Abstract
Background
To determine the effects in adult offspring of maternal exposure to stress and alcohol during pregnancy, we imaged striatal and midbrain dopamine transporter (DAT) binding by positron emission tomograpahy (PET) in rhesus monkeys (Macaca mulatta). We also evaluated the relationship between DAT binding and behavioral responses previously found to relate to dopamine D2 receptor density (responsivity to tactile stimuli, performance on a learning task, and behavior during a learning task).
Methods
Subjects were adult offspring derived from a 2×2 experiment in which pregnant monkeys were randomly assigned to control, daily mild stress exposure (acoustic startle), voluntary consumption of moderate level alcohol, or both daily stress and alcohol. Adult offspring (n = 38) were imaged by PET with the DAT ligand [18F]FECNT.
Results
Results showed that prenatal stress yielded an overall increase of 15% in [18F]FECNT binding in the striatum (p = 0.016), 17% greater binding in the putamen (p = 0.012), and 13% greater binding in the head of the caudate (p = 0.028) relative to animals not exposed to prenatal stress. Striatal [18F]FECNT binding correlated negatively with habituation to repeated tactile stimulation and positively with tactile responsivity. There were no significant effects of prenatal alcohol exposure on [18F]FECNT binding.
Conclusions
Maternal exposure to mild daily stress during pregnancy yielded increases in striatal DAT availability that were apparent in adult offspring, and were associated with behavioral characteristics reflecting tactile hyperresponsivity, a condition associated with problem behaviors in children.
Keywords: Prenatal stress, Primate, Brain, Dopamine transporter, Striatum, Tactile responsivity
INTRODUCTION
Chronic psychological stress during pregnancy is associated with a range of adverse developmental outcomes, including low birth weight and shorter gestation duration, reduced neonatal attention and habituation, impaired motor function and executive function, and increased risk for ADHD, autism, schizophrenia, depression, and anxiety (1–4). Nonhuman primates are excellent models for studying prenatal stress with experimental control because of the similarity to humans in complex cognitive and social behaviors as well as in brain structures and biological processes of stress reactions (5).
In this study, pregnant rhesus monkeys were experimentally exposed to a daily mild stressor, moderate level alcohol, or both stress and alcohol in order to measure the effects in offspring on brain and behavior. The adult offspring were studied with non-invasive in vivo molecular imaging by positron emission tomography (PET) to determine if prenatal stress and/or alcohol exposure altered dopamine transporter (DAT) availability. Because of the importance of the dopamine (DA) system in regulating mood, affect, motivation and reward responses, as well as the initiation and control of motor behaviors, this information could assist in developing treatments for individuals from prenatally stressed pregnancies (6; 7). To measure DAT availability, we used [18F]FECNT, which has 25 and 156 fold greater affinity for human DAT than for the serotonin and norepinehrine transporters respectively (8). Test-retest reproducibility of the [18F]FECNT PET target to cerebellum specific uptake ratio is 2.6%, and this PET measure correlates very well with postmortem rhesus striatal DAT immunoreactivity intensity measurements (R2 = 0.83) (9).
We also examined whether DAT availability would be related to behavioral characteristics in these monkeys. More specifically, we assessed behavioral responses to repeated tactile stimuli to determine the relationship between dopamine function and responsivity to repeated non-noxious tactile stimuli. Sensory processing disorders, characterized by under- or over-responsivity to non-noxious sensory stimuli, occur in approximately 5% of the general population (10). Sensory processing disorders pose unique challenges for people with developmental disabilities (11). The core deficits in sensory processing disorders are regarded as difficulty suppressing irrelevant sensory input, coupled with inappropriately high responsiveness to those stimuli. The basal ganglia and thalamocortical circuits are considered potential contributors to developmental disorders that are characterized by reduced inhibitory control and difficulty filtering information appropriately (12). Our previous studies showed that PET measures of striatal dopamine functioning (ratio of dopamine D2 receptor (D2R) availability to DA synthesis) were negatively correlated with behavioral inhibition or suppression of irrelevant action during cognitive testing (13). In addition, we found that binding of the D2R ligand [18F]fallypride in striatum was negatively related to habituation to repeated tactile stimulation and positively related to the magnitude of tactile responsivity (14).
DAT is a membrane-bound presynaptic protein that rapidly clears DA that has been released into the extracellular space and thereby limits the amplitude and duration of DA signaling. DAT’s role in limiting synaptic dopamine is complex, and there is evidence that D2 receptors stimulate expression of DAT on the surfaces of cells (15). In addition, activation of D2 receptors stimulates DAT function and dopamine clearance, whereas D2 antagonists block dopamine uptake (see (16) for a review).
Experimental animal studies have shown that both the DA system and DA-regulated behaviors are especially vulnerable to prenatal environmental influences (17). We found only one published study examining the effect of prenatal stress on DAT; it showed reduced DAT expression in the midbrain and striatum of prenatally stressed mice (18). Previously we found that monkeys exposed to prenatal stress had higher striatal D2R availability compared to non-stressed animals (13). Therefore, we expected that striatal DAT density would also be increased by prenatal stress.
The subjects were the offspring of female rhesus macaques from an experiment that independently manipulated exposure to daily prenatal stress and moderate dose prenatal alcohol. The animals in the four treatment groups (daily prenatal stress, moderate dose prenatal alcohol exposure, combination of prenatal stress and alcohol exposure, and control) have been studied longitudinally since birth (5). Animals in all four conditions have been subjected to identical experimental protocols. We tested the hypothesis that prenatal stress and/or prenatal alcohol, would yield increases in DAT availability in adulthood compared with offspring not exposed to prenatal stress or alcohol. PET was used with the ligand [18F]FECNT to measure DAT availability in the midbrain (substantia nigra and ventral tegmental area) and striatum (putamen, caudate nucleus, and nucleus accumbens), brain regions rich in DA cell bodies and DAergic innervation respectively. We also examined the relationships of ligand binding to behaviors that in our previous work had shown effects of dopamine system alterations (13; 14).
METHODS & MATERIALS
Subjects
This experiment was approved by the University of Wisconsin-Madison Animal Care and Use Committee. The subjects were 38 adult rhesus monkeys (Macaca mulatta, 21F:17M, 13.8 ± 1.0 years, 12.0 – 14.7 years), members of a longitudinal experiment investigating moderate level prenatal alcohol exposure and prenatal stress. Healthy female monkeys from the breeding colony were screened for willingness to consistently and voluntarily consume 0.6g/kg, 6% v/v alcohol solution sweetened with aspartame (300 mg/100 ml, NutraSweet, Chicago) daily for 2 weeks; 68% of the colony females tested fell into this category. This dosage, which is at the low end of the range used in primate and rodent teratogenic studies (19), is comparable to an average-sized woman consuming approximately two alcoholic drinks, and yielded blood concentrations (BACs) of 20–50 mg/dl 60 minutes after consumption (blood samples were obtained prior to pregnancy to avoid stress given that prenatal stress was a variable of interest). Alcohol-consuming females were randomly assigned to one of four groups in a 2 (Prenatal Stress) × 2 (Prenatal Alcohol) factorial design.
The pregnant females in the prenatal alcohol-only and alcohol + stress groups voluntarily consumed 0.6 g/kg in a 6% alcohol solution sweetened with aspartame (300 mg/100 ml) daily throughout gestation at 1600h. The alcohol treatment began 5 days before breeding and ended at parturition. All animals were fed Purina Monkey Chow (St. Louis) daily at 0600h and were given a fresh fruit supplement on Monday, Wednesday, and Friday at 1300h. There was normally no Chow left when the alcohol solution was introduced. Water was available ad libitum, including when alcohol was available. The control and prenatal stress-only mothers consumed a sucrose solution approximately equivolemic and equicaloric (8g/100 ml water) to the alcohol solution.
The pregnant females in the prenatal stress and prenatal stress + alcohol groups were exposed to stress 5 times per week at approximately 1530h during gestational days 90 through 145. The stress treatment involved removal from the home cage to a darkened room where three noise bursts (1300 Hz, 115 dB at 1 m) were randomly administered over a 10-minute period (5). With this treatment, plasma cortisol levels have been shown to increase from 25.2 ± 2.2 μg/dl at baseline to 34.8 ± 2.4 μg/dl 30 minutes post-stress treatment (mean ± SEM) (20).
The offspring in this study consisted of 12 controls (9F:3M), 8 prenatal-stressed (2F:6M), 10 prenatal alcohol-exposed (7F:3M), and 8 prenatal alcohol + stress-exposed monkeys (3F:5M). The rearing conditions and previous testing of animals in all conditions are described in detail elsewhere (21). Briefly, all infants were housed with their mothers in individual cages during the first 6 months except for four brief separations during the first month for neurobehavioral testing (21). At 6 months, they were separated from their mothers for weaning and then reared in mixed-sex peer groups consisting of 5–6 monkeys from similar prenatal conditions (22). At the time of the present study, the animals were pair-housed with same-sex peers from similar treatment groups. All housing conditions were controlled (lights on: 0600 – 2000h; 21 ± 0.5 °C).
Behavioral measures
We found earlier that binding of the D2 receptor ligand [18F]fallypride in the striatum was significantly related to responsivity to repeated tactile stimulation and to behavior during a non-match-to-sample learning task (13). Therefore, in the present study we examined the correlations between [18F]FECNT binding in striatum and these two behavioral measures. The behavioral procedures are described in detail elsewhere (14; 23). Briefly, at age 32 to 34 months the animals described in the present study were trained to criterion of 90% correct on the non-match-to-sample task, and behaviors during the learning task were rated. At age 5 to 7 years the animals were tested for responsivity to repeated tactile stimulation (14). This scale was developed by adapting procedures from sensory processing assesments for children (24; 25). Responsivity on each of the 18 trials (6 trials each of 3 diferent textures) was rated by an observor. The ‘sensory magnitude’ score reported below was the mean responsivity over all 18 trials. The ‘habituation’ score was the average of the linear trend for responsivity over the 6 repeated trials for two of the textures (cotton and feather). Magnitude and habituation are moderately negatively correlated (r = −0.314) but capture different aspects of sensory responsivity.
Radiotracer
The cocaine analog [18F]2β-carbomethoxy-3β-(4-chlorophenyl)-8-(2-fluoroethyl)-nortropane ([18F]FECNT) was prepared by the reaction of the precursor 3-β-(4-chlorophenyl)nortropane-2-β-carboxylic acid methyl ester (ABX, Radeberg, Germany) with [18F]1-bromo-2-fluoroethane (26).
PET scanning protocol
Subjects were anesthetized with ketamine (10 mg/kg im, 50 ± 11 minutes prior to radiotracer injection), transported to the scanner, intubated, and maintained on isoflurane (1.5 ± 0.1 % in oxygen) throughout scanning. Subjects were positioned prone in a microPET P4 scanner (27) (Siemens, Knoxville) with their heads face down and fixed with ear bars, a tooth bar, and a pad pressing on the top of the head such that transaxial scanner planes corresponded to axial brain slices. A 57Co transmission scan was acquired (120–125 keV energy window), and a 150 minute emission scan was begun (350–650 keV, 6 ns coincidence window, 3D list mode). 60 seconds after scan start, [18F]FECNT was injected intravenously (activity: 188 ± 11 MBq, specific activity: 37 ± 25 MBq/nmol, injected mass per body weight: 0.91 ± 0.71 pmol/g).
Image reconstruction
Images were reconstruced using the scanner vendor’s software (Siemens, microPET Manager 2.4.1.1). Emission list data were binned into 3D sinograms at 5×1 + 5×2 + 3×5 + 12×10 minutes (4B integers, 168 projection angles × 192 bins, span 3, ring difference 31, hist.exe 2.338). To generate smooth accurate μ maps for attenuation and scatter corrections, 57Co transmission list data were binned into 3D sinograms, which were then reconstructed by filtered back projection (FBP, full transaxial FOV). The resulting transmission images were smoothed (4 mm FWHM Gaussian), calibrated so brain tissue had an average value of μ = 0.095 cm−1, segmented so pixels of μ > 0.095/2 cm−1 were assigned μ = 0.095 cm−1 and others zero, and forward projected into 3D sinograms (ASIPro 6.7.1.2). Emission images were reconstructed with FBP (fourier 2D rebinning, pixel size 0.47 mm × 0.47 mm in-plane × 1.21mm slice thickness, ramp filter, recon.exe 2.330). The resulting images included corrections for detector sensitivity, deadtime, decay, attenuation, and scatter (scatter_alpha.exe).
Image processing
Images were aligned to a common space as follows. Regions of interest (ROIs) from a digitized version of a rhesus brain atlas (28) were aligned to a rhesus MRI template (29). To better visualize whole brain, static 0- to 14-min post-injection [18F]FECNT images were created. The 0–14 min image of one control subject was manually aligned to the MRI template by 6 degrees of freedom (df). The remaining 11 control images were aligned to this image by 6 df using a coregistration algorithm with a correlation ratio cost function (FSL FLIRT, version 5; Oxford Centre for Functional MRI of the Brain). Each of these 12 control images was divided by its mean value in whole brain, and the resulting 12 whole brain normalized images were averaged. Values in this image were determined for caudate, putamen, nucleus accumbens, substantia nigra and ventral tegmental area, and remainder of brain, and these values were assigned to the ROIs in the digitized atlas, which was then smoothed (2.5 mm FWHM Gaussian) to approximate the scanner resolution, thus creating a synthetic target. The average control image was aligned to the synthetic target by 9 df. The control images were aligned to this image first by 6 and then by 9 df, and they were averaged creating a 0–14 minute control template. Finally, the 0–14 minute image for each of the 38 subjects in the study was whole brain normalized and aligned to the control template by first 6 and then 9 df using a normalized correlation ratio cost function. Images were inspected and in 2 cases further adjusted manually with 6 df.
For each subject, the resulting transformation matrices were multiplied and the product was applied to the corresponding 0–150 minute dynamic image. To check for head motion, the aligned dynamic images were viewed in cine mode and TACs were inspected. The aligned dynamic images were time averaged and inspected for agreement with the ROIs. The time averaged images were whole brain normalized, averaged within group, and inspected for systematic differences in alignment or anatomy associated with maternal treatment.
Pharmacokinetic modeling
[18F]FECNT binding was determined using the Logan graphical reference tissue method (30). The following atlas-based ROIs were used: striatum, with sub-ROIs of nucleus accumbens, putamen, caudate, head of caudate, body of caudate, and tail of caudate. A radioactivity-based ROI was delineated in midbrain to encompass substantia nigra and ventral tegmental area (SN/VTA). The reference region was a cerebellar grey matter area drawn to exclude vermis and avoid spillover from adjoining regions. Time-activity curves (TACs) were determined for each of the ROIs. The target to reference ratio rose continuously and plateaued at approximately 90 minutes (Supplement: Figure S3) in agreement with results reported elsewhere (9). Therefore, target-to-reference distribution volume ratios (DVR) were calculated as the slope of ∫target/target vs ∫reference/target for the period 90–150 min using in-house software (dyanakc.py v20100406). Under the assumption that the reference region was devoid of specific binding, the binding potential of specifically bound [18F]FECNT with respect to nondisplaceable tracer in tissue was reported as BPND = DVR − 1 (31).
Statistical analysis
Prenatal treatment effects on [18F]FECNT binding were evaluated for the midbrain and striatum ROIs in a 2 (Stress) × 2 (Alcohol) factorial analysis of variance (ANOVA). Effects meeting p < 0.05 were considered significant. We used a hierarchical strategy, in which the sub-ROIs of the striatum were tested contingent on significant treatment effects on the striatum. Pearson correlations were calculated between the behavioral variables that were previously found to be significantly related to [18F]fallypride binding in striatum.
RESULTS
Observed [18F]FECNT binding
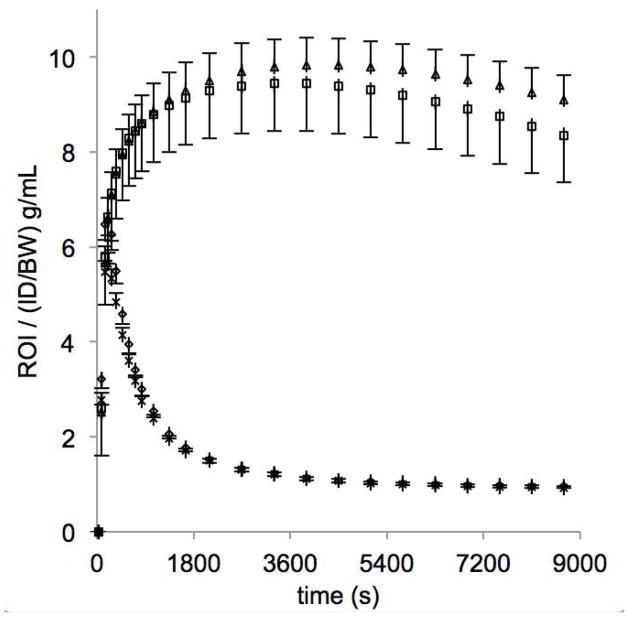
For all four prenatal treatment groups, high radioactivity was observed in regions expected to be rich in DAT, especially striatum and midbrain (Fig. 1). Mean time-activity curves are shown in Fig. 2 for prenatal stress and no prenatal stress, which indicate similar behavior in the cerebellar reference region and differences in the striatum. Logan plots for a typical animal are shown in Fig. 3. Mean [18F]FECNT binding potentials in striatal and midbrain regions are shown for each of the groups in Table 1 along with radiotracer parameters. Control subject means are consistent with [18F]FECNT binding measures in rhesus macaques reported by others (9). Detailed imaging results are shown in Supplement: Figures S1 and S2.
Figure 1. [18F]FECNT image.
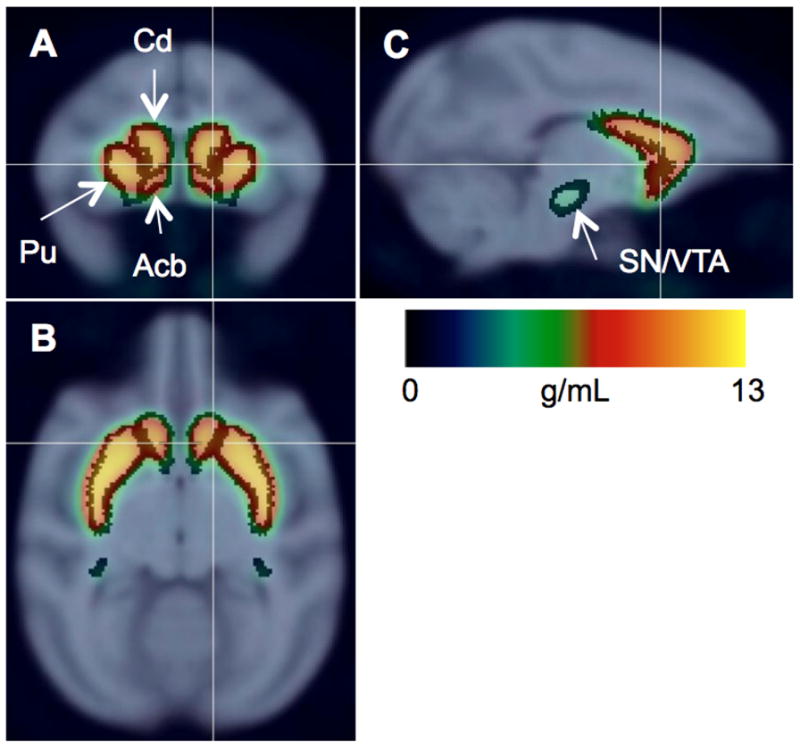
Radioactivity images of control subjects (n = 12, 0–150 min) scaled to injected dose/body weight, averaged, and overlaid on MRI template with regions of interest shown for nucleus accumbens (Acb), caudate (Cd), putamen (Pu), and substantia nigra/ventral tegmental area (SN/VTA). Coronal (a), axial (b), and sagittal (c) slices shown at right striatum.
Figure 2. Time-Activity curves.
Mean radioactivity normalized to injected dose per body weight for monkeys not exposed to prenatal stress (n = 22) and monkeys exposed to prenatal stress (n = 16). Curves are shown for the cerebellar reference region (no stress: ◇, stress: X) and striatum (no stress: □, stress: △). Error bars indicate SEM.
Figure 3. Logan plots for a control subject.
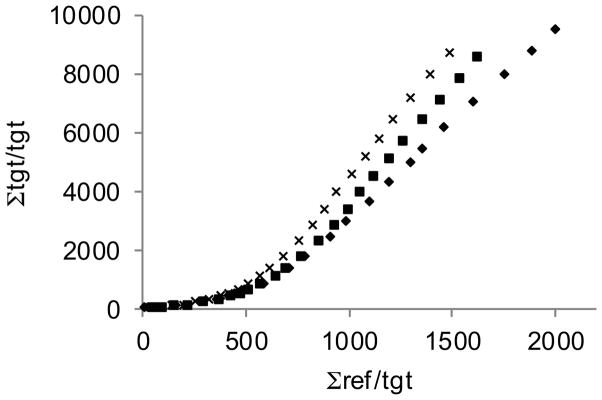
[18F]FECNT binding is determined using the slope of the straight line fit to the last 6 data points, corresponding to 90–150 minutes post-injection of tracer. (putamen: x, head of caudate: ■, nucleus accumbens: ◆)
Table 1.
In utero treatment and PET measures of [18F]FECNT binding in striatal and midbrain regions.
| [18F]FECNT BPND |
In Utero Maternal Treatment, Mean (sd)
|
Marginal Means (se) | Main Effect of Stress
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Alcohol | Stress | Alcohol + Stress | Control & Alcohol | Stress & Alcohol+Stress | difference | F | p | |
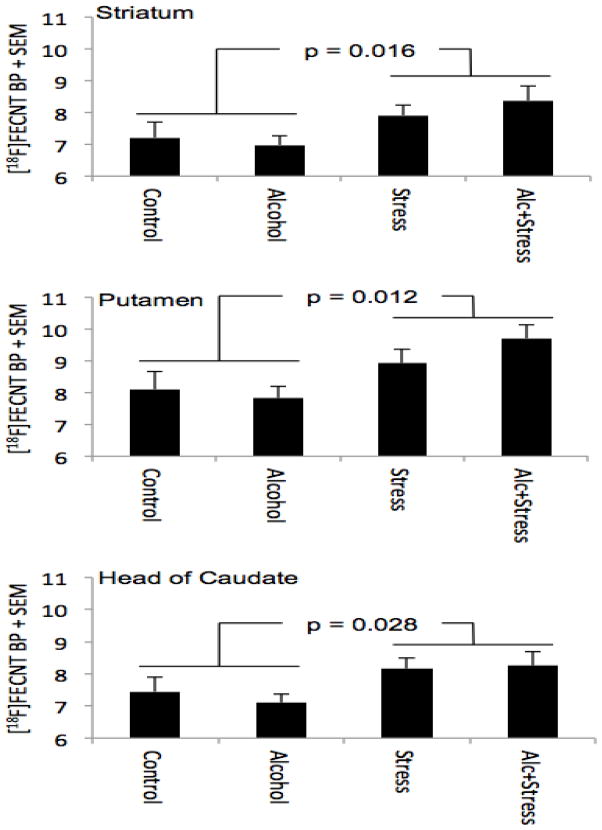
| Striatum | 7.22(1.64) | 6.98(0.86) | 7.91(0.87) | 8.38(1.33) | 7.10(0.27) | 8.15(0.31) | +15% | 6.43 | 0.016* |
| Putamen | 8.10(1.96) | 7.84(1.11) | 8.93(1.22) | 9.71(1.59) | 7.97(0.33) | 9.32(0.39) | +17% | 7.01 | 0.012* |
| Caudate | 6.40(1.40) | 6.16(0.77) | 6.98(0.77) | 7.10(1.13) | 6.28(0.23) | 7.04(0.27) | +12% | 4.49 | 0.041* |
| Head | 7.44(1.61) | 7.11(0.84) | 8.16(0.98) | 8.26(1.25) | 7.28(0.26) | 8.21(0.31) | +13% | 5.23 | 0.028* |
| Body | 4.54(0.93) | 4.59(1.07) | 4.93(1.26) | 5.06(1.39) | 4.56(0.24) | 5.00(0.29) | +10% | 1.33 | 0.257 |
| Tail | 3.14(0.93) | 3.11(0.39) | 3.18(0.41) | 3.44(0.71) | 3.12(0.14) | 3.31(0.17) | +6% | 0.71 | 0.406 |
| Acb | 5.14(0.92) | 5.31(0.73) | 5.59(0.72) | 5.80(0.84) | 5.23(0.17) | 5.69(0.20) | +9% | 2.99 | 0.093 |
| SN/VTA | 1.20(0.20) | 1.25(0.11) | 1.24(0.10) | 1.29(0.20) | 1.22(0.03) | 1.26(0.04) | +3% | 0.51 | 0.480 |
| Subject Characteristics | |||||||||
| F:M | 9:3 | 7:3 | 2:6 | 3:5 | |||||
| Age (yr) | 14.1(0.6) | 13.9(0.8) | 13.7(1.2) | 13.1(1.2) | 14.00(0.20) | 13.39(0.24) | −4% | 3.72 | 0.062 |
| Weight (kg) | 9.7(1.9) | 8.7(2.1) | 10.1(1.0) | 9.9(1.5) | 9.23(0.37) | 10.01(0.44) | +9% | 1.87 | 0.180 |
| Radiotracer Parameters | |||||||||
| ID (MBq) | 189(13) | 188(7) | 185(15) | 189(9) | 188.3(2.4) | 186.8(2.9) | −1% | 0.15 | 0.700 |
| ID/BW (kBq/g) | 20.1(3.8) | 22.7(6.5) | 18.5(3.2) | 19.6(3.5) | 21.4(1.0) | 19.0(1.1) | −11% | 2.54 | 0.121 |
| SA (MBq/nmol) | 29(24) | 34(19) | 28(18) | 54(25) | 31.3(4.6) | 41.0(5.4) | +31% | 1.82 | 0.186 |
| IM/BW (pmol/g) | 1.23(1.03) | 0.92(0.54) | 0.89(0.48) | 0.53(0.46) | 1.07(0.15) | 0.71(0.18) | −34% | 2.37 | 0.133 |
| Aref (g/mL) | 0.94(0.20) | 1.04(0.20) | 0.97(0.14) | 0.96(0.14) | 0.99(0.04) | 0.96(0.04) | −3% | 0.22 | 0.642 |
| Mref (pmol/mL) | 1.14(1.00) | 0.98(0.67) | 0.85(0.42) | 0.47(0.33) | 1.06(0.15) | 0.66(0.18) | −38% | 2.94 | 0.095 |
Acb = nucleus accumbens, SN/VTA = substantia nigra/ventral tegmental area; ID = injected dose of [18F]FECNT; ID/BW = injected dose per body weight; SA = specific activity of radiotracer at injection; IM/BW = injected mass per body weight; Aref = reference region radioactivity/(injected dose/body weight) 90–150 min after injection; Mref = reference region radioactivity/SA = reference region tracer mass concentration 90–150 min after injection; main effect of stress: difference = (stress/no stress) − 1, 2 (Stress) × 2 (Alcohol) ANOVA, F(1, 34), p: 2-tailed,
p<0.05.
Effects of prenatal treatment
Analysis of [18F]FECNT binding revealed a main effect of prenatal stress (Table 1). Prenatal stress resulted in an overall increase of 15% in DAT availability in the striatum compared to no prenatal stress (p = 0.016). Testing the sub-ROIs of the striatum showed 17% greater [18F]FECNT binding in putamen (p = 0.012) and 13% greater binding in head of caudate (p = 0.028) (Fig. 4). There were no significant effects of prenatal alcohol exposure (smallest p = 0.62), nor were there significant stress x alcohol interactions (smallest p = 0.31).
Figure 4. Maternal stress during pregnancy increases DAT availability in striatal regions of adult offspring.
[18F]FECNT binding potentials (BP, supressed zero) in striatum, putamen, and head of caudate are plotted for the four in utero treatment groups. P values indicate significance of main effect of stress from 2 (Stress) × 2 (Alcohol) analysis of variance.
Relation of FECNT binding to behavioral measures
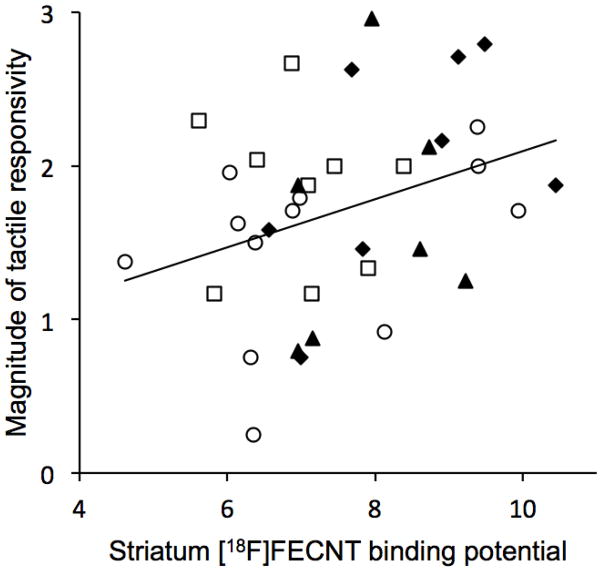
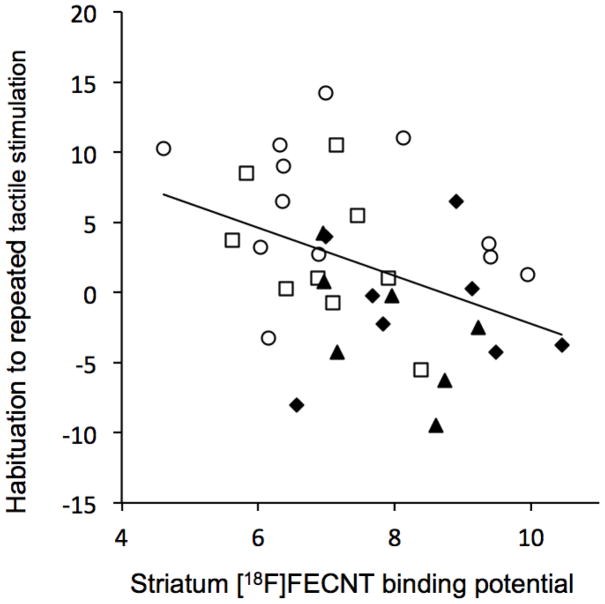
The correlations between [18F]FECNT binding in striatum and the non-match-to-sample learning task and the tactile sensitivity measures are given in Table 2. The relationship between [18F]FECNT binding in striatum and overall tactile responsivity (magnitude) was positive and significant, and the relationship between binding and habituation to repeated tactile stimulation was negative and significant (see Figures 5 and 6). These regressions did not differ significantly between stress and non-stress animals (ps = 0.10 and 0.40, for magnitude and habituation, respectively). The relationships of [18F]FECNT binding with trials-to-criterion in the non-match-to-sample task and ratings of inhibition in the task were not significant.
Table 2.
Correlations between DAT binding in striatum and behavioral measures previously found to be significantly related to D2R binding in striatum.
| r | p | N | |
|---|---|---|---|
| Sensory magnitude | 0.332 | 0.045 | 35 |
| Gentle habituation | −0.404 | 0.013 | 35 |
| NMS trials to criterion | −0.15 | 0.383 | 35 |
| NMS inhibition | −0.087 | 0.615 | 35 |
NMS = non-match-to-sample; Pearson r, 2-tailed p,
p < 0.05.
Figure 5.
Magnitude of tactitle responsivity is positively related to DAT availability in striatum as indexed by [18F]FECNT binding. (r = 0.332, p = 0.045). ○ = control, □ = alcohol, ▲ = stress, ◆ = alcohol + stress.
Figure 6.
Habitutation to repeated tactile stimulation is negatively related to DAT availability in striatum as indexed by [18F]FECNT binding. (r = −0.404, p = 0.013). ○ = control, □ = alcohol, ▲ = stress, ◆ = alcohol + stress.
Sensitivity analysis of potential sex effects
Because mothers were randomly assigned to treatment groups, the numbers of offspring in the treatment groups were not balanced with respect to sex. To gauge sensitivity to potential sex effects, sex was included in the model (2×2×2 ANOVA, Supplement: Table S1). This reduced the significance of the main effect of stress, which still tended to show increased DAT availability of approximately the same magnitude in striatum (12%, p = 0.056), putamen (15% p = 0.037), and head of caudate (10% p = 0.102). Other findings, however, support the interpretation of the 2×2 ANOVA results (Table 1) to be a consequence of prenatal treatment rather than an artifact of the uneven distribution of sex acrosss conditions: the data presented here reveal neither a significant main effect of sex nor sex x stress interaction, rat studies show lower striatal DAT availability in males (32), and furthermore, human studies show either no gender effect (32; 33) or lower striatal DAT availability in males (34–38).
DISCUSSION
This is the first study, to our knowledge, demonstrating that experimentally-controlled exposure to prenatal stress produces alterations of DAT that are evident in adulthood in primates. In human correlational research, many factors differ across life that may be correlated with prenatal experiences such as maternal stress. In contrast, for the animals in the present experiment, although many intervening events have occurred, the animals in all four conditions were exposed to identical research protocols since birth. In the present study, prenatally-stressed adult rhesus monkeys showed significantly increased striatal DAT binding compared with non-prenatally-stressed monkeys even as mature adults. Moreover, the inference of DAT availability in striatum was made using a radioligand that is highly selective for DAT ([18F]FECNT) in conjunction with high resolution PET (1.8 mm full width at half maximum).
In the mammalian brain, DAT binding sites are most dense in the striatum and midbrain (39). The significant differences in striatal DAT availability in the prenatal stress conditions of the present experiment were focused in the putamen and head of the caudate. These findings are consistent with evidence linking prenatal stress to impaired cognitive and motor function in infants and children (40). Human lesion studies link the caudate to regulation of complex cognitive functioning and the putamen to motor functions (41). Interestingly, we previously reported that the prenatally-stressed monkeys in this experiment showed reduced neonatal attention and motor maturity and reduced executive function during adolescence compared with non-stressed monkeys (5). Our findings support a body of literature suggesting that fronto-striatal dopamine dysfunction may contribute to the cognitive and behavioral impairments reported in children from prenatally stressed pregnancies. For instance, ADHD has been associated with prenatal stress in children (1). Although results are mixed, ADHD patients have exhibited alterations in striatal DAT binding of similar magnitude to that reported here in a primate model (42; 43). Moreover, ADHD is commonly treated with methylphenidate, which blocks DAT and thereby raises synaptic levels of DA (7).
Prenatal stress exposure has been shown to affect the dopamine system in rodents. In rats, prenatal stress has been reported to alter dopaminergic indices including DA cell number, DA turnover, and D2 receptor (D2R) density (44–48). However, only one previous study has assessed DAT in prenatally-stressed animals, finding a reduction in DAT in midbrain and striatum of mice (18). This inconsistency with our results could be due to a number of factors such as the nature and timing of the prenatal stressor, time of DAT assessment, and species differences in DAT. Indeed, while the genetic sequence of DAT is highly conserved across species, human DAT is 92% identical to the rat and 98% identical to the monkey (49; 50).
Earlier work on the monkeys examined in this study showed that prenatal stress increased the ratio between D2R availability (indexed by [18F]fallypride binding) and presynaptic DA synthesis (indexed by 6-[18F]fluoro-L-m-tyrosine uptake) compared to non-stressed subjects, primarily as a consequence of 26% greater striatal D2R availability (13). The relations between DAT, extracellular DA, and D2R are complex. Increased striatal DAT, as found in the present study, is likely accompanied by increased DA clearance, which then will result in reduced extracellular DA levels. Reduced extracellular DA may then cause upregulation of D2R. But the causal direction is unclear -- upregulated D2R binding, in principle, may cause DAT upregulation because D2R activation can also activate DAT (16).
Given the importance of the striatum in sensory and motor integration (51) and our previous finding that tactile responsivity was related to D2R density, it is reasonable to expect lower habituation and higher responsivity to repeated tactile stimulation to be related to DAT binding. The striatum, part of the basal ganglia, mediates a range of functions, which includes inhibitory control (52), learning (53), and attention (54). We found that lower habituation and higher magnitude of responsivity to repeated tactile stimuli were associated with higher striatal DAT binding. Thus, altered functioning of the DAergic neuromodulatory circuits may underlie the phenotypic expression of sensory defensiveness, a common condition in the general population (10). It was interesting that high striatal DAT binding was associated with reduced habituation to tactile stimuli and increased responsivity, but not to standard measures on a cognitive task requiring inhibitory control. This suggests that our behavioral measures of sensory responsivity may be more closely related to DAT function than some standard learning tasks.
The mechanisms by which prenatal events may program the DA system are not well understood. Maternal stress likely acts through a combination of neuroendocrine, immune/inflammatory, and vascular pathways to alter the maternal-placental-fetal system to influence a variety of birth outcomes (3). For example, studies with both humans and nonhuman primates have shown that maternal release of catecholamines during a stressful event can constrict placental blood vessels and cause fetal hypoxia, which can alter brain development (55). Maternal stress hormones can cross the placenta and thereby compromise fetal brain development (56–58). In rodents the effects of prenatal stress can be mimicked by administering glucocorticoids or ACTH and abolished by maternal adrenalectomy (58). Also prenatal stress can afffect the activity of the placental barrier enzyme 11B-HSD2, which converts cortisol to the inactive cortisone, potentially affecting the neurodevelopmental outcome of the offspring (59–62). Interestingly, there appear to be no in vivo or in vitro studies that have directly examined the effect of cortisol on DAT expression or activity, and only a very few studies have indirectly investigated associations between cortisol and DAT (63; 64).
The following methodological considerations and sensitivity analyses are presented in greater detail in the Supplement. Tracer mass. There was 61% greater tracer mass concentration observed in the reference region for the subjects not exposed to prenatal stress because of unintentional differences in specific activity of injected [18F]FECNT (Table 1, Mref, p = 0.095). This had the potential to artificially decrease [18F]FECNT binding in those subjects due to occupancy of DAT in the target regions by non-radioactive FECNT. Given the measured affiinity of [11C]methylphenidate in rat striatum (65), a rough calculation suggests that FECNT occupancy may be negligible (Supplement). Nevertheless, we performed an ANCOVA with tracer mass in the reference region, Mref, included as a covariate. The fractional difference in striatal binding for stress vs no stress decreased by 3% when adjusted for the effect of mass (12%, p = 0.055). Although fitting the model with both sex and mass may not be justified (66), the resulting differences in binding for stress vs no stress were further decreased and lost significance (Supplement). Tracer metabolite. An inactive metabolite of [18F]FECNT may cross the blood brain barrier, distribute evenly in the brain, and thus reduce the apparent binding potential of [18F]FECNT determined by reference tissue methods (67; 68). The radioactivity observed in the cerebellar reference region scaled to injected dose/body weight differed by only 3% between the prenatally stressed and non-stressed groups (Table 1, Aref), which suggests that any confound due to differences in radiotracer metabolism would be small compared to the observed effects. Analysis of striatal binding at earlier time points (60–120 minutes), when the relative concentration of metabolite would be expected to be lower, yielded a stress effect similar to that reported in Table 1 (12%, p = 0.033). Injected radioactivity. The stress groups on average received 11% lower injected radioactivity per body weight, which would be expected to yield higher statistical noise. It has been shown that the Logan method is susceptible to noise, such that increasing noise systematically yields lower target to reference distribution volume ratios (69). In this case, this confound is of reduced concern because in fact the stress groups exhibit higher binding. DAT trafficking. Isoflurane anesthesia has been shown in vitro to cause DAT to traffic intracellularly, and [18F]FECNT binding in rhesus putamen decreased by 63% as isoflurane was increased from 1.0 to 2.0% (70). To reduce noise introduced by this effect, we held the inhaled isoflurane concentration stable across subjects at 1.5 ± 0.1%.
Conclusion
Changes in striatal DAT availability due to experimental exposure to prenatal stress may represent a fundamental neuroadaptation in the dopamine system. Perturbations in levels of DAT in adulthood are also related to reduced habituation and increased magnitude in response to repeated tactile stimuli. Our findings underscore the role of prenatal stress on the functioning of inhibitory circuits, which could mediate certain behavioral effects of prenatal stress, including sensory defensiveness, reduced attention, motor function, and executive function, as well as susceptibility to neuropsychiatric disorders. Further study of this model may lead to improved therapeutic strategies for neuropsychiatric disorders, such as ADHD, which have been linked to dopaminergic dysfunction as well as prenatal stress.
Supplementary Material
Acknowledgments
The authors are grateful to Kacey V. Kronenfeld for scanner operation. This work was primarily supported by NIH grants R01AA12277 and R01AA10079 to MLS. Additional support was provided by NIH grants S10RR015801 and P30HD003352.
Footnotes
FINANCIAL DISCLOSURES
All authors report no biomedical financial interests or potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Van den Bergh BRH, Marcoen A. High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems, and anxiety in 8-and 9-year-olds. Child development. 2004;75:1085–1097. doi: 10.1111/j.1467-8624.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- 2.Glover V. Prenatal stress and the origins of psychopathology: an evolutionary perspective. Journal of Child Psychology and Psychiatry. 2011;52:356–367. doi: 10.1111/j.1469-7610.2011.02371.x. [DOI] [PubMed] [Google Scholar]
- 3.Wadhwa PD, Federenko IS. Prenatal stress influences human fetal development and birth outcomes: Implications for development origins of health and disease. In: Hodgson DM, Coe CL, editors. Perinatal Programming: Early Life Determinants of Adult Health and Disease. London: Taylor and Francis Group; 2006. pp. 29–46. [Google Scholar]
- 4.Kinney DK, Munir KM, Crowley DJ, Miller AM. Prenatal stress and risk for autism. Neuroscience and biobehavioral reviews. 2008;32:1519–1532. doi: 10.1016/j.neubiorev.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneider ML, Moore CF, Dejesus OT, Converse AK. Prenatal stress influences on neurobehavior, stress reactivity, and dopaminergic function in rhesus macaques. In: Burbacher T, Sackett GP, Grant KS, editors. Primate Models of Children’s Health and Developmental Disabilities. New York: Elsevier; 2008. pp. 231–258. [Google Scholar]
- 6.Bozarth MA. The mesolimbic dopamine system as a model reward system. In: Willner P, Scheel-Kruger J, editors. The Mesolimbic Dopamine System: From Motivation to Action. Chichester, England: Wiley; 1991. pp. 302–330. [Google Scholar]
- 7.Bannon MJ. The dopamine transporter: role in neurotoxicity and human disease. Toxicology and applied pharmacology. 2005;204:355–60. doi: 10.1016/j.taap.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Goodman MM, Kilts CD, Keil R, Shi B, Martarello L, Xing DX, et al. 18F-labeled FECNT: A selective radioligand for PET imaging of brain dopamine transporters. Nuclear medicine and biology. 2000;27:1–12. doi: 10.1016/s0969-8051(99)00080-3. [DOI] [PubMed] [Google Scholar]
- 9.Masilamoni G, Votaw J, Howell L, Villalba RM, Goodman M, Voll RJ, et al. 18F-FECNT: Validation as PET dopamine transporter ligand in parkinsonism. Experimental neurology. 2010;226:265–273. doi: 10.1016/j.expneurol.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahn RR, Miller LJ, Milberger S, McIntosh DN. Prevalence of parents’ perceptions of sensory processing disorders among kindergarten children. The American Journal of Occupational Therapy. 2004;58:287–93. doi: 10.5014/ajot.58.3.287. [DOI] [PubMed] [Google Scholar]
- 11.Baranek GT. Efficacy of sensory and motor interventions for children with autism. Journal of Autism and Developmental Disorders; Meeting of the Committee-on-Education-Interventions-for-Children-with-Autism. 2002;32:397–422. doi: 10.1023/a:1020541906063. [DOI] [PubMed] [Google Scholar]
- 12.Casey BJ. Disruption of inhibitory control in developmental disorders: A mechanistic model of implicated fronto-striatal circuitry. In: McClelland JL, Siegler RS, editors. Mechanisms of cognitive development: Behavioral and neural perspectives. Mahwah, NJ: Erlbaum; 2001. pp. 327–349. [Google Scholar]
- 13.Roberts AD, Moore CF, Dejesus OT, Barnhart TE, Larson JA, Mukherjee J, et al. Prenatal stress, moderate fetal alcohol exposure, and dopamine system function in rhesus monkeys. Neurotoxicology and teratology. 2004;26:169–178. doi: 10.1016/j.ntt.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Schneider ML, Moore CF, Gajewski LL, Larson JA, Roberts AD, Converse AK, DeJesus OT. Sensory processing disorder in a primate model: Evidence from a longitudinal study of prenatal alcohol and prenatal stress effects. Child development. 2008;79:100–113. doi: 10.1111/j.1467-8624.2007.01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bolan EA, Kivell B, Jaligam V, Oz M, Jayanthi LD, Han Y, et al. D2 receptors regulate dopamine transporter function via an extracellular signal-regulated kinases 1 and 2-dependent and phosphoinositide 3 kinase-independent mechanism. Molecular pharmacology. 2007;71:1222–32. doi: 10.1124/mol.106.027763. [DOI] [PubMed] [Google Scholar]
- 16.Zhu J, Reith MEA. Role of the dopamine transporter in the action of psychostimulants, nicotine, and other drugs of abuse. CNS & neurological disorders drug targets. 2008;7:393–409. doi: 10.2174/187152708786927877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodrigues A-J, Leao P, Carvalho M, Almeida OFX, Sousa N. Potential programming of dopaminergic circuits by early life stress. Psychopharmacology. 2011;214:107–120. doi: 10.1007/s00213-010-2085-3. [DOI] [PubMed] [Google Scholar]
- 18.Son GH, Chung S, Geum D, Kang SS, Choi WS, Kim K, Choi S. Hyperactivity and alteration of the midbrain dopaminergic system in maternally stressed male mice offspring. Biochemical and biophysical research communications. 2007;352:823–9. doi: 10.1016/j.bbrc.2006.11.104. [DOI] [PubMed] [Google Scholar]
- 19.Schneider ML, Moore CF, Adkins MM. The effects of prenatal alcohol exposure on behavior: rodent and primate studies. Neuropsychology review. 2011;21:186–203. doi: 10.1007/s11065-011-9168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider ML, Moore CF. Effects of prenatal stress on development: A nonhuman primate model. In: Nelson C, editor. Minnesota Symposium on Child Psychology. Lawrence Erlbaum; 2000. pp. 201–244. [Google Scholar]
- 21.Schneider ML, Roughton EC, Lubach GR. Moderate alcohol consumption and psychological stress during pregnancy induce attention and neuromotor impairments in primate infants. Child development. 1997;68:747–759. doi: 10.1111/j.1467-8624.1997.tb01959.x. [DOI] [PubMed] [Google Scholar]
- 22.Schneider ML, Moore CF, Kraemer GW. Moderate level alcohol during pregnancy, prenatal stress, or both and limbic-hypothalamic-pituitary-adrenocortical axis response to stress in rhesus monkeys. Child development. 2004;75:96–109. doi: 10.1111/j.1467-8624.2004.00656.x. [DOI] [PubMed] [Google Scholar]
- 23.Schneider ML, Moore CF, Kraemer GW. Moderate alcohol during pregnancy: Learning and behavior in adolescent rhesus monkeys. Alcoholism-Clinical and Experimental Research. 2001;25:1383–1392. [PubMed] [Google Scholar]
- 24.Baranek GT, Berkson G. Tactile defensiveness in children with developmental disabilities: responsiveness and habituation. Journal of autism and developmental disorders. 1994;24:457–71. doi: 10.1007/BF02172128. [DOI] [PubMed] [Google Scholar]
- 25.Miller LJ, McIntosh DN, McGrath J, Shyu V, Lampe M, Taylor AK, et al. Electrodermal responses to sensory stimuli in individuals with fragile X syndrome: a preliminary report. American journal of medical genetics. 1999;83:268–79. [PubMed] [Google Scholar]
- 26.Murali D, Barnhart TE, Vandehey NT, Christian BT, Nickles RJ, Converse AK, et al. An efficient synthesis of dopamine transporter tracer [(18)F]FECNT. Applied radiation and isotopes: including data, instrumentation and methods for use in agriculture, industry and medicine. 2013;72:128–32. doi: 10.1016/j.apradiso.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tai YC, Chatziioannou A, Siegel S, Young J, Newport D, Goble RN, et al. Performance evaluation of the microPET P4: a PET system dedicated to animal imaging. Physics in Medicine and Biology. 2001;46:1845–1862. doi: 10.1088/0031-9155/46/7/308. [DOI] [PubMed] [Google Scholar]
- 28.Paxinos G, Huang X-F, Petrides M, Toga AW. The Rhesus Monkey Brain in Stereotaxic Coordinates. 2. Academic Press; 2009. p. 416. [Google Scholar]
- 29.McLaren DG, Kosmatka KJ, Oakes TR, Kroenke CD, Kohama SG, Matochik JA, et al. A population-average MRI-based atlas collection of the rhesus macaque. NeuroImage. 2009;45:52–9. doi: 10.1016/j.neuroimage.2008.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 1996;16:834–40. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Innis RB, Cunningham VJ, Delforge J, Fujita M, Giedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. Journal of Cerebral Blood Flow and Metabolism. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 32.Best SE, Sarrel PM, Malison RT, Laruelle M, Zoghbi SS, Baldwin RM, et al. Striatal dopamine transporter availability with [123I]beta-CIT SPECT is unrelated to gender or menstrual cycle. Psychopharmacology. 2005;183:181–9. doi: 10.1007/s00213-005-0158-5. [DOI] [PubMed] [Google Scholar]
- 33.Van Dyck CH, Seibyl JP, Malison RT, Laruelle M, Wallace E, Zoghbi SS, et al. Age-related decline in striatal dopamine transporter binding with iodine-123-beta-CITSPECT. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 1995;36:1175–81. [PubMed] [Google Scholar]
- 34.Lavalaye J, Booij J, Reneman L, Habraken JB, Van Royen EA. Effect of age and gender on dopamine transporter imaging with [123I]FP-CIT SPET in healthy volunteers. European journal of nuclear medicine. 2000;27:867–9. doi: 10.1007/s002590000279. [DOI] [PubMed] [Google Scholar]
- 35.Eusebio A, Azulay J-P, Ceccaldi M, Girard N, Mundler O, Guedj E. Voxel-based analysis of whole-brain effects of age and gender on dopamine transporter SPECT imaging in healthy subjects. European journal of nuclear medicine and molecular imaging. 2012;39:1778–83. doi: 10.1007/s00259-012-2207-8. [DOI] [PubMed] [Google Scholar]
- 36.Mozley LH, Gur RC, Mozley PD, Gur RE. Striatal dopamine transporters and cognitive functioning in healthy men and women. American Journal of Psychiatry. 2001;158:1492–1499. doi: 10.1176/appi.ajp.158.9.1492. [DOI] [PubMed] [Google Scholar]
- 37.Staley JK, Krishnan-Sarin S, Zoghbi S, Tamagnan G, Fujita M, Seibyl JP, et al. Sex differences in [123I]beta-CIT SPECT measures of dopamine and serotonin transporter availability in healthy smokers and nonsmokers. Synapse (New York, NY) 2001;41:275–84. doi: 10.1002/syn.1084. [DOI] [PubMed] [Google Scholar]
- 38.Wong KK, Müller MLTM, Kuwabara H, Studenski SA, Bohnen NI. Gender differences in nigrostriatal dopaminergic innervation are present at young-to-middle but not at older age in normal adults. Journal of clinical neuroscience: official journal of the Neurosurgical Society of Australasia. 2012;19:183–4. doi: 10.1016/j.jocn.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 39.Varrone A, Halldin C. Molecular Imaging of the Dopamine Transporter. Journal of Nuclear Medicine. 2010;51:1331–1334. doi: 10.2967/jnumed.109.065656. [DOI] [PubMed] [Google Scholar]
- 40.Sandman CA, Davis EP, Buss C, Glynn LM. Exposure to Prenatal Psychobiological Stress Exerts Programming Influences on the Mother and Her Fetus. Neuroendocrinology. 2011;95:8–21. doi: 10.1159/000327017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhatia KP, Marsden CD. The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain: a journal of neurology. 1994;117:859–76. doi: 10.1093/brain/117.4.859. [DOI] [PubMed] [Google Scholar]
- 42.Spencer TJ, Biederman J, Madras BK, Dougherty DD, Bonab AA, Livni E, et al. Further evidence of dopamine transporter dysregulation in ADHD: A controlled PET imaging study using altropane. Biological psychiatry. 2007;62:1059–1061. doi: 10.1016/j.biopsych.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Volkow ND, Wang G-J, Kollins SH, Wigal TL, Newcorn JH, Telang F, et al. Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA: the journal of the American Medical Association. 2009;302:1084–91. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alonso SJ, Navarro E, Santana C, Rodriguez M. Motor lateralization, behavioral despair and dopaminergic brain asymmetry after prenatal stress. Pharmacology Biochemistry and Behavior. 1997;58:443–448. doi: 10.1016/s0091-3057(97)00009-9. [DOI] [PubMed] [Google Scholar]
- 45.Choy KHC, De Visser YP, Van den Buuse M. The effect of “two hit” neonatal and young-adult stress on dopaminergic modulation of prepulse inhibition and dopamine receptor density. British journal of pharmacology. 2009;156:388–396. doi: 10.1111/j.1476-5381.2008.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fride E, Weinstock M. Alterations in behavioral and striatal dopamine asymmetries induced by prenatal stress. Pharmacology, biochemistry, and behavior. 1989;32:425–30. doi: 10.1016/0091-3057(89)90174-3. [DOI] [PubMed] [Google Scholar]
- 47.Henry C, Guegant G, Cador M, Arnauld E, Arsaut J, Le Moal M, Demotes-Mainard J. Prenatal stress in rats facilitates amphetamine-induced sensitization and induces long-lasting changes in dopamine-receptors in the nucleus-accumbens. Brain research. 1995;685:179–186. doi: 10.1016/0006-8993(95)00430-x. [DOI] [PubMed] [Google Scholar]
- 48.McClure WO, Ishtoyan A, Lyon M. Very mild stress of pregnant rats reduces volume and cell number in nucleus accumbens of adult offspring: some parallels to schizophrenia. Developmental Brain Research. 2004;149:21–28. doi: 10.1016/j.devbrainres.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 49.Miller GM, Yatin SM, De La Garza R, Goulet M, Madras BK. Cloning of dopamine, norepinephrine and serotonin transporters from monkey brain: relevance to cocaine sensitivity. Molecular Brain Research. 2001;87:124–143. doi: 10.1016/s0169-328x(00)00288-6. [DOI] [PubMed] [Google Scholar]
- 50.Chen NH, Reith MEA. Structure and function of the dopamine transporter. European journal of pharmacology. 2000;405:329–339. doi: 10.1016/s0014-2999(00)00563-x. [DOI] [PubMed] [Google Scholar]
- 51.Fernagut P-O, Chalon S, Diguet E, Guilloteau D, Tison F, Jaber M. Motor behaviour deficits and their histopathological and functional correlates in the nigrostriatal system of dopamine transporter knockout mice. Neuroscience. 2003;116:1123–30. doi: 10.1016/s0306-4522(02)00778-9. [DOI] [PubMed] [Google Scholar]
- 52.Joti P, Kulashekhar S, Behari M, Murthy A. Impaired inhibitory oculomotor control in patients with Parkinson’s disease. Experimental brain research. Experimentelle Hirnforschung Expérimentation cérébrale. 2007;177:447–57. doi: 10.1007/s00221-006-0687-0. [DOI] [PubMed] [Google Scholar]
- 53.Gaffan D. Memory, action and the corpus striatum: Current developments in the memory-habit distinction. Seminars In The Neurosciences. 1996;8:33–38. [Google Scholar]
- 54.Jackson S, Houghton G. Sensorimotor selection and the basal ganglia: A neruonal network model. In: Houk JC, Davis JL, Beiser DG, editors. Models of information processing in the basal ganglia. Cambridge, Mass: MIT Press; 1995. pp. 337–369. [Google Scholar]
- 55.Teixeira JMA, Fisk NM, Glover V. Association between maternal anxiety in pregnancy and increased uterine artery resistance index: cohort based study. British medical journal. 1999;318:153–157. doi: 10.1136/bmj.318.7177.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takahashi LK, Turner JG, Kalin NH. Prolonged stress-induced elevation in plasma corticosterone during pregnancy in the rat: Implications for prenatal stress studies. Psychoneuroendocrinology. 1998;23:571–581. doi: 10.1016/s0306-4530(98)00024-9. [DOI] [PubMed] [Google Scholar]
- 57.Gitau R, Cameron A, Fisk NM, Glover V. Fetal exposure to maternal cortisol. Lancet. 1998;352:707–708. doi: 10.1016/S0140-6736(05)60824-0. [DOI] [PubMed] [Google Scholar]
- 58.Barbazanges A, Piazza PV, Le Moal M, Maccari S. Maternal glucocorticoid secretion mediates long-term effects of prenatal stress. J Neurosci. 1996;16:3943–3949. doi: 10.1523/JNEUROSCI.16-12-03943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Benediktsson R, Calder AA, Edwards CRW, Seckl JR. Placental 11 beta-hydroxysteroid dehydrogenase: a key regulator of fetal glucocorticoid exposure. Clinical endocrinology. 1997;46:161–166. doi: 10.1046/j.1365-2265.1997.1230939.x. [DOI] [PubMed] [Google Scholar]
- 60.Shams M, Kilby MD, Somerset DA, Howie AJ, Gupta A, Wood PJ, et al. 11Beta-hydroxysteroid dehydrogenase type 2 in human pregnancy and reduced expression in intrauterine growth restriction. Human Reproduction. 1998;13:799–804. doi: 10.1093/humrep/13.4.799. [DOI] [PubMed] [Google Scholar]
- 61.Welberg LAM, Thrivikraman KV, Plotsky PM. Chronic maternal stress inhibits the capacity to up-regulate placental 11beta-hydroxysteroid dehydrogenase type 2 activity. Journal of Endocrinology. 2005;186:R7–R12. doi: 10.1677/joe.1.06374. [DOI] [PubMed] [Google Scholar]
- 62.Lucassen PJ, Bosch OJ, Jousma E, Kroemer SA, Andrew R, Seckl JR, Neumann ID. Prenatal stress reduces postnatal neurogenesis in rats selectively bred for high, but not low, anxiety: possible key role of placental 11beta-hydroxysteroid dehydrogenase type 2. European Journal of Neuroscience. 2009;29:97–103. doi: 10.1111/j.1460-9568.2008.06543.x. [DOI] [PubMed] [Google Scholar]
- 63.Isovich E, Mijnster MJ, Flugge G, Fuchs E. Chronic psychosocial stress reduces the density of dopamine transporters. European Journal of Neuroscience. 2000;12:1071–1078. doi: 10.1046/j.1460-9568.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- 64.Cyr M, Morissette M, Barden N, Beaulieu S, Rochford J, Di Paolo T. Dopaminergic activity in transgenic mice underexpressing glucocorticoid receptors: Effect of antidepressants. Neuroscience. 2001;102:151–158. doi: 10.1016/s0306-4522(00)00444-9. [DOI] [PubMed] [Google Scholar]
- 65.Sossi V, Dinelle K, Jivan S, Fischer K, Holden JE, Doudet D. In vivo dopamine transporter imaging in a unilateral 6-hydroxydopamine rat model of Parkinson disease using 11C-methylphenidate PET. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2012;53:813–22. doi: 10.2967/jnumed.111.101436. [DOI] [PubMed] [Google Scholar]
- 66.Raab GM. Selecting Confounders from Covariates. Journal of the Royal Statistical Society Series A (Statistics in Society) 1994;157:271. [Google Scholar]
- 67.Zoghbi SS, Shetty HU, Ichise M, Fujita M, Imaizumi M, Liow J-S, et al. PET imaging of the dopamine transporter with 18F-FECNT: A polar radiometabolite confounds brain radioligand measurements. Journal of Nuclear Medicine. 2006;47:520–527. [PubMed] [Google Scholar]
- 68.Price JC, Lopresti BJ, Meltzer CC, Smith GS, Mason NS, Huang Y, et al. Analyses of [(18)F]altanserin bolus injection PET data. II: consideration of radiolabeled metabolites in humans. Synapse (New York, NY) 2001;41:11–21. doi: 10.1002/syn.1055. [DOI] [PubMed] [Google Scholar]
- 69.Ichise M, Toyama H, Innis RB, Carson RE. Strategies to improve neuroreceptor parameter estimation by linear regression analysis. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2002;22:1271–81. doi: 10.1097/01.WCB.0000038000.34930.4E. [DOI] [PubMed] [Google Scholar]
- 70.Votaw J, Byas-Smith M, Hua J, Voll R, Martarello L, Levey AI, et al. Interaction of isoflurane with the dopamine transporter. Anesthesiology. 2003;98:404–11. doi: 10.1097/00000542-200302000-00021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.