Abstract
The degree to which the MTL system contributes to effective language skills is not well delineated. We sought to determine if the MTL plays a role in single-word decoding in healthy, normal skilled readers. The experiment follows from the implications of the dual-process model of single-word decoding, which provides distinct predictions about the nature of MTL involvement. The paradigm utilized word (regular and irregularly spelled words) and pseudoword (phonetically regular) stimuli that differed in their demand for non-lexical as opposed lexical decoding. The data clearly showed that the MTL system was not involved in single word decoding in skilled, native English readers. Neither the hippocampus, nor the MTL system as a whole showed significant activation during lexical or non-lexical based decoding. The results provide evidence that lexical and non-lexical decoding are implemented by distinct but overlapping neuroanatomical networks. Non-lexical decoding appeared most uniquely associated with cuneus and fusiform gyrus activation biased toward the left hemisphere. In contrast, lexical decoding appeared associated with right middle frontal and supramarginal, and bilateral cerebellar activation. Both these decoding operations appeared in the context of a shared widespread network of activations including bilateral occipital cortex and superior frontal regions. These activations suggest that the absence of MTL involvement in either lexical or non-lexical decoding appears likely a function of the skilled reading ability of our sample such that whole-word recognition and retrieval processes do not utilize the declarative memory system, in the case of lexical decoding, and require only minimal analysis and recombination of the phonetic elements of a word, in the case of non-lexical decoding.
Index terms: reading, medial temporal lobe, language, non-lexical decoding, lexical decoding
INTRODUCTION
The medial temporal lobe (MTL) is traditionally viewed as a key substrate for declarative memory. Recent work (Bartha et al., 2005; Tracy & Boswell, 2008; Hamberger & Seidel, 2009) has suggested the MTL is also involved in aspects of language processing such as naming, fluency, and comprehension. These studies utilized data from patients in whom the mesial temporal lobe is damaged or patholoic (e.g. epilepsy). Though these studies clearly suggest the MTL has some role in language, because they are based on lesions, these studies are not able to infer how healthy a MTL contributes to language. As part of their role in declarative memory, MTL structures such as the hippocampus are known to be involved in retrieval of individual items or exemplars from episodic or semantic stores (for a review see: Agosta, et al., 2009). Work showing MTL activation during retrieval of individual word exemplars (Dolan & Fletcher, 1997; Patterson et al., 2007) supports this view. Other work suggests that the hippocampus is disposed to the parsing, combining and binding of elements, such as words, as part of its role in relational memory (see Cohen et al., 1999). Still more work shows hippocampal activation during the early stages of syntax learning (Optiz and Friederici, 2003), and during the detection of semantically “mismatched” sentence material, activities that both require the analysis of words and word elements.
These features potentially offer a role for the MTL system in aspects of language processing (for a review see Tracy & Boswell, 2008). However, studies examining these MTL functionalities have not examined the process of single word reading. In this project, we examine single word decoding and based upon the differential cognitive demands inherent in the reading of different word types (e.g., phonetically regular versus irregular) tested for selective MTL involvement. The goal was, one, to determine if the MTL system is active in significant ways during single word decoding and, two, clarify which functionalities of the MTL system noted above might contribute to efficient reading.
Reading decoding is the process of taking the visual, written representation of a word (i.e. the words in this sentence) and accessing it’s phonological representation (e.g. Decode- Dee-kohd or dē-kōd'). This phonological representation may be linked to meaning. There are three types of written linguistic stimuli that must be decoded: orthographic (regular), non-orthographic (irregular), and novel (Lee, 2009). There are a number of models of single word decoding (for a review see: Asakawa, 2008; Colheart et al., 1993, Colheart et al., 2001), with the dual-route model of single word reading among the most prominent (Colheart et al., 1977; Colheart et al., 2001). This model postulates that all written linguistic stimuli are decoded by either whole-word based (lexical, also referred to in the literature as exemplar) or rule-based (non-lexical) mechanisms. According to this model, regular words (ex. ‘Word’) and novel, linguistic, orthographic stimuli (i.e. pseudowords that follow regular phonetic rules, ex. ‘Spal’) are decoded by breaking down the visual stream into linguistic units, converting the phonemes into pronounceable units using rules (orthographic to phoneme conversion), combining the units into a pronounceable whole, and then accessing meaning. Accordingly, for competent readers both routes become automatized in that they come to require few cognitive resources and deliberation, and are initiated through bottom-up, stimulus-driven processing. Also, in the model, all words are initially learned via a ruled-based, sublexical approach. Eventually, with high exposure and/or practice, by the non-lexical route, the reading of regular words leads to orthographic recognition and direct application of the rules that parse the word into phonetic units. In contrast, for words that do not follow these orthographic rules, or words that are not pronounced as they are spelled (ex. ‘Gnat’, ‘Know’), reading involves recognition and access to the entire word without parsing or applying orthographic rules. By this route, reading competency arises from the memorization of individual words (exemplars) and the act of decoding involves retrieval of the whole word from lexical stores as an entire unit, and utilizing letter recognition strategies or semantic meaning as clues to recover (“guess at” ) the pronunciation.
A competing theory for the decoding of linguistic stimuli, involving just a single route, has also been supported. According to this model, through repeated writing, reading and speaking, a set of connections is laid down between the orthographic and phonological representations of a word, allowing both orthographic and nonorthographic inputs to be decoded using the same network. Initially, this model was composed of a single pathway mapping orthography to phonology (Seidenberg and McClelland, 1989). More recently, this model (now called the triangular model) has been elaborated upon, with the addition of a semantic processing mechanism (Xiang, Fonteijn, Norris, & Hagoort, 2010; Nagai, Inui, & Iwata, 2010; Chouinard & Goodale, 2010; Kibby et al., 2009). Despite these additions, the triangle model still argues for a single mechanism, a mechanism that integrates orthographic, phonological and, more recently, semantic information in parallel (Harm and Seidenberg, 2004). The dual route and triangle models lead to different predictions about the underlying brain neuroanatomy. The models may make similar predictions about the substrates for orthographic and phonologic processing (see Binder et al., 2005), but the dual-route, committed to seeing lexical processing as characteristic of a separate route, is compelled to predict that this whole word form (semantic) processing generates a separable, more distinct neuroanatomical network.
The dual-mechanism model of word reading has recently been tested using fMRI. Mechelli et al. (2005) was perhaps the first to identify partially distinct anatomical substrates for semantic (lexical) and phonological (non-lexical) contributions to reading. Both involved the left dorsolateral prefrontal cortex, but phonological processing appeared to be carried out by the posterior aspect, involving the premotor cortex, whereas lexical decoding of irregular words was carried out by the inferior frontal gyrus pars triangularis, an area known to be involved in semantic processing (Xiang, Fonteijn, Norris, & Hagoort, 2010; Nagai, Inui, & Iwata, 2010; Chouinard & Goodale, 2010; Kibby et al., 2009). Wilson and Iacoboni (2006) found evidence for unique, but overlapping, neuroanatomical substrates for each of the decoding routes (lexical versus non-lexical). After a meta-analysis of 35 neuroimaging studies,Jobard et al. (2003) also found support for two overlapping but distinct anatomical pathways during reading. The left visual word form area (VWFA) appeared to be activated by both lexical and non-lexical linguistic stimuli. However, non-lexical decoding more distinctly relied on left superior temporal areas, supramarginal gyrus, and the opercular part of the inferior frontal gyrus. In contrast, lexical decoding relied on a basal inferior temporal area, the posterior part of the middle temporal gyrus, and the triangular part of inferior frontal gyrus. This meta-analysis focused on the difference between word and pseudoword reading, therefore potentially reflecting the semantic processing difference and not strictly the difference between lexical and non-lexical decoding. A recent study (Levy et al. 2009), correlating the fMRI activation associated with reading competence, found that the anatomical pathways for word reading (i.e., lexical route) and pseudoword processing (non-lexical) were similar, but weighted quite differently in terms of the inter-correlations, or effective connectivity, between the involved anatomical regions (i.e., middle occipital gyrus, the left occipito-temporal junction, left parietal cortex, and left inferior frontal gyrus). A study which sought to elucidate the neural network involved in the skilled reading of familiar words found distinct networks involved in lexical (left posterior occipito-temporal and right inferior parietal) and non-lexical (left anterior occipito-temporal and left ventral inferior frontal) decoding, with activation in each network increasing as a function of faster reading speed (Seghier et al., 2008). The above studies suggest there exist two at least partially distinct networks, and therefore converge to support the dual-route processing model.
The two distinct decoding routes provide different predictions about the potential role of the MTL in reading. The non-lexical route, because of its reliance on parsing of the visual stream, linking the orthographic units to phonemes, and combining the phonemes into pronounceable units, could take advantage of the MTL system’s skill in parsing and binding information. Though typically thought of as implementing this skill in the context of large semantic units, this MTL functionality may make a subtle contribution to reading by the non-lexical route. On the other hand, the lexical route, because of its reliance on access to whole-word exemplars from lexical storage, could take advantage of the MTL system’s role in either the conscious recollection (hippocampal) and/or familiarity (perirhinal) component of recognition memory (Suchan et al., 2008; Gilboa et al., 2005; Gilboa et al., 2006). Though typically thought of as implementing this skill in the context of declarative autobiographical/episodic memory, the above effects on recollection are long-lasting (Suchan et al., 2008) suggesting this MTL functionality may contribute to the lexical route of reading that could readily draw on declarative semantic memory.
We designed an experiment that would follow from the implications of the dual process model and its distinct predictions about the nature of MTL involvement in the two routes for reading. We chose a single word reading paradigm utilizing word stimuli that differed in their demands for lexical as opposed to non-lexical decoding, and make distinct predictions about the contribution of the MTL to each of these cognitive algorithms. We should note that previous studies using similar experimental paradigms (Binder et al., 2005; Dietz et al., 2005; Wilson et al., 2006) seeking to test aspects of the dual route model, did not specify a priori involvement of any specific anatomical substrate such as the hippocampus. We chose a relatively short word exposure time (1.5 sec) to sharpen the focus on early decoding mechanisms while reducing involvement of secondary semantic or ancillary word association responses that can easily be initiated if subjects are given more time to read (n.b., the Binder et al. utilized 3.0 seconds). This exposure time is not short with respect to the chronometry of initial access to the phonologic or semantic aspects of a stimulus, which is on the order of miliseconds, but it ensures a reading response, allows for a sufficient signal to emerge detectable by fMRI, and should, as noted, preclude the development of secondary semantic associations. We also chose covert, silent reading in order to avoid the large motor artifacts associated with speech in the MRI, but fully acknowledge that reading aloud versus silently likely differ in their neuroanatomies (Dietz et al, 2005). Our decision to use covert reading also served to reduce the auditory feedback of speaking. Speaking would potentially add another step to the algorithm used for word decoding and, therefore, would likely complicate rather than simplify the isolation of activation associated solely with the lexical versus non-lexical decoding.
In the context of competent, native English readers we suspected that both routes to reading were well learned, and that MTL involvement would come as part of involvement in a broader network of lexical access and decoding. Differential involvement of the MTL in lexical versus non-lexical decoding would be taken as evidence that it contributes a specific functionality to the reading of single words. That is, potentially implementing the parsing, binding, and re-combining of phonetic units in the case of non-lexical decoding, or, in contrast, implementing the whole-word recognition memory and retrieval demands (accessing lexical/semantic stores) that are inherent to the process of lexical decoding.
METHODS
Subjects
Subjects (n=17) were native English speakers, healthy adults between the ages of 21 and 27 years (mean 25.1 years (sd=2.7)). There were 10 male and 7 female participants, most were post-graduate or medical students. All subjects were required to complete an Edinburgh Handedness Scale to determine the extent of their right hand use following their self-reported right hand dominance. All were strongly right-handed (mean 89.0 (sd=4.5) (Oldfield, 1971). Prior to scanning, all subjects were screened for MRI compatibility and exclusion of psychiatric disorders, and medical or neurologic conditions with a central nervous system impact. Each participant described English as their first learned and primary language. Prior to scanning, participants were tested on their ability to efficiently read and decode regular, pseudo, and irregular words using well-known and established tests: the Test of Irregular Word Reading Efficiency (TIWRE; Reynolds & Kamphaus, 2007; abbreviated Tir), and the test of Word Reading Efficiency (TOWRE; Torgesen, Wagner, & Rashotte, 1999), with the abbreviation Tr referring to the Sight Word Efficiency subtest that involves reading regular words, and the abbreviation Tp referring to the Phonemic Decoding Efficiency subtest that requires reading phonemically regular, non-meaningful pseudowords. When used as a pre-scan baseline measure, these tasks were administered with the standardized test instructions provided by the test manual. Participants were required to read aloud as quickly and accurately as possible the list of stimulus words presented in columns on a 8’ × 11’ sheet. In accordance with the manual, the score utilized for the Tr and Tp measures was the total number of words correctly pronounced in 45 seconds. The score utilized for the Tir measure was the total number of words correctly pronounced in a list of 50 words read aloud. Based on these tests, it was determined that all subjects were free from any reading disorder and were highly practiced readers, clearly competent at rapidly decoding all three types of stimuli presented in our study. As with any college-educated sample, through standard schooling all subjects in our sample had exposure to at least one language outside of English. Importantly, this exposure never occurred at home (no subject was raised bilingual) and for the vast majority of subjects this exposure was in high school and adolescence, well beyond the critical periods for language development. No subject was fluent, nor even proficient, in another language. Therefore, it is not likely that exposure to this second language effected the neural representation of their native English language, nor influenced English reading decoding procedures. Finally, it should be emphasized that all subjects were screened for a history of neurologic disorder or reading disability (or any learning disability) prior to inclusion in the current study.
All subjects were recruited according to Institutional Review Board guidelines from the Thomas Jefferson University community, provided written informed consent, and were paid for participation.
fMRI Scanning Parameters
Whole-brain scans were collected using a Philips Achieva 3.0 Tesla clinical scanner using an 8 channel head-coil. Single shot echoplanar gradient echo imaging sequence acquiring T2* signal was used with the following parameters: 34 axial slices acquired parallel to the AC-PC line, TE= 35 ms, TR= 2.5 seconds (interleaved, contiguous collections), FOV= 256mm, 128×128 data matrix isotropic voxels, flip angle=90°, bandwidth = 1.802(+/−241.1kHz). The in-plane resolution was 2 mm^3. Prior to collection of the T2* images, T1-weighted images (256 slices) were collected using an MPRage sequence (256 × 256 isotropic voxels; TR= 640ms, TE= 3.2 ms, FOV 256 mm, flip angle 8°) in positions identical to the functional scans to provide an anatomical reference. The in-plane resolution for each T1 slice was 1 mm^3 (axial oblique; angle following anterior, posterior commissure line). Subjects lay in a foam pad to comfortably stabilize the head and were instructed to remain still throughout the task. Survey and field reference inhomogeneity images were collected prior to the start of the study. Each EPI imaging series started with three discarded scans to allow for T1 signal stabilization.
Stimuli and Experimental Task Procedures
Subjects were pre-instructed before entering the scanner to silently read the words presented to them on the video screen. Subjects were told to not memorize, speak, verbalize, or respond in any other way to the stimuli presented. During rest periods the participant was instructed to look at the fixation cross on the center of the screen.
Each subject was presented with four different kinds of experimental stimuli which form our four key experimental conditions: Regular Words, Pseudowords, Irregular Words, and Symbols. As defined by the TOWRE examiner’s manual (Torgesen, Wagner, & Rashotte, 1999), a Regular Word is a word that is spelled orthographically so that it is readily pronounceable following English phonetic rules, i.e. ‘frequent,’ ‘prudent,’ or ‘invoice.’ A Pseudoword is a “word” that has no actual meaning but is readily pronounceable following English phonetic rules, i.e. ‘thundelp,’ ‘klup,’ and ‘skad.’ Words like ‘oblige,’ ‘talk’, and ‘conscience’ are considered “Irregular Words” because they are non-orthographic (Reynolds & Kamphaus, 2007), and do not follow regular grapheme-phoneme and stress rules (McGurn, Starr, & Tropfer et al., 2004). The words from the TOWRE and TIWRE used for the baseline and experimental scanning sessions differed. Words were 4 to 6 syllables in length. An analysis of potential word frequency differences between the regular and irregular words was conducted utilizing the British National College word frequency lists for written words (see Kilgarriff, 1997). The regular and irregular words used for this study showed no difference in this regard ((mean frequency, regular words=79(8.3); mean frequency, irregular words=74(4.8); paired t-test, p=.115)). The words used as experimental stimuli during scanning, while still high in frequency, yielded a frequency estimate of 76% when compared to all words in the English language (n.b., 100% would represent the most common words in English).
As noted, the TOWRE and TIWRE are widely used, well-normed tests of regular, irregular, and pseudoword decoding. The linguistic stimuli were extensively tested and validated (Reynolds & Kamphaus, 2007; McGurn, Starr, & Tropfer et al., 2004) as representative of the word stimulus categories they are intended to represent (regular, irregular, and pseudowords). Separate analyses of orthographic neighborhood (Colheart’s N) and bigram frequency revealed that irregular words showed significantly lower values on these variables compared to both regular and pseudo words, with no difference evident between regular and pseudowords (see Table 1) (Medler & Binder, 2005). This suggests that the more familiar (i.e., frequent) and readily recognizable phonetic patterns of regular words will bias the processing of these stimuli toward the non-lexical route, a bias that is absent in the irregular words. In this sense the stimuli used in this study do express an expected bias, reflecting inherent differences between regularly (regular and pseudo) and irregularly spelled words (i.e. regularity produces reliable co-occurrences in bigrams and regular words are generally more frequent in English language usage)( Massaro & Lucas, 1984). This bias supports reliance on different decoding routes, fully in accord with the dual-route model and our stated hypotheses; namely, that regular words would more likely rely on the non-lexical and irregular words on the lexical route.
Table 1.
Summary Statistics (mean and SD) for experimental stimuli
| Orth.Neigh. | Big.Freq. | |
|---|---|---|
| Regular | 4(11)a | 560(292)c |
| Irregular | 1.2(1.5)b | 2426(5810)d |
| Pseudo | 3(4.5)a | 1026(987)c |
Orth.Neigh.: Orthographic Neighborhood Size, Big.Freq.: Bigram Frequency The One-Way ANOVA?s predicting Orthographic Neighborhood Size (F(2, 125)=11) and Bigram Frequency (F(2, 125)=3.6) were statistically significant. Note, levels of the factor (regular, irregular, pseudo words) with different superscript letters are significantly different at p<.05 based on Scheffe post-hoc tests.
An array of symbols that neither resembled any real object or pronounceable lexical unit, nor carried any semantic value served as a control condition. Lastly, a rest condition was also used, consisting of passive viewing of a fixation cross (constant and un-flashing).
Stimuli were presented in white lower-case, 36 point, bolded Ariel font on a black background in the center of the field of view. Stimuli subtended approximately 5 degrees of visual angle at the center of the field of view. More precisely, it is important to note that the visual angle does, of course, vary slightly depending on the length of the individual word stimulus, though none of the words varied by more than one degree of visual angle. The above estimate of 5 degrees reflects the modal visual angle, which varied depending on additional factors such as the subject’s head size. The symbol control condition used the same font and visual parameters as the word stimuli. All stimuli were presented through E-Prime 1.8 (Psychology Software Tools, Inc.) administered through the Invivo Corp’s Eloquence system, which utilizes a patient display hood with a high resolution 15" LCD monitor (30° FOV).
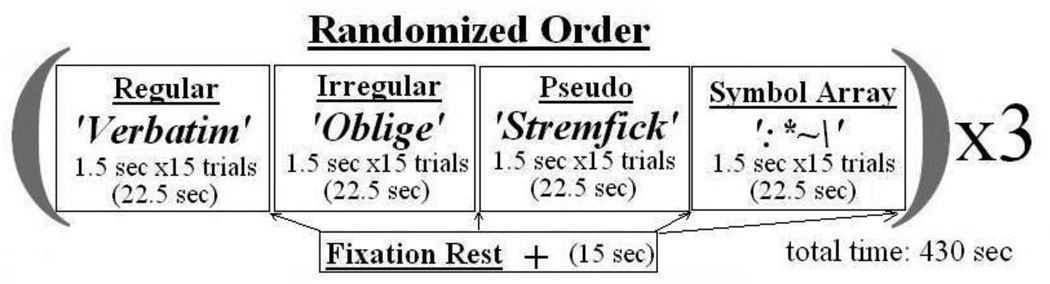
The experiment utilized a blocked, randomized, counterbalanced design. The stimuli for each experimental condition (Regular, Irregular, Pseudoword, Symbols) were presented in separate blocks, with a total of 12 blocks administered for each condition. Each block consisted of 15 stimuli with a duration of 1.5 seconds per stimulus, yielding a total duration of 22.5 seconds per block. Within each block the separate stimuli were randomized and the order of blocks was randomized and counterbalanced (see Figure 1). With the scanning parameters described above, this experimental design resulted in a scanning session of 7 minutes, 30 seconds, and the collection of 180 total brain volumes.
Figure 1.
Depiction of experimental task design with examples of experimental stimuli.
Image Processing and Statistical Analysis
SPM5 (http://www.fil.ion.ucl.ac.uk/spm/software/spm5) was utilized for the post-processing all images. Slice timing correction was used to adjust for variable acquisition time over slices in a volume, with the middle slice in every volume used as reference. A six parameter variance cost function rigid body affine registration was used to realign all images within a session to the first volume, after the initial 3 volumes were discarded to account for stabilization of T1 signal. Motion regressors were computed and later used as a regressor of no interest in the first level, subject specific analysis. To maximize mutual information, coregistration between functional scans and the NMI305 template was carried out using six iterations and resampled with a 7th-Degree B-Spline interpolation. Functional images were then normalized and wrapped into standard space (NMI305) to allow for signal averaging across subjects. We utilized the standard normalization method in SPM5, which minimizes the sum-of-squared differences between the subject's image and the template (MNI305), while maximizing the prior probability of the transformation. This spatial normalization begins by determining the optimum twelve-parameter affine transformation to account for differences in position, orientation and overall brain size. After affine transformation, a nonlinear transformation is applied to correct for gross differences in head shape that were not accounted for by the affine transformation. The nonlinear deformations are described by the lowest frequency components of a three-dimensional discrete cosine transform basis functions (Ashburner & Friston, 1999). The three major parameter estimation settings: nonlinear frequency cutoff, nonlinear regularization and the number of nonlinear iterations, were all set to the SPM defaults, namely: 25mm, medium regularization, and 16 nonlinear iterations, respectively. Next, a 128 Hz high-pass temporal filter was applied to remove low frequency fluctuations. All images were smoothed by convolution with a Gaussian kernel, with a full width at half maximum of 8mm in all directions to increase the signal to noise ratio and to meet the assumptions of the statistical tests (e.g., normality). The general linear model (GLM) procedure of SPM5 was used to create single-subject statistical models containing double gamma HRF sinusoid boxcar waveforms representing all stimulus blocks and the rest condition.
Paired t-tests representing the relevant contrasts were run to identify whole brain activation at the single subject level. A total of eleven subject-specific statistical comparisons (contrasts) were run. The contrasts can be grouped into two categories, those highlighting activation associated with lexical and non-lexical processing, with the lexical contrasts being further divided into those capturing regular (meaningful/semantic) and pseudoword (non-meaningful/non-semantic) processing. The contrasts highlighting activation associated with non-lexical reading were: Regular minus Symbol, Regular minus Irregular, Pseudo minus Symbol, Pseudo minus Irregular. A contrast comparing non-versus lexical decoding was also run ((Regular plus Pseudo) minus Irregular). The contrasts capturing activation associated with lexical (irregular word) reading were: Irregular minus Symbol, Irregular minus Regular, Irregular minus Pseudo, Irregular minus Regular and Pseudo. A contrast showing activation shared by all three experimental conditions (Regular plus Irregular plus Pseudo) compared to the Symbol control was run (referred to as the Linguistic Decoding minus Symbols contrast). We also examined the activation associated with meaningful versus nonmeaningful word stimuli ((Regular plus Irregular) minus Pseudo); ((Regular plus Irregular) minus Symbol). These subject-specific activation maps were then taken to a random effects, group level analysis, utilizing a one sample T-test. We report results corrected at a specificity of at least p ≤ 0.01 (family-wise error rate) and a corrected extent threshold consistent with image smoothness (i.e. expected voxels per cluster) (Friston & Ashburner, 2004).
To test more specifically for MTL system involvement, we conducted a region-of-interest (ROI) analysis using the Wake Forest University Pickatlas (Maldjian et al, 2003). Six ROIs were used: left hippocampus, right hippocampus, bilateral hippocampus, left MTL, right MTL, and bilateral MTL. The structures included in the MTL ROI were the hippocampus, amygdala, and parahippocampal gyri including the entorhinal cortices (n.b., this combination of substrates is typically considered to constitute the entire MTL system, both functionally and anatomically). We used both constrained (hippocampal) and broader medial temporal lobe (MTL) ROIs because the functional involvement of the hippocampus is crucial to our hypotheses, and the hippocampus works and depends on other structures in the MTL system to complete many of its functions. For instance, all information reaching the hippocampus must first pass through the entorhinal cortex (for a description of the direct and indirect pathways to the hippocampus, see work from our lab, Tracy et al., 2008). Thus, to tap the functionality of an integrated neural system we felt it important to include not just the hippocampus, but the MTL system as a whole. These ROIs were investigated in the context of the key experimental contrasts, noted above, for their role in lexical and non-lexical decoding.
To examine activation associated with the relative strength of non-lexical and lexical decoding, we examined the activation associated with a covariate-of-interest involving the subject’s baseline TOWRE and TIWRE scores (Tr and Tp for non-lexical and Tir for lexical processing; age corrected standard scores, mean centered, were used). Each covariate was run separately in the context of each of the above key experimental contrasts. This group level, random effects analysis utilized a one sample T-test. As with our whole-brain analyses, all covariate results were corrected at a specificity of at least p ≤ 0.01 (family wise error) and a corrected extent threshold consistent with image smoothness.
Results
Baseline reading Measure
On the baseline measure of Regular word reading (Tr) the sample mean age-corrected standard score was 125 (sd=5.23, >90th percentile). On the baseline measure of Pseudoword reading (Tp) the sample mean age corrected standard score was 123 (sd=7.01, >90th percentile). On the baseline measure of Irregular-word reading (Tir), the sample mean raw score was 48 (sd=1, >84th percentile, n.b.: this is the highest percentile listed in the manual). As noted, these scores demonstrate that our subjects are free from any reading disorder, and indicate that they are highly practiced readers, clearly capable of efficiently decoding all three types of stimuli presented in our study.
Pearson correlational analyses revealed the three reading measures were significantly related, but that the three tasks do show independence. As expected, the two measures of non-lexical reading were strongly related to each other (Tr and Tp, r =.7, p<.01) and more weakly related to the measure of lexical reading (for both Tr and Tp the correlation with Tir was .3, p<.01). Note, the squared correlations showed that no two tests shared more than 25% of their variance.
Activation Associated with Non-Lexical Decoding
Contrasts highlighting activation during Regular word decoding compared to the Symbol control (see Table 2) showed bilateral occipital lobe activation (IOG, LG; BA 17, 18). This contrast showed additional activation of bilateral MTG (BA 22, 39), medial bilateral SFG (BA 6), and left anterior STG/IFG (BA 44). The covariate analysis examining the strength of Regular word reading (Tr) in the context of this contrast revealed that better reading efficiency scores were associated with an increase in the spatial extent of activation in the occipital region, and increased intensity (maxima t score increase) in the left superior temporal/ inferior frontal gyri.
Table 2.
Statistical results of all whole brain experimental contrasts with brain localization labels.
| Contrast | Substrate | Fig. | Sig. | L/R | Cluster | Talairach | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-Lexical Decoding | Corr. | z | Ke | x | y | z | BA | |||
| Regular-Symbol | IOG | 4a | Tr | L/R | 5.98 | 8130 | −14 | −88 | −7 | 17 |
| SFG | 4b | Tr | L/R | 4.33 | 836 | 0 | 5 | 51 | 6 | |
| MTG | L | 3.86 | 990 | −65 | −37 | 2 | 21/39 | |||
| STG/IFG | Tr | L | 3.8 | 771 | −48 | 7 | −5 | 38/44 | ||
| MTG | R | 3.78 | 622 | 63 | −35 | 2 | 22 | |||
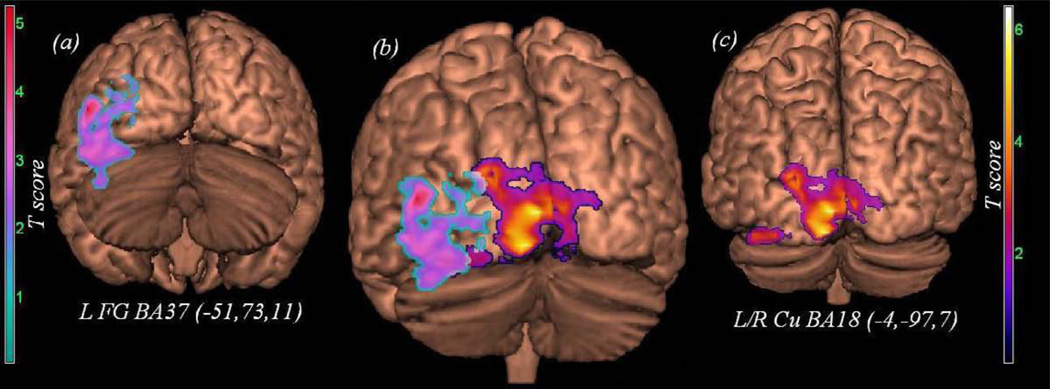
| Regular-Irregular | Cu | 2c | Tr | L/R | 4.24 | 2586 | −4 | −97 | 7 | 18 |
| Pseudo-Symbol | LG | 4a | Tp | L/R | 4.66 | 634 | 12 | −88 | −4 | 17 |
| IOG | 4a | Tp | L/R | 4.38 | 1120 | −42 | −86 | −6 | 18 | |
| SFG | 4b | Tp | L/R | 3.97 | 220 | 2 | 6 | 49 | 6 | |
| Pseudo-Irregular | FG | 2a | Tp | L | 5.25 | 2174 | −51 | −73 | 11 | 37 |
| Regular&Pseduo-lrregular | NULL | |||||||||
| Lexical Decoding | ||||||||||
| Irregular-Symbol | SFG | 4b | Tir | L/R | 4.8 | 1231 | −2 | 6 | 49 | 6 |
| LG | 4a | Tir | L/R | 4.59 | 6857 | 14 | −88 | −4 | 17 | |
| IFG | Tir | L | 4.28 | 869 | −55 | 4 | 11 | 44 | ||
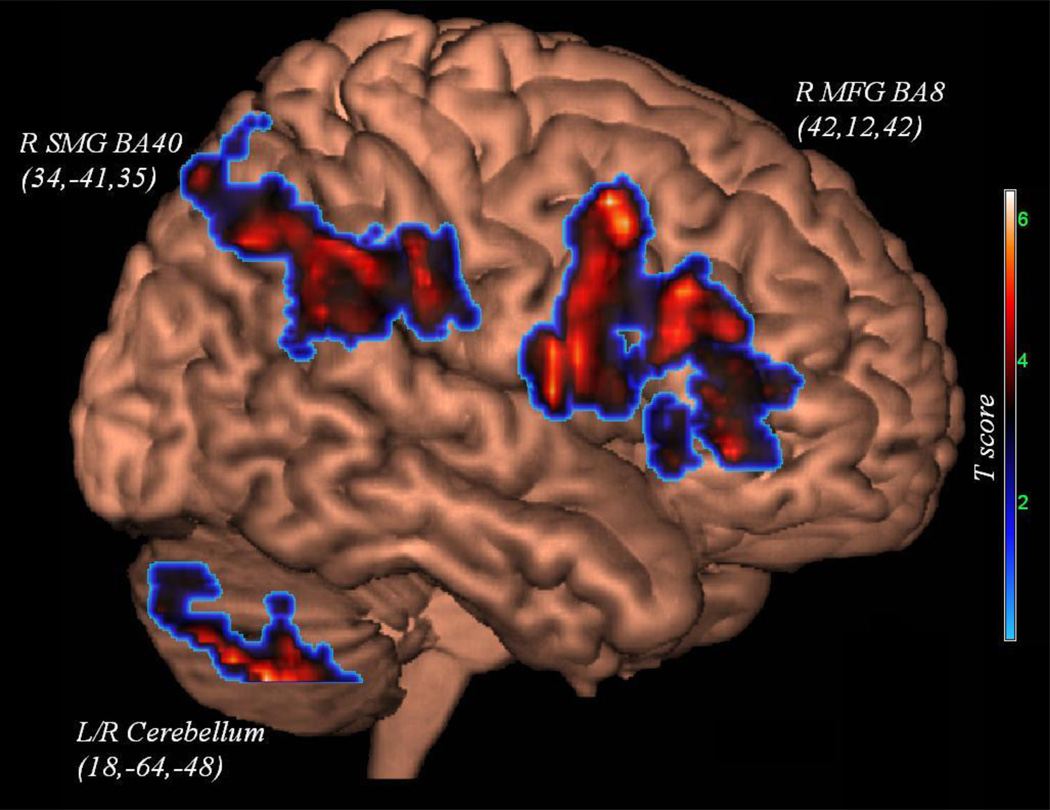
| Irregular-Regular | MFG | 3a | Tir | R | 6.38 | 1731 | 42 | 12 | 42 | 8 |
| SMG | 3b | Tir | R | 6.41 | 1266 | 34 | −41 | 35 | 40 | |
| Uvula (CB) | 3c | L/R | 5.34 | 726 | 18 | −64 | −48 | |||
| Irregular-Pseudo | NULL | |||||||||
| Irregular-Regular&Pseudo | P.L. (CB) | L/R | 4.62 | 2381 | 0 | −60 | −36 | |||
| MFG/PRG | Tir | R | 3.72 | 5227 | 53 | −1 | 17 | 6/8 | ||
| Linguistic Decoding minus Symbols | ||||||||||
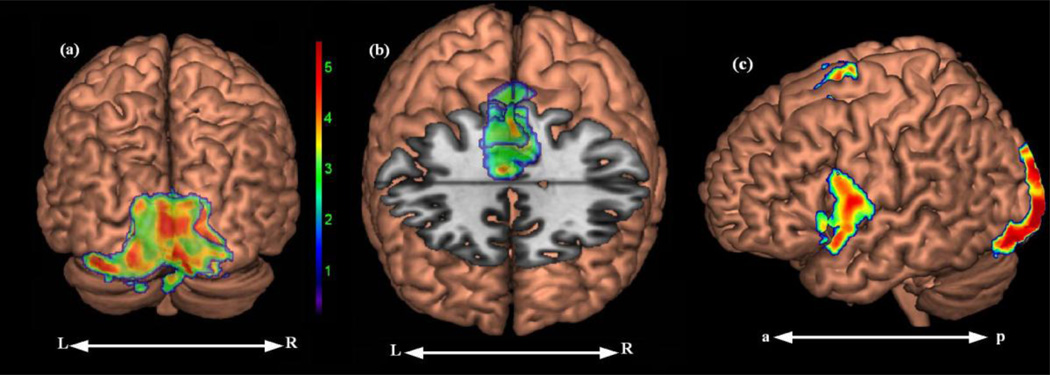
| Lexical Decoding-Symbols | IOG | All | L/R | 5.12 | 3643 | −14 | −90 | −7 | 18 | |
| SFG | All | L/R | 4.36 | 344 | 0 | 5 | 51 | 6 | ||
| Meaningful Words-Symbols | IOG | Tr/Tir | L/R | 7.03 | 8929 | −14 | −94 | −14 | 18 | |
| SFG | Tr/Tir | L/R | 6.26 | 1256 | 0 | 2 | 56 | 6 | ||
| IFG | 2c | Tr/Tir | L | 4.7 | 802 | −58 | 6 | 14 | 44 | |
All results corrected (FWE) to p<.01; corrected extent threshold consistent with image smoothness
Contrasts highlighting activation during Pseudoword decoding compared to the Symbol control (see Table 2) showed bilateral occipital lobe activation (IOG, LG; BA 17, 18) and activation of bilateral, medial SFG (BA 6). The covariate analysis examining the strength of Pseudoword reading (Tp) in the context of this contrast revealed that better reading efficiency scores were associated with these two clusters of activation, with no substantive change in either the spatial extent or intensity of the clusters.
The contrasts highlighting non-lexical compared to lexical decoding (see Table 2 and Figure 2) revealed activation of bilateral cuneus (Regular vs. Irregular: BA 18, Figure 2 panel c) and left fusiform gyrus (Pseudo vs. Irregular: BA 37, Figure 3, panel a; note, panel b depicts the overlap between these two contrasts). The covariate analysis examining the strength of regular (Tr) and pseudoword reading (Tp) in the context of these contrasts revealed that more efficient reading was associated with the increases in both the spatial extent and intensity of the same regional activations (Tr with an increase in bilateral cuneus; Tp with an increases in the left fusiform).
Figure 2.
Activation unique to non-lexical decoding. Panel [a] shows the result of the contrast Pseudo minus Irregular, Panel [c] shows the result of the contrast Regular minus Irregular, Panel [b] shows the overlap of these two contrasts.
Figure 3.
Activation unique to lexical decoding.
The ROI analyses that tested for involvement of strictly MTL structures (left hippocampus, right hippocampus, bilateral hippocampus, left MTL, right MTL, and bilateral MTL) all produced a null result. In order to understand the nature of the null results, percent signal change analysis was conducted on these ROIs in the context of the key experimental contrasts for non-lexical decoding. These null findings were the result of weak signal change (n.b., all contrasts reflected a signal change of 1% or less). There were no signs of constrained variance when BOLD signal variability within each experimental condition was visually examined.
Activation Associated with Lexical Decoding
The contrast highlighting activation during Irregular word decoding compared to the Symbol control showed bilateral occipital lobe activation (LG; BA 17), bilateral, medial SFG (BA 6), and the left IFG (BA 44), the latter was nearly identical to the IFG activation seen during Regular word decoding (see Table 2). The covariate analysis examining the strength of Irregular word reading (Tir) in the context of this contrast revealed that more efficient reading was associated with an increase in the intensity of the bilateral occipital lobe and the left IFG activation.
The contrast highlighting lexical decoding (Irregular-Regular) showed activation of right middle frontal gyrus (BA 8), right supramarginal gyrus (BA 40), and bilateral uvula of the cerebellum (see Figure 3 for all three clusters). The covariate analysis examining the strength of Irregular word reading (Tir) in the context of this contrast revealed that more efficient reading was associated with an increase in the spatial extent of the above supramarginal and middle frontal gyrus activations such that they converged to form one large cluster. The contrast Irregular minus Pseudo was null, while the contrast Irregular minus Regular and Pseudo revealed activation of the bilateral posterior lobe of the cerebellum and the right precentral gyrus (BA 6) extending anteriorly to the middle frontal gyrus (BA 8). Baseline Irregular word reading (Tir) as a covariate was associated with an increase in the intensity of the above prefrontal/middle frontal gyrus activation but the cerebellum did not appear related to baseline Irregular word reading.
The ROI analyses that tested for involvement of strictly MTL structures (left hippocampus, right hippocampus, bilateral hippocampus, left MTL, right MTL, and bilateral MTL) all produced a null result. In order to understand the nature of the null results, percent signal change analysis was conducted on these ROIs in the context of the key experimental contrasts for lexical decoding. These null findings were the result of weak signal change (n.b., all contrasts reflected a signal change of 1% or less). There were no signs of constrained variance when BOLD signal variability within each experimental condition was visually examined.
Activation Associated with Linguistic Decoding
Contrasts highlighting activation common to all experimental conditions (Decoding versus Symbols) showed bilateral occipital lobe activation (IOG, LG; BA 17, 18), and medial, bilateral SFG (BA 6) (see Table 2, Figure 4, panels a & b), similar to the activation obtained for each individual lexical stimulus type compared to Symbols. To examine the activation patterns associated with the strength of baseline decoding, we ran separate covariate analyses in the context of this contrast. The results showed that each of the three covariates produced virtually the same pattern of bilateral occipital activation as the underlying contrast (Decoding versus Symbols) noted above.
Figure 4.
Panels [a] and [b] display the activation resulting from the Lexical Decoding minus Symbols contrast (Regular plus Irregular plus Pseudo minus Symbols. Panel [c] displays activation associated with meaningful as compared to non-meaningful stimuli (Regular and Irregular minus Symbols).
A final analysis was conducted to examine the effect of word meaning by comparing activation associated with the semantically meaningful stimuli (Regular plus Irregular minus Pseudo; Regular plus Irregular minus Symbol). The comparisons with Pseudo were both null, but the combined comparison against Symbols resulted in an area of left IFG activation (see Figure 4, panel c). This same left IFG activation was observed when examining the activations associated with the Tr and Tir covariates in the context of this contrast.
Discussion
The data did not provide support for our hypothesis regarding MTL system involvement in single word decoding in native English speakers. Neither the hippocampus, nor the MTL system as a whole, showed significant activation during non-lexical or lexical decoding. The results, however, do provide evidence that non-lexical and lexical decoding are implemented by distinct neuroanatomical networks. Non-lexical decoding appeared most uniquely associated with cuneus and fusiform gyrus activation biased toward the left hemisphere. In contrast, lexical decoding appeared associated with right middle frontal/precentral and supramarginal, and bilateral cerebellar activation. Both these decoding operations appeared in the context of a shared widespread network of activations including bilateral occipital cortex and superior frontal regions that are shown in Figure 4. Regarding the lack of temporal lobe activation during the decoding of all three types of linguistic stimuli, it should be noted that our sample was composed of skilled readers (as demonstrated by baseline TOWRE and TIWRE scores). It is important to note that studies using similar passive paradigms have failed to find temporal lobe involvement in skilled reading (Levy et al., 2009), yet this does not preclude a role for the temporal lobe in more active reading tasks, such as those involving semantic judgment (Olichney et al., 2010).
Compared to the decoding of irregular words, regular words showed increased activation in the cuneus (BA 18), and the activation in this area varied with the efficiency of regular word decoding (Tr scores). This activation suggests a more intense and widespread area of posterior activation is present during the reading of regular words, beyond the shared activation evident in the other reading conditions. This finding is consistent with other research on the cuneus showing that this area is involved in the initial decoding of visual, linguistic stimuli (Jessen et al., 1999), acts as a substrate for early and elementary recognition procedures (Levy et al., 2008), and is a substrate whose activity is enhanced when phonemic regularity is present.
Compared to decoding of irregular words, pseudowords showed increased activation in left fusiform gyrus (BA37). A recent meta-analysis of neuroimagng findings implicated the visual word form area (which includes the inferior portion of the fusiform gyrus), as a common neuroanatomical substrate for non-lexical decoding (Jobard et al., 2003; see also Levy et al., 2009). This conclusion is consistent with our finding for non-lexical decoding (see areas, BA 18 and BA 37 in activation Figure 2). Because the cuneus and fusiform were not associated with irregular word decoding, we suspect the unique responses in these areas did not emerge from word form recognition (for review of visual word form area see McCandliss, Cohen & Dehaene, 2003; Gaillard et al., 2006; Schurz et al., 2010). Our data implicates these areas in some other aspect of decoding and the application of phonetic rules. One possible explanation involves the process of matching and testing phonetic rules. Support for this possibility is provided by a recent study which found the left fusiform gyrus to be reliably active during rule identification (Tachibana et al., 2009). Using an fMRI experiment where subjects had to identify the correct rule for the presentation of sequenced stimuli,Tachibana et al. (2009) found that medial frontal cortex, caudate nucleus, fusiform gyrus, and middle temporal cortex exhibited significant activation during rule identification. Thus, the fusiform activation we observed during pseudoword decoding may represent a testing of stored phonemic rules to see if they match incoming linguistic stimuli. It is worth noting that the presence of this effect in our sample of skilled readers and its association with regular word reading efficiency suggests the rule application mechanism we are observing in the cuneus and fusiform is fairly automatic in nature. An important study byMechelli et al. (2005) demonstrated that within the left fusiform gyrus there may be important distinctions within the decoding network (see also: Deitz et al., 2005; Binder et al., 2005; Binder et al., 2006; Vinkier et al., 2007; Starrfelt & Gerlach, 2007). Mechelli et al. (2205) found that the anterior fusiform was active during irregular word decoding, and the posterior during pseudoword decoding. Indeed, our fusiform finding for pseudowords does predominantly involve the posterior section, suggesting this area may be preferentially sensitive to non-lexical decoding.
Regular word decoding also involved activation of the posterior part of bilateral middle temporal gyri (BA 21, 22, 39) including Wernicke’s area. Wernicke’s area has traditionally been shown to be involved in comprehension of spoken language; however, Tanner (2007) redefines it as an important conduit to language comprehension regardless of modality. Anatomically, this is supported by the large level of connectivity between Wernicke’s area and visual cortex via the angular gyrus. A study in support of this isStrange et al. (2001) who used a lexical decision task to show an increased bilateral temporoparietal (BA 39) response to reading real words. It is important to note that the Strange et al. study used only high-frequency words in the English language, and high frequency implies regularity (Hanna, 1966).
Compared to the decoding of regular words, irregular word decoding activated the right middle frontal and supramarginal, and bilateral cerebellar regions. Several studies have found right hemisphere involvement in lexical decoding (Seghier et al., 2008), most commonly inferior frontal regions. For instance, previous research using English and non-English irregular words in normal (Binder et al., 2005; Cai et al., 2006; De Diego Balaguer et al., 2006; Senaha et al., 2005) and reading impaired subjects (Bolger et al., 2008) has implicated bilateral inferior frontal regions in the decoding of irregular words. Also, there has been some evidence that a right frontal response is associated with the difficulty of irregular word decoding (Binder et al., 2005; also see Levy et al., 2008). Despite these findings, it must be said that the right-hemisphere activation we observed for irregular words is unusual, and not typical of the literature (Binder et al., 2005; Levy et al., 2009). It is important to note that in our data, the right frontal activation was responsive to the efficiency of irregular word decoding (Tir scores). This provides a clue that this right frontal activity may be arising from some other mechanism, perhaps related to the skill or speed of processing.
During lexical encoding, the word is not parsed into phonetic elements but retrieved in its entirety with letter recognition and semantic associations brought to bear to identify and match the word to stored exemplars, a pronunciation “guess” is then generated (Daehene, 2009). Our data, contrary to our hypothesis, indicates that this process does not involve the hippocampus and MTL, key structures involved in declarative semantic memory. The right frontal cortex has been implicated in declarative memory retrieval (Frackowiak, 1994; Tulving, 1972) but its contributions to memory processing are beginning to be worked out. For instance, hemispheric differences in the encoding of linguistic stimuli have been observed in the DLPFC, with the right encoding veridically and the left abstractly (Evans & Federmieri, 2007). In the context of the activity of single word reading, veridical encoding would imply that cognitive operations are applied to the unparcellated whole word rather than phonetic units, keeping the whole-word (veridical percept) intact from the start of processing.
In our data, right supramarginal gyrus activation was also evident during irregular word decoding (Figure 3). This activation was not related to reading efficiency (Tir). Drawing support from recent neuroimaging findings and parietal lesion studies, Olson & Berryhill (2009) make a convincing argument for the role of the parietal lobe in both the encoding and retrieval of working and semantic memory. In the context of our study this suggests that access to exemplars during reading is similar to the access of other types of semantic knowledge. This may suggest that the supramarginal gyrus works in concert with the right middle frontal cortex to implement a cognitive mechanism common to working and semantic memory, a mechanism that can be utilized in reading; namely, access to whole word meaning. Thus, the right middle frontal and supramarginal activations we observed may represent this veridical whole-word processing, reflecting either conscious retrieval of the exemplar from lexical/semantic memory (the recollection component of recognition memory) or a familiarity response (the familiarity component) based on the veridical matching of the word stimulus to a stored exemplar. Again, it should be noted that this right hemisphere finding and our interpretation is not the norm in the decoding literature (Binder et al., 2005; Levy et al., 2009).
Lastly, the activation unique to lexical decoding involved the bilateral cerebellum; particularly the uvula. A PET study attempting to disambiguate word and facial recognition byKim et al. (1999), found that the cerebellum is consistently active during all types of recognition memory, suggesting that the cerebellum may serve as the coordinator of recognition memory. This may suggest the cerebellum invokes a mechanism common to the many types of recognition responses. One such mechanism may involve the dichotomous recognition decision that a match (or non-match) exists between the target stimulus and stored exemplar on grounds of visual or phonetic codes (e.g., whether the guess at whole-word pronunciation is deemed correct). The fact that this activation was not associated with the baseline reading task (Tir) suggests that the cerebellum does not provide a parametric or graded response, but instead acts in a dichotomized, on or off fashion, consistent with its functional principle of feedforward processing whereby cerebellar output involves a clear response to its inputs and does not generate or add a complex set of reverberatory processes (Eccles, Ito, & Szentágothai, 1967).
Though we did not explicitly look for differences between meaningful and non-meaningful stimuli, it appears that there were two activation areas present for both regular and irregular words, but not pseudowords. The first was the left IFG (see Figure 4c), with this activation varying as a function of the strength of baseline skills in reading these word types. This finding is consistent with several others in the literature showing that the left IFG has a role in the retrieval of semantic meaning of decoded words (see: Binder et al., 2005; Fiez et al., 1999; Wilson et al., 2006). Most notably, Heim et al. 2009 suggested the left IFG is involved in the decoding of both regular and pseudowords and that the lexical decision regarding the presence of meaning is assigned here. Our study, however, failed to show this region’s involvement in pseudoword decoding. This discrepancy may be accounted for by task differences. In the case of theHeim et al. (2009) study, subjects were asked to assign meaning to the pseudowords and our instructions were simply to read (covertly pronounce) the stimuli. The second area of activation associated with the decoding of semantically meaningful as opposed to pseudowords was located in the left precuneus (BA7). Though pseudowords follow the phoneme rules of the English language, it is plausible to think of them as unique and novel stimuli. For instance,Bitan et al. (2005) showed that activation in the superior parietal lobules decreased with practice and training in the reading of nonsensical, artificial scripts, suggesting these areas have a role in the learning of novel linguistic stimuli.
Before ending, more must be said about our data showing no support for MTL system involvement during either lexical or non-lexical decoding.Meyer et al. (2005) using evoked potentials in healthy controls during the detection of “mismatched” sentence material suggested the hippocampus is involved in controlled reanalysis and repair during comprehension, a process considered non-automatic, and dependent on declarative memory. This suggests the MTL’s skill in the parsing and binding of material, crucial to is contribution to declarative memory and present during the comprehension of spontaneous, unpredictable speech, is not needed in skilled readers decoding single words. That is, single word reading may represent a well-worn aspect of one’s existing knowledge and, as such, the parsing, binding, re-analyzing capabilities of the MTL system that may be used to understand ongoing, spontaneous, unpredictable speech (or applying phonetic rules) are not needed in the case of single words as this parsing/binding/re-combining work has already been done. Similarly, the whole-word recognition and retrieval demands engaged by exemplar decoding may be obviated in skilled readers, where the familiarity and frequency of exposure have automated the recognition and retrieval processes.
Several caveats about our findings are warranted. First, our results may not apply to single words read aloud. A study byDietz et al. (2005) showed that reading words aloud versus silently increases activity in bilateral motor, auditory, and extrastriate visual cortices. Though we did observe striate and extrastriate activation in our silent reading data the other areas were not observed. Thus, are results are consistent with the notion that reading under these two conditions are not homologous or interchangeable. This is an important confound to consider when designing an experiment mimicking the ethological process of reading, a process which is usually silent, even though phonological access is usually involved in decoding (Frost et al., 2009). Second, there is a possible concern regarding word association effects. Clearly, using a study paradigm with a prolonged stimulus exposure and response time (Binder et al., 2005; Dietz et al., 2005) introduces a possible confound related to a strong word association effects (Karni et al., 2005). Discrimination of lexical and non-lexical stimuli can occur as early as 230 msec (Mariol et al., 2008) and primary semantic associations can occur as early as 300 msec (Kissler et al., 2007; Solomyak & Marantz, 2009). Secondary semantic associations can begin to occur between 700 and 900 ms and continue long after (Meyer & Federmeier, 2007). We sought to minimize these secondary word associations by using a relatively short stimulus exposure time, but still must acknowledge that some of the activation observed (particularly the left IFG and bilateral SFG) may be related to these additional semantic processes.
In this study we sought to examine what role, if any, the MTL system has in single word decoding in healthy, native-English readers. Based on the unique cognitive demands that emerge during the reading of different types of words, we tested for selective MTL involvement. We did not observe any evidence of MTL system contribution to single word decoding under conditions of either non- or lexical decoding. However, we did observe distinct regional involvements during lexical and non-lexical decoding, and based on our findings propose these two routes to successful decoding are implemented by at least partially distinct neuroanatomical networks.
All single-word decoding relies on visual recognition of word forms in the bilateral occipital lobe, but the application of phonetic rules associated with the non-lexical routed appears to invoke a more widespread set of activation in this area. Our data also suggests the fusiform is important to non-lexical decoding, potentially implementing the process of testing and matching known, stored phonetic rules against the incoming stimulus. Our data also supports previous findings of posterior-anterior disentanglement in the fusiform gyrus, perhaps suggesting the posterior section is more sensitive to non-lexical decoding.
In contrast, the lexical route enlists the right middle frontal cortex to implement a more veridical recognition process than is obtained during non-lexical decoding in the sense that the whole word rather than phonetic parts are operated upon during processing. The right middle frontal cortex, along with the right supramarginal gyrus, may be involved in the recollection and/or familiarity components of recognition memory that occur during identification and retrieval of whole-word exemplars from semantic memory. Lastly, the bilateral cerebellum is recruited to execute a dichotomous recognition response regarding the target stimulus and stored exemplar to determine whether the “guess” at the pronunciation of the whole word is accurate.
Our data can be seen as supporting a variety of neuroimaging studies, including dynamic causal modeling, neural network modeling, and meta-analysis, that suggest at least a partial disambiguation of the neural substrates for lexical and non-lexical linguistic processing (Jobard et al., 2003; Levy et al., 2009; Seghier et al., 2008; Mechelli et al., 2005; Fiez et al., 1999; Schurtz et al., 2010; Joubert et al., 2004; Mechelli, Gorno-Tempini, & Price, 2003; Binder et al., 2005). Our data provides grounds for distinguishing between lexical and non-lexical decoding on both anatomical and cognitive grounds, and in this sense strengthens the accumulating evidence for partial differentiation between the two routes as promulgated by the dual route model. It may be, however, that there are no brain structures unequivocally dedicated to each route. This may be due to various factors such as language transparency, the regularity and frequency attributes of the stimuli used in experiments, different experimental paradigms, etc.. Indeed, the endeavor of isolating the two neural routes is still precarious due to the complexity and overlap of the underlying mechanisms involved. More advanced data analysis, better temporal resolution, along with directional inferences about information trafficking, will be needed.
The absence of MTL involvement in either lexical or non-lexical decoding appears likely a function of the skilled status of our sample in terms of their reading ability such that whole-word recognition and retrieval processes need not utilize declarative memory system (in the case of lexical), and the need to analyze and recombine phonetic elements of the word is at a minimum (in the case of non-lexical). Our finding, however, leaves open the possibility that in non-skilled (e.g., learning disabled) or non-native readers there is a reliance on MTL substrates during single word decoding.
This research advances the literature on single word decoding by bolstering evidence for making anatomical distinctions between the lexical and non-lexical routes for reading, providing information on the neuroanatomical correlates of specific types of single word decoding (regular, irregular, and pseudowords), reporting important negative results regarding the role of the MTL system in reading, and suggesting important contributions for the less understood aspects of the reading response in the brain, such as the potential role of the right hemisphere in single word decoding.
Acknowledgements
This work was supported, in part, by NINDS R21 NS056071-01A1 to Joseph I. Tracy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agosta F, Viskontas IV, Gorno-Tempini ML. FMRI of Memory. Neuromethods. 2009;41:379–409. [Google Scholar]
- Asakawa S. Mixtures of experts: As an attempt to integrate the dual route cascaded and the triangle models for reading english words. Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics) 2008;4985 LNCS(PART 2):749–758. [Google Scholar]
- Bartha L, Marien P, Brenneis C, Trieb T, Kremser C, Ortler M, et al. Hippocampal formation involvement in a language-activation task in patients with mesial temporal lobe epilepsy. Epilepsia. 2005;46(11):1754–1763. doi: 10.1111/j.1528-1167.2005.00292.x. [DOI] [PubMed] [Google Scholar]
- Binder JR, Medler DA, Desai R, Conant LL, Liebenthal E. Some neurophysiological constraints on models of word naming. NeuroImage. 2005;27(3):677–693. doi: 10.1016/j.neuroimage.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Binder JR, Medler DA, Westbury CF, Liebenthal E, Buchanan L. Tuning of the human left fusiform gyrus to sublexical orthographic structure. NeuroImage. 2006;33(2):739–748. doi: 10.1016/j.neuroimage.2006.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T, Manor D, Morocz I, Karni A. Effects of alphabeticality, practice and type of instruction on reading an artificial script: An fMRI study. Cognitive Brain Research. 2005;25(1):90–106. doi: 10.1016/j.cogbrainres.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Bolger DJ, Minas J, Burman DD, Booth JR. Differential effects of orthographic and phonological consistency in cortex for children with and without reading impairment. Neuropsychologia. 2008;46(14):3210–3224. doi: 10.1016/j.neuropsychologia.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C, Kochiyama T, Kagawa H, Michihara R, Osaka K, Wu J. Paper presented at the 2885–2890. 2006. Neural substrates of passively listening to japanese and english words, nonsense words by japanese subjects: An fMRI study. [Google Scholar]
- Chouinard PA, Goodale MA. Category-specific neural processing for naming pictures of animals and naming pictures of tools: An ALE metaanalysis. Neuropsychologia. 2010;48(2):409–418. doi: 10.1016/j.neuropsychologia.2009.09.032. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Ryan J, Hunt C, Romine L, Wszalek T, Nash C. Hippocampal system and declarative (relational) memory: Summarizing the data from functional neuroimaging studies. Hippocampus. 1999;9(1):83–98. doi: 10.1002/(SICI)1098-1063(1999)9:1<83::AID-HIPO9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Coltheart M, Davelaar E, Jonasson JT, Besner D. Access to internal lexicon. In: Dornic S, editor. Attention and Performance V1. New York, NY: Academic Press; 1977. pp. 535–555. [Google Scholar]
- Coltheart M, Rastle K, Perry C, Langdon R, Ziegler J. DRC: A dual route cascaded model of visual word recognition and reading aloud. Psychological Review. 2001;108(1):204–256. doi: 10.1037/0033-295x.108.1.204. [DOI] [PubMed] [Google Scholar]
- De Diego Balaguer R, Rod -Fornells A, Rotte M, Bahlmann J, Heinze H, Munte T. Neural circuits subserving the retrieval of stems and grammatical features in regular and irregular verbs. Human Brain Mapping. 2006;27(11):874–888. doi: 10.1002/hbm.20228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S. Reading in the brain. New York: Penguin; 2009. [Google Scholar]
- Dietz N, Jones K, Gareau L, Zeffiro T, Eden G. Phonological decoding involves left posterior fusiform gyrus. Human Brain Mapping. 2005;26(2):81–93. doi: 10.1002/hbm.20122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan RJ, Fletcher PC. Dissociating prefrontal and hippocampal function in episodic memory encoding. Nature. 1997;388(6642):582–585. doi: 10.1038/41561. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Ito M, Szentágothai J. The Cerebellum as a Neuronal Machine. Springer-Verlag; 1967. [Google Scholar]
- Evans KM, Federmeier KD. The memory that's right and the memory that's left: Event-related potentials reveal hemispheric asymmetries in the encoding and retention of verbal information. Neuropsychologia. 2007;45(8):1777–1790. doi: 10.1016/j.neuropsychologia.2006.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez JA, Balota DA, Raichle ME, Petersent SE. Effects of lexicality, frequency, and spelling-to-sound consistency on the functional anatomy of reading. Neuron. 1999;24(1):205–218. doi: 10.1016/s0896-6273(00)80833-8. [DOI] [PubMed] [Google Scholar]
- Frackowiak R. Functional mapping of verbal memory and language. Trends in Neurosciences. 1994;17(3):109–115. doi: 10.1016/0166-2236(94)90119-8. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J. Generative and recognition models for neuroanatomy. NeuroImage. 2004;23(1):21–24. doi: 10.1016/j.neuroimage.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Frost SJ, Landi N, Mencl WE, Sandak R, Fulbright RK, Tejada ET, et al. Phonological awareness predicts activation patterns for print and speech. Annals of Dyslexia. 2009;59(1):78–97. doi: 10.1007/s11881-009-0024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard R, et al. Direct intracranial, fMRI, and lesion evidence for the causal role of left inferotemporal cortex in reding. Neuron. 2006;50:191–204. doi: 10.1016/j.neuron.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Ramirez J, Köhler S, Westmacott R, Black SE, Moscovitch M. Retrieval of autobiographical memory in Alzheimer's disease: Relation to volumes of medial temporal lobe and other structures. Hippocampus. 2005;15(4):535–550. doi: 10.1002/hipo.20090. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Winocur G, Rosenbaum RS, Poreh A, Gao F, Black SE, et al. Hippocampal contributions to recollection in retrograde and anterograde amnesia. Hippocampus. 2006;16(11):966–980. doi: 10.1002/hipo.20226. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, Seidel WT. Localization of cortical dysfunction based on auditory and visual naming performance. Journal of the International Neuropsychological Society. 2009;15(4):529–535. doi: 10.1017/S1355617709090754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna P, Hanna J, Hodges R, Rudorf E. Doc.OE-32008. Washington, D.C.: U.S. Dept. of Health, Education, and Welfare. USGPO; 1966. Phoneme Grapheme Correspondences as Cues to Spelling Improvement. [Google Scholar]
- Harm MW, Seidenberg MS. Computing the meanings of words in reading: cooperative division of labor between visual and phonological processes. Psychological Review. 2004;111:662–720. doi: 10.1037/0033-295X.111.3.662. [DOI] [PubMed] [Google Scholar]
- Heim S, Eickhoff SB, Ischebeck AK, Friederici AD, Stephan KE, Amunts K. Effective connectivity of the left BA 44, BA 45, and inferior temporal gyrus during lexical and phonological decisions identified with DCM. Human Brain Mapping. 2009;30(2):392–402. doi: 10.1002/hbm.20512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F, Erb M, Klose U, Lotze M, Grodd W, Heun R. Activation of human language processing brain regions after the presentation of random letter strings demonstrated with event-related functional magnetic resonance imaging. Neuroscience Letters. 1999;270(1):13–16. doi: 10.1016/s0304-3940(99)00453-x. [DOI] [PubMed] [Google Scholar]
- Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: A metanalysis of 35 neuroimaging studies. NeuroImage. 2003;20(2):693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Joubert S, Beauregard M, Walter N, Bourgouin P, Beaudoin G, Leroux J, et al. Neural correlates of lexical and sublexical processes in reading. Brain and Language. 2004;89(1):9–20. doi: 10.1016/S0093-934X(03)00403-6. [DOI] [PubMed] [Google Scholar]
- Karni A, Morocz IA, Bitan T, Shaul S, Kushnir T, Breznitz Z. An fMRI study of the differential effects of word presentation rates (reading acceleration) on dyslexic readers' brain activity patterns. Journal of Neurolinguistics. 2005;18(2 SPEC. ISS.):197–219. [Google Scholar]
- Kibby MY, Kroese JM, Krebbs H, Hill CE, Hynd GW. The pars triangularis in dyslexia and ADHD: A comprehensive approach. Brain and Language. 2009;111(1):46–54. doi: 10.1016/j.bandl.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgarriff A. Putting Frequencies in the Dictionary. International Journal of Lexicography. 1997;10 (2):135–155. [Google Scholar]
- Kim JJ, Andreasen NC, O'Leary DS, Wiser AK, Boles Ponto LL, Watkins GL, et al. Direct comparison of the neural substrates of recognition memory for words and faces. Brain. 1999;122(6):1069–1083. doi: 10.1093/brain/122.6.1069. [DOI] [PubMed] [Google Scholar]
- Kissler J, Herbert C, Peyk P, Junghofer M. Buzzwords: Early cortical responses to emotional words during reading: Research report. Psychological Science. 2007;18(6):475–480. doi: 10.1111/j.1467-9280.2007.01924.x. [DOI] [PubMed] [Google Scholar]
- Lee CH. Testing the role of phonology in reading: Focus on sentence processing. Journal of Psycholinguistic Research. 2009;38(4):333–344. doi: 10.1007/s10936-008-9092-0. [DOI] [PubMed] [Google Scholar]
- Levy J, Pernet C, Treserras S, Boulanouar K, Aubry F, Démonet J, et al. Testing for the dual-route cascade reading model in the brain: An fMRI effective connectivity account of an efficient reading style. PLoS ONE. 2009;4(8) doi: 10.1371/journal.pone.0006675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J, Pernet C, Treserras S, Boulanouar K, Berry I, Aubry F, et al. Piecemeal recruitment of left-lateralized brain areas during reading: A spatio-functional account. NeuroImage. 2008;43(3):581–591. doi: 10.1016/j.neuroimage.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mariol M, Jacques C, Schelstraete M, Rossion B. The speed of orthographic processing during lexical decision: Electrophysiological evidence for independent coding of letter identity and letter position in visual word recognition. Journal of Cognitive Neuroscience. 2008;20(7):1283–1299. doi: 10.1162/jocn.2008.20088. [DOI] [PubMed] [Google Scholar]
- Massaro DW, Lucas PA. Typing letter strings varying in orthographic structure. Acta Psychologica. 1984;57(2):109–131. doi: 10.1016/0001-6918(84)90038-6. [DOI] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S. The visual word form area: Expertise for reading in the fusiform gyrus. Trends in Cognitive Sciences. 2003;7(7):293–299. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- McGurn B, Starr JM, Topfer JA, Pattie A, Whiteman MC, Lemmon HA, et al. Pronunciation of irregular words is preserved in dementia, validating premorbid IQ estimation. Neurology. 2004;62(7):1184–1186. doi: 10.1212/01.wnl.0000103169.80910.8b. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Long S, Friston KJ, Lambon Ralph MA, Patterson K, et al. Dissociating reading processes on the basis of neuronal interactions. Journal of Cognitive Neuroscience. 2005;17(11):1753–1765. doi: 10.1162/089892905774589190. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Gorno-Tempini ML, Price CJ. Neuroimaging studies of word and pseudoword reading: Consistencies, inconsistencies, and limitations. Journal of Cognitive Neuroscience. 2003;15(2):260–271. doi: 10.1162/089892903321208196. [DOI] [PubMed] [Google Scholar]
- Medler DA, Binder JR. MCWord: An On-Line Orthographic Database of the English Language. 2005 http://www.neuro.mcw.edu/mcword/
- Meyer A, Federmeier K. The effects of context, meaning frequency, and associative strength on semantic selection: Distinct contributions from each cerebral hemisphere. Brain Research. 2007;1183(1):91–108. doi: 10.1016/j.brainres.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai C, Inui T, Iwata M. Role of broca's subregions in syntactic processing: A comparative study of japanese patients with lesions in the pars triangularis and opercularis. European Neurology. 2010;63(2):79–86. doi: 10.1159/000276397. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The edinburgh \ inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olichney JM, Taylor JR, Chan S, Yang J, Stringfellow A, Hillert DG, et al. FMRI responses to words repeated in a congruous semantic context are abnormal in mild alzheimer's disease. Neuropsychologia. 2010;48(9):2476–2487. doi: 10.1016/j.neuropsychologia.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson I, Berryhill M. Some surprising findings on the involvement of the parietal lobe in human memory. Neurobiology of Learning and Memory. 2009;91(2):155–165. doi: 10.1016/j.nlm.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz B, Friederici AD. Interactions of the hippocampal system and the prefrontal cortex in learning language-like rules. NeuroImage. 2003;19(4):1730–1737. doi: 10.1016/s1053-8119(03)00170-8. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? the representation of semantic knowledge in the human brain. Nature Reviews Neuroscience. 2007;8(12):976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Reynolds C, Kamphaus R. Test of Irregular Word Reading Efficiency. Austin, TX: Pro-Ed.; 2007. [Google Scholar]
- Schurz M, Sturm D, Richlan F, Kronbichler M, Ladurner G, Wimmer H. A dual-route perspective on brain activation in response to visual words: Evidence for a length by lexicality interaction in the visual word form area (VWFA) NeuroImage. 2010;49(3):2649–2661. doi: 10.1016/j.neuroimage.2009.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Lee HL, Schofield T, Ellis CL, Price CJ. Inter-subject variability in the use of two different neuronal networks for reading aloud familiar words. NeuroImage. 2008;42(3):1226–1236. doi: 10.1016/j.neuroimage.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenberg MS, McClelland JL. A distributed, developmental model of word recognition and naming. Psychological Review. 1989;96:523–568. doi: 10.1037/0033-295x.96.4.523. [DOI] [PubMed] [Google Scholar]
- Senaha MLH, Martin MGM, Amaro E, Jr, Campi C, Caramelli P. Patterns of cerebral activation during lexical and phonological reading in portuguese. Brazilian Journal of Medical and Biological Research. 2005;38(12):1847–1856. doi: 10.1590/s0100-879x2005001200013. [DOI] [PubMed] [Google Scholar]
- Solomyak O, Marantz A. Lexical access in early stages of visual word processing: A single-trial correlational MEG study of heteronym recognition. Brain and Language. 2009;108(3):191–196. doi: 10.1016/j.bandl.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Starrfelt R, Gerlach C. The visual what for area: Words and pictures in the left fusiform gyrus. NeuroImage. 2007;35(1):334–342. doi: 10.1016/j.neuroimage.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Strange B, Henson R, Friston K, Dolan R. Anterior prefrontal cortex mediates rule learning in humans. Cerebral Cortex. 2001;11(11):1040–1046. doi: 10.1093/cercor/11.11.1040. [DOI] [PubMed] [Google Scholar]
- Suchan B, Gayk AE, Schmid G, Köster O, Daum I. Hippocampal involvement in recollection but not familiarity across time: A prospective study. Hippocampus. 2008;18(1):92–98. doi: 10.1002/hipo.20371. [DOI] [PubMed] [Google Scholar]
- Tachibana K, Suzuki K, Mori E, Miura N, Kawashima R, Horie K, et al. Neural activity in the human brain signals logical rule identification. Journal of Neurophysiology. 2009;102(3):1526–1537. doi: 10.1152/jn.90659.2008. [DOI] [PubMed] [Google Scholar]
- Tanner DC. Redefining wernicke's area: Receptive language and discourse semantics. Journal of Allied Health. 2007;36(2):63–66. [PubMed] [Google Scholar]
- Torgesen J, Wagner R, Rashotte C. Test of word reading efficiency. Austin, TX: Pro-Ed.; 1999. [Google Scholar]
- Tracy J, Boswell S. Modeling the Interaction between Language and Memory: The Case of Temporal Lobe Epilepsy. In: Stemmer B, Whitaker H, editors. Handbook of the Neuroscience of Language. San Diego, CA: Academic Press; 2008. pp. 319–328. [Google Scholar]
- Tsubomi H, Ikeda T, Hanakawa T, Hirose N, Fukuyama H, Osaka N. Connectivity and signal intensity in the parieto-occipital cortex predicts top-down attentional effect in visual masking: An fMRI study based on individual differences. NeuroImage. 2009;45(2):587–597. doi: 10.1016/j.neuroimage.2008.11.028. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson W, editors. Organisation of memory. New York, NY: Academic Press; 1972. pp. 381–403. [Google Scholar]
- Vinckier F, Dehaene S, Jobert A, Dubus JP, Sigman M, Cohen L. Hierarchical coding of letter strings in the ventral stream: Dissecting the inner organization of the visual word-form system. Neuron. 2007;55(1):143–156. doi: 10.1016/j.neuron.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Iacoboni M. Neural responses to non-native phonemes varying in producibility: Evidence for the sensorimotor nature of speech perception. NeuroImage. 2006;33(1):316–325. doi: 10.1016/j.neuroimage.2006.05.032. [DOI] [PubMed] [Google Scholar]
- Xiang H, Fonteijn HM, Norris DG, Hagoort P. Topographical functional connectivity pattern in the perisylvian language networks. Cerebral Cortex. 2010;20(3):549–560. doi: 10.1093/cercor/bhp119. [DOI] [PubMed] [Google Scholar]