Abstract
Biomedical research often requires primary cultures of specific cell types, which are challenging to obtain at high purity in a reproducible fashion. Here we engineered the murine Rosa26 locus by introducing the diphtheria toxin receptor flanked by loxP sites. The resultant strain was nicknamed the Terminator mouse. This approach results in diphtheria toxin receptor expression in all non-Cre expressing cell types, making these cells susceptible to diphtheria toxin exposure. In primary cultures of kidney cells derived from the Terminator mouse, over 99.99% of cells were dead within 72 hours of diphtheria toxin treatment. After crossing the Terminator with the Podocin-Cre (podocyte specific) mouse or the Ggt-Cre (proximal tubule specific) mouse, diphtheria toxin treatment killed non-Cre expressing cells but spared podocytes and proximal tubule cells, respectively, enriching the primary cultures to over 99% purity based on both Western blotting and immunostaining of marker proteins. Thus, the Terminator mouse can be a useful tool to selectively and reproducibly obtain even low abundant cell types at high quantity and purity.
Integrated Introduction, Results, and Discussion
An enormous amount of research is currently performed using immortalized cell lines that have been isolated from the organs of various mammalian species and maintained in culture for long periods. These cell lines provide a ready platform for the in vitro dissection of signaling pathways, metabolic cycle analysis, cell proliferative and survival responses, channel function, etc. However, this approach is continuously confounded by two problems: 1) immortalized cells exhibit altered proliferative and apoptotic responses as a result of either abnormal gene expression (SV40 e.g.) or selection (MDCK cells, e.g.); and 2) continuous cell culture typically leads to significant epigenetic and phenotypic changes and the dysregulated expression of many genes when compared to the in vivo expression profile of the parent cell type1, 2, 3.
These problems can be partially overcome using primary cultures of freshly isolated cells. However, these cells are hard to be efficiently isolated in large numbers with high purity, and attempts to expand primary cultures to obtain large numbers of cells are frequently unsuccessful or lead to either replicative senescence or the selective expansion of abnormal clonal populations. Low abundant cell types are particularly difficult to be purified and analyzed in primary culture.
Generation of the Terminator mouse
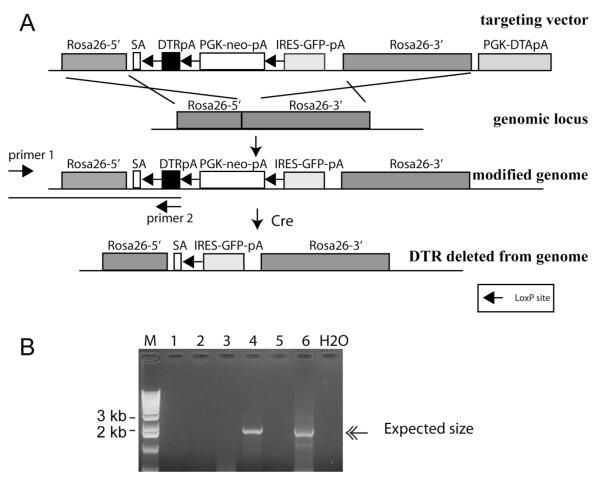
To overcome these problems and provide rapid and efficient isolation of large numbers of primary cells of any type, we have developed a mouse in which the diphtheria toxin receptor (DTR) gene is knocked into the Rosa26 locus, thus ubiquitously expressed, but can be turned off by expression of the Cre recombinase4 (Figure 1A). By mating this mouse, nicknamed the Terminator mouse (Rosa-DTRflox), to an appropriate Cre strain, DTR expression is permanently removed from cells that express Cre at any time prior to diphtheria toxin selection. Upon Cre mediated recombination, GFP expression is designed to be turned on, driven by the Rosa26 promoter (Figure 1A)4. After gene targeting in embryonic stem cells, a total of 8 clones were identified that carried the correctly modified Rosa26 locus and two were subjected to blastocyst microinjection to establish chimeras. Four male chimeras were generated and 3 had germ line transmission. The Terminator mouse can be genotyped by a pair of primers that amplify the Rosa26 promoter and the inserted DTR, with the sense primer complementing a genomic area outside of the targeting vector (Figure 1B). The Terminator mice are viable, fertile, and healthy (>14 months) without any macroscopic or microscopic abnormalities observed (data not shown).
Figure 1. Generation of the Terminator mouse.
A. The targeting vector was constructed to contain the DTR-polyA fragment flanked by a pair of loxP sites. PGK-Neo-polyA fragment was placed between the DTR and the IRES-GFP-polyA to serve as a positive selection marker during ES targeting; B, long template PCR to screen for clones that have undergone proper homologous recombination. See Panel A for primer location. An example of 6 ES clones is shown, with #4 and 6 exhibiting successful recombination. SA, splicing acceptor sequence.
DTR is ubiquitously expressed
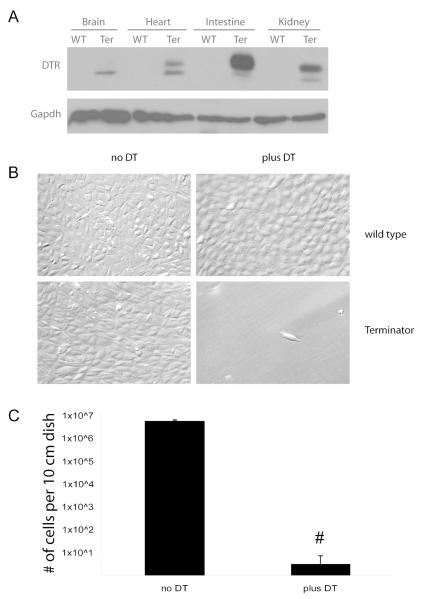
Multiple organs including the brain, heart, intestine, kidney, liver, lung, pancreas, skeletal muscle, gonads, and spleen were isolated and subjected to Western blotting. DTR is detected in all tested organs from the Terminator mouse, while wild type mice do not have detectable levels of DTR (Figure 2A, and data not shown).
Figure 2. Functional expression of DTR in the Terminator mouse.
A. Western analysis of tissues from the listed organs immunoblotted with α-DTR. The variation in size of the DTR in different organs is believed to be due to different degrees of O-linked glycosylation8. WT, wild type control; Ter, the Terminator mouse.; B, Primary cultures of cells derived from whole kidney digest of wild type and Terminator mice treated with DT (100ng/ml) for 72 hours; C, quantification of cell numbers of cultures treated as in (B); n=4 for no DT control, and n=6 for plus DT group; # P<0.0001.
To test the sensitivity of cells isolated from the Terminator mouse to diphtheria toxin (DT), kidneys were minced, digested in collagenase and the resultant cells seeded onto multiple 10 cm dishes. DT (100ng/ml) was added to the culture medium starting on day 2 and cultures washed every 48 hours to remove dead cells. While wild type kidney cells remained healthy after DT treatment, the vast majority of kidney cells from the Terminator (Rosa-DTRflox) mouse had detached within 24 hours after DT treatment. By 72-96 hrs, the treated dishes had fewer than 10 viable cells/dish remaining, while the untreated dishes were nearly confluent (2B, C). This suggests that the DTR is expressed by nearly all renal cells in the Terminator mouse, but that rare cells do survive DT selection even in the absence of Cre expression.
Primary culture of podocytes using the Podocin-Cre;Rosa-DTRflox mouse
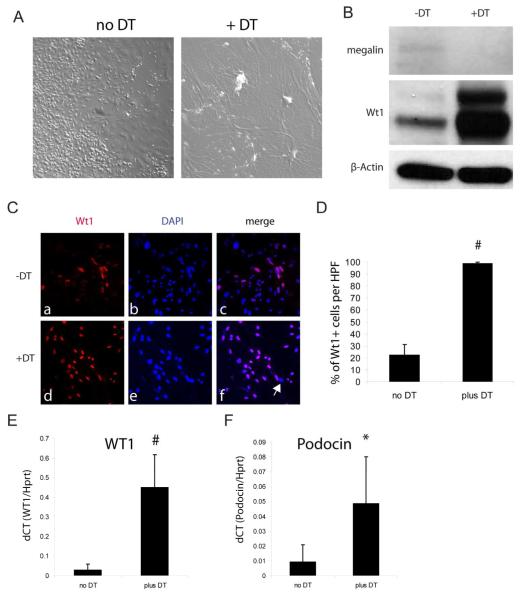
To test our hypothesis that the Terminator mouse can assist in the enrichment of primary cultures of a particular type of cells, primary cultures were examined from whole kidneys of Podocin-Cre;Rosa-DTRflox double transgenic mice5. In the absence of DT treatment, whole kidney cultures exhibited a typical mixed phenotypic picture (Figure 3A). DT treatment resulted in death of many of the adherent cells by 96 hours, and the surviving cells exhibited typical podocyte features (Figure 3A). Western blot analysis of surviving cells revealed enriched expression of the podocyte marker Wt1 and down regulation of the proximal tubule marker megalin in the DT treated samples (Figure 3B). Immunofluorescence revealed that greater than 99% of the cells that survived DT treatment exhibited Wt1+ expression, whereas untreated cultures have approximately 22% WT1+ cells (Figure 3C, D). Podocyte enrichment was further confirmed by quantitative RT-PCR analysis of Wt1 and podocin expression (Figure 3E, F).
Figure 3. Generation of primary podocyte cultures using the Terminator mouse.
A. DT treated primary cell cultures derived from Podocin-Cre;Rosa-DTRflox exhibit typical morphology of cultured podocytes as compared to the mixed phenotype seen in control cultures in the absence of DT; B. Western analysis of whole cell lysates from control and DT treated primary kidney cultures derived from the Podocin-Cre;Rosa-DTRflox mouse; C. Podocyte cultures derived as in (A) were passaged ×1 and immunostained for Wt1 and DAPI. Arrow in panel f points to a single cell that does not have detectable WT1 expression; D, quantification of percentage of WT1 positive cells in control vs DT treated cultures as in (C), n=5 each; E~F, Quantitative RT-PCR analysis of Wt1 and podocin gene expression in cultured cells normalized to Hprt1. n=5 each; #P<0.005, *P<0.05
Primary culture of proximal tubular cells using the Ggt-Cre; Rosa-DTRflox mouse
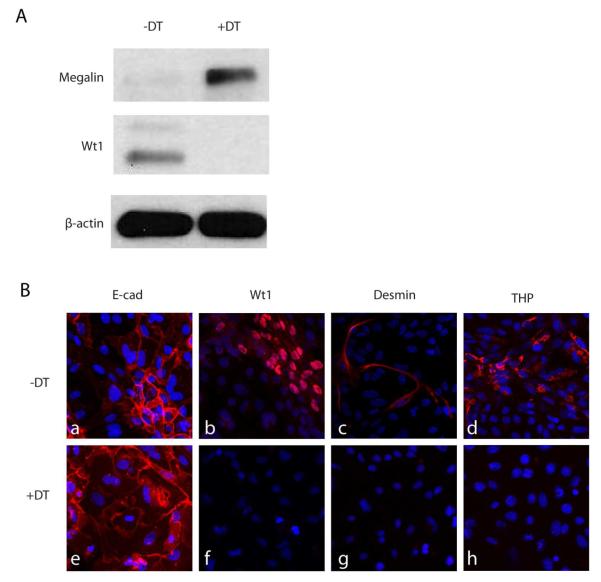
To test the efficacy of cell type specific enrichment of another common cell type, Terminator mice were mated to Ggt-Cre mice to delete DTR expression in proximal tubular cells6. Total kidney digests from Ggt-Cre;Rosa-DTRflox mice were seeded onto multiple culture dishes. On day 2, half of the plates were treated with DT (100ng/ml). 96 hrs later, cells from DT treated dishes had increased expression of megalin and markedly reduced expression of Wt1 as compared to control dishes (Figure 4A). Immunofluorescence staining of whole kidney cultures from Ggt-Cre;Rosa-DTRflox mice following DT treatment revealed loss of cells with detectable desmin expression (mesangial/interstitial cells), Wt1 expression (podocytes), and Tamm-Horsfall protein expression (thick ascending limb cells), whereas the number of E-cadherin expressing epithelial cells was enriched (Figure 4B). In contrast, control cultures continued to show the expected mix of all cell types. Of note, GFP expression was not detected in any cells, suggesting that the IRES-GFP cassette does not express at sufficiently high levels to be easily detected.
Figure 4. Generation of primary proximal tubular cell culture using the Terminator mouse.
A. Primary cultures of proximal tubular cells derived from Ggt-Cre;Rosa-DTRflox mice were treated ± DT for 96 hours, lysed and subjected to Western analysis of megalin and Wt1 expression; B, fluorescent immunostaining of Ecadherin (epithelial marker), Desmin (interstitial/mesangial marker), Wt1 (podocyte marker) and THP protein (thick ascending limb marker) expression by passage zero cells treated as in (A).
In summary, the Terminator mouse provides a powerful genetic tool to rapidly and efficiently enrich mixed cell cultures for individual types as long as an appropriate promoter is available for selective Cre expression. To our knowledge, this is the first mouse tool for such purposes. This approach will allow investigators to rapidly derive purified cell lines from wild-type or transgenic mice for in vitro studies, and obviate the need to immortalize these cells or use them at high passage. Kabgani et al reported primary culture of podocytes with proven origin by cell sorting7. Our approach has a few advantages over that method, including a higher degree of initial cell purity and avoidance of the stress of cell sorting. This technology can also be applied to isolation of very rare cell types as long as a tissue specific Cre mouse strain is available, as well as isolation of common cell types in an organ specific manner (cardiac endothelial cells using the Tie2-Cre, e.g.). Moreover, embryonic stem cells (ESC) carrying the Rosa-DTRflox locus and an appropriate Cre promoter (Pax2-Cre;Rosa-DTRflox for embryonic kidney, e.g.) would assist in high-throughput screening of small compounds to induce efficient ESC differentiation toward a desired cell lineage. However, it should be noted that the DT selection process does result in transient exposure to large numbers of detached and dying cells that could release factors that at least temporarily influence the phenotype of the surviving DTR negative cells.
Supplemental Concise Methods
Animals
Mouse protocols were approved by the Yale University Institutional Animal Care and Use Committee (IACUC). The Ggt-Cre mouse was kindly provided by Dr. Eric Neilson (Feinberg School of Medicine, Chicago, IL), and the Podocin-Cre mouse by Dr. Lawrence Holzman (University of Pennsylvania, Philadelphia, PA).
ES cell targeting and blastocyst injection
To allow for potential transplantation studies using C57Bl/6 recipients or any other experiments calling for a defined genetic background, C57Bl/6 ES cell line (obtained from ATCC) was used for gene targeting following standard protocols. Identified ESC clones were verified for chromosome integrity by karyotyping before being injected.
Primary culture of podocytes and proximal tubular cells
Donor mice (6~8 weeks old) were anesthetized with ketamine and perfused with 1×PBS via left ventricle before kidneys were isolated. Kidney capsule was removed, minced into ~2 mm × 2 mm × 2 mm cubes and incubated with collagenase B (Roche, 0.2 mg/ml) or Liberase TM (Roche, 0.2mg/ml) for 45 min with occasional agitation at 37°C. The digested mixture was then filtered through a 40 μM cell strainer and seeded onto multiple plates. For podocyte culture, plates were pre-coated with rat tail collagen. Podocytes were grown in RPMI 1640 medium supplemented with 10% FBS, penicillin/streptomycin, HEPES (10 mM), sodium pyruvate (1 mM) and sodium bicarbonate (1mM), while proximal tubular cells were cultured in DMEM high glucose medium, supplemented with 10% FBS and penicillin/streptomycin.
48 hrs after seeding, DT containing medium (Sigma, 100ng/ml) was applied to the culture. After starting DT treatment the medium was changed every 48hrs ×2, followed by every 72 hours until harvest (~2 weeks).
Histology and Fluorescent Immunostaining
After sacrifice, kidneys were fixed in 1×PBS buffered formalin (3.7% formaldehyde) for 12 hours, embedded in paraffin, and sectioned at 3 microns. To review histology, slides were stained by hematoxylin & eosin (H&E). Immunostaining of cultured cells was performed following standard protocols. Rabbit anti-E-cadherin was purchased from Cell Signaling Technology (Danvers, MA); Anti-Desmin, anti-Wt1, and anti-THP antibodies were from Santa Cruz Biotechnology. Megalin antibody was a kind gift from Dr. Daniel Biemesderfer (Yale University, New Haven, CT). All fluorescent images were acquired using a Zeiss LCM 710/Duo system.
Acknowledgements
We thank Dr. Richard Young (Cincinnati Children’s Hospital) for kindly providing the DTR fragment, Dr. Philippe Soriano (Mount Sinai Medical School, NYC) for sharing with us the Rosa26-1 vector, and Dr. Eric Neilson (Chicago, IL) for the Ggt-Cre mouse. The anti-megalin rabbit polyclonal antibody was a generous gift from Dr. Daniel Biemesderfer (New Haven, CT). We also thank Mr. Al Mennone for excellent technical support for imaging on the Zeiss LCM 710/Duo system (Center for Cellular and Molecular Imaging, Department of Cell Biology, Yale Medical School). Alan Shan was supported by a summer student program from Yale George O’Brien Center (NIH/NIDDK P30DK079310). This study was supported by the following grants: NIDDK-DK075464 (JKG), NIDDK-DK65109 (LGC) and a pilot grant from the Connecticut Stem Cell Fund (LGC).
Grant numbers and source of funding: NIH/NIDDK grants: DK075464 (JKG); NIDDK-DK65109 & CT stem cell fund (LGC).
Footnotes
Disclosure The authors disclose no financial interest/conflict of interests.
References
- 1.Giles RE, Boyce FM, Brockman WW. Evaluation of the mutagenic effects of SV40 in mouse, hamster, and mouse-human hybrid cells. Somat Cell Mol Genet. 1991;17:327–339. doi: 10.1007/BF01233058. [DOI] [PubMed] [Google Scholar]
- 2.Manoharan H, Babcock K, Pitot HC. Changes in the DNA methylation profile of the rat H19 gene upstream region during development and transgenic hepatocarcinogenesis and its role in the imprinted transcriptional regulation of the H19 gene. Mol Carcinog. 2004;41:1–16. doi: 10.1002/mc.20036. [DOI] [PubMed] [Google Scholar]
- 3.Tommasi S, Zheng A, Weninger A, et al. Mammalian cells acquire epigenetic hallmarks of human cancer during immortalization. Nucleic Acids Res. 2013;41:182–195. doi: 10.1093/nar/gks1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 5.Moeller MJ, Sanden SK, Soofi A, et al. Podocyte-specific expression of cre recombinase in transgenic mice. Genesis. 2003;35:39–42. doi: 10.1002/gene.10164. [DOI] [PubMed] [Google Scholar]
- 6.Iwano M, Plieth D, Danoff TM, et al. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kabgani N, Grigoleit T, Schulte K, et al. Primary cultures of glomerular parietal epithelial cells or podocytes with proven origin. PLoS One. 2012;7:e34907. doi: 10.1371/journal.pone.0034907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hranitzky KW, Durham DL, Hart DA, et al. Role of glycosylation in expression of functional diphtheria toxin receptors. Infect Immun. 1985;49:336–343. doi: 10.1128/iai.49.2.336-343.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]