induced CD8+ T cells control AIDS virus replication. Nature. 2012;491(7422):129–33. doi: 10.1038/nature11443. This study described a correlation between the magnitude of vaccine-elicited CD8+ T cell responses to Mamu-B*08-restricted epitopes in the SIV Vif and Nef proteins and containment of chronic phase viral loads after SIV challenge. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TY, Pietzsch J, Fenyo D, Abadir A, Velinzon K, et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333:1633–7. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu X, Zhou T, Zhu J, Zhang B, Georgiev I, Wang C, Chen X, Longo NS, Louder M, McKee K, et al. Focused Evolution of HIV-1 Neutralizing Antibodies Revealed by Structures and Deep Sequencing. Science. 2011;333(6049):1593–602. doi: 10.1126/science.1207532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, Wang SK, Ramos A, Chan-Hui PY, Moyle M, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477(7365):466–70. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pejchal R, Doores KJ, Walker LM, Khayat R, Huang PS, Wang SK, Stanfield RL, Julien JP, Ramos A, Crispin M, et al. A Potent and Broad Neutralizing Antibody Recognizes and Penetrates the HIV Glycan Shield. Science. 2011;334(6059):1097–103. doi: 10.1126/science.1213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McLellan JS, Pancera M, Carrico C, Gorman J, Julien JP, Khayat R, Louder R, Pejchal R, Sastry M, Dai K, et al. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature. 2011;480(7377):336–43. doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walker LM, Sok D, Nishimura Y, Donau O, Sadjadpour R, Gautam R, Shingai M, Pejchal R, Ramos A, Simek MD, et al. Rapid development of glycan-specific, broad, and potent anti–HIV-1 gp120 neutralizing antibodies in an R5 SIV/HIV chimeric virus infected macaque. Proc Natl Acad Sci. 2011;108(50):20125–9. doi: 10.1073/pnas.1117531108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, Imamichi H, Bailer RT, Chakrabarti B, Sharma SK, et al. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature. 2012;491(7424):406–12. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *51.Hoot S, McGuire AT, Cohen KW, Strong RK, Hangartner L, Klein F, Diskin R, Scheid JF, Sather DN, Burton DR, Stamatatos L. Recombinant HIV envelope proteins fail to engage germline versions of anti-CD4bs bNAbs. PLOS Pathog. 2013;9(1):e1003106. doi: 10.1371/journal.ppat.1003106. This study show that HIV Envelope do not bind to the germline B cell receptor versions of broadly neutralizing antibodies, thus providing a novel explanation for the difficulties in eliciting these antibodies with recombinant soluble Env immunogens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liao HX, Lynch R, Zhou T, Gao F, Alam SM, Boyd SD, Fire AZ, Roskin KM, Schramm CA, Zhang Z, et al. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature. 2013 Apr 25;496(7446):469–76. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moore PL, Gray ES, Wibmer CK, Bhiman JN, Nonyane M, Sheward DJ, Hermanus T, Bajimaya S, Tumba NL, Abrahams MR, et al. Evolution of an HIV glycan–dependent broadly neutralizing antibody epitope through immune escape. Nat Med. 2012 Nov;18(11):1688–92. doi: 10.1038/nm.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 55.Hessel AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CM, Landucci G, Forthal DN, Parren PW, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–105. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 56.Moldt B, Rakasz EG, Schultz N, Chan-Hui PY, Swiderek K, Weisgrau KL, Piaskowski SM, Bergman Z, Watkins DI, Poignard P, et al. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc Natl Acad Sci USA. 2012 Nov 13;109(46):18921–5. doi: 10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jardine J, Julien JP, Menis S, Ota T, Kalyuzhniy O, McGuire A, Sok D, Huang PS, MacPherson S, Jones M, et al. Rational HIV immunogen design to target specific germline B cell receptors. Science. 2013;340(6133):711–6. doi: 10.1126/science.1234150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *58.Lindqvist M, van Lunzen J, Soghoian DZ, Kuhl BD, Ranasinghe S, Kranias G, Flanders MD, Cutler S, Yudanin N, Muller MI, et al. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J Clin Invest. 2012;122:3271–80. doi: 10.1172/JCI64314. One of three studies showing that while follicular helper T cells are important targets for HIV/SIV infection, their number are relatively expanded compared to other CD4+ T cell subsets. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cubas RA, Mudd JC, Savoye AL, Perreau M, van Grevenynghe J, Metcalf T, Connick E, Meditz A, Freeman GJ, Abesada-Terk G, Jr, et al. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat Med. 2013;19(4):494–9. doi: 10.1038/nm.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Herbeuval JP, Nilsson J, Boasso A, Hardy AW, Kruhlak MJ, Anderson SA, Dolan MJ, Dy M, Andersson J, Shearer GM. Differential expression of IFN-alpha and TRAIL/DR5 in lymphoid tissue of progressor versus nonprogressor HIV-1-infected patients. Proc Natl Acad Sci U S A. 2006;103(18):7000–5. doi: 10.1073/pnas.0600363103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hyrcza MD, Kovacs C, Loutfy M, Halpenny R, Heisler L, Yang S, Wilkins O, Ostrowski M, Der SD. Distinct transcriptional profiles in ex vivo CD4+ and CD8+ T cells are established early in human immunodeficiency virus type 1 infection and are characterized by a chronic interferon response as well as extensive transcriptional changes in CD8+ T cells. J Virol. 2007;81(7):3477–86. doi: 10.1128/JVI.01552-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bosinger SE, Li Q, Gordon SN, Klatt NR, Duan L, Xu L, Francella N, Sidahmed A, Smith AJ, Cramer EM, et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest. 2009;119:3556–72. doi: 10.1172/JCI40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jacquelin B, Mayau V, Targat B, Liovat AS, Kunkel D, Petitjean G, Dillies MA, Roques P, Butor C, Silvestri G, et al. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest. 2009;119(12):3544–55. doi: 10.1172/JCI40093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fernandez S, Tanaskovic S, Helbig K, Rajasuriar R, Kramski M, Murray JM, Beard M, Purcell D, Lewin SR, Price P, French MA. CD4+ T-cell deficiency in HIV patients responding to antiretroviral therapy is associated with increased expression of interferon-stimulated genes in CD4+ T cells. J infect Dis. 201;204(12):1927–35. doi: 10.1093/infdis/jir659. [DOI] [PubMed] [Google Scholar]
- 65.Vanderford TH, Slichter C, Rogers KA, Lawson BO, Obaede R, Else J, Villinger F, Bosinger SE, Silvestri G. Treatment of SIV-infected sooty mangabeys with a type-I IFN agonist results in decreased virus replication without inducing hyperimmune activation. Blood. 2012;119(24):5750–7. doi: 10.1182/blood-2012-02-411496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pillai SK, Abdel-Mohsen M, Guatelli J, Skasko M, Monto A, Fujimoto K, Yukl S, Greene WC, Kovari H, Rauch A, et al. Role of retroviral restriction factors in the interferon-α-mediated suppression of HIV-1 in vivo. Proc Natl Acad Sci U S A. 2012;109(8):3035–40. doi: 10.1073/pnas.1111573109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Azzoni L, Foulkes AS, Papasavvas E, Mexas AM, Lynn KM, Mounzer K, Tebas P, Jacobson JM, Frank I, Busch MP, et al. Pegylated Interferon-α2A mono-therapy results in suppression of HIV-1 replication and decreased cell-associated HIV DNA integration. J Infect Dis. 2013;207(2):213–222. doi: 10.1093/infdis/jis663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Katlama C, Deeks SG, Autran B, Martinez-Picado J, van Lunzen J, Rouzioux C, Miller M, Vella S, Schmitz JE, Ahlers J, Richman DD, Sekaly RP. Barriers to a cure for HIV: new ways to target and eradicate HIV-1 reservoirs. Lancet. 2013;381(9883):2109–17. doi: 10.1016/S0140-6736(13)60104-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hunt PW. HIV and inflammation: mechanisms and consequences. Curr HIV/AIDS Rep. 2012;9(2):139–47. doi: 10.1007/s11904-012-0118-8. [DOI] [PubMed] [Google Scholar]
- 70.Ganesan A, Crum-Cianflone N, Higgins J, Qin J, Rehm C, Metcalf J, Brandt C, Vita J, Decker CF, Sklar P, et al. High dose atorvastatin decreases cellular markers of immune activation without affecting HIV-1 RNA levels: results of a double-blind randomized placebo controlled clinical trial. J Infect Dis. 2011;203(6):756–64. doi: 10.1093/infdis/jiq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Piconi S, Parisotto S, Rizzardini G, Passerini S, Terzi R, Argenteri B, Meraviglia P, Capetti A, Biasin M, Trabattoni D, et al. Hydroxychloroquine drastically reduces immune activation in HIV-infected, antiretroviral therapy-treated immunologic nonresponders. Blood. 2011;118(12):3263–72. doi: 10.1182/blood-2011-01-329060. [DOI] [PubMed] [Google Scholar]
- 72.Deeks SG, Autran B, Berkhout B, Benkirane M, Cairns S, Chomont N, Chun TW, Churchill M, Di Mascio M, Katlama C, et al. Towards an HIV cure: a global scientific strategy. Nat Rev Immunol. 2012;12(8):607–14. doi: 10.1038/nri3262. [DOI] [PMC free article] [PubMed] [Google Scholar]


