Abstract
Engineered tissue strategies for central nervous system (CNS) repair have the potential for localizing treatment using a wide variety of cells or growth factors. However, these strategies are often limited by their ability to address only one aspect of the injury. Here we report the development of a novel alginate construct that acts as a multi-functional tissue scaffold for CNS repair, and as a localized growth factor delivery vehicle. We show that the surface of this alginate construct acts as an optimal growth environment for neural progenitor cell (NPC) attachment, survival, migration, and differentiation. Importantly, we show that tailor-made alginate constructs containing brain-derived neurotrophic factor or neurotrophin-3 differentially direct lineage fates of NPCs and may therefore be useful in treating a wide variety of injuries. It is this potential for directed differentiation of a scaffold prior to implantation at the injury site that we explore here.
Keywords: Alginate, neural progenitor cell, neurotrophic factor, genetically engineered fibroblasts, differentiation, central nervous system
Introduction
Injury to the central nervous system (CNS) is devastating due to inflammation, scarring, and the presence of inhibitory molecules in the glial scar that limit self-regenerative capacity. Bioengineered systems for growth factor delivery and cell transplantation have become popular due to their high potential to enhance CNS repair1–4. Delivery of neurotrophic factors, such as brain derived neurotrophic factor (BDNF) and neurotrophin −3 (NT-3), to CNS injury site has been shown to reduce neuronal death and promote neuronal regeneration in several different models5–8. Transplantation of genetically engineered fibroblasts has been shown to act as a controlled delivery system to continuously supply the required neurotrophic factors9–11. However, the transplanted cells often elicit adverse immune responses. We have shown that encapsulation inside microcapsules possessing a size-controlling membrane can eliminate the need for immune suppression usually required with the use of allogeneic cells12. This strategy is continued in this study.
Transplantation of stem and neural progenitor cells (NPCs) to injury models has also shown success under certain circumstances13. This is especially noted in the spinal cord where transplantation has been shown to facilitate cell survival, proliferation, differentiation, and regeneration14–17. A crucial advantage appears to be the generation of oligodendrocytes that remyelinate spared axons in the vicinity of a lesion18. Critical to the use of NPCs will be the ability to understand their differentiation capacity in a given bioengineered solution. As Obermair et al. point out, one of the challenges to stem cell transplantation is to develop methods of optimizing cell characteristics prior to grafting and development of methods to produce adequate commercial-scale quantities19, a challenge we address here. Both haptotactic such as those initiated by interaction with hydrogel scaffolds and chemotactic stimuli induced by cytokines, drugs and neuropeptides have been shown to act in concert in both a spatial and temporal fashion 20–22 . In some cases NPCs have shown the capacity for remyelination 23–24 . Nakajima and coworkers reported that the effects of growth factors could be altered depending on the scaffold indicating synergistic effects between adhesion and growth factor signals 25 .
To capitalize on these repair strategies, developing a graft that can both protect allogeneic engineered cells by encapsulation, and provide a growth permissive surface for support of cells for directed tissue regeneration is important. These grafts require the use of biologically safe materials 26–28 and in this regard alginate is a promising candidate. Alginate is a water soluble copolymer derived from brown seaweed. An important characteristic of alginate is that its linear chains consist of repeating monomeric units of α-(1→ 4) linked L-guluronic acid and β-(1→ 4) D-mannuronic acid residues that can form highly cross-linked hydrogels with multivalent cations (with the exception of Mg 2+). The gel properties are governed by the proportion of the different monomer units and the molecular weight. In addition, alginate belongs to a group of compounds that have been generally regarded as safe by the FDA. Previous reports have shown that a freeze-dried version can enhance the effects of neurotrophic factors while helping to prevent glial scar tissue formation in spinal cord injury models29,30 . The surface of alginate gels can also be modified with peptides for cell attachment or coated with poly-L-ornithine (PLO) for immunoprotection 31,12 . It has been shown by various researchers (e.g., Goosen et al 1985) that a polycation membrane influences diffusion of proteins and that the molecular weight cut-off for exclusion can be controlled by the nature of the polycation32. We have shown that our encapsulation methods allow passage of BDNF but prevent rejection of allografts in vivo5,12. The encapsulated fibroblasts were shown to survive in culture, secrete bioactive BDNF and continue to grow for at least two months 12.
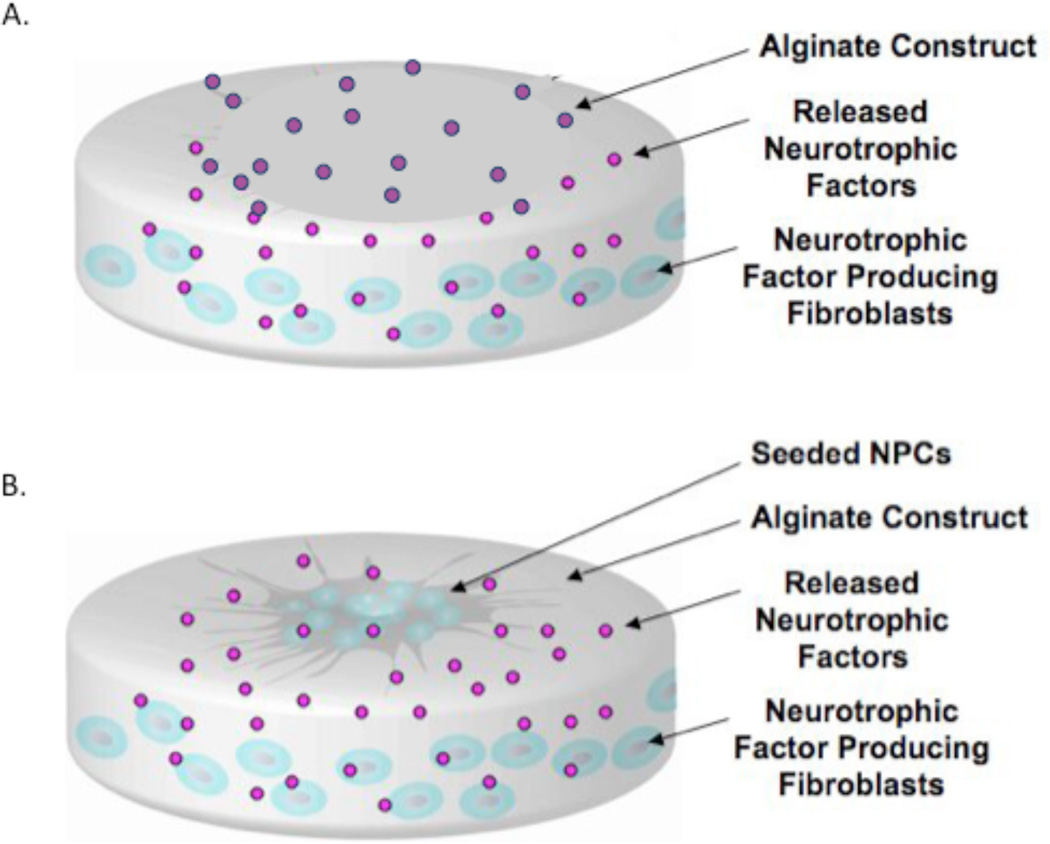
Here, we report the development of an alginate construct, depicted in graphic form in Figure 1., that acts as a multi-functional, tissue engineered graft for CNS repair utilizing NPCs, as well as a localized growth factor delivery vehicle consisting of encapsulated genetically engineered fibroblasts producing neurotrophic factors BDNF or NT-3, which we denote as Fb/BDNF and Fb/NT-3, respectively. This approach represents a promising bioengineered solution for neural repair that integrates NPCs and a growth factor delivery system tailored for lineage specific differentiation prior to implantation.
Figure 1.
A). Representation of the multifunctional alginate construct (grey) containing genetically modified fibroblasts (blue) encapsulated within the body of the construct while continually delivering neurotrophic factors (pink). B) Progenitor cells (NPCs) (Dark grey/blue) can be seeded on the surface of the construct and allowed to differentiate.
Experimental section
Cell culture
Rat abdominal skin fibroblasts genetically engineered to release brain derived nerve growth factor (Fb/BDNF) or neurotrophin-3 (Fb/NT-3) were kindly provided by Dr. Itzhak Fisher, Drexel University College of Medicine33. The retroviral vector in the fibroblasts has the human BDNF or NT-3 transgene linked to a reporter gene LacZ, which codes for β-galactosidase. Fibroblasts were cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented media with 10% FBS (Gibco, Rockville, MD), antibiotics (Penicillin 100 IU/ml, Streptomycin 50 µg/ml, Sigma, St. Louis, MO), at 37°C and 5% CO2. The cells were passaged using 0.25% trypsin-EDTA (Sigma) when they attained 70–80% confluency.
Neural progenitor cells (NPCs) derived from the telencephalon of embryonic day 14 mice and cultured DMEM/Ham’s F-12 (50:50, Gibco) supplemented with 2% B-27 (Gibco), 20 ng/ml bFGF (basic fibroblast growth factor, Invitrogen) and 20 ng/ml EGF (epidermal growth factor, Invitrogen) at 37°C and 5% CO2. NPCs were cultured as colonies and passaged at 7 days after all colonies were visibly spherical using trypsin-EDTA.
Cell viability assay
For cell viability the Live/Dead Reduced Biohazard Viability/Cytotoxicity Kit #1(Invitrogen) was used. NPCs were incubated for 15 minutes at room temperature with Component A and Component B. Cells were then fixed in 4% glutaraldehyde (Sigma) for 1 hour at room temperature. Images were acquired using an Olympus IX71 fluorescence microscope. Images were processed using SPOT image software to adjust intensity levels. Images of stained cells in 10 adjacent fields were then counted blindly for each marker.
Hydrogel construct preparation
Calcium carbonate crosslinked alginate constructs - A high guluronic acid alginate was received as a gift from FMC Biopolymer (Drammen, Norway) (LF200M, Batch# S15596). A slow gelling method based on that of Kuo and Ma, in which calcium carbonate (Sigma) is gradually solubilized under mildly acid conditions generated by slow hydrolysis of glucono-delta-lactone (GDL) was used to prepare constructs34. Five ml of a 1% (w/v) sterile filtered (0.45 micron bottle top filter) alginate solution was poured into a sterile 15 ml centrifuge tube. Aqueous calcium carbonate (2.5 ml of 56 mM CaCO3) was added and the solution was mixed, followed by addition of 2.5 ml of a 157 mM aqueous GDL solution, creating a slurry with a final GDL concentration of 80 mM. All aqueous solutions were first sterilized by autoclave. A 2 ml aliquot of the slurry was poured into each well of a 6 well culture plate and allowed to incubate at 37°C for 24 hours to harden. The resulting constructs were washed with N-[2-hydroxyethylpiperazine-N’–[2-ethanesulfonic acid] sodium salt (HEPES) (Sigma), buffer (pH7.4) yielding disc with surface area of 9.6 cm2 and a depth of 0.2 cm.
The original genetically engineered Fb-BDNF and Fb-NT3 secrete the neurotrophic factors at a rate of 12.8 ng and 47.7 ng/106 cells/24 h, respectively35,36 . We have shown previously that encapsulation reduces this amount to about 62% of the original levels37.
Surface Coating of hydrogel constructs
Alginate discs were coated with fresh, filter-sterilized (0.2µm cellulose acetate filter) poly-L-ornithine (PLO), molecular weight 15,000 – 30,000 (Sigma Chemical Co., St. Louis, MO), 0.5mg/ml in HEPES buffer for 6 minutes by gentle swirling using 6 times the volume of gel used for making the discs. The constructs were then washed with HEPES buffer to remove any unreacted PLO. The discs were then exposed to 1ml of a sterile 25% solution of natural mouse laminin 111 derived from an EHS sarcoma (Invitrogen, Carlsbad, CA), for 24 hours. Finally the constructs were washed with HEPES buffer to remove excess unreacted laminin 111.
Encapsulation of genetically engineered fibroblasts in alginate constructs
Fibroblasts (Fb/BDNF or Fb/NT-3) were harvested from culture at about 80% confluency using 0.25% trypsin for 3 minutes, and resuspended in sterile HEPES buffer. The cells were added to filter-sterilized (0.45 µm) alginate solution to obtain a 1% (w/v) alginate solution with a cell concentration of about 3 × 106 cells/ml of alginate solution. Alginate discs containing the Fb/BDNF or Fb/NT-3 were prepared and coated as outlined above. Fibroblast growth medium was added to the alginate discs and incubated at 37°C
Cell Seeding
NPCs were seeded on the hydrogel scaffold coated with laminin 111 as described above. The final cell concentration of the NPCs on the surface of the scaffold was 200,000 cells/ml of alginate.
Quantification of NPC migration distance
Migration distance of NPCs was quantified as a ratio of the radius of the original NPC colony and the radius of the farthest migration distance of the cells out of the original colony. These two values were compared (Migrated Radius: Original Radius) to normalize all NPC colony sizes. 10 adjacent fields were quantified for migration distance for each type of construct.
Immunohistochemistry
For immunostaining, cells were fixed in 2% paraformaldehyde (Sigma) (PFA) for 30 minutes at room temperature. Fixed cells were blocked for a minimum of 30 min in PBS containing 0.1% Triton X-100 (Sigma) and 10% normal goat serum followed by incubation overnight with the relevant primary antibodies at 4°C. DAPI counter-staining was used when required, and images were acquired using an Olympus IX71 fluorescence microscope. Images were processed using SPOT image software to adjust intensity levels. The following antibodies were used for immunohistochemistry: anti-p75 (mouse IgG2a, 1:100), anti-Trk B (mouse IgG2a, 1:100), anti-Trk C (mouse IgG2a, 1:100), anti-neuronal class III tubulin (mouse IgG2a,1:1000), anti- MAP-2 kinase (mouse IgG2a,1:500), anti-GFAP (rabbit polyclonal, 1:200), anti-S100b (rabbit polyclonal, 1:200), anti-GalC (rabbit polycolonal, 1:200), and anti CNPase (rabbit polycolonal, 1:200). Appropriate Alexa Fluor 488 and Alexa Fluor 568 – conjugated IgG (1:100 – 1:500) (Invitrogen, Carlsbad, CA), were used as secondary antibodies. Images of stained cells in adjacent fields were then counted blindly for markers for each phenotype.
β-Galactosidase staining
Fb/BDNF and Fb/NT-3 were stained for β –galactosidase, the product of the marker gene, using an X-Gal Staining Kit (Genlantis, San Diego, CA). After alginate discs containing Fb/BDNF or Fb/NT-3 had been implanted in mice for 7 days, they were recovered and washed with PBS (pH 7.4). Fixing Buffer was then added to each disc and incubated at room temperature for 15 minutes. The Fixing Buffer was then removed and the disc was then washed sufficiently with PBS. X-Gal solution was then added to each disc and incubated at 37°C for 18 hours to ensure proper staining. The X-Gal solution was then removed and the discs were washed with PBS. Photographs of the discs were taken using an Olympus IX71 fluorescence microscope.
Statistical analysis
Statistical analysis for all data for comparison was performed by one-way analysis of variance (ANOVA) followed by a Tukey-Kramer test using Graphpad PRISM software. p>0.05 was used for significance. All data were analyzed in a blinded manner.
Results
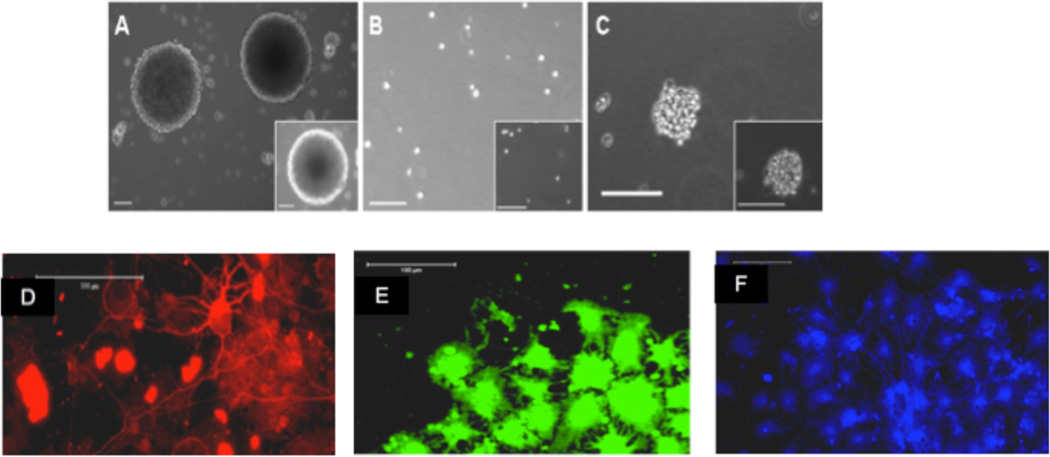
The long-term goal of this work is to create a multi-functional, implantable hydrogel construct for the delivery of trophic factors to the injured CNS that is seeded with neuronal cells with a predetermined lineage. This study is designed to demonstrate conditions under which the construct has the ability to support cell attachment and preserve viability, and to investigate the relationships between cell adhesion on the hydrogel and growth factor signaling in NPCs. We utilize NPCs, which have stem cell-like properties as noted in Figure 2.
Figure 2.
Light and fluorescent micrographs validating the stem cell like properties of the NPCs used for experimentation. NPCs were grown as spherical colonies. (A), dissociated into single cells (B), and reformed into spherical colonies after 7 days (C). Additionally, spherical colonies were grown and differentiated into all three distinct lineages on poly-L-Lysine (PLL) coated coverslips and were positive for β-III tubulin (D), CNPase (E) and GFAP (F). Scale bar = 100 µm.
Neural Progenitor Cell Adhesion and survival on Hydrogel Constructs
In order to test the potential of alginate as a scaffolding material for NPC in conjunction with an intrinsic continuous supply of neurotrophic factors, we first quantified NPC adhesion to alginate scaffolds that were either uncoated or coated with poly-L-ornithine and laminin 111. Constructs were formed into the shape of discs and coated with poly-L-ornithine and laminin 111 (a heterotrimeric extracellular matrix (ECM) protein). For NPC attachment, ~200,000 cells were seeded on both plain and laminin 111-coated alginate, discs, with and without encapsulated Fb/BDNF and Fb/NT-3.
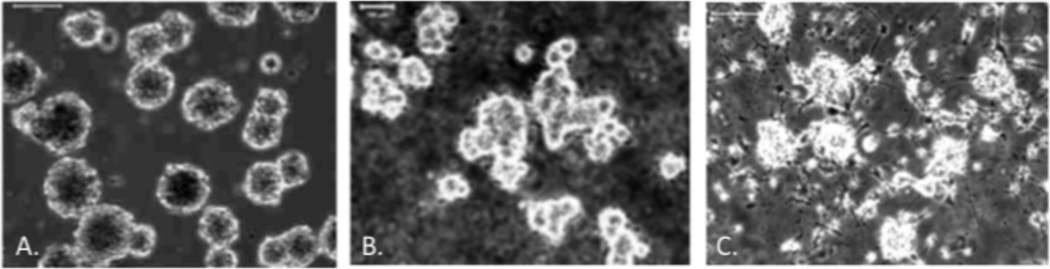
NPCs that were attached to the laminin coated alginate discs showed differentiation and cell migration out of the neurospheres after day 1 (Fig. 3.A), day 3 (Fig 3B.) and 5 days (Fig. 3C).
Figure 3.
Micrograph of NPC’s attached to a laminin 111 coated alginate disc 1, 3 and 5 days in culture, Scale bar – 100 µm. Disc day 1 (A), Disc day 3(B), Disc day 5 (C)
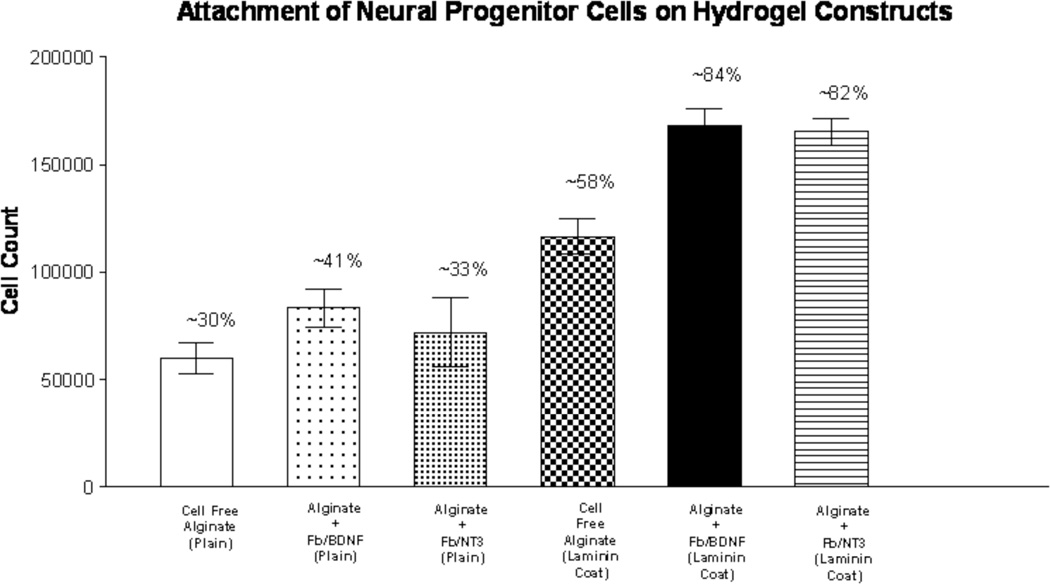
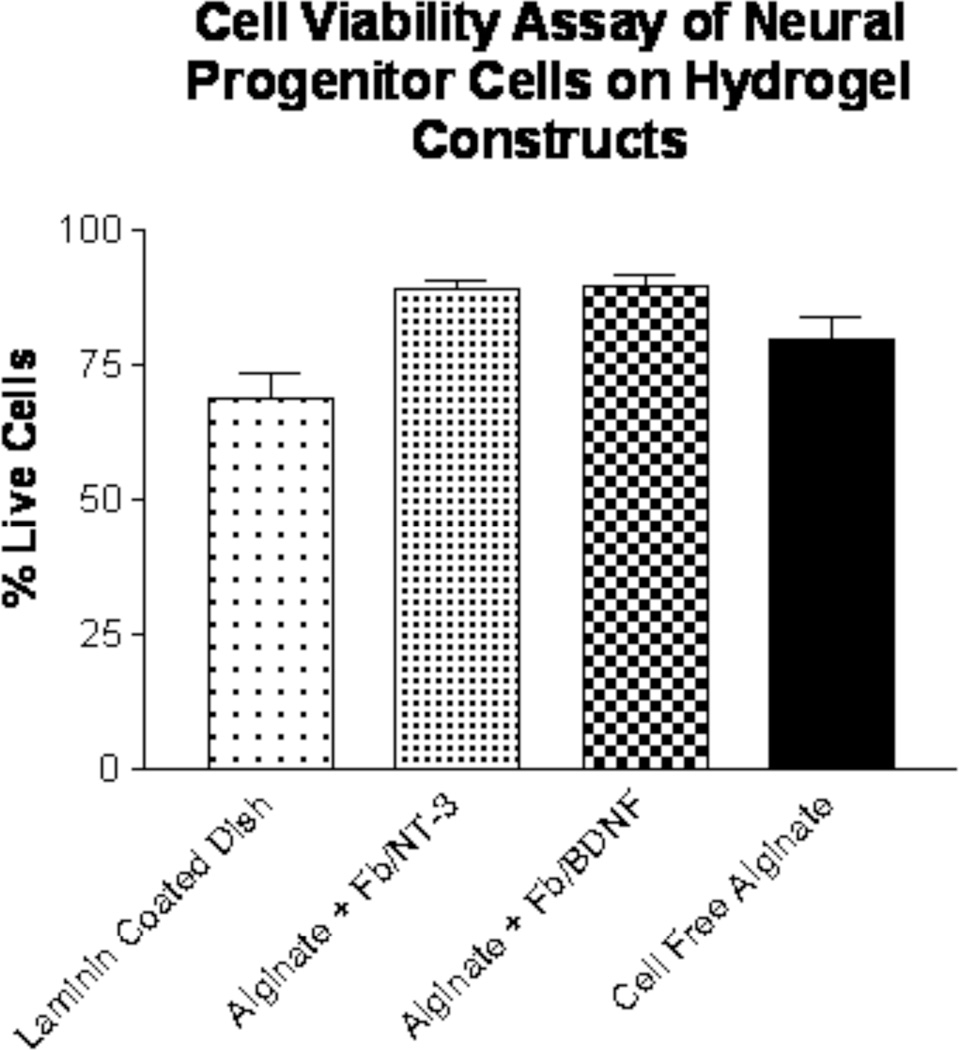
The best NPC attachment (Figure 4) was seen when neurotrophin-releasing cells, Fb/BDNF (~84%) or Fb/NT-3 (~82%) were encapsulated within laminin-111 coated alginate constructs. This was in stark contrast to results obtained with gellan gum that had been used for constructs. Gellan has a similar charged nature, but statistically significant fewer cells attached (p < 0.001), while agarose, a neutral gel, showed no attachment whatsoever (data not shown). In addition, alginate constructs with and without encapsulated Fb/BDNF or Fb/NT-3 showed the highest NPC survival after 7 days in culture (cells alive on fibroblast free construct: 79.8 ± 9.3%; Fb/NT-3 construct: 89.0 ± 3.1%; Fb/BDNF construct: 89.6 ± 4.6%;), significantly more than control laminin 111 coated culture dishes (cells alive: 69.0 ± 10.5%; p< 0.001), which are shown in figure 5. These results demonstrate that alginate is a suitable biomaterial to promote NPC attachment and survival.
Figure 4.
Effect of construct composition on NPC attachment on hydrogel discs. Constructs with or without laminin 111 coat and with or without encapsulated neurotrophic factor producing fibroblasts evaluated by calculating the percentage of attached and unattached NPCs seeded on alginate).  Cell free disc (no coat),
Cell free disc (no coat), Disc + encap. Fb/BDNF, (no coat),
Disc + encap. Fb/BDNF, (no coat),  Disc + encap. Fb/NT-3 (no coat),
Disc + encap. Fb/NT-3 (no coat),  Cell free disc (laminin coated),
Cell free disc (laminin coated),  Disc + Fb/BDNF (laminin coated),
Disc + Fb/BDNF (laminin coated),  Disc + Fb/NT-3 (laminin coated) (n=10).
Disc + Fb/NT-3 (laminin coated) (n=10).
Figure 5.
Cell viability of NPCs on hydrogel constructs of alginate (n=10). Live/dead assay performed after 7 days in culture on laminin-coated discs compared to control of laminn coated tissue culture plates.
Neurotrophic Factors Increase Neural Progenitor Cell Migration On Alginate Constructs
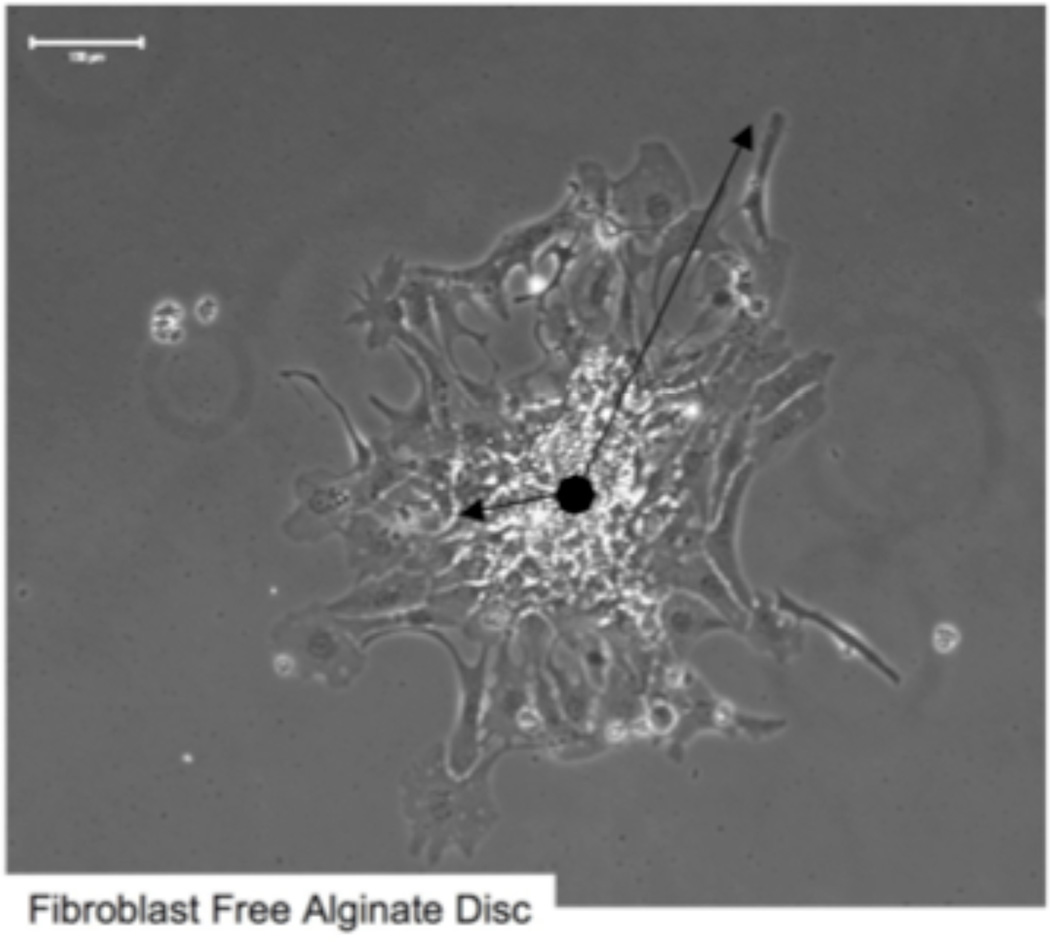
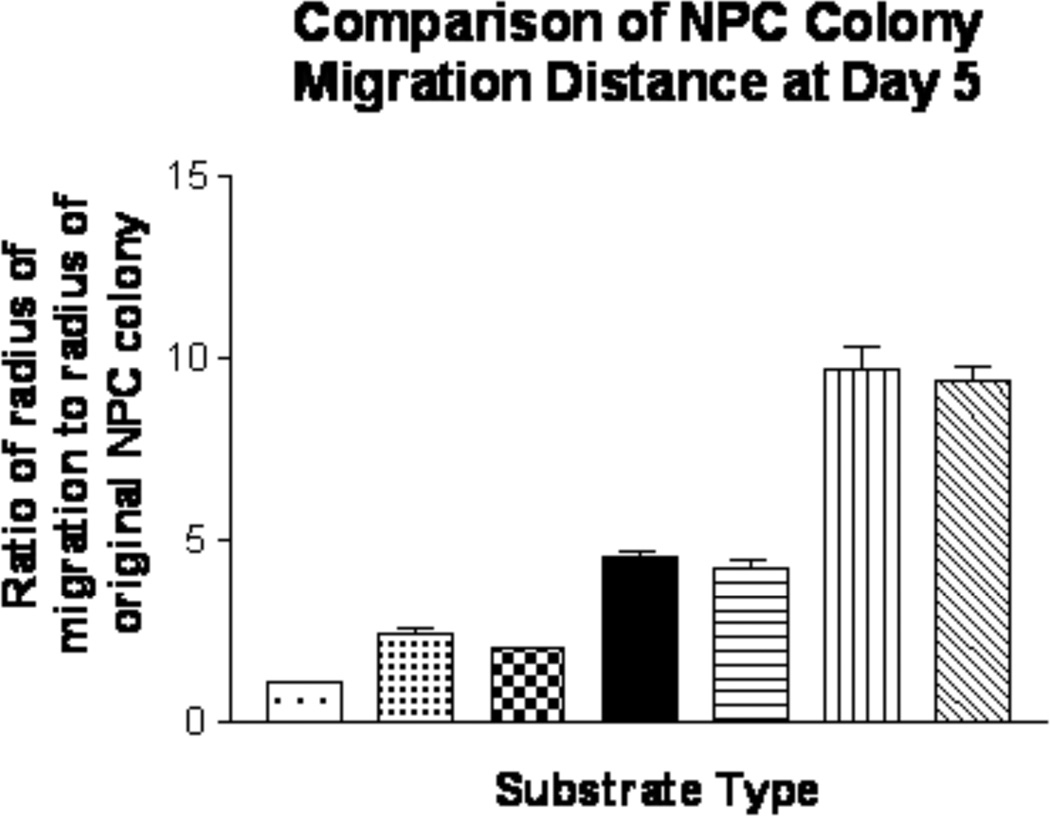
Once implanted it is important that NPCs migrate to the site of injury. To test this potential, migration of NPCs on PLO and laminin-coated alginate constructs was evaluated for a 5 day period. Measured distances were normalized using the ratio of the migration distance of the cells out of the original colony to the radius of the original colony to account for colony size (method shown for the example of a fibroblast-free control disc in Figure 6). Plain alginate constructs. (control) promoted minimal migration of NPCs, with the average the average distance migrated being only 1.1 ± 0.1 times the original radius (p>0.05) (Figure 7). However, laminin 111-coated alginate constructs and control laminin 111-coated culture dishes promoted more extensive NPC migration (2.5 ± 0.3 and 2.0 ± 0.2 times the distance of the original radius, respectively; p<0.05). The incorporation of Fb/BDNF or Fb/NT-3- within the alginate scaffold increased migration distance of NPCs to 4.5 ± 0.6 and 9.8 ±1.9 times the original radius, respectively (p<0.01). The addition of a PLO rate-controlling membrane coat to the alginate constructs with encapsulated Fb/BDNF and Fb/NT-3 did not significantly affect migration distance (p >0.05). Thus, encapsulation of neurotrophic factor-producing cells in the matrix appreciably promoted migration, with Fb/NT3 being superior to Fb/BDNF.
Figure 6.
Migration distance calculation example. Photomirograph of a seeded colony of NPCs. Migration distance of NPCs was calculated as a ratio of outer migration distance to radius of original NPC colony/ Scale bar = 100 µm
Figure 7.
Extent of NPC migration on different alginate constructs. Cell migration distance was calculated as a ratio of the original NPC colony radius to the final migration. All quantification was done after NPCs had attached and differentiated for 5 days in culture (n=10).  Plain alginate disc (no coat),
Plain alginate disc (no coat),  Laminin coated alginate disc,
Laminin coated alginate disc,  Laminin coated culture dish (no alginate),
Laminin coated culture dish (no alginate),  Laminin coated disc + encap. Fb/BDNF,
Laminin coated disc + encap. Fb/BDNF,  Laminin +PLO + Fb/BDNF disc,
Laminin +PLO + Fb/BDNF disc,  Laminin coated disc + encap. Fb/NT3,
Laminin coated disc + encap. Fb/NT3,  Laminin+PLO alginate disc + Fb/NT3
Laminin+PLO alginate disc + Fb/NT3
BDNF and NT-3 Differentially Affect the Fate of Neural Progenitor Cells on Alginate Constructs
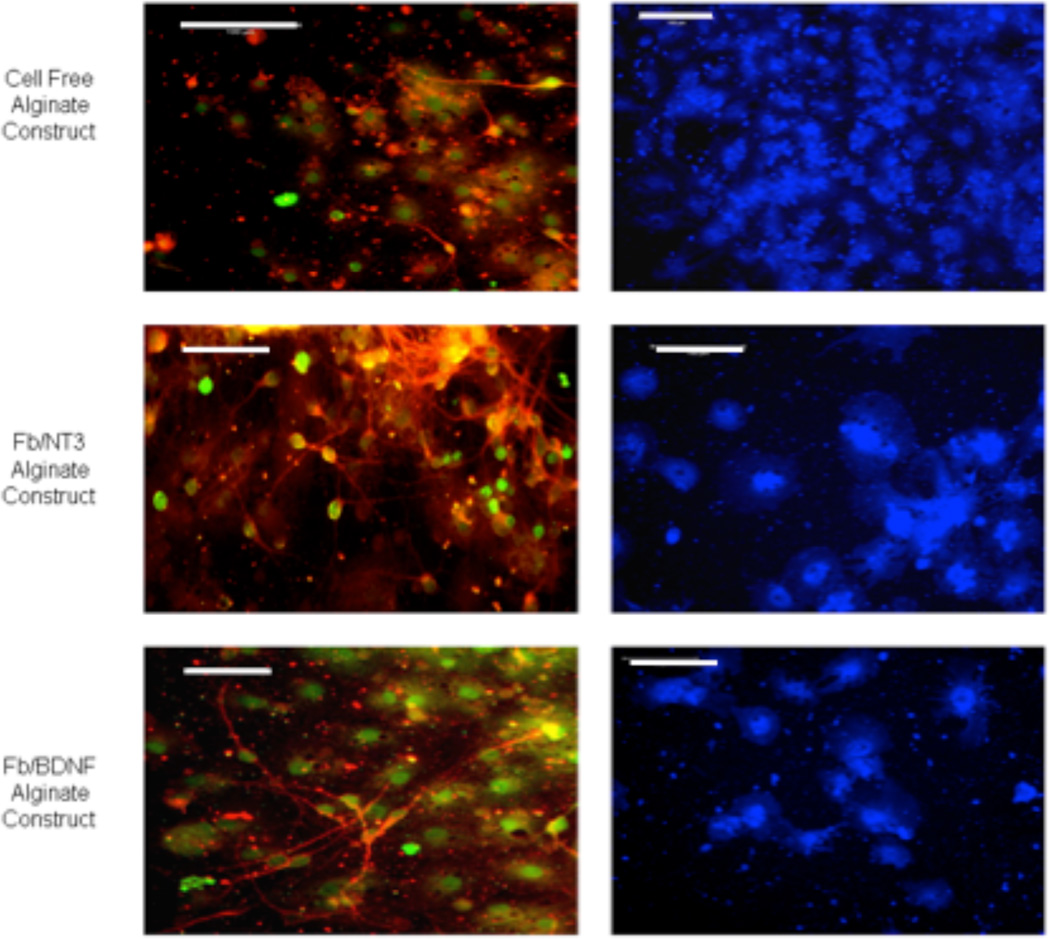
After 5 days in culture, cells derived from NPCs seeded on alginate constructs with or without encapsulated neurotrophic factor-producing fibroblasts had generated differentiated cells that included all three major CNS cell types. In all cultures there were some cells immunoreactive with antibodies against either βIII-tubulin (a neuron-specific protein), MBP (an oligodendrocyte-specific protein), and GFAP (an astrocyte-specific protein) indicating that the alginate constructs allowed NPCs to differentiate into all three distinct cell lineages (Figure 8).
Figure 8.
Fluorescence micrographs showing multi-lineage differentiation of NPCs on laminin 111 and PLO coated alginate discs with or without encapsulated Fb/NT-3 or Fb/BDNF. After 5 days in culture, cells were stained with the following antibodies: anti- -tubulin (red), anti-GALc (green), and anti-GFAP (blue), scale bar = 100 m.
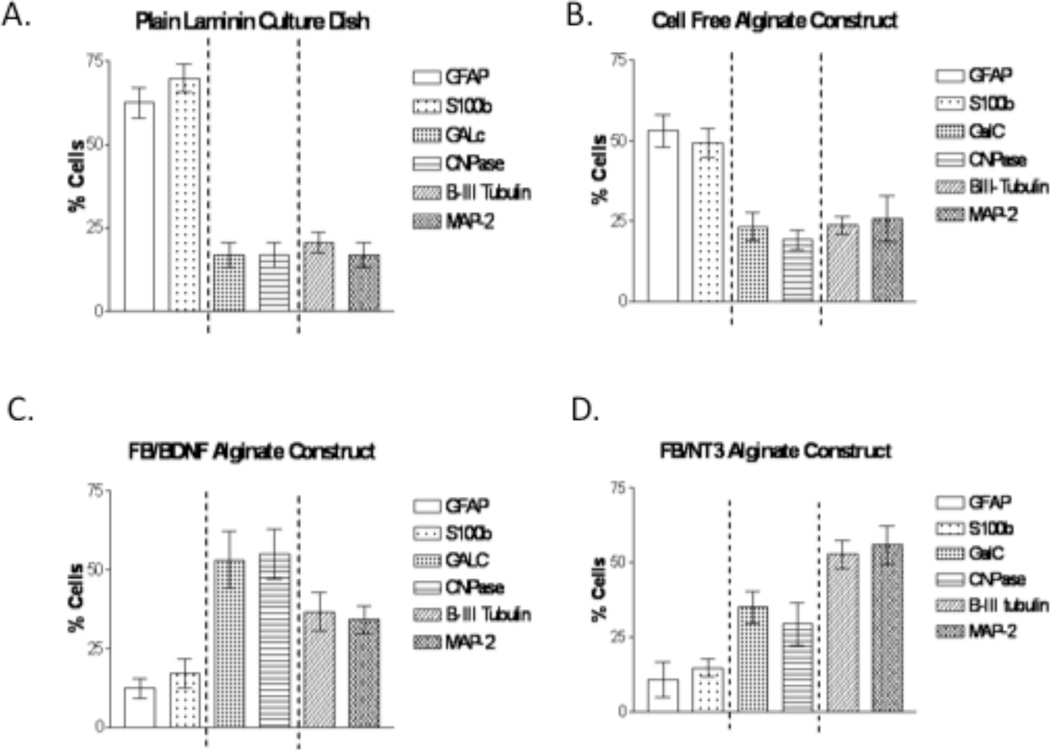
To quantify the effects BDNF and NT-3, on the fate of NPCs grown on the surface of alginate constructs, we determined the percentages of cells that were positively differentiated into neurons (βIII-tubulin, MAP-2), oligodendrocytes (GalC, CNPase), and astrocytes (GFAP, S100β) (Figure 9). The first disparity that was observed was in astrocyte production; plain alginate supported significantly (p<0.01) fewer astrocytes than did coated tissue culture plates by both measures of detection (GFAP: 53.0 ± 5.1% for the construct compared to 62.5 ± 4.5% for culture plates and S100β: 49.2 ± 4.4% compared with 69.9 ± 4.1% respectively). Differences are even more marked when neurotrophic factor-releasing fibroblasts are entrapped in the alginate, showing 12.3 ± 3.3% (GFAP positive) and 17.1 ± 4.5% (S100β positive) for Fb/BDNF and 10.79 ± 5.9% (GFAP positive) and 14.7 ± 2.9% (S100 β positive) for Fb/NT-3, significantly different than cells seeded in culture dishes or on cell-free alginate constructs (p<0.01). However, of the cells seeded on alginate constructs with encapsulated Fb/BDNF 53.1 ± 9.1% were GalC positive and 54.9 ± 8.0% were CNPase positive indicating that BDNF promotes an oligodendrocyte fate. In contrast, only 35.0 ± 5.4% and 29.3 ± 7.2% of the cells measured on alginate constructs with Fb/NT-3 expressed GalC and CNPase, respectively, whereas 52.7 ± 4.7% of the cells were β III-tubulin positive and 55.9 ± 6.5% were MAP-2 positive indicating that NT-3 favors a neuronal fate. There was no statistically significant difference between the results obtained for the duplicate markers, namely for neurons there was no difference between βIII-tubulin and MAP-2, for oligodendrocytes between GalC and CNPase, and for astrocytes between GFAP and S100β (p>0.05). The results are summarized in Table 1. These data demonstrate that not only does plain alginate affect the fate of NPC s growing on its surface but also the notable ability of encapsulated FB/BDNF and Fb/NT3 to dramatically reduce the proportion of NPCs that differentiate into astrocytes.
Figure 9.
Quantification of NPC differentiation profiles on alginate constructs after 5 days in culture. A. Laminin coated culture dish, B. Cell free alginate construct, C. Alginate construct encapsulating FB/BDNF, D. Alginate construct encapsulating Fb/NT3. (n=10).
Table 1.
Effect of substrate and encapsulated fibroblasts on NPC lineage differentiation
| Astrocytes | Oligoes | Neurons | |||||
|---|---|---|---|---|---|---|---|
| Antibody | GFAP | S100b | GalC | CNPase | beta Tub | MAP 2 | |
| Construct type | |||||||
| Plates | 62.5±4.5% | 69.9±4.1% | 16.9±3.3% | 16.8±3.3% | 20.6±3.0% | 16.9±3.3% | |
| PlainAlginate | 53±5.1% | 49.2±4.4% | 23.3±4.3% | 19.2±3.1% | 23.7±2.8% | 17.7±3.7% | |
| Fb/BDNF | 12.3±3.3% | 17.1±4.5% | 53.1±9.1% | 54.9±8.0% | 36.7±5.6% | 34.0±3.9% | |
| Fb/NT3 | 10.79±5.9% | 14.7±2.9% | 35±5.4% | 29.3±7.2% | 52.7±4.7% | 55.9±6.5% | |
Discussion
Our results demonstrate that both an alginate substrate and delivery of neurotrophic factors from that substrate have the capacity to influence the behavior and lineage of NPCs seeded on its surface. For cell attachment, survival and migration both plain alginate and laminin-coated alginate performed better than laminin-coated tissue culture plates, and incorporation of neurotrophic factor - releasing fibroblasts within the matrix enhanced the effect. Specifically, they promote the attachment, survival and migration of NPCs. Laminin also plays a role but was not essential for attachment and proliferation of NPCs on alginate.
To date, many studies reported in literature have explored the potential of NPCs for regeneration, proliferation, and lineage differentiation after direct implantation of undifferentiated cells into an injury site38,14. NPCs and other cells have also been encapsulated within different hydrogels and the matrices implanted into injured CNS regions27,39–42. The potential drawback of these immobilization strategies is that cell encapsulation significantly increases the time required for these cells to begin their regenerative/differentiation process because the encapsulating agent has to be sufficiently degraded before they can be released from the matrix and integrate into the host tissue. This in turn can lead to limited cell proliferation and can potentially reduce the regenerative capacity of this delivery strategy for NPCs and other cell lines39–42. Prang et al. have reported encapsulation of NPCs in alginate in the form of “anisotropic capillary hydrogels” which have the advantage of having a hexagonally structured anisotropic capillary gel structure which promotes directed axonal regrowth43. Others, including Ashton et al., have encapsulated degrading enzymes within the matrix in order to allow for sufficient NPC release44. However, the in vivo consequences of the delivery of this foreign enzyme remain unclear.
Certain cells are known to adjust their stiffness to match the substrate upon which they are growing45. It has also been shown that integrin-mediated linkage between the cytoskeleton and extracellular matrix are reinforced on application of force46. Stem cells are known to have surface integrins and recent studies elegantly demonstrated the propensity of substrate elasticity to direct stem cell lineage specification47,48. Discher and his group at the University of Pennsylvania have convincingly demonstrated the propensity of substrate elasticity to direct stem cell lineage specification48. In a recent study, Huebsch et al. demonstrated a more dynamic situation with mesenchymal stem cells encapsulated in a 3D matrix which suggests that the cells themselves can be utilized to transform the matrix into structures that influence cell fate50. Here we show that not only do NPCs attach well to alginate but also that the type of neurotrophin supplied from genetically engineered fibroblasts encapsulated within the matrix can influence lineage determination of NPCs growing on the surface.
In addition to the regenerative capacity of NPCs, this construct continuously releases neurotrophic factors to further aid in injury recovery. The alginate constructs developed in this study favor NPC attachment while simultaneously increasing the differentiation potential of the cells. NPCs seeded on laminin 111-coated alginate constructs also showed multi-lineage differentiation and migration, a normal characteristic of NPC colonies. The constructs can act as both neural tissue-engineered scaffolds and as neurotrophic factor delivery vehicles that can be implanted at selected sites of injury. Using this delivery system, future choice of cells and excreted bioactive factors have the potential to produce constructs tailored for different applications. Various ECM substrates for NPC growth, as well as cell-free administration of neurotrophic factors on NPCs in culture have been reported to show a potential for directing neural differentiation51–58. As early as 1997 Lanchyankar et al. showed that treatment of neurospheres cultured from embryonal striatum with neurotrophin-3 or ciliary neurotrophic factor resulted in bipolar neuronal cells and oligodendrocytes51. Later, Cladwell et al.55 , reported that neurospheres grown on PLO-coated cover slips responded to the addition of NT3 and NT4, by producing an increase in the number of neurons of 15 and 18% respectively, and a decrease in the number of astrocytes to 40 and 35%, respectively. However no significant effects on either neuronal or glial cell number were observed with BDNF. Silva et al. have recently shown rapid differentiation of NPCs into neurons with 35% of total cells staining positive for β-tubulin inside self assembling nanotubes composed of IKVAV-containing peptide ampiphiles59. Interestingly, our alginate constructs were able to direct the differentiation of NPCs by greater amounts than in other studies while at the same time substantially increasing NPC migration. Alginate constructs with and without encapsulated neurotrophic factor-producing fibroblasts greatly reduced the number of cells that exhibit an astrocytic phenotype. Selective differentiation of NPCs was possible on these constructs in that cells seeded on constructs with encapsulated Fb/BDNF exhibited a predominant oligodendrocyte phenotype, while those seeded on constructs with NT-3-producing fibroblasts acquired substantial neuronal phenotype. Since normal differentiation profiles require a large quantity of astrocytes as the framework for neurons to grow and survive, the alginate construct itself may be acting here in a similar supporting capacity.
Acknowledgements
The authors would like to thank Prof. Charles ffrench-Constant (University of Edinburgh) for guidance and helpful discussions on the manuscript. J.D.L. was supported by the NIH-Cambridge Graduate Partnership Program. M.A.W. is supported by HL 52901 and the Bryon Reisch Paralysis Foundation
References
- 1.Nomura H, Tator CH, Shoichet MS. Bioengineered strategies for spinal cord repair. J. Neurotrauma. 2006;23(3–4):496–507. doi: 10.1089/neu.2006.23.496. [DOI] [PubMed] [Google Scholar]
- 2.Dang JM, Leong KW. Natural polymers for gene delivery and tissue engineering. Adv. Drug Deliv. Rev. 2006;58(4):487–499. doi: 10.1016/j.addr.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Pearse DD, Bunge MB. Designing cell- and gene-based regeneration strategies to repair the injured spinal cord. J. Neurotrauma. 2006;23(3–4):438–452. doi: 10.1089/neu.2006.23.437. [DOI] [PubMed] [Google Scholar]
- 4.Teng YD, Lavik EB, Qu X, Park KI, Ourednik J, Zurakowski D, Langer R, Snyder EY. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc. Natl. Acad. Sci. U S A. 2002;99(5):3024–3029. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tobias CA, Dhoot NO, Wheatley MA, Tessler A, Murray MM, Fischer I. Grafting of encapsulated BDNF-producing fibroblasts into the injured spinal cord without immune suppression in adult rats. J. Neurotrauma. 2001;18(3):287–301. doi: 10.1089/08977150151070937. [DOI] [PubMed] [Google Scholar]
- 6.Tuszynski MH, Grill R, Jones LL, Brant A, Blesch A, Lo K, Lacroix S, Lu P. NT-3 gene delivery elicits growth of chronically injured corticospinal axons and modestly improves functional deficits after chronic scar resection. Exp. Neurol. 2003;181(1):47–56. doi: 10.1016/s0014-4886(02)00055-9. [DOI] [PubMed] [Google Scholar]
- 7.Katz A, Meiri N. Brain-derived neurotrophic factor is critically involved in thermal-experience-dependent developmental plasticity. J. Neurosci. 2006;26(15):3899–3907. doi: 10.1523/JNEUROSCI.0371-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hapner SJ, Nielsen KM, Chaverra M, Esper RM, Loeb JA, Lefcort F. NT-3 and CNTF exert dose-dependent, pleiotropic effects on cells in the immature dorsal root ganglion: Neuregulin-mediated proliferation of progenitor cells and neuronal differentiation. Dev Biol. 2006;297:182–197. doi: 10.1016/j.ydbio.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Nakahara Y, Gage FH, Tuszynski MH. Grafts of fibroblasts genetically modified to secrete NGF, BDNF, NT-3, or basic FGF elicit differential responses in the adult spinal cord. Cell Transplant. 1996;5(2):191–204. doi: 10.1177/096368979600500209. [DOI] [PubMed] [Google Scholar]
- 10.Kim DH, Gutin PH, Noble LJ, Nathan D, Yu JS, Nockels RP. Treatment with genetically engineered fibroblasts producing NGF or BDNF can accelerate recovery from traumatic spinal cord injury in the adult rat. Neuroreport. 1996;7(13):2221–2225. doi: 10.1097/00001756-199609020-00033. [DOI] [PubMed] [Google Scholar]
- 11.Jin Y, Tessler A, Fischer I, Houle JD. Fibroblasts genetically modified to produce BDNF support regrowth of chronically injured serotonergic axons. Neurorehabil. Neural Repair. 2000;14(4):311–317. doi: 10.1177/154596830001400407. [DOI] [PubMed] [Google Scholar]
- 12.Tobias CA, Han SSW, Shumsky JS, Kim D, Tumolo M, Dhoot NO, Wheatley MA, Fischer I, Tessler A, Murray M. Alginate encapsulated BDNF-producing fibroblast grafts permit recovery of function after spinal cord injury in the absence of immune suppression. J. Neurotrauma. 2005;22(1):138–156. doi: 10.1089/neu.2005.22.138. [DOI] [PubMed] [Google Scholar]
- 13.Madhavan L, Ourednik V, Ouredni J. Neural Stem/Progenitor cells initiate the formation of cellular networks that provide neuroprotection by growth factor-modulated antioxidant expression. Stem Cells. 2008;26:254–265. doi: 10.1634/stemcells.2007-0221. [DOI] [PubMed] [Google Scholar]
- 14.Wu S, Suzuki Y, Kitada M, Kitaura M, Kataoka K, Takahashi J, Ide C, Nishimura Y. Migration, integration, and differentiation of hippocampus-derived neurosphere cells after transplantation into injured rat spinal cord. Neurosci. Lett. 2001;312(3):173–176. doi: 10.1016/s0304-3940(01)02219-4. [DOI] [PubMed] [Google Scholar]
- 15.Cummings BJ, Uchida N, Tamaki SJ, Anderson AJ. Human neural stem cell differentiation following transplantation into spinal cord injured mice: association with recovery of locomotor function. Neurol. Res. 2006;28(5):474–481. doi: 10.1179/016164106X115116. [DOI] [PubMed] [Google Scholar]
- 16.Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Morshead CM, Fehlings MG. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J. Neurosci. 2006;26(13):3377–3389. doi: 10.1523/JNEUROSCI.4184-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korochkin LI, Revishchin AV, Okhotin VE. Neural stem cells and their role in recovery processes in the nervous system. Neurosci. Behav. Physiol. 2006;36(5):499–512. doi: 10.1007/s11055-006-0047-3. [DOI] [PubMed] [Google Scholar]
- 18.Enzmann GU, Benton RL, Talbott JF, Cao Q, Whittemore SR. Functional considerations of stem cell transplantation therapy for spinal cord repair. J Neurotrauma. 2006;23:479–495. doi: 10.1089/neu.2006.23.479. [DOI] [PubMed] [Google Scholar]
- 19.Obermair F-J, Schröter A, Thallmair M. Endogenous neural progenitor cells as therapeutic target after spinal cord injury. Physiol. 2008;23:296–304. doi: 10.1152/physiol.00017.2008. [DOI] [PubMed] [Google Scholar]
- 20.Dillon GP, Yu XJ, Sridharan A, Ranieri JP, Bellamkonda RV. The influence of physical structure and charge on neurite extension in a 3D hydrogel scaffold. J. Biomater. Sci. Polym. Ed. 1998;9:1049–1069. doi: 10.1163/156856298x00325. [DOI] [PubMed] [Google Scholar]
- 21.Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, Stupp SI. Selective differentiation of neural progenitor cells by high epitope density nanofibers. Science. 2004;303:1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 22.Teixeira, Duckworth, Hermanson, Teixeira AI, Duckworth JK, Hermanson O. Getting the right stuff: Controlling neural stem cell state and fate in vivo and in vitro with biomaterials. Cell Res. 2007;17:56–61. doi: 10.1038/sj.cr.7310141. 2007. [DOI] [PubMed] [Google Scholar]
- 23.Akiyamaa Y, Honmou O, Kato T, Uede T, Kazuo Hashi K, Kocsis JD. Transplantation of Clonal Neural Precursor Cells Derived from Adult Human Brain Establishes Functional Peripheral Myelin in the Rat Spinal Cord. Exp. Neurol. 2001;167(1):27–39. doi: 10.1006/exnr.2000.7539. [DOI] [PubMed] [Google Scholar]
- 24.Cummings BJ, Uchida >N, Tamaki SJ, Salazar DL, Hooshmand M, Summers R, Levenberg S, Burdick JA, Kraehenbuehl T, Langer R. Neurotrophin-induced differentiation of human embryonic stem cells on three-dimensional polymeric scaffolds. Tissue Eng. 2005;11(3–4):506–512. doi: 10.1089/ten.2005.11.506. [DOI] [PubMed] [Google Scholar]
- 25.Nakajima M, Ishimuro T, Kato K, Ko I-K, Hiratac I, Arimaa Y, Iwata H. Combinatorial protein display for the cell-based screening of biomaterials that direct neural stem cell differentiation. Biomaterials. 2007;28:1048–1060. doi: 10.1016/j.biomaterials.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Novikov LN, Novikova LN, Mosahebi A, Wiberg M, Terenghi G, Kellerth J-O. A novel biodegradable implant for neuronal rescue and regeneration after spinal cord injury. Biomaterials. 2002;23(16):3369–3376. doi: 10.1016/s0142-9612(02)00037-6. [DOI] [PubMed] [Google Scholar]
- 27.Novikova LN, Novikov LN, Kellerth JO. Biopolymers and biodegradable smart implants for tissue regeneration after spinal cord injury. Curr. Opin. Neurol. 2003;16(6):711–715. doi: 10.1097/01.wco.0000102620.38669.3e. [DOI] [PubMed] [Google Scholar]
- 28.Novikova LN, Mosahebi A, Wiberg M, Terenghi G, Kellerth J-O, Novikov LN. Alginate hydrogel and matrigel as potential cell carriers for neurotransplantation. J. Biomed. Mater. Res . A. 2006;77(2):242–252. doi: 10.1002/jbm.a.30603. [DOI] [PubMed] [Google Scholar]
- 29.Kataoka K, et al. Kataoka K, Suzuki Y, Kitada M, Ohnishi K, Suzuki K, Tanihara M, Ide C, Endo K, Nishimura Y. Alginate a bioresorbable material derived from brown seaweed, enhances elongation of amputated axons of spinal cord in infant rats. J. Biomed. Mater. Res. 2001;54(3):373–384. doi: 10.1002/1097-4636(20010305)54:3<373::aid-jbm90>3.0.co;2-q. 2001. [DOI] [PubMed] [Google Scholar]
- 30.Kataoka K, Suzuki Y, Kitada M, Hashimoto T, Chou H, Bai H, Ohta M, Wu S, Suzuki K, Ide C. Alginate enhances elongation of early regenerating axons in spinal cord of young rats. Tissue Eng. 2004;10(3–4):493–504. doi: 10.1089/107632704323061852. [DOI] [PubMed] [Google Scholar]
- 31.Dhoot, et al. Dhoot NO, Tobias CA, Fischer IA, Wheatley M. Peptide-modified alginate surfaces as a growth permissive substrate for neurite outgrowth. J. Biomed. Mater. Res. A. 2004;71(2):191–200. doi: 10.1002/jbm.a.30103. 2004. [DOI] [PubMed] [Google Scholar]
- 32.Goosen MFA, O’Shea GM, Gharapetian HM, Chou S, Sun AM. Optimization of Microencapsulation Parameters: Semipermeable Microcapsules as a Bioartificial Pancreas. Biotechnol Bioeng. 1985;XXVI:146–150. doi: 10.1002/bit.260270207. [DOI] [PubMed] [Google Scholar]
- 33.Kuo CK, Ma PX. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: part 1. Structure, gelation rate and mechanical properties. Biomaterials. 2001;22(6):511–521. doi: 10.1016/s0142-9612(00)00201-5. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Himes T, Tryon B, Moul J, Chow SY, Jin H, Murray M, Tessler A, Fischer I. Intraspinal grafting of fibroblasts genetically modified by recombinant adenoviruses. Neuroreport. 1998;9(6):1075–1079. doi: 10.1097/00001756-199804200-00021. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Kim D, Himes BT, Chow SY, Schallert T, Murray M, Tessler A, Fischer I. Transplants of fibroblasts genetically modified to express BDNF promote regeneration of adult rat rubrospina axons and recovery of forelimb function. J. Neurosci. 1999;19:4370–4387. doi: 10.1523/JNEUROSCI.19-11-04370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tobias CA, Shumsky JS, Shibata M, Tuszynski MH, Fischer I, Tessler A, Murray M. Delayed grafting of BDNF and NT-3 producing fibroblasts into the injured spinal cord stimulates sprouting, partially rescues axotomized red nucleus neurons from loss and atrophy, and provides limited regeneration. Exp. Neurol. 2003;84:97–113. doi: 10.1016/s0014-4886(03)00394-7. [DOI] [PubMed] [Google Scholar]
- 37.Kanakasabai S. Alginate Strings and Their Applications in Spinal Cord Regeneration. Drexel University; 2007. PhD Thesis. [Google Scholar]
- 38.Vroemen M, Aigner L, Winkler J, Weidner N. Adult neural progenitor cell grafts survive after acute spinal cord injury and integrate along axonal pathways. Eur. J. Neurosci. 2003;18(4):743–745. doi: 10.1046/j.1460-9568.2003.02804.x. [DOI] [PubMed] [Google Scholar]
- 39.Read TA, Stensvaag V, Vindenes H, Ulvestad E, Bjerkvig R, Thorsen F. Cells encapsulated in alginate: a potential system for delivery of recombinant proteins to malignant brain tumours. Int. J. Dev. Neurosci. 1999;17(5–6):653–663. doi: 10.1016/s0736-5748(99)00052-0. [DOI] [PubMed] [Google Scholar]
- 40.Markusen JF, Mason C, Hull DA, Town MA, Tabor AB, Clements M, Boshoff CH, Dunnill P. Behavior of adult human mesenchymal stem cells entrapped in alginate-GRGDY beads. Tissue Eng. 2006;12(4):821–830. doi: 10.1089/ten.2006.12.821. [DOI] [PubMed] [Google Scholar]
- 41.Dar A, Shachar M, Leor J, Cohen S. Optimization of cardiac cell seeding and distribution in 3D porous alginate scaffolds. Biotechnol Bioeng. 2002;80(3):305–312. doi: 10.1002/bit.10372. [DOI] [PubMed] [Google Scholar]
- 42.Ma HL, Hung S-C, Lin S-Y, Chen Y-L, Lo W-H. Chondrogenesis of human mesenchymal stem cells encapsulated in alginate beads. J. Biomed. Mater. Res. A. 2003;64(2):273–281. doi: 10.1002/jbm.a.10370. [DOI] [PubMed] [Google Scholar]
- 43.Prang P, Müller R, Eljaouhari A, Heckmann K, Kunz W, Weber T, Faber C, Vroemen M, Bogdahn U, Weidner N. The promotion of oriented axonal regrowth in the injured spinal cord by alginate-based anisotropic capillary hydrogels. Biomaterials. 2006;27:3560–3569. doi: 10.1016/j.biomaterials.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 44.Ashton RS, Banerjee A, Punyani S, Schaffer D, Kane R. Scaffolds based on degradable alginate hydrogels and poly(lactide-coglycolide) microspheres for stem cell culture. Biomaterials. 2007;28(36):5518–25. doi: 10.1016/j.biomaterials.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 45.Solon, Solon J, Levental I, Sengupta K, Georges PC, Janmey PA. Fibroblast Adaptation and Stiffness Matching to Soft Elastic Substrates. Biophys. J. 2007;93:4453–4461. doi: 10.1529/biophysj.106.101386. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.von Wichert, et al. von Wichert G, Haimovich B, Feng GS, Sheetz MP. Force-dependent integrin-cytoskeleton linkage formation requires downregulation of focal complex dynamics by Shp2. EMBO J. 2003;22:5023–5035. doi: 10.1093/emboj/cdg492. 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kong HJ, Polte TR, Alsberg E, Mooney DJ. FRET measurements of cell-traction forces and nano-scale clustering of adhesion ligands varied by substrate stiffness. Proc. Natl. Acad. Sci. USA. 2005;102:4300–4305. doi: 10.1073/pnas.0405873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 49.Kearns SM, Laywell ED, Kukekov VK, Steindler DA. Extracellular matrix effects on neurosphere cell motility. Exp. Neurology. 2003;182:240–244. doi: 10.1016/s0014-4886(03)00124-9. [DOI] [PubMed] [Google Scholar]
- 50.Huebsch N, Arany PR, Mao AS, Shvartsman D, Omar A, Ali OA, Sidi A, Bencherif1 José Rivera-Feliciano, Mooney DA. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nature Mat. 2010;9:518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lachyankar MB, Condon PJ, Quesenberry PJ, Litofsky NS, Recht LD, Ross AH. Embryonic precursor cells that express Trk receptors: induction of different cell fates by NGF, BDNF, NT-3, and CNTF. Exp. Neurol. 1997;144(2):350–360. doi: 10.1006/exnr.1997.6434. [DOI] [PubMed] [Google Scholar]
- 52.Englund U, Bjorklund A, Wictorin K. Migration patterns and phenotypic differentiation of clong-term expanded human neural progenitor cells after transplantation into the adult rat brain. Brain Res. Dev Brain Res. 2002;134(1–2):123–141. doi: 10.1016/s0165-3806(01)00330-3. [DOI] [PubMed] [Google Scholar]
- 53.Ohyama K, Ellis P, Kimura S, Placzek M. Directed differentiation of neural cells to hypothalamic dopaminergic neurons. Development. 2005;132(23):5185–5197. doi: 10.1242/dev.02094. [DOI] [PubMed] [Google Scholar]
- 54.Jones B, Erin N, Mallapragada SK. Directed growth and differentiation of stem cells towards neural cell fates using soluble and surface-mediated cues. J. Biomater. Sci. Polym. Ed. 2007;18(8):999–1015. doi: 10.1163/156856207781494449. [DOI] [PubMed] [Google Scholar]
- 55.Caldwell MA, He X, Wilkie N, Pollack S, Marshall G, Wafford KA, Svendsen CN. Growth factors regulate the survival and fate of cells derived from human neurospheres. Nat. Biotechnol. 2001;19(5):475–479. doi: 10.1038/88158. [DOI] [PubMed] [Google Scholar]
- 56.Tabar V, Panagiotakos G, Greenberg ED, Chan BK, Sadelain M, Gutin PH, Stude L. Migration and differentiation of neural precursors derived from human embryonic stem cells in the rat brain. Nat. Biotechnol. 2005;23(5):601–606. doi: 10.1038/nbt1088. [DOI] [PubMed] [Google Scholar]
- 57.Goetz AK, Scheffler B, Chen H-X, Wang S, Suslov OH, Brüstle O, Roper SN, Steindler DA. Temporally restricted substrate interactions direct fate and specification of neural precursors derived from embryonic stem cells. Proc. Natl Acad Sci U S A. 2006;103(29):11063–11068. doi: 10.1073/pnas.0510926103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levenberg S, Burdick JA, Kraehenbuehl T, Langer R. Neurotrophin-induced differentiation of human embryonic stem cells on three-dimensional polymeric scaffolds. Tissue Eng. 2005;11(3–4):506–512. doi: 10.1089/ten.2005.11.506. [DOI] [PubMed] [Google Scholar]
- 59.Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, Stupp SI. Selective Differentiation of Neural Progenitor Cells by High–Epitope Density Nanofibers. Science. 2008;303:1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]