Cancer immunotherapy has been hampered by complex, cumbersome agents and cells; individualized and laborious preparations; and questionable clinical efficacy. Recent reports of a new class of monoclonal antibodies (MoAbs) have garnered tremendous enthusiasm for the field, based on the interruption of suppressive signals that are delivered to the adaptive immune system and the demonstration of clinical efficacy within the setting of off-the-shelf systemic immunotherapy. These "checkpoint" receptor inhibitors such as anti-programmed cell death protein 1 (PD-1/PD-L1) block inhibitory receptor signaling in T cells from a variety of cancers and widen the vista for the accelerated development of this emerging modality of cancer therapy.
Tumor-infiltrating lymphocytes (TILs) are major effector cells in the dynamic host immune responses to tumor-associated antigens (TAs) within the tumor microenvironment (TME).1 However, the TME constitutes a potentially hostile milieu, which may induce T-cell dysfunction to avoid immune cell attack, thus permitting tumor progression.2 One of the mechanisms of tumor-induced inhibition of TILs involves a receptor-ligand interaction in which cytolytic activity is determined by inhibitory signaling generated by immune checkpoint inhibitory receptors such as PD-1. The family of T-cell inhibitory receptors3–7 limits T-cell functions by negatively regulating signals in immune cells (eg, T cells and natural killer cells), including activating signals mediated by the T cell receptor (TCR).8 Our emerging understanding of translational tumor immunology has indicated that effective antitumor immunity can be achieved using MoAbs to block these inhibitory receptors, including anticytotoxic T lymphocyte antigen-4 (CTLA-4) (targeted by the MoAb ipilimumab, which has been newly approved by the US Food and Drug Administration), as well as the recently reported PD-1 pathway-blocking MoAb.9,10 Studies have demonstrated that this T-cell functional impairment can be restored through the blockade of these inhibitory receptors,5–7 leading to the clinical efficacy that has been observed recently.
Several lines of preclinical evidence also have demonstrated that PD-1 expression inhibits the activity of cluster of differentiation (CD)8+ T cells in chronic viral infections, including the human immunodeficiency virus, hepatitis C virus, and hepatitis B virus.11–13 Moreover, recent studies have highlighted that in the TME, in which chronic inflammatory conditions including dynamic tumor antigen presentation either by tumor- and antigen-presenting cells and various inflammatory cytokine release occur, immune checkpoint inhibitory receptors such as CTLA-4, PD-1, and T cell immunoglobulin mucin-3 (TIM-3) are upregulated and impair antitumor functions of TIL.14–16 Collectively, these results suggest that chronic exposure of antigens and inflammation are involved in the expression of immune checkpoint inhibitory receptors on T cells, forming functionally exhausted T cells.
PD-1 expression on T cells normally represents a protective effect to reduce damage to uninvolved normal tissue during acute immune responses and therefore provides a mechanism of escape when tumor cells become capable of expressing PD-L1 and thus exert an immunosuppressive effect to "turn off" the antitumor activity of TA-specific T lymphocytes (Figure 1). Based on the temporal and phenotypic differences in this inhibitory/checkpoint receptor:ligand pair the subsets of T lymphocytes that are reactivated when disruptive therapeutic MoAbs are used would be expected, including variability in the immune toxicities (generation of autoimmune reactions) to normal, uninvolved, nonmalignant tissues. Expression patterns of PD-L2 are being studied.
Figure 1.
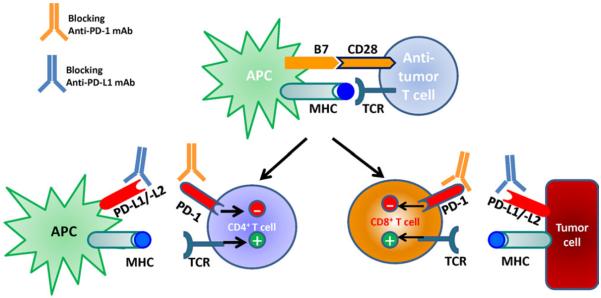
Cancer immunotherapy is administered through antibody approaches to inhibit programmed cell death protein 1 (PD-1)/PD-L1 checkpoint receptor-ligand signaling to reactivate antitumor T cells. A blocking monoclonal antibody (MoAb) disrupts negative regulatory PD-1 signaling on tumor-infiltrating T lymphocytes, permitting effector T-cell activation in the tumor microenvironment, in which suppressive PD-L1 signals are mediated by intratumoral antigen-presenting cells (APC) or the tumor cells themselves. MHC indicates major histocompatibility complex; TCR, T cell receptor; CD4, cluster of differentiation 4; −, negative; +, positive; CD28, cluster of differentiation 28; CD8, cluster of differentiation 8. Expression of PD-L2 on tumor cells is currently under investigation.
Clinical experience with the anti-PD-1 MoAb (MDX-1106/BMS-936558/ONO-4538) was recently reported at the 2012 annual meeting of the American Society of Clinical Oncology and in 2 companion articles published in June 20129,10 in which PD-L1 and PD-1 were targeted in separate clinical trials. Significant endogenous TA-specific immunity was observed, including clinical tumor regression after checkpoint pathway inhibition. The new mechanism of oncologic efficacy acts by reversing immune resistance, through this blockade of adaptive resistance mediated by tumor cells selected for their capacity to induce suppressive via the PD-1/PDL1 pathway. This strategy might synergize with other treatments, and longer follow-up is required to confirm their durability after removal of pathway blockade. Traditionally, immune-based therapeutic approaches have garnered enthusiasm for their capacity to induce durable memory responses to cope with the evolution and adaptability of tumor cells through genomic and pathway instability. It is interesting to note that remarkable similarities between clinical patterns of antitumor activity were observed when targeting anti-PD-L1 and anti–PD-1 MoAbs, reinforcing the importance of this pathway and acquired tumor cell escape from immune surveillance. However, head-to-head randomized trial comparisons or sequential crossover-type designs might be envisioned in the future to define the overlapping and nonoverlapping subsets of lymphocytes and, by extension, select cancer patient populations who might benefit from these related therapeutic strategies.
It is interesting to note that the anti-PD-1 MoAb demonstrated clinical objective responses in patients with previously presumed "nonimmunogenic" tumor types, including non-small cell lung cancer (NSCLC) and ovarian cancer, in whom durable partial responses (PRs) and stable disease were observed.10 Three of 52 patients with melanoma demonstrated a complete response, whereas 9 patients demonstrated a PR. PRs were also noted in 2 of 17 patients with renal cancer and 5 of 49 patients with NSCLC. Of the latter group of patients, 4 of 5 of the NSCLCs were of nonsquamous histology. NCI Grade 3 or 4 toxic effects were found to be related to treatment in 9% of patients. Of those patients treated with anti–PD-1, 236 patients were eligible for response evaluation and the cumulative response rates were reported to be 18% among patients with NSCLC (14 of 76 patients), 28% among patients with melanoma (26 of 94 patients), and 27% among patients with renal cell carcinoma (9 of 33 patients). Long-term durable responses occurred in approximately two-thirds of patients and lasted longer than 1 year.
In summary, blockade of the PD-1/PD-L1 pathway is a clinically effective strategy for interrupting inhibitory receptors expressed by tumor-infiltrating T lymphocytes. Reversal of immune escape and acquired immune resistance have now achieved demonstrable clinical success, with a favorable immune-related toxicity profile. On the heels of US Food and Drug Administration approval in 2011 of the anti–CTLA-4 MoAb for the treatment of melanoma (ipilumumab), historical enthusiasm for immunotherapy, particularly "off-the-shelf" approaches that are available in routine oncologic clinical practice, now appears to be a reality. Further work is warranted to harness the enthusiasm of physicians and their patients, including determining the appropriate dose for maximum clinical efficacy versus the toxicity profile, identifying/validating biomarkers of response (such as PD-L1 expression, which was found to be expressed only in clinically responding patients), and investigating combination approaches to enhance and expand the objective response rate. In addition, the integration of these therapies into the upfront, previously untreated population is now warranted, particularly in combination with traditional cytotoxic chemotherapy, radiotherapy, and/or TA-specific MoAb therapy,17 which have recently been demonstrated to induce TA release from dying tumor cells and help reprogram the TME to be biased toward antitumor activity. Indeed, cancer immunotherapy in the future appears compelling, despite likely future hurdles that will need to be overcome. The field of cancer immunotherapy is now outgrowing the disappointment traditionally associated with this type of therapy, which previously was limited to a small cadre of believers, and is earning its name as the "fourth" therapeutic modality.
Acknowledgments
FUNDING SUPPORT
Supported by National Institutes of Health grant R01 DE19727.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The author made no disclosures.
REFERENCES
- 1.Ngiow SF, Teng MW, Smyth MJ. Prospects for TIM3-targeted antitumor immunotherapy. Cancer Res. 2011;71:6567–6571. doi: 10.1158/0008-5472.CAN-11-1487. [DOI] [PubMed] [Google Scholar]
- 2.Radoja S, Frey AB. Cancer-induced defective cytotoxic T lymphocyte effector function: another mechanism how antigenic tumors escape immune-mediated killing. Mol Med. 2000;6:465–479. [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmadzadeh M, Johnson LA, Heemskerk B, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapon M, Randriamampita C, Maubec E, et al. Progressive upregulation of PD-1 in primary and metastatic melanomas associated with blunted TCR signaling in infiltrating T lymphocytes. J Invest Dermatol. 2011;131:1300–1307. doi: 10.1038/jid.2011.30. [DOI] [PubMed] [Google Scholar]
- 5.Fourcade J, Sun Z, Benallaoua M, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010;107:7875–7880. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vazquez-Cintron EJ, Monu NR, Frey AB. Tumor-induced disruption of proximal TCR-mediated signal transduction in tumor-infiltrating CD8+ lymphocytes inactivates antitumor effector phase. J Immunol. 2010;185:7133–7140. doi: 10.4049/jimmunol.1001157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boni C, Fisicaro P, Valdatta C, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81:4215–4225. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 13.Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol. 2007;81:9249–9258. doi: 10.1128/JVI.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson AC. Tim-3, a negative regulator of anti-tumor immunity. Curr Opin Immunol. 2012;24:213–216. doi: 10.1016/j.coi.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Baitsch L, Baumgaertner P, Devevre E, et al. Exhaustion of tumor-specific CD8+ T cells in metastases from melanoma patients. J Clin Invest. 2011;121:2350–2360. doi: 10.1172/JCI46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mumprecht S, Schurch C, Schwaller J, Solenthaler M, Ochsenbein AF. Programmed death 1 signaling on chronic myeloid leukemia-specific T cells results in T-cell exhaustion and disease progression. Blood. 2009;114:1528–1536. doi: 10.1182/blood-2008-09-179697. [DOI] [PubMed] [Google Scholar]
- 17.Ferris RL, Jaffee EM, Ferrone S. Tumor antigen-targeted, monoclonal antibody-based immunotherapy: clinical response, cellular immunity, and immunoescape. J Clin Oncol. 2010;28:4390–4399. doi: 10.1200/JCO.2009.27.6360. [DOI] [PMC free article] [PubMed] [Google Scholar]


