Abstract
Introduction
Omacetaxine mepesuccinate (omacetaxine) is a first-in-class cephalotaxine that has demonstrated efficacy in CML. In this analysis we evaluated omacetaxine in CML patients with resistance or intolerance to 2 or more tyrosine kinase inhibitors (TKIs).
Patients and Methods
Data were pooled from 2 phase II trials of subcutaneous omacetaxine, administered at 1.25 mg/m2 twice daily for 14 consecutive days every 28 days until response, then for 7 days every 28 days as maintenance. Patients with resistance or intolerance to imatinib and at least 1 other approved TKI (dasatinib and/or nilotinib) were included; results for patients in chronic phase (CP) are reported here. Major cytogenetic response (MCyR) was the primary end point.
Results
Eighty-one patients with CML-CP (median age, 59 years; range, 26–83 years) were included in the analysis. All patients previously received imatinib, 69 (85%) previously received dasatinib, and 48 (59%) previously received nilotinib. Median omacetaxine exposure was 7.5 months (range, 0.03–38.6 months), with 13 patients ongoing. MCyR was reported in 16 patients (20%; one-sided 95% lower confidence limit, 12.8%), including 8 complete responses; median duration was 17.7 months (95% confidence interval, 4.1 months – not reached). Fifty-six patients (69%) achieved and/or maintained hematologic response for at least 8 weeks; median duration was 12.2 months (range, 8.4–26.2 months). Median failure-free and overall survival were 9.6 months and 34 months, respectively. Toxicity was mainly hematologic: the most common grade 3/4 adverse events were thrombocytopenia (67%), neutropenia (47%), and anemia (37%).
Conclusion
Omacetaxine produced clinically meaningful responses with acceptable tolerability in patients with CML-CP previously treated with 2 or more TKIs.
Keywords: Dasatinib, Homoharringtonine, Intolerance, Nilotinib, Resistance
Introduction
Tyrosine kinase inhibitors (TKIs) that target the Bcr-Abl oncoprotein are the current standard of care for patients with chronic myeloid leukemia (CML). TKIs currently approved for first-line treatment include imatinib and the second-generation agents nilotinib and dasatinib. In newly diagnosed CML patients, the complete cytogenetic response (CCyR) rate achieved with these agents ranges from 65% to 82%,1–5 with a progression-free survival (PFS) rate ≥ 94% at 24 months.6,7 Within 5 years, however, at least 25% of patients discontinue imatinib because of unsatisfactory response and/or toxicity.8,9 Discontinuation rates for nilotinib or dasatinib as first-line therapy are 16% to 18% at 12 months4,5 and 23% to 26% at 24 months.6,10
Patients failing first-line TKI therapy with imatinib typically receive dasatinib or nilotinib. The CCyR rate is ≤ 50% in this setting, and the estimated PFS rate at 24 months is 75% to 80%.11–17 In patients who demonstrate resistance or intolerance to second-line TKI therapy, treatment options include allogeneic stem cell transplantation or a third TKI. Resistance to TKI-based therapy might arise as a result of a point mutation in the BCR-ABL gene or BCR-ABL gene amplification, and Bcr-Abl–independent mechanisms of resistance.18–20 Non-TKI therapeutic options that act independently of Bcr-Abl might have the potential to overcome these mechanisms of resistance and offer improved prognosis in patients resistant to multiple TKIs.
Omacetaxine mepesuccinate is a protein synthesis inhibitor that induces apoptosis in leukemic cells by reducing levels of multiple oncoproteins, including Bcr-Abl and MCL-1.21 In vitro studies show that omacetaxine can induce apoptosis of leukemic stem cells,21 which distinguishes it from TKIs and indicates clinical potential in persistent CML. Indeed, omacetaxine has shown promising activity in CML patients with resistance or intolerance to TKI therapy, including patients with TKI resistance driven by a T315I BCR-ABL mutation.8,22–24
Here we evaluate the safety and efficacy of subcutaneous omacetaxine in chronic-phase CML patients with resistance or intolerance to 2 or more approved TKIs via a pooled analysis of data from 2 open-label phase II studies.25,26
Patients and Methods
Study Group
Data from 2 multicenter, international, single-arm, open-label studies (CML-202 and CML-203) were included in this pooled analysis. All patients enrolled in the CML-202 study had resistance or intolerance to imatinib therapy and a history of the T315I BCR-ABL mutation. Patients enrolled in the CML-203 study had resistance or intolerance to imatinib and at least 1 other approved TKI (dasatinib and/or nilotinib). Patients in either study with resistance or intolerance to 2 or more approved TKIs (ie, imatinib followed by dasatinib and/or nilotinib) were eligible for inclusion in this analysis. Only data from patients with chronic-phase CML are reported here. The outcome of patients with the T315I BCR-ABL mutation treated with omacetaxine is part of a separate analysis to be reported elsewhere.
TKI resistance was defined as: (1) lack or loss of complete hematologic response by 12 weeks; (2) lack or loss of any cytogenetic response (CyR) by 24 weeks (ie, 100% Philadelphia chromosome-positive [Ph+] metaphases); (3) lack or loss of major CyR (MCyR) by 52 weeks (> 35% Ph+ metaphases); or (4) progressive leukocytosis. TKI intolerance was defined as: (1) grade 3/4 nonhematologic toxicity that did not resolve with adequate intervention; (2) grade 4 hematologic toxicity lasting more than 7 days; or (3) persistent grade ≥ 2 toxicity that was unacceptable to the patient.
In both studies, eligible patients were ≥ 18 years of age and had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2. Exclusion criteria included New York Heart Association class III or IV heart disease, uncontrolled hypertension, congestive heart failure, or other uncontrolled cardiac condition; another active malignancy (excluding squamous or basal cell skin cancer and in situ cervical cancer); or HIV or human T-lymphotropic virus type I/II positive status. The CML-202 study also excluded patients who were candidates for allogeneic bone marrow or blood stem cell transplantation at the time of enrollment.
Treatment
Identical treatment regimens were used in the CML-202 and -203 studies. Induction therapy consisted of subcutaneous omacetaxine 1.25 mg/m2 twice daily for 14 consecutive days every 28 days, until hematologic response (ie, complete or partial response, or hematologic improvement). Patients who showed no signs of clinical response after 6 cycles of induction therapy were considered for discontinuation from omacetaxine treatment. Patients who achieved a response during induction therapy were switched to maintenance dosing consisting of omacetaxine 1.25 mg/m2 twice daily for 7 consecutive days every 28 days. Maintenance therapy was continued beyond 24 months at the physician’s discretion if the patient was experiencing benefit from taking omacetaxine.
Doses were adjusted for toxicity. For hematologic toxicity, the number of consecutive days of induction and/or maintenance therapy was decreased in 2-day increments to maintain absolute neutrophil count > 0.5 × 109/L and platelet count > 50 × 109/L; no adjustment was made in patients with white blood cell (WBC) count > 10 × 109/L, or absolute blast count > 5 × 109/L. Administration of hematopoietic growth factors was allowed only for treatment of febrile neutropenia; use of erythropoietin or darbepoetin alfa was permitted for treatment of anemia. For non-hematologic toxicity, treatment interruption was permitted for grade ≥ 2 toxicities that were unresponsive to supportive care; when resolved, treatment was resumed with a reduction in the number of consecutive days of treatment. Omacetaxine could be discontinued in patients with moderate to severe adverse events (AEs) considered possibly or probably related to treatment, as judged by the clinical investigator.
End Points and Assessments
An independent data monitoring committee was responsible for adjudicating the response of each patient. The primary efficacy end point was the rate of MCyR, defined as a CCyR (0% Ph+ meta-phases) or partial CyR (1%–35% Ph+ metaphases). Secondary end points included the proportion of patients with a hematologic response that was maintained for ≥ 8 weeks (defined as WBC count ≤ 10 × 109/L, platelets < 450 × 109/L, < 20% basophils in peripheral blood, the absence of blasts or promyelocytes in peripheral blood, < 5% myelocytes plus metamyelocytes in peripheral blood, and the absence of extramedullary involvement), and rates of non-MCyRs (minor: 36%–65% Ph+ metaphases; minimal: 66%–95% Ph+ metaphases). Other secondary end points included time to onset of response, duration of response, failure-free survival (FFS), overall survival (OS; including long-term follow-up for survival in a telephone survey after study discontinuation), and safety.
Complete blood counts were measured at baseline, every 7 days during induction, and every 14 days during maintenance. Conventional bone marrow cytogenetic analyses were performed at baseline and every 3 months. AEs were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. AEs were coded per protocol using the Medical Dictionary for Regulatory Affairs version 10.0, relative to the system organ class and preferred term.
Statistical Analysis
This is a supplemental analysis of pooled patient data from 2 studies. Patients who received any study drug were included in the safety population. All efficacy and safety analyses were conducted in the safety population. Descriptive statistics were used for response and safety end points. One-sided 95% lower confidence limits (95% LCLs) were based on exact (Clopper-Pearson) confidence limits and calculated for response rates where appropriate. Two subgroup analyses were conducted, 1 based on the number of approved TKIs previously received (2 or 3), and 1 based on resistance to vs. intolerance of previous TKIs.
Medians and 95% confidence intervals (CIs) were estimated using Kaplan-Meier analysis for the following variables: duration of response, FFS, and OS. Duration of response was defined as the time from the onset of hematologic or CyR until the date of objective evidence of disease progression, relapse, or death; patients with ongoing response and patients who discontinued treatment for reasons other than AEs, progression, or death were censored at the last examination date. FFS was defined as the time from initiation of treatment until the date of death from any cause, development of accelerated-phase or blast-phase CML, loss of hematologic response or MCyR, or discontinuation because of toxicity or lack of efficacy; patients without progression and patients who discontinued treatment for reasons other than AEs, progression, or death were censored at the last examination date. OS was defined as the time from initiation of treatment until death from any cause at any time; patients alive at the time of analysis were censored at the last recorded contact or evaluation.
Results
Patient Characteristics
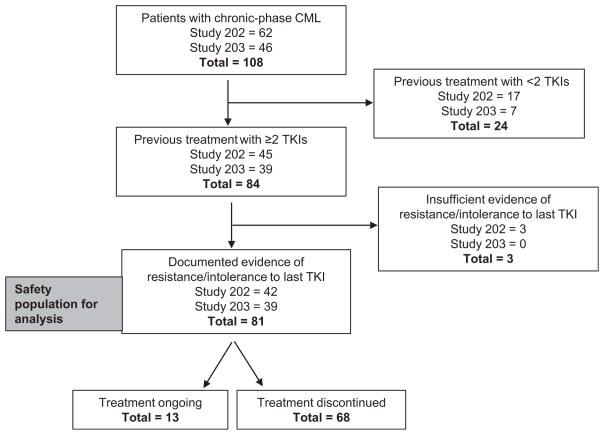
One hundred and eight patients with chronic-phase CML were treated with omacetaxine in the CML-202 (n = 62) and CML-203 (n = 46) studies. Eighty-one patients had received at least 2 approved TKIs and had documented evidence of resistance or intolerance, and were included in this analysis (CML-202, n = 42; CML-203, n = 39; Figure 1). Median age was 59 years (range, 26–83 years); 98% had an ECOG performance status of 0 or 1 (Table 1). Thirty percent of patients had a hematologic response at study entry.
Figure 1. Patient Disposition.
Abbreviations: CML = chronic myeloid leukemia; TKI = tyrosine kinase inhibitor.
Table 1.
Patient Demographic and Baseline Characteristics
| Patients (n = 81) | |
|---|---|
| Male patients, n (%) | 49 (61) |
| Median age, years (range) | 59 (26–83) |
| Age ≥ 65 y, n (%) | 25 (31) |
| Race, n (%) | |
| White | 66 (82) |
| Black | 4 (5) |
| Asian | 3 (4) |
| Hispanic | 3 (4) |
| Other | 5 (6) |
| ECOG performance status, n (%) | |
| 0 | 54 (67) |
| 1 | 25 (31) |
| 2 | 2 (3) |
| NYHA Classification, n (%) | |
| I | 79 (98) |
| II | 2 (3) |
| Hematologic response present at study entry, n (%) | |
| Yes | 24 (30) |
| No | 57 (70) |
| Hydroxyurea use at study entry, n (%) | |
| Yes | 43 (53) |
| No | 38 (47) |
| Previous leukemia treatment, n (%) | |
| Imatinib | 81 (100) |
| Dasatinib | 69 (85) |
| Nilotinib | 48 (59) |
| Investigational drug | 1 (1) |
| Other neoplastic agentsa | 11 (14) |
| Approved TKIs received, n (%) | |
| Imatinib and dasatinib | 33 (41) |
| Imatinib and nilotinib | 12 (15) |
| Imatinib, dasatinib, and nilotinib | 36 (44) |
| Median (range) time from diagnosis to omacetaxine, mo | 75.4 (7.9–234.3) |
| Median (range) time from last TKIs to omacetaxine, mo | 1.3 (0.2–28.0) |
Abbreviations: ECOG = Eastern Cooperative Oncology Group; NYHA = New York Heart Association; TKI = tyrosine kinase inhibitor.
Alkyl sulfonates (busulfan), anthracyclines and related substances, interferon alpha, rituximab, nitrogen mustard analogues, nitrosoureas, other antineoplastic agents (anagrelide, homoharringtonine [omacetaxine mepesuccinate is a semisynthetic formulation of the plant alkaloid homoharringtonine], hydroxycarbamide), etoposide, purine analogs, or pyrimidine analogs.
All patients had previously received imatinib, 69 (85%) had previously received dasatinib, 48 (59%) had previously received nilotinib, and 11 (14%) had previously received other antineoplastic agents (Table 1). Forty-five patients (56%) had received 2 approved TKIs and 36 patients (44%) had received 3 approved TKIs. Sixty-nine patients (85%) demonstrated resistance to at least 2 approved TKIs, 7 patients (9%) demonstrated intolerance to at least 2 approved TKIs, and 5 patients (6%) demonstrated intolerance to 1 approved TKI and resistance to another.
Omacetaxine Exposure
Patients received a median of 6 treatment cycles (range, 1–36 cycles) with a median treatment duration of 7.5 months (range, 0.03–38.6 months). Median number of treatment days per cycle was 14 and 12 days in induction cycles 1 and 2, respectively, and 6 to 7 days in all subsequent cycles.
Thirteen patients were still receiving omacetaxine at the time of data cutoff; these patients had received a median of 17 (range, 8–36) cycles to date and had been receiving therapy for a median of 26.8 months (range, 10.2–46.0 months). Sixty-eight patients discontinued treatment; the most common reason was progressive disease (ie, loss of hematologic response, loss of MCyR, or development of accelerated-phase or blast-phase CML) (Table 2). Seven patients (9%) discontinued treatment because of an AE, including pancytopenia (n = 2), and aplasia, sepsis, gout, tachyarrhythmia, and diplopia (n = 1 each).
Table 2.
Reasons for Omacetaxine Discontinuation
| n (%) | |
|---|---|
| Patients continuing therapy | 13 (16) |
| Patients discontinued therapy | 68 (84) |
| Reason for discontinuation | |
| Progressive disease | 24 (30) |
| Withdrawal by requesta | 11 (14) |
| Lack of efficacy | 10 (12) |
| Adverse event | 7 (9) |
| Deathb | 5 (6) |
| Noncompliance | 2 (3) |
| Lost to follow-up | 1 (1) |
| Otherc | 8 (10) |
Includes withdrawals at the request of the patient, principal investigator, sponsor, or regulatory authority.
Includes deaths occurring up to 30 days after last dose.
Reasons specified were stem cell transplant (n = 5), donor lymphocyte infusion, hematologic resistance, and medical decision.
Efficacy
Hematologic response was achieved and/or maintained for at least 8 weeks in 56 patients (69%; 1-sided 95% LCL, 59.6%); 18 patients (22%) had no hematologic response, and 7 (9%) were unevaluable because of missing data. Among patients without hematologic response at baseline (n = 57), hematologic response was achieved in 37 (65%). Median duration of hematologic response for the entire cohort was 12.2 months (95% CI, 8.4–26.2 months).
Major CyR was reported in 16 patients (20%; 1-sided 95% LCL, 12.8%), including CCyR in 8 patients (10%) (Table 3). In an ad hoc analysis, the arithmetic median time from initiation of treatment to onset of MCyR among patients who achieved this response was 2.6 months (range, 0–6.3 months). MCyR was subsequently lost in 6 patients with a median duration of MCyR of 17.7 months (95% CI, 4.1 months-not reached). Overall, 28 patients (34.6%) had “any” CyR (major, minor, or minimal). The CyR rate was higher in patients who had received 2 previous TKIs than in those who had received 3 previous TKIs (MCyR rate 27% vs. 11%), and was also higher in patients with intolerance to at least 2 TKIs than in those with resistance to at least 2 TKIs (29% vs. 19%; Table 3). A total of 76 patients with chronic-phase CML were included in an ad hoc efficacy analysis that served as the basis for US Food and Drug Administration (FDA) approval (2 patients were excluded because of the presence of MCyR at baseline, and 3 patients for administrative reasons); the MCyR rate in this population was 18% (14/76; 95% CI, 10.5%–29.0%), with a CCyR rate of 8% (6/76). Notably, 1 of the patients excluded from analysis because of baseline MCyR later achieved a CCyR while receiving omacetaxine treatment.
Table 3.
Best Cytogenetic Response to Treatment With Omacetaxine
| n | Major Cytogenetic Response,a n (%) | Other Cytogenetic Response, n (%) | Total Cytogenetic Response, n (%) | ||||
|---|---|---|---|---|---|---|---|
| Complete | Partial | Total MCyR | Minor | Minimal | |||
| All patients | 81b | 8 (10) | 8 (10) | 16 (20)c | 4 (5) | 8 (10) | 28 (35) |
| Response by number of previous TKIs | |||||||
| 2 Previous TKIs | 45 | 6 (13) | 6 (13) | 12 (27) | 2 (4) | 5 (11) | 19 (42) |
| 3 Previous TKIs | 36 | 2 (6) | 2 (6) | 4 (11) | 2 (6) | 3 (8) | 9 (25) |
| Response by previous resistance to or intolerance of TKIs | |||||||
| Resistance to ≥ 2 TKIs | 69 | 7 (10) | 6 (9) | 13 (19) | 3 (4) | 7 (10) | 23 (33) |
| Intolerance of ≥ 2 TKIs | 7 | 0 | 2 (29) | 2 (29) | 1 (14) | 1 (14) | 4 (57) |
| Resistance to 1 TKI and intolerance of 1 TKI | 5 | 1 (20) | 0 | 1 (20) | 0 | 0 | 1 (20) |
Abbreviations: MCyR = major cytogenetic response; TKI = tyrosine kinase inhibitor.
Includes confirmed and unconfirmed responses.
Twenty-one patients (26%) were not evaluable for response.
One-sided 95% lower confidence limit = 12.8%.
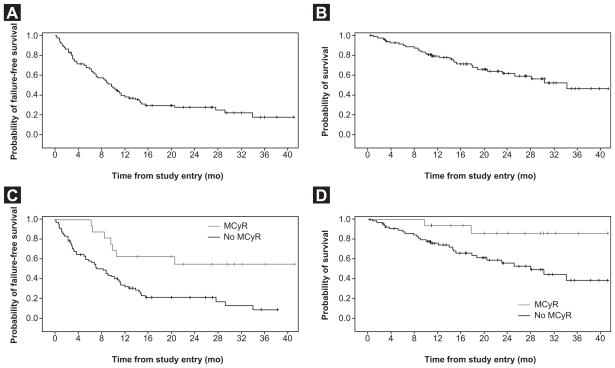
With a median follow-up time of 19.5 months, median FFS and OS were 9.6 months (95% CI, 6.8–11.3 months) and 33.9 months (95% CI, 22.9 months-not reached), respectively (Figure 2A and B). Among patients who achieved MCyR, median FFS and OS were not reached (Figure 2C and D).
Figure 2.
(A) Failure-free and (B) Overerall Survival in All Patients; and (C) Failure-Free and (D) Overall Survival in Patients With or Without Major Cytogenetic Response (MCyR)
Median FFS was longer in patients who had received 2 previous TKIs (10.5 months; 95% CI, 8.8–13.8 months) than in patients who had received 3 previous TKIs (6.5 months; 95% CI, 3.0–14.4 months). Median OS was 30.1 months (95% CI, 18.6 months-not reached) in patients who had received 2 previous TKIs and was not reached (95% CI, 14.4 months-not reached) in patients who had received 3 previous TKIs.
Safety
Adverse events occurring in ≥ 15% of patients are summarized in Table 4. The most common grade ≥ 3 AEs were hematologic, including thrombocytopenia (67%), neutropenia (46%), anemia (38%), and leukopenia (24%); these were typically reversible with each cycle. Incidence of grade 3/4 thrombocytopenia increased from cycle 1 to 2 (33% and 48%, respectively) and declined thereafter (17% in cycle 6 and ≤ 10% in cycles 7 or later). Incidence of grade 3/4 neutropenia was highest in cycle 1 (33%) and then declined (19% in cycle 6 and ≤ 10% in cycles 7 or later). Median time to nadir ranged from 15 to 22 days for platelets and neutrophils in the first 6 cycles. Median time to resolution of grade 3/4 thrombocytopenia and neutropenia to grade ≤ 1 was 31 days and 19 days, respectively; assessment of laboratory values over time indicated that 63% (44/70) of patients with grade 3/4 thrombocytopenia and 62% (42/68) with grade 3/4 neutropenia experienced recurrence in later cycles. Colony-stimulating factors were administered in 21 patients (26%) and erythropoietic stimulating agents in 19 patients (24%); transfusions were required in 59 patients (73%).
Table 4.
Treatment-Emergent Adverse Events Occurring in ≥ 15% of Patients
| Event, n (%) | All Grades | Grade ≥ 3 |
|---|---|---|
| Hematologic | ||
| Thrombocytopenia | 61 (75) | 54 (67) |
| Anemia | 51 (63) | 31 (38) |
| Neutropenia | 39 (48) | 37 (46) |
| Leukopenia | 21 (26) | 19 (24) |
| Pancytopenia | 18 (22) | 16 (20) |
| Nonhematologic | ||
| Infectiona | 37 (46) | 10 (12) |
| Diarrhea | 35 (43) | 1 (1) |
| Nausea | 29 (36) | 1 (1) |
| Fatigue | 23 (28) | 5 (6) |
| Asthenia | 18 (22) | 1 (1) |
| Fever | 17 (21) | 1 (1) |
| Arthralgia | 16 (20) | 1 (1) |
| Headache | 16 (20) | 1 (1) |
| Injection site erythema | 15 (19) | 0 |
| Constipation | 13 (16) | 0 |
| Epistaxis | 12 (15) | 1 (1) |
| Extremity pain | 12 (15) | 1 (1) |
Includes all preferred terms in system organ class “Infections and Infestations.”
Nonhematologic AEs were primarily grades 1 and 2, most commonly infection, diarrhea, nausea, and fatigue (Table 4). Infection (12%), fatigue (6%), and gastrointestinal hemorrhage (4%) were the most common grade ≥ 3 nonhematologic AEs. Treatment-emergent hypertension was observed in 7 patients (9%), but did not exceed grade 2 in severity. Of note, there were no posttreatment corrected QT interval values ≥ 500 milliseconds. Generally, AEs occurred at higher rates in early cycles when patients were receiving higher doses of omacetaxine, and fewer AEs occurred when dosage was reduced to maintenance levels (eg, grade 3/4 AEs were reported in 58%–69% of patients in cycles 1–3, 29%–36% in cycles 4–6, and 0–24% in cycles 7–18).
Among 65 patients who received more than 1 omacetaxine treatment cycle, 56 (86%) had 1 or more treatment delays, with a median of 4 (range, 0–20) treatment delays per patient. In the first 6 cycles of treatment, the median duration of delay was 13 days and the most common reasons for delay were thrombocytopenia (36% of delays), pancytopenia (19%), and neutropenia (15%).
Serious AEs were reported in 43 patients (53%) and included pancytopenia (n = 13), thrombocytopenia (n = 9), febrile neutropenia (n = 6), infection (n = 7), pyrexia (n = 3), neoplasms (n = 3), gastrointestinal hemorrhage (n = 3), anemia (n = 2), febrile bone marrow aplasia (n = 2), and back pain (n = 2). Two patients died within 30 days of receiving the study drug (1 from disease progression and 1 from multiorgan failure considered unrelated to the study drug). An additional 29 patients died during long-term follow-up. Most deaths were a result of disease progression (n = 16) or unknown causes (n = 7). Two patients died in long-term follow-up as a result of treatment-emergent AEs considered related to the study drug. One of these 2 patients discontinued the study after 6 cycles of omacetaxine because of pancytopenia and sepsis; the patient died 71 days after the last dose of study drug. The other patient developed pancytopenia in the first cycle of treatment and treatment was discontinued; the pancytopenia was not resolved and the patient died of sepsis 239 days (7.9 months) after the last dose of study drug.
Discussion
This analysis evaluated the activity of omacetaxine, a protein synthesis inhibitor, in chronic-phase CML patients with documented resistance or intolerance to 2 or more TKIs, using data pooled from 2 phase II trials. Omacetaxine demonstrated clinical activity in this heavily pretreated population, producing or maintaining hematologic response in 69% and MCyR in 20% of patients. Furthermore, responses were durable: median duration of hematologic response and MCyR were 12.2 months and 17.7 months, respectively.
In addition to patients with MCyR, 12 (15%) patients in this analysis experienced a minor CyR during omacetaxine treatment. A recent analysis of 165 consecutive patients treated with a TKI as second-line therapy or beyond after failure of previous imatinib treatment demonstrated that achievement of lesser CyRs (including minor response) was associated with a survival benefit compared with no CyR.27 These results suggest that the benefit of lesser CyRs should be recognized in this setting and evaluated against other treatments available to a given patient before a change in therapy is recommended.
Omacetaxine provides a unique mechanistic approach to the treatment of patients with CML, representing a new class of therapy with the ability to produce a CyR outside of allogeneic stem cell transplantation; to date, the only medications reported to produce a CyR include interferon, TKIs, and now omacetaxine. Other non-TKIs such as hydroxyurea offer little if any likelihood of producing a CyR after TKI failure. Furthermore, there is some evidence that omacetaxine treatment might resensitize the disease to TKIs in some patients, offering the potential for rechallenge with a TKI after omacetaxine therapy.28,29
Overall, the efficacy of omacetaxine observed in this study is comparable with that of second-generation TKIs nilotinib and dasatinib when used in the third-line setting. CCyR rates of 12% to 24% were reported in several small studies of these agents in chronic-phase CML patients in whom previous treatment with imatinib/dasatinib or imatinib/nilotinib had failed30–32; however, these responses tended to be short-lived, particularly among patients with previous resistance to TKIs.30 In the current study, similar response rates were observed, with a CCyR rate of 13% in patients who have received 2 previous TKIs, with encouraging duration of response and OS.
The recent approvals of ponatinib (a multikinase inhibitor) and bosutinib (a v-yes-1 Yamaguchi sarcoma viral related oncogene homolog/Bcr-Abl dual TKI) provide additional TKI options as second- and third-line treatment. Bosutinib and ponatinib have also been investigated in the third-line setting in patients in whom at least 2 previous TKIs had failed. Reports indicate a CCyR rate of 24% with bosutinib (26 of 118 patients) and 63% with ponatinib (27 of 43 patients) in the third-line setting.33–35 Ponatinib has the distinct benefit of having preclinical and clinical activity against T315I BCR-ABL. The current treatment algorithm includes the use of a TKI as initial therapy for nearly all patients. Patients who experience resistance or intolerance to 1 TKI will most frequently receive a second TKI, with most patients experiencing clinical benefit. If a T315I mutation is present, the TKI of choice would be ponatinib. Even with these additional TKI options, it is likely that some patients will not achieve an optimal outcome with TKI therapy, either because of treatment resistance or intolerance. Patients experiencing resistance or intolerance to TKIs have few non-TKI treatment options beyond stem cell transplantation. Omacetaxine provides an additional treatment option for these patients, and could be considered when a patient has received 2 or 3 TKIs without adequate clinical outcome, or for a patient with T315I who is not a good candidate for ponatinib. With the availability of more treatment options, further studies are required to determine how to best sequence treatment with TKIs, how much cross-resistance and cross-intolerance will be observed among TKIs, and at what point a non-TKI approach would be warranted.
Toxicity with omacetaxine is primarily hematologic, with most grade 3/4 AEs related to myelosuppression, including thrombocytopenia, neutropenia, and anemia. Hematologic toxicity was usually reversible with dosing delays and appropriate supportive care. Discontinuation of treatment for hematologic toxicity alone was required in only 2% of patients, although cycle delays were required for most patients. Reductions in the number of days of omacetaxine administration per cycle appeared to alleviate hematologic toxicity as indicated by lower rates of myelosuppression in later cycles, although this might be in part a result of selection of patients who were tolerating therapy well. Grade 3/4 nonhematologic AEs were rare, with the exception of infection in 12% of patients. Cardiovascular toxicity was infrequent and typically mild with this dosing schedule, although 1 patient discontinued treatment because of tachyarrhythmia. The dosing regimen used in this trial was based on previous studies of intravenous omacetaxine; investigation of different dosing schedules, including once-daily dosing, might be worthwhile. Additional studies focused on the development of a more individualized treatment schema, including dose adjustments based on WBC and platelet counts, might improve efficacy and tolerability.
Conclusion
Durable hematologic and CyRs were observed after omacetaxine use in patients previously treated with 2 or more TKIs, with a relatively long median OS. Hematologic toxicity was common, but typically reversible with appropriate management. These results support omacetaxine as a treatment option for chronic-phase CML patients with resistance or intolerance to 2 or more TKIs.
Clinical Practice Points
Omacetaxine provides a unique mechanistic approach to the treatment of patients with CML, representing a new class of therapy with the ability to produce a CyR.
Omacetaxine provides a novel treatment option in patients who are intolerant of TKIs or have TKI-resistant disease.
Hematologic toxicity associated with omacetaxine is most frequent in initial induction cycles and can typically be managed by dose adjustments (ie, a reduction in the number of dosing days per cycle) and supportive care.
Omacetaxine-associated toxicities unrelated to myelosuppression include diarrhea, nausea, and fatigue, and are generally mild to moderate.
The results of this analysis formed the basis for FDA approval of omacetaxine for chronic-phase CML patients with resistance or intolerance to 2 or more TKIs.
Acknowledgments
The authors thank the investigators in the Omacetaxine-202 and -203 Study Groups: Sikander Ailawadhi, Maria Baer, Charles Chuah, Valérie Coiteux, Dan Douer, Robert Emmons, Gabriel Etienne, Thierry Facon, Agnès Guerci, François Guilhot, Andrzej Hellmann, Françoise Huguet-Rigal, Pierre Laneuville, Philipp Le Coutre, Laurence Legros, Armin Leitner, Frédéric Maloisel, David Marin, Tomas Masszi, Purvish Parikh, Delphine Réa, Candido Rivera, Philippe Rousselot, Lydia Roy, Richard Van Etten, Krzysztof Warzocha, and Peter Wiernik; Dan Jones for his critical review of the manuscript; Peter Brown, DPhil, of Teva Pharmaceuticals for his critical review of the data and manuscript; and Glen Davis of Teva Pharmaceuticals for his support with collection and review of additional clinical data.
This study was sponsored by ChemGenex Pharmaceuticals, an indirect wholly owned subsidiary of Teva Pharmaceutical Industries Ltd. Financial support for medical editorial assistance was provided by Teva Pharmaceutical Industries Ltd. Dr. Cortes’ participation in this study was supported in part by MD Anderson Cancer Center Support Grant CA016672 and Award Number P01 CA049639 from the National Cancer Institute.
Footnotes
Disclosure
J. Cortes received research support from Novartis, BMS, Pfizer, Ariad, Deciphera, and ChemGenex, and served as a consultant for Novartis, BMS, Pfizer, Ariad, and Teva; F.E. Nicolini received research support from Novartis, received honoraria from Novartis, Ariad, BMS, and Pfizer, and served as a consultant for Novartis and Ariad; M. Wetzler received research funding, honoraria, and served as a consultant for ChemGenex and Teva; J.H. Lipton served as a consultant for Teva; L. Akard received research funding from ChemGenex, Novartis, and Pfizer, and received honoraria from Novartis, BMS, and Celgene; A. Craig, N. Nanda, and A.-C. Benichou were employed by ChemGenex and consulted for Teva; J. Leonoudakis provided medical writing assistance in the production of the manuscript, funded by Teva; H.J. Khoury received research funding from Novartis, BMS, Pfizer, Ariad, Deciphera, and ChemGenex; A. Hochhaus received research funding from and served as a consultant for ChemGenex, Novartis, BMS, MSD, Ariad, and Pfizer; M. Baccarani served as a consultant for Novartis, BMS, and Pfizer; and H.M. Kantarjian received research support from ChemGenex.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cortes JE, Baccarani M, Guilhot F, et al. Phase III, randomized, open-label study of daily imatinib mesylate 400 mg versus 800 mg in patients with newly diagnosed, previously untreated chronic myeloid leukemia in chronic phase using molecular end points: tyrosine kinase inhibitor optimization and selectivity study. J Clin Oncol. 2010;28:424–30. doi: 10.1200/JCO.2009.25.3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Lavallade H, Apperley JF, Khorashad JS, et al. Imatinib for newly diagnosed patients with chronic myeloid leukemia: incidence of sustained responses in an intention-to-treat analysis. J Clin Oncol. 2008;26:3358–63. doi: 10.1200/JCO.2007.15.8154. [DOI] [PubMed] [Google Scholar]
- 3.Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–17. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362:2260–70. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 5.Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362:2251–9. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian HM, Shah NP, Cortes JE, et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION) Blood. 2012;119:1123–9. doi: 10.1182/blood-2011-08-376087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larson RA, Kim D, Rosti G, et al. Comparison of nilotinib and imatinib in patients (pts) with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP): ENESTnd 24-month follow-up [Abstract] J Clin Oncol. 2011;29:6511. [Google Scholar]
- 8.de Lavallade H, Khorashad JS, Davis HP, et al. Interferon-alpha or homoharringtonine as salvage treatment for chronic myeloid leukemia patients who acquire the T315I BCR-ABL mutation. Blood. 2007;110:2779–80. doi: 10.1182/blood-2007-06-094508. [DOI] [PubMed] [Google Scholar]
- 9.Kantarjian H, O’Brien S, Jabbour E, et al. Impact of treatment end point definitions on perceived differences in long-term outcome with tyrosine kinase inhibitor therapy in chronic myeloid leukemia. J Clin Oncol. 2011;29:3173–8. doi: 10.1200/JCO.2010.33.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kantarjian HM, Hochhaus A, Saglio G, et al. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. Lancet Oncol. 2011;12:841–51. doi: 10.1016/S1470-2045(11)70201-7. [DOI] [PubMed] [Google Scholar]
- 11.Apperley JF. Part II: management of resistance to imatinib in chronic myeloid leukaemia. Lancet Oncol. 2007;8:1116–28. doi: 10.1016/S1470-2045(07)70379-0. [DOI] [PubMed] [Google Scholar]
- 12.Hochhaus A, Baccarani M, Deininger M, et al. Dasatinib induces durable cytogenetic responses in patients with chronic myelogenous leukemia in chronic phase with resistance or intolerance to imatinib. Leukemia. 2008;22:1200–6. doi: 10.1038/leu.2008.84. [DOI] [PubMed] [Google Scholar]
- 13.Hochhaus A, Kantarjian HM, Baccarani M, et al. Dasatinib induces notable hematologic and cytogenetic responses in chronic-phase chronic myeloid leukemia after failure of imatinib therapy. Blood. 2007;109:2303–9. doi: 10.1182/blood-2006-09-047266. [DOI] [PubMed] [Google Scholar]
- 14.Kantarjian H, Giles F, Wunderle L, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med. 2006;354:2542–51. doi: 10.1056/NEJMoa055104. [DOI] [PubMed] [Google Scholar]
- 15.Kantarjian HM, Giles F, Gattermann N, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance. Blood. 2007;110:3540–6. doi: 10.1182/blood-2007-03-080689. [DOI] [PubMed] [Google Scholar]
- 16.Shah NP, Kim DW, Kantarjian H, et al. Potent, transient inhibition of BCR-ABL with dasatinib 100 mg daily achieves rapid and durable cytogenetic responses and high transformation-free survival rates in chronic phase chronic myeloid leukemia patients with resistance, suboptimal response or intolerance to imatinib. Haematologica. 2010;95:232–40. doi: 10.3324/haematol.2009.011452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354:2531–41. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 18.Branford S, Rudzki Z, Walsh S, et al. High frequency of point mutations clustered within the adenosine triphosphate-binding region of BCR/ABL in patients with chronic myeloid leukemia or Ph-positive acute lymphoblastic leukemia who develop imatinib (STI571) resistance. Blood. 2002;99:3472–5. doi: 10.1182/blood.v99.9.3472. [DOI] [PubMed] [Google Scholar]
- 19.Gorre ME, Mohammed M, Ellwood K, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–80. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 20.Shah NP, Nicoll JM, Nagar B, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2:117–25. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- 21.Allan EK, Holyoake TL, Craig AR, et al. Omacetaxine may have a role in chronic myeloid leukaemia eradication through downregulation of Mcl-1 and induction of apoptosis in stem/progenitor cells. Leukemia. 2011;25:985–94. doi: 10.1038/leu.2011.55. [DOI] [PubMed] [Google Scholar]
- 22.Legros L, Hayette S, Nicolini FE, et al. BCR-ABL(T315I) transcript disappearance in an imatinib-resistant CML patient treated with homoharringtonine: a new therapeutic challenge? Leukemia. 2007;21:2204–6. doi: 10.1038/sj.leu.2404772. [DOI] [PubMed] [Google Scholar]
- 23.O’Brien S, Kantarjian H, Keating M, et al. Homoharringtonine therapy induces responses in patients with chronic myelogenous leukemia in late chronic phase. Blood. 1995;86:3322–6. [PubMed] [Google Scholar]
- 24.Quintas-Cardama A, Kantarjian H, Garcia-Manero G, et al. Phase I/II study of subcutaneous homoharringtonine in patients with chronic myeloid leukemia who have failed prior therapy. Cancer. 2007;109:248–55. doi: 10.1002/cncr.22398. [DOI] [PubMed] [Google Scholar]
- 25.Cortes J, Lipton JH, Rea D, et al. Phase 2 study of subcutaneous omacetaxine mepesuccinate after TKI failure in patients with chronic-phase CML with T315I mutation. Blood. 2012;120:2573–80. doi: 10.1182/blood-2012-03-415307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nanda N, Cortes C, Lipton J, et al. Treatment of chronic phase (CP) chronic myeloid leukemia (CML) patients who harbor the BCR-ABL T315I mutation with subcutaneous omacetaxine results in improved survial compared to historical data [Abstract] Haematologica. 2011;96:422–3. [Google Scholar]
- 27.Cortes J, Quintas-Cardama A, Jabbour E, et al. The clinical significance of achieving different levels of cytogenetic response in patients with chronic phase chronic myeloid leukemia after failure to front-line therapy: is complete cytogenetic response the only desirable endpoint? Clin Lymphoma Myeloma Leuk. 2011;11:421–6. doi: 10.1016/j.clml.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicolini FE, Chomel JC, Roy L, et al. The durable clearance of the T315I BCR-ABL mutated clone in chronic phase chronic myelogenous leukemia patients on omacetaxine allows tyrosine kinase inhibitor rechallenge. Clin Lymphoma Myeloma Leuk. 2010;10:394–9. doi: 10.3816/CLML.2010.n.073. [DOI] [PubMed] [Google Scholar]
- 29.Coude MM, Luycx O, Cariou ME, et al. Undetectable molecular residual disease after omacetaxine and nilotinib combination therapy in an imatinib-resistant chronic myeloid leukaemia patient harbouring the BCR-ABL1 T315I gatekeeper mutation. Br J Haematol. 2012;157:407–10. doi: 10.1111/j.1365-2141.2011.09016.x. [DOI] [PubMed] [Google Scholar]
- 30.Garg RJ, Kantarjian H, O’Brien S, et al. The use of nilotinib or dasatinib after failure to 2 prior tyrosine kinase inhibitors: long-term follow-up. Blood. 2009;114:4361–8. doi: 10.1182/blood-2009-05-221531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giles FJ, Abruzzese E, Rosti G, et al. Nilotinib is active in chronic and accelerated phase chronic myeloid leukemia following failure of imatinib and dasatinib therapy. Leukemia. 2010;24:1299–301. doi: 10.1038/leu.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quintas-Cardama A, Kantarjian H, Jones D, et al. Dasatinib (BMS-354825) is active in Philadelphia chromosome-positive chronic myelogenous leukemia after imatinib and nilotinib (AMN107) therapy failure. Blood. 2007;109:497–9. doi: 10.1182/blood-2006-07-035493. [DOI] [PubMed] [Google Scholar]
- 33.Cortes JE, Kantarjian HM, Shah N, et al. Subset analysis of response to treatment of chronic phase CML in a phase 1 study of ponatinib in refractory hematologic malignancies [Abstract] Blood (ASH Annual Meeting Abstracts) 2011;118:602. [Google Scholar]
- 34.Khoury HJ, Cortes JE, Kantarjian HM, et al. Bosutinib is active in chronic phase chronic myeloid leukemia after imatinib and dasatinib and/or nilotinib therapy failure. Blood. 2012;119:3403–12. doi: 10.1182/blood-2011-11-390120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cortes JE, Kantarjian H, Shah NP, et al. Ponatinib in refractory Philadelphia chromosome-positive leukemias. N Engl J Med. 2012;367:2075–88. doi: 10.1056/NEJMoa1205127. [DOI] [PMC free article] [PubMed] [Google Scholar]