Abstract
We describe a patient with semantic variant of frontotemporal dementia who received longitudinal clinical evaluations and structural MRI scans and subsequently came to autopsy. She presented with early behavior changes and semantic loss for foods and people and ultimately developed a pervasive semantic impairment affecting social-emotional as well as linguistic domains. Imaging revealed predominant atrophy of the right temporal lobe, with later involvement of the left, and pathology confirmed bilateral temporal involvement. Findings support the view that left and right anterior temporal lobes serve as semantic hubs that may be affected differentially in semantic variant by early, relatively unilateral damage.
Keywords: semantic dementia, frontotemporal dementia, right temporal variant of FTD, temporal lobe, TDP-43
1. Introduction
Frontotemporal dementia (FTD) comprises a group of clinical syndromes characterized by progressive impairment of behavior and/or language. These syndromes include a behavioral variant (bvFTD), the nonfluent/agrammatic variant of primary progressive aphasia (nfvPPA), and the semantic variant of PPA (svPPA). FTD subtypes have been the subject of a growing body of research, which has revealed a distinct clinical, neuroanatomical, and pathological profile for each syndrome. A majority of patients with semantic variant present with progressive loss of word and object knowledge in the context of left greater than right anterior temporal lobe atrophy. Patients with more prominent right temporal involvement are less well-characterized and have been described as presenting with behavior and personality changes and loss of person-specific knowledge (Chan et al., 2009; Edwards-Lee et al., 1997; Gainotti, Barbier, & Marra, 2003; Joubert et al., 2006; Miller, Chang, Mena, Boone, & Lesser, 1993; Seeley et al., 2005; Thompson, Patterson, & Hodges, 2003; Tyrrell, Warrington, Frackowiak, & Rossor, 1990).
Numerous studies of svPPA and of semantic processing in healthy individuals implicate the left temporal lobe in semantic processing for words and objects (e.g., Hodges et al., 1992; Mummery et al., 2000). The role of the right temporal lobe has been less thoroughly characterized, however there is increasing evidence for its role in empathy and emotion processing (Rankin, Kramer, & Miller, 2005; Rankin et al., 2006; Rosen et al., 2002); abstract conceptual social knowledge (Zahn et al., 2009); multimodal semantic knowledge for persons (Joubert et al., 2003; Joubert et al., 2006; Snowden, Thompson, & Neary, 2004) and food (Gorno-Tempini et al., 2004c); as well as taste recognition (Small, Jones-Gotman, Zatorre, Petrides, & Evans, 1997) and eating behavior (Uher & Treasure, 2005).
Whereas these studies tend to implicate the left and right temporal lobes in distinct cognitive roles, another view holds that both anterior temporal lobes (ATLs) play a role in semantic processing (Lambon Ralph, McClelland, Patterson, Galton, & Hodges, 2001; Mion et al., 2010). According to this view, each ATL has differential connectivity to other cortical regions, which may result in different patterns of impairment when one or the other is primarily affected by disease. For instance, the left ATL has greater connectivity with phonological and orthographic representations (also housed in the left hemisphere) and therefore, damage to the left ATL is likely to cause a greater degree of verbal/linguistic impairment (Lambon Ralph et al., 2001), as is observed in individuals with left greater than right temporal atrophy and the “classic” presentation of semantic variant PPA. Likewise, it may be the case that the right ATL serves to bind sensory representations recruited for social and emotional processes and therefore, disease that predominantly affects the right ATL may produce greater impairments in social and behavioral domains (Olson, Plotzker, & Ezzyat, 2007). This is consistent with findings that the right anterior temporal lobe plays a role in supporting abstract social conceptual knowledge in healthy individuals (Ross & Olson, 2010; Zahn et al., 2007) as well as patients with neurodegenerative disease (Zahn et al., 2009).
There are relatively few within-subjects longitudinal studies examining the clinical and anatomical evolution of FTD syndromes (Brambati et al., 2007; Brambati et al., 2009; Gorno-Tempini, Murray, Rankin, Weiner, & Miller, 2004b; Janssen et al., 2005; Seeley et al., 2005; Whitwell, Anderson, Scahill, Rossor, & Fox, 2004). In svPPA, previous longitudinal clinical (Seeley et al., 2005) and neuroimaging (Brambati et al, 2007) analyses suggest that disease spreads from one anterior temporal lobe to the other, with bilateral involvement of the amygdalae as well as later atrophy of frontal, insular and inferoposterior temporal cortices. The clinical picture tends to reflect the topography of cortical atrophy. As such, in individuals with predominant right temporal atrophy, problems with social functioning and emotion processing outweigh deficits in word and object semantics early on; in those with left-predominant atrophy, the opposite pattern is observed. As atrophy spreads from one temporal lobe to the other, the deficits less prominent at presentation become more apparent (Seeley et al., 2005). Regardless of laterality, patients with svPPA most often show a common pathological profile characterized by Type C TDP-43 positive, tau-negative inclusions (Davies et al., 2005; Deramecourt et al., 2010; Hodges et al., 2004; Hodges et al., 2010; Mackenzie et al., 2011; Rohrer et al., 2010).
Few studies to date have examined the progression of behavior deficits and atrophy in FTD patients who later came to autopsy (Sanchez-Valle, Forman, Miller, & Gorno-Tempini, 2006). In this study, we examine longitudinal neuropsychological, behavioral, and neuroimaging data for a patient, JT, who presented with svPPA caused by right greater than left temporal lobe atrophy. JT was described previously, with a focus on her cognitive and behavioral profile at presentation in 2002, approximately three years after symptom onset (Gorno-Tempini et al., 2004c). Here we explore the behavioral and neuroanatomical progression of her disease by reviewing data from annual evaluations spanning three years. In addition, we provide information regarding neuropathological diagnosis and distribution of pathology at autopsy.
2. Methods
JT presented to the UCSF Memory and Aging Center in May of 2002 at the age of 67. She underwent annual neuropsychological, speech and language, and neuroimaging evaluations in 2002, 2003, and 2004.
2.1 Cognitive and behavioral assessments
As outlined in the previous case report, language was assessed using the Western Aphasia Battery (Kertesz, 1982), as well as tests examining semantic and syntactic skills, reading, and motor speech. Performance on neuropsychological and speech-language measures was compared with data from normal controls (previously published in Gorno-Tempini et al., 2004a,c; Kramer et al., 2003) or with published norms. Performance greater than 1.5 standard deviations below the normal control mean was considered to be impaired.
Behavioral and personality changes were characterized using the Neuropsychiatric Inventory (NPI, Cummings et al., 1994), as well as informant-based questionnaires (administered at years 1 and 2 only) examining social and personality factors. These questionnaires, completed by the patient’s daughter, included the Interpersonal Adjectives Scale (IAS, Wiggins, 1995), a measure of interpersonal aspects of personality, and the Interpersonal Reactivity Index (IRI, Davis, 1983), a multifaceted measure of empathy.
2.2 Neuroimaging
High-resolution 3D structural MR images were obtained within four months of each clinical assessment. Images were segmented into grey matter, white matter, and cerebrospinal fluid and first normalized to standard space using the unified segmentation procedure in SPM5 (Ashburner & Friston, 2005) with subsequent registration using the DARTEL toolbox (Ashburner, 2007). Modulated, smoothed gray matter images were derived from each of the patient’s three MRI scans as well as for 32 age-matched healthy female controls. In order to examine regional gray matter atrophy over time, gray matter volume in each of JT’s scans was compared to that in the control group using t-tests.
2.3 Autopsy
JT died and underwent brain autopsy 4 years and 8 months after her third clinical evaluation. An autopsy limited to brain and spinal cord was performed 6.1 hours after death. The fresh brain, weighing 930 grams, was cut into 8-10 mm-thick coronal slabs, which were alternately fixed for 72 hours in 4% paraformaldehyde or rapidly frozen. Regions of interest were dissected from the fixed slabs, embedded in paraffin wax, and cut at 8 microns following previously described procedures (Tartaglia et al., 2010). Hematoxylin and eosin staining was performed on every block to allow a regional survey of nonspecific neurodegenerative changes. Immunohistochemical staining for amyloid-beta, hyperphosphorylated tau, TDP-43, and alpha-synuclein was performed on a standard subset of regions, as previously detailed (Tartaglia et al., 2010).
3. Results
As outlined in detail in the previous case report, JT’s symptoms began at age 62 with prominent behavioral and personality changes and multimodal semantic loss for foods and people (Gorno-Tempini et al., 2004c). At that time, there was no evidence of verbal semantic deficits (e.g., word-finding difficulty, word comprehension deficits, or surface dysgraphia). By the time that she was seen for her first clinical evaluation three years later, JT showed additional semantic loss affecting words and objects. Demographic information and neuropsychological data from her three yearly evaluations are presented in Table 1.
Table 1.
Demographic and neuropsychological data
| Maximum score | 2002 | 2003 | 2004 | |
|---|---|---|---|---|
| Age | 65 | 66 | 67 | |
| Education | 15 | 15 | 15 | |
| MMSE | 30 | 24* | 12* | 7* |
| Visuospatial functions | ||||
| Benson Figure Copy | 17 | 16 | 13 | 11* |
| VOSP Number Location | 10 | 6* | 9 | NC |
| Benton Facial Recognition Test | 54 | 42 | NC | NC |
| Calculations (multiplication, subtraction, addition) | 5 | 5 | 2*† | 2*† |
| Visual memory | ||||
| Benson Figure Recall (10 minute delay) | 17 | 5* | 0* | NC |
| Benson Figure Recognition | 1 | 1 | 0* | 0* |
| Verbal memory | ||||
| CVLT 30 sec. Free Recall | 9 | 1* | 2* | NC |
| CVLT 10 min. Free Recall | 9 | 0* | 0* | NC |
| CVLT 10 min. Recognition | 9 | 0* | 0* | NC |
| Executive functions | ||||
| Backward digit span | 7 | 6 | 6 | 2* |
indicates impaired performance relative to normal controls from Gorno Tempini et al., 2004a/c or
NC = not collected
MMSE = Mini Mental State Examination (Folstein, Folstein, & McHugh, 1975)
Figure Copy, Recall, and Recognition = Benson Figure (Possin, Laluz, Alcantar, Miller, & Kramer, 2011)
VOSP = Visual Object and Space Perception Battery (Warrington & James, 1991)
Facial Recognition Test = Benton Facial Recognition Test (Benton & Van Allen, 1968)
WMS III = Wechsler Memory Scale III (Wechsler, 1997)
CVLT = California Verbal Learning Test (Delis, Kramer, Kaplan, & Ober, 2000)
Note: only a subset of tests could be administered in 2004, due to the severity of the patient’s language impairment
Behavioral and personality measures from year 1 were reported in detail in the initial case report. Here we will compare results from years 1 and 2 to estimates of premorbid functioning provided by JT’s daughter. The IAS personality scale revealed that, relative to her premorbid personality, JT showed a progressive reduction in warm, extraverted, and ingenuous behaviors. Assured behaviors had decreased considerably at year 1, but increased somewhat at year 2 (however, not to premorbid levels). Cold-hearted behaviors did not increase at year 1 from already high premorbid levels, but showed a relative increase at year 2. The IAS also revealed a reduction in the ability to flexibly respond to social situations in an appropriate manner, with an increasing tendency toward stereotyped, inappropriate behaviors (Gorno-Tempini et al., 2004c). The IRI revealed significant decreases in cognitive aspects of empathy over time, including perspective-taking and tendency to fantasize. The IRI also revealed a decrease in emotional empathy, i.e. empathic concern for others, accompanied by increased “personal distress,” which occurs when one is unable to cope effectively with others’ emotions (Rankin et al., 2005). The NPI revealed early agitation and disinhibition, along with aberrant motor behavior and eating and sleep disorders (year 1). In addition to these behaviors, irritability and apathy were noted at year 2; delusions, anxiety and euphoria were endorsed at year 3.
As the disease progressed, JT’s verbal semantic impairment became more pervasive. Results from speech and language assessments (Table 2) revealed, at year 1, prominent naming difficulty for both objects and famous people and poor performance on both verbal and nonverbal semantic association tasks. These deficits were observed in the context of spared fluency, repetition, single-word comprehension, syntactic processing, and motor speech. At year 2, JT demonstrated a further decline in performance on naming and other verbal semantic tasks, and showed development of single-word comprehension deficits and surface dyslexia. These deficits became more prominent at year 3. Syntactic comprehension was spared until the final assessment, at which point severe lexical deficits impeded sentence comprehension. By contrast, non-semantic aspects of communication, such as fluency, repetition and motor speech, remained relatively spared at the third evaluation.
Table 2.
Speech, language, and nonverbal semantic assessments
| Maximum score | 2002 | 2003 | 2004 | |
|---|---|---|---|---|
| WAB Fluency | 10 | 9* | 9* | 9* |
| WAB Information Content | 10 | 9* | 8* | 6* |
| WAB Repetition | 100 | 100 | 98* | 97* |
| WAB Auditory Word Recognition | 60 | 58* | 50* | 28* |
| Pyramids and Palm Trees (words) | 52 | 43* | 27* | NC |
| Pyramids and Palm Trees (pictures) | 52 | 27* | 22* | NC |
| Boston Naming Test | 60 | 6* | 3* | NC |
| Experimental Famous Face Battery (Gorno-Tempini et al., 2004b): | ||||
| • Famous Face Naming | 20 | 0* | NC | NC |
| • Famous Face Recognition | 20 | 14* | NC | NC |
| • Famous Face Semantic Association | 20 | 5* | NC | NC |
| • Famous Name-to-Face Matching | 20 | 6* | NC | NC |
| WAB Sequential Commands | 80 | 80 | 78* | 2* |
| CYCLE (Sentence Comprehension) Subtests: | ||||
| • Cycle 2,3 (declaratives, possession) | 10 | 10 | 9 | 5* |
| • Cycle 4 (active & passive voice; double embedding) | 15 | 14* | 15 | 6* |
| • Cycle 5,7 (passive voice, subject relatives) | 10 | 10 | 10 | 5* |
| • Cycle 8 (object clefting; object relative clauses) | 10 | 10 | 10 | NC |
| • Cycle 9 (object relatives; relative pronouns) | 10 | 9 | 7* | 1* |
| PALPA Reading Regular Words | 30 | 30 | 30 | 14† |
| PALPA Reading Exception Words | 30 | 27† | 20† | 6† |
| Motor Speech Evaluation (Wertz et al., 1984) | -- | WNL | WNL | WNL |
indicates impaired performance relative to normal controls from Gorno Tempini et al., 2004a/c or
published norms
NC = not collected
WAB = Western Aphasia Battery (Kertesz, 1982)
Boston Naming Test (Kaplan, Goodglass, & Weintraub, 2001)
CYCLE = Curtiss-Yamada Comprehensive Language Evaluation- Receptive (Curtiss & Yamada, 1988)
PALPA = Psycholinguistic Assessment of Language Processing in Aphasia (Kay, Lesser, & Coltheart, 1992)
WNL = within normal limits
Note: only a subset of tests could be administered in 2004, due to the severity of the patient’s language impairment
Voxel-based morphometry (VBM; Figure 1) revealed spreading asymmetric atrophy (right greater than left) over a three-year period, with early involvement of the right amygdala and hippocampus, parahippocampal and fusiform gyri, and temporal pole and less prominent atrophy in the left amygdala and parahippocampal gyrus. With disease progression, atrophy extended to additional right, then left cortical regions, including posterior and inferolateral temporal cortex and the insula. Frontal lobe involvement remained relatively mild.
Figure 1.
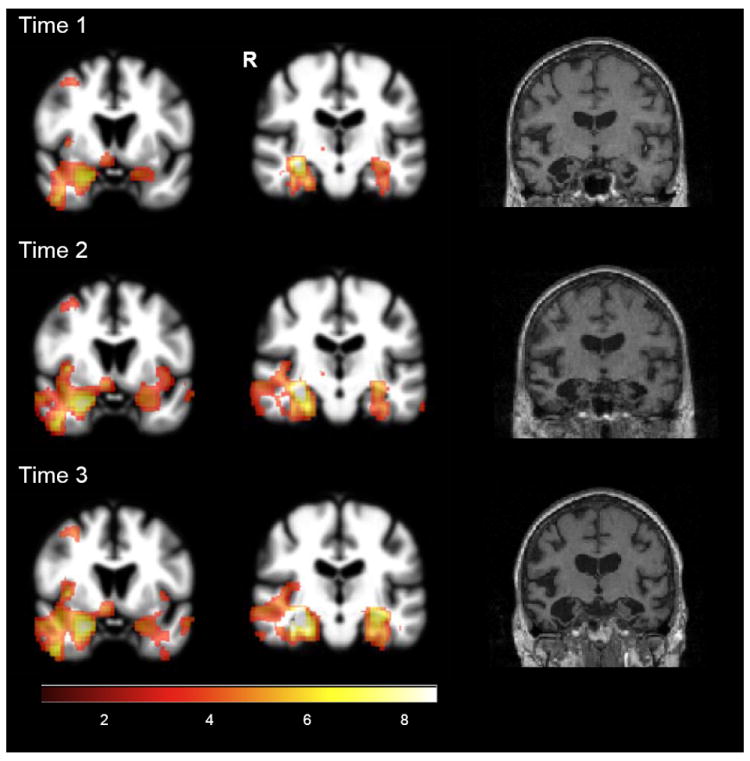
Voxel-based morphometry findings at three time points (JT vs. 32 normal controls; p < 0.001, uncorrected. Color bar indicates t-values).
The pattern of gross atrophy at autopsy (Figure 2) mirrored that identified with MRI. Bilateral anterior and inferoposterior temporal cortices were thinned to less than 1mm, with blurring of the margins between gray and white matter. The amygdalae were flattened. Hematoxylin and eosin staining revealed prominent microvacuolation, astrogliosis, and neuronal loss in anterior inferior temporal, subgenual anterior cingulate, agranular mid-insular, and entorhinal/parahippocampal cortices, as well as in nucleus accumbens and putamen (Table 3). White matter subjacent to anterior and inferior temporal regions were pale. The hippocampus was relatively spared. Immunohistochemical analyses revealed TDP-43 immunoreactive neuronal inclusions, primarily taking the form of long dystrophic neurites in superficial greater than deep layers of affected cortices, although abundant round, circumscribed neuronal cytoplasmic inclusions were seen in ventral striatum and dentate gyrus. The morphology and distribution of TDP-43 inclusions was consistent with FTLD-TDP, Type C (Mackenzie et al., 2011). Incidentally noted was mild medial temporal lobe tau-immunoreactive neurofibrillary pathology (Braak stage 2) and scattered diffuse amyloid plaques in frontal, parietal, and occipital cortex. No alpha-synuclein-immunoreactive pathology was observed.
Figure 2.
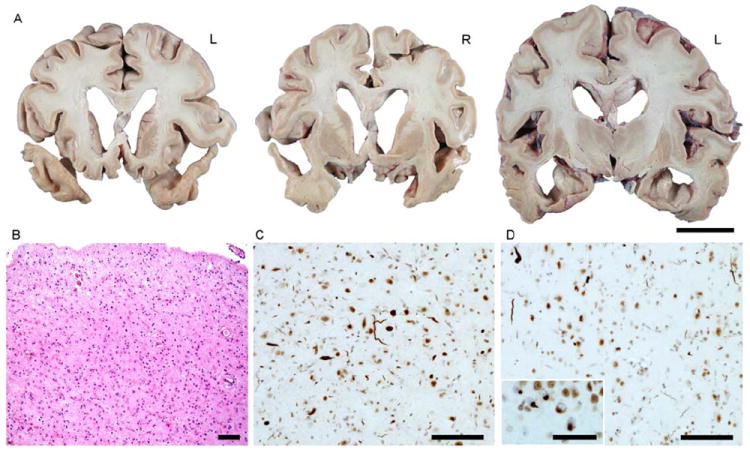
Neuropathology. (A) Gross findings. (B) Severe astrocytic gliosis and neuronal loss in right amygdala. Hematoxylin and eosin stain.(C-D) TDP-43 immunohistochemistry reveals frequent dystrophic neurites of varying caliber in amygdala (C) and anterior inferior temporal gyrus (D). Inset in (D) shows neuronal cytoplasmic inclusions in dentate gyrus granule cells. Scale bars indicate 3 cm (A), 100 μM (B-D), and 50 μM (D inset).
Table 3.
Regional distribution of histopathology
| Region | Subregion | Microvacuolation | Astrogliosis | TDP-43 DNs | TDP-43 NCIs |
|---|---|---|---|---|---|
| Frontal | Frontal pole | 0 | 0 | n.a. | n.a. |
| Anterior orbital gyrus | + | + | n.a. | n.a. | |
| Pregenual ACC | + | ++ | +++ | + | |
| Middle frontal gyrus | 0 | + | n.a. | n.a. | |
| Inferior frontal gyrus, oper | + | + | n.a. | n.a. | |
| Subgenual ACC | ++ | +++ | +++ | + | |
| Precentral gyrus | + | + | +++ | + | |
| Superior frontal sulcus | + | + | n.a. | n.a. | |
| Insula Temporal | Middle insula | + | ++ | ++ | + |
| Entorhinal cortex | ++ | +++ | ++ | + | |
| Inferior temporal gyrus | ++ | +++ | +++ | + | |
| Parietal | Postcentral gyrus | + | + | + | 0 |
| Posterior cingulate cortex | + | + | n.a. | n.a. | |
| Angular Gyrus | + | + | n.a. | n.a. | |
| Occipital Limbic | Striate cortex | 0 | 0 | n.a. | n.a. |
| Amygdala | n.a. | +++ | +++ | ++ | |
| Dentate gyrus | n.a. | 0 | + | +++ | |
| CA3-4 | n.a. | 0 | 0 | 0 | |
| CA2 | n.a. | 0 | + | 0 | |
| CA1/Subiculum | n.a. | 0 | + | 0 | |
| Subcortical | Nucleus accumbens | n.a. | +++ | + | +++ |
| Putamen | n.a. | ++ | + | +++ | |
| Globus Pallidus | n.a. | + | 0 | 0 | |
| Thalamus | n.a. | + | n.a. | n.a. | |
| Claustrum | n.a. | 0 | + | 0 | |
| Cerebellum | Dentate nucleus | n.a. | 0 | n.a. | n.a. |
| Folia | n.a. | 0 | n.a. | n.a. | |
| Midbrain | Substantia Nigra | n.a. | 0 | 0 | 0 |
| Tectum | n.a. | 0 | + | 0 | |
| Periaqueductal gray | n.a. | 0 | + | 0 | |
| Oculomotor nucleus | n.a. | 0 | 0 | 0 | |
| Pons | Locus ceruleus | n.a. | 0 | n.a. | n.a. |
| Median raphe | n.a. | 0 | n.a. | n.a. | |
| Abducens nucleus | n.a. | 0 | n.a. | n.a. | |
| Facial nucleus | n.a. | 0 | n.a. | n.a. | |
| Medulla | Hypoglossal nucleus | n.a. | 0 | 0 | 0 |
| Dorsal motor nucleus, vagus | n.a. | 0 | 0 | 0 | |
| Nucleus of the solitary tract | n.a. | 0 | 0 | 0 | |
| Olive, inferior | n.a. | 0 | 0 | 0 | |
| Spinal cord | Cervical anterior horn cells | n.a. | 0 | 0 | 0 |
| Cervical corticospinal tract | n.a. | 0 | 0 | 0 | |
| Thoracic anterior horn cells | n.a. | 0 | 0 | 0 | |
| Thoracic IML cell column | n.a. | 0 | 0 | 0 | |
| Lumbar anterior horn cells | n.a. | 0 | 0 | 0 | |
| Sacral anterior horn cells | n.a. | 0 | + | 0 |
ACC, anterior cingulate cortex; DNs, dystrophic neurites; n.a., not assessed, NCIs, neuronal cytoplasmic inclusions
4. Conclusion
JT’s pattern of atrophy and histopathology echoes that previously reported in temporal lobe variants of FTD. As in the majority of previous svPPA reports, here autopsy revealed TDP-43 histopathology, Type C. Atrophy, detected by in-vivo imaging and confirmed at autopsy, showed a predilection for the anterior and inferior temporal lobes, including the temporal pole, middle/inferior temporal gyri, and anterior fusiform gyrus, as well as the amygdalae. With disease progression, atrophy extended to the contralateral (left) hemisphere and spread caudally within the temporal lobe. JT’s clinical features were a reflection of the distribution of atrophy/histopathology, which increasingly involved the left anterior temporal lobe in addition to the right. Initial deficits, as revealed by clinical history, revealed semantic loss specific to social-emotional domains and person/food knowledge (Gorno-Tempini et al., 2004c). With spread of disease into the left temporal lobe, the clinical picture evolved into a pronounced, multi-modal semantic impairment additionally involving knowledge of objects and words. Even at the third and final evaluation, however, non-semantic aspects of speech and language remained relatively intact. For example, spoken language was strikingly fluent and grammatically correct, even in the context of markedly impaired lexical retrieval.
It is noteworthy that JT showed a modality discrepancy (words > pictures) on the Pyramids and Palm Trees Test (Howard & Patterson, 1992), a measure of semantic association that involves matching either words or pictures based on semantic relatedness. This modality discrepancy is consistent with findings of domain-specific effects of atrophy in the right temporal lobe for the picture version of the test and in the left temporal lobe for the word version (Butler, Brambati, Miller, & Gorno-Tempini, 2009). Whereas domain-specific effects may suggest an access problem, rather than a central semantic deficit, JT’s performance was impaired regardless of modality. Relatively worse performance on picture stimuli. however, may be indicative of a deficit in semantically-driven visuoperceptual processing (Butler et al., 2009).
In svPPA, diffusion tensor imaging has revealed prominent white matter abnormalities in the inferior longitudinal fasciculus (ILF) and the uncinate fasciculus (UF) (Agosta et al., 2010; Galantucci et al., 2011; Whitwell et al., 2010). Likewise, at autopsy, our case demonstrated severe white matter degeneration in regions subjacent to anterior and inferior temporal cortex. Damage to the left ILF may contribute to the picture naming, object recognition and reading impairments observed in svPPA by disconnecting posterior temporo-occipital cortex involved in lexical and visual/orthographic processing from the anterior temporal lobe. ILF damage in the right hemisphere may, likewise, disconnect posterior visual and auditory association areas from the right anterior temporal lobe, with a resultant inability to process emotional and person-specific stimuli. Damage to white matter connecting frontal and anterior temporal regions (i.e., the UF) may play an additional role in behavioral deficits observed in svPPA.
The pattern of social, emotional, and cognitive-linguistic impairments observed in this case supports the hypothesis that anterior temporal cortex may function as an amodal semantic hub that binds information from cortical sensory, motor, and language regions (Lambon Ralph, Sage, Jones, & Mayberry, 2010; Mion et al., 2010; Patterson, Nestor, & Rogers, 2007; Pobric, Jefferies, & Lambon Ralph, 2010). Different patterns of impairment in individuals with left versus right predominant atrophy in the ATL, however, suggest that these hubs may have distinct patterns of connectivity. Patients with left-predominant ATL atrophy show disproportionate impairment on verbal semantic tasks such as naming and word comprehension and show object recognition deficits (Hodges & Patterson, 2007), suggesting that the left anterior temporal lobe may be a cortical association hub for word and object meaning. Conversely, patients with right-predominant ATL atrophy show greater impairment in social and emotional domains, with abnormal scores on measures of empathy (Rankin et al., 2006) and impaired performance on tasks that tap abstract conceptual social knowledge (Zahn et al., 2009). Thus, the right anterior temporal lobe may construct social and emotional meaning by binding information about viscero-autonomic inputs, people, and non-verbal aspects of communication (e.g., emotional prosody). Whereas these hubs may be affected differentially by early, relatively unilateral damage, in svPPA, progression of disease typically involves spread of atrophy to the contralateral temporal lobe, resulting in a bitemporal clinical picture.
Acknowledgments
This work was supported by NIH (NIDCD F32DC010945, NIA P50 AG023501, NIA P01 AG019724, NIA R01 AG033017, NINDS R01 NS050915), Alzheimer’s Disease Research Center of California (03-75271 DHS/ADP/ARCC); Larry L. Hillblom Foundation; John Douglas French Alzheimer’s Foundation; Koret Family Foundation; McBean Family Foundation; and the Consortium for Frontotemporal Dementia Research.
References
- Agosta F, Henry RG, Migliaccio R, Neuhaus J, Miller BL, Dronkers NF, et al. Language networks in semantic dementia. Brain : A Journal of Neurology. 2010;133(Pt 1):286–299. doi: 10.1093/brain/awp233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Benton AL, Van Allen MW. Impairment in facial recognition in patients with cerebral disease. Cortex: A Journal Devoted to the Study of the Nervous System and Behavior. 1968;4:344–348. [Google Scholar]
- Brambati SM, Rankin KP, Narvid J, Seeley WW, Dean D, Rosen HJ, et al. Atrophy progression in semantic dementia with asymmetric temporal involvement: A tensor-based morphometry study. Neurobiology of Aging. 2009;30(1):103–111. doi: 10.1016/j.neurobiolaging.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambati SM, Renda NC, Rankin KP, Rosen HJ, Seeley WW, Ashburner J, et al. A tensor based morphometry study of longitudinal gray matter contraction in FTD. NeuroImage. 2007;35(3):998–1003. doi: 10.1016/j.neuroimage.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler CR, Brambati SM, Miller BL, Gorno-Tempini ML. The neural correlates of verbal and non-verbal semantic processing deficits in neurodegenerative disease. Cognitive and Behavioral Neurology: Official Journal of the Society for Behavioral and Cognitive Neurology. 2009;22(2):73–80. doi: 10.1097/WNN.0b013e318197925d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan D, Anderson V, Pijnenburg Y, Whitwell J, Barnes J, Scahill R, et al. The clinical profile of right temporal lobe atrophy. Brain. 2009;132(5):1287–1298. doi: 10.1093/brain/awp037. [DOI] [PubMed] [Google Scholar]
- Cummings JL. The neuropsychiatric inventory: Comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Curtiss S, Yamada J. The Curtiss–Yamada comprehensive language evaluation (CYCLE) 1988 Unpublished Test. [Google Scholar]
- Davies RR, Hodges JR, Kril JJ, Patterson K, Halliday GM, Xuereb JH. The pathological basis of semantic dementia. Brain. 2005;128(9):1984–1995. doi: 10.1093/brain/awh582. [DOI] [PubMed] [Google Scholar]
- Davis MH. Measuring individual differences in empathy: Evidence for a multidimensional approach. Journal of Personality and Social Psychology. 1983;44(1):113–126. [Google Scholar]
- Delis D, Kramer J, Kaplan E, Ober B. California Verbal Learning Test. 2nd. San Antonio, Tx: The Psychological Corporation; 2000. [Google Scholar]
- Deramecourt V, Lebert F, Debachy B, Mackowiak-Cordoliani MA, Bombois S, Kerdraon O, et al. Prediction of pathology in primary progressive language and speech disorders. Neurology. 2010;74(1):42–49. doi: 10.1212/WNL.0b013e3181c7198e. [DOI] [PubMed] [Google Scholar]
- Edwards-Lee T, Miller BL, Benson DF, Cummings JL, Russell GL, Boone K, et al. The temporal variant of frontotemporal dementia. Brain. 1997;120(6):1027–1040. doi: 10.1093/brain/120.6.1027. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gainotti G, Barbier A, Marra C. Slowly progressive defect in recognition of familiar people in a patient with right anterior temporal atrophy. Brain. 2003;126(4):792–803. doi: 10.1093/brain/awg092. [DOI] [PubMed] [Google Scholar]
- Galantucci S, Tartaglia MC, Wilson SM, Henry ML, Filippi M, Agosta F, et al. White matter damage in primary progressive aphasias: A diffusion tensor tractography study. Brain. 2011;134(10):3011–3029. doi: 10.1093/brain/awr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, et al. Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology. 2004a;55(3):335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Murray RC, Rankin KP, Weiner MW, Miller BL. Clinical, cognitive and anatomical evolution from nonfluent progressive aphasia to corticobasal syndrome: A case report. Neurocase : Case Studies in Neuropsychology, Neuropsychiatry, and Behavioural Neurology. 2004b;10(6):426–436. doi: 10.1080/13554790490894011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Rankin KP, Woolley JD, Rosen HJ, Phengrasamy L, Miller BL. Cognitive and behavioral profile in a case of right anterior temporal lobe neurodegeneration. Cortex. 2004c;40(4-5):631–644. doi: 10.1016/s0010-9452(08)70159-x. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Davies RR, Xuereb JH, Casey B, Broe M, Bak TH, et al. Clinicopathological correlates in frontotemporal dementia. Annals of Neurology. 2004;56(3):399–406. doi: 10.1002/ana.20203. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Mitchell J, Dawson K, Spillantini MG, Xuereb JH, McMonagle P, et al. Semantic dementia: Demography, familial factors and survival in a consecutive series of 100 cases. Brain. 2010;133(1):300–306. doi: 10.1093/brain/awp248. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K. Semantic dementia: A unique clinicopathological syndrome. The Lancet Neurology. 2007;6(11):1004–1014. doi: 10.1016/S1474-4422(07)70266-1. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia: Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115(6):1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson K. Pyramids and palm trees: A test of semantic access from pictures and words. Bury St.Edmunds, UK: Thames Valley Test Company; 1992. [Google Scholar]
- Janssen JC, Schott JM, Cipolotti L, Fox NC, Scahill RI, Josephs KA, et al. Mapping the onset and progression of atrophy in familial frontotemporal lobar degeneration. commentary. Journal of Neurology, Neurosurgery and Psychiatry. 2005;76(2):162–168. doi: 10.1136/jnnp.2003.032201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert S, Felician O, Barbeau E, Ranjeva JP, Christophe M, Didic M, et al. The right temporal lobe variant of frontotemporal dementia. Journal of Neurology. 2006;253(11):1447–1458. doi: 10.1007/s00415-006-0232-x. [DOI] [PubMed] [Google Scholar]
- Joubert S, Felician O, Barbeau E, Sontheimer A, Barton JJ, Ceccaldi M, et al. Impaired configurational processing in a case of progressive prosopagnosia associated with predominant right temporal lobe atrophy. Brain. 2003;126(11):2537–2550. doi: 10.1093/brain/awg259. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia: Lippincott, Williams and Wilkins; 2001. [Google Scholar]
- Kay J, Lesser R, Coltheart M. PALPA: Psycholinguistic assessments of language processing in aphasia. Hove: Lawrence Erlbaum Associates Ltd; 1992. [Google Scholar]
- Kertesz A. The western aphasia battery. New York: Grune & Stratton; 1982. [Google Scholar]
- Kramer JH, Jurik J, Sha SJ, Rankin KP, Rosen HJ, Johnson JK, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and alzheimer disease. Cognitive and Behavioral Neurology. 2003;16(4):211–218. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Sage K, Jones RW, Mayberry EJ. Coherent concepts are computed in the anterior temporal lobes. Proceedings of the National Academy of Sciences. 2010;107(6):2717–2722. doi: 10.1073/pnas.0907307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph M, McClelland J, Patterson K, Galton C, Hodges J. No right to speak? the relationship between object naming and semantic impairment: Neuropsychological evidence and a computational model. Journal of Cognitive Neuroscience. 2001;13(3):341–356. doi: 10.1162/08989290151137395. [DOI] [PubMed] [Google Scholar]
- Mackenzie IRA, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathologica. 2011;169(4):1343–1352. doi: 10.1007/s00401-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BL, Chang L, Mena I, Boone K, Lesser IM. Progressive right frontotemporal degeneration: Clinical, neuropsychological and SPECT characteristics. Dementia and Geriatric Cognitive Disorders. 1993;4(3-4):204–213. doi: 10.1159/000107324. [DOI] [PubMed] [Google Scholar]
- Mion M, Patterson K, Acosta-Cabronero J, Pengas G, Izquierdo-Garcia D, Hong YT, et al. What the left and right anterior fusiform gyri tell us about semantic memory. Brain. 2010;133(11):3256–3268. doi: 10.1093/brain/awq272. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RS, Hodges JR. A voxel-based morphometry study of semantic dementia: Relationship between temporal lobe atrophy and semantic memory. Annals of Neurology. 2000;47(1):36–45. [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The enigmatic temporal pole: A review of findings on social and emotional processing. Brain. 2007;130(7):1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? the representation of semantic knowledge in the human brain. Nature Reviews Neuroscience. 2007;8(12):976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Pobric G, Jefferies E, Lambon Ralph MA. Category-specific versus category-general semantic impairment induced by transcranial magnetic stimulation. Current Biology. 2010;20(10):964–968. doi: 10.1016/j.cub.2010.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possin KL, Laluz VR, Alcantar OZ, Miller BL, Kramer JH. Distinct neuroanatomical substrates and cognitive mechanisms of figure copy performance in Alzheimer’s disease and behavioral variant frontotemporal dementia. Neuropsychologia. 2011;49(1):43–48. doi: 10.1016/j.neuropsychologia.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin KP, Gorno-Tempini ML, Allison SC, Stanley CM, Glenn S, Weiner MW, et al. Structural anatomy of empathy in neurodegenerative disease. Brain. 2006;129(11):2945–2956. doi: 10.1093/brain/awl254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin KP, Kramer JH, Miller BL. Patterns of cognitive and emotional empathy in frontotemporal lobar degeneration. Cognitive and Behavioral Neurology. 2005;18(1):28–36. doi: 10.1097/01.wnn.0000152225.05377.ab. [DOI] [PubMed] [Google Scholar]
- Rohrer JD, Geser F, Zhou J, Gennatas ED, Sidhu M, Trojanowski JQ, et al. TDP-43 subtypes are associated with distinct atrophy patterns in frontotemporal dementia. Neurology. 2010;75(24):2204–2211. doi: 10.1212/WNL.0b013e318202038c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Perry RJ, Murphy J, Kramer JH, Mychack P, Schuff N, et al. Emotion comprehension in the temporal variant of frontotemporal dementia. Brain. 2002;125(10):2286–2295. doi: 10.1093/brain/awf225. [DOI] [PubMed] [Google Scholar]
- Ross LA, Olson IR. Social cognition and the anterior temporal lobes. NeuroImage. 2010;49(4):3452–3462. doi: 10.1016/j.neuroimage.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Valle R, Forman MS, Miller BL, Gorno-Tempini ML. From progressive nonfluent aphasia to corticobasal syndrome: A case report of corticobasal degeneration. Neurocase. 2006;12(6):355–359. doi: 10.1080/13554790600977218. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Bauer AM, Miller BL, Gorno-Tempini ML, Kramer JH, Weiner M, et al. The natural history of temporal variant frontotemporal dementia. Neurology. 2005;64(8):1384–1390. doi: 10.1212/01.WNL.0000158425.46019.5C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Jones-Gotman M, Zatorre RJ, Petrides M, Evans AC. A role for the right anterior temporal lobe in taste quality recognition. The Journal of Neuroscience. 1997;17(13):5136–5142. doi: 10.1523/JNEUROSCI.17-13-05136.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Thompson JC, Neary D. Knowledge of famous faces and names in semantic dementia. Brain. 2004;127(4):860–872. doi: 10.1093/brain/awh099. [DOI] [PubMed] [Google Scholar]
- Tartaglia MC, Thai JN, See T, Kuo A, Harbaugh R, Raudabaugh B, et al. Pathologic evidence that the T188R mutation in PRNP is associated with prion disease. Journal of Neuropathology & Experimental Neurology. 2010;69(12):1220–1227. doi: 10.1097/NEN.0b013e3181ffc39c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SA, Patterson K, Hodges JR. Left/right asymmetry of atrophy in semantic dementia: Behavioral-cognitive implications. Neurology. 2003;61(9):1196–1203. doi: 10.1212/01.wnl.0000091868.28557.b8. [DOI] [PubMed] [Google Scholar]
- Tyrrell PJ, Warrington EK, Frackowiak RS, Rossor MN. Progressive degeneration of the right temporal lobe studied with positron emission tomography. Journal of Neurology, Neurosurgery & Psychiatry. 1990;53(12):1046–1050. doi: 10.1136/jnnp.53.12.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R, Treasure J. Brain lesions and eating disorders. Journal of Neurology, Neurosurgery & Psychiatry. 2005;76(6):852–857. doi: 10.1136/jnnp.2004.048819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington EK, James M. The Visual Object and Space Perception Battery. Bury St. Edmunds, Suffolk, UK: Thames Valley Test Company; 1991. [Google Scholar]
- Wechsler D. Weschler Memory Scale – 3rd edition (WMS III) San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- Whitwell JL, Anderson VM, Scahill RI, Rossor MN, Fox NC. Longitudinal patterns of regional change on volumetric MRI in frontotemporal lobar degeneration. Dementia and Geriatric Cognitive Disorders. 2004;17(4):307–310. doi: 10.1159/000077160. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Avula R, Senjem ML, Kantarci K, Weigand SD, Samikoglu A, et al. Gray and white matter water diffusion in the syndromic variants of frontotemporal dementia. Neurology. 2010;74(16):1279–1287. doi: 10.1212/WNL.0b013e3181d9edde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins JS. Interpersonal Adjective Scales: Professional manual. Odessa, FL: Psychological Assessment Resources; 1995. [Google Scholar]
- Zahn R, Moll J, Iyengar V, Huey ED, Tierney M, Krueger F, et al. Social conceptual impairments in frontotemporal lobar degeneration with right anterior temporal hypometabolism. Brain. 2009;132(3):604–616. doi: 10.1093/brain/awn343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R, Moll J, Krueger F, Huey ED, Garrido G, Grafman J. Social concepts are represented in the superior anterior temporal cortex. Proceedings of the National Academy of Sciences. 2007;104(15):6430–6435. doi: 10.1073/pnas.0607061104. [DOI] [PMC free article] [PubMed] [Google Scholar]


