Abstract
Background
Testosterone regulates numerous physiological processes, and evidence suggests that it plays a critical role in male aging. It has yet to be determined whether the heritability of testosterone varies in accordance with its diurnal rhythm. Similarly, it is unclear whether changes in testosterone level throughout the day are genetically influenced. The aim of the present study was to determine the degree to which genetic and environmental factors contribute to individual differences in testosterone throughout the day in middle-aged men.
Methods
Saliva-based measures of free testosterone, sampled at multiple time-points both at-home and in-lab, were collected from 783 male twins (193 monozygotic pairs, 196 dizygotic pairs, 5 unpaired twins) as part of the Vietnam Era Twin Study of Aging (VETSA). The average age of participants was 55.9 years (SD=2.6).
Results
Testosterone levels declined substantially over the course of the day, with 32%–39% of the change occurring in the first 30 minutes after waking. Heritability estimates for specific time-points ranged from .02 to .39. The heritability of the average at-home and in-lab testosterone values were notably higher (.42 and .47 respectively). Daily rates of change showed some evidence of genetic influence, with heritability estimates ranging from .15 to .29, whereas there were no observable genetic influences on coefficients of variation.
Conclusions
Genetic influences account for a significant proportion of the variance in average testosterone levels, while environmental factors account for the majority of intra-individual variability. These results highlight the need to explore both genetic and individual-specific environmental factors as determinants of free testosterone levels in aging men.
Keywords: Testosterone, Heritability, Twin Study, Diurnal Variation, Aging, Men, Middle-Age
The primary male androgen testosterone regulates numerous physiological processes, and has been related to a variety of health and behavioral outcomes. Although frequently linked to levels of aggression, physical strength, and sexual functioning in men (Zitzmann and Nieschlag, 2001), increasing evidence suggests that testosterone, as well as the broader hypothalamic-pituitary-gonadal (HPG) axis, plays a critical role in male aging. In adult men, testosterone levels begin to decline as early as the fourth decade of life (Feldman et al., 2002; Ferrini and Barrett-Connor, 1998; Harman et al., 2001), leading to functional changes in androgen receptor-regulated tissues (Stanworth and Jones, 2008). Lower or substantial declines in testosterone levels in aging men have been associated with increased risk for cardiovascular disease (Zmuda et al., 1997), metabolic syndrome (Saad and Gooren, 2009), physical frailty (Hyde et al., 2010), depression (Joshi et al., 2010), and overall mortality (Laughlin et al., 2008). The hormone has also been repeatedly linked to age-related changes in cognitive functioning (Holland et al., 2011), as well as disorders of cognition such as mild cognitive impairment and Alzheimer’s disease (Chu et al., 2008; Hogervorst et al., 2004; Moffat et al., 2004). Genetic studies have further shown that testosterone interacts with variants of the apolipoprotein-E (APOE) gene to influence cognition and brain structure (Panizzon et al., 2010; Raber, 2008), and that the hormone may regulate the genetic determinants of brain structures such as the hippocampus (Panizzon et al., 2012). Given the apparent wide-ranging effects of testosterone on male physiology and aging, there is a clear need to elucidate the determinants, both biological and environmental, of individual differences in testosterone levels in aging men.
To date, a number of twin and family studies have examined the heritability of male testosterone levels (i.e., the degree to which genetic factors contribute to individual differences in the hormone level). These studies have overwhelmingly examined measures of total testosterone – representing the sum total of hormone that is unbound, bound to sex hormone binding globulin (SHBG), and loosely bound to other proteins like albumin (Stanworth and Jones, 2008) – and with few exceptions have been largely consistent with one another, establishing a moderate level of heritability in the range of .40 to .60 (Bogaert et al., 2008; Harris et al., 1998; Hoekstra et al., 2006; Hong et al., 2001; Kuijper et al., 2007; Meikle et al., 1987; Ring et al., 2005; Sluyter et al., 2000; Storgaard et al., 2006). In contrast, only three studies have examined the heritability of free testosterone, hormone that is not bound to SHBG and is therefore physiologically active. Meikle and colleagues estimated the heritability of free testosterone at .34 in a sample of men ages 20 to 60 (Meikle et al., 1987), whereas Hoekstra and colleagues observed a substantially larger heritability of .52 in a sample of twelve-year-old boys (Hoekstra et al., 2006). More recently, Caramaschi and colleagues found evidence for no heritability of free testosterone in five-month-old infants; however, their analyses combined results for male and female twin pairs, thus results for male infants alone were not provided (Caramaschi et al., 2012). Clearly, there is limited information as to the degree to which genetic and environmental factors contribute to the level of free testosterone in adult men.
It is also the case that the majority of studies that have examined the heritability of testosterone, regardless of the type, have to date been based on one measurement taken exclusively during the morning, typically between 8 and 11 a.m. Although less pronounced than the well-documented diurnal rhythm of cortisol (Hellhammer et al., 2007; Stone et al., 2001), the HPG axis secretes testosterone with a significant diurnal variation, with levels reaching their highest point early in the morning followed by a gradual decline throughout the day (Diver et al., 2003). This diurnal rhythm has been shown to become blunted with increasing age, suggesting that aging may be associated with reductions in both overall testosterone output and daily variation (Bremner et al., 1983). Within the clinic, it has been recommended that testosterone be assessed only during the morning hours in order to avoid the potential confounding effects of this diurnal variation (Brambilla et al., 2009). Epidemiological studies of testosterone and male hypogonadism have tended to use this same approach. It has yet to be determined whether changes in testosterone level throughout the day are to some extent genetically driven; moreover, it remains to be seen whether the heritability of testosterone varies in accordance with substantial changes in the level of the hormone that are observed throughout the day. Filling these knowledge gaps will clarify whether testosterone level is more or less vulnerable to environmental influences over the course of the day, as well as clarify whether genetically informative studies of testosterone are utilizing the optimal (i.e., most heritable) phenotype.
The goal of the present study was to determine the degree to which genetic and environmental influences contribute to individual differences in testosterone level in a sample of middle-aged male twins (ages 51 to 60). Utilizing multiple time-points of data across multiple collection days, we sought to establish whether the heritability of free testosterone remained constant throughout the day, or whether the degree of genetic and environmental influences differ as function of collection time. In addition, we examined the degree to which two measures of intra-individual variation in free testosterone, the rate of change (slope) and the coefficient of variation, were influenced by genetic and environmental factors. By utilizing a middle-aged sample with a relatively narrow age range, the present study aims to provide estimates of the degree to which both genes and the environment contribute to individual differences in testosterone during what is known to be a critical transition period in the aging process.
Methods
Participants
Data were obtained as part of the Vietnam Era Twin Study of Aging (VETSA), a longitudinal study of aging with baseline in midlife (Kremen et al., 2006). VETSA participants were sampled from the Vietnam Era Twin (VET) Registry, a nationally distributed sample of male-male twin pairs who served in the United States military at some point between 1965 and 1975 (Goldberg et al., 2002). Although all VETSA participants are military veterans, the vast majority did not experience combat situations during their military careers. In total, 1237 men ages 51 to 60 participated in the primary VETSA project. The average age was 55.4 years (SD = 2.5). Participants were predominantly Caucasian (89.7%), with an average education of 13.8 years (SD = 2.1). In comparison to U.S. census data, VETSA participants are similar in demographic and health characteristics to American men in their age range (Centers for Disease Control and Prevention, 2003). Zygosity for 92% of the sample was determined by analysis of 25 microsatellite markers obtained from blood samples. For the remainder of the sample zygosity was determined through a combination of questionnaire and blood group methods. A comparison of these two approaches in the .VETSA sample has demonstrated an agreement rate of 95%.
As part of the VETSA project, participants traveled to either the University of California San Diego or Boston University for a daylong series of physical, psychosocial, and neurocognitive assessments. To be eligible both members of a twin pair had to agree to participate and be between the ages of 51 and 59 at the time of recruitment. In the third year of the project levels of testosterone, cortisol, and dehyrdoepiandrosterone sulfate (DHEAS) were obtained via saliva samples (N = 783, 193 monozygotic pairs, 196 dizygotic pairs, 5 unpaired twins). Approval from local institutional review boards was obtained for each study site, and all participants provided signed informed consent prior their participation.
Testosterone Collection and Assay
All hormone measurements were obtained on two days at home during a participant’s typical week, as well as on the assessment day. The at-home samples were collected approximately two weeks prior to the assessment day in order to avoid disruption of normal schedules that could be caused by travel to the testing site. Saliva was collected at waking, 30 minutes after waking (wake +30), 10:00 a.m., 3:00 p.m., and evening/bedtime on all days. These time-points were selected primarily to capture diurnal changes in cortisol levels. Participants were mailed a saliva collection kit that included individualized instructions, labeled 4.5 ml Cryotube vials, Trident original sugarless gum, straws, tissues, a daily log, pen, reminder watch, and a storage container with an electronic track cap for determining compliance with the protocol. Precise times of sample collection were recorded by the participant, and were later confirmed against data from the electronic track cap. Once collected, samples were sent via overnight mail to the University of California, Davis for assay.
Prior to assay, saliva samples were centrifuged at 3000 rpm for 20 minutes to separate the aqueous component from mucins and other suspended particles. Salivary concentrations of free testosterone were determined in duplicate using commercial radioimmunoassay kits (Beckman Coulter Inc., formerly Diagnostics Systems Laboratories, Webster, TX). Assay procedures were identical to those described by Granger and colleagues (Granger et al., 1999). Intra-assay and inter-assay coefficients of variation were 3.141 pg/ml and 4.878 pg/ml, respectively. The least detectable concentration for the assay was 1.3697 pg/ml. All samples from each participant were assayed together; and data from one to three individuals were included in each assay batch. Assays were always performed without knowledge of the zygosity of the twin pairs. Values greater than three standard deviations above the mean waking testosterone level, the highest value of the day, were set to missing in order to eliminate outlying data points. Scores for missing values were imputed only if the participant had no more than one missing value on a day. In order to impute missing data, we calculated the full samples’ mean change in testosterone level between the time-point with the missing value and the adjacent time-point. We then added or subtracted the mean change in testosterone for those two points from the participant’s non-missing time-point. This was done for less than 1% of all available hormone samples. Data from participants who reported taking testosterone supplements or other medications known to alter testosterone levels were excluded (N = 4).
For the present analyses, at-home testosterone values were averaged at corresponding times in order to create a single value for analysis at each time-point. In addition to individual time-points, we also examined the average testosterone levels across all time-points for the at-home and in-lab collection days separately. Variation within the day was measured by the rate of change in testosterone level (i.e., the slope). Due to a notable differences in the rate of change in testosterone over the course of the day (see Figure 1), we utilized three slope measures: 1) wake to evening, 2) wake to wake +30, and 3) wake +30 to evening. In addition, we calculated a coefficient of variation for each individual, defined as the standard deviation of each individual’s data divided by his average testosterone level. This provided an indicator of daily intra-individual variability that might not be reflected in a measure of simple linear decline, as well as a measure of variability that was independent of the individual’s average hormone level. Since testosterone levels possessed marginal positive skewness all measures were square-root transformed prior to analysis in order to normalize the distributions. Average testosterone values, rates of change, and coefficients of variation were calculated based on the raw variables and were then later transformed if necessary.
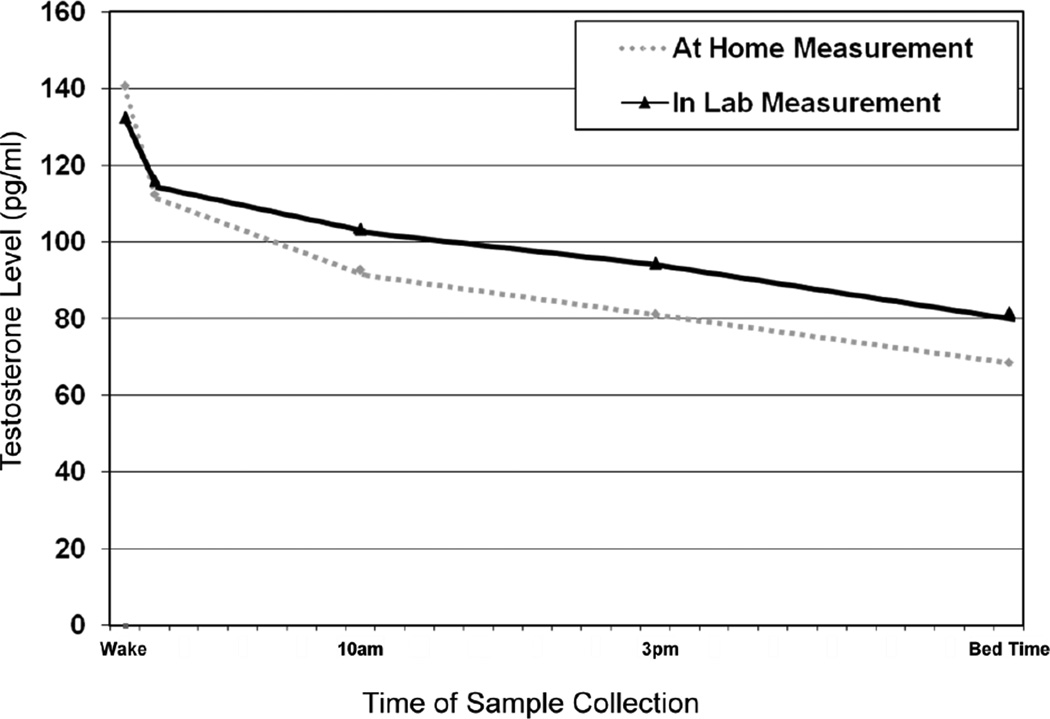
Figure 1.
Average testosterone levels across at-home and in-lab assessment days. Standard errors for the points of measurement ranged from 0.99 to 1.9, and not presented in the figure due to the overall scale. All in-lab testosterone measurements are significantly different from the corresponding at-home level at the p < .05 level.
Data Analysis
In the classical twin design the variance of any phenotype can be decomposed into the proportion attributed to additive genetic (A) influences, common or shared environmental (C) influences (environmental factors that make both members of a twin pair similar to one another), and unique environmental (E) influences (environmental factors that make members of a twin pair different from one another, including measurement error) (Eaves et al., 1978). Monozygotic (MZ) twins are assumed to correlate perfectly with respect to their additive genetic influences, as they share 100% of their DNA. Dizygotic (DZ) twins, in contrast, share on average 50% of their segregating DNA, and are therefore assumed to correlate .50 for additive genetic influences. The common environment is assumed to correlate perfectly between members of a twin pair regardless of zygosity.
In order to account for the partial nesting of twin pairs within assay batches, as well as the nesting of batches within twin pairs, estimates of relative genetic and environmental influence for each testosterone measure were determined within a Bayesian framework using Markov Chain Monte Carlo methods with the publically available WinBUGS software package (Spiegelhatlter et al., 2004). Detailed descriptions of our analytic approach and requisite assumptions have been published elsewhere (Franz et al., 2010; Prom-Wormley et al., 2011). Briefly, the estimated testosterone measure of the jth twin of the ith pair is denoted as Yijk, where k indicates the batch in which the value was assayed.
We then let:
Yijk = μ + τij + βk
where μ represents the measured testosterone level, τij represents the random differences between twins, conceivably correlated between pairs, and βk the random differences between batches. For each type of twin (MZ and DZ) we assume that the twin effects (τij) are bivariate normal with standard deviation στ and intraclass correlations of ρMZ for MZ and ρDZ for DZ pairs, respectively. The batch effects are assumed to have a normal distribution. Samples from the posterior distribution of variance components allowed for the estimation of their 95% confidence intervals.
The contribution of additive genetic influences to the observed variance (i.e., the heritability) was primarily calculated as twice the difference between the MZ and DZ intraclass correlations, A=2(ρMZ - ρDZ). The contribution of the common environment was estimated as twice the DZ correlation minus the MZ correlation, C=2ρDZ - ρMZ. Unique environmental influences were calculated as 1 minus the MZ correlation, E=1 - ρMZ. Note that estimates of C may be negative if there are large non-additive genetic effects, as would be indicated by an MZ correlation that is more than twice the DZ correlation. In such a scenario estimates of A can be dramatically and erroneously inflated (e.g., an MZ correlation of .75 and a DZ correlation of .20 would result in a heritability estimate of 110%). To avoid this bias, negative estimates of C were constrained to zero, and A was instead allowed to be equal to the MZ correlation. Similarly, in cases where the DZ correlation exceeded the MZ correlation, A was constrained to zero, and C was allowed to be equal to the MZ correlation. This ensured that the combined total of all variance components did not exceed 1.0.
Results
Participants who provided saliva-based hormone samples were not statistically different from the rest of the VETSA sample (p>.05) in terms of years of education, body mass index, smoking status, self-reported symptoms of depression, or self-reported classification of overall health status. A small but nevertheless significant difference in age was observed (Present Sample: Age=55.9; Remaining VETSA Sample: Age=54.6: p<.0001). We observed no significant differences in testosterone levels between participants who described themselves as Caucasian versus non-Caucasian. As anticipated, levels of testosterone both at home and on the day of testing were highest at waking, and declined substantially over the course of the day (see Figure 1). The average waking testosterone level was significantly higher for at-home measures in comparison to the assessment day (p<.0001); however, for all other time-points testosterone level on the day of testing was significantly greater than the corresponding at-home measurement (p values ranged from .048 to <.0001). A pronounced decrease in testosterone level was noted from waking to wake +30, accounting for roughly 39% of the total daily change for the at-home measures and 32% of the change on the day of testing. The rate of change between waking and wake +30 was significantly greater than the two other rate of change measures (all p values <.0001).
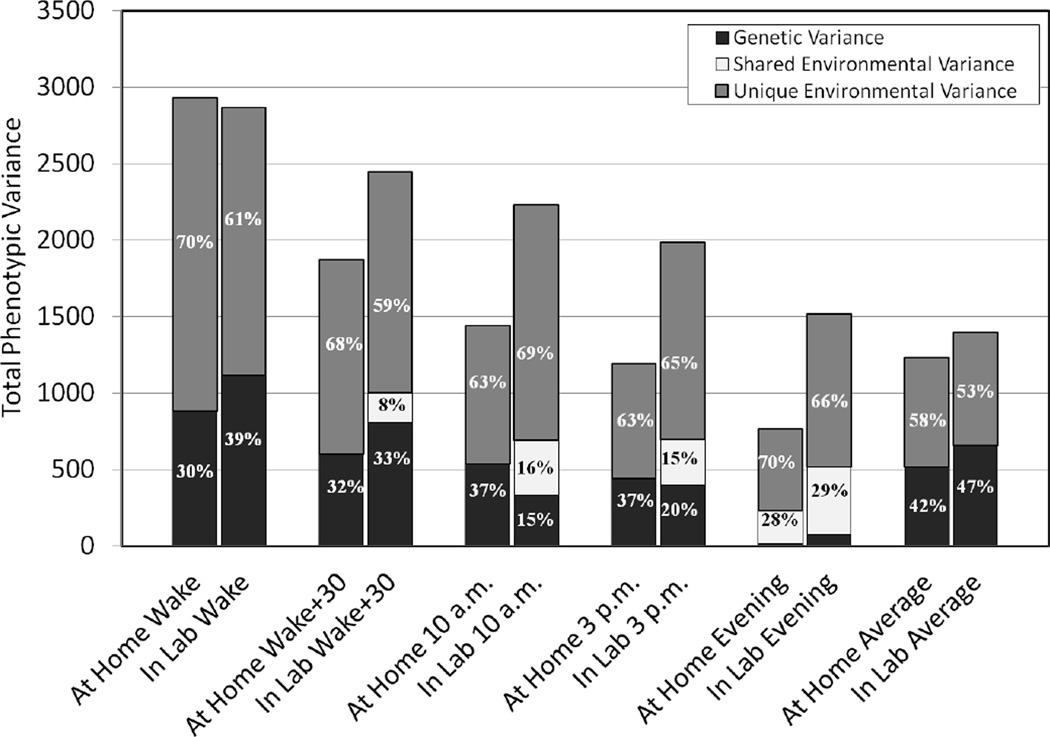
Genetic and environmental variance components for each collection time-point, as well as the daily averages for the at-home and day of testing measurements are presented in Table 1. These values are also presented in Figure 2 relative to the overall phenotypic variance of each measurement. As can be seen in Figure 2, across both the at-home and in-lab assessment days, the phenotypic variance of the testosterone measurements declined dramatically from waking to evening, corresponding to the decline in observed hormone level. With the exception of the waking measurement, the variance of in-lab measurements was consistently higher than at-home measurements. At-home heritability estimates ranged from a low of .02 in the evening to a high of .37 for both 10 a.m. and 3 p.m. Genetic factors on the day of testing were at their weakest in the evening (Heritability= .05), and had their strongest effect at waking (Heritability=.39). Midday heritability estimates on the day of testing, those taken at 10 a.m. and 3 p.m., were nearly half the magnitude of the at-home measures, and substantial but non-significant effects of the shared environment were observed. Adjusting for variability in waking time, as well as variability in the time of all other sample collections, did not alter the contributions of the genetic or environmental variance components at the specific time-points. The heritability of the average at-home testosterone value was .42, notably higher than the estimates for the individual time-points. Similar to the at-home results, the heritability of the average day-of-testing testosterone level was .47, and showed no evidence of shared environmental influences.
Table 1.
Summary statistics and variance component estimates for At-Home and Day-of-Testing testosterone levels
| Testosterone | Collection | Correlations |
Variance Components |
||||
|---|---|---|---|---|---|---|---|
| Measure | level pg/ml (SD) | Time (SD) | rmz (95% CI) | rdz (95% CI) | A (95% CI) | C (95% CI) | E (95% CI) |
| At Home (Days 1 & 2) | |||||||
| Wake | 140.65 (54.15) | 0631 h (2.25) | .30 (.15; .44) | .13 (.01; .29) | .30 (.15; .44) | -- | .70 (.56; .85) |
| Wake +30* | 112.47 (43.27) | 0684 h (2.26) | .32 (.17; .47) | .18 (.04; .32) | .32 (.17; .47) | -- | .68 (.53; .83) |
| 10 a.m. | 92.74 (38.0) | 1019 h (1.56) | .37 (.23; .50) | .14 (.04; .27) | .37 (.23; .50) | -- | .63 (.50; .77) |
| 3 p.m. | 81.12 (34.52) | 1502 h (1.66) | .37 (.23; .49) | .12 (.03; .26) | .37 (.23; .49) | -- | .63 (.51; .77) |
| Evening | 68.51 (27.71) | 2040 h (3.36) | .30 (.15; .44) | .29 (.15; .43) | .02 (−.36; .39) | .28 (−.02; .59) | .70 (.57; .85) |
| Overall Mean | 100.58 (35.10) | - | .42 (.27; .55) | .17 (.03; .32) | .42 (.27; .55) | -- | .58 (.45; 73) |
| Day of Testing | |||||||
| Wake | 132.43 (53.53) | 0589 h (0.78) | .39 (.26; .51) | .16 (.04; .31) | .39 (.26; .40) | -- | .61 (.49; .74) |
| Wake +30 | 115.90 (49.44) | 0644 h (0.87) | .41 (.27; .52) | .25 (.10; .38) | .33 (−.02; .67) | .08 (−.21; .37) | .59(.48; .73) |
| 10 a.m. | 103.24 (47.23) | 0973 h (0.58) | .31 (.15; .45) | .23 (.08; .38) | .15 (−.24; .52) | .16 (−.16; .46) | .69 (.55; .85) |
| 3 p.m. | 94.39 (44.59) | 1493 h (0.98) | .35 (.20; .48) | .25 (.09; .40) | .20 (−.18; .58) | .15 (−.17; .46) | .65 (.52; .80) |
| Evening | 81.48 (38.94) | 2058 h (4.67) | .34 (.20; .46) | .31 (.15; .46) | .05 (−.32; .42) | .29 (−.04; .59) | .66 (.54; .80) |
| Overall Mean | 107.45 (37.35) | -- | .47 (.33; .58) | .23 (.06; .38) | .47 (.33; .58) | -- | .53 (.42; .67) |
Results from AE models are presented in cases where the shared environment (C) was positive, but nevertheless accounted for less than 5 percent of the phenotypic variance. rmz = Monozygotic twin correlation; rdz = Dizygotic twin correlation; A = Additive genetic influences; C = Shared environmental influences; E = Unique environmental influences.
Figure 2.
Genetic and environmental contributions to the phenotypic variance of free testosterone level. The vertical axis represents the total phenotypic variance for the raw (untransformed) free testosterone measures.
Heritability estimates for the daily rates of change and coefficients of variation in testosterone level are presented in Table 2. For the at-home measures, a small but nevertheless significant genetic influence was observed for the wake to evening slope (Heritability =.16), while the wake to wake +30 slope was not heritable. The rate of change between wake +30 and evening, however, was significantly heritable (Heritability = .23). On the day of testing, all rate of change measures were significantly heritable, with estimates ranging from .15 to .29. Both the at-home and in-lab assessment day coefficients of variation demonstrated no genetic influences, suggesting that nearly all variability in this measure is due to unique environmental influences.
Table 2.
Standardized variance components for rates of change and coefficients of variation
| Correlation |
Variance Components |
||||
|---|---|---|---|---|---|
| Measure | rmz (95% CI) | rdz (95% CI) | A (95% CI) | C (95% CI | E (95% CI) |
| At Home (Days 1 & 2) | |||||
| Wake to Evening* | .16 (.01; .30) | .09 (.02; .25) | .16 (.01; .49) | -- | .84 (.70; .99) |
| Wake to Wake +30 | .04 (.00; .12) | .10 (.02; .24) | -- | .04 (.00; .12) | .96 (.88; 1.0) |
| Wake +30 to Evening | .23 (.08; .37) | .07 (.02; .19) | .23 (.08; .37) | -- | .77 (.63; .92) |
| Coefficient of Variation | .17 (.02; .31) | .23 (.09; .38) | -- | .17 (.02; .31) | .83 (.69; .98) |
| Day of Testing | |||||
| Wake to Evening | .29 (.15; .42) | .06 (.01; .19) | .29 (.15; .42) | -- | .71 (.58; .85) |
| Wake to Wake +30 | .15 (.04; .28) | .07 (.01; .19) | .15 (.04; .28) | -- | .85 (.72; .96) |
| Wake +30 to Evening* | .28 (.14; .41) | .15 (.02; .31) | .28 (.14; .41) | -- | .72 (.59; .86) |
| Coefficient of Variation | .08 (.01; .20) | .12 (.02; .25) | -- | .08 (.01; .20) | .92 (.80; .99) |
Results from AE models are presented in cases where the shared environment (C) was positive, but nevertheless accounted for less than 5 percent of the phenotypic variance. rmz = Monozygotic twin correlation; rdz = Dizygotic correlation; A = Additive genetic influences; C = Shared environmental influences; E = Unique environmental influences.
Secondary Analyses
In order to determine whether factors that have been associated with, or shown to alter, testosterone levels influenced the observed heritability estimates, all analyses were repeated after adjusting for the following covariates: age, body mass index (BMI), alcohol consumption (number of drinks consumed over the past two weeks), smoking status (current smoker versus non-smoker), sleep quality, and self-reported health. Results from these analyses are presented in Supplementary Tables 1 and 2. Sleep quality was assessed with the total score from the Pittsburgh Sleep Quality Index, a self-report instrument that examines numerous dimensions of sleep (e.g., quality, duration, efficiency) over the past month (Buysse et al., 1989). Self-reported health was assessed with the Charlson Comorbidity Index, a composite score reflecting the total number of diagnosed medical conditions known to negatively influence mortality (Charlson et al., 1994; Charlson et al., 1987).
Heritability estimates for the adjusted at-home testosterone levels declined slightly, but maintained the same overall pattern observed for the unadjusted measures. For the individual time-points, decreases in the heritability ranged from .01 to .04 in magnitude. The most prominent change in heritability was for the average at-home testosterone level, which declined from .42 to .33. None of these adjusted heritability estimates significantly differed from the unadjusted estimates based on the overlapping 95% confidence intervals. Similarly, small decreases ranging from .00 to .04 were observed for the heritability estimates of the at-home rate of change measures and the at-home coefficient of variation. More noticeable differences were observed for the in-lab heritability estimates. The wake +30, 3 p.m., and daily average heritability estimates all decreased by .10 or more; however, they did not significantly differ from the unadjusted estimates based on the 95% confidence intervals. Heritability estimates for the other in-lab time points showed little or no changes, with the exception of the evening estimate which increased from .05 to .14, but was still not significantly different from zero. The heritability estimate did not change for the wake to evening slope; however, for the wake to wake +30 slope, and the wake +30 to evening slope, genetic influences dropped to zero after accounting for the covariates.
Given the potential opposing actions of testosterone and cortisol (Viau, 2002), additional analyses were preformed in which corresponding measures of cortisol were included in addition to the other covariates. These results are presented in Supplementary Tables 3 and 4. Organizing the analyses in this way allowed us to determine the degree to which cortisol influenced the heritability of testosterone above and beyond the other potential covariates. At-home and in-lab cortisol levels as well as measures of intra-individual variability in cortisol were calculated in the identical fashion as the corresponding testosterone measures. Detailed descriptions of the cortisol data cleaning procedures, as well as the heritability estimates for these measures have been previously published (Franz et al., 2010). Correlations between the corresponding testosterone and cortisol measures ranged from .07 (at-home coefficients of variation) to .36 (in-lab evening sample).
After adding cortisol as a covariate no clear pattern of change in the resulting heritability estimates was observed. For 15 of the 20 measures we observed no or small changes in the heritability estimates ranging from −.05 to +.04 in magnitude. The at-home wake +30 estimate fell from .28 to .16. The in-lab evening sample and the at-home wake to evening slope heritability estimates declined from .14 to .05 and .12 to .04, respectively. These estimates were not significantly different from the previous adjusted or unadjusted estimates based on the 95% confidence intervals. The wake to wake +30 and the wake +30 to evening slopes both showed increases in their heritability estimates from .00 to .14 and from .00 to .11, respectively. Of these two measures, only the heritability estimate for the wake to wake +30 slope was significantly different from zero.
Discussion
The results of the present study demonstrate that while a substantial portion of the variability in average daily free testosterone level is determined by genetic influences, at distinct time-points throughout the day the magnitude of these genetic influences can vary dramatically. Both at home and on the assessment day, the heritability of testosterone level was moderate in the early morning (ranging from .30 to .39), and declined to near zero by the evening. These heritability estimates for morning free testosterone levels are consistent with the estimate provide by Meikle and colleagues (Meikle et al., 1987), the only other twin study to examine free testosterone in adult men of which we are aware. During the in-lab assessment heritability estimates were noticeably lower at mid-day, while contributions of the common environment were higher than at other time-points. The smaller standard deviations for the in-lab collection times clearly indicate that there was less variability in the timing of sample collections when participants were in the lab; thus, the observed differences may be the result of the structure and coordination of the participants’ experiences over the course of the assessment day.
Heritability estimates for the average testosterone levels both at home and on the assessment day were greater than all individual estimates. The discrepancy between the average testosterone levels and those from specific time-points was likely due to the reduction in measurement error, and therefore reduction in unique environmental variance, brought about by averaging across multiple data points. This suggests that repeated measurement of free testosterone over the course of a day may be necessary in order to establish reliable and robust estimates of genetic influence, as well as more reliable estimates of the overall hormone level.
After controlling for age, BMI, alcohol consumption, smoking status, sleep quality, self-reported health, as well as corresponding measures of cortisol we observed generally small changes in heritability estimates for individual time-points and overall daily averages. These changes, however, were not consistent in their magnitude or direction, and the resulting heritability estimates did not significantly differ from the unadjusted estimates.
The importance of diurnal variation in hormone levels has primarily been the topic of cortisol research (Hellhammer et al., 2007; Stone et al., 2001). In contrast, little attention has been given to the patterns of daily variation in testosterone. We found evidence of significant genetic factors accounting for approximately one-fifth of the variance in all of the rate-of-change (slope) measures from early morning to evening. There were, however, no observable genetic influences on the coefficient of variation for testosterone levels, a measure of intra-individual variability that is independent of the hormone level. This might suggest that testosterone is much more reactive to environmental factors than has previously been considered. It remains to be seen whether measures of intra-individual variability in total testosterone, which when sampled in the morning provides heritability estimates that are larger than those reported here, would demonstrate the same pattern of effects as free testosterone.
Intriguingly, we observed a substantial drop in testosterone level between awakening and 30 minutes after awakening, a drop which accounted for 32%–39% of the overall daily change. This finding has been observed in past studies examining the effects of sleep on testosterone level (Axelsson et al., 2005; Diver et al., 2003), although its potential meaning has not been explored further. There has been substantial attention paid to the cortisol awakening response, another aspect of diurnal variation. Cortisol and testosterone are thought to have opposing actions (Viau, 2002); thus, it is of interest that this substantial decline in free testosterone coincides with a period that is typically characterized by a sharp increase in cortisol (Hellhammer et al., 2007). In the present study, corresponding measurements of testosterone and cortisol were all positively correlated with one another; moreover, the rates of change from wake to wake +30 for both hormones were correlated .35 for the at-home assessments and .14 for the in-lab assessment. Although no negative associations were observed, due to the opposing signs of the testosterone and cortisol wake to wake +30 slopes, these results indicate that a greater decline in testosterone after waking is associated with a smaller cortisol awakening response. Despite the somewhat larger correlation, there was no observable effect of cortisol on the heritability of the wake to wake +30 slope for the at-home assessments. In contrast, after adding cortisol to the other covariates the heritability for the slope from the in-lab assessment day went from .00 to a significant heritability of .14. Associations such as these suggest that while the two awakening responses are somewhat related, other contributing factors are likely at work. These differences might be due to differences in sleep quality, individual difference in metabolism, factors that differ between at-home and day-of-testing, or they could simply reflect error variance. However, in the absence of supporting data such hypotheses are purely speculative. In any case, the clinical significance of the testosterone awakening response remains unclear.
It remains to be seen what specific genetic factors may be contributing to free testosterone level in late middle age. A recent genome-wide association study of serum-based testosterone levels identified two single nucleotide polymorphisms associated with the sex hormone binding globulin (SHBG) gene, associations that proved significant for both overall level of testosterone and the classification of low-testosterone (Ohlsson et al., 2011). An association has also been found between the nuclear receptor coactivator-3 (NCOA3) gene with free testosterone but not total testosterone level in late middle-aged men (Sheu et al., 2006). The APOE gene has been associated with free testosterone levels, specifically men with the ε4 allele have been found to have higher levels of free testosterone relative to non-carriers (Berteau-Pavy et al., 2007). Finally, variation in the androgen receptor (AR) gene, specifically the number of trinucleotide repeats on exon 1, may also influence testosterone level, suggesting that genetically determined androgen sensitivity contributes in part to the degree of hormone production (Huhtaniemi et al., 2010).
The present results should be considered in the context of the following limitations. The primarily Caucasian composition of the VETSA sample and the narrow age range, limits our ability to generalize these results to other populations. It is also the case that the cross-sectional design of the present study does not allow us to determine whether the observed genetic and environmental influences of free testosterone are long-standing, or represent changes from earlier periods of life. It is entirely plausible that the factors that lead to age-related declines in testosterone, whether genetic or environmental, are independent from those that influence the hormone earlier in life. Thus, although heritability estimates may be comparable, the specific genetic and environmental factors that influence testosterone may be very different in a younger cohort of men. It is also well established that testosterone levels rebound during sleep, and that disruption of the sleep cycle can impact testosterone levels in otherwise healthy individuals (Luboshitzky et al., 2001). It remains to be seen if testosterone levels during sleep may be more genetically influenced than daytime samples or if the rate of change between bedtime and waking testosterone levels is itself heritable. It is important to note that measures of testosterone which are based off of a single assessment day are likely to be less reliable than estimates which are based off of multiple assessment days. Therefore, it is possible that heritability estimates from the in-lab assessment day are fundamentally less reliable, than those based off of multiple at-home assessment days. In the present study the in-lab assessment had to be limited to one day, thus, we are unable to test this hypothesis.
Finally, the present study is limited by the fact that we cannot elucidate what role SHBG may play in the present results. Free-testosterone by definition represents hormone that is not bound to SHBG; therefore, one would anticipate that they would be correlated with one another. Like measures of total and free testosterone, SHBG has been found to be heritable in adult men (Kuijper et al., 2007; Ring et al., 2005). Whether the genetic determinants of SHBG and free-testosterone are the same or different remains to be seen. We are aware of only two studies that have examined the genetic and environmental overlap between free testosterone and SHBG. Bogaert et al (2008) observed a week phenotypic correlation between the two hormones (rp=. −06) and a correspondingly week genetic correlation (rg=.14) in young adult men (age range 25 to 45). Coviello et al (2011) found a significant genetic correlation between the two hormones (rg=−.60) in adult women, however, the findings also suggest that both hormones are influenced by unshared genetic factors. It should be noted that both of these studies were based on extended family designs, and were not twin studies; thus, their estimates of heritability and genetic correlation are likely to be less precise than those derived from a twin sample. Additional studies are needed in order to determine whether free testosterone levels, and changes in free testosterone levels with increasing age, are influenced by the same or difference genetic determinants as those influencing SHBG.
In summary, the present study demonstrates that while measures of average daily free testosterone provide robust heritability estimates, the magnitude of the genetic influences of the hormone are not constant throughout the day. Moreover, while daily rates of change in testosterone demonstrated some genetic influence, the overall intra-individual variability in the hormone appears to be primarily due to individual specific environmental factors. These results highlight the importance of both genetic and environmental factors in regulating the functioning of the hypothalamic-pituitary-gonadal axis, as well as the need to explore both genetic and individual-specific environmental factors as potential determinants of age-related testosterone changes.
Supplementary Material
Acknowledgements
The Cooperative Studies Program of the U.S. Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. Numerous organizations have provided invaluable assistance in the conduct of this study, including: Department of Defense; National Personnel Records Center, National Archives and Records Administration; the Internal Revenue Service; National Opinion Research Center; National Research Council, National Academy of Sciences; the Institute for Survey Research, Temple University. This material was, in part, the result of work supported with resources of the VA San Diego Center of Excellence for Stress and Mental Health Healthcare System. Most importantly, the authors gratefully acknowledge the continued cooperation and participation of the members of the VET Registry and their families. Without their contribution this research would not have been possible. We also appreciate the time and energy of many staff and students on the VETSA projects.
Role of Funding Source
The VETSA project is supported by grants from NIH/NIA (R01 AG018386, R01 AG018384, R01 AG022381, and R01 AG022982). The content of the present manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA or the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflicts of interest.
Contributors All authors have contributed to and have approved the final manuscript. Dr. Panizzon conducted the data analyses, managed the literature review, and was the primary author of the manuscript. Dr. Hauger assisted in the design of the study, the literature review, and writing of the manuscript. Drs. Eaves, York, and Prom-Wormley developed the data analytic approach, and assisted in the editing and revision of the manuscript. Dr. Mendoza assisted in the design of the study and conducted the hormone assays. Mrs. McKenzie assisted in the literature review and writing of the manuscript. Drs. Jacobson, Grant, Xian, and Franz assisted in study design, data collection and management, and assisted in the editing and revising of the manuscript. Drs. Lyons and Kremen are the principal investigators of the VETSA project. They designed the VETSA project and provided assistance in the drafting and revising of the present manuscript.
References
- Axelsson J, Ingre M, Akerstedt T, Holmback U. Effects of acutely displaced sleep on testosterone. Journal of Clinical Endocrinology and Metabolism. 2005;90:4530–4535. doi: 10.1210/jc.2005-0520. [DOI] [PubMed] [Google Scholar]
- Berteau-Pavy F, Park B, Raber J. Effects of sex and APOE epsilon4 on object recognition and spatial navigation in the elderly. Neuroscience. 2007;147:6–17. doi: 10.1016/j.neuroscience.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Bogaert V, Taes Y, Konings P, Van Steen K, De Bacquer D, Goemaere S, Zmierczak H, Crabbe P, Kaufman JM. Heritability of blood concentrations of sex-steroids in relation to body composition in young adult male siblings. Clinical Endocrinology. 2008;69:129–135. doi: 10.1111/j.1365-2265.2008.03173.x. [DOI] [PubMed] [Google Scholar]
- Brambilla DJ, Matsumoto AM, Araujo AB, McKinlay JB. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. Journal of Clincal Endocrinology and Metabolism. 2009;94:907–913. doi: 10.1210/jc.2008-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. Journal of Clinical Endocrinology and Metabolism. 1983;56:1278–1281. doi: 10.1210/jcem-56-6-1278. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Caramaschi D, Booij L, Petitclerc A, Boivin M, Tremblay RE. Genetic and environmental contributions to saliva testosterone levels in male and female infant twins. Psychoneuroendocrinology. 2012;37:1954–1959. doi: 10.1016/j.psyneuen.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Public health and aging: Trends in aging--United States and worldwide. MMWR CDC Surveillance Summaries. 2003;52:101–106. [Google Scholar]
- Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. Journal of Clinical Epidemiology. 1994;47:1245–12451. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Diseases. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Chu LW, Tam S, Lee PW, Wong RL, Yik PY, Tsui W, Song Y, Cheung BM, Morley JE, Lam KS. Bioavailable testosterone is associated with a reduced risk of amnestic mild cognitive impairment in older men. Clin Endocrinol. 2008;68:589–598. doi: 10.1111/j.1365-2265.2007.03094.x. [DOI] [PubMed] [Google Scholar]
- Coviello AD, Zhuang WV, Lunetta KL, Bhasin S, Ulloor J, Zhang A, Karasik D, Kiel DP, Vasan RS, Murabito JM. Circulating testosterone and SHBG concentrations are heritable in women: the Framingham Heart Study. Journal of Clinical Endocrinology and Metabolism. 2011;96:1491–1495. doi: 10.1210/jc.2011-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diver MJ, Imtiaz KE, Ahmad AM, Vora JP, Fraser WD. Diurnal rhythms of serum total, free and bioavailable testosterone and of SHBG in middle-aged men compared with those in young men. Clinical Endocrinology. 2003;58:710–717. doi: 10.1046/j.1365-2265.2003.01772.x. [DOI] [PubMed] [Google Scholar]
- Eaves LJ, Last KA, Young PA, Martin NG. Model-fitting approaches to the analysis of human behavior. Heredity. 1978;41:249–320. doi: 10.1038/hdy.1978.101. [DOI] [PubMed] [Google Scholar]
- Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB. Age trends in the level of serum testosterone and other hormones in middle-aged men: Longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589–598. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- Ferrini RL, Barrett-Connor E. Sex hormones and age: a cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. American Journal of Epidemiology. 1998;147:750–754. doi: 10.1093/oxfordjournals.aje.a009519. [DOI] [PubMed] [Google Scholar]
- Franz CE, York TP, Eaves LJ, Mendoza SP, Hauger RL, Hellhammer DH, Jacobson KC, Levine S, Lupien SJ, Lyons MJ, Prom-Wormley E, Xian H, Kremen WS. Genetic and environmental influences on cortisol regulation across days and contexts in middle-aged men. Behavior Genetics. 2010;40:467–479. doi: 10.1007/s10519-010-9352-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J, Curran B, Vitek ME, Henderson WG, Boyko EJ. The Vietnam Era Twin Registry. Twin Research and Human Genetics. 2002;5:476–481. doi: 10.1375/136905202320906318. [DOI] [PubMed] [Google Scholar]
- Granger DA, Schwartz EB, Booth A, Arentz M. Salivary testosterone determination in studies of child health and development. Horm Behav. 1999;35:18–27. doi: 10.1006/hbeh.1998.1492. [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. The Journal of Clinical Endocrinology and Metabolism. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- Harris JA, Vernon PA, Boomsma DI. The heritability of testosterone: a study of Dutch adolescent twins and their parents. Behavior Genetics. 1998;28:165–171. doi: 10.1023/a:1021466929053. [DOI] [PubMed] [Google Scholar]
- Hellhammer J, Fries E, Schweisthal OW, Schlotz W, Stone AA, Hagemann D. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: state- and trait components. Psychoneuroendocrinology. 2007;32:80–86. doi: 10.1016/j.psyneuen.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Hoekstra RA, Bartels M, Boomsma DI. Heritability of testosterone levels in 12-year-old twins and its relation to pubertal development. Twin Research and Human Genetics. 2006;9:558–565. doi: 10.1375/183242706778025071. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Bandelow S, Combrinck M, Smith AD. Low free testosterone is an independent risk factor for Alzheimer's disease. Exp Gerontol. 2004;39:1633–1639. doi: 10.1016/j.exger.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Holland J, Bandelow S, Hogervorst E. Testosterone levels and cognition in elderly men: a review. Maturitas. 2011;69:322–337. doi: 10.1016/j.maturitas.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Hong Y, Gagnon J, Rice T, Perusse L, Leon AS, Skinner JS, Wilmore JH, Bouchard C, Rao DC. Familial resemblance for free androgens and androgen glucuronides in sedentary black and white individuals: the HERITAGE Family Study. Health, Risk Factors, Exercise Training and Genetics. Journal of Endocrinology. 2001;170:485–492. doi: 10.1677/joe.0.1700485. [DOI] [PubMed] [Google Scholar]
- Huhtaniemi IT, Pye SR, Holliday KL, Thomson W, O'Neill TW, Platt H, Payne D, John SL, Jiang M, Bartfai G, Boonen S, Casanueva FF, Finn JD, Forti G, Giwercman A, Han TS, Kula K, Lean ME, Pendleton N, Punab M, Silman AJ, Vanderschueren D, Labrie F, Wu FC. Effect of polymorphisms in selected genes involved in pituitarytesticular function on reproductive hormones and phenotype in aging men. Journal of Clinical Endocrinology and Metabolism. 2010;95:1898–1908. doi: 10.1210/jc.2009-2071. [DOI] [PubMed] [Google Scholar]
- Hyde Z, Flicker L, Almeida OP, Hankey GJ, McCaul KA, Chubb SA, Yeap BB. Low free testosterone predicts frailty in older men: the health in men study. Journal of Clinical Endocrinology and Metabolism. 2010;95:3165–3172. doi: 10.1210/jc.2009-2754. [DOI] [PubMed] [Google Scholar]
- Joshi D, van Schoor NM, de Ronde W, Schaap LA, Comijs HC, Beekman AT, Lips P. Low free testosterone levels are associated with prevalence and incidence of depressive symptoms in older men. Clinical Endocrinology. 2010;72:232–240. doi: 10.1111/j.1365-2265.2009.03641.x. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Thompson-Brenner H, Leung YJ, Grant MD, Franz CE, Eisen SA, Jacobson KC, Boake C, Lyons MJ. Genes, environment, and time: The Vietnam Era Twin Study of Aging (VETSA) Twin Res Hum Genet. 2006;9:1009–1022. doi: 10.1375/183242706779462750. [DOI] [PubMed] [Google Scholar]
- Kuijper EA, Lambalk CB, Boomsma DI, van der Sluis S, Blankenstein MA, de Geus EJ, Posthuma D. Heritability of reproductive hormones in adult male twins. Human Reproduction. 2007;22:2153–2159. doi: 10.1093/humrep/dem145. [DOI] [PubMed] [Google Scholar]
- Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. Journal of Clinical Endocrinology and Metabolism. 2008;93:68–75. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luboshitzky R, Zabari Z, Shen-Orr Z, Herer P, Lavie P. Disruption of the nocturnal testosterone rhythm by sleep fragmentation in normal men. Journal of Clinical Endocrinology and Metabolism. 2001;86:1134–1139. doi: 10.1210/jcem.86.3.7296. [DOI] [PubMed] [Google Scholar]
- Meikle AW, Bishop DT, Stringham JD, West DW. Quantitating genetic and nongenetic factors that determine plasma sex steroid variation in normal male twins. Metabolism. 1987;35:1090–1095. doi: 10.1016/0026-0495(86)90020-x. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Metter EJ, Kawas C, Blackman MR, Harman SM, Resnick SM. Free testosterone and risk for Alzheimer disease in older men. Neurology. 2004;62:188–193. doi: 10.1212/wnl.62.2.188. [DOI] [PubMed] [Google Scholar]
- Ohlsson C, Wallaschofski H, Lunetta KL, Stolk L, Perry JR, Koster A, Petersen AK, Eriksson J, Lehtimaki T, Huhtaniemi IT, Hammond GL, Maggio M, Coviello AD, Ferrucci L, Heier M, Hofman A, Holliday KL, Jansson JO, Kahonen M, Karasik D, Karlsson MK, Kiel DP, Liu Y, Ljunggren O, Lorentzon M, Lyytikainen LP, Meitinger T, Mellstrom D, Melzer D, Miljkovic I, Nauck M, Nilsson M, Penninx B, Pye SR, Vasan RS, Reincke M, Rivadeneira F, Tajar A, Teumer A, Uitterlinden AG, Ulloor J, Viikari J, Volker U, Volzke H, Wichmann HE, Wu TS, Zhuang WV, Ziv E, Wu FC, Raitakari O, Eriksson A, Bidlingmaier M, Harris TB, Murray A, de Jong FH, Murabito JM, Bhasin S, Vandenput L, Haring R. Genetic determinants of serum testosterone concentrations in men. PLoS Genetics. 2011;7:e1002313. doi: 10.1371/journal.pgen.1002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzon MS, Hauger R, Dale AM, Eaves LJ, Eyler LT, Fischl B, Fennema-Notestine C, Franz CE, Grant MD, Jak AJ, Jacobson K, Lyons MJ, Mendoza SP, Neale MC, Prom-Wormley E, Seidman L, Tsuang MT, Xian H, Kremen WS. Testosterone modifies the effect of APOE genotype on hippocampal volume in middle-aged men. Neurology. 2010;75:874–880. doi: 10.1212/WNL.0b013e3181f11deb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzon MS, Hauger RL, Eaves LJ, Chen CH, Dale AM, Eyler LT, Fischl B, Fennema-Notestine C, Franz CE, Grant MD, Jacobson KC, Jak AJ, Lyons MJ, Mendoza SP, Neale MC, Prom-Wormley E, Seidman LJ, Tsuang MT, Xian H, Kremen WS. Genetic influences on hippocampal volume differ as a function of testosterone level in middle-aged men. Neuroimage. 2012;59:1123–1131. doi: 10.1016/j.neuroimage.2011.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prom-Wormley EC, York TP, Jacobson KC, Eaves LJ, Mendoza SP, Hellhammer D, Maninger N, Levine S, Lupien S, Lyons MJ, Hauger R, Xian H, Franz CE, Kremen WS. Genetic and environmental effects on diurnal dehydroepiandrosterone sulfate concentrations in middle-aged men. Psychoneuroendocrinology. 2011;36:1441–1452. doi: 10.1016/j.psyneuen.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J. AR, apoE, and cognitive function. Hormones and Behavior. 2008;53:706–715. doi: 10.1016/j.yhbeh.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring HZ, Lessov CN, Reed T, Marcus R, Holloway L, Swan GE, Carmelli D. Heritability of plasma sex hormones and hormone binding globulin in adult male twins. The Journal of Endocrinology and Metabolism. 2005;90:3653–3658. doi: 10.1210/jc.2004-1025. [DOI] [PubMed] [Google Scholar]
- Saad F, Gooren L. The role of testosterone in the metabolic syndrome: a review. The Journal of Steroid Biochemistry and Molecular Biology. 2009;114:40–43. doi: 10.1016/j.jsbmb.2008.12.022. [DOI] [PubMed] [Google Scholar]
- Sheu YT, Zmuda JM, Cauley JA, Moffett SP, Rosen CJ, Ishwad C, Ferrell RE. Nuclear receptor coactivator-3 alleles are associated with serum bioavailable testosterone, insulin-like growth factor-1, and vertebral bone mass in men. Journal of Clinical Endocrinology and Metabolism. 2006;91:307–312. doi: 10.1210/jc.2005-0864. [DOI] [PubMed] [Google Scholar]
- Sluyter F, Keijser JN, Boomsma DI, van Doornen LJ, van den Oord EJ, Snieder H. Genetics of testosterone and the aggression-hostility-anger (AHA) syndrome: a study of middle-aged male twins. Twin Research and Human Genetics. 2000;3:266–276. [PubMed] [Google Scholar]
- Spiegelhatlter D, Thomas A, Best N, Lunn D. WinBUGS version 2.0 users manual. Cambridge: MRC Biostatistics Unit; 2004. [Google Scholar]
- Stanworth RD, Jones TH. Testosterone for the aging male; current evidence and recommended practice. Clinical Interventions in Aging. 2008;3:25–44. doi: 10.2147/cia.s190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AA, Schwartz JE, Smyth J, Kirschbaum C, Cohen S, Hellhammer D, Grossman S. Individual differences in the diurnal cycle of salivary free cortisol: A replication of flattened cycles for some individuals. Psychoneuroendocrinology. 2001;26:295–306. doi: 10.1016/s0306-4530(00)00057-3. [DOI] [PubMed] [Google Scholar]
- Storgaard L, Bonde JP, Ernst E, Andersen CY, Spano M, Christensen K, Petersen HC, Olsen J. Genetic and environmental correlates of semen quality: a twin study. Epidemiology. 2006;17:674–681. doi: 10.1097/01.ede.0000239730.47963.4e. [DOI] [PubMed] [Google Scholar]
- Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. Journal of Neuroendocrinology. 2002;14:506–513. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- Zitzmann M, Nieschlag E. Testosterone levels in healthy men and the relation to behavioural and physical characteristics: facts and constructs. European Journal of Endocrinology. 2001;144:183–197. doi: 10.1530/eje.0.1440183. [DOI] [PubMed] [Google Scholar]
- Zmuda JM, Cauley JA, Kriska A, Glynn NW, Gutai JP, Kuller LH. Longitudinal relation between endogenous testosterone and cardiovascular disease risk factors in middle-aged men. A 13-year follow-up of former Multiple Risk Factor Intervention Trial participants. American Journal of Epidemiology. 1997;146:609–617. doi: 10.1093/oxfordjournals.aje.a009326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.