Abstract
Aims
To determine if naltrexone affects smoking behaviours in smokers preparing to quit, and whether such pre-quit responses predict post-quit date outcomes.
Design
Double-blind, placebo-controlled, randomized study. Current study focused on smoking-related outcomes in the pre-quit phase, which was one week prior to the quit date, and these findings were linked with reductions in same outcomes demonstrated in the post-quit phase previously published for this RCT in mediation analyses.
Setting
Community sample of adult smokers desiring to quit in Chicago, Illinois USA.
Participants
Participants were 315 smokers randomized to naltrexone (n=161; mean age=42.6 years; 60% white) or placebo (n=154; mean age=41.3 years; 55% white).
Measurements
Difference from baseline in the number of cigarettes smoked during pre-quit phase interval was the primary outcome. Secondary pre-quit outcomes were assessed using Likert scales of subjective responses and consumption of cigarettes, alcohol, and food. Number of cigarettes smoked, alcoholic drinks consumed, and the Brief Questionnaire of Smoking Urges were assessed in the post-quit phase.
Findings
Relative to placebo, naltrexone decreased the number of cigarettes smoked (−4.21 vs. −2.93, p<.05), smoking urge (p=.02), and number of alcoholic drinks consumed (p=.04). Exploratory mediation analyses linking outcomes of the pre quit and post quit phases found that naltrexone’s effects on reducing smoking urge, cigarettes smoked and alcoholic drinks consumed in the pre-quit phase demonstrated full mediation of their respective effects during the post-quit phase.
Conclusions
Naltrexone taken in the week before a quit attempt appears to reduce cigarette consumption, urges to smoke and alcohol consumption relative to placebo. The size of the effect statistically mediates the size of similar effects after the quit date.
Keywords: naltrexone, opioid antagonist, cigarette smoking, cotinine, smoking urge, alcohol
INTRODUCTION
Currently, 19% of adults in the United States smoke cigarettes and tobacco use remains the leading preventable cause of death (1). Despite the fact that more than two of every three smokers report a desire to quit smoking (2), most are unsuccessful in achieving this goal even when using currently approved pharmacotherapies (3). As there is evidence for interactions between the nicotinic and endogenous opioid systems (4, 5), a potential target for novel treatments may involve antagonism of opioid receptors to alter cigarette reward and related consummatory behaviors, such as alcohol consumption, that often precedes smoking (6) and reduces likelihood of treatment success (7–9). Naltrexone, a mu opioid receptor antagonist that is approved for the treatment of opioid and alcohol dependencies, has shown efficacy to improve quit rates and decrease smoking behaviors in some trials (10–14), but not in others (15, 16). Naltrexone may also decrease women’s long-term weight gain associated with quitting smoking (17). The 2006 Cochrane Report (18) evaluating the evidence for naltrexone in smoking cessation concluded that data are insufficient to make recommendations for its use in treating nicotine dependence. A greater understanding of specific biobehavioral mechanisms of naltrexone effects on smoking and other consummatory behaviors would enable more targeted use of the drug to optimize outcomes and allow comparisons with novel medications. The current study examined the effects of naltrexone on behavioral, objective, and subjective responses among treatment-seeking smokers participating in a clinical trial evaluation of naltrexone for smoking cessation during the naltrexone dose initiation phase prior to their quit date.
Results from acute human laboratory studies examining naltrexone effects on smoking behaviors have been mixed. Some studies have shown naltrexone attenuation in the number of cigarettes smoked, responses to smoking cues, or self-report craving (19–23), but other studies have failed to find effects of naltrexone on these indices (19, 24–26). While acute human laboratory paradigms are an important tool to characterize drug effects (27), conflicting results for naltrexone on cigarette smoking may be the result of assessments being limited to several hours after a single drug administration and samples consisting largely of non-treatment seekers. Examining naltrexone effects in a clinically-relevant paradigm and on a variety of domains in smokers desiring to quit may enable better elucidation of the mechanisms facilitating the opioid system to smoking behavior change, and allow for comparisons with other therapeutics.
The current study examined the effects of naltrexone on smoking and related behaviors among treatment-seeking smokers participating in a randomized, placebo-controlled, double-blinded trial of naltrexone for smoking cessation during the naltrexone dose initiation phase prior to their quit date. As this was the period of medication initiation, the dose was gradually up-titrated to minimize adverse effects, with the full therapeutic dose (50 mg) taken on the fourth through sixth day of the pre-quit phase. Naltrexone was hypothesized to reduce the number of cigarettes smoked in this phase (19, 21, 23). Naltrexone was also hypothesized to reduce smoking subjective effects including cigarette urge, taste, and pleasure and the number of alcoholic drinks consumed in the pre-quit phase (20–23, 28, 29). Exploratory analyses were conducted to examine whether naltrexone effects during the pre-quit week mediated outcomes after the quit date.
METHODS
Participant screening
Candidates were recruited by advertisements on print and radio media, mass transit, the Internet, and by word-of-mouth referrals. In-person screening included completion of questionnaires, and psychiatric and medical screening (for details, see King et al., 2012(13)). Participants were eligible if they were 18–65 years old; smoked 12–40 cigarettes daily for at least 2 years and reported a desire to quit smoking; had a body mass index of 19–38 kg/m2; had hepatic transaminase concentrations within normal range (<2.5 times normal); were able to read and write English; were not currently taking opioid or psychotropic medications; and did not have a past-year history of a major medical or psychiatric disorder, lifetime diagnosis of opioid abuse or dependence, and were not nursing or pregnant.
Treatments and Procedures
This pre-quit interval study was designed a priori as part of a larger smoking cessation trial. The study was located at three Chicago area sites, including the University of Chicago (58% of sample), as well as the Respiratory Health Association (26%) and the Howard Brown Health Center (16%). Participants were enrolled from June 2006–March 2009, with follow-ups completed by April 2010. All participants consented to randomization to receive either naltrexone or placebo, attend behavioral counseling, and take open-label nicotine patch after the quit date (13). Details of the computer-generated randomization and post-quit phase results are reported in King et al., 2012 (13). Naltrexone or placebo group assignment was stratified by sex. The study was fully approved by the University of Chicago Institutional Review Board.
Participants were given their assigned tablets in a daily pill box organizer one week prior to the quit date. The titrated dose included: 12.5 mg on day one, 25 mg daily on days two and three, and 50 mg on days four through six. The 50 mg dose was also continued daily on the quit date and throughout the post quit date phase to be consistent with the FDA-approved dose of naltrexone for alcohol and opioid dependence. To decrease nausea and other adverse effects, participants were encouraged to consume food prior to taking each tablet.
Participants completed a short questionnaire each evening prior to going to sleep during the pre-quit phase. The primary dependent measure was the number of cigarettes smoked (“today, how many cigarettes did you smoke?”). Other secondary measures included subjective ratings of smoking urge (“what was your urge to smoke?”), cigarette pleasure and taste (“what was your pleasure or enjoyment of smoking”, “what was the appealing taste of your cigarettes?”), and other consummatory behaviors including alcohol, eating, and caffeine consumption (the latter as a control item). To standardize consumption quantifications, subjects were informed that a cigarette included a single puff up to an entire cigarette, a caffeinated drink was 8 oz coffee/tea or a 12 oz caffeinated soda, and a standard alcoholic drink was 1½ ounces of liquor, 5 ounces of wine, or 12 ounces of beer. The subjective effects were rated on 5-point scales from a lot less than usual (1) to a lot more than usual (5). Adverse effects were each rated from none (1) to severe (5) and included three common naltrexone effects: “today, how much did you feel” to assess the items light-headed/dizzy, tired/sedated, and nauseated. As a check of naltrexone effects on general functioning, three additional items were included to assess anxious mood, depressed/sad mood, and the amount of sleep.
Tablet adherence was assessed by interview on the quit date and by collection of any unused tablets, and quantified as each participant’s ratio of the number of tablets taken to number disbursed. Adherence to naltrexone was also confirmed by objective measures, i.e., a urine and saliva test to determine naltrexone and its main metabolite, 6-β-naltrexol. The samples were collected by each participant on the morning of day seven, i.e., the designated quit date. The instructions included having the participant void upon awakening to empty the bladder. This was followed by tablet administration and collection of their urine for the next 180 minutes, and their saliva at 90 minutes. The participant brought all their samples to the study visit on the quit date and were compensated $35.
Assay methods
The methods for identification of naltrexone and its major metabolite, 6-β-naltrexol in the saliva or urine sample were performed by Ammon Laboratories (Linden, NJ) using the Immunalysis Naltrexone Direct ELISA Kit (Immunalysis Corporation, Pomona CA), and confirmed by gas chromatography-mass spectrometry (Agilent Technologies, Santa Clara, CA).
Post quit date procedures
As stated earlier, the pre-quit interval was the six days leading to each participant’s designated quit date. During the pre-quit interval, no instructions were given on smoking behaviors. However, starting on the quit date, the participant was expected to achieve abstinence from smoking. Participants initiated open-label nicotine patch starting on the quit date, and attended once weekly behavioral counseling, both of which ended four weeks after the quit date. Nicotine patch was included to reduce potential withdrawal-like effects that may be augmented in smokers given an opioid antagonist (30) and reduce drop out rates which have been high in trials of naltrexone alone (11). During weeks 5–12, naltrexone or placebo groups continued as a monotherapy for relapse prevention. Details of the post-quit phase portion of the trial can be found in King et al., 2012(13).
Statistical Analyses
Analysis was based on intent-to-treat sample (n=161 naltrexone, n=154 placebo), which we a priori defined as a subject who took at least one tablet (see CONSORT diagram, Figure 1). Twenty participants (6%) did not complete any data recording for the questionnaire, so mean imputation was used separately for participants in the naltrexone and placebo groups to replace these missing values. For other pre-quit measures, baseline values were used to replace missing values. Linear regression was used to assess the medication effect on the primary outcome (the change in number of cigarettes smoked during full dose phase from baseline) and the secondary outcomes (ratings for subjective effects, the change in number of alcoholic or caffeinated drinks versus baseline levels). Analyses included the unadjusted differences between groups and then were repeated in several hierarchical models adjusting for demographic variables (sex, age, education and race), adverse effects, and baseline smoking variables (nicotine dependence, carbon monoxide, number of prior quit attempts and smoking duration) For the latter, only ratings of nausea were included as the adverse effect since nausea is the most widely reported adverse effect of naltrexone and to avoid collinearity as ratings of nausea, dizziness and sedation were significantly intercorrelated (rs≥0.36, ps < .01). The intent-to-treat sample was used in all analyses except for analysis of the number of alcoholic drinks, which included only current drinkers, i.e., the 75% of the sample who drank at least one alcoholic beverage in the two weeks prior to enrollment.
Figure 1.
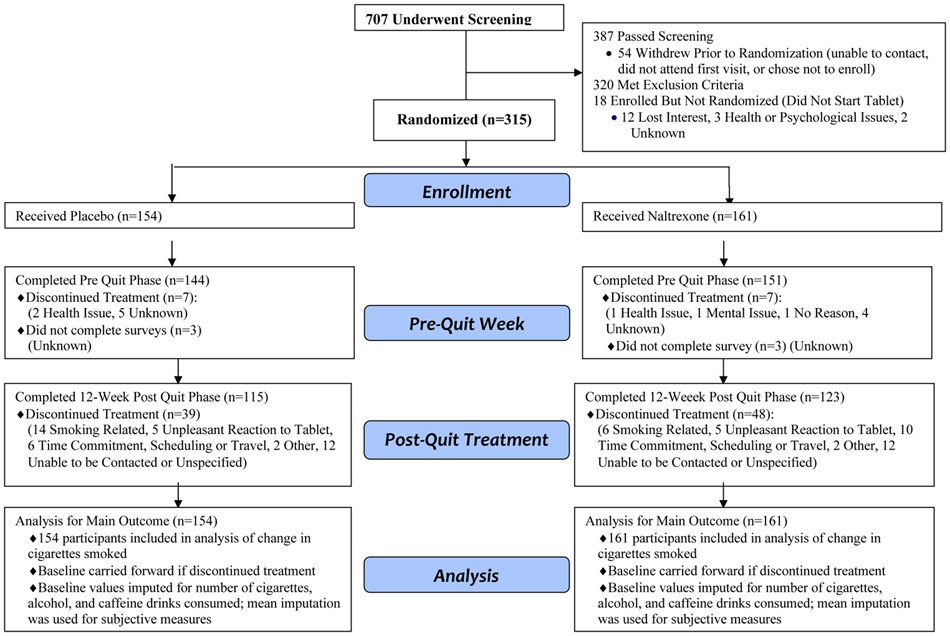
CONSORT Diagram. Flow diagram of the process through the phases of a randomized trial of naltrexone versus placebo groups.
Mediation analyses (31) were conducted to determine if various pre-quit effects served as mediators of their corresponding post-quit outcomes (13). For each post-quit outcome, three regression models were conducted sequentially for testing mediation (31), including examination of whether the medication effect was associated with the post-quit outcome and whether the medication effect was associated with the pre-quit measure. If these were significant, then the third model was conducted to examine whether the medication effect on the post quit outcome was still significant while including pre-quit measure, with mediation demonstrated if the medication effect was no longer significant.
This study was powered to detect an estimated hazard ratio of 1.97 (power=0.80; α=.05) for the second phase outcome of the comparison of prolonged abstinence quit rates at 12 weeks for the interactions of medication and sex(32).
RESULTS
Demographic and background characteristics of the intent-to-treat sample are included in Table 1. Tablet adherence during the pre-quit week was high for both groups with full adherence reported in 94% of those in the placebo group and 93% of those in the naltrexone group. Further, in the naltrexone group, 96% of participants were confirmed positive for detection of naltrexone or the metabolite 6-β-naltrexol.
Table 1.
Demographic and Smoking Characteristics
| Placebo (N=154) | Naltrexone (N=161) | |
|---|---|---|
|
| ||
| Demographic Variables: | ||
|
| ||
| Age (yrs) | 42.58 (10.87) | 41.32 (11.61) |
| Education (yrs) | 15.04 (2.19) | 15.12 (2.37) |
| Sex (male) | 71 (46) | 76 (47) |
| Marital status (married/living with partner) | 53 (34) | 49 (30) |
| Race (Caucasian) | 93 (60) | 88 (55) |
|
| ||
| Smoking and Alcohol Variables: | ||
|
| ||
| Smoking Urge (baseline B-QSU score) | 26.17 (12.30) | 28.30 (13.01) |
| Nicotine dependence (Fagerström score) | 5.31 (2.03) | 5.11 (1.73) |
| Baseline carbon monoxide level (ppm) | 24.35 (13.00) | 22.09 (10.90) |
| Number of prior quit attempts a | 4.08 (5.47) | 4.56 (8.93) |
| Cigarettes smoked per day | 16.55 (5.33) | 15.99 (5.12) |
| Smoking duration (years) | 24.88 (11.36) | 23.36 (11.90) |
| Drinks per drinking occasion b | 3.53 (2.29) | 3.41 (2.38) |
| Alcohol drinking days per week b | 2.39 (1.53) | 2.25 (1.57) |
Note: Data are Mean (SD) or N (%) as indicated.
Number of prior quit attempts defined as each attempt of intending to quit and remaining smoke-free for at least 12 hours.
Drinks per drinking occasion and alcohol drinking days per week were based on the one month timeline follow back interview prior to enrollment and summarized for current drinkers only (n=123 naltrexone, 109 placebo).
Adverse Effects and Moods
Naltrexone did not affect general mood or health states, including anxiety, depressed mood, or amount of sleep. However, naltrexone did increased adverse effects that have been reported previously in alcohol dependence studies (33), including nausea, dizziness, and sedation. Nausea was reported in 45% of naltrexone participants versus 22% of placebo participants and when it was experienced, it was most often rated as mild, i.e., in 88% of naltrexone and 94% of placebo participants. Further, a comparison of cigarette smoking (number of cigarettes smoked, smoking urge, pleasure and taste) in a median split of high and low nausea participants showed no differences (|t|s≤1.38, ps≥0.17).
Outcomes
In unadjusted analyses, naltrexone, compared with placebo, significantly reduced the number of cigarettes smoked (see Table 2 for estimated effect size). The reduction in smoking in the naltrexone group was 4.21 less cigarettes daily (26% reduction) compared with a 2.93 less cigarettes (17% reduction) in the placebo group. In terms of secondary outcomes, naltrexone also reduced smoking urge, cigarette taste and pleasure ratings, food pleasure, appetite, amount of sweet foods consumed, and number of alcoholic drinks consumed (Table 2). Caffeine use was unaffected by naltrexone.
Table 2.
Pre Quit Phase Cigarette Smoking and Other Behavioral and Subjective Effects
| Pre-Quit Phase | Linear Regression Models Testing Medication Effect | ||||||
|---|---|---|---|---|---|---|---|
| Placebo (n=154) | Naltrexone (n=161) | Mean Difference (95% CI) | p values | ||||
| Unadjusted: Medication Effect Only | Adjusted for Demographic Characteristics | Adjusted for Demographics and Nausea | Adjusted for Demographics, Nausea and Baseline | ||||
| Primary Outcomea | |||||||
| Cigarettes per day | −2.93 (4.54) | −4.21 (4.45) | 1.28 (0.28 to 2.27) | 0.01 | 0.02 | <0.05 | 0.02 |
| Secondary Behavioral Outcomes a | |||||||
| Alcoholic drinks per drinking day | −1.98 (2.98) | −1.94 (2.77) | 0.95 (0.21 to 1.70) | 0.01 | 0.02 | 0.01 | 0.04 |
| Caffeinated drinks per day | −1.22 (2.84) | −1.35 (2.21) | 0.13 (−0.46 to 0.71) | 0.7 | 0.83 | 0.95 | 0.9 |
| Secondary Subjective Effects b: | |||||||
| Smoking Urge | 2.58 (0.64) | 2.32 (0.65) | 0.25 (0.11 to 0.40) | <0.01 | <0.01 | 0.01 | 0.02 |
| Cigarette Pleasure | 2.40 (0.62) | 2.22 (0.63) | 0.18 (0.04 to 0.32) | 0.01 | 0.01 | 0.06 | 0.07 |
| Cigarette Taste | 2.32 (0.61) | 2.12 (0.63) | 0.19 (0.05 to 0.33) | 0.01 | 0.01 | 0.04 | 0.08 |
| Appetite ratings | 2.93 (0.58) | 2.72 (0.66) | 0.20 (0.07 to 0.34) | <0.01 | <0.01 | 0.09 | 0.42 |
| Food Pleasure | 2.88 (0.48) | 2.72 (0.58) | 0.17 (0.05 to 0.28) | 0.01 | 0.01 | 0.08 | 0.30 |
| Sweet food consumption | 2.83 (0.65) | 2.64 (0.69) | 0.19 (0.05 to 0.34) | 0.01 | 0.01 | 0.08 | 0.11 |
Note: Data are Mean change score (SD), or Mean score (SD).
Behavioral outcomes (cigarettes, alcoholic and caffeinated drinks) are expressed as change scores during pre-quit days 4–6 from baseline (2 weeks prior to enrollment).
Subjective effects were rating average on the 5-point scale and did not have a pre-treatment baseline.
In analyses adjusting for demographic characteristics only, all of the aforementioned effects of naltrexone remained. However, when adjusting for both demographic characteristics and nausea, the effects of naltrexone remained for number of cigarettes smoked, smoking urge, cigarette taste, and number of alcoholic drinks consumed but effects on cigarette pleasure, food pleasure, appetite, and sweet food consumption were no longer significant (Table 2). In the final set of analyses adjusting for demographic characteristics, nausea and baseline smoking variables, naltrexone’s significant reduction in smoking urge and number of cigarettes smoked and alcoholics drinks consumed remained (Table 2).
Mediators of Treatment Outcomes
As the first step of mediation, effects of naltrexone versus placebo during the pre quit and post quit phases were examined by regression analyses. Only those pre-quit effects significantly reduced by naltrexone in the final adjusted analyses (smoking urge and change in number of cigarettes and alcoholic drinks) were considered. For post-quit date outcomes, naltrexone significantly reduced the number of cigarettes smoked at four weeks post quit compared with placebo (2.6 vs. 5.2 cigarettes per week, respectively) and reduced smoking urge ratings (BQSU peak scores 25.4 vs. 28.7 placebo). Naltrexone also reduced the number of alcoholic drinks consumed (19.2 drinks consumed over first four weeks for naltrexone vs. 25.5 for placebo). In addition, naltrexone reduced number of alcoholic drinks consumed at 12 weeks (53.1 drinks consumed over twelve weeks for naltrexone vs. 68.4 for placebo). The final step of mediation analyses for these variables showed that the pre-quit effects fully mediated the naltrexone effects in the post-quit phase (Table 3).
Table 3.
Mediation Models of Pre-Quit Effects of Naltrexone to Post-Quit Outcomes
| Outcomes | Naltrexone predicting outcome Post Quit Phasea | Naltrexone predicting effect in Pre Phase Quit | Prequit outcome as Mediator | Mediation ? | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | se | p | B | se | p | B | se | p | ||
| At 4 Weeks after Quit Date | ||||||||||
| Δ Cigarettes Smoked | −2.60 | 1.24 | .04 | −1.28 | 0.51 | .01 | −2.31 | 1.25 | .07 | YES |
| Smoking Urge Ratings (B-QSU) | −3.27 | 1.49 | .02 | −0.25 | 0.07 | <.01 | −2.50 | 1.43 | .08 | YES |
| Δ Alcohol Drinks Consumed | −6.25 | 2.98 | .04 | −0.95 | 0.38 | .01 | −4.86 | 2.98 | .10 | YES |
| At 12 weeks after Quit Date | ||||||||||
| Δ Alcohol Drinks Consumed | −15.28 | 7.78 | .05 | −0.95 | 0.38 | .01 | −12.41 | 7.82 | .11 | YES |
Note. Data are the B, se, and p values in regression models for mediation analyses.
Post Quit phase refers to the period four weeks after the quit date for number of cigarettes smoked and smoking urge peak rating (B-QSU). For alcohol drinks consumed the post quit phase includes both four and twelve weeks after the quit date, as indicated.
DISCUSSION
To our knowledge, this study represents the first translational study of the acute mechanisms of opioid antagonism in nicotine dependent participants examined in a clinically relevant context. The findings elucidated naltrexone’s mechanism of action on smoking and other related indices. Consistent with our hypothesis, compared with placebo, naltrexone significantly reduced the number of cigarettes smoked. Naltrexone (vs. placebo) also decreased smoking urge and cigarette hedonics (pleasure, taste) during these final few days before the designated quit date. Naltrexone also reduced other consummatory behaviors during the pre-quit phase including alcohol drinking, appetite, and food pleasure ratings but after controlling for nausea, only the reduction in alcohol drinking remained. Naltrexone had no effects on mood, sleep, or caffeine use, suggesting that the medication did not produce malaise or dampening of all consummatory behaviors. Caffeine, a methylzanthine and central nervous system stimulant, binds primarily to adenosine receptors and is not directly involved with the opioid system (34) so neurobiologically, an opioid receptor antagonist would not be expected to alter intake within this substance class. On the other hand, pre-clinical research does implicate the role of the endogenous opioid system in feeding behaviors, food hedonics, and sucrose intake (35–39). However, these effects appear to be associated with nausea as they were no longer significant in the adjusted analyses. Given that gut and gastrointestinal processes are inherently tied to feeding behaviors and appetite, it was not surprising that naltrexone-induced nausea was associated with those effects, even if the nauseated feelings were mild and tolerable. Overall, the behavioral and subjective effects observed in the present study are supported by the neurobiological circuitry of the opioid system and its connections to dopaminergic pathways underlying motivational salience and hedonic pleasurable effects of nicotine, alcohol and eating behaviors (35, 40–42).
The present study findings have translational significance, as pre-quit measures of number of cigarettes smoked and smoking urge mediated naltrexone’s effects on their corresponding early post-quit outcomes. This demonstrates that smokers who are more sensitive to opioid antagonist effects before the target quit date may be likely to benefit, at least initially, from this pharmacotherapy in treatment. The findings are important because prior research has shown that markers early in treatment (43, 44) have better associations to post treatment outcomes than do pretreatment markers, so the challenges of identifying mediators of outcomes has been longstanding.
The results lend support to initiating naltrexone during a pre-quit interval not only to reduce adverse effects but also to enable a potential extinction phase of smoking reinforcement. The one-week pre-quit week titration schedule for naltrexone in the current study represents the longest interval examined to date in trials examining the threshold dose of 50 mg naltrexone in treatment (11, 12, 14). This interval was chosen a priori to facilitate the current investigation, as well as to reduce unpleasant side effects and to match the duration as recommended for other smoking cessation medications such as bupropion and varenicline. It is plausible that a longer pre-quit initiation of naltrexone might further enhance post quit date outcomes, as extended preloading could provide a longer extinction phase before the target quit date. For example, a recent study with varenicline demonstrated pre-quit medication for four weeks improved post quit date outcomes compared to the standard one-week dosing before the quit date (45). However, data are needed to determine if this is the case with naltrexone.
This study included several strengths, including both behavioral and subjective measures of smoking and other consummatory behaviors in a large sample and demonstration of the clinical significance of early sensitivity to naltrexone before the quit date to initial treatment outcomes after the quit date. However, there are some limitations worth noting. First, baseline data prior to randomization were included for behavioral measures but not for subjective measures. However, the groups did not differ on other measures of smoking at baseline, including nicotine dependence scores from the Fagerström scale, number of cigarettes smoked, number of prior quit attempts, and BQSU smoking urge ratings(13). Also, saliva and urine samples for medication adherence confirmation was collected by participants in their own environment which may have affected validity but was chosen to avoid undue burden on participants to have an extended visit on quit day considering their already stressful pre-quit week and demands on their time. Second, the main dependent variables were assessed during a period of behavior change with declines in many measures even in the placebo group. This was not entirely unexpected since participants were anticipating and preparing for their quit date and taking a tablet daily without knowing whether or not it was the active medication. Finally, only one dose of naltrexone was examined (50 mg) so the study could not determine dose-ranging effects.
In sum, the current study demonstrated novel findings with regard to naltrexone effects on smoking indices and other behaviors in smokers preparing to quit. Naltrexone reduced cigarette smoking and urges and alcohol consumption before the designated quit date, and these effects mediated the medication’s effect on these outcomes in the post quit phase. The current findings lend preclinical support for continued research to evaluate the potential role for naltrexone as a treatment adjunct for smoking cessation on numerous clinically-relevant outcomes, and to potentially extend pre-quit date medication initiation to examine if effects after the quit date might be augmented with a longer extinction-type phase. Future research examining biomarkers in those more sensitive to naltrexone, such as OPRM1 and other genetic factors(46) is warranted to determine if genetic factors can help identify those most likely to benefit from naltrexone in smoking treatment, as well as continued study of naltrexone effects on smoking and drinking outcomes in those with co-use of these substances (47–49). Naltrexone is generally well-tolerated and approved for the treatment of alcohol and opioid dependencies, and there may be a role for repurposing the medication in the treatment of nicotine dependence. Further understanding of mechanisms may enable targeted and more effective use of this medication and/or facilitate comparisons with novel therapeutics.
Acknowledgments
This study was supported by a grant from the National Institute of Drug Abuse (#R01-DA016834). We thank the Howard Brown Health Center and Respiratory Health Association of Metropolitan Chicago for their overall support and for providing satellite study locations. Appreciation is also extended to Gerard Meenan, Ammon Laboratories for biological assays. Finally, we thank Dr. Tracie Wilcox for medical oversight and Ryan Stachoviak for assistance with data collection and database management.
Footnotes
Conflict of interest:
Authors reported no conflict of interest.
Clinicaltrials.gov identifier: Efficacy of Naltrexone in Women’s Smoking Cessation: http://clinicaltrials.gov/ct2/show/NCT00271024?term=NCT00271024&rank=1 NCT00271024
References
- 1.CDC. Current Cigarette Smoking Prevalence Among Working Adults—United States, 2004–2010. Morbidity and Mortality Weekly Report. 2011;60:1305–9. [PubMed] [Google Scholar]
- 2.CDC. Quitting Smoking Among Adults---United States, 2001—2010. Morbidity and Mortality Weekly Report. 2011;60:1513–9. [PubMed] [Google Scholar]
- 3.Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Clinical Practice Guideline. Rockville, MD: US Department of Health and Human Services Public Health Service; 2008. May, Treating tobacco use and dependence: 2008 update. [Google Scholar]
- 4.Pomerleau OF. Endogenous opioids and smoking: a review of progress and problems. Psychoneuroendocrinology. 1998 Feb;23(2):115–30. doi: 10.1016/s0306-4530(97)00074-7. [DOI] [PubMed] [Google Scholar]
- 5.Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine Tob Res. 2000 Feb;2(1):19–37. doi: 10.1080/14622200050011277. [DOI] [PubMed] [Google Scholar]
- 6.McKee SA, Krishnan-Sarin S, Shi J, Mase T, O’Malley SS. Modeling the effect of alcohol on smoking lapse behavior. Psychopharmacology (Berl) 2006 Dec;189(2):201–10. doi: 10.1007/s00213-006-0551-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borland R. Slip-ups and relapse in attempts to quit smoking. Addict Behav. 1990;15(3):235–45. doi: 10.1016/0306-4603(90)90066-7. [DOI] [PubMed] [Google Scholar]
- 8.Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. J Consult Clin Psychol. 1996 Apr;64(2):366–79. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- 9.Shiffman S, Gwaltney CJ, Balabanis MH, Liu KS, Paty JA, Kassel JD, et al. Immediate antecedents of cigarette smoking: an analysis from ecological momentary assessment. J Abnorm Psychol. 2002 Nov;111(4):531–45. doi: 10.1037//0021-843x.111.4.531. [DOI] [PubMed] [Google Scholar]
- 10.Byars JA, Frost-Pineda K, Jacobs WS, Gold MS. Naltrexone augments the effects of nicotine replacement therapy in female smokers. J Addict Dis. 2005;24(2):49–60. doi: 10.1300/J069v24n02_05. [DOI] [PubMed] [Google Scholar]
- 11.Covey LS, Glassman AH, Stetner F. Naltrexone effects on short-term and long-term smoking cessation. J Addict Dis. 1999;18(1):31–40. doi: 10.1300/J069v18n01_04. [DOI] [PubMed] [Google Scholar]
- 12.King A, de Wit H, Riley RC, Cao D, Niaura R, Hatsukami D. Efficacy of naltrexone in smoking cessation: a preliminary study and an examination of sex differences. Nicotine Tob Res. 2006 Oct;8(5):671–82. doi: 10.1080/14622200600789767. [DOI] [PubMed] [Google Scholar]
- 13.King AC, Cao D, O’Malley SS, Kranzler HR, Cai X, Dewit H, et al. Effects of naltrexone on smoking cessation outcomes and weight gain in nicotine-dependent men and women. J Clin Psychopharmacol. 2012 Oct;32(5):630–6. doi: 10.1097/JCP.0b013e3182676956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Malley SS, Cooney JL, Krishnan-Sarin S, Dubin JA, McKee SA, Cooney NL, et al. A controlled trial of naltrexone augmentation of nicotine replacement therapy for smoking cessation. Arch Intern Med. 2006 Mar 27;166(6):667–74. doi: 10.1001/archinte.166.6.667. [DOI] [PubMed] [Google Scholar]
- 15.Toll BA, White M, Wu R, Meandzija B, Jatlow P, Makuch R, et al. Low-dose naltrexone augmentation of nicotine replacement for smoking cessation with reduced weight gain: a randomized trial. Drug Alcohol Depend. 2010 Oct 1;111(3):200–6. doi: 10.1016/j.drugalcdep.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong GY, Wolter TD, Croghan GA, Croghan IT, Offord KP, Hurt RD. A randomized trial of naltrexone for smoking cessation. Addiction. 1999 Aug;94(8):1227–37. doi: 10.1046/j.1360-0443.1999.948122713.x. [DOI] [PubMed] [Google Scholar]
- 17.King AC, Cao D, Zhang L, O’Malley S. Naltrexone Reduction of Long-Term Smoking Cessation Weight Gain in Women: A Randomized Controlled Trial. Am J Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.09.025. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.David S, Lancaster T, Stead LF, Evins AE. Opioid antagonists for smoking cessation. Cochrane Database Syst Rev. 2006;(4):CD003086. doi: 10.1002/14651858.CD003086.pub2. [DOI] [PubMed] [Google Scholar]
- 19.Epstein AM, King AC. Naltrexone attenuates acute cigarette smoking behavior. Pharmacol Biochem Behav. 2004 Jan;77(1):29–37. doi: 10.1016/j.pbb.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Hutchison KE, Monti PM, Rohsenow DJ, Swift RM, Colby SM, Gnys M, et al. Effects of naltrexone with nicotine replacement on smoking cue reactivity: preliminary results. Psychopharmacology (Berl) 1999 Feb;142(2):139–43. doi: 10.1007/s002130050872. [DOI] [PubMed] [Google Scholar]
- 21.King AC, Meyer PJ. Naltrexone alteration of acute smoking response in nicotine-dependent subjects. Pharmacol Biochem Behav. 2000 Jul;66(3):563–72. doi: 10.1016/s0091-3057(00)00258-6. [DOI] [PubMed] [Google Scholar]
- 22.Lee YS, Joe KH, Sohn IK, Na C, Kee BS, Chae SL. Changes of smoking behavior and serum adrenocorticotropic hormone, cortisol, prolactin, and endogenous opioids levels in nicotine dependence after naltrexone treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2005 Jun;29(5):639–47. doi: 10.1016/j.pnpbp.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Wewers ME, Dhatt R, Tejwani GA. Naltrexone administration affects ad libitum smoking behavior. Psychopharmacology (Berl) 1998 Nov;140(2):185–90. doi: 10.1007/s002130050756. [DOI] [PubMed] [Google Scholar]
- 24.Brauer LH, Behm FM, Westman EC, Patel P, Rose JE. Naltrexone blockade of nicotine effects in cigarette smokers. Psychopharmacology (Berl) 1999 Apr;143(4):339–46. doi: 10.1007/s002130050957. [DOI] [PubMed] [Google Scholar]
- 25.Rohsenow DJ, Monti PM, Hutchison KE, Swift RM, MacKinnon SV, Sirota AD, et al. High-dose transdermal nicotine and naltrexone: effects on nicotine withdrawal, urges, smoking, and effects of smoking. Exp Clin Psychopharmacol. 2007 Feb;15(1):81–92. doi: 10.1037/1064-1297.15.1.81. [DOI] [PubMed] [Google Scholar]
- 26.Sutherland G, Stapleton JA, Russell MA, Feyerabend C. Naltrexone, smoking behaviour and cigarette withdrawal. Psychopharmacology (Berl) 1995 Aug;120(4):418–25. doi: 10.1007/BF02245813. [DOI] [PubMed] [Google Scholar]
- 27.Plebani JG, Ray LA, Morean ME, Corbin WR, Mackillop J, Amlung M, et al. Human Laboratory Paradigms in Alcohol Research. Alcohol Clin Exp Res. 2012 Feb 6; doi: 10.1111/j.1530-0277.2011.01704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davidson D, Swift R, Fitz E. Naltrexone increases the latency to drink alcohol in social drinkers. Alcohol Clin Exp Res. 1996 Jun;20(4):732–9. doi: 10.1111/j.1530-0277.1996.tb01679.x. [DOI] [PubMed] [Google Scholar]
- 29.Anton RF, Drobes DJ, Voronin K, Durazo-Avizu R, Moak D. Naltrexone effects on alcohol consumption in a clinical laboratory paradigm: temporal effects of drinking. Psychopharmacology (Berl) 2004 Apr;173(1–2):32–40. doi: 10.1007/s00213-003-1720-7. [DOI] [PubMed] [Google Scholar]
- 30.Krishnan-Sarin S, Rosen MI, O’Malley SS. Naloxone challenge in smokers. Preliminary evidence of an opioid component in nicotine dependence. Arch Gen Psychiatry. 1999 Jul;56(7):663–8. doi: 10.1001/archpsyc.56.7.663. [DOI] [PubMed] [Google Scholar]
- 31.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986 Dec;51(6):1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 32.Schoenfeld DA. Sample-size formula for the proportional-hazards regression model. Biometrics. 1983 Jun;39(2):499–503. [PubMed] [Google Scholar]
- 33.Croop RS, Faulkner EB, Labriola DF. The safety profile of naltrexone in the treatment of alcoholism. Results from a multicenter usage study. The Naltrexone Usage Study Group. Arch Gen Psychiatry. 1997 Dec;54(12):1130–5. doi: 10.1001/archpsyc.1997.01830240090013. [DOI] [PubMed] [Google Scholar]
- 34.Nehlig A, Daval JL, Debry G. Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res Brain Res Rev. 1992 May-Aug;17(2):139–70. doi: 10.1016/0165-0173(92)90012-b. [DOI] [PubMed] [Google Scholar]
- 35.Kenny PJ. Common cellular and molecular mechanisms in obesity and drug addiction. Nat Rev Neurosci. 2011 Nov;12(11):638–51. doi: 10.1038/nrn3105. [DOI] [PubMed] [Google Scholar]
- 36.Glass MJ, Billington CJ, Levine AS. Opioids and food intake: distributed functional neural pathways? Neuropeptides. 1999 Oct;33(5):360–8. doi: 10.1054/npep.1999.0050. [DOI] [PubMed] [Google Scholar]
- 37.Pecina S, Smith KS. Hedonic and motivational roles of opioids in food reward: implications for overeating disorders. Pharmacol Biochem Behav. 2010 Nov;97(1):34–46. doi: 10.1016/j.pbb.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 38.Wong KJ, Wojnicki FH, Corwin RL. Baclofen, raclopride, and naltrexone differentially affect intake of fat/sucrose mixtures under limited access conditions. Pharmacol Biochem Behav. 2009 May;92(3):528–36. doi: 10.1016/j.pbb.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeomans MR, Gray RW. Opioid peptides and the control of human ingestive behaviour. Neurosci Biobehav Rev. 2002 Oct;26(6):713–28. doi: 10.1016/s0149-7634(02)00041-6. [DOI] [PubMed] [Google Scholar]
- 40.Kenny PJ. Reward mechanisms in obesity: new insights and future directions. Neuron. 2011 Feb 24;69(4):664–79. doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993 Sep-Dec;18(3):247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 42.Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008 Oct 12;363(1507):3137–46. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Higgins ST, Heil SH, Dumeer AM, Thomas CS, Solomon LJ, Bernstein IM. Smoking status in the initial weeks of quitting as a predictor of smoking-cessation outcomes in pregnant women. Drug Alcohol Depend. 2006 Nov 8;85(2):138–41. doi: 10.1016/j.drugalcdep.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 44.Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter D, Baker TB. Predicting smoking cessation. Who will quit with and without the nicotine patch. JAMA. 1994 Feb 23;271(8):589–94. doi: 10.1001/jama.271.8.589. [DOI] [PubMed] [Google Scholar]
- 45.Hajek P, McRobbie HJ, Myers KE, Stapleton J, Dhanji AR. Use of varenicline for 4 weeks before quitting smoking: decrease in ad lib smoking and increase in smoking cessation rates. Arch Intern Med. 2011 Apr 25;171(8):770–7. doi: 10.1001/archinternmed.2011.138. [DOI] [PubMed] [Google Scholar]
- 46.Oslin DW, Berrettini WH, O’Brien CP. Targeting treatments for alcohol dependence: the pharmacogenetics of naltrexone. Addict Biol. 2006 Sep;11(3–4):397–403. doi: 10.1111/j.1369-1600.2006.00036.x. [DOI] [PubMed] [Google Scholar]
- 47.King A, Cao D, Vanier C, Wilcox T. Naltrexone decreases heavy drinking rates in smoking cessation treatment: an exploratory study. Alcohol Clin Exp Res. 2009 Jun;33(6):1044–50. doi: 10.1111/j.1530-0277.2009.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Malley SS, Krishnan-Sarin S, McKee SA, Leeman RF, Cooney NL, Meandzija B, et al. Dose-dependent reduction of hazardous alcohol use in a placebo-controlled trial of naltrexone for smoking cessation. Int J Neuropsychopharmacol. 2009 Jun;12(5):589–97. doi: 10.1017/S146114570800936X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fucito LM, Park A, Gulliver SB, Mattson ME, Gueorguieva RV, O’Malley SS. Cigarette Smoking Predicts Differential Benefit from Naltrexone for Alcohol Dependence. Biol Psychiatry. 2012 Apr 25; doi: 10.1016/j.biopsych.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]


