Abstract
This review examines the role that respiratory plasticity has in the maintenance of breathing stability during sleep in individuals with sleep apnea. The initial portion of the review considers the manner in which repetitive breathing events may be initiated in individuals with sleep apnea. Thereafter, the role that two forms of respiratory plasticity, progressive augmentation of the hypoxic ventilatory response and long-term facilitation of upper airway and respiratory muscle activity, might have in modifying breathing events in humans is examined. In this context, present knowledge regarding the initiation of respiratory plasticity in humans during wakefulness and sleep is addressed. Also, published findings which reveal that exposure to intermittent hypoxia promotes breathing instability, at least in part, because of progressive augmentation of the hypoxic ventilatory response and the absence of long-term facilitation, are considered. Next, future directions are presented and are focused on the manner in which forms of plasticity that stabilize breathing might be promoted while diminishing destabilizing forms, concurrently. These future directions will consider the potential role of circadian rhythms in the promotion of respiratory plasticity and the role of respiratory plasticity in enhancing established treatments for sleep apnea.
Keywords: progressive augmentation, long-term facilitation, circadian rhythms, upper airway muscles, intermittent hypoxia
1. Introduction
Prior reviews from this laboratory focused in part, on basic mechanisms responsible for initiating two forms of respiratory plasticity, progressive augmentation of the hypoxic ventilatory response and long-term facilitation (Fig. 1). In addition, protocols and preparations used to initiate these forms of respiratory plasticity have been addressed (Mateika and Narwani, 2009; Mateika and Sandhu, 2011). To avoid repeating information extensively, we refer readers to these prior publications and to other publications to gain an essential understanding of respiratory plasticity, if necessary (Mahamed and Mitchell, 2007; Mateika and Narwani, 2009; Mateika and Sandhu, 2011; Mitchell et al. 2001; Mitchell and Johnson, 2003). The goal of this review is to focus principally on a hypothetical scenario outlined previously (Mateika and Narwani, 2009), in order to present recent empirical evidence that adds to development of the hypothesis. This scenario is focused on the impact that enhancement of progressive augmentation of the hypoxic ventilatory response, induced by alterations in chemoreflex properties, and long-term facilitation of respiratory and upper airway muscles, have on breathing stability in individuals with sleep apnea. We also address a second hypothetical scenario outlined in our previous review (Mateika and Narwani, 2009). This hypothesis is focused on the possible role that intermittent hypoxia has in the depression of upper airway muscle activity and its ultimate impact on breathing stability. In this case, less detail is presented because the hypothesis has not been empirically tested in humans.
Figure 1.
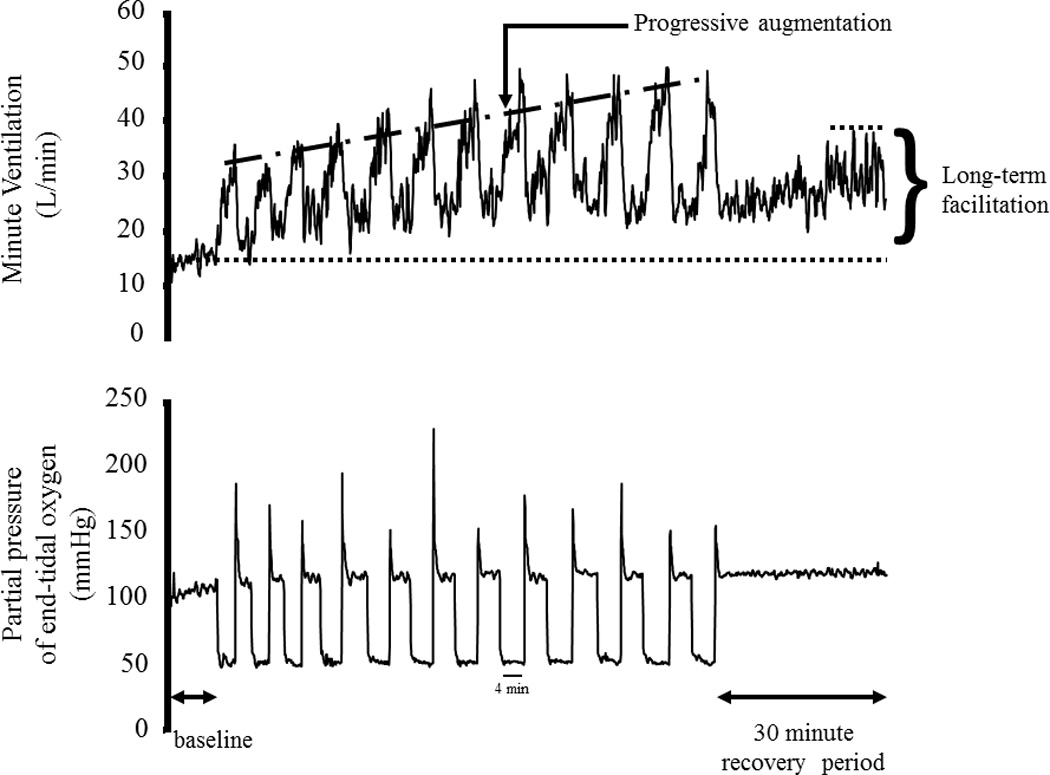
Breath-by-breath minute ventilation values recorded from a human participant before, during and following exposure to 12 episodes of hypoxia. Each hypoxic episode and subsequent recovery period was 4 min in duration with the exception of the last recovery period, which was 30 min in duration. Note that during exposure to intermittent hypoxia the ventilatory response to hypoxia gradually increased from the initial hypoxic episode to the final hypoxic episode. This phenomenon is referred to as progressive augmentation. Also note that during exposure to intermittent hypoxia minute ventilation gradually increased during the normoxic recovery periods so that it was substantially higher during the final recovery period compared with baseline. This phenomenon is referred to as long-term facilitation. Republished from Mateika and Narwani (2009), Exp. Physiol. 91, 89–102.
The review that follows will initially provide brief definitions of the various forms of plasticity that may contribute to breathing stability. Thereafter, the hypothetical scenario and updated empirical evidence reinforcing the hypothesis will be presented. In presenting the published experimental findings we will also include a section that addresses side issues (see “Research Aside”) related to the data, which might be of interest to the reader and may provoke additional research studies. A substantial amount of the review will also focus on future experimental directions centered principally on identifying methods to promote mechanisms of respiratory plasticity that ultimately lead to improved breathing stability. These directions will include the potential role of circadian rhythms in the promotion of respiratory plasticity and the role of respiratory plasticity in enhancing established treatments for sleep apnea.
2. Sleep apnea and breathing instability
As shown in Fig. 2, the initiation of a single apnea often leads to a cycle of recurrent breathing events that is the hallmark of breathing instability. Initiation of this cycle is linked in part to the hypoxia and possibly hypercapnia that transpires during apneic events, which ultimately leads to activation of peripheral and perhaps central chemoreceptors. Activation of these receptors, usually coupled with arousal from sleep, leads to increases in ventilation in order to correct for blood gas alterations. However, the increase in ventilation that occurs is frequently inappropriate for the accompanying metabolic rate. The result is the development of hypocapnia, which under conditions of wakefulness may not have a significant impact on ventilation because of the presence of “wakefulness stimuli” (Shea, 1996). Conversely, if sleep is re-established, as is often the case, the presence of hypocapnia leads to a reduction or abolition of central respiratory drive. This often occurs during non-rapid eye movement sleep because ventilation is believed to be controlled primarily by input from the peripheral and central chemoreceptors (Phillipson and Bowes, 1986). These receptors have a firing threshold that is sensitive to carbon dioxide levels. If carbon dioxide levels are beneath the chemoreflex threshold, central respiratory drive and chest wall respiratory muscle activity are eliminated culminating in a central apnea. The carbon dioxide level that demarcates the point at which ventilation is abolished has been deemed the apneic threshold (Dempsey, 2005; Xie et al. 2002; Zhou et al. 2000). The absence of central drive is also coupled to a reduction in upper airway muscle activity and ultimately to partial or complete closure of the upper airway (Badr et al. 1995; Badr and Kawak, 1996; Badr et al. 1997). This sequence of events often repeats throughout the night.
Figure 2.
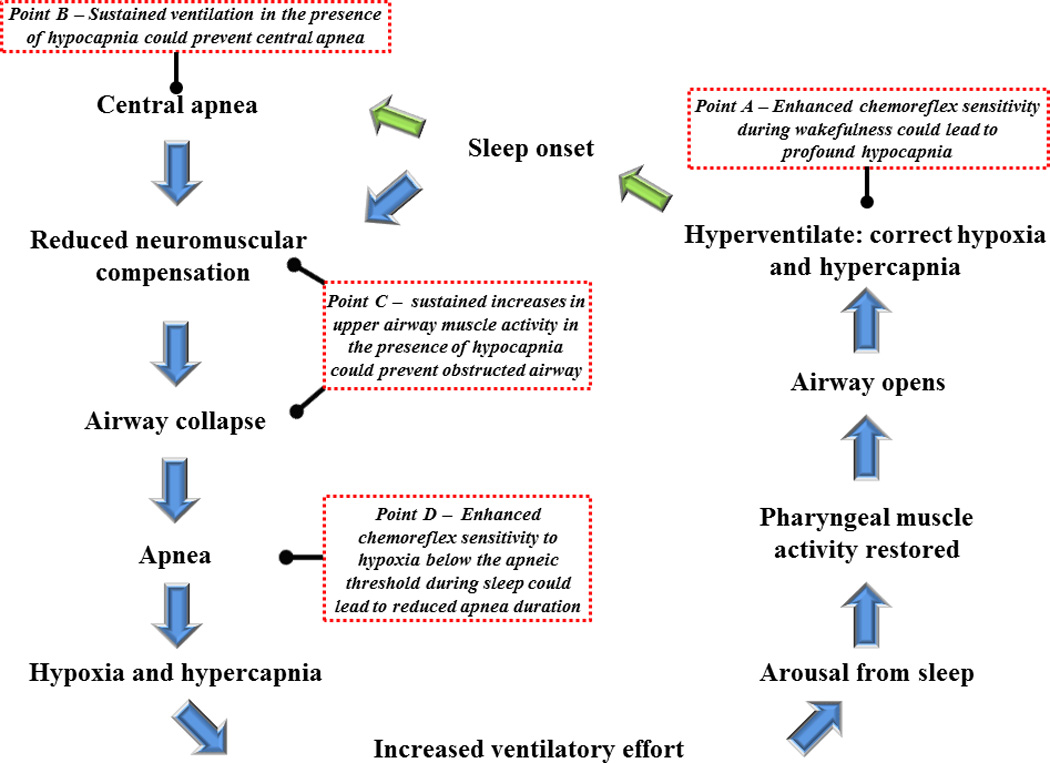
Schematic diagram showing the sequence of events leading to the development of a central and/or obstructive apnea, and subsequent events that ultimately results in re-establishing patency of the upper airway. In addition, various points along the pathway (see Points A–D) are highlighted. These points highlight outcomes that might occur in response to the manifestation of progressive augmentation of the hypoxic ventilatory response or long term facilitation of minute ventilation or upper airway muscle activity.
There are points within the breathing stability cycle where mechanistic adaptations that impact on the control of breathing could potentially ameliorate breathing instability, while modifications at other points could potentiate instability. More specifically, enhancement of peripheral and possibly central chemoreflex sensitivity, particularly during wakefulness (see Point A - Fig. 2), would likely induce profound hypocapnia, resulting in carbon dioxide levels that are significantly below the apneic threshold. This enhanced sensitivity, which might be coupled with a reduction in the carbon dioxide reserve [i.e. the difference between the carbon dioxide value that demarcates the apneic threshold and normal resting values - see Fig. 2 in (Dempsey, 2005) or Fig. 4 in (Mateika and Narwani, 2009) for additional clarification of carbon dioxide reserve if required], could ultimately result in a prolonged central and obstructive apnea during sleep because of the time required for carbon dioxide to build and exceed the apneic threshold. Alternatively, if respiratory muscle activity was enhanced by a mechanism that was independent of chemoreflex input (see Respiratory Plasticity for this discussion) and continued to persist in the presence of hypocapnia (Point B – Fig 1), the development of a central apnea could be prevented. Similarly, the initiation of a subsequent obstructive event (Point C – Fig. 2) would be avoided if upper airway muscle activity was augmented and able to persist in the presence of hypocapnia. Interestingly, although enhancement of chemoreflex sensitivity during wakefulness would likely contribute to perpetuating apnea, a similar alteration in sensitivity during sleep might minimize the duration of an apneic event (Point D – Fig. 2), since increased receptor feedback could elicit an arousal or increase upper airway muscle activity. For this hypothesis to be correct, it is necessary that the peripheral chemoreflex responds to hypoxia in the presence of hypocapnia induced by hyperventilation during wakefulness. However, previous studies in humans suggest that this may not be the case (Duffin, 2007; Jounieaux et al. 2002; Mohan and Duffin, 1997). If the peripheral chemoreflex does not respond to hypoxia in the presence of hypocapnia, it is likely that apnea duration would be prolonged, despite enhancement of the peripheral chemoreflex, because of the duration of time required for carbon dioxide to slowly build to the point that the apneic threshold is exceeded.
3. Respiratory Plasticity
3.1 Sleep apnea and long-term facilitation of ventilation and upper airway muscle activity
There is much interest in establishing the stimuli and/or conditions that promote mechanisms that stabilize breathing, while minimizing mechanisms that foster breathing instability (Mahamed and Mitchell, 2007; Mateika and Narwani, 2009;White, 2007). This attraction led to the hypothesis that respiratory plasticity may have a role in preventing cyclical events associated with sleep apnea. Respiratory plasticity is defined as the capacity for continuous alteration of neural pathways and synapses of the nervous system involved in generating respiratory activity in response to prior experience (Mitchell and Johnson, 2003). This hypothesis was initially proposed after a number of studies showed that exposure to brief episodes of intermittent hypoxia led to sustained elevations in phrenic nerve/diaphragmatic muscle activity, as well as hypoglossal nerve/genioglossus muscle activity, after removal of the stimulus in a variety of animal species (Bach and Mitchell, 1996; Cao et al. 1992; Fregosi and Mitchell, 1994; Hayashi et al. 1993; Mateika and Fregosi, 1997; McKay et al. 2004; Millhorn et al. 1980a; Mitchell et al. 2001; Peng and Prabhakar, 2003; Peng et al. 2006; Sokolowska and Pokorski, 2006; Turner and Mitchell, 1997; Zabka et al. 2001). This form of respiratory plasticity was referred to as long-term facilitation (Fig. 1). Some of the neuromodulators responsible for initiating long-term facilitation, including serotonin (Fregosi and Mitchell, 1994;Mateika and Narwani, 2009;Millhorn et al. 1980b; Mitchell et al. 2001), norepinephrine (Mody et al. 2011; Stettner et al. 2012) and adenosine (Golder et al. 2008; Hoffman et al. 2010; Hoffman and Mitchell, 201; Hoffman et al. 2012; Nichols et al. 2012) have been identified. In addition, many components of the cellular pathways involved in the initiation of long-term facilitation have been determined (Dale-Nagle et al. 2010; Hoffman etal. 2012; Macfarlane et al. 2008; Macfarlane and Mitchell, 2009; Macfarlane et al. 2009; Satriotomo et al. 2012).
The finding that intermittent hypoxia induced long-term facilitation was intriguing to many because this stimulus is a hallmark of sleep apnea. Therefore, it was further postulated that exposure to intermittent hypoxia throughout the night might lead to initiation of long-term facilitation of respiratory and upper airway muscle activity, which could mitigate the cyclical events characteristic of breathing instability. If this was the case, long-term facilitation of respiratory and upper airway muscle would have to persist in the presence of hypocapnia, given that this blood gas condition contributes to the perpetuation of cyclic events. If so, long-term facilitation of respiratory muscle activity might contribute to preventing the development of a central apnea (Point B - Fig. 2), while long-term facilitation of upper airway muscle activity could prevent the subsequent obstructive event (Point C - Fig. 2).
Given these speculations, it was imperative to demonstrate that long-term facilitation of ventilation and upper airway muscle activity could be induced in humans following exposure to intermittent hypoxia. However, initial studies indicated that these phenomena could not be induced in healthy males and females (Harris et al. 2006; Jordan et al. 2002; Mateika et al. 2004; Morelli et al. 2004), and in individuals with sleep apnea following exposure to intermittent hypoxia during wakefulness (Khodadadeh et al. 2006). We eventually determined that long-term facilitation did not manifest itself because of the presence of hypocapnia (Harris et al. 2006) and showed that the phenomenon was clearly evident once carbon dioxide was sustained above baseline levels. Specifically, we demonstrated that long-term facilitation of ventilation and genioglossus muscle activity could be induced by intermittent hypoxia in healthy males and females (Harris et al. 2006; Wadhwa et al. 2008). Likewise, we found that this phenomenon could be activated in individuals with obstructive sleep apnea (Lee et al. 2009) and that the magnitude of the response was greater compared to healthy individuals (Lee et al. 2009) because of repeated daily exposure to intermittent hypoxia (often referred to as chronic intermittent hypoxia) (Gerst et al. 2011). We are convinced that the phenomenon induced in these populations was a consequence of exposure to intermittent hypoxia. A number of sham studies were completed in which sustained levels of carbon dioxide coupled with a single breath of hyperoxic gas, mimicking the single breath of hyperoxic gas we used to re-establish normoxia after each hypoxic episode in our intermittent hypoxia protocols, did not result in increases in ventilation that approached values measured when individuals were exposed to intermittent hypoxia (Gerst et al. 2011; Harris et al. 2006; Syed et al. 2013). Our findings are robust since they have been subsequently confirmed in other studies (Griffin et al. 2012). Thus, we established that long-term facilitation of ventilation and genioglossus muscle activity could be induced and were excited because of its potential to stabilize breathing at two locations within the breathing stability cycle (Point B & C - Fig. 2).
3.1.1. Research Aside
We were also excited that exposure to intermittent hypoxia in combination with sustained hypercapnia could initiate respiratory plasticity, because it may be years before the field is able to determine the appropriate route, dose and interaction of potential neuromodulators (e.g. serotonin, adenosine, norepinephrine) that are capable of initiating respiratory plasticity in humans without significant complications. Thus, the ability to initiate respiratory plasticity using a simple stimulus and method could have important translational implications (see Future Clinical Studies and Translational Relevance for further discussion). Likewise, our findings also revealed that the intermittent hypoxia protocol employed was capable of initiating forms of plasticity that could serve to mitigate apnea. However, additional work is required to determine the optimal intensity, duration and frequency of hypoxia that will initiate the greatest magnitude of ventilatory and genioglossus muscle long-term facilitation. Further work is also required to develop an intermittent hypoxia profile that reflects the cyclic variations in oxygen saturation that accompanies apneic events in order to determine if long-term facilitation can be induced naturally. This may prove to be difficult since the variability in duration, number and frequency of each episode of hypoxia is likely to vary significantly within and between individuals with sleep apnea across the night.
3.1.2 Long-term facilitation of ventilation and upper airway muscle activity during sleep
Although demonstrating the presence of long-term facilitation during wakefulness was important, the presence of this phenomenon during sleep was required if it was to impact on breathing stability during this state. Badr and colleagues completed a series of experiments designed to demonstrate that long-term facilitation could be induced during sleep. Many of the findings were intriguing. Initial findings indicated that long-term facilitation of ventilation could be induced in flow limited but not in non-flow limited individuals (Babcock et al. 2003; Babcock and Badr, 1998). Subsequent studies suggested that the increased ventilation observed in flow limited individuals was principally a result of long-term facilitation of upper airway muscle activity and not diaphragmatic activity (Aboubakr et al. 2001; Shkoukani et al. 2002). Further studies are required to provide additional support for these latter findings, since diaphragmatic activity was measured with surface electrodes, a method of recording that lacks specificity, and long-term facilitation of upper airway muscle activity during sleep in flow limited individuals has not been measured to date. In subsequent studies, long-term facilitation of ventilation was induced in non-flow limited individuals (Chowdhuri et al. 2010; Pierchala et al. 2008). The investigators surmised that long-term facilitation was induced in these latter studies because the duration of hypoxic episodes was reduced from 5 to 1 minute or because carbon dioxide levels were more tightly controlled than in previous studies. We favor the latter explanation, since we showed that long-term facilitation of ventilation is consistently evident in both healthy males and females, and males and females with sleep apnea during wakefulness and non-rapid eye movement sleep in the presence of sustained hypercapnia (Syed et al. 2013).
3.1.3 Research Aside
The measurement of diaphragm and upper airway muscle activity using indwelling electrodes in individuals with obstructive sleep apnea during sleep would be of interest because it could establish the threshold of activity of these separate muscles during and following exposure to intermittent hypoxia. The initial hypothesis put forth by Badr and colleagues, coupled with their findings, indicated that the threshold of activity (i.e. the point at which phasic respiratory activity is observed) of both the diaphragm and genioglossus muscle activity are similar during sleep. Their hypothesis also suggested that long-term facilitation of upper airway muscle activity was evident and long-term facilitation of diaphragmatic activity was absent, during sleep. The finding that respiratory and upper airway muscle activity thresholds are similar is contrary to previous studies in sleeping humans (Pillar et al. 2000), human infants (Carlo et al. 1988) and other animals (Brouillette and Thach,1980; Haxhiu et al. 1984; Horner et al. 2002; Parisi et al. 1987), which showed that the threshold of activity for upper airway muscles is typically higher compared to the diaphragm. If dissimilar thresholds exist in humans, it would be expected that under normocapnic conditions sustained alterations in diaphragmatic activity would occur while alterations in genioglossus muscle activity may not manifest because carbon dioxide levels are below levels required to achieve sustainable increases in phasic muscle activity. Additional studies are required to explore these possibilities.
3.2 Sleep apnea and progressive augmentation of the hypoxic ventilatory response
Although we demonstrated that beneficial forms of plasticity were induced by intermittent hypoxia we also reported that potential detrimental forms of respiratory plasticity were introduced during and following exposure to intermittent hypoxia. More specifically, we established that the ventilatory response to hypoxia progressively increased from the initial to final hypoxic episode, when exposure to intermittent hypoxia occurred during wakefulness (Harris et al. 2006; Lee et al. 2009; Syed et al. 2013; Wadhwa et al. 2008) but not during sleep (Syed et al. 2013). We also showed that the ventilatory response to hypercapnia and sustained hypoxia was enhanced during wakefulness following exposure to intermittent hypoxia (Khodadadeh et al. 2006; Mateika et al. 2004; Morelli et al. 2004). This gradual enhancement of the hypoxic ventilatory response has been deemed progressive augmentation (Fig. 1) and is a phenomenon observed in both humans and other animals (Bautista et al. 2012; Fregosi and Mitchell, 1994; Peng et al. 2006; Turner and Mitchell, 1997). However, unlike long-term facilitation, the neuromodulator responsible for initiating this form of respiratory plasticity remains elusive. In some animals studies it has been reported that serotonin plays a significant role in initiating the phenomenon (Bautista et al. 2012;Peng et al. 2006), while this was shown not to be the case in other studies (Fregosi and Mitchell, 1994; Nichols et al. 2012). Moreover, the cellular pathways ultimately responsible for the phenomenon have not been explored. As outlined previously progressive augmentation could be detrimental because the enhanced sensitivity to hypoxia and hypercapnia could lead to a higher level of ventilation following an apneic event, resulting in carbon dioxide levels that are substantially below the apneic threshold. Moreover, since progressive augmentation of the hypoxic ventilatory response was clearly evident during wakefulness (Harris et al. 2006; Lee et al. 2009;Wadhwa et al. 2008) but not during sleep (Syed et al. 2013), alterations in chemoreflex sensitivity to hypoxia may not contribute to shortening apnea duration as proposed above (Point D - Fig. 2).
4. Impact of Respiratory Plasticity on Breathing Stability (Hypothesis # 1)
Once we demonstrated that both beneficial and detrimental forms of plasticity could be induced in response to exposure to intermittent hypoxia, we then considered how these forms of plasticity impacted breathing stability. As outlined in Fig. 3, if long-term facilitation of ventilation and upper airway muscle activity could be induced despite the existence of hypocapnia, the self-perpetuating breathing instability cycle (Fig. 2) could be broken and events ameliorated. At the present time, supporting evidence is not strong for this hypothesis because only a few studies have addressed it in humans. Rowley and colleagues (Rowley et al. 2007) reported that exposure to intermittent hypoxia did not alter the critical closing pressure of the upper airway in humans. One factor that impacts on the critical closing pressure is upper airway muscle activity (i.e. genioglossus muscle), and thus it seemed unlikely that long-term facilitation of upper airway muscle activity was induced. However, upper airway resistance was reduced following exposure (Rowley et al. 2007). This led to the suggestion that long-term facilitation of upper airway muscle activity was induced and had its greatest impact on the airway at the onset of inspiration, as reflected in the measure of airway resistance. In contrast, Rowley and colleagues (Rowley et al. 2007) suggested that the measure of critical closing pressure reflected the behavior and properties of the upper airway later in the inspiratory cycle when flow limitation developed. Although this scenario is possible, one would anticipate that the critical closing pressure should be altered, at least in some individuals, if long-term facilitation of upper airway muscle activity was induced. We hypothesize that this lack of effect may be related to the carbon dioxide level that was sustained throughout the protocol. Given our previous findings, which highlighted the importance of carbon dioxide in the manifestation of long-term facilitation (Harris et al. 2006), sustained and elevated levels of carbon dioxide may be necessary before the impact of long-term facilitation on critical closing pressure becomes clearly evident. However, additional studies are required to further establish the role that long-term facilitation of respiratory or upper airway muscle activity has on breathing stability.
Figure 3.
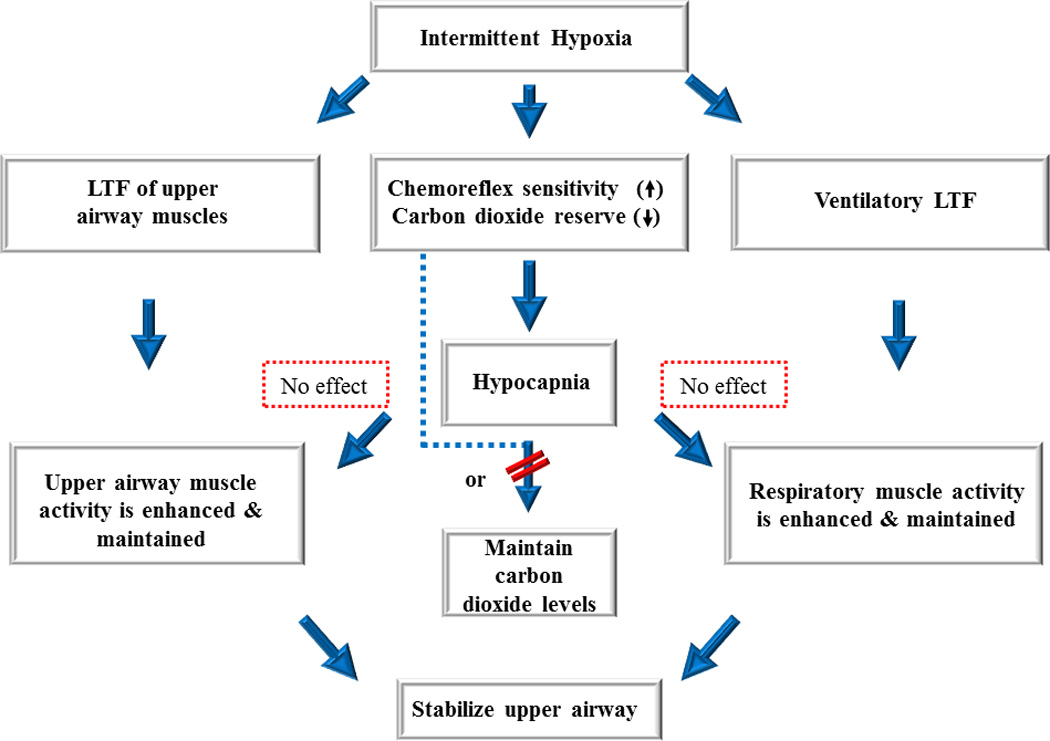
A schematic diagram showing that initiation of long-term facilitation of upper airway muscle activity and minute ventilation may serve to maintain upper airway patency and respiratory muscle activity. Ultimately, this integrated response promotes breathing and potentially mitigates the severity of apnea. In order for this scenario to occur long-term facilitation must persist despite reductions in carbon dioxide levels (i.e. hypocapnia) (see dotted red boxes). If long-term facilitation cannot persist in the presence of hypocapnia, as suggested by published findings, then alterations in chemoreflex properties must be minimized or eliminated (indicated by dashed line and diagonal red lines) to ensure the maintenance of desired carbon dioxide levels. Adapted from Mateika and Narwani (2009), Exp. Physiol. 91, 89–102.
If our findings are correct and the impact of long-term facilitation does not manifest in the presence of hypocapnia then detrimental forms of plasticity might predominate and apnea severity could potentially increase after exposure to intermittent hypoxia (Fig. 2 & 4). To investigate this possibility, we exposed individuals with obstructive sleep apnea to a brief protocol of intermittent hypoxia for ten consecutive days (Gerst et al. 2011; Yokhana et al. 2012). Exposure to the protocol occurred at night on the first and tenth day. Immediately following exposure, participants completed a sleep study, with no additional experimental interventions. Following completion of these sleep studies, the type, number and duration of breathing events were characterized. Our results showed that, independent of sleep stage (i.e. the data compared was obtained from N2 sleep), the number and duration of breathing events increased following exposure to intermittent hypoxia on both the first and last day of the protocol compared to baseline (Yokhana et al. 2012). We recently provided additional support for this finding by showing that the apnea hypopnea index one hour after exposure to intermittent hypoxia was increased compared to measures obtained after exposure to a sham protocol in individuals with sleep apnea (Syed et al. 2013). These simple findings indicate that detrimental forms of plasticity may predominate under conditions in which carbon dioxide levels are not maintained.
Figure 4.
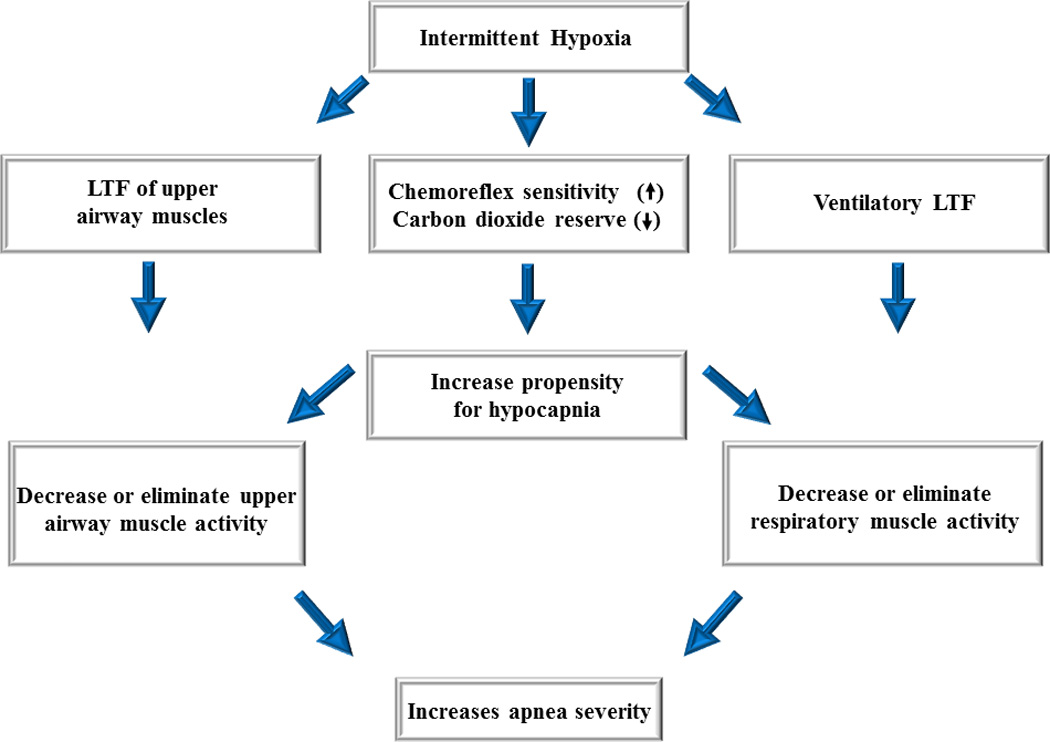
A diagram outlining a scenario in which long-term facilitation is induced in upper airway muscles following exposure to intermittent hypoxia but ultimately is ineffective in mitigating apnea because decreases in carbon dioxide reserve and increases in chemoreflex sensitivity increase the propensity for developing hypocapnia. The induced hypocapnia restrains long-term facilitation of upper airway muscle activity and respiratory muscle activity, ultimately resulting in the promotion of apnea. Adapted from Mateika and Narwani (2009), Exp. Physiol. 91, 89–102.
We hypothesized that the increased number of events were a consequence of enhanced chemoreflex sensitivity and a reduction in the carbon dioxide reserve. We did not obtain these measures in our study because we were interested in documenting breathing events in the absence of encumbrances typically associated with more sophisticated experimental interventions. Nonetheless, our postulations are supported by other investigations which have shown that exposure to intermittent hypoxia induces an increase in chemoreflex sensitivity and a decrease in the carbon dioxide reserve during sleep in healthy individuals (Chowdhuri et al. 2010). More importantly, Salloum and colleagues (Salloum et al. 2010) reported that measures of chemoreflex sensitivity were increased and the carbon dioxide reserve was decreased during sleep in untreated individuals with sleep apnea compared to matched controls. These alterations could be due to the nighttime exposure to intermittent hypoxia. Although further studies are required to obtain direct support for this suggestion, Salloum and colleagues (Salloum et al. 2010) did demonstrate that measures of chemoreflex sensitivity and the carbon dioxide reserve in sleep apnea participants were similar to matched controls after six weeks of treatment with continuous positive airway pressure.
In summary, it appears that exposure to intermittent hypoxia in an experimental setting is capable of enhancing chemoreflex sensitivity and reducing the carbon dioxide reserve, and that these alterations play an important role in promoting breathing instability. Likewise, it seems that long-term facilitation of upper airway and respiratory muscle activity may not be induced, or if induced does not have a significant impact on stabilizing breathing in the presence of hypocapnia. If our findings accurately translate to the natural state, chemoreflex sensitivity might increase from the beginning to the end of the night, at least in some individuals, because of repeated exposure to hypoxia, and this increase may be accompanied by increases in apnea severity. Indeed, some studies have reported that the ventilatory response to hypoxia measured during wakefulness after a night of sleep was greater than measures obtained before the sleep period (Mahamed et al. 2005). Likewise, clinical studies have reported that apnea severity increases from the beginning to the end of the night (Charbonneau et al. 1994; Fanfulla et al. 1997; Sforza et al. 1998). Nonetheless, additional well designed studies are required to solidify these relationships.
4.1. Research Aside
It is important to note that a specific group of individuals with sleep apnea was targeted for many of the studies which explored the link between respiratory plasticity and breathing instability. The recruited participants were relatively young, did not suffer from other comorbid conditions (i.e. diabetes, cardiovascular disease and obesity), and were typically exposed to mild hypoxemia at night. Consequently, the participants recruited did not reflect many sleep clinic patients, which are older, obese and often suffer from other co-morbid conditions (e.g. diabetes and/or hypertension). This phenotypic group of individuals was employed because it is one of the better experimental approaches to examine the link between respiratory plasticity and sleep apnea while accounting for a variety of co-morbidities which could impact on the measurements. Once the link between respiratory plasticity and breathing instability in a group of sleep apnea patients without comorbidities is established it will serve as a basis for comparison with other phenotypes. Indeed, we contend that the responses we have described may be dissimilar to outcomes observed in untreated individuals with sleep apnea that are older and have been exposed to severe levels of intermittent hypoxia (see below Impact of Respiratory Plasticity on Breathing Stability - Hypothesis # 2).
4.2. Future Clinical Studies and Translational Relevance
Our published results might be viewed as disappointing, since the potential benefits of long-term facilitation on breathing stability did not manifest in the studies published to date. However, we remain optimistic that long-term facilitation can serve to stabilize breathing under the appropriately controlled conditions. Accordingly, we suggest that long-term facilitation of upper airway and chest wall muscle activity will serve to stabilize breathing in the presence of sustained hypercapnia, particularly if the detrimental impact of an increased chemoreflex sensitivity and reduced carbon dioxide reserve are diminished or eliminated (Fig. 3 - see dotted line and red diagonal lines). Thus, the optimal situation as it relates to breathing stability is to maintain levels of carbon dioxide during and following exposure to intermittent hypoxia while dampening or eliminating the impact that alterations in chemoreflex properties might have on breathing instability (Fig. 3). This scenario could be achieved by administering intermittent hypoxia and sustained hypercapnia with continuous positive airway pressure. The application of sustained hypercapnia would ensure the manifestation of long-term facilitation. Likewise, the detrimental influence of a reduced carbon dioxide reserve would be minimized because of the presence of sustained hypercapnia, and the role that chemoreflex sensitivity may have in perpetuating breathing events will be diminished because of the absence of apnea and persistent arousal associated with the application of continuous positive airway pressure. The combination of intermittent hypoxia and sustained hypercapnia may have some important translational applications. The sustained hypercapnia in conjunction with continuous positive airway pressure could be effective in those individuals with predominantly obstructive sleep apnea (i.e. apnea events comprised of both a central and obstructive component), since carbon dioxide alone would enhance upper airway muscle activity as well as increase the carbon dioxide reserve. The additional application of intermittent hypoxia would initiate long term facilitation of upper airway muscle activity as well as long-term facilitation of minute ventilation. Ultimately, these forms of plasticity in combination with continuous positive airway pressure might prove to be a simple and cost effective method of improving breathing stability and reducing the therapeutic pressure required for treatment leading to improved compliance.
5. Respiratory Plasticity and Circadian Rhythms
In conjunction with the treatment studies described above (Future Clinical Studies and Translational Relevance) we have begun to explore the possibility that the impact that intermittent hypoxia has on the magnitude of respiratory plasticity may be modulated by a circadian rhythm (Fig. 5). This possibility is based partly on our results which showed that the magnitude of ventilatory long-term facilitation was greater in the early evening compared to the morning during wakefulness on the initial two days and final two days of exposure to a brief intermittent hypoxia protocol (Gerst et al. 2011). In contrast, progressive augmentation of the hypoxic ventilatory response appeared to be greater in the morning compared to the evening particularly on the final two days of the protocol (Gerst et al. 2011). Although further investigation is required, because other masking effects could be responsible for our previous finding, it is possible that the manifestation and maintenance of various forms of respiratory plasticity during a given stage of sleep may be modulated by a circadian rhythm. This possibility is supported by studies which have shown that serotonin (Agren et al. 1986; Mateos et al. 2009; Sun et al. 2002), brain derived neurotrophic factor and phosphorylated extracellular regulated kinases (Serchov and Heumann, 2006), which all have a role in initiating phrenic and hypoglossal long term facilitation in rats, are modulated by a circadian rhythm. In addition, 5-HT2A receptor mRNA levels in rats differ between two time points, being higher at the onset of the active phase (6–7 pm) compared to the onset of the rest period (8–9 am) (see Volgin et al. in this Special Issue of Sleep and Breathing). Likewise, work in humans and other animals indicate that the magnitudes of other forms of plasticity are mediated by a circadian rhythm (Raghavan et al. 1999; Sale et al. 2010). Given this possibility, the magnitude of various forms of plasticity that promote breathing stability may be greater while other forms of plasticity that tend to promote breathing instability may be diminished concurrently at specific points within the day/night cycle. However, these hypotheses are largely untested.
Figure 5.

A schematic diagram outlining the potential impact of an endogenous circadian rhythm on breathing stability during nighttime (red arrows) and daytime sleep (blue arrows) as a consequence of oscillations in (i) chemoreflex properties and neuromuscular control of the upper airway and (ii) the magnitude of respiratory plasticity. The diagram also shows the role that an endogenous circadian rhythm, either alone or in combination with the initiation of respiratory plasticity, may have in determining the magnitude of continuous positive airway pressure. The overlying theme of the diagram is that an endogenous circadian rhythm may promote mechanisms (e.g. neuromuscular control of upper airway muscle activity) and forms of plasticity that stabilize breathing, while simultaneously minimizing mechanisms that destabilize breathing (i.e. increases in the ventilatory response to hypoxia or hypercapnia and decreases in the carbon dioxide reserve) during daytime compared to nighttime sleep. The increases (+, ++) and decreases (−, − −) shown are meant to reflect changes that might occur during nighttime compared to daytime sleep as a consequence of an endogenous circadian rhythm. For example, it is hypothesized that the apneic threshold will decrease (−) (i.e. move further away from resting levels of carbon dioxide) and chemoreflex sensitivity will decrease (−) leading to an increase in the carbon dioxide reserve (+) and ultimately breathing stability (+) during daytime compared to nighttime sleep. + = increase; − = decrease; ++ = a greater increase; − − = a greater decrease.
5.1 Research Aside
The possibility that respiratory plasticity is modulated by a circadian rhythm is an extension of a postulate that chemoreflex sensitivity and the carbon dioxide reserve, as well as, upper airway collapsibility, are influenced by a circadian rhythm (Fig. 5). If these mechanisms are modulated by a circadian rhythm then breathing instability during sleep could be heightened at some point during the day/night cycle and ameliorated at other points. Indeed, computer modeling simulations have predicted a reduction in apneic events during daytime versus nighttime sleep (Stephenson, 2004). Moreover, these simulations indicated that an increased propensity toward breathing instability during nighttime sleep could result from a shift in the apneic threshold and a consequent decrease in the carbon dioxide reserve, coupled with a small or absent decrease in peripheral chemoreflex sensitivity, which is normally present in healthy individuals (Stephenson, 2004).
Although well-designed studies are necessary to support these simulations, there is some experimental evidence from a small number of hypertensive men which showed that the apnea-hypopnea index was reduced during sleep in the day compared to the night (Scharf et al. 1990). Moreover, experimental findings, including our own work, indicate that variations in the chemoreflex threshold or sensitivity to hypoxia and/or hypercapnia in healthy humans (Cummings et al. 2007; Raschke and Moller,1989; Spengler et al. 2000; Stephenson et al. 2000; Stephenson, 2003) and in humans with sleep apnea (Fuse et al. 1999; Gerst et al. 2011) during wakefulness may be modulated by a circadian rhythm. Although the results were not consistent, because of differences in experimental design (which introduced masking effects in some cases), methodology and the composition of populations recruited for each study, the majority of studies did report variations in chemoreflex properties throughout the 24 hour cycle.
Despite these findings, no studies to our knowledge have examined if the apneic threshold, carbon dioxide reserve or chemoreflex sensitivity (i.e. hypocapnic or hypoxic ventilatory response) is modulated by a circadian rhythm during sleep. Moreover, previous studies have indicated that force and strength of limb muscles are subject to modulation by a circadian rhythm and are typically greater in the late afternoon compared to the early morning (Martin et al. 1999; Zhang et al. 2009). Consequently, it is possible that upper airway muscle activity, which is one mechanism that contributes to maintaining upper airway patency, is also modulated by a circadian rhythm. Thus, mechanisms that typically contribute to breathing instability might have a greater influence during sleep at different points throughout the day/night cycle. However, these possibilities have not been firmly established. Once established the results might lead to an understanding that therapeutic airway pressure determined at a given time of night may not be effective at other points during the night or day; leading to a more careful determination of pressures at different points throughout the 24 hour cycle or improved refinement of auto continuous positive airway pressure to provide more effective treatment. Moreover, findings from future studies could lead to the understanding that targeting periods of increased breathing stability via the implementation of prescribed sleep schedules and the promotion of daytime naps, may lead to more effective treatment of sleep apnea and improved compliance with continuous positive airway pressure.
6. Impact of Respiratory Plasticity on Breathing Stability (Hypothesis# 2)
It is well known that the response to sustained hypoxia is dependent on the pattern, duration and intensity of exposure (Powell et al. 1998). Exposure to sustained hypoxia over a short period of time is typically accompanied by increases in minute ventilation; however a gradual decline is evident as the duration of hypoxic exposure is extended. Similarly, exposure to intermittent hypoxia and the subsequent initiation of long-term facilitation of respiratory and upper airway motor neurons might not always translate into sustained increases in respiratory or upper airway muscle activity and force production (Fig. 6). It is probable that exposure to mild hypoxia, particularly over an acute period of time, initiates long term facilitation of hypoglossal nerve activity and that this translates to sustained increases in genioglossus muscle activity and force production in humans. Chronic exposure to mild hypoxia over a longer period of time might also induce a similar response. These responses may be limited to individuals with obstructive sleep apnea that are relatively young, and experience mild to moderate apnea, in regards to both frequency of events and intensity of hypoxia. Given these characteristics, hypoxic depression of ventilation and detrimental upper airway responses, either myogenic or non-myogenic, are avoided.
Figure 6.

A diagram summarizing a situation in which exposure to intermittent hypoxia leads to fatigue of the upper airway muscles which results in the promotion of apnea, despite alterations in chemoreflex properties that could potentially mitigate apnea. Adapted from Mateika and Narwani (2009), Exp. Physiol. 91, 89–102.
In contrast, exposure to severe levels of hypoxia over many hours, days, months or years may depress the ventilatory response to hypoxia (Powell et al. 1998) and impair hypoglossal motor neuronal and/or upper airway muscle function, resulting in the loss of capacity to develop the force necessary to overcome a resistive load applied to the upper airway (Fig. 6). This outcome may be most obvious in older untreated humans with sleep apnea that experience excessive number of apneas each night for many years accompanied by severe reductions in oxygen saturation. Overall, studies completed in animals other than humans provide support for this hypothesis, since studies in rats have shown that exposure to chronic intermittent hypoxia induced changes in both structure and function of the geniohyoid and sternohyoid (Bradford et al. 2005), which are pharyngeal dilator muscles. Specifically, chronic intermittent hypoxia led to increased fatigability of pharyngeal dilator muscles (Bradford et al. 2005). In contrast, Ray and colleagues (Ray et al. 2007) reported that fatigability of the sternohyoid muscle was not altered by chronic intermittent hypoxia, indicating that non-myogenic rather than myogenic mechanisms might contribute, at least in part, to the absence of long-term facilitation of hypoglossal nerve activity, and reduced muscle function following chronic exposure to severe levels of hypoxia. Independent of the site of impairment, reduced muscle function following long-term exposure to intermittent hypoxia might ultimately occur as a consequence of the accumulation of reactive oxygen species either at the level of the muscle (Bradford et al. 2005) or at the hypoglossal motor nucleus (Veasey et al. 2004).
In contrast to studies in other animals, the results obtained from humans are less definitive with regards to functional outcome of upper airway muscle activity in individuals with sleep apnea. However, some studies have reported clear differences in upper airway function. Ramchadren and colleagues (Ramchandren et al. 2010) reported that hypoglossal compound muscle action potentials were reduced in patients with obstructive sleep apnea. Likewise, Saboisky and colleagues (Saboisky et al. 2012) noted in a recent review that task failure during an isometric tongue protrusion task may occur sooner in patients with obstructive sleep apnea compared to control. In addition, in vitro studies using strips of genioglossus muscle revealed greater fatigability in patients with obstructive sleep apnea compared to control (Carrera et al. 1999), while the recovery of maximal force capacity following submaximal tongue protrusions was prolonged in patients with obstructive sleep apnea (Carrera et al. 1999). These outcome studies are complemented by other studies which showed changes in the timing of the neural drive to genioglossus muscle (Saboisky et al. 2007; Saboisky et al. 2012), and increases in the duration and area of genioglossus motor unit action potentials in obstructive sleep apnea patients (Saboisky et al. 2012;Wang et al. 2010). These increases may occur in response to denervation and subsequent reinnervation following axonal loss. It is also well established that muscle fiber typing is altered in individuals with obstructive sleep apnea. The majority of studies have reported increases in Type II fibers in individuals with sleep apnea (Saboisky et al. 2012). This alteration in fiber typing could be advantageous because it could increase force generating capacity. Conversely, this fiber type is less resistant to fatigue. Whether or not alterations in force production or neural drive are specifically linked to exposure to intermittent hypoxia is not well established. However, there is evidence that treatment with continuous positive airway pressure reverses the fatigability of upper airway muscles and reduces the number of type II fibers (Ramchandren et al. 2010), indicating a causal link between myogenic and non-myogenic alterations and apneic episodes. It is also interesting to note that the findings reported above were obtained primarily from older individuals with severe obstructive sleep apnea.
Long term exposure to hypoxia could also lead to reductions in the sensitivity to hypoxia or hypercapnia. The possibility that the duration and intensity of exposure to intermittent hypoxia might impact on the hypoxic ventilatory response is supported indirectly by studies that have examined the ventilatory response to hypoxia at high altitude. These studies showed that although exposure to continuous hypoxia initially leads to enhancement of the ventilatory response (Hupperets et al. 2004; Sato, Severinghaus et al. 1992), eventually a reduction is observed [i.e. hypoxic desensitization - (Powell et al. 1998; Weil, 1986; Weil, 1994)]. Thus, one might anticipate that the sensitivity to hypoxia would be reduced in older individuals with severe sleep apnea. This reduction in sensitivity is a form of plasticity that could promote breathing stability (Fig. 2), since inappropriately high levels of ventilation following arousal could be avoided. However, impairment of upper airway muscle function could ultimately overpower any influence that alterations in ventilatory sensitivity or threshold might have on the severity of apnea. For example, we speculated above that exposure to severe hypoxia over a prolonged period of time might dampen ventilatory sensitivity, which could mitigate apnea. However, in the presence of upper airway muscle fatigue, alterations in chemoreflex properties may have little impact.
7. Summary
Present knowledge indicates that progressive augmentation and long-term facilitation can be induced in humans during and following exposure to intermittent hypoxia. The manifestation of long-term facilitation is dependent on sustained levels of carbon dioxide that are above normocapnic values. These responses may be limited to humans that have not been previously exposed to intermittent hypoxia (healthy individuals) or individuals exposed to mild forms of intermittent hypoxia. In contrast, the hypoxic ventilatory responses and long-term facilitation of upper airway muscle activity may be reduced or depressed in individuals exposed to severe forms of intermittent hypoxia over long durations of time (i.e. untreated older individuals with severe sleep apnea). In those individuals in which long-term facilitation can be potentially induced, exposure to intermittent hypoxia is accompanied by increased breathing instability, if carbon dioxide levels are not maintained. This increase may be the result of enhanced chemoreflex sensitivity. Ongoing studies in our laboratory are now concentrated on approaches that promote the beneficial effects of long-term facilitation while minimizing or eliminating the detrimental effects of enhanced chemoreflex sensitivity. We are focused on using intermittent hypoxia and sustained hypercapnia as an adjunct therapy in combination with continuous positive airway pressure to achieve this goal. Likewise, we are pursuing if the effectiveness of this form of respiratory plasticity is modulated by a circadian rhythm.
Highlights.
The following topics are addressed
-
➢
Repetitive breathing events and sleep apnea.
-
➢
Initiation of respiratory plasticity in humans during wakefulness and sleep.
-
➢
Respiratory plasticity and the maintenance of ventilation in sleep apnea.
-
➢
Future directions focused on promoting forms of plasticity that stabilize breathing.
-
➢
Potential role of circadian rhythms in the promotion of respiratory plasticity.
ACKNOWLEDGEMENTS
This work is supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and a National Heart, Lung, and Blood Institute Grant (R01-HL-085537).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aboubakr SE, Taylor A, Ford R, Siddiqi S, Badr MS. Long-term facilitation in obstructive sleep apnea patients during NREM sleep. J Appl Physiol. 2001;91:2751–2757. doi: 10.1152/jappl.2001.91.6.2751. [DOI] [PubMed] [Google Scholar]
- Agren H, Koulu M, Saavedra JM, Potter WZ, Linnoila M. Circadian covariation of norepinephrine and serotonin in the locus coeruleus and dorsal raphe nucleus in the rat. Brain Res. 1986;397:353–358. doi: 10.1016/0006-8993(86)90638-4. [DOI] [PubMed] [Google Scholar]
- Babcock M, Shkoukani M, Aboubakr SE, Badr MS. Determinants of long-term facilitation in humans during NREM sleep. J.Appl.Physiol. 2003;94:53–59. doi: 10.1152/japplphysiol.00476.2002. [DOI] [PubMed] [Google Scholar]
- Babcock MA, Badr MS. Long-term facilitation of ventilation in humans during NREM sleep. Sleep. 1998;21:709–716. [PubMed] [Google Scholar]
- Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir.Physiol. 1996;104:251–260. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- Badr MS, Kawak A. Post-hyperventilation hypopnea in humans during NREM sleep. Respir Physiol. 1996;103:137–145. doi: 10.1016/0034-5687(95)00083-6. [DOI] [PubMed] [Google Scholar]
- Badr MS, Kawak A, Skatrud JB, Morrell MJ, Zahn BR, Babcock MA. Effect of induced hypocapnic hypopnea on upper airway patency in humans during NREM sleep. Respir Physiol. 1997;110:33–45. doi: 10.1016/s0034-5687(97)00072-8. [DOI] [PubMed] [Google Scholar]
- Badr MS, Toiber F, Skatrud JB, Dempsey J. Pharyngeal narrowing/occlusion during central sleep apnea. J Appl Physiol. 1995;78:1806–1815. doi: 10.1152/jappl.1995.78.5.1806. [DOI] [PubMed] [Google Scholar]
- Bautista TG, Xing T, Fong AY, Pilowsky PM. Recurrent laryngeal nerve activity exhibits a 5-HT-mediated long-term facilitation and enhanced response to hypoxia following acute intermittent hypoxia in rat. J. Appl.Physiol. 2012;112:1144–1156. doi: 10.1152/japplphysiol.01356.2011. [DOI] [PubMed] [Google Scholar]
- Bradford A, McGuire M, O'Halloran KD. Does episodic hypoxia affect upper airway dilator muscle function? Implications for the pathophysiology of obstructive sleep apnoea. Respir Physiol Neurobiol. 2005;147:223–234. doi: 10.1016/j.resp.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Brouillette RT, Thach BT. Control of genioglossus muscle inspiratory activity. J Appl Physiol. 1980;49:801–808. doi: 10.1152/jappl.1980.49.5.801. [DOI] [PubMed] [Google Scholar]
- Cao KY, Zwillich CW, Berthon-Jones M, Sullivan CE. Increased normoxic ventilation induced by repetitive hypoxia in conscious dogs. J. Appl.Physiol. 1992;73:2083–2088. doi: 10.1152/jappl.1992.73.5.2083. [DOI] [PubMed] [Google Scholar]
- Carlo WA, Martin RJ, Difiore JM. Differences in CO2 threshold of respiratory muscles in preterm infants. J.Appl.Physiol. 1988;65:2434–2439. doi: 10.1152/jappl.1988.65.6.2434. [DOI] [PubMed] [Google Scholar]
- Carrera M, Barbe F, Sauleda J, Tomas M, Gomez C, Agusti AG. Patients with obstructive sleep apnea exhibit genioglossus dysfunction that is normalized after treatment with continuous positive airway pressure. Am.J.Respir.Crit Care Med. 1999;159:1960–1966. doi: 10.1164/ajrccm.159.6.9809052. [DOI] [PubMed] [Google Scholar]
- Charbonneau M, Marin JM, Olha A, Kimoff RJ, Levy RD, Cosio MG. Changes in obstructive sleep apnea characteristics through the night. Chest. 1994;106:1695–1701. doi: 10.1378/chest.106.6.1695. [DOI] [PubMed] [Google Scholar]
- Chowdhuri S, Shanidze I, Pierchala L, Belen D, Mateika JH, Badr MS. Effect of episodic hypoxia on the susceptibility to hypocapnic central apnea during NREM sleep. J.Appl.Physiol. 2010;108:369–377. doi: 10.1152/japplphysiol.00308.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings KJ, Swart M, Ainslie PN. Morning attenuation in cerebrovascular CO2 reactivity in healthy humans is associated with a lowered cerebral oxygenation and an augmented ventilatory response to CO2. J Appl.Physiol. 2007;102:1891–1898. doi: 10.1152/japplphysiol.01437.2006. [DOI] [PubMed] [Google Scholar]
- Dale-Nagle EA, Hoffman MS, Macfarlane PM, Mitchell GS. Multiple pathways to long-lasting phrenic motor facilitation. Adv.Exp.Med.Biol. 2010;669:225–230. doi: 10.1007/978-1-4419-5692-7_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey JA. Crossing the apnoeic threshold: causes and consequences. Exp.Physiol. 2005;90:13–24. doi: 10.1113/expphysiol.2004.028985. [DOI] [PubMed] [Google Scholar]
- Duffin J. Measuring the ventilatory response to hypoxia. J.Physiol. 2007;584:285–293. doi: 10.1113/jphysiol.2007.138883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanfulla F, Patruno V, Bruschi C, Rampulla C. Obstructive sleep apnoea syndrome: is the "half-night polysomnography" an adequate method for evaluating sleep profile and respiratory events? Eur.Respir J. 1997;10:1725–1729. doi: 10.1183/09031936.97.10081725. [DOI] [PubMed] [Google Scholar]
- Fregosi RF, Mitchell GS. Long-term facilitation of inspiratory intercostal nerve activity following carotid sinus nerve stimulation in cats. J.Physiol. 1994;477(Pt 3):469–479. doi: 10.1113/jphysiol.1994.sp020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuse K, Satoh M, Yokota T, Ohdaira T, Muramatsu Y, Suzuki E, Arakawa M. Regulation of ventilation before and after sleep in patients with obstructive sleep apnoea. Respirology. 1999;4:125–130. doi: 10.1046/j.1440-1843.1999.00163.x. [DOI] [PubMed] [Google Scholar]
- Gerst DG, III, Yokhana SS, Carney LM, Lee DS, Badr MS, Qureshi T, Anthouard MN, Mateika JH. The hypoxic ventilatory response and ventilatory long-term facilitation are altered by time of day and repeated daily exposure to intermittent hypoxia. J.Appl.Physiol. 2011;110:15–28. doi: 10.1152/japplphysiol.00524.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Ranganathan L, Satriotomo I, Hoffman M, Lovett-Barr MR, Watters JJ, Baker-Herman TL, Mitchell GS. Spinal adenosine A2a receptor activation elicits long-lasting phrenic motor facilitation. J.Neurosci. 2008;28:2033–2042. doi: 10.1523/JNEUROSCI.3570-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin HS, Pugh K, Kumar P, Balanos GM. Long-term facilitation of ventilation following acute continuous hypoxia in awake humans during sustained hypercapnia. J.Physiol. 2012;590:5151–5165. doi: 10.1113/jphysiol.2012.236109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DP, Balasubramaniam A, Badr MS, Mateika JH. Long-term facilitation of ventilation and genioglossus muscle activity is evident in the presence of elevated levels of carbon dioxide in awake humans. Am.J Physiol Regul.Integr.Comp Physiol. 2006;291:R1111–R1119. doi: 10.1152/ajpregu.00896.2005. [DOI] [PubMed] [Google Scholar]
- Haxhiu MA, van LE, Mitra J, Cherniack NS. Responses to chemical stimulation of upper airway muscles diaphragm in awake cats. J Appl.Physiol. 1984;56:397–403. doi: 10.1152/jappl.1984.56.2.397. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Coles SK, Bach KB, Mitchell GS, McCrimmon DR. Time-dependent phrenic nerve responses to carotid afferent activation: intact vs. decerebellate rats. Am.J.Physiol. 1993;265:R811–R819. doi: 10.1152/ajpregu.1993.265.4.R811. [DOI] [PubMed] [Google Scholar]
- Hoffman MS, Golder FJ, Mahamed S, Mitchell GS. Spinal adenosine A2(A) receptor inhibition enhances phrenic long term facilitation following acute intermittent hypoxia. J.Physiol. 2010;588:255–266. doi: 10.1113/jphysiol.2009.180075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MS, Mitchell GS. Spinal 5-HT7 receptor activation induces long-lasting phrenic motor facilitation. J.Physiol. 2011;589:1397–1407. doi: 10.1113/jphysiol.2010.201657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MS, Nichols NL, Macfarlane PM, Mitchell GS. Phrenic long-term facilitation after acute intermittent hypoxia requires spinal ERK activation but not TrkB synthesis. J.Appl.Physiol. 2012;113:1184–1193. doi: 10.1152/japplphysiol.00098.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner RL, Liu X, Gill H, Nolan P, Liu H, Sood S. Effects of sleep-wake state on the genioglossus vs.diaphragm muscle response to CO(2) in rats. J Appl.Physiol. 2002;92:878–887. doi: 10.1152/japplphysiol.00855.2001. [DOI] [PubMed] [Google Scholar]
- Hupperets MD, Hopkins SR, Pronk MG, Tiemessen IJ, Garcia N, Wagner PD, Powell FL. Increased hypoxic ventilatory response during 8 weeks at 3800 m altitude. Respir Physiol Neurobiol. 2004;142:145–152. doi: 10.1016/j.resp.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Jordan AS, Catcheside PG, O'Donoghue FJ, McEvoy RD. Long-term facilitation of ventilation is not present during wakefulness in healthy men or women. J.Appl.Physiol. 2002;93:2129–2136. doi: 10.1152/japplphysiol.00135.2002. [DOI] [PubMed] [Google Scholar]
- Jounieaux V, Parreira VF, Aubert G, Dury M, Delguste P, Rodenstein DO. Effects of hypocapnic hyperventilation on the response to hypoxia in normal subjects receiving intermittent positive-pressure ventilation. Chest. 2002;121:1141–1148. doi: 10.1378/chest.121.4.1141. [DOI] [PubMed] [Google Scholar]
- Khodadadeh B, Badr MS, Mateika JH. The ventilatory response to carbon dioxide and sustained hypoxia is enhanced after episodic hypoxia in OSA patients. Respir.Physiol Neurobiol. 2006;150:122–134. doi: 10.1016/j.resp.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Lee DS, Badr MS, Mateika JH. Progressive augmentation and ventilatory long-term facilitation are enhanced in sleep apnoea patients and are mitigated by antioxidant administration. J Physiol. 2009;587:5451–5467. doi: 10.1113/jphysiol.2009.178053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane PM, Mitchell GS. Episodic spinal serotonin receptor activation elicits long-lasting phrenic motor facilitation by an NADPH oxidase-dependent mechanism. J.Physiol. 2009;587:5469–5481. doi: 10.1113/jphysiol.2009.176982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane PM, Satriotomo I, Windelborn JA, Mitchell GS. NADPH oxidase activity is necessary for acute intermittent hypoxia-induced phrenic long-term facilitation. J Physiol. 2009;587:1931–1942. doi: 10.1113/jphysiol.2008.165597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane PM, Wilkerson JE, Lovett-Barr MR, Mitchell GS. Reactive oxygen species and respiratory plasticity following intermittent hypoxia. Respir.Physiol Neurobiol. 2008;164:263–271. doi: 10.1016/j.resp.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahamed S, Hanly PJ, Gabor J, Beecroft J, Duffin J. Overnight changes of chemoreflex control in obstructive sleep apnoea patients. Respir Physiol Neurobiol. 2005;146:279–290. doi: 10.1016/j.resp.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp.Physiol. 2007;92:27–37. doi: 10.1113/expphysiol.2006.033720. [DOI] [PubMed] [Google Scholar]
- Martin A, Carpentier A, Guissard N, van HJ, Duchateau J. Effect of time of day on force variation in a human muscle. Muscle Nerve. 1999;22:1380–1387. doi: 10.1002/(sici)1097-4598(199910)22:10<1380::aid-mus7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Mateika JH, Fregosi RF. Long-term facilitation of upper airway muscle activities in vagotomized and vagally intact cats. J.Appl.Physiol. 1997;82:419–425. doi: 10.1152/jappl.1997.82.2.419. [DOI] [PubMed] [Google Scholar]
- Mateika JH, Mendello C, Obeid D, Badr MS. Peripheral chemoreflex responsiveness is increased at elevated levels of carbon dioxide after episodic hypoxia in awake humans. J.Appl.Physiol. 2004;96:1197–1205. doi: 10.1152/japplphysiol.00573.2003. [DOI] [PubMed] [Google Scholar]
- Mateika JH, Narwani G. Intermittent hypoxia and respiratory plasticity in humans and other animals: does exposure to intermittent hypoxia promote or mitigate sleep apnoea? Exp.Physiol. 2009;94:279–296. doi: 10.1113/expphysiol.2008.045153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateika JH, Sandhu KS. Experimental protocols and preparations to study respiratory long term facilitation. Respir.Physiol Neurobiol. 2011;176:1–11. doi: 10.1016/j.resp.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos SS, Sanchez CL, Paredes SD, Barriga C, Rodriguez AB. Circadian levels of serotonin in plasma and brain after oral administration of tryptophan in rats. Basic Clin.Pharmacol.Toxicol. 2009;104:52–59. doi: 10.1111/j.1742-7843.2008.00333.x. [DOI] [PubMed] [Google Scholar]
- McKay LC, Janczewski WA, Feldman JL. Episodic hypoxia evokes long-term facilitation of genioglossus muscle activity in neonatal rats. J Physiol. 2004;557:13–18. doi: 10.1113/jphysiol.2004.064006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by a new central neural mechanism. Respir.Physiol. 1980a;41:87–103. doi: 10.1016/0034-5687(80)90025-0. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by endogenous central serotonin. Respir.Physiol. 1980b;42:171–188. doi: 10.1016/0034-5687(80)90113-9. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr. Invited review: Intermittent hypoxia and respiratory plasticity. J.Appl.Physiol. 2001;90:2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J.Appl.Physiol. 2003;94:358–374. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Powell FL, Hopkins SR, Milsom WK. Time domains of the hypoxic ventilatory response in awake ducks: episodic and continuous hypoxia. Respir.Physiol. 2001;124:117–128. doi: 10.1016/s0034-5687(00)00197-3. [DOI] [PubMed] [Google Scholar]
- Mody P, Rukhadze I, Kubin L. Rats subjected to chronic-intermittent hypoxia have increased density of noradrenergic terminals in the trigeminal sensory and motor nuclei. Neurosci.Lett. 2011;505:176–179. doi: 10.1016/j.neulet.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan R, Duffin J. The effect of hypoxia on the ventilatory response to carbon dioxide in man. Respir.Physiol. 1997;108:101–115. doi: 10.1016/s0034-5687(97)00024-8. [DOI] [PubMed] [Google Scholar]
- Morelli C, Badr MS, Mateika JH. Ventilatory responses to carbon dioxide at low and high levels of oxygen are elevated after episodic hypoxia in men compared with women. J.Appl.Physiol. 2004;97:1673–1680. doi: 10.1152/japplphysiol.00541.2004. [DOI] [PubMed] [Google Scholar]
- Nichols NL, Dale EA, Mitchell GS. Severe acute intermittent hypoxia elicits phrenic long-term facilitation by a novel adenosine-dependent mechanism. J.Appl.Physiol. 2012;112:1678–1688. doi: 10.1152/japplphysiol.00060.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi RA, Neubauer JA, Frank MM, Edelman NH, Santiago TV. Correlation between genioglossal and diaphragmatic responses to hypercapnia during sleep. Am.Rev.Respir.Dis. 1987;135:378–382. doi: 10.1164/arrd.1987.135.2.378. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Prabhakar NR. Reactive oxygen species in the plasticity of respiratory behavior elicited by chronic intermittent hypoxia. J Appl.Physiol. 2003;94:2342–2349. doi: 10.1152/japplphysiol.00613.2002. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Yuan G, Jacono FJ, Kumar GK, Prabhakar NR. 5-HT evokes sensory long-term facilitation of rodent carotid body via activation of NADPH oxidase. J Physiol. 2006;576:289–295. doi: 10.1113/jphysiol.2006.116020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipson E, Bowes G. Control of breathing during sleep. chapt. 19. In: Cherniack NS, Widdicombe JG, editors. Handbook of Physiology, Section 3. The Respiratory System-Vol II: Control of Breathing, Part 2. Vol. 2. Bethesda, MD: American Physiological Society; 1986. pp. 649–689. [Google Scholar]
- Pierchala LA, Mohammed AS, Grullon K, Mateika JH, Badr MS. Ventilatory long-term facilitation in non-snoring subjects during NREM sleep. Respir.Physiol Neurobiol. 2008;160:259–266. doi: 10.1016/j.resp.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Pillar G, Malhotra A, Fogel RB, Beauregard J, Slamowitz DI, Shea SA, White DP. Upper airway muscle responsiveness to rising PCO(2) during NREM sleep. J Appl.Physiol. 2000;89:1275–1282. doi: 10.1152/jappl.2000.89.4.1275. [DOI] [PubMed] [Google Scholar]
- Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir.Physiol. 1998;112:123–134. doi: 10.1016/s0034-5687(98)00026-7. [DOI] [PubMed] [Google Scholar]
- Raghavan AV, Horowitz JM, Fuller CA. Diurnal modulation of long-term potentiation in the hamster hippocampal slice. Brain Res. 1999;833:311–314. doi: 10.1016/s0006-8993(99)01523-1. [DOI] [PubMed] [Google Scholar]
- Ramchandren S, Gruis KL, Chervin RD, Lisabeth LD, Concannon M, Wolfe J, Albers JW, Brown DL. Hypoglossal nerve conduction findings in obstructive sleep apnea. Muscle Nerve. 2010;42:257–261. doi: 10.1002/mus.21690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschke F, Moller KH. [The diurnal rhythm of chemosensitivity and its contribution to nocturnal disorders of respiratory control] Pneumologie. 1989;43(Suppl 1):568–571. [PubMed] [Google Scholar]
- Ray AD, Magalang UJ, Michlin CP, Ogasa T, Krasney JA, Gosselin LE, Farkas GA. Intermittent hypoxia reduces upper airway stability in lean but not obese Zucker rats. Am.J Physiol Regul.Integr.Comp Physiol. 2007;293:R372–R378. doi: 10.1152/ajpregu.00038.2007. [DOI] [PubMed] [Google Scholar]
- Rowley JA, Deebajah I, Parikh S, Najar A, Saha R, Badr MS. The influence of episodic hypoxia on upper airway collapsibility in subjects with obstructive sleep apnea. J.Appl.Physiol. 2007;103:911–916. doi: 10.1152/japplphysiol.01117.2006. [DOI] [PubMed] [Google Scholar]
- Saboisky JP, Butler JE, Gandevia SC, Eckert DJ. Functional role of neural injury in obstructive sleep apnea. Front Neurol. 2012;3:95. doi: 10.3389/fneur.2012.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saboisky JP, Butler JE, McKenzie DK, Gorman RB, Trinder JA, White DP, Gandevia SC. Neural drive to human genioglossus in obstructive sleep apnoea. J.Physiol. 2007;585:135–146. doi: 10.1113/jphysiol.2007.139584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale MV, Ridding MC, Nordstrom MA. Circadian modulation of neuroplasticity in humans and potential therapeutic implications. Rev.Neurosci. 2010;21:55–66. doi: 10.1515/revneuro.2010.21.1.55. [DOI] [PubMed] [Google Scholar]
- Salloum A, Rowley JA, Mateika JH, Chowdhuri S, Omran Q, Badr MS. Increased propensity for central apnea in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure. Am.J.Respir.Crit Care Med. 2010;181:189–193. doi: 10.1164/rccm.200810-1658OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Severinghaus JW, Powell FL, Xu FD, Spellman MJ., Jr. Augmented hypoxic ventilatory response in men at altitude. J.Appl.Physiol. 1992;73:101–107. doi: 10.1152/jappl.1992.73.1.101. [DOI] [PubMed] [Google Scholar]
- Satriotomo I, Dale EA, Dahlberg JM, Mitchell GS. Repetitive acute intermittent hypoxia increases expression of proteins associated with plasticity in the phrenic motor nucleus. Exp.Neurol. 2012;237:103–115. doi: 10.1016/j.expneurol.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf SM, Garshick E, Brown R, Tishler PV, Tosteson T, McCarley R. Screening for subclinical sleep-disordered breathing. Sleep. 1990;13:344–353. [PubMed] [Google Scholar]
- Serchov T, Heumann R. Constitutive activation of ras in neurons: implications for the regulation of the mammalian circadian clock. Chronobiol.Int. 2006;23:191–200. doi: 10.1080/07420520500521970. [DOI] [PubMed] [Google Scholar]
- Sforza E, Krieger J, Petiau C. Nocturnal evolution of respiratory effort in obstructive sleep apnoea syndrome: influence on arousal threshold. Eur.Respir J. 1998;12:1257–1263. doi: 10.1183/09031936.98.12061257. [DOI] [PubMed] [Google Scholar]
- Shea SA. Behavioural and arousal-related influences on breathing in humans. Exp.Physiol. 1996;81:1–26. doi: 10.1113/expphysiol.1996.sp003911. [DOI] [PubMed] [Google Scholar]
- Shkoukani M, Babcock MA, Badr MS. Effect of episodic hypoxia on upper airway mechanics in humans during NREM sleep. J Appl Physiol. 2002;92:2565–2570. doi: 10.1152/japplphysiol.00938.2001. [DOI] [PubMed] [Google Scholar]
- Sokolowska B, Pokorski M. Ventilatory augmentation by acute intermittent hypoxia in the rabbit. J Physiol Pharmacol. 2006;57(Suppl 4):341–347. [PubMed] [Google Scholar]
- Spengler CM, Czeisler CA, Shea SA. An endogenous circadian rhythm of respiratory control in humans. J Physiol. 2000;526(Pt 3):683–694. doi: 10.1111/j.1469-7793.2000.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson R. Do circadian rhythms in respiratory control contribute to sleep-related breathing disorders? Sleep Med.Rev. 2003;7:475–490. doi: 10.1016/s1087-0792(03)90002-5. [DOI] [PubMed] [Google Scholar]
- Stephenson R. A theoretical study of the effect of circadian rhythms on sleep-induced periodic breathing and apnoea. Respir.Physiol Neurobiol. 2004;139:303–319. doi: 10.1016/j.resp.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Stephenson R, Mohan RM, Duffin J, Jarsky TM. Circadian rhythms in the chemoreflex control of breathing. Am.J Physiol Regul.Integr.Comp Physiol. 2000;278:R282–R286. doi: 10.1152/ajpregu.2000.278.1.R282. [DOI] [PubMed] [Google Scholar]
- Stettner GM, Fenik VB, Kubin L. Effect of chronic intermittent hypoxia on noradrenergic activation of hypoglossal motoneurons. J.Appl.Physiol. 2012;112:305–312. doi: 10.1152/japplphysiol.00697.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Deng J, Liu T, Borjigin J. Circadian 5-HT production regulated by adrenergic signaling. Proc.Natl.Acad.Sci U.S.A. 2002;99:4686–4691. doi: 10.1073/pnas.062585499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed Z, Lin HS, Mateika JH. The impact of arousal state, sex, and sleep apnea on the magnitude of progressive augmentation and ventilatory long-term facilitation. J.Appl.Physiol. 2013;114:52–65. doi: 10.1152/japplphysiol.00985.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DL, Mitchell GS. Long-term facilitation of ventilation following repeated hypoxic episodes in awake goats. J.Physiol. 1997;499(Pt 2):543–550. doi: 10.1113/jphysiol.1997.sp021947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veasey SC, Zhan G, Fenik P, Pratico D. Long-term intermittent hypoxia: reduced excitatory hypoglossal nerve output. Am.J Respir.Crit Care Med. 2004;170:665–672. doi: 10.1164/rccm.200403-261OC. [DOI] [PubMed] [Google Scholar]
- Wadhwa H, Gradinaru C, Gates GJ, Badr MS, Mateika JH. Impact of intermittent hypoxia on long-term facilitation of minute ventilation and heart rate variability in men and women: do sex differences exist? J Appl.Physiol. 2008;104:1625–1633. doi: 10.1152/japplphysiol.01273.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Kang J, Kong D. The central motor conductivity of genioglossus in obstructive sleep apnoea. Respirology. 2010;15:1209–1214. doi: 10.1111/j.1440-1843.2010.01858.x. [DOI] [PubMed] [Google Scholar]
- Weil JV. Ventilatory control at high altitude. In: NS Cherniack, JG Widdicombe., editors. Handbook of Physiology, Section 3: The Respiratory System-Vol II: Control of Breathing, Part 2. Bethesda, MD: American Physiological Society; 1986. pp. 703–728. [Google Scholar]
- Weil JV. "Ventilatory responses to CO2 and hypoxia after sustained hypoxia in awake cats". J Appl.Physiol. 1994;76:2251–2252. doi: 10.1152/jappl.1994.76.6.2251. [DOI] [PubMed] [Google Scholar]
- White DP. Long-term facilitation (LTF) and obstructive sleep apnea. Respir.Physiol Neurobiol. 2007;158:112–113. doi: 10.1016/j.resp.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie A, Skatrud JB, Puleo DS, Rahko PS, Dempsey JA. Apnea-hypopnea threshold for CO2 in patients with congestive heart failure. Am J Respir Crit Care Med. 2002;165:1245–1250. doi: 10.1164/rccm.200110-022OC. [DOI] [PubMed] [Google Scholar]
- Yokhana SS, Gerst DG, Jr., Lee DS, Badr MS, Qureshi T, Mateika JH. Impact of repeated daily exposure to intermittent hypoxia and mild sustained hypercapnia on apnea severity. J.Appl.Physiol. 2012;112:367–377. doi: 10.1152/japplphysiol.00702.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabka AG, Behan M, Mitchell GS. Selected contribution: Time-dependent hypoxic respiratory responses in female rats are influenced by age and by the estrus cycle. J Appl.Physiol. 2001;91:2831–2838. doi: 10.1152/jappl.2001.91.6.2831. [DOI] [PubMed] [Google Scholar]
- Zhang X, Dube TJ, Esser KA. Working around the clock: circadian rhythms and skeletal muscle. J.Appl.Physiol. 2009;107:1647–1654. doi: 10.1152/japplphysiol.00725.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XS, Shahabuddin S, Zahn BR, Babcock MA, Badr MS. Effect of gender on the development of hypocapnic apnea/hypopnea during NREM sleep. J.Appl.Physiol. 2000;89:192–199. doi: 10.1152/jappl.2000.89.1.192. [DOI] [PubMed] [Google Scholar]


