Abstract
Oxytocin has been shown to affect human social information processing including recognition memory for faces. Here we investigated the neural processes underlying the effect of oxytocin on memorizing own-race and other-race faces in men and women. In a placebo-controlled, doubleblind, between-subject study, participants received either oxytocin or placebo before studying own-race and other-race faces. We recorded event-related potentials (ERPs) during both the study and recognition phase to investigate neural correlates of oxytocin’s effect on memory encoding, memory retrieval, and perception. Oxytocin increased the accuracy of familiarity judgments in the recognition test. Neural correlates for this effect were found in ERPs related to memory encoding and retrieval but not perception. In contrast to its facilitating effects on familiarity, oxytocin impaired recollection judgments, but in men only. Oxytocin did not differentially affect own-race and other-race faces. This study shows that oxytocin influences memory, but not perceptual processes, in a face recognition task and is the first to reveal sex differences in the effect of oxytocin on face memory. Contrary to recent findings in oxytocin and moral decision making, oxytocin did not preferentially improve memory for own-race faces.
Keywords: oxytocin, recognition memory, remember/know, own-race faces, other-race faces, ERP, sex differences
1 Introduction
Oxytocin has been shown to affect human social information processing including recognition memory for faces (Guastella et al., 2008b; Herzmann et al., 2012; Rimmele et al., 2009; Savaskan et al., 2008). Recognition memory consists of two sub-processes: familiarity (i.e., a face feels familiar) and recollection (i.e., a face is remembered with details from the study episode) (Yonelinas, 2002). In studies with male subjects, oxytocin has been shown to enhance familiarity judgments (Guastella et al., 2008b; Rimmele et al., 2009) but to impair the detailed recollection of faces (Herzmann et al., 2012). Event-related potentials (ERPs) measure neural correlates for familiarity and recollection processes (Rugg & Curran, 2007) and for perceptual functions. Here we used ERPs together with behavioral measures to identify the neural processes through which oxytocin influences recognition memory for faces. We were particularly interested in whether oxytocin would modulate both memory and perceptual processes or only memory processes. Our study included men and women and tested recognition memory for own-race and other-race faces because recent reviews suggested that aspects of the individual, like sex, and aspects of the testing situation, like the race of a face, moderate the effects of oxytocin (Bartz et al., 2011; De Dreu 2012; Van IJzendoorn & Bakermans-Kranenburg, 2012).
To identify the brain processes of oxytocin’s effect on human face memory, we investigated ERPs related to memory encoding, memory retrieval, and perception. The ERP difference due to memory (Dm) is taken to reflect memory encoding. It is measured in the study phase of a memory task (Paller et al., 1987) and obtained by sorting study-phase ERPs according to the participant’s memory performance in the subsequent recognition test. Prefrontal, medial-temporal, and parietal areas have been identified as brain regions related to subsequent memory effects in fMRI studies (Spaniol et al., 2009). The FN400 and parietal old/new effect are ERP correlates of memory retrieval. The FN400 distinguishes hits from correct rejections without being influenced by the recollection of details from the study episode (Curran 2000). It is thus taken as a correlate of familiarity processes and likely generated in the prefrontal cortex (Rugg & Curran, 2007). The parietal old/new effect varies with the amount of recollected information from the study episode (Vilberg et al., 2006; Wilding 2000) and thus measures recollection processes. It is most likely generated in the parietal cortex (Rugg & Curran, 2007). We assessed the effects of oxytocin on perceptual processes by measuring the P100, N170, and P200. The P100 correlates with the processing of basic physical characteristics of a stimulus like luminance or contrast (Luck, 2005). The N170 has been associated with the initial basic-level categorization of a stimulus as a face by activating neural face representations and also the coding of individual face representations (Rossion & Jacques, 2011). The P200 has been related to more detailed perceptual analyses of faces like the processing of metric distances between facial features (Latinus & Taylor, 2006). We expected to find effects of oxytocin in the Dm because oxytocin has been suggested to influence memory encoding (Guastella et al., 2008b; Rimmele et al., 2009). Oxytocin has also been shown to affect eye movements when looking at faces (Guastella et al., 2008a) and might thus affect perceptual ERPs related to face processing, especially the N170 and P200.
Face recognition research has shown that memory performance depends on the race of a face. This so-called other-race effect can be seen as better memory for faces from one’s own race than for faces from a different race. Recently, studies have indicated that oxytocin affects the processing of own-race and other-race faces differently. A preference for members from subjects’ own-group was found in studies investigating the effect of oxytocin on social behavior and moral decision making (De Dreu et al., 2011; Van IJzendoorn & Bakermans-Kranenburg, 2012). Oxytocin was suggested to facilitate in-group coordination and cooperation (De Dreu 2012). Our study included own-race and other-race faces to test the hypothesis that oxytocin would enhance the recognition of own-race faces when studied together with other-race faces. These enhancements were hypothesized to be associated with effects of oxytocin on perception (N170 and P200) and memory encoding (Dm).
Subjects in our study were young, healthy men and women. The great majority of oxytocin studies included only men and thus excluded half of the human population (MacDonald & MacDonald, 2010; Van IJzendoorn & Bakermans-Kranenburg, 2012). In contrast to most previous studies, we fully crossed oxytocin/placebo administration with the subjects’ sex. One previous oxytocin study on face memory (Savaskan et al., 2008) included men and women but did not find sex differences. That study administered oxytocin after, rather than before the study phase, as is commonly done (Guastella et al., 2008b; Rimmele et al., 2009) and done here. This might have prevented Savaskan et al. (2008) from obtaining sex differences. Animal research has shown that oxytocin can affect males and females differently (Carter, 2007). In addition, human studies that investigated the effect of oxytocin on amygdala reactivity reported sex differences in oxytocin effectiveness (Domes et al., 2007, 2010; Zink & Meyer-Lindenberg, 2012); therefore, sex differences might emerge in the present study because the amygdala plays a role in processing facial expressions, facial attractiveness, and trustworthiness which have been suggested to affect face memory (Tsukiura 2012).
2 Materials and Methods
2.1 Subjects
Fifty-two healthy, young, right-handed, non-smoking adults volunteered in this study. Half of the participants were women (aged 18 to 28, M = 22.3, SD = 3.1) and the other half were men (aged 18 to 29, M = 23.1, SD = 3.4). All participants were Caucasians apart from one African American man (placebo group), one Hispanic woman (oxytocin group), and one African American woman (placebo group). The performance of these three subjects with regard to the other-race effect was not different from the Caucasian participants; and the results did not change when the three subjects were excluded. Furthermore, Gross (2009) found that recognition performance for African American and Hispanic subjects was the same for own-race and Caucasian faces. We therefore decided to leave the three Non-Caucasian subjects in the sample. The literature on the other-race effect uses the standard terms “own-race” and “other-race,” to conform to this practice and to enhance comprehension of the present paper we refer to the Caucasian faces as “own-race faces” even though not all of our subjects were Caucasian.
None of the participants had ever been diagnosed with any neurological, psychiatric, or medical illness or were on any medication, as determined in a self-report interview by an experimenter. Women were not pregnant as determined by a pregnancy test administered before the study. The study was approved by the Institutional Review Board and the Scientific Advisory Research Committee of the University of Colorado and was conducted in accordance with the Declaration of Helsinki. All participants gave written informed consent and were paid for participation.
2.2 Design
The experimental design included the between-subject factors oxytocin/placebo (double-blind) and subject sex as well as the within-subject factors of stimulus race (Caucasian, Chinese), memory status of the stimuli (old, new), and emotional expression of the stimuli (happy, neutral). Thirteen men (mean age 23.6, SD = 3.7, range 19 to 29) and 13 women (mean age 21.6, SD = 2.7, range 18 to 28) were randomly selected to receive oxytocin, the other 13 men (mean age 22.5, SD = 3.1, range 18 to 29) and 13 women (mean age 23.0, SD = 3.3, range 18 to 28) received placebo. There were no significant age differences across the four groups (ps > .17). Oxytocin has been shown to affect sexual arousal (Carmichael et al., 1987, 1994). Thus, female subjects were run by female experimenters and male subjects by male experimenters to avoid any influence of experimenter sex. Oxytocin might interact with hormonal variations caused by the female menstrual cycle or by birth control pills. Women in the drug and placebo condition did not differ with regard to the time since their last menstrual cycle (p = .84) or whether or not they used birth control (p = .13). A total of 54% of the female subjects used birth control. No participant reported any adverse side effects.
2.3 Stimuli
320 grayscale Caucasian (i.e., own-race) faces (Color FERET database, Phillips et al., 2000) and 320 grayscale Chinese (i.e., other-race) faces (CAS-PEAL database, Gao et al., 2004) were used as stimuli. Face stimuli were portrait pictures (5.3 cm × 7.0 cm) that showed hair, necks, and background. Half of the faces had neutral and the other half had smiling facial expressions. Half of the faces were female.
2.4 Procedure
The study consisted of one four-hour session, which was conducted between 8 am and 4 pm. Time of day of testing did not vary significantly across the subject groups (all ps > .10). Participants were instructed to abstain from beverages with caffeine or alcohol 24 hours before the study day and to maintain a regular sleep-wake cycle two nights before the study day, with sleep periods between about 11 pm and 7 am. The study was conducted under close medical supervision provided by the Clinical Translational Research Center at the University of Colorado Boulder. Nurses administered oxytocin or placebo and monitored heart rate and blood pressure throughout the session.
On the study day, participants received 24 IU of oxytocin (Syntocinon Spray; Novartis; three puffs per nostril; each puff with 4 IU of oxytocin) or a placebo (saline nasal spray, three puffs per nostril) intranasally. This dose of oxytocin was chosen because the same dose was used in previous studies of oxytocin’s effects on memory (Guastella et al., 2008b; Herzmann et al., 2012; Savaskan et al., 2008; Rimmele et al., 2009). Forty minutes after administration, when central nervous oxytocin levels reached the plateau of their highest concentration (Born et al., 2002), participants studied 160 Caucasian and 160 Chinese faces intermixed in 16 blocks. Short breaks were allowed after every 20 faces. In the study phase, each picture was presented for two seconds on a light gray background in the middle of a 17-inch monitor. Participants were told to memorize all stimuli as well as possible and to make attractiveness ratings (1 = very unattractive to 7 = very attractive) without time limit on a computer keyboard to foster memory encoding. The prompt for the attractiveness rating appeared immediately after the stimulus had disappeared to separate memory encoding from attractiveness ratings and keep the presentation time for all items constant. One second after the response, the next stimulus was presented. A fixation cross was shown in the response-to-stimulus interval. The study phase lasted about 40 minutes.
After the study phase, participants completed an unrelated temporal discounting task, which will be reported elsewhere (de la Vega, Chatham, Herzmann, and Munakata, unpublished observations). The recognition test started about 30 minutes after the end of the study phase (i.e., 110 minutes after the administration of the nasal spray). All 320 studied items intermixed with 160 new Caucasian and 160 new Chinese faces were tested. Short breaks were allowed after every 20 faces. Each stimulus was presented for 1.5 seconds. The response options then appeared below the stimulus, and participants were asked, without time limit, to make memory judgments by pressing the corresponding key on a computer keyboard. They were told to judge the items as “recollected” when they could remember the presented item together with specific details about learning this item in the study phase (such as a thought that came to mind or something that happened in the room). In the case that they did not recollect a face, they were asked to rate its familiarity. They were told to use “definitely familiar” or “maybe familiar” if they believed that they had seen the item in the study phase but could not consciously remember anything particular about its appearance or the experience of learning it. “Maybe unfamiliar” or “definitely unfamiliar” were to be used if they did not recognize the item from the study phase (Woodruff et al., 2006). Before the beginning of the study phase, participants practiced making recollect/familiar judgments to verify, as judged by the experimenter, that they fully understood the differences between the meanings of these memory judgments.
Possible oxytocin-related changes in attention (assessed with a computerized Continuous Performance Task, CPT), wakefulness (assessed with the wakefulness scale of an English version of the Multidimensional Mood Questionnaire, MDMQ, Steyer et al., 1997), and mood (assessed with the Positive and Negative Affect Scale, PANAS, Watson et al., 1988) were tracked over the course of the study session. Measurements were taken right before drug/placebo administration, right before the start of the study phase (40 minutes after drug/placebo administration), and right before the start of the recognition test phase (110 minutes after drug/placebo administration). Participants also completed the Cambridge Face Memory Test (Duchaine & Nakayama, 2006) at the end of the session to measure their general face memory ability.
A self-report questionnaire of the participants’ beliefs about whether they had received oxytocin or placebo was completed at the end of the session.
The sequence of tasks and questionnaires, the sequence of trials within tasks, and the assignment of stimuli to old/new conditions was kept constant for all subjects to ensure comparability of task demands.
2.5 Performance measurement
“Recollect” responses were assumed to directly reflect the proportion of trials associated with recollection for both hits and false alarms. However, the raw “familiar” condition, comprising memory judgments “maybe familiar” and “definitely familiar,” cannot be taken as a direct reflection of familiarity because these responses are contingent on non-recollection. Using the so-called independent remember-know procedure (IRK, Yonelinas, 2002), familiarity hits and false alarms were calculated as the probability of responding “familiar” to an item provided that the item was not given a “recollect” response (i.e., for hit rates and false alarms, respectively, IRK “familiar” = “familiar”/(1-“recollect”)). Discrimination indices of recollection and familiarity were estimated separately as hits minus false alarms using “recollect” and IRK “familiar” responses. Qualitatively similar results were obtained using d’ rather than hits minus false alarms as a discrimination measure, but only hits minus false alarms are reported.
High-confidence correct classifications of distracters (high-confidence correct rejections), incorrect classification of distracters (high-confidence false alarms), and correct classifications of targets (high-confidence “familiar” hits) were used as additional measures of face memory. High-confidence correct rejections were calculated as the proportion of “definitely new”/(“definitely new”+”maybe new”) responses, high-confidence false alarms and high-confidence “familiar” responses were calculated as “definitely familiar”/(“definitely familiar”+”maybe familiar”) for new items and targets, respectively.
2.6 Event-related potential recording and measurement
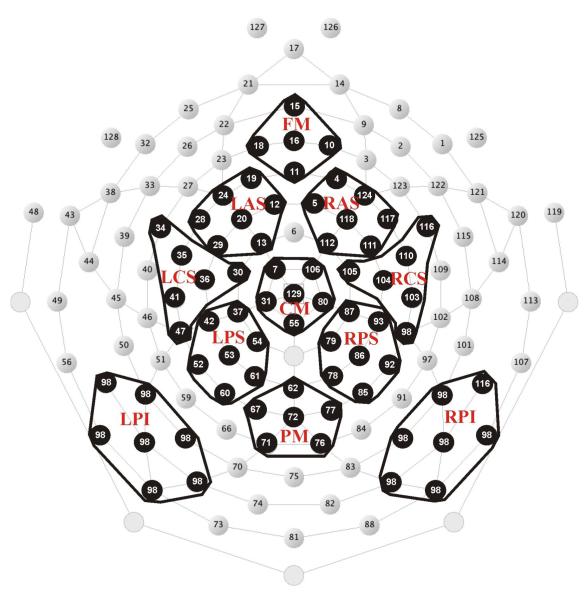
The EEG was recorded in the study and recognition test phase with a 128-channel Geodesic Sensor Net™ (HydroCel GSN 128 1.0, Tucker, 1993, Fig. 1) connected to an AC-coupled, 128-channel, high-input impedance amplifier (200 MΩ, Net Amps™, Electrical Geodesics Inc., Eugene, OR). Amplified analog voltages (0.1-100 Hz bandpass) were digitized at 250 Hz. The recording reference was the vertex channel (Cz). Individual sensors were adjusted until impedances were less than 50 kΩ.
Figure 1.
Geodesic sensor net layout. Electrode sites are numbered. Highlighted clusters are regions of interest included in analyses. L = left, R = right, F = frontal, A = anterior, C = central, P = parietal, M = medial, S = superior, I = inferior.
Epochs of 1100 ms for study-phase items and 1300 ms for test-phase items, each starting 100 ms before stimulus onset, were generated offline from the continuous record. Horizontal and vertical eye movements were corrected using the ocular correction ICA transformation in Brain Vision Analyzer 2.0.1 (Brain Products GmbH, Munich, Germany). Trials with non-ocular artifacts were discarded. ERPs were aligned to a 100-ms baseline before target onset, averaged separately for each channel and condition, digitally low-pass filtered at 40 Hz, and recalculated to average reference. A minimum of 15 trials per condition was ensured for each subject.
Time segments and regions of interest (ROIs) were defined according to visual inspection and previous research (Herzmann & Curran, 2011; Herzmann et al., 2011) (Fig. 1). Mean amplitudes were computed by averaging the channels within each ROI for each condition and subject. The Dm was divided into three time segments: 250-500 ms, 500-750 ms, and 750-1000 ms. The FN400 was measured between 300 and 500 ms and the parietal old/new effect between 500 and 800 ms and 800 and 1200 ms. ROIs for the FN400 were the frontal-medial and left and right anterior superior channel groups (FM, LAS, and RAS, Fig. 1). ROIs for the Dm and the parietal old/new effects were the medial as well as the left and right superior channel groups over frontal, central, and parietal regions (FM, LAS, RAS, CM, LCS, RCS, PM, LPS, and RPS, Fig. 1).
Perceptual ERP components, the P100, N170, and P200, were identified in the right and left hemispheres on posterior-temporal ROIs (LPI and RPI, Fig. 1), where these components were most pronounced across all conditions. Time segments were chosen as follows: for the P100, 100-132 ms in the study and test phases, for the N170, 140-184 ms in the study phase and 144-192 ms in the test phase, and for the P200, 192-272 ms in the study phase and 188-260 ms in the test phase.
2.7 Data analysis
Behavioral effects of oxytocin were analyzed in mixed model analyses of variance (ANOVA) with repeated measures on the within-subject factors, stimulus race (Caucasian, Chinese) and emotional expression (neutral, happy) and the between-subject factors, drug (oxytocin, placebo) and subject sex (female, male). For analyses of control variables, the same ANOVAs were calculated, but the factors of stimulus race and emotional expression were excluded.
For analyses of the ERP measures, the within-subject factor of emotional expression was excluded because too few trials per condition would have resulted from considering this factor. Three additional within-subject factors were instead included: frontal-parietal (anterior to posterior gradient of ROIs), left-right (laterality gradient of ROIs), and memory judgment. The last factor assessed memory effects related to recollection by contrasting ERPs to “recollected” vs. “familiar” faces and those related to familiarity by contrasting ERPs to “familiar” vs. forgotten faces in the study phase and “familiar” vs. correctly rejected new items in the recognition test phase, as traditionally done for the analysis of Dm and old/new effects.
To identify relationships between behavioral and ERP measures, multivariate analyses of covariance (MANCOVA) were conducted. In MANCOVAs, performance measures of oxytocin’s effect on recognition memory from the recognition test phase were used as independent variables. Performance measures were controlled for individual differences in general face memory by regressing the performance in the CFMT out of the measure from the recognition test phase. The residuals of these regressions were used as individual indicators of oxytocin’s effect on face memory. Only performance measures that were found to be influenced by oxytocin were considered in MANCOVAs with ERPs.
Post-tests that followed up on any significant main effect or interaction were Bonferroni-corrected for multiple comparisons. All p-values associated with more than one degree of freedom were corrected according to the Huynh-Feldt (1976) procedure for sphericity violations although we report uncorrected degrees of freedom. All effects remain when the more robust overall error/df term is used.
3 Results
For behavioral and ERP data, only significant drug effects or interactions are reported.
3.1 Memory performance
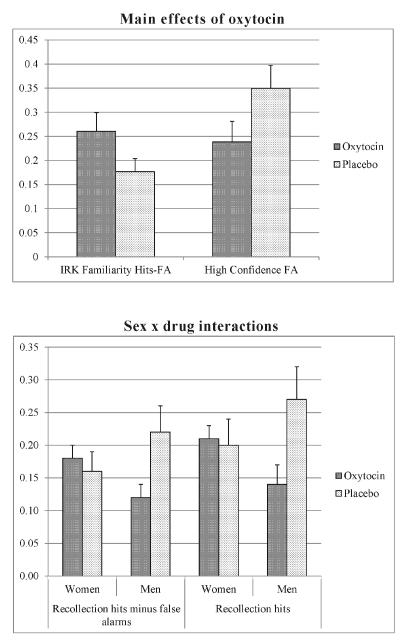
Table 1 shows memory performance for women and men in the oxytocin and placebo groups. The most important behavioral results are highlighted in Figure 2. Two main effects of oxytocin showed that the drug increased accurate IRK familiarity hits minus false alarms (oxytocin, M = 0.26, SD = 0.14 vs. placebo, M = 0.18, SD = 0.10), F(1,48) = 7.154, p = .010, and lowered high-confidence false alarms (oxytocin, M = 0.24, SD = 0.16 vs. placebo, M = 0.35, SD = 0.17), F(1,48) = 6.877, p = .012 (Fig. 2). There was no main effect of drug on recollection, all ps > .11.
Table 1.
Recognition memory performance for own-race and other-race faces for women and men in the oxytocin and placebo group. Data is averaged across happy and neutral faces. Asterisks indicate significant drug effects for pair-wise comparisons (p < .05) separately for women and men
| Women | Men | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Oxytocin | Placebo | Oxytocin | Placebo | |||||||
|
|
||||||||||
| Caucasian faces | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | ||
| IRK familiarity hits minus false alarms | 0.31 | 0.03 | 0.26 | 0.04 | n.s. | 0.38 | 0.05 | 0.26 | 0.02 | * |
| IRK familiarity hits | 0.56 | 0.04 | 0.48 | 0.04 | n.s. | 0.62 | 0.04 | 0.54 | 0.05 | n.s. |
| IRK familiarity false alarms | 0.25 | 0.04 | 0.23 | 0.04 | n.s. | 0.23 | 0.03 | 0.29 | 0.05 | n.s. |
| Recollection hits minus false alarms | 0.22 | 0.02 | 0.21 | 0.04 | n.s. | 0.15 | 0.02 | 0.27 | 0.05 | * |
| Recollection hits | 0.25 | 0.03 | 0.24 | 0.04 | n.s. | 0.17 | 0.03 | 0.31 | 0.05 | * |
| Recollection false alarms | 0.03 | 0.01 | 0.03 | 0.01 | n.s. | 0.02 | 0.02 | 0.04 | 0.01 | n.s. |
| High-confidence “familiar” hits | 0.56 | 0.04 | 0.50 | 0.03 | n.s. | 0.47 | 0.04 | 0.55 | 0.04 | n.s. |
| High-confidence correct rejections | 0.49 | 0.06 | 0.58 | 0.07 | n.s. | 0.43 | 0.05 | 0.50 | 0.07 | n.s. |
| High-confidence false alarms | 0.33 | 0.05 | 0.40 | 0.05 | n.s. | 0.18 | 0.03 | 0.37 | 0.05 | * |
|
| ||||||||||
| Chinese faces | ||||||||||
|
| ||||||||||
| IRK familiarity hits minus false alarms | 0.15 | 0.03 | 0.11 | 0.03 | n.s. | 0.19 | 0.04 | 0.08 | 0.01 | * |
| IRK familiarity hits | 0.60 | 0.03 | 0.56 | 0.05 | n.s. | 0.62 | 0.04 | 0.63 | 0.03 | n.s. |
| IRK familiarity false alarms | 0.45 | 0.03 | 0.44 | 0.06 | n.s. | 0.43 | 0.05 | 0.55 | 0.03 | * |
| Recollection hits minus false alarms | 0.13 | 0.02 | 0.11 | 0.02 | n.s. | 0.08 | 0.01 | 0.16 | 0.03 | * |
| Recollection hits | 0.16 | 0.02 | 0.15 | 0.03 | n.s. | 0.11 | 0.03 | 0.23 | 0.05 | * |
| Recollection false alarms | 0.03 | 0.01 | 0.04 | 0.01 | n.s. | 0.03 | 0.02 | 0.07 | 0.02 | n.s. |
| High-confidence “familiar” hits | 0.43 | 0.03 | 0.46 | 0.03 | n.s. | 0.36 | 0.04 | 0.45 | 0.04 | n.s. |
| High-confidence correct rejections | 0.25 | 0.05 | 0.38 | 0.09 | n.s. | 0.20 | 0.04 | 0.28 | 0.07 | n.s. |
| High-confidence false alarms | 0.27 | 0.04 | 0.29 | 0.04 | n.s. | 0.17 | 0.05 | 0.34 | 0.05 | * |
Figure 2.
Most important behavioral effects of oxytocin. Top: Main effects of oxytocin show increased IRK familiarity hits minus false alarms and reduced high-confidence false alarms for oxytocin as compared to placebo. Data is averaged across the factors subject sex, stimulus race, and stimulus facial expression. Bottom: Sex × drug interactions show that oxytocin significantly reduced recollection hits minus false alarms and recollection hits for men but not for women. Data is averaged across the factors stimulus race and stimulus facial expression.
Oxytocin interacted with subject sex for recollection hits minus false alarms, F(1,48) = 4.384, p = .042, and recollection hits, F(1,48) = 3.978, p = .050 (Fig. 2). Only in men, oxytocin as compared to placebo impaired recollection judgments measured as lower hits minus false alarms (oxytocin, M = 0.11, SD = 0.07 vs. placebo, M = 0.22, SD = 0.14), F(1,24) = 5.839, p = .024, and lower hit rates (oxytocin, M = 0.14, SD = 0.10 vs. placebo, M = 0.27, SD = 0.18), F(1,24) = 4.989, p = .035. There was no sex × drug interaction for familiarity, all ps > .17. When tested for women separately, there were no differences in familiarity measures between the oxytocin and placebo, all ps > .27 (Tab. 1). For men, there was a difference in familiarity measured as hits minus false alarms between oxytocin (M = 0.28, SD = 0.16) and placebo (M = 0.17, SD = 0.07), F(1,24) = 7.765, p = .010 (Tab. 1).
Oxytocin had a stronger effect on faces with happy emotional expression, indicated by a drug × happy/neutral interaction for IRK familiar false alarms, F(1,48) = 6.194, p = .016. For the oxytocin group a one-way ANOVA contrasting happy and neutral faces showed that fewer IRK familiar false alarms were made for happy faces, F(1,24) = 12.041, p = .002 (happy, M = 0.32, SD = 0.12 vs. neutral, M = 0.36, SD = 0.12). In the placebo group, happy (M = 0.37, SD = 0.13) and neutral (M = 0.36, SD = 0.13) faces did not differ from one another, p = .618.
Oxytocin did not affect memory for own-race and other-race faces differently when measured across all participants nor did it have an effect when measured for women and men separately. Oxytocin did not differentially affect memory for female and male faces.
3.2 ERPs in the study phase
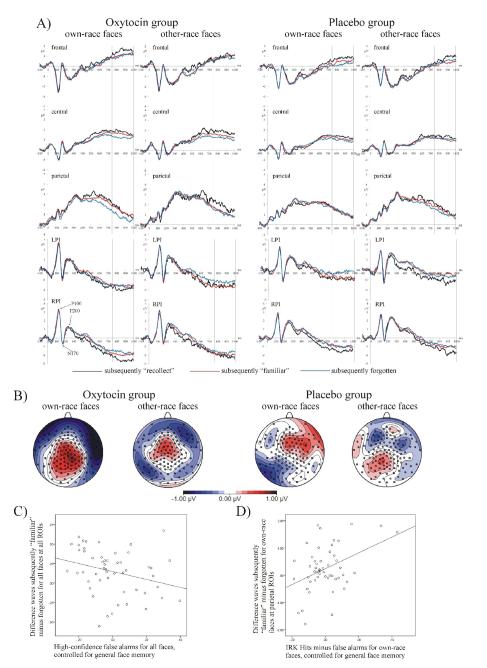
Effects of oxytocin on memory encoding were only found for encoding effects related to familiarity (i.e., subsequently “familiar” vs. subsequently forgotten faces) between 750-1000 ms (Fig. 3A). No other Dms in any other time window showed drug effects. No sex × drug interactions were found. Oxytocin, but not placebo, led to a significant difference between subsequently “familiar” and subsequently forgotten faces, F(1,48) = 5.963, p = .018, across all subjects. Post-hoc tests are significant for men, F(1,24) = 4.213, p = .050, but not for women, F(1,24) = 1.751, p = .198. The MANCOVA showed that this effect was associated with reduced high-confidence false alarms in the recognition test, F(1,50) = 4.607, p = .037 (Fig. 3C). Oxytocin-induced familiar-minus-forgotten Dm effects interacted with stimulus race and the frontal-parietal gradient of the ERPs, F(2,96) = 5.425, p = .016 (Fig. 3B). This familiarity-based encoding interaction arose from the drug × stimulus race interaction being observed only over parietal regions, F(1,50) = 6.460, p = .014. At parietal areas, a significant encoding effect was only found for own-race faces in the oxytocin group, F(1,25) = 13.039, p = .001. This oxytocin-induced, familiarity-related encoding effect for own-race faces over parietal areas was associated with more accurate familiarity judgments (measured as hits minus false alarms) for own-race faces in the subsequent recognition test F(1,50) = 7.180, p = .010 (Fig. 3D).
Figure 3.
Effects of oxytocin on ERPs from the study phase. A) Memory encoding is shown as mean amplitudes at frontal, central, and parietal regions for subsequently “recollected,” subsequently “familiar,” and subsequently forgotten own-race and other-race faces in the oxytocin and placebo group. For these three regions ERPs are averaged across medial and left and right superior regions of interest. Vertical lines indicate the time window between 750-1000 ms where effects of oxytocin were observed. Here, only oxytocin caused a significant memory encoding effect between subsequently “familiar” and subsequently forgotten faces. Over parietal areas, this effect was only found for own-race faces (see also panel B for topographical maps). Perceptual ERPs are shown at LPI and RPI. No effects of oxytocin were observed for perceptual ERPs. B) Topographical maps of the ERP difference of subsequently “familiar” minus subsequently forgotten items between 750-1000 ms showing significant Dms for own-race and other-race faces over frontal and central areas, but a significant Dm over parietal areas only for own-race faces. C) Scatter plot (regression equation: Y = −0.29 * X + 0.15, R2 = .084, p = .037) depicting the negative correlation between high-confidence false alarms, which are controlled for differences in general memory, and the familiarity-related encoding effect (ERP difference wave of subsequently “familiar” minus subsequently forgotten items) averaged across own-race and other-race faces and all regions of interest. D) Scatter plot (regression equation: Y = 0.35 * X + 0.24, R2 = .126, p = .010) depicting the positive correlation between familiarity judgments measured as hits minus false alarms, which are controlled for differences in general memory, and the familiarity-related encoding effect (ERP difference wave of subsequently “familiar” minus subsequently forgotten items) for own-race faces averaged across all parietal regions of interest.
Oxytocin did not affect memory encoding related to recollection (i.e., ERPs for subsequently “recollect” vs. subsequently “familiar” faces), did not cause differences in memory encoding between men and women, and also did not influence perceptual ERPs (i.e., P100, N170, and P200; see Fig. 3A) in the study phase.
3.3 ERPs in the recognition test phase
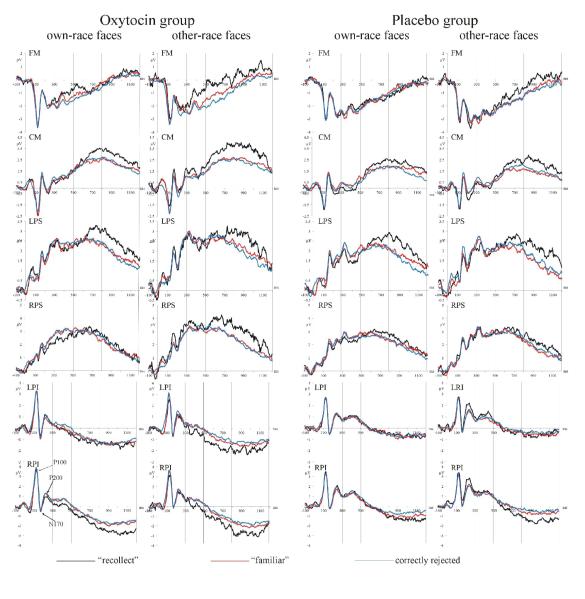
Oxytocin affected ERPs in the recognition test phase. In the time window of the FN400 between 300 and 500 ms, only the oxytocin, but not the placebo group, showed old/new effects related to recollection (i.e., difference between “recollected” and “familiar” faces), F(1,48) = 6.839, p = .012, and familiarity (i.e., difference between “familiar” old and correctly rejected new faces), F(2,96) = 4.196, p = .018, the latter interacted with ROI indicating that familiarity-related old/new effects were only significant over frontal-medial regions (Fig. 4), as is consistent with the typical mid-frontal distribution of FN400 old/new effects (Rugg & Curran, 2007).
Figure 4.
Effects of oxytocin on ERPs from the recognition test phase. Shown are mean amplitudes for “recollected” old, “familiar” old, and correctly rejected new own-race and other-race faces in the oxytocin and placebo group. Vertical lines indicate the time windows for statistical analyses. Only oxytocin led to significant recollection- and familiarity-related old/new effects between 300 and 500 ms. It also caused generally higher mean amplitudes starting at 500 ms (best seen at CM). Perceptual ERPs are shown at LPI and RPI. No effects of oxytocin were observed for perceptual ERPs.
Starting at 500 ms and continuing until 1200 ms, the oxytocin group showed generally higher mean amplitudes than the placebo group across all conditions and all ROIs, Fs(1,48) > 4.6, ps < .037 (best seen at CM in Fig. 4). The parietal old/new effect, the difference between “recollected” and “familiar” faces, was not affected by oxytocin.
Between 800 and 1200 ms, old/new effects related to familiarity showed a significant drug × memory judgment × stimulus race interaction, F(1,48) = 5.493, p = .023, which was further qualified by an interaction with subject sex, F(1,48) = 7.044, p = .011. Familiarity-related old/new effects were significant for own-race but not for other-race faces in the placebo group, F(1,25) = 6.620, p = .016. Similarly, women in the oxytocin group showed significant old/new effects related to familiarity for own-race but not for other-race faces, F(1,12) = 8.919, p = .011. In contrast, men treated with oxytocin showed these familiarity effects only for other-race faces, F(1,12) = 9.568, p = .009.
MANCOVAs did not yield any significant relationships of the observed drug effects in test-phase ERPs with performance in the recognition test.
Oxytocin did not affect perceptual ERPs (i.e., P100, N170, and P200; see Fig. 4) in the test phase.
3.4 Control variables
None of the included control variables showed any systematic variation with oxytocin administration. No significant differences between the oxytocin and placebo group were found in general memory performance (CFMT, p > .87), attention (accuracies and reaction times of the Continuous Performance Test, ps > .16), wakefulness (wakefulness scale of the MDMQ, p > .24), or mood (positive and negative affect scales of the PANAS, ps > .10). No group differences were found for the frequencies (i.e., how often faces were judged as attractive, neutral, or unattractive) or reaction times of attractiveness ratings to faces in the study phase (all ps > .10) (see Supplementary Material Table 1 for descriptive statistics on attractiveness ratings). There were also no interactions of oxytocin/placebo group with stimulus race, stimulus sex, or subject sex for attractiveness ratings. At the end of the study, subjects were unable to identify whether they had received oxytocin or placebo (p = .08) as indicated by a Χ2 test. This marginal group difference showed that the majority of subjects believed they had received placebo (14 of 26 in the oxytocin group, 21 of 26 in the placebo group).
4 Discussion
This study replicated previous findings. It showed that familiarity judgments to studied faces are more accurate after oxytocin administration (Rimmele et al., 2009). Oxytocin enhanced memory performance for faces with happy emotional expression (Guastella et al., 2008b). And, it impaired recollection from episodic memory in men (Herzmann et al., 2012). In addition, the present study yielded several novel findings. Oxytocin affected face memory in women and men differently, but it did not influence memory for own-race and other-race faces differently. Neural correlates for the memory-enhancing effect on familiarity were found for memory-related but not perceptual ERPs in the study phase and recognition-test phase indicating that oxytocin directly affects memory. The specificity of its effects on memory is further supported by the fact that none of the control variables influenced the effects of oxytocin.
Oxytocin facilitated face memory by making faces in memory more familiar (Rimmele et al., 2009) as indicated by more accurate familiarity judgments to previously encountered faces and reduced high-confidence false alarms to new faces (Guastella et al., 2008b; Rimmele et al., 2009; Savaskan et al., 2008). Table 1 reports that these effects were only significant in men, but go in the same direction for women. In fact, there was no sex × drug interaction for familiarity measures. It is possible that the results in women may also become significant if the sample size is increased. The old/new discrimination was especially improved for happy faces for which oxytocin reduced false alarms of familiarity judgments. This finding suggests that oxytocin enhances memory of faces with positive facial expressions (Guastella et al., 2008b).
Oxytocin also impaired memory, but only for men. Replicating the memory-impairing effect of oxytocin on recollection when men were previously tested alone (Herzmann et al., 2012), this is the first finding of sex differences in oxytocin’s effects on memory. In fact, the present results (Table 1) yielded no significant effects of oxytocin on women when tested separately. These sex differences in the effect of oxytocin are not influenced by baseline sex-differences in memory performance as indicated by the similar performance of men and women in the CFMT. Only one previous study (Savaskan et al., 2008) on oxytocin and face memory included men and women, but did not find sex differences. That study administered oxytocin after, rather than before the study phase as done in our study. Thus, in Savaskan et al.’s investigation the highest levels of oxytocin concentration in the brain would have been reached at the beginning of the recognition test phase (Born et al., 2002). This difference in the procedure suggests that the existence of sex differences might depend on when oxytocin is administered. FMRI studies of face processing (Domes et al., 2007, 2010; Zink & Meyer-Lindenberg, 2012) have shown opposite patterns of amygdala activation in women and men following oxytocin administration and speculated about a possible interaction of oxytocin and gonadal steroid hormones. It is possible that sex differences in oxytocin’s effect on the amygdala influenced recognition memory for faces and contributed to our observed sex effects (Tsukiura, 2012). Animal research has also shown significant sex differences in oxytocin function that could be expected to influence memory formation and recognition. For example, higher binding of oxytocin receptors in the medial prefrontal cortex have been found in female than male prairie voles (Smeltzer et al., 2006). In rats, higher binding for female than male rats was observed in the hypothalamus (Uhl-Bronner et al., 2005). Independent from the mechanism that might have led to the observed sex-specific effects of oxytocin, the present results emphasize the necessity of including both women and men when investigating oxytocin’s effects. Studying women and men might also provide clues as to the biological basis of behavioral disorders like depression, autism, and schizophrenia, which have been connected to oxytocin innervation and show sex differences in their occurrence (de Vries, 2008).
Oxytocin affected memory for own-race and other-race faces in the same way. A preference for members from one’s own race was found in previous studies that tested the effect of oxytocin on social behavior (De Dreu 2012; Van IJzendoorn & Bakermans-Kranenburg, 2012). Specifically, De Dreu 2012 proposed that oxytocin may influence human behavior in three ways: social categorization processes, evaluative judgments, and self-sacrificial tendencies. Whereas past research has investigated the influence of oxytocin on evaluative judgments and self-sacrificial tendencies (De Dreu 2012), the effect on social categorization processes involved in learning and remembering faces from in-group and out-group members has not yet been tested. The present study makes an important contribution to this aspect by suggesting that oxytocin is unlikely to influence social categorization processes, at least in the context of recognition memory.
Previous experiments on oxytocin and social behavior (De Dreu 2012; Van IJzendoorn & Bakermans-Kranenburg, 2012) used other-race groups which possessed a negative connotation for the tested subjects. For our participants, Chinese faces might not have possessed the same negative associations that either German or Arabic individuals possess for Dutch participants (De Dreu et al., 2011). This might also explain the absence of race-dependent effects of oxytocin in our behavioral data. It is important to note that the lack of different effects of oxytocin on own-race and other-race faces is not due to an unsuccessful manipulation of race differences in the stimulus material. This is proven by the significant other-race effects in memory performance which show generally poorer memory for Chinese than Caucasian faces (Tab. 1).
Two hypotheses have been proposed to explain the other-race effect in face memory. The perceptual expertise hypothesis holds that people are experts for faces with which they have the highest amount of experience (own-race faces) and perform poorly with faces with which they have very little experience (other-race faces) (Meissner & Brigham, 2001). The social-cognitive hypothesis postulates that people are able to perform similarly well with all faces but that they do not employ this ability for other-race faces because of social factors like prejudice or motivational disregard towards other-race faces (Hugenberg et al, 2010). With regard to the expertise hypothesis, the present data suggests that oxytocin influences face memory independent from the base level of performance. This is interesting because one could have expected that faces associated with poorer performance (other-race faces) would profit more from a memory-facilitating drug than faces for which recognition skills are already at ceiling. This finding further suggests that oxytocin’s effect on face memory is independent from the task difficulty, opposite to current opinion (Bartz et al., 2011).
With regard to the social-cognitive hypothesis, our findings suggest that oxytocin does not interact with the social factors postulated to decrease memory for other-race faces. This is striking because oxytocin is assumed to influence social processing (De Dreu 2012; MacDonald & MacDonald, 2010; Van IJzendoorn & Bakermans-Kranenburg, 2012). However, one limitation is that Chinese faces might not have led to strong prejudice or motivational disregard for our subjects. Future studies that investigate the effect of oxytocin on memory for a different group of other-race faces will provide valuable information whether or not oxytocin interacts with the social factors that affect memory for other-race faces.
Neural correlates for the familiarity-enhancing effect of oxytocin were found in ERPs of memory encoding and memory retrieval. No effects of oxytocin on perceptual processes were observed. In the context of a recognition memory task, oxytocin did not affect general, low-level, visual processing (P100), the structural encoding of faces (N170), or the detailed encoding of facial features and their relations to each other (P200). It is possible that oxytocin affects face perception in other tasks that depend more heavily on perceptual processes of faces like the identifying the emotional expression of a face (cf. Bartz et al., 2011).
Oxytocin significantly influenced memory encoding measured by the Dm. Only the oxytocin group showed familiarity-related Dms, which were associated with lowered high-confidence false alarms (Figure 3C) and thus better discrimination of new from old faces. Dm studies of face memory that used the remember/know procedure (Duarte et al., 2004; Voss & Paller, 2009) have typically reported no familiarity-related Dm, as was also observed here for our placebo group. The selective existence of a familiarity-related Dm in the present oxytocin group suggests that oxytocin facilitated memory encoding by enhancing brain processes that are associated with subsequently improved familiarity. Prefrontal, medial-temporal, and parietal areas have been identified as brain regions associated with subsequent memory effects in fMRI studies (Spaniol et al., 2009). Our results suggest that these brain areas are likely to be affected by oxytocin during memory encoding.
Another ERP-correlate for the familiarity enhancement during memory encoding was found selectively for own-race faces. For these faces, oxytocin led to a widespread Dm that was also present over parietal regions, where familiarity-related Dms were absent for all other conditions. Brain-behavior relationships showed a correlation of the parietal familiarity Dm with more accurate familiarity judgments in the recognition test (Figure 3D). This finding indicates that memory encoding of own-race faces under oxytocin involved a wider network of brain areas which subsequently led to more accurate memory judgments. This result also suggests that memory encoding of own-race and other-race faces is differentially affected by oxytocin but that these differences are not associated with performance differences. It could be speculated that activating more brain areas for own-race faces under oxytocin might indicate that these faces received special attention during memory encoding. This view has some overlap with the hypothesis that oxytocin enhances in-group favoritism and would suggest an influence of oxytocin on social categorization (De Dreu et al., 2011; Van IJzendoorn & Bakermans-Kranenburg, 2012), even though the increased, oxytocin-induced memory encoding of own-race faces did not lead to superior performance for own-race than other-race faces in the present study.
Oxytocin affected memory retrieval processes measured as the FN400 over prefrontal areas. During memory retrieval in the recognition-test phase, only the oxytocin group showed an FN400 for “familiar” faces. Although the FN400 typically indicates more accurate discrimination of “familiar” old from new items (Curran, 2000), no such brain-behavior correlations were found. This might suggest that oxytocin’s effects on the FN400 are not directly related to individual differences in its effects on memory performance. Nevertheless these findings indicate that oxytocin affects prefrontal areas which are involved in generating the FN400 (Rugg & Curran, 2007).
An influence of oxytocin on familiarity-related old/new effects were also found between 800-1200 ms. Significant old/new effects were found for own-race faces in the placebo group and in women of the oxytocin group. Men from the oxytocin group, however, showed these effects for other-race faces. Again, no brain-behavior relationships were found. This result is difficult to reconcile with the present behavioral data. It might be a possible consequence of large variability in the ERP data when considering sex effects because analyses of sex × drug interactions decreased the sample size to N = 13, which is on the low side for ERP experiments. These low sample sizes could also be the reason for the lack of observing ERP correlates for oxytocin’s memory-impairing effects on recollection in men.
Oxytocin also affected test-phase ERPs in two additional ways. The oxytocin group showed an early onset of recollection-related old/new effects between 300 and 500 ms. Early recollection effects are somewhat unusual but not unprecedented (MacKenzie & Donaldson, 2007) in recognition experiments with faces. The oxytocin group showed also increased mean amplitudes starting at 500 ms. The importance and interpretation of these effects must remain open as they neither correspond with the behavioral data, nor with assumptions about the effects of oxytocin. Furthermore, oxytocin levels will have started to decline and returned to baseline during the recognition test. Future research will show whether or not these effects are observed reliably.
In conclusion, the present findings suggest that oxytocin, when administered before the study phase, leads to more successful memory encoding of subsequently “familiar” own-race and other-race faces. This behavioral effect has its neural correlate in the study phase between 750 and 1000 ms after stimulus presentation, where the Dm for familiarity was enhanced in the oxytocin group. During recognition, “familiar” faces were more easily discriminated from new faces as reflected in the FN400. This effect is likely a consequence from the facilitated memory encoding because oxytocin levels were highest during the study phase. Importantly, oxytocin did not affect perceptual ERPs in the study or test phase. This suggests that, in the context of a recognition memory task, oxytocin selectively enhances processes of memory but not perception. Contrary to current opinion, oxytocin affected memory performance for own-race and other-race faces similarly. This finding suggests that oxytocin-induced memory effects are independent from both perceptual training and social cognition which have been suggested to underlie the other-race effect in face memory. Finally, oxytocin led to a memory-impairing effect on recollection, but only in men. These sex differences in oxytocin’s effect on face memory require further investigation.
Supplementary Material
Acknowledgments
Portions of the research in this paper use the Color FERET database of facial images collected under the FERET program (Phillips et al., 2000). We thank Patrick White, Paula Mathews, Debra Coady, Mary Jo Reiling, Candice Morris, Phil Rhodes, Elizabeth Connick, Krystin Corby, and Lucas Ellison for research assistance.
Role of the Funding Source Funding for this research was provided by NIH Grants MH64812 and MH096698, NIH/NCRR Colorado CTSI Grant Number UL1 RR025780 to the Clinical Translational Research Center at the University of Colorado, and NSF grant #SBE-0542013 to the Temporal Dynamics of Learning Center (an NSF Science of Learning Center. None of the funding agencies had a further role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. Contents are the authors’ sole responsibility and do not necessarily represent official views of any funding agencies.
Footnotes
Conflict of Interest All authors declare that they have no conflicts of interest.
Contributors Grit Herzmann managed the literature searches, supervised the data collection, undertook the statistical analyses, and wrote the first draft of the manuscript. Grit Herzmann and Tim Curran designed the study and wrote the protocol. Megan Freeman and Christopher W. Bird collected the data. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends in Cogn. Sci. 2011;15:301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat. Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Carmichael MS, Humbert R, Dixen J, Palmisano G, Greenleaf W, Davidson JM. Plasma oxytocin increases in the human sexual response. J Clin Endocrinol Metab. 1987;64:27–31. doi: 10.1210/jcem-64-1-27. [DOI] [PubMed] [Google Scholar]
- Carmichael MS, Warburton VL, Dixen J, Davidson JM. Relationships among cardiovascular, muscular, and oxytocin responses during human sexual activity. Arch Sex Behav. 1994;23:59–79. doi: 10.1007/BF01541618. [DOI] [PubMed] [Google Scholar]
- Carter CS. Sex differences in oxytocin and vasopressin: implications for autism spectrum disorders? Behav Brain Res. 2007;176:170–186. doi: 10.1016/j.bbr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Curran T. Brain potentials of recollection and familiarity. Mem Cognit. 2000;28:923–938. doi: 10.3758/bf03209340. [DOI] [PubMed] [Google Scholar]
- De Dreu CKW. Oxytocin modulates cooperation within and competition between groups: an integrative review and research agenda. Horm Behav. 2012;61:419–428. doi: 10.1016/j.yhbeh.2011.12.009. [DOI] [PubMed] [Google Scholar]
- De Dreu CKW, Greer LL, Van Kleef GA, Shalvi S, Handgraaf MJJ. Oxytocin promoted human ethnocentrism. Proc. Natl. Acad. Sci. USA. 2011;108:1262–1266. doi: 10.1073/pnas.1015316108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries GJ. Sex differences in vasopression and oxytocin innervtion of the brain. Prog Brain Res. 2008;170:17–27. doi: 10.1016/S0079-6123(08)00402-0. [DOI] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpetz SC. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35:83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Gläscher J, Büchel C, Braus DF, Herpertz S. Oxytocin attenuates amygdala response to emotional faces regardless of valence. Biol. Psychiatry. 2007;62:1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Duarte A, Ranganath C, Winward L, Hayward D, Knigth RT. Dissociable neural correlates for familiarity and recollection during the encoding and retrieval of pictures. Cogn Brain Res. 2004;18:255–272. doi: 10.1016/j.cogbrainres.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Duchaine B, Nakayama K. The Cambridge Face Memory Test: Results for neurologically intact individuals and an investigation of its validity using inverted face stimuli and prosopagnosic participants. Neuropsychologia. 2006;44:576–585. doi: 10.1016/j.neuropsychologia.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Gao W, Cao B, Shan S, Zhou D, Zhang X, Zhao D. The CAS-PEAL Large-Scale Chinese Face Database and Evaluation Protocols. Joint Research & Development Laboratory; CAS: 2004. Technical Report No. JDL_TR_04_FR_001. 2004. [Google Scholar]
- Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biol. Psychiatry. 2008a;63:3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Mathews F. Oxytocin enhances the encoding of positive social memories in humans. Biol. Psychiatry. 2008b;64:256–258. doi: 10.1016/j.biopsych.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Herzmann G, Curran T. Experts’ memory: an ERP study of perceptual expertise effects on encoding and recognition. Mem Cognit. 2011;39:412–432. doi: 10.3758/s13421-010-0036-1. [DOI] [PubMed] [Google Scholar]
- Herzmann G, Willenbockel V, Tanaka JW, Curran T. The neural correlates of memory encoding and recognition for own-race and other-race faces. Neuropsychologia. 2011;49:3103–3115. doi: 10.1016/j.neuropsychologia.2011.07.019. [DOI] [PubMed] [Google Scholar]
- Herzmann G, Young B, Bird CW, Curran T. Oxytocin can impair memory for social and non-social visual objects: A within-subject investigation of oxytocin’s effects on human memory. Brain Res. 2012;1451:65–73. doi: 10.1016/j.brainres.2012.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenberg K, Young SG, Bernstein MJ, Sacco DF. The categorization-individuation model: An integrative account of the other-race recognition deficit. Psychol Rev. 2010;117:1168–1187. doi: 10.1037/a0020463. [DOI] [PubMed] [Google Scholar]
- Huynh H, Feldt LS. Estimation of the Box correction for degrees of freedom from sample data in randomized block and split-plot designs. Journal of Educational Statistics. 1976;1:69–82. [Google Scholar]
- Latinus M, Taylor MJ. Face processing stages: Impact of difficulty and the separation of effects. Brain Res. 2006;1123:179–187. doi: 10.1016/j.brainres.2006.09.031. [DOI] [PubMed] [Google Scholar]
- Luck SJ. An introduction to the event-related potentials technique. MIT Press; Cambridge: 2005. [Google Scholar]
- MacDonald K, MacDonald TM. The peptide that binds: a systematic review of oxytocin and its prosocial effects in humans. Harv. Rev. Psychiat. 2010;18:1–21. doi: 10.3109/10673220903523615. [DOI] [PubMed] [Google Scholar]
- MacKenzie G, Donaldson DI. Dissociating recollection from familiarity: electrophysiological evidence that familiarity for faces is associated with a posterior old/new effect. Neuroimage. 2007;36:454–463. doi: 10.1016/j.neuroimage.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Meissner CA, Brigham JC. Thirty years of investigating the own-race bias in memory for faces: A meta-analytic review. Psychology, Public Policy, and Law. 2001;7:3–35. [Google Scholar]
- Paller KA, Kutas M, Mayes AR. Neural correlates of encoding in an incidental learning paradigm. Electroencephalogr Clin Neurophysiol. 1987;67:360–371. doi: 10.1016/0013-4694(87)90124-6. [DOI] [PubMed] [Google Scholar]
- Phillips PJ, Moon H, Rizvi SA, Rauss PJ. The FERET evaluation methodology for face recognition algotithms. IEEE T. Pattern Anal. 2000;22:1090–1104. [Google Scholar]
- Rimmele U, Hediger K, Heinrichs M, Klaver P. Oxytocin makes a face in memory familiar. J. Neurosci. 2009;29:38–42. doi: 10.1523/JNEUROSCI.4260-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossion B, Jacques C. The N170: Understanding the time course of face perception in the human brain. In: Luck SJ, Kappenman ES, editors. The Oxford Handbook of Event-Related Potential Components. Oxford University Press; 2011. pp. 115–141. [Google Scholar]
- Rugg MD, Curran T. Event-related potentials and recognition memory. Trends in Cogn. Sci. 2007;11:251–257. doi: 10.1016/j.tics.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Savaskan E, Ehrhardt R, Schulz A, Walter M, Schächinger Post-learning intranasal oxytocin modulates human memory for facial identity. Psychoneuroendocrinology. 2008;33:368–374. doi: 10.1016/j.psyneuen.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Smeltzer MD, Curtis JT, Aragona BJ, Wang Z. Dopamine, oxytocin, and vasopressin receptor binding in the medial prefrontal cortex of monogamous and promiscuous voles. Neurosci Lett. 2006;394:146–151. doi: 10.1016/j.neulet.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Davidson PSR, Kim ASN, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: Meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Steyer R, Schwenkmezger P, Notz P, Eid M. Der Mehrdimensionale Befindlichkeitsbogen (MDBF) Hogrefe; Göttingen: 1997. [Google Scholar]
- Tsukiura T. Neural mechanisms underlying the effects of face-based affective signals on memory for faces: a tentative model. Front Integr Neurosci. 2012;2012:6–50. doi: 10.3389/fnint.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker DM. Spatial sampling of head electrical fields: the geodesic sensor net. Electroencephalogr Clin Neurophysiol. 1993;87:154–163. doi: 10.1016/0013-4694(93)90121-b. [DOI] [PubMed] [Google Scholar]
- Uhl-Bronner S, Waltisperger E, Martinez-Lorenzana G, Condes Lara M, Freund-Mercier MJ. Sexually dimorphic expression of oxytocin binding sites in forebrain and spinal cord of the rat. Neuroscience. 2005;135:147–154. doi: 10.1016/j.neuroscience.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Van IJzendoorn MH, Bakermans-Kranenburg MJ. A sniff of trust: meta-analysis of the effects of intranasal oxytocin administration on face recognition, trust to in-group, and trust to out-group. Psychoneuroendocrinology. 2012;37:438–443. doi: 10.1016/j.psyneuen.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Vilberg KL, Moosavi RF, Rugg MD. The relationship between electrophysiological correlates of recollection and amount of information retrieved. Brain Res. 2006;1122:161–170. doi: 10.1016/j.brainres.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Paller KA. Remembering and knowing: Electrophysiological distinctions at encoding but not retrieval. NeuroImage. 2009;46:280–289. doi: 10.1016/j.neuroimage.2009.01.048. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Pers. Social Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wilding EL. In what way does the parietal ERP old/new effect index recollection? Int. J. Psychophysiol. 2000;35:81–87. doi: 10.1016/s0167-8760(99)00095-1. [DOI] [PubMed] [Google Scholar]
- Woodruff CC, Hayama HR, Rugg MD. Electrophysiological dissociation of the neural correlates of recollection and familiarity. Brain Res. 2006;1100:125–135. doi: 10.1016/j.brainres.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: a review of 30 years of research. J. Mem. Lang. 2002;46:441–517. [Google Scholar]
- Zink CF, Meyer-Lindenberg A. Human neuroimaging of oxytocin and vasopression in social cognition. Horm Behav. 2012;61:400–409. doi: 10.1016/j.yhbeh.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.