Abstract
Adolescence is characterized behaviorally by increased impulsivity and risk-taking that declines in parallel with maturation of the prefrontal cortex and executive function. In the brain, the receptor for advanced glycation end products (RAGE) is critically involved in neurodevelopment and neuropathology. In humans, the risk of alcoholism is greatly increased in those who begin drinking between 13 and 15 years of age, and adolescents binge drink more than any other age group. We have previously found that alcoholism is associated with increased expression of neuroimmune genes. This manuscript tested the hypothesis that adolescent binge drinking upregulates RAGE and Toll-like receptor (TLR) 4 as well as their endogenous agonist, high-mobility group box 1 (HMGB1). Immunohistochemistry, Western blot, and mRNA analyses found that RAGE expression was increased in the human post-mortem alcoholic orbitofrontal cortex (OFC). Further, an earlier age of drinking onset correlated with increased expression of RAGE, TLR4, and HMGB1. To determine if alcohol contributed to these changes, we used an adolescent binge ethanol model in rats (5.0 g/kg, i.g., 2-day on/2-day off from postnatal day [P] 25 to P55) and assessed neuroimmune gene expression. We found an age-associated decline of RAGE expression from late adolescence (P56) to young adulthood (P80). Adolescent intermittent ethanol exposure did not alter RAGE expression at P56, but increased RAGE in the young adult PFC (P80). Adolescent intermittent ethanol exposure also increased TLR4 and HMGB1 expression at P56 that persisted into young adulthood (P80). Assessment of young adult frontal cortex mRNA (RT-PCR) found increased expression of proinflammatory cytokines, oxidases, and neuroimmune agonists at P80, 25 days after ethanol treatment. Together, these human and animal data support the hypothesis that an early age of drinking onset upregulates RAGE/TLR4-HMGB1 and other neuroimmune genes that persist into young adulthood and could contribute to risk of alcoholism or other brain diseases associated with neuroinflammation.
Keywords: Ethanol, neuroimmune, innate immunity, cytokine, adolescence, alcoholism, Toll-like receptor, HMGB1, binge drinking
Introduction
The innate immune system has increasingly been implicated in neurodevelopment and neurodegeneration. Both the receptor for advanced glycation end products (RAGE) and Toll-like receptors (TLRs) are involved in neurogenesis and neurite outgrowth (Chou et al. 2004; Okun et al. 2011), partly through interactions with amphoterin (i.e., high-mobility group box 1 [HMGB1]; Chou et al. 2004; Merenmies et al. 1991). Indeed, blockade of RAGE in P19 embryonic stem cells prevents neuronal differentiation and inhibits neurite outgrowth, which appear to be mediated through downstream Rac1/Cdc42 pathways (Wang et al. 2008). In a similar fashion, TLRs exert a neuromodulatory role in neural development as knockout of TLR2 impairs hippocampal neurogenesis while TLR4 knockout facilitates proliferation and neuronal differentiation (Rolls et al. 2007). Activation of the innate immune system is also linked to CNS disease (Hanisch et al. 2008; Lehnardt 2010). Recent studies find that alcoholism is associated with increased brain microglial markers and expression of the proinflammatory cytokine CCL2 (MCP-1 [He and Crews 2008]) as well as increased TLR and HMGB1 expression (Crews et al. 2013). In addition, increased expression of neuroimmune genes is associated with depression, neurodegeneration, and many other brain diseases (Forrest et al. 2012; Garate et al. 2013; Hanisch et al. 2008; Maczurek et al. 2008; Suchankova et al. 2012). Signaling through RAGE can also contribute to neuroinflammation. For instance, extracellular HMGB1 activation of RAGE contributes to increased transcription of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) increasing expression of proinflammatory cytokines, oxidases, and other neuroimmune genes (Han et al. 2011). Once activated in the CNS, neuroimmune signals often remain elevated well beyond resolution of the activating event in the periphery (Qin et al. 2008; Qin et al. 2007), which is consistent with the insidious nature of many brain diseases. Thus, alcohol-induced changes in RAGE/TLR-HMGB1 expression may contribute to brain disease.
Adolescence is a developmental period in humans and other mammalian species characterized by high levels of impulsivity, thrill seeking, and risk-taking that parallels maturation of the prefrontal cortex and executive functions. For instance, there is substantial refinement of prefrontal synapses during adolescence, involving pruning of excitatory glutamatergic inputs (Huttenlocher 1984) and fine-tuning of dopaminergic and serotonergic innervations (Kalsbeek et al. 1988; Rosenberg and Lewis 1994). Unfortunately, adolescence is also a time when the majority of young people initiate alcohol use (Windle et al. 2008), with current statistics indicating that approximately 12% of 13-14 year old 8th graders, 22% of 10th graders, and 28% of 12th graders report heavy episodic drinking (i.e., >5 consecutive alcohol drinks per binge drinking episode) in the past two weeks (Masten et al. 2008; Windle et al. 2008). Interestingly, an early age of drinking onset (i.e., <14 to 21 years of age) is associated with 4- to 5-fold greater risk of developing alcoholism within the lifetime and increased lifetime problems while drinking (Grant et al. 2001; Hingson et al. 2001; Hingson et al. 2000). Thus, we tested the hypothesis that binge drinking during adolescence alters RAGE/TLR4-HMGB1 expression that persists, and could contribute to alcoholism or other brain diseases.
We report here that RAGE mRNA and protein expression is increased in the post-mortem human alcoholic orbitofrontal cortex (OFC), and that an earlier age of drinking onset is correlated with increased expression of RAGE, TLR4, and HMGB1. Using a rodent model to determine if adolescent binge ethanol exposure contributed to these changes, we found maturational changes in RAGE, TLR4, and HMGB1 expression in the developing rat prefrontal cortex. Adolescent binge ethanol (5.0 g/kg, i.g. from postnatal day [P] 25 to P55) increased RAGE expression in the young adult (P80) 25 days after the last ethanol binge, while levels of TLR4 and HMGB1 were persistently upregulated from late adolescence (P56) to young adulthood (P80). Further, we found increased expression of proinflammatory cytokines, oxidases, and neuroimmune agonists in the young adult frontal cortex. These data support the hypothesis that adolescent binge ethanol persistently upregulated RAGE, TLR4, HMGB1, and other neuroimmune markers that could contribute to the development of alcoholism and other brain diseases associated with neuroinflammation.
Materials and methods
Post-mortem Human Samples
Human post-mortem paraffin sections and frozen samples of the OFC (see Figure 1A) were obtained from the New South Wales Tissue Resource Centre at the University of Sydney, supported by the National Health and Medical Research Council of Australia-Schizophrenia Research Institute and the National Institute of Alcohol Abuse and Alcoholism (NIH [NIAAA] R24AA012725). Subject information was collected through personal interviews, next-of-kin interviews, and medical records, and is presented in Table 1. Alcoholic patients reported an average age of drinking onset of 18±1 years of age, which were compared with age-matched moderate drinking controls whose average age of drinking onset was 25±1. Age of drinking onset was determined through personal interviews, medical records, and next-of-kin interviews, or recorded as 25 years of age if the age of drinking onset was unclear from these sources (Sheedy et al. 2008). Only individuals with alcohol dependence uncomplicated by liver cirrhosis and/or nutritional deficiencies were included. All psychiatric and alcohol use disorder diagnoses were confirmed using the Diagnostic Instrument for Brain Studies that complies with the Diagnostic and Statistical Manual of Mental Disorders (Dedova et al. 2009).
FIGURE 1. SAMPLE SELECTION AND EXPERIMENTAL DESIGN.
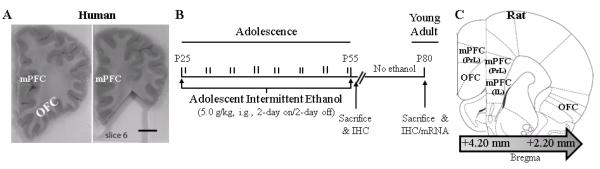
(A) Representative image depicting human orbitofrontal cortex (OFC) sectioning by New South Wales Tissue Resource Centre at the University of Sydney. Left: Image of intact alcoholic frontal cortex tissue sample containing OFC. Right: Image depicting sampling of OFC from alcoholic volunteer. Scale bar = 1 cm. (B) Male Wistar rats were treated from postnatal day (P)25 to P55 with either ethanol (5.0 g/kg, 20% ethanol w/v, i.g.) or a comparable volume of water on a 2-day on/2-day off administration schedule. Blood ethanol concentrations (BECs) were measured one hr after ethanol treatment on P38 and P54. Subjects were sacrificed either 24 hr after the completion of AIE treatment (i.e., P56) or 25 days after the conclusion of AIE treatment (i.e., P80). (C) Representative micrographs of the regions of interest assessed for neuroimmune agonist and receptor expression in the prefrontal cortex. Receptor for advanced glycation end product expression was measured in subregions of the rat medial prefrontal cortex (mPFC), including the prelimbic cortex (PrL; Bregma: +4.20 mm to +2.20 mm) and infralimbic cortex (IL; Bregma: +3.20 mm to +2.20 mm) as well as the orbitofrontal cortex (OFC; Bregma: +4.20 mm to +2.20 mm) according to the atlas of Paxinos and Watson (1998). Toll-like receptor 4 and high-mobility group box 1 immunoreactivity was measured in the OFC (Bregma: +4.20 mm to +2.20 mm) according to the atlas of Paxinos and Watson (1998).
Table 1.
Case characteristics of human subjects.
| Group | Age at Death |
Gender | PMI | Clinical Cause of Death | Age of Drinking Onset |
|---|---|---|---|---|---|
| Control | 24 | Male | 43 | Undetermined (Consistent with idiopathic cardiac arrhythmia) |
20 |
| Control | 43 | Male | 66 | Aspiration pneumonia | 25 |
| Control | 44 | Male | 50 | Ischemic heart disease | 25 |
| Control | 46 | Male | 29 | Acute myocardial infarction | 25 |
| Control | 50 | Male | 30 | Coronary artery disease | 25 |
| Control | 50 | Male | 40 | Haemopericardium | 25 |
| Control | 53 | Male | 16 | Dilated cardiomyopathy | 25 |
| Control | 60 | Male | 28 | Ischemic heart disease | 25 |
| Control | 62 | Male | 46 | Ischemic heart disease | 25 |
| Control | 48 | Male | 24 | Ischemic heart disease | 29 |
|
| |||||
| Alcoholic | 49 | Male | 16 | Coronary artery thrombosis | 14 |
| Alcoholic | 45 | Male | 7 | Drowning | 15 |
| Alcoholic | 49 | Male | 44 | Ischemic heart disease | 16 |
| Alcoholic | 61 | Male | 59 | Myocarditis | 16 |
| Alcoholic | 51 | Male | 27 | Gastrointestinal hemorrhage | 17 |
| Alcoholic | 61 | Male | 23 | Atherosclerotic cardiovascular disease | 17 |
| Alcoholic | 42 | Male | 41 | Combined bromoxynil and alcohol toxicity |
18 |
| Alcoholic | 50 | Male | 17 | Ischemic heart disease | 18 |
| Alcoholic | 44 | Male | 15 | Ischemic heart disease | 20 |
| Alcoholic | 43 | Male | 43 | Carbon monoxide intoxication/Alcohol intoxication |
25 |
PMI: post-mortem interval. Mean age of drinking onset for moderate drinking controls was (25±1) and for alcoholics was (18±1). Alcoholic individuals tended to report an earlier age of drinking onset than moderate drinking controls.
Western Blot Determination of Human RAGE Expression
For human Western blots, 70 mg of frozen OFC tissue was homogenized in 0.8 ml RIPA lysis buffer (Sigma-Aldrich, St. Louis, MO) containing protease inhibitor (1:100; Sigma-Aldrich) and centrifuged at 14000 rpm at 4°C for 20 min. After centrifugation, protein content in the supernatant was assessed using a Bradford reagent kit (Bio-Rad, Hercules, CA), and 7 μg of protein from each denatured sample was loaded into precast polyacrylamide mini-gel (4-15%; Bio-Rad), and blotted onto immunoblot PVDF membranes (Bio-Rad). Immunoblot membranes were blocked in Odyssey blocking buffer (LiCOR Biosciences, Lincoln, NE), and incubated in the primary antibody solution (rabbit anti-RAGE [1:500] and mouse anti-Tubulin [1:1000]; Abcam, Cambridge, MA) for 24 hr at 4°C. After washing, the immunoblot membranes were incubated in appropriate fluorescent secondary antibodies (Rockland Immunochemicals, Gilbertsville, PA), and bands were scanned with an Odyssey Infrared Imager (LiCOR Bioscience, Lincoln, NE). Band intensity was quantified using Bioquant Imaging software and normalized to Tubulin band intensity.
Animals
Young time-mated pregnant female Wistar rats (embryonic day 17; Harlan Sprague-Dawley, Indianapolis, IN) were acclimated to our animal facility prior to birthing. P1 (24 hr after birth), litters were culled to 10 pups. Dams were housed in a temperature- (20°C) and humidity-controlled vivarium on a 12 h/12 h light/dark cycle (light onset at 700 h), and provided ad libitum access to food and water. All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of North Carolina at Chapel Hill, and conducted in accordance with National Institute of Health (NIH) regulations for the care and use of animals in research.
AIE Treatment
On P21, male Wistar rats were weaned and randomly assigned to either: (i) adolescent intermittent ethanol (AIE) or (ii) water-treated control (CON). From P25 to P55, AIE animals received intragastric (i.g.) administration of ethanol (5.0 g/kg, 20% ethanol w/v) and CON subjects received comparable volumes of H2O on a 2-day on/2-day off schedule (Figure 1B). Body weight (g) was measured for the duration of experimentation. Tail blood was collected to assess blood ethanol content (BEC) one hour after ethanol administration on P38 and P54. Tail BECs were calculated using a GM7 Analyzer (Analox; London, UK). On P38 and P54, mean BECs (±SEM) were 134±8 mg/dL and 165±17 mg/dL, respectively. For the duration of experimentation, subjects evidenced dramatic increases in body weight that were not significantly affected by AIE treatment (all p’s>0.1 [P25: CON=80±1 g, AIE=80±2 g; P55: CON=306±8 g; AIE=290±5 g; P80: CON=428±14 g; AIE=427±14 g]).
Immunohistochemistry
Human post-mortem paraffin sections were deparaffinized, washed in TBS, and antigen retrieval performed by incubation in Citra solution (BioGenex, San Ramon, CA) for 1 hr at 70°C. Following incubation in blocking solution with normal goat serum (MP Biomedicals, Solon, OH), slides were processed in primary antibody solution (rabbit anti-RAGE [1:500]; Abcam) for 24 hr at 4°C. Slides were incubated for 1 hr in biotinylated secondary antibody (1:200; Vector Laboratories, Burlingame, CA), and then incubated for 1 hr in avidin-biotin complex solution (Vectastain ABC Kit; Vector Laboratories). The chromogen, nickel-enhanced 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma-Aldrich, St. Louis, MO), was used to visualize immunoreactivity. Slides were dehydrated and coverslipped. Negative control for non-specific binding was conducted on separate sections employing the above-mentioned procedures with the exception that the primary antibody was omitted.
On P56 and P80, male Wistar rats were anesthetized with sodium pentobarbital (100 mg/kg, i.p.) and transcardially perfused with 0.1 M phosphate-buffered saline (PBS, pH 7.4), followed by 4.0% paraformaldehyde in PBS. Coronal section (40 μm) were cut on a sliding microtome (MICROM HM450; ThermoScientific, Austin, TX), and sections were sequentially collected into well plates and stored at −20°C in a cryoprotectant solution (30% glycol/30% ethylene glycol in PBS) for immunohistochemistry. Free-floating frontal cortex sections were processed similar to previously described methods (Vetreno and Crews 2012). Sections were incubated in primary antibody (rabbit anti-RAGE [1:1000], rabbit anti-TLR4 [1:200], or rabbit anti-HMGB1 [1:500]; Abcam) for 24 hr at 4°C. Slides were then rinsed in TBS, and processed identically to the previously described methodology.
Microscopic Quantification and Image Analysis
In the human study, eight regions of interest within the OFC were randomly assessed for RAGE immunoreactivity (+IR). In the animal studies, assessment of RAGE +IR was performed in the PrL (Bregma: +4.70 mm to +2.20 mm), IL (Bregma: +3.20 mm to +2.20 mm), and OFC (Bregma: +4.70 mm to +2.20 mm) according to the atlas of Paxinos and Watson (1998). For all animal immunohistochemical protocols, sections were assessed in a 1:6 series containing the PFC (see Figure 1C). In each cortical region, +IR was assessed in 4 randomly selected regions of interest per hemisphere, for a total of 8 samples per section.
Across experiments, BioQuant Nova Advanced Image Analysis (R&M Biometric, Nashville, TN) was used for image capture and quantification. Representative images were captured using an Olympus BX50 microscope and Sony DXC-390 video camera linked to a computer. Since RAGE expression was dense and homogenously distributed throughout the rodent tissue samples, pixel density was used to quantify RAGE+IR within the region of interest and thresholded to normalize pixel intensity. The outlined region of interest was determined and staining density calculated by dividing the pixel count by the overall area (mm2). In contrast to RAGE, TLR4 and HMGB1+IR was heterogeneous, and quantification was performed using a modified stereological profile cell counting method within the regions of interest (Crews et al. 2004; Vetreno and Crews 2012), and data are expressed as cells per mm2. In the human experiments, RAGE+IR was quantified similarly to the protocol described above. For each measure, the microscope, camera, and software were background corrected and normalized to preset light levels to ensure fidelity of data acquisition.
Double Immunofluorescence and Confocal Analysis
To determine co-expression of RAGE with NeuN in the human OFC, post-mortem paraffin-embedded sections were washed in TBS, incubated in 0.3% H2O2, antigen retrieval performed by incubation in Citra solution (BioGenex), and blocked with normal horse serum (MP Biomedicals). Slides were incubated for 48 hr at 4°C in a primary antibody cocktail of rabbit anti-RAGE (1:1000; Abcam) with an antibody against neurons (mouse anti-NeuN [1:500; Millipore, Temecula, CA]). Slides were then rinsed in TBS and incubated for 2 hr at room temperature in the secondary antibody cocktail (Alexa Fluor 594 and Alexa Fluor 488; Invitrogen, Carlsbad, CA). Slides were coverslipped using Prolong Gold Anti-fade mounting media (Life Technologies, Grand Island, NY).
Free-floating frontal cortex sections from P80 rats were processed similar to previously reported methods (see Vetreno and Crews 2012). Briefly, sections were incubated for 48 hr at 4°C in a primary antibody cocktail of rabbit anti-RAGE (1:1000; Abcam) with an antibody against neurons (mouse anti-NeuN [1:500; Millipore]), microglia (1:1000 goat anti-Iba-1 [Abcam]), or astrocytes (1:1000 goat anti-GFAP [Abcam]). Sections were washed in TBS, and processed identically to the previously described immunofluorescence methodology. Confocal analysis was performed using an inverted Zeiss 510 Meta laser scanning confocal microscope (Carl Zeiss MicroImaging, Thomwood, NY) in the Michael Hooker microscopy facility at UNC equipped with standard high-power objectives and corresponding software (LSM 510 META). Single optical Z-stacks (1 μm; 1-airy unit) were collected, and the number of RAGE cells that co-localized with NeuN were counted (approximately 100 RAGE+IR cells per subject).
RNA Extraction and Reverse Transcription Polymerase Chain Reaction
For human analysis, RNA was extracted from frozen OFC tissue by homogenization in TRIzol (Invitrogen). Tissue samples from the rat were collected anterior of the union of the corpus callosum (approximately +1.60 mm from Bregma) according to the atlas of Paxinos and Watson (1998), and reverse transcribed as previously described (see Vetreno and Crews 2012; Zou et al. 2012). The primer sequences are presented in Table 2. Differences in primer expression between groups were expressed as cycle time (Ct) values normalized with β-actin, and relative differences between groups were calculated and expressed as the percent difference relative to CONs.
Table 2.
List of primers for RT-PCR.
| Primer | Species | Forward | Reverse |
|---|---|---|---|
| RAGE | Human | 5′-CTA CCG AGT CCG TGT CTA CCA-3′ | 5′-CAT CCA AGT GCC AGC TAA GAG-3′ |
| Β-actin | Human | 5′-GAT GCA GAA GGA GAT CAC TGC-3′ | 5′-ATA CTC CTG CTT GCT GAT CCA-3′ |
| RAGE | Rat | 5′-AAC TAC CGA CTC CGA GTL TAC C-3′ | 5′-ACA ACT GTC CCT TTG CCA TCA-3′ |
| TNFα | Rat | 5′-ATG TGG AAC TGG CAG AGG AG -3′ | 5′-ACG AGC AGG AAT GAG AAG AAG-3′ |
| IL-1β | Rat | 5′-GAA ACA GCA ATG GTC GGG AC-3′ | 5′-AAG ACA CGG GTT CCA TGG TG-3′ |
| MCP1 | Rat | 5′-TCA CGC TTC TGG GCC TGT TG-3′ | 5′-CAG CCG ACT CAT TGG GAT CAT C-3′ |
| NOX2 | Rat | 5′-AAG CCA TTG GAT CAC AAC CT-3′ | 5′-GGC TTC TAC TGT AGC GTT CA-3′ |
| COX2 | Rat | 5′-CCG GGT TGC TGG GGG AAG GA-3′ | 5′-CCA CCA GCA GGG CGG GAT ACA G-3′ |
| iNOS | Rat | 5′-CAA TGG CTT GAG GCA GAA GC-3′ | 5′-GCC ACC TCG GATATC TCT TGC-3′ |
| Fbn | Rat | 5′-GAA AGG CAA CCA GCA GAG TC-3′ | 5′-CTG GAG TCA AGC CAG ACA CA-3′ |
| MyD88 | Rat | 5′-GAC TGC CAG AAA TAC ATA CG-3′ | 5′-ATC TCC TGC ACA AAC TCA A-3′ |
| S100β | Rat | 5′-GGG AAT TAG GAT GTC TGA GCT GGA GAA G-3′ |
5′-GCG GAT CCA CTC CTG GAA GT CACA CTC C-3′ |
| β-actin | Rat | 5′-CTA CAA TGA GCT GCG TGT GGC-3′ | 5′-CAG GTC CAG ACG CAG GAT GGC-3′ |
Statistical Analysis
Statistical analysis was performed using SPSS (Chicago, IL). Analysis of variance (ANOVA) was used to determine the effects of AIE on BEC, immunohistochemistry, Western blot protein analysis, and real time PCR mRNA expression. Repeated measure ANOVAs were used to assess the effects of AIE on body weight. Pearson’s r correlations were used to determine the association between neuroimmune receptor (i.e., RAGE and TLR4) and HMGB1 expression in the animal experiment, and neuroimmune expression (i.e., RAGE, TLR4, and HMGB1) with age of drinking onset in the human experiment. All values are reported as mean ± SEM, and significance was defined at p≤0.05.
Results
RAGE is Elevated in the Post-mortem Human Alcoholic Orbitofrontal Cortex
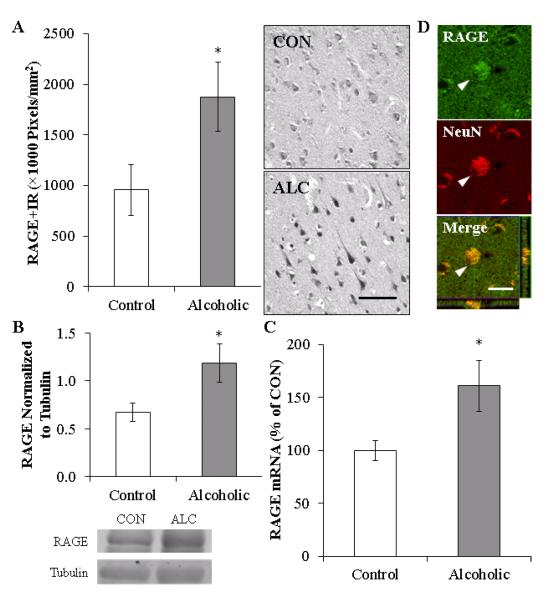
To determine if RAGE expression was increased in the human alcoholic OFC, we performed immunohistochemistry, Western blot, and RT-PCR analysis. In moderate drinking controls, RAGE+IR was characterized by lightly stained large and small cells, whereas RAGE+IR in the alcoholic was characterized by darkly stained large, neuron-like cells and smaller glia-like cells. Microscopic quantification found an approximate 2-fold increase in RAGE+IR (196%±36%) in the alcoholic OFC, relative to moderate drinking controls (one-way ANOVA: F=4.7, p<0.05 [see Figure 2A]). Similar to the immunohistochemical data, Western blot analysis of RAGE protein expression found a 176%±29% increase in the human alcoholic OFC, relative to moderate drinking controls (one-way ANOVA: F=5.5, p<0.05, see Figure 2B). Further, expression of RAGE mRNA was increased by 161%±24% in the human post-mortem alcoholic OFC (one-way ANOVA: F=5.5, p<0.05, see Figure 2C). Given our findings of increased RAGE expression, we used confocal microscopy to assess co-expression of RAGE on neurons, and found that 77%±9% of RAGE+IR colocalized with NeuN-positive neurons in the human OFC (see Figure 2D). Thus, we find increased RAGE mRNA and protein (+IR and Western analysis) in the human post-mortem alcoholic OFC that is primarily localized on neurons.
FIGURE 2. RECEPTOR FOR ADVANCED GLYCATION END PRODUCT (RAGE) EXPRESSION IS INCREASED IN THE HUMAN POST-MORTEM ORBITOFRONTAL CORTEX (OFC) AND IS COLOCALIZED ON NEURONS.
(A) RAGE pixel density (×1000 Pixels/mm2) was increased in the alcoholic OFC (n=10), relative to moderate drinking controls (n=10). Representative photomicrographs of RAGE+IR in a moderate drinking control and alcoholic post-mortem OFC. Scale bar = 20 μm. (B) Western blot analyses found increased RAGE protein concentration in the human alcoholic OFC, relative to moderate drinking controls (n=9). Representative lanes from Western blot analysis of RAGE. CON is moderate drinking control and ALC alcoholic subject. Western blot analyses were run in triplicate, and the mean was reported. (C) RT-PCR assessment found increased RAGE mRNA in the human alcoholic OFC, relative to moderate drinking controls (n=8). RT-PCR analyses were run in triplicate, and the mean was reported. (D) Top and Middle: High magnification photomicrographs of RAGE (green) colocalization with the neuronal marker NeuN (red) in the post-mortem human alcoholic OFC. White arrowheads indicate a RAGE+IR cell that colocalized with NeuN. Bottom: Z-stack demonstrating colocalization of RAGE+IR cell with NeuN (yellow). Scale bar = 20 μm. * indicates p<0.05.
Expression of RAGE, TLR4, and HMGB1 Correlates with Age of Drinking Onset in Humans
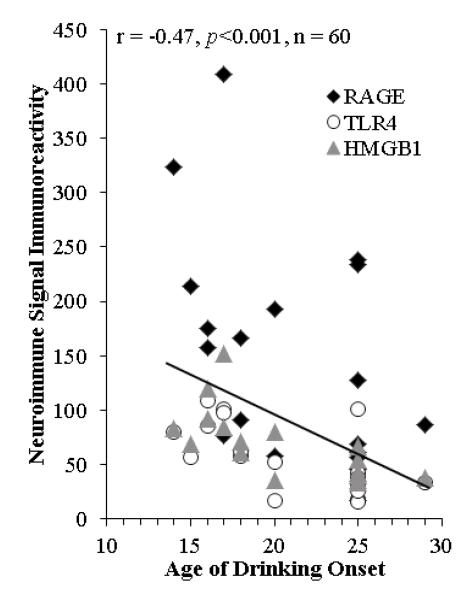
Since an earlier age of drinking onset is associated with increased risk of alcohol dependence across the lifetime of the individual (Grant and Dawson 1998; Gruber et al. 1996), we correlated age of drinking onset with expression of RAGE, TLR4, and HMGB1. Age of drinking onset was negatively correlated with expression of RAGE (r=-0.52, p<0.05, n=20), TLR4 (r=-0.66, p<0.01, n=20), and HMGB1 (r=-0.72, p<0.01, n=20) in the human post-mortem OFC. As depicted in Figure 3, a younger age of drinking onset is associated with increased expression of RAGE, TLR4, and HMGB1, with extremely high levels of RAGE expression only found in alcoholics that reported an earlier age of drinking onset (18±1). Since establishing an accurate age of drinking onset is important for this analysis, trained clinical nurses and psychologists from the New South Wales Tissue Resource Centre performs extensive interviews with human study volunteers and their families when volunteers indicate that they will become post-mortem brain donors. The age of drinking onset is derived from personal interviews with the volunteer as well as medical records and next-of-kin interviews. In cases where the age of drinking onset was not recalled or unclear, an age of 25 was given (Sheedy et al. 2008). It is possible that individuals who could not recall the age they began drinking impacted this correlation, so we removed those assigned an age of 25. However, we found that age of drinking onset was still negatively correlated with neuroimmune signal expression in the post-mortem human OFC (r=-0.34, p=0.05, n=33). It is interesting to note that 90% of alcoholics were able to recall the age at which drinking commenced while only 20% of moderate drinking controls were able to provide accurate drinking age onset. Thus, the expression of RAGE, TLR4, and HMGB1 in the OFC, either independently or grouped, show a significant correlation with age of drinking onset.
FIGURE 3. RECEPTOR FOR ADVANCED GLYCATION END PRODUCTS (RAGE), TOLL-LIKE RECEPTOR 4 (TLR4), AND HIGH-MOBILITY GROUP BOX 1 (HMGB1) EXPRESSION IN THE HUMAN ALCOHOLIC POST-MORTEM ORBITOFRONTAL CORTEX IS CORRELATED WITH AGE OF DRINKING ONSET.
Depicted are individual self and family reports of age of drinking onset vs. RAGE (circle; × 100000 Pixels/mm2), TLR4 (square; Cells/mm3), and HMGB1 (triangle; Cells/mm3) immunoreactivity. Across subjects, age of drinking onset negatively correlated with neuroimmune immunoreactivity (r = −0.47, p<0.001). Note: Each subject’s immunoreactivity data (i.e., RAGE, TLR4, and HMGB1) lie on vertical points over each subject’s reported age of drinking onset. Moderate alcohol drinking controls tended to self-report a later age of drinking onset (25±1 years of age) in comparison to individuals that met criteria for alcoholism (18±1 years of age).
Adolescent Intermittent Ethanol Exposure Disrupts the Maturational Trajectory of RAGE Expression in the Young Adult Rat Prefrontal Cortex
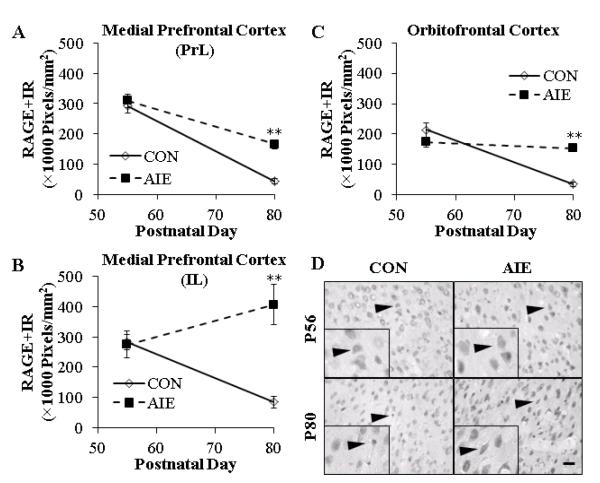
The association of RAGE induction with age of drinking onset prompted investigation of the effect of ethanol during adolescence on expression of RAGE. We modeled adolescent drinking using an intermittent schedule that is consistent with known human adolescent patterns of heavy binge drinking on weekends, but not daily drinking observed in alcoholic adults. In our AIE model, male Wistar rats were treated intermittently with ethanol (5.0 g/kg, i.g., 20% ethanol w/v; 2-day on/2-day off) throughout adolescence (i.e., P25 – P55). Receptor for advanced glycation end product expression was determined immunohistochemically in medial prefrontal cortex sections (i.e., PrL and IL) and the OFC of adolescent (P56) rats one day after the conclusion of AIE and 25 days after treatment in young adulthood (P80). Assessment of RAGE+IR cells in CON- and AIE-treated adolescent prefrontal cortex revealed widespread homogeneous distribution, characterized by darkly stained nuclei and hollow neuron-like silhouettes (see Figure 4D). In CON animals, we found a significant age-associated maturational decline of RAGE+IR from adolescence (P56) to young adulthood (P80) in the PrL (85%; one-way ANOVA: F=73.0, p<0.01), IL (70%; one-way ANOVA: F=40.9, p<0.01), and OFC (84%; one-way ANOVA: F=60.7, p<0.01). Adolescent binge ethanol exposure did not affect RAGE+IR in the adolescent PrL, IL, or OFC relative to the CON subjects (all p’s>0.1). In young adulthood (P80), AIE increased RAGE+IR by 280%±36% in the PrL (one-way ANOVA: F=43.3, p<0.01), 375%±80% in the IL (one-way ANOVA: F=22.0, p<0.01), and 340%±36% in the OFC (one-way ANOVA: F=65.3, p<0.01), relative to CONs (see Figure 4A-4C). Measures of RAGE mRNA in young adult (P80) frontal cortex tissue samples from AIE-treated animals did not reveal any differences relative to CONs (data not shown). Together, these data suggest that RAGE expression in the developing adolescent prefrontal cortex undergoes an age-associated maturational decline that is reduced by adolescent binge ethanol exposure, resulting in increased expression in the young adult OFC.
FIGURE 4. THE MATURATIONAL TRAJECTORY OF RECEPTOR FOR ADVANCED GLYCATION END PRODUCTS (RAGE) EXPRESSION IN THE YOUNG ADULT PREFRONTAL CORTEX IS DISRUPTED FOLLOWING ADOLESCENT BINGE ETHANOL EXPOSURE IN THE RAT.
(A) RAGE pixel density (×1000 Pixels/mm2) was unchanged in the adolescent prelimbic cortex (PrL) following adolescent intermittent ethanol (AIE) treatment, but was increased by 280% in the young adult, relative to CONs. (B) AIE treatment did not affect RAGE+IR in the adolescent infralimbic cortex (IL), but did increase expression by 375% in the young adult, relative to CONs. (C) AIE did not affect RAGE pixel density (×1000 Pixels/mm2) in the adolescent orbitofrontal cortex, but did increase RAGE+IR by 340% in the young adult, relative to CONs. Note: There is a maturational reduction of RAGE+IR in the CON PrL, IL, and OFC from adolescence to young adulthood. (D) Representative photomicrographs of RAGE+IR in the OFC of adolescent (P56) and young adult (P80) CON- and AIE-treated rats. Black arrowheads indicate RAGE+IR cells. Data are presented as mean ± SEM. ** indicates p<0.01, relative to CON rats. Scale bar = 50 μm.
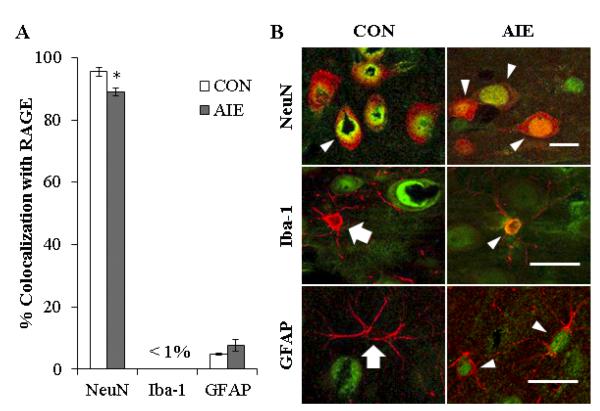
To determine the cellular phenotype of cells expressing RAGE in the young adult prefrontal cortex (P80), we used double immunofluorescence to assess colocalization of RAGE with NeuN (a neuronal marker), Iba-1 (a microglial marker), and GFAP (an astrocyte marker). Employing confocal microscopy, we found that 96%±3% and 89%±2% of RAGE+IR colocalized with NeuN (one-way ANOVA: F=12.7, p<0.05) in the CON- and AIE-treated prefrontal cortex (i.e., PrL, IL, and OFC), respectively. Assessment of Iba-1 yielded very low levels of colocalization in either treatment group. In contrast, we found that 5%±0.3% and 8%±2% of RAGE-immunopositive cells colocalized with the astrocytic marker GFAP (p>0.05) in CON- and AIE-treated animals, respectively (see Figures 5A and 5B). Thus, similar to the human OFC, the majority of RAGE+IR is colocalized in neurons (NeuN+IR) in the rat prefrontal cortex.
FIGURE 5. EXPRESSION OF RECEPTOR FOR ADVANCED GLYCATION END PRODUCTS (RAGE) IS HIGHLY COLOCALIZED WITH NEURONS IN THE RODENT YOUNG ADULT PREFRONTAL CORTEX.
(A) Quantification of RAGE colocalization with neuronal (NeuN), microglial (Iba-1), and astrocytic (GFAP) markers. Data are presented as mean ± SEM. * indicates p<0.05, relative to CON rats. (B) High magnification photomicrographs of RAGE (green) colocalization with the cellular marker NeuN, Iba-1, or GFAP (red) in the prefrontal cortex of young adult CON- and AIE-treated rats. White arrowheads indicate RAGE+IR cells that colocalized with a cellular marker (yellow). White arrows emphasize cell makers that did not colocalize with RAGE. All scale bars = 20 μm.
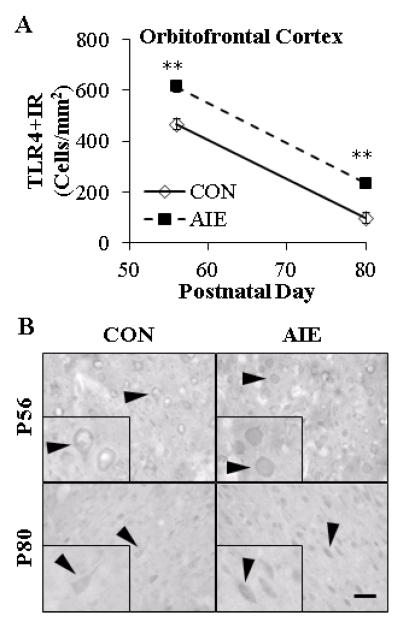
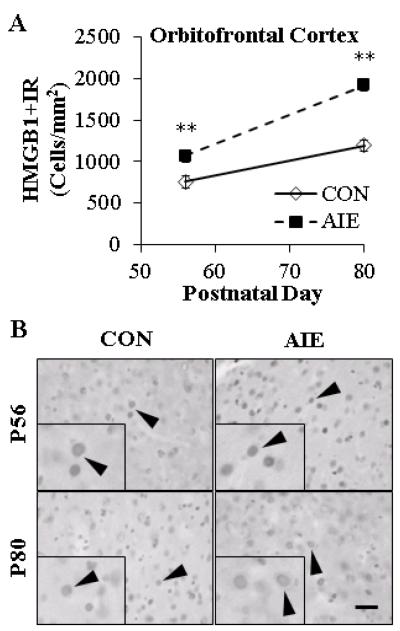
Binge Ethanol Exposure During Adolescence Persistently Increases TLR4 and HMGB1 Expression in the Young Adult Orbitofrontal Cortex
Our findings in human OFC prompted investigation of how RAGE induction by AIE relates to expression of TLR4+IR and HMGB1+IR in the rat OFC. Expression profiles of TLR4-positive cells in CONs during adolescence (P56) was characterized by distinct hollow neuron-like silhouettes that declined significantly with age from P56 to P80 (one-way ANOVA: F=529.7, p<0.01). Assessment of AIE-treated rats revealed more TLR4+IR cells (see Figure 6B). Adolescent binge ethanol exposure increased TLR4+IR by 32%±2% in the adolescent at P56 (one-way ANOVA: F=106.3, p<0.01) and 141%±40% in the young adult OFC at P80 (one-way ANOVA: F=11.5, p<0.01), relative to CONs (see Figure 6A). Across treatment and age, HMGB1+IR cells were homogeneously expressed, appearing as solid nuclei and hollow somas (see Figure 7B). In CONs, we found increased HMGB1+IR in young adult (P80) OFC relative to adolescents (P56 [one-way ANOVA: F=18.1, p<0.01]). Quantification of HMGB1+IR in the AIE-treated animals revealed significantly increased HMGB1+IR cells in the adolescent OFC (41%±5% [one-way ANOVA: F=80.1, p<0.01]) that persisted into young adulthood (61%±7% [one-way ANOVA: F=69.1, p<0.01]), relative to CONs (see Figure 7A). Together, these data support the hypothesis that AIE treatment of rats increases RAGE, TLR4, and HMGB1+IR in the young adult OFC.
FIGURE 6. ADOLESCENT INTERMITTENT ETHANOL (AIE) TREATMENT PERSISTENTLY INCREASED EXPRESSION OF TOLL-LIKE RECEPTOR 4 (TLR4) IN THE ADOLESCENT (P56) AND ADULT (P80) ORBITOFRONTAL CORTEX.
(A) Profile counts (Cells/mm2) revealed that AIE exposure increased TLR4+IR cells by 32% in the adolescent and 141% in the young adult orbitofrontal cortex (OFC), relative to CONs. Note: There is a maturational reduction of TLR4+IR in the CON OFC from adolescence to young adulthood. ** indicates p<0.01, relative to CON rats. Scale bar = 50 μm. (B) Representative photomicrographs of TLR4+IR in OFC of adolescent (P56) and young adult (P80) CON- and AIE-treated rats. Black arrowheads indicate TLR4+IR cells. Data are presented as mean ± SEM. ** indicates p<0.01, relative to CON rats. Scale bar = 50 μm.
FIGURE 7. ADOLESCENT INTERMITTENT ETHANOL (AIE) EXPOSURE INCREASES HIGH-MOBILITY GROUP BOX 1 (HMGB1) EXPRESSION IN THE ADOLESCENT (P56) ORBITOFRONTAL CORTEX THAT PERSISTS INTO YOUNG ADULTHOOD (P80).
(A) Profile counts (Cells/mm2) revealed that AIE treatment increased HMGB1+IR by 41% in the adolescent and 61% in the young adult orbitofrontal cortex (OFC), relative to CONs. Note: There is a maturational increase of HMGB1+IR in the CON OFC from adolescence to young adulthood. ** indicates p<0.01, relative to CON rats. Scale bar = 50 μm. (B) Representative photomicrographs of HMGB1+IR in OFC of adolescent (P56) and young adult (P80) CON- and AIE-treated rats. Black arrowheads indicate HMGB1+IR cells. Data are presented as mean ± SEM. ** indicates p<0.01, relative to CON rats. Scale bar = 50 μm.
Previous studies have hypothesized that positive loops of activation underlie neuroimmune gene induction (Crews et al. 2011). This hypothesis would predict a relationship between RAGE and HMGB1 expression in the prefrontal cortex. In AIE-treated rats, expression of HMGB1 was correlated with RAGE in the young adult prefrontal cortex (see Table 3). Interestingly, the strongest correlation of HMGB1 with RAGE was in the OFC, although all brain regions measures showed positive correlations. These findings are consistent with RAGE-HMGB1 upregulation contributing to their own induction as well as the amplification and persistence of neuroimmune expression in brain.
Table 3.
Correlation of receptor for advanced glycation end products (RAGE) expression with the agonist high-mobility group box 1 (HMGB1) in the young adult prefrontal cortex following adolescent binge ethanol exposure.
| RAGE |
||||
|---|---|---|---|---|
| PrL | IL | OFC | ||
|
|
||||
| HMGB1 | PrL | 0.64 * | 0.51 | 0.78 ** |
|
|
||||
| IL | 0.80 ** | 0.78 ** | 0.86 *** | |
|
|
||||
| OFC | 0.64 * | 0.57 * | 0.83 *** | |
|
|
||||
Pearson’s r correlations were conducted for the three PFC regions assessed for RAGE and HMGB1+IR cells. HMGB1+IR cell counts in the prelimbic cortex (PrL) and infralimbic cortex (IL) have been reported previously (Vetreno and Crews 2012). Although correlations do not prove linkage, statistically significant associations were observed between RAGE+IR cells and HMGB1+IR cells in the PFC. These correlations provide further evidence that HMGB1-RAGE signaling perpetuates brain regional expression of neuroimmune signaling. Pearson’s r correlation coefficients were used with two-tailed significance (* p<0.05, ** p<0.01, *** p<0.001). OFC: orbitofrontal cortex.
Adolescent Binge Ethanol Upregulates Downstream Cytokine, Oxidase, and Neuroimmune Agonist Expression in the Young Adult Prefrontal Cortex
Since HMGB1 is an agonist at RAGE and TLR4 that converge upon NF-κB transcription of innate immune genes, we assessed expression of proinflammatory cytokines, oxidases, and other innate immune genes in the young adult frontal cortex (P80) 25 days after the last binge ethanol exposure. Assessment of cytokine mRNA revealed an AIE-induced increase of TNFα (one-way ANOVA: F=25.3, p<0.01) and MCP-1 (one-way ANOVA: F=58.3, p<0.01) by approximately three-fold, but did not change expression of IL-1β (p>0.2), relative to CONs (see Figure 8A). Measures of proinflammatory oxidase mRNA revealed an approximate three-fold increase of NOX2 (one-way ANOVA: F=62.2, p<0.01) and a two-fold increase in COX2 (one-way ANOVA: F=6.7, p<0.05), relative to CONs. Levels of iNOS were unchanged in the young adult frontal cortex following AIE exposure (p>0.4 [see Figure 8B]). Measurement of neuroimmune signaling molecule mRNA found a two-fold increase of fibronectin (one-way ANOVA: F=42.4, p<0.01) and an 80% increase of S100β (one-way ANOVA: F=7.5, p<0.05) in the young adult frontal cortex, relative to CONs. Assessment of MyD88, a downstream adaptor protein of the proinflammatory TLR4 and RAGE cascade, found a 44% increase relative to CONs (one-way ANOVA: F=6.2, p<0.05 [see Figure 8C]). These data indicate that AIE exposure increases neuroimmune gene mRNA consistent with increased RAGE/TLR4-HMGB1 expression inducing innate immune gene upregulation in the prefrontal cortex.
FIGURE 8. ADOLESCENT INTERMITTENT ETHANOL (AIE) EXPOSURE INCREASES MRNA LEVELS OF PROINFLAMMATORY CYTOKINES, OXIDASES, AND NEUROIMMUNE AGONIST MARKERS IN THE YOUNG ADULT FRONTAL CORTEX.
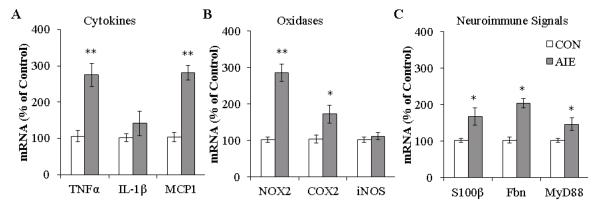
(A) RT-PCR assessment of proinflammatory cytokine mRNA in frontal cortex tissue samples found an approximate three-fold increase of tumor necrosis factor α (TNFα), no change in Interleukin-1β (IL-1β), and an approximate three-fold increase of monocyte chemotactic protein-1 (MCP-1), relative to CONS. (B) RT-PCR assessment of proinflammatory oxidases in frontal cortex tissue samples found an approximate three-fold increase of NADPH oxidase (NOX2) mRNA, an approximate 80% increase of cyclooxygenase-2 (COX2) mRNA, and no change in inducible nitric oxide synthase (iNOS) mRNA, relative to CONS. (C) RT-PCR assessment of neuroimmune agonist mRNA in frontal cortex tissue samples found an approximate 180% increase of S100 calcium binding protein B (S100β), a two-fold increase of fibronectin (Fbn), and a 44% increase of myeloid differentiation primary response gene 88 (MyD88), relative to CONS. RT-PCR analyses were run in triplicate. * indicates p<0.05, ** indicates p<0.01, relative to CON rats.
Discussion
We report here for the first time that RAGE is upregulated in the post-mortem human alcoholic OFC, and an earlier age of drinking onset is correlated with increased RAGE, TLR4, and HMGB1 expression. The association between an early age of drinking onset and increased expression of RAGE, TLR4, and HMGB1 in the human OFC prompted investigation into the persistence of ethanol-induced neuroimmune upregulation in an animal model of adolescent binge drinking. From adolescence to young adulthood, we observed a maturational shift in neuroimmune expression, with levels of RAGE and TLR4 decreasing while HMGB1 increased, in the rat prefrontal cortex. Adolescent binge ethanol in rats produced a maturational shift in prefrontal cortex RAGE expression culminating in increased levels by young adulthood, while AIE upregulated TLR4 and HMGB1 in the adolescent that persisted into young adulthood. The persistence of RAGE, TLR4, and HMGB1 in the rat prefrontal cortex may be due in part to activation of proinflammatory positive loops as evidenced by increased expression of proinflammatory cytokines (i.e., TNFα and MCP-1), oxidases (COX2 and NOX2), and innate immune agonists (i.e., HMGB1, S100ß, and fibronectin). Together, these data support the hypothesis that adolescent binge drinking persistently upregulates neuroimmune gene expression in the prefrontal cortex that might contribute to the development of alcohol dependence and other brain diseases.
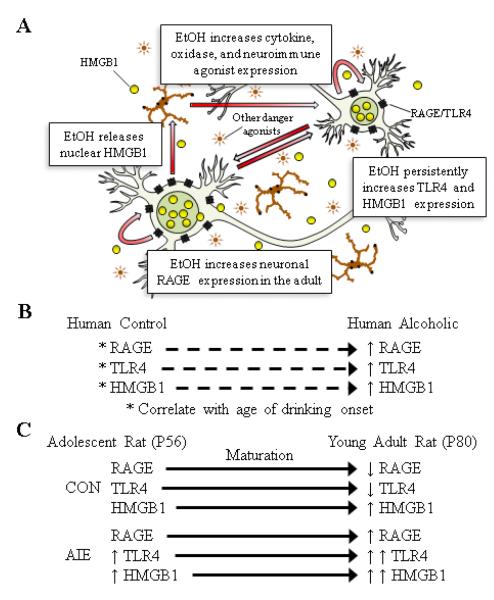
While the mechanisms underlying ethanol induction of neuroimmune genes are complex, the correlation of AIE-induced RAGE and TLR4 upregulation (Vetreno and Crews 2012) with HMGB1 is consistent with these molecules signaling through positive loops of amplification that increase neuroimmune gene expression (see Figure 9A). Although we found an AIE-induced increase of HMGB1expression in the adult OFC and medial prefrontal cortex (Vetreno and Crews 2012), the subcellular distribution of upregulated HMGB1 is not clear. Previous studies using monocytes (Bonaldi et al. 2003) and hepatocytes (Evankovich et al. 2010) suggest that HMGB1 cycles between nuclear and cytosolic forms with cell activation releasing cytosolic forms from vesicles. In the present study, we investigated HMGB1 distribution in brain cells, but do not feel we can determine nuclear vs. cytosolic distribution reliably from our immunohistochemistry. Regardless, we previously found increased HMGB1 protein expression in both cell lysate and the media derived from hippocampal brain slice culture exposed to ethanol (Crews et al. 2013), supporting that HMGB1 is released from cells exposed to ethanol. Increased NF-κB transcription of proinflammatory signaling includes TLR4 and RAGE genes that are induced during proinflammatory cascades (Rouhiainen et al. 2013; Tewari et al. 2012). We have previously reported that ethanol treatment increases brain HMGB1 and TLR4 mRNA, indicating that gene induction occurs during ethanol treatment. Chronic alcohol exposure has previously been found to increase NF-κB-DNA binding as well as increased expression of proinflammatory target genes, such as TNFα, IL-1β, NOX, and iNOS (Crews et al. 2013; Crews and Vetreno 2011; Qin and Crews 2012; Vetreno and Crews 2012; Zou and Crews 2012). In addition to HMGB1, adolescent binge ethanol exposure leads to long-term upregulation of other innate immune receptor agonists, such as S100B and fibronectin, which are agonists at RAGE (Han et al. 2011) and TLR4 (Ribes et al. 2010), respectively. The induction of TLR4-HMGB1 protein persists into adulthood during long periods of abstinence, which are consistent with persistently increased signaling. Receptor for advanced glycation end product expression was not increased during ethanol treatment, but increased during the abstinent maturation period into young adulthood. Upregulation of RAGE is induced by NF-κB, consistent with TLR4-HMGB1 signaling leading to persistent and progressive changes in RAGE. Thus, it is possible that RAGE upregulation occurs downstream of TLR4-HMGB1 signaling.
FIGURE 9. ADOLESCENT INTERMITTENT ETHANOL (AIE) EXPOSURE ALTERS THE DEVELOPMENTAL TRAJECTORY OF THE NEUROIMMUNE SIGNALING SYSTEM, LEADING TO UPREGULATION THAT PERSISTS INTO YOUNG ADULTHOOD.
(A) A simplified schematic of AIE-induced cyclic activation of the innate immune signaling system in the prefrontal cortex that persists into young adulthood. Ethanol (EtOH) exposure causes nuclear release of high-mobility group box 1 (HMGB1 [Crews et al. 2013]) , which becomes a proinflammatory cytokine (Yang et al. 2005). Ethanol also activates glial cells (Qin and Crews 2012) to release proinflammatory cytokines (i.e., TNFα, MCP-1) and oxidases (i.e., NOX2, COX2) that increase innate immune signal agonist expression (i.e., fibronectin, S100β). These proinflammatory cytokines, oxidases, and neuroimmune signaling agonists increase the expression of Toll-like receptor 4 (TLR4), which facilitate further release of HMGB1 and other proinflammatory signals establishing the cycle of TLR4/HMGB1 innate immunity signaling (Hohne et al. 2013). Once the inflammagen (i.e., EtOH) is removed from this system, the activated innate immune signaling system continues to release proinflammatory signals leading to upregulation of the receptor for advanced glycation end products (RAGE) on neurons. (B) Schematic of the correlation of age of drinking onset with neuroimmune signaling in the human post-mortem alcoholic orbitofrontal cortex, relative to moderate drinking controls. (C) Schematic of the adolescent maturational trajectory of neuroimmune signaling. (Top) Depicted is the maturational trajectory of neuroimmune signaling from adolescence to young adulthood. (Bottom) Depicted are AIE-induced changes to the maturational trajectory of neuroimmune signaling.
The innate immune system is activated in alcoholism and alcohol dependence is associated with an earlier age of drinking onset. Receptor for advanced glycation end products is well known to be induced during innate immune activation (Rouhiainen et al. 2013). In the human alcoholic, we found increased RAGE mRNA and protein expression in the OFC. Similar to our human data, chronic ethanol exposure in young adult mice (i.e., 5 months) resulted in elevated levels of RAGE in the cerebellum (Lippai et al. 2013). These results are consistent with previous work from our laboratory that found activated microglia as well as upregulated expression of MCP-1, TLR4, and HMGB1 in the post-mortem human alcoholic brain (Crews et al. 2013; He and Crews 2008). In addition to alcoholics, RAGE expression was found to increase in the human Parkinsonian substantia nigra (Sathe et al. 2012) as well as in the temporal lobe of patients with epilepsy (Iori et al. 2013; Zurolo et al. 2011). In the present study, our observation that an earlier age of drinking onset in humans (i.e., during early adolescence) correlated with increased RAGE, TLR4, and HMGB1 expression may represent a mechanism contributing to the increased risk for alcohol dependence in individuals that start drinking at an younger age (Grant 1998). Adolescence is a period of considerable prefrontal cortex maturation involving fine-tuning of afferent and efferent projections, and refinement of neurotransmitter systems to adult levels (Ernst et al. 2009; Spear 2000). Initiation of alcohol use typically commences during adolescence (Johnston et al. 2009), and adolescent individuals are capable of binge drinking because they are less sensitive to the sedative effects of alcohol (Silveri and Spear 1998). As a consequence of binge drinking and higher blood alcohol contents, there is a greater risk of alcohol-induced neurotoxicity (Crews et al. 2007; Monti et al. 2005) and activation of the innate immune system in the brain that may alter adolescent-typical cortical maturation. Once activated, the innate immune system remains elevated for long periods in the CNS (Qin et al. 2008; Qin et al. 2007), and may contribute to the risk of later alcohol dependence. Although we found a correlation between age of drinking onset and neuroimmune expression in the human alcoholic OFC, previous studies also found a correlation between neuroimmune signal expression and lifetime alcohol consumption (Crews et al. 2013). Thus, our findings indicate that adolescent exposure can induce long-term changes in adult brain, but do not establish the adolescent brain response as unique since adult chronic ethanol treatment also induces neuroimmune genes (Crews et al. 2011). However, this is the first study to report an effect of ethanol on RAGE expression. The AIE model was designed to replicate human adolescent drinking patterns that involve heavy binge drinking on weekends (i.e., intermittent, high levels of exposure), which is not common in adult human alcoholics. Additional studies are needed to determine whether chronic ethanol induces RAGE in adult rats, but we found that RAGE expression is increased in the adult alcoholic human brain. The human and rodent data in the present study support the hypothesis that adolescent binge drinking is capable of persistently upregulating neuroimmune expression, which might contribute to increased drinking later in life. In support of this hypothesis, a previous study found that binge ethanol exposure during early adolescence (i.e., P30 to P43) increased ethanol consumption later in life while late adolescent exposure (i.e., P45 to P58) did not impact adult drinking behavior (Alaux-Cantin et al. 2013). Further, in a series of elegant studies, Blednov and colleagues provide compelling evidence supporting the hypothesis that innate immune genes regulate ethanol drinking. Across multiple strains of transgenic mice with innate immune gene deletions, these animals universally drink significantly less ethanol than matched controls across multiple ethanol drinking paradigms (Blednov et al. 2005; Blednov et al. 2012). In further support, activation of the innate immune system with lipopolysaccharide, the prototypical TLR4 agonist, induced long-lasting increases in ethanol consumption in mice (Blednov et al. 2011). Thus, the increased vulnerability of the adolescent brain to alcohol-induced neurotoxicity and neuroimmune gene induction might contribute to the development of alcohol dependence.
Although we are beginning to unravel the effects of ethanol exposure on neuroimmune signaling in the adult brain, little is known regarding the consequences of ethanol-induced activation of the innate immune signaling system during adolescent maturation. This is of particular import given the potential involvement of TLR4 and RAGE in the maturation of the adolescent prefrontal cortex as well as the quantities of alcohol consumed by adolescents during this period (Deas et al. 2000). In the rat, we found that levels of RAGE immunoreactivity were unchanged 24 hours following the conclusion of AIE treatment, but after an extended period of abstinence, RAGE was increased in the young adult prefrontal cortex. In contract, TLR4 and HMGB1 expression was increased at the conclusion of adolescent binge ethanol exposure (P56) and remained elevated into young adulthood (P80), an effect that was previously observed in the medical prefrontal cortex (Vetreno and Crews 2012). Although the mechanisms underlying the delay in RAGE upregulation are unknown, it might involve an ethanol-induced maturational shift in RAGE expression. It is also possible that RAGE upregulation occurs downstream of TLR4-HMGB1 signaling. Consistent with our animal findings, increased RAGE expression is delayed in a mouse model of Parkinson’s disease (Sathe et al. 2012). Interestingly, we found that the majority of RAGE immunoreactivity colocalized with NeuN-positive neurons in both the post-mortem human OFC and in the young adult rat prefrontal cortex. In other studies, RAGE expression was predominantly observed on neurons (Iori et al. 2013; Zurolo et al. 2011), with some increase in astrocytic colocalization (Iori et al. 2013), which complements our human and animal data. The increase in neuronal RAGE may lead to hyperexcitability (Iori et al. 2013) resulting in frontal cortical deactivation (Crews et al. 2006) and consequent behavioral inflexibility (Gruber et al. 2010). Thus, adolescent binge ethanol induction of the innate immune system may contribute to increased risk of alcohol dependence through hyperexcitability-induced diminution of cortical behavioral control and behavioral inflexibility.
Involvement of the innate immune system in adolescent maturation has not been explored, but work in the embryonic brain implicates both RAGE and TLRs in neural development. During brain development, RAGE modulates neuronal development and neurite outgrowth (Wang et al. 2008) via interactions with HMGB1 (amphoterin) and S100B (Huttunen et al. 1999; Huttunen et al. 2000). Similarly, TLR signaling exerts a neuromodulatory role in neural development, neurogenesis, and neurite outgrowth (Fang et al. 2012; Trudler et al. 2010). Given the involvement of these receptors in brain development, RAGE and TLR4 might also contribute to maturation of the prefrontal cortex during adolescence. In CON subjects, we found a maturational reduction of RAGE- and TLR4-immunopositive cells as the adolescent (P56) transitioned to young adulthood (P80). These data also suggest that RAGE and TLR4 might undergo age-associated pruning similar to neurotransmitter receptor diminution that occurs between adolescence and adulthood (see e.g., Coleman et al. 2011). In contrast, we found that HMGB1 expression in CONs increased in an age-dependent manner from adolescence (P56) to young adulthood (P80). HMGB1 has been shown to exert its physiological effects through interactions with RAGE and TLR4 to modulate neurite outgrowth and neurogenesis (see e.g., Huttunen et al. 1999). Taken together, these data indicate that these pathways might play an important physiological role in neuroplasticity and/or neurodevelopment, and highlight the need to further elucidate the involvement of the innate immune signaling system in brain development and maturation.
In conclusion, RAGE expression is increased in the human post-mortem alcoholic OFC, and importantly, humans that started drinking at an earlier age had the highest expression of RAGE, TLR4, and HMGB1 (see Figure 9B). Using a rat model of adolescent binge drinking, we observed developmental changes in neuroimmune expression, with RAGE and TLR4 levels reducing with age while HMGB1 levels increased in the prefrontal cortex. These novel data suggest that the observed developmental changes in RAGE, TLR4, and HMGB1 expression might reflect maturational changes in the prefrontal cortex. We found that adolescent binge ethanol persistently alters expression of TLR4 and HMGB1, whereas RAGE expression only changed later in development suggesting that ethanol might alter the maturational reductions in RAGE expression (see Figure 9C). These data reveal that early life insults exert long-lasting neuroimmune changes in the developing CNS that might contribute to increased risk of developing alcohol dependence and other psychopathologies.
Highlights.
RAGE expression is increased in human post-mortem alcoholic orbitofrontal cortex
RAGE, TLR4, and HMGB1 induction correlates with human age of drinking onset
Adolescent binge ethanol leads to a maturational increase in adult RAGE expression
Adolescent binge ethanol persistently upregulates neuroimmune receptors in brain
Acknowledgements
This work was supported in part by the National Institutes of Health, National Institute on Alcoholism and Alcohol Abuse (AA019767, AA11605, AA007573, and AA021040), the Neurobiology of Adolescent Drinking in Adulthood (NADIA [AA020023, AA020024, and AA020022]), and the Bowles Center for Alcohol Studies. The authors thank Diana Lotito for help with preparation of the manuscript.
Abbreviations
- AIE
adolescent intermittent ethanol
- +IR
immunoreactivity
- PMI
post-mortem interval
- RAGE
receptor for advanced glycation end products
- TLR
Toll-like receptor
- HMGB1
high-mobility group box 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest The authors report no conflicts of interest.
References
- Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, Vilpoux C, et al. Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology. 2013;67:521–31. doi: 10.1016/j.neuropharm.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Geil C, Perra S, Morikawa H, Harris RA. Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behav Immun. 2011;25(Suppl 1):S92–S105. doi: 10.1016/j.bbi.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Bergeson SE, Walker D, Ferreira VM, Kuziel WA, Harris RA. Perturbation of chemokine networks by gene deletion alters the reinforcing actions of ethanol. Behav Brain Res. 2005;165:110–25. doi: 10.1016/j.bbr.2005.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Ponomarev I, Geil C, Bergeson S, Koob GF, Harris RA. Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addict Biol. 2012;17:108–20. doi: 10.1111/j.1369-1600.2010.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, et al. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. Embo J. 2003;22:5551–60. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou DK, Zhang J, Smith FI, McCaffery P, Jungalwala FB. Developmental expression of receptor for advanced glycation end products (RAGE), amphoterin and sulfoglucuronyl (HNK-1) carbohydrate in mouse cerebellum and their role in neurite outgrowth and cell migration. J Neurochem. 2004;90:1389–401. doi: 10.1111/j.1471-4159.2004.02609.x. [DOI] [PubMed] [Google Scholar]
- Coleman LG, Jr., He J, Lee J, Styner M, Crews FT. Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice. Alcohol Clin Exp Res. 2011;35:671–88. doi: 10.1111/j.1530-0277.2010.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86:189–99. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F, Nixon K, Kim D, Joseph J, Shukitt-Hale B, Qin L, et al. BHT blocks NF-kappaB activation and ethanol-induced brain damage. Alcohol Clin Exp Res. 2006;30:1938–49. doi: 10.1111/j.1530-0277.2006.00239.x. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K, Wilkie ME. Exercise reverses ethanol inhibition of neural stem cell proliferation. Alcohol. 2004;33:63–71. doi: 10.1016/j.alcohol.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Crews FT, Qin L, Sheedy D, Vetreno RP, Zou J. High mobility group box 1/Toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biol Psychiatry. 2013;73:602–12. doi: 10.1016/j.biopsych.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP. Addiction, adolescence, and innate immune gene induction. Front Psychiatry. 2011;2:19. doi: 10.3389/fpsyt.2011.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Zou J, Qin L. Induction of innate immune genes in brain create the neurobiology of addiction. Brain Behav Immun. 2011;25(Suppl 1):S4–S12. doi: 10.1016/j.bbi.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deas D, Riggs P, Langenbucher J, Goldman M, Brown S. Adolescents are not adults: developmental considerations in alcohol users. Alcohol Clin Exp Res. 2000;24:232–7. [PubMed] [Google Scholar]
- Dedova I, Harding A, Sheedy D, Garrick T, Sundqvist N, Hunt C, et al. The importance of brain banks for molecular neuropathological research: The New South wales tissue resource centre experience. Int J Mol Sci. 2009;10:366–84. doi: 10.3390/ijms10010366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Romeo RD, Andersen SL. Neurobiology of the development of motivated behaviors in adolescence: a window into a neural systems model. Pharmacol Biochem Behav. 2009;93:199–211. doi: 10.1016/j.pbb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Evankovich J, Cho SW, Zhang R, Cardinal J, Dhupar R, Zhang L, et al. High mobility group box 1 release from hepatocytes during ischemia and reperfusion injury is mediated by decreased histone deacetylase activity. J Biol Chem. 2010;285:39888–97. doi: 10.1074/jbc.M110.128348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang P, Schachner M, Shen YQ. HMGB1 in development and diseases of the central nervous system. Mol Neurobiol. 2012;45:499–506. doi: 10.1007/s12035-012-8264-y. [DOI] [PubMed] [Google Scholar]
- Forrest CM, Khalil OS, Pisar M, Smith RA, Darlington LG, Stone TW. Prenatal activation of Toll-like receptors-3 by administration of the viral mimetic poly(I:C) changes synaptic proteins, N-methyl-D-aspartate receptors and neurogenesis markers in offspring. Mol Brain. 2012;5:22. doi: 10.1186/1756-6606-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garate I, Garcia-Bueno B, Madrigal JL, Caso JR, Alou L, Gomez-Lus ML, et al. Stress-induced neuroinflammation: role of the Toll-like receptor-4 pathway. Biol Psychiatry. 2013;73:32–43. doi: 10.1016/j.biopsych.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Grant BF. The impact of a family history of alcoholism on the relationship between age at onset of alcohol use and DSM-IV alcohol dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. Alcohol Health Res World. 1998;22:144–7. [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age of onset of drug use and its association with DSM-IV drug abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1998;10:163–73. doi: 10.1016/s0899-3289(99)80131-x. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Harford TC. Age at onset of alcohol use and DSM-IV alcohol abuse and dependence: a 12-year follow-up. J Subst Abuse. 2001;13:493–504. doi: 10.1016/s0899-3289(01)00096-7. [DOI] [PubMed] [Google Scholar]
- Gruber AJ, Calhoon GG, Shusterman I, Schoenbaum G, Roesch MR, O’Donnell P. More is less: a disinhibited prefrontal cortex impairs cognitive flexibility. J Neurosci. 2010;30:17102–10. doi: 10.1523/JNEUROSCI.4623-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber E, DiClemente RJ, Anderson MM, Lodico M. Early drinking onset and its association with alcohol use and problem behavior in late adolescence. Prev Med. 1996;25:293–300. doi: 10.1006/pmed.1996.0059. [DOI] [PubMed] [Google Scholar]
- Han SH, Kim YH, Mook-Jung I. RAGE: the beneficial and deleterious effects by diverse mechanisms of actions. Mol Cells. 2011;31:91–7. doi: 10.1007/s10059-011-0030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch UK, Johnson TV, Kipnis J. Toll-like receptors: roles in neuroprotection? Trends Neurosci. 2008;31:176–82. doi: 10.1016/j.tins.2008.01.005. [DOI] [PubMed] [Google Scholar]
- He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol. 2008;210:349–58. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson R, Heeren T, Zakocs R. Age of drinking onset and involvement in physical fights after drinking. Pediatrics. 2001;108:872–7. doi: 10.1542/peds.108.4.872. [DOI] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Jamanka A, Howland J. Age of drinking onset and unintentional injury involvement after drinking. Jama. 2000;284:1527–33. doi: 10.1001/jama.284.12.1527. [DOI] [PubMed] [Google Scholar]
- Hohne C, Wenzel M, Angele B, Hammerschmidt S, Hacker H, Klein M, et al. High mobility group box 1 prolongs inflammation and worsens disease in pneumococcal meningitis. Brain. 2013 doi: 10.1093/brain/awt064. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synapse elimination and plasticity in developing human cerebral cortex. Am J Ment Defic. 1984;88:488–96. [PubMed] [Google Scholar]
- Huttunen HJ, Fages C, Rauvala H. Receptor for advanced glycation end products (RAGE)-mediated neurite outgrowth and activation of NF-kappaB require the cytoplasmic domain of the receptor but different downstream signaling pathways. J Biol Chem. 1999;274:19919–24. doi: 10.1074/jbc.274.28.19919. [DOI] [PubMed] [Google Scholar]
- Huttunen HJ, Kuja-Panula J, Sorci G, Agneletti AL, Donato R, Rauvala H. Coregulation of neurite outgrowth and cell survival by amphoterin and S100 proteins through receptor for advanced glycation end products (RAGE) activation. J Biol Chem. 2000;275:40096–105. doi: 10.1074/jbc.M006993200. [DOI] [PubMed] [Google Scholar]
- Iori V, Maroso M, Rizzi M, Iyer AM, Vertemara R, Carli M, et al. Receptor for Advanced Glycation Endproducts is upregulated in temporal lobe epilepsy and contributes to experimental seizures. Neurobiol Dis. 2013 doi: 10.1016/j.nbd.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HB. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol. 1988;269:58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- Lippai D, Bala S, Petrasek J, Csak T, Levin I, Kurt-Jones EA, et al. Alcohol-induced IL-1beta in the brain is mediated by NLRP3/ASC inflammasome activation that amplifies neuroinflammation. J Leukoc Biol. 2013 doi: 10.1189/jlb.1212659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maczurek A, Shanmugam K, Munch G. Inflammation and the redox-sensitive AGE-RAGE pathway as a therapeutic target in Alzheimer’s disease. Ann N Y Acad Sci. 2008;1126:147–51. doi: 10.1196/annals.1433.026. [DOI] [PubMed] [Google Scholar]
- Masten AS, Faden VB, Zucker RA, Spear LP. Underage drinking: a developmental framework. Pediatrics. 2008;121(Suppl 4):S235–51. doi: 10.1542/peds.2007-2243A. [DOI] [PubMed] [Google Scholar]
- Merenmies J, Pihlaskari R, Laitinen J, Wartiovaara J, Rauvala H. 30-kDa heparin-binding protein of brain (amphoterin) involved in neurite outgrowth. Amino acid sequence and localization in the filopodia of the advancing plasma membrane. J Biol Chem. 1991;266:16722–9. [PubMed] [Google Scholar]
- Monti PM, Miranda R, Jr., Nixon K, Sher KJ, Swartzwelder HS, Tapert SF, et al. Adolescence: booze, brains, and behavior. Alcohol Clin Exp Res. 2005;29:207–20. doi: 10.1097/01.alc.0000153551.11000.f3. [DOI] [PubMed] [Google Scholar]
- Okun E, Griffioen KJ, Mattson MP. Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci. 2011;34:269–81. doi: 10.1016/j.tins.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 1998. [DOI] [PubMed]
- Qin L, Crews FT. NADPH oxidase and reactive oxygen species contribute to alcohol-induced microglial activation and neurodegeneration. J Neuroinflammation. 2012;9:5. doi: 10.1186/1742-2094-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflammation. 2008;5:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–62. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribes S, Ebert S, Regen T, Czesnik D, Scheffel J, Zeug A, et al. Fibronectin stimulates Escherichia coli phagocytosis by microglial cells. Glia. 2010;58:367–76. doi: 10.1002/glia.20929. [DOI] [PubMed] [Google Scholar]
- Rolls A, Shechter R, London A, Ziv Y, Ronen A, Levy R, et al. Toll-like receptors modulate adult hippocampal neurogenesis. Nat Cell Biol. 2007;9:1081–8. doi: 10.1038/ncb1629. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA. Changes in the dopaminergic innervation of monkey prefrontal cortex during late postnatal development: a tyrosine hydroxylase immunohistochemical study. Biol Psychiatry. 1994;36:272–7. doi: 10.1016/0006-3223(94)90610-6. [DOI] [PubMed] [Google Scholar]
- Rouhiainen A, Kuja-Panula J, Tumova S, Rauvala H. RAGE-mediated cell signaling. Methods Mol Biol. 2013;963:239–63. doi: 10.1007/978-1-62703-230-8_15. [DOI] [PubMed] [Google Scholar]
- Sathe K, Maetzler W, Lang JD, Mounsey RB, Fleckenstein C, Martin HL, et al. S100B is increased in Parkinson’s disease and ablation protects against MPTP-induced toxicity through the RAGE and TNF-alpha pathway. Brain. 2012;135:3336–47. doi: 10.1093/brain/aws250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheedy D, Garrick T, Dedova I, Hunt C, Miller R, Sundqvist N, et al. An Australian Brain Bank: a critical investment with a high return. Cell Tissue Bank. 2008;9:205–16. doi: 10.1007/s10561-008-9076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22:670–6. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Suchankova P, Klang J, Cavanna C, Holm G, Nilsson S, Jonsson EG, et al. Is the Gly82Ser polymorphism in the RAGE gene relevant to schizophrenia and the personality trait psychoticism? J Psychiatry Neurosci. 2012;37:122–8. doi: 10.1503/jpn.110024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari R, Choudhury SR, Ghosh S, Mehta VS, Sen E. Involvement of TNFalpha-induced TLR4-NF-kappaB and TLR4-HIF-1alpha feed-forward loops in the regulation of inflammatory responses in glioma. Journal of molecular medicine. 2012;90:67–80. doi: 10.1007/s00109-011-0807-6. [DOI] [PubMed] [Google Scholar]
- Trudler D, Farfara D, Frenkel D. Toll-like receptors expression and signaling in glia cells in neuro-amyloidogenic diseases: towards future therapeutic application. Mediators Inflamm. 2010;2010 doi: 10.1155/2010/497987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Crews FT. Adolescent binge drinking increases expression of the danger signal receptor agonist HMGB1 and Toll-like receptors in the adult prefrontal cortex. Neuroscience. 2012;226:475–88. doi: 10.1016/j.neuroscience.2012.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Li S, Jungalwala FB. Receptor for advanced glycation end products (RAGE) mediates neuronal differentiation and neurite outgrowth. J Neurosci Res. 2008;86:1254–66. doi: 10.1002/jnr.21578. [DOI] [PubMed] [Google Scholar]
- Windle M, Spear LP, Fuligni AJ, Angold A, Brown JD, Pine D, et al. Transitions into underage and problem drinking: developmental processes and mechanisms between 10 and 15 years of age. Pediatrics. 2008;121(Suppl 4):S273–89. doi: 10.1542/peds.2007-2243C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Wang H, Czura CJ, Tracey KJ. The cytokine activity of HMGB1. J Leukoc Biol. 2005;78:1–8. doi: 10.1189/jlb.1104648. [DOI] [PubMed] [Google Scholar]
- Zou J, Crews FT. Inflammasome-IL-1beta Signaling Mediates Ethanol Inhibition of Hippocampal Neurogenesis. Front Neurosci. 2012;6:77. doi: 10.3389/fnins.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Vetreno RP, Crews FT. ATP-P2X(7) receptor signaling controls basal and TNFalpha-stimulated glial cell proliferation. Glia. 2012;60:661–73. doi: 10.1002/glia.22302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurolo E, Iyer A, Maroso M, Carbonell C, Anink JJ, Ravizza T, et al. Activation of Toll-like receptor, RAGE and HMGB1 signalling in malformations of cortical development. Brain. 2011;134:1015–32. doi: 10.1093/brain/awr032. [DOI] [PubMed] [Google Scholar]