Abstract
Previous evidence in an animal model of drug self-administration and drug seeking showed that acute oxytocin decreased methamphetamine (meth) seeking in male rats, suggesting potential clinical efficacy for the treatment of psychostimulant addiction. However, based on the well-established role of oxytocin in reproduction and pair bond formation, it is important to know how this effect extrapolates to females. Here, we tested whether oxytocin (1 mg/kg, IP) would decrease meth seeking in female rats across various stages of the estrous cycle (Experiment 1). Freely cycling Long Evans female rats self-administered meth (IV) in 2-h daily sessions, followed by daily extinction sessions. Following extinction, rats received oxytocin (0, 0.3, or 1 mg/kg, IP) 30 min before a meth priming injection (1 mg/kg, IP) to assess reinstatement of meth seeking. Next, we examined the effects of oxytocin on motivated meth- and sucrose-taking and seeking in male and female rats. In separate experiments, males and females self-administered meth (Experiment 2) or sucrose (Experiment 3) until responding was stabilized along a fixed ratio (FR) 5 schedule of reinforcement. Subsequently, rats received either oxytocin or vehicle prior to self-administration along a progressive ratio (PR) schedule of reinforcement. Rats were subsequently tested for cue-, meth-, and stress-induced reinstatement after pretreatment with oxytocin or vehicle. While oxytocin reduced meth seeking in females, we found that estrous cycle stage (as determined from vaginal cytology) did not influence meth-primed reinstatement or the ability of oxytocin to decrease reinstatement of meth seeking. Oxytocin reduced PR responding for meth only in females. Females responded more than males during cue-induced reinstatement of meth and sucrose seeking, and oxytocin reduced this responding only in meth females. In both sexes, oxytocin attenuated meth seeking in response to a meth prime and yohimbine (a pharmacological stressor). The results suggest that oxytocin may have efficacy as a treatment of meth addiction in both sexes; however, females may show greater response to oxytocin treatment for the prevention of relapse.
Keywords: methamphetamine, self-administration, oxytocin, sex differences, reinstatement, progressive ratio
1. Introduction
Oxytocin is an endogenous peptide that is primarily synthesized in magnocelluar neurons of the supraoptic and paraventricular nuclei of the hypothalamus and is secreted through the posterior pituitary for systemic circulation. In addition, oxytocin is produced in parvocelluar neurons in the paraventicular nucleus, which project to various brain regions. Oxytocin binds to the Gq class of G protein-coupled receptors activating phopholipase C and subsequently increasing neuronal firing and neurotransmitter release through this second messenger cascade (Gimpl and Fahrenholz, 2001). In both males and females, central oxytocin regulates social and sexual bonding, as well as other behaviors such as aggression, stress, and anxiety (Baskerville and Douglas, 2010). In females, systemic oxytocin plays an important role in reproduction by inducing uterine contractions for childbirth and facilitating lactation. Oxytocin receptors are distributed throughout the brain (Gimpl and Fahrenholz, 2001), including regions of the mesocorticolimbic dopamine system critically involved in reward processing (Baskerville and Douglas, 2010). For example, oxytocin fibers innervate dopamine-containing nuclei in the ventral tegmental area. Cell bodies of dopamine neurons that make up the mesocorticolimbic dopamine pathways originate in this area, suggesting that oxytocin may subsequently affect dopamine release into target regions (e.g., nucleus accumbens,amygdala, prefrontal cortex). Additionally, hypothalamic oxytocin cells express dopamine receptors (Baskerville et al., 2009), suggesting that dopamine may mediate oxytocin release. Combined, this extensive interaction of the dopamine and oxytocin systems has expanded the interest in the role of oxytocin in drug addiction.
Based on such evidence, oxytocin has potential for treatment of psychostimulant addiction (McGregor and Bowen, 2012) and may play a regulatory role in attenuating drug tolerance, dependence, and withdrawal via actions in the mesolimbic dopamine reward pathways (Baskerville and Douglas, 2010). Oxytocin blocked cocaine-induced dopamine release in the nucleus accumbens (Kovacs et al., 1990) and decreased cocaine intake during self-administration (Sarnyai and Kovacs, 1994). Additionally, recent preclinical evidence suggests that oxytocin may have therapeutic benefits in preventing relapse to methamphetamine (meth) use (Carson et al., 2010a). Of particular note, oxytocin decreased meth seeking in an animal model of addiction (Carson et al., 2010a) and reduced meth-conditioned reward (Qi et al., 2009; Baracz et al., 2012) in males. Oxytocin may be a critical regulator in drug addiction via modulation of dopaminergic transmission in corticolimbic structures (Baskerville and Douglas, 2010; Sarnyai and Kovacs, 1994). For example, oxytocin decreased dopamine release and receptor binding in mesolimbic brain structures (Sarnyai and Kovacs, 1994). Support for the use of oxytocin as an addiction treatment has gained momentum, due in part to the ability of some drugs to enhance social interactions (McGregor et al., 2008; Dumont et al., 2009). Further, neural circuits mediating social bonding and drug reward may overlap (Burkett and Young, 2012).
To date, the ability of oxytocin to ameliorate meth seeking or conditioned reward in female rats is unknown. However, this is an important question given that gonadal hormones regulate oxytocin’s binding affinity and receptor density in various brain regions (Schumacher et al., 1990; Patchev et al., 1993). Further, progesterone and estrogen fluctuate throughout the estrous cycle in female rats, rendering studies with females a necessity to determine oxytocin’s full potential as a pharmacotherapeutic treatment for meth addiction. Clinical research in meth addiction indicates numerous sex differences in terms of meth use patterns and response to treatment (reviewed in (Dluzen and Liu, 2008). For example, women tend to initiate meth use at a younger age (Dluzen and Liu, 2008), transition faster to dependence, exhibit greater dependence (Rawson et al., 2005), and have greater comorbidity with other neuropsychiatric disorders (Hser et al., 2005; Yen and Chong, 2006). While clinical evidence has shown gender differences, preclinical research investigating meth addiction and potential pharmacotheraputic treatments has primarily focused only on male subjects. This pattern is problematic, as females may respond differently to treatments when compared to males, suggesting a need for gender-specific therapies. Consistent with clinical populations, animal models indicate that female rodents have an increased sensitivity to meth’s psychomotor stimulating effects (Schindler et al., 2002; Milesi-Halle et al., 2007).
Animal models of drug self-administration incorporate various aspects of the addiction cycle, including motivation to consume a drug and/or drug seeking behavior in the absence of reinforcement. Self-administration models require animals to learn to press an operandum (typically a lever) for an intravenous drug infusion. In these models, more female rats acquire meth self-administration relative to males (Roth and Carroll, 2004), exhibit higher meth intake (Reichel et al., 2012), and exert more effort for meth as a primary reward (Roth and Carroll, 2004). Meth seeking can be inferred by the reinstatement of lever responding following a period of non-reinforced responding. These reinstatement tests incorporate various trigger factors (e.g., cues, drug-priming, or stress) for the testing of pharmacotherapies that may block reinstatement (Yahyavi-Firouz-Abadi and See, 2009).
Here, we assessed whether systemic oxytocin may be a potential treatment for meth addiction in females and males. To this end, we first determined the effects of oxytocin (0.3 and 1 mg/kg) on meth-primed reinstatement of meth seeking throughout the various stages of the estrous cycle in female rats. Second, we determined whether oxytocin (1 mg/kg, the dose previously shown to reduce meth-primed reinstatement in females) would impact motivation for meth in both males and females during meth self-administration and in response to meth associated cues, meth priming, or pharmacological stress (yohimbine) induced reinstatement. We utilized yohimbine (an α2-adrenergic receptor antagonist) as a pharmacological stressor as it can elicit anxiety states in clinical studies (Bremner et al., 1996), craving in drug dependent subjects (Greenwald et al., 2013), and reinstatement of drug seeking in rats (See and Waters, 2010). Finally, we determined whether oxytocin effects would extend to natural reinforcement (i.e., sucrose pellets) in males and females.
2. Methods
2.1. Subjects
A total of 22 male and 39 female Long-Evans rats (Charles River, Raleigh, NC) weighing 250–300 g and 180–200 g, respectively, at the time of arrival, were used. All rats were individually housed on a reversed 12:12 light-dark cycle in a temperature and humidity controlled vivarium. Water was available ad libitum throughout the study and rat chow (Harlan, Indianapolis, IN, USA) was provided daily until SA stabilized, after which time food was provided ad libitum. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina and were in accordance with the “Guide for the Care and Use of Laboratory Rats” of the Institute of Laboratory Animal Resources on Life Sciences, National Research Council.
2.2. Surgery and drugs
Rats were anesthetized with IP injections of ketamine (66 mg/kg; Vedco Inc, St. Joseph, MO, USA), xylazine (1.3 mg/kg; Lloyd Laboratories, Shenandoah, IA, USA), and Equithesin (0.5 ml/kg; sodium pentobarbital 4 mg/kg, chloral hydrate 17 mg/kg, 21.3 mg/kg magnesium sulfate heptahydrate dissolved in 44% propylene glycol, 10% ethanol solution). Ketorolac (2.0 mg/kg, IP; Sigma Chemical, St. Louis, MO) was given before surgery as an analgesic. Catheters (constructed with Silastic tubing, Dow Corning Corporation, Midland, MI) were inserted 33 mm into the right jugular vein and secured with silk sutures. The opposite end ran subcutaneously and exited through a small incision on the back below the shoulder blades and was attached to an infusion harness (Instech Solomon, Plymouth Meeting, PA). During recovery from surgery, catheters were flushed once daily for 5 days with 0.1 ml of Timentin (24 mg/0.1; GlaxoSmithKline, Research Triangle Park, NC) and 10 U/ml of heparinized saline (Elkins-Sinn, Cherry Hill, NJ). During meth self-administration (methamphetamine hydrochloride; Sigma Chemical, St. Louis, MO), catheters were flushed with 0.1 ml of 10 U/ml heparinized saline before and after every self-administration session. Catheter patency was periodically verified with methohexital sodium (10 mg/ml dissolved in 0.9% saline; Sigma Chemical, St. Louis, MO), a short acting barbiturate that produces a rapid loss in muscle tone when administered intravenously. Oxytocin (1 mg/ml dissolved in ddH20; Cell Sciences, Canton, MA) was administered prior to progressive ratio (PR) and reinstatement testing and yohimbine hydrochloride (2.5 mg/ml dissolved in ddH20; Sigma Chemical, St. Louis, MO) was used for stress-induced reinstatement.
2.3. Self-administration
Self-administration procedures were based on recent studies of meth self-administration in males (Reichel et al., 2012; Reichel and See, 2010). All self-administration experiments were conducted during the rats’ dark cycle in standard Plexiglas self-administration chambers (30 × 20 × 20 cm) that were enclosed in sound attenuating cubicles with a ventilation fan (Med Associates, St. Albans, Vermont) and linked to a computerized data collection program (MED PC, Med Associates). Each chamber was equipped with two retractable levers with a white stimulus light above each lever, house light, and tone generator. For meth self-administration, infusion tubing enclosed in steel spring leashes (Plastics One Inc., Roanoke, VA) was connected to the infusion harness and a weighted swivel apparatus (Instech, Plymouth Meeting, PA) was suspended above the box to allow for free movement within the chamber.
Self-administration sessions were conducted 6 days/week to criterion (14 sessions > 10 infusions). The house light remained on throughout the sessions and a response on the active lever resulted in activation of the pump and delivery of a 2-sec meth infusion (17.5 µg/50 µl bolus for females and 20 µg/50 µl bolus for males) and a 5-sec presentation of a stimulus complex (illumination of the white stimulus light over the active lever and activation of tone generator; 78 dB, 4.5 kHz), followed by a 20-sec timeout. During the time-out period, responses on the active and inactive levers were recorded, but had no scheduled consequences.
2.4. Progressive ratio schedule of reinforcement
PR tests were conducted when stable self-administration was evident based on the criteria of 14 days with more than 10 reinforcers earned. Before tests, rats were injected with oxytocin (1 mg/kg) or vehicle. Test order was counterbalanced, and between tests, rats received a minimum of 2 self-administration sessions.During these tests,reinforcement was contingent upon an increasing number of responses which incrementally increased through the following progression: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, 603 (Richardson and Roberts, 1996). Lever presses, infusions, and breakpoints were assessed for each test. Breakpoint was defined as the highest number of lever presses required by the rat to receive a single infusion. The session terminated if a rat failed to receive an infusion or sucrose pellet for 1 h or after a total of 5 h.
2.5. Extinction and reinstatement
Following self-administration, rats underwent 2 h daily extinction sessions for a minimum of 10 days, where responses on both the active and inactive levers were recorded, but had no scheduled consequences. Extinction criterion consisted of <25%responding on the active lever relative to the last 5 days of self-administration. Upon reaching criteria, rats underwent cue-, meth-, and yohimbine-induced reinstatement testing. Prior to all reinstatement tests, rats received either an injection of oxytocin (0.3 or 1 mg/kg) or vehicle (IP) 30 min before testing (see specific experimental methods detailed below). For cue-induced reinstatement, responding on the active lever resulted in presentation of the light+tone stimulus complex along a FR 5 schedule of reinforcement, whereby 5 lever presses were required to receive the stimulus complex.For drug-primed reinstatement tests an injection of meth (1 mg/kg dissolved in 0.9%physiological saline, IP) was given immediately prior to testing. Prior to stress-induced reinstatement tests, an injection of yohimbine hydrochloride (2.5 mg/kg, IP) was given after oxytocin or vehicle, 30 min prior to testing. During both meth-primed and yohimbine-induced reinstatement tests, responses on either lever were recorded, but did not elicit any programmed consequences. Between all reinstatement tests, rats experienced a minimum of 2 extinction sessions, or until extinction criterion was met.
2.6. Estrous cycle monitoring
Female rats were habituated to vaginal cytology procedures during self-administration. Vaginal lumen samples were collected by gently flushing 30 µl of ddH20 with a sterile saline-dipped pipette tip and extracting the sample using a micropipette before rats were placed in the self-administration chambers. Collected samples were smeared on to a glass slide, stained with Quik-Dip Hematology Stain (Mercedes Medical, FL), and examined for classification of cycle phase (estrus, proestrus, and diestrus I/II) based on previously published criteria (Marcondes et al., 2002; Feltenstein et al., 2011) with a light microscope set at 10x magnification.
2.7. Experiment 1: Oxytocin effects on meth-primed reinstatement across the estrous cycle
Female rats (n=10) self-administered meth along a FR 1 schedule of reinforcement throughout the self-administration phase. Following self-administration and extinction, rats underwent nine meth-primed reinstatement tests with pretreatment of 0, 0.3, and 1 mg/kg oxytocin in order to determine an effective dose in females. When possible, rats were tested during each of the phases of their estrous cycle (estrus, proestrus, diestrus I/II). The number of animals that tested in each condition follows: Control: Diestrus I/II n=8, Proestrus n=4, Estrus n=8; 0.3 mg/kg Oxytocin: Diestrus I/II n=10, Proestrus N=8, Estrus n=10; and 1 mg/kg Oxytocin: Diestrus I/II n=10, Proestrus N=7, Estrus n=10. Reinstatement tests were conducted in randomized order with a minimum of 2 extinction sessions occurring between tests, or until subjects returned to criterion.
2.8. Experiment 2: Oxytocin effects on meth self-administration and reinstatement
Male (n=15) and female (n=17) rats initially self-administered meth along a FR1 schedule of reinforcement (1 lever press resulted in a meth infusion) until they reached criterion of a minimum of 5 days with >10 infusions (with <25% change in the number of infusions for 2 consecutive days). Rats then moved to a FR3 schedule for a minimum of 3 days, followed by a FR5 schedule for the remainder of the self-administration sessions. Testing on the PR reinforcement schedule followed stable responding on the FR5. Following these tests, extinction criterion was met and all rats underwent 3 types of reinstatement tests in the following order: cue-induced, meth-primed, and yohimbine-induced, each counterbalanced for oxytocin (1 mg/kg) or vehicle pretreatment for a total of 6 reinstatement tests. Test order did not interact with reinstatement responding. Between all reinstatement tests, rats experienced a minimum of 2 extinction sessions, or until extinction criterion was met.
2.9. Experiment 3: Oxytocin effects on sucrose self-administration and reinstatement
Males (n=7) and females (n=12) underwent identical procedures as rats in Experiment 2, with the following exceptions. Instead of meth, sucrose pellets (45 mg, Noyes pellets, Fisher Scientific) served as the primary reinforcer. Sucrose rats did not undergo surgery. Additionally, during sucrose primed reinstatement tests, rats received one non-contingent pellet every 2 min for the first 10 min of the session and one pellet every 30 min thereafter.
2.10. Data analysis
The number of lever responses, breakpoint, and infusions during the progressive ratio were the primary dependent variables and were analyzed using analysis of variance (ANOVA). For reinstatement testing during the estrous cycle, lever responses were analyzed using a two-way ANOVA with oxytocin dose and cycle phase as between subject factors. Planned and post hoc comparisons were conducted using a Bonferroni’s correction for family wise error when applicable, with the alpha set at 0.05. All data are expressed as the mean ± SEM.
3. Results
3.1. Experiment 1: Estrous cycle effects on meth-primed reinstatement with oxytocin pretreatment
Figure 1 depicts meth-primed reinstatement at three doses of oxytocin (0, 0.3, and 1 mg/kg) during the three phases of the estrous cycle (proestrus, diestrus I/II, estrus).Overall, oxytocin dose did not interact with estrous cycle, nor was there a main effect of estrous cycle. However, there was a main effect of oxytocin dose [Figure 1b, F(2,66)=4.63, p<0.05]. Specifically, 1 mg/kg oxytocin decreased responding relative to vehicle [p<0.05].
Figure 1.
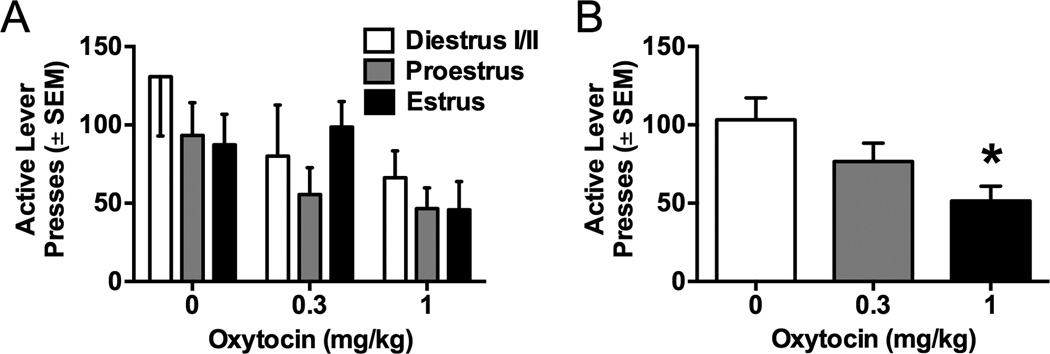
Meth-primed reinstatement to meth seeking in females following oxytocinpretreatment. A) Active lever responding during reinstatement by estrous cycle phaseand dose of oxytocin. No effect of cycle phase was seen during meth-primed reinstatement with or without oxytocin. B) Active lever responding during reinstatement by dose of oxytocin. Oxytocin (1 mg/kg) significantly decreased active lever responding as compared to vehicle (*p<0.05).
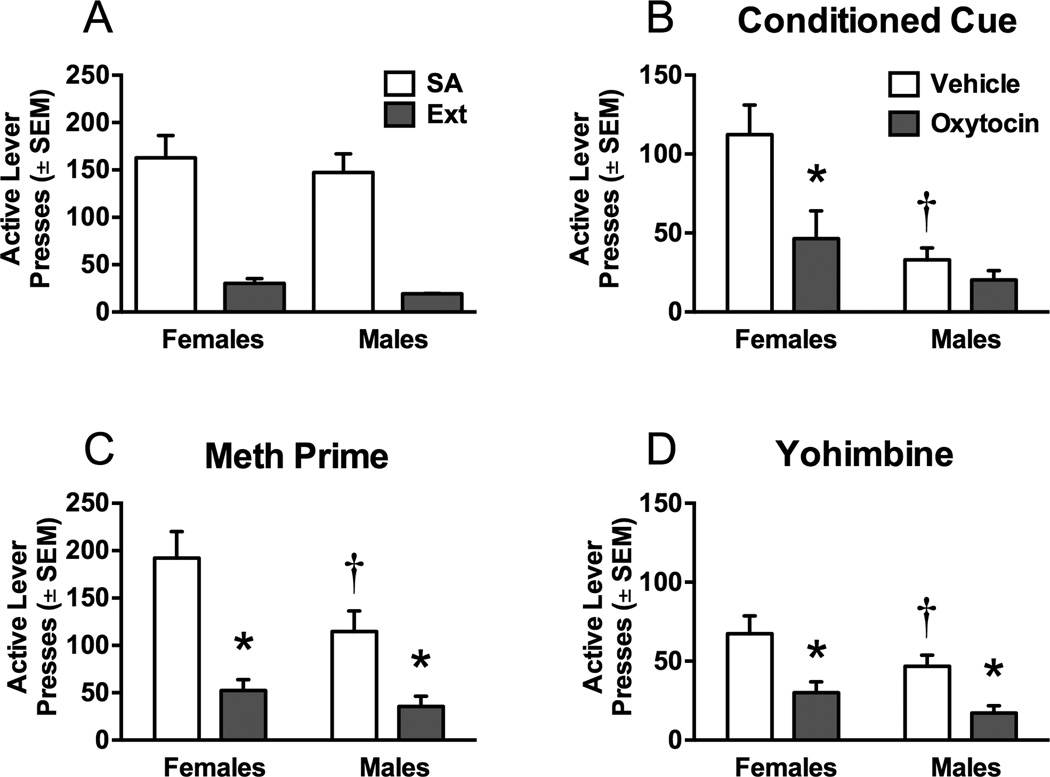
3.2. Experiment 2: Oxytocin effects on meth self-administration and reinstatement
Figure 2 depicts the impact of oxytocin on PR performance (lever presses, break points, and infusions) for males and females during meth self-administration. Although sex did not interact with oxytocin treatment [F(1,28)=3.13, p=0.09], females had, overall,more active [Figure 2A, sex main effect, F(1,28)=6.26, p<0.05] and inactive (Table 1, sex main effect, F(7,28)=4.48, p<0.05] lever responses as compared to males. Oxytocin decreased active lever responding [treatment main effect, F(1,28)=10.8, p<0.001].However this decrease was only significantly relevant to controls in females [Bonferroni,p<0.05]. Consistently, females had a higher breakpoint than males [Figure 2B, sex main effect, F(1,28)=5.68, p<0.05], but sex did not interact with oxytocin [F(1,28)=2.42,p=0.07]. Oxytocin decreased breakpoints [treatment main effect, F(1,28)=10.31, p<0.001], but only significantly in females relative to saline controls [Bonferroni, p<0.05]. The number of infusions earned during progressive ratio responding did not interact between males and females [F(1,28)=3.41, p=0.09]. Males and females earned similar meth infusions (Figure 2C), and oxytocin pretreatment decreased infusions earned [treatment main effect, F(1,28)=14.08, p<0.001]. This decrease in meth infusions was only significant in females [Bonferroni, p<0.05].
Figure 2.
Progressive ratio (PR) responding in males and females during meth self-administration. A) Active lever responding in males and females after oxytocin or vehicle. Females responded more on the active lever during the PR test relative to males. Oxytocin decreased active lever responding for females, but not males. B) Breakpoint during PR tests. Females had a higher breakpoint relative to males and oxytocin (1 mg/kg) reduced the breakpoint in females, but not males. C) Number of infusions during PR tests. Oxytocin decreased the number of infusions for females, but not males. Significant differences from same sex control (*p<0.05) or between males and females treated with vehicle (†p<0.05) are indicated.
Table 1.
Mean (± SEM) inactive lever responding during meth self-administration, progressive ratio, and reinstatement tests.
| Females | Males | |||
|---|---|---|---|---|
| Self-administration | 50.8 (± 18.3) | 29.3 (± 6.5) | ||
| Extinction | 21.4 (± 10.4) | 14.7 (± 3.5) | ||
| Vehicle | Oxytocin | Vehicle | Oxytocin | |
| Progressive ratio + | 130.8 (± 36.7) | 98.2 (± 30.5) | 43.7 (± 12.9) | 31.0 (± 10.1) |
| Reinstatement Test | ||||
| Cue * | 31.7 (± 10.7) | 10.4 (± 3.3) | 15.4 (± 3.8) | 10.9 (± 2.4) |
| Prime * | 57.1 (± 21.3) | 24.3 (± 12.5) | 19.8 (± 4.2) | 12.5 (± 3.9) |
| Yohimbine * | 22.6 (± 8.9) | 14.3 (± 4.1) | 16.5 (± 3.3) | 10.0 (± 2.0) |
Significant difference between males and females
Significant difference between oxytocin and vehicle
Figure 3 depicts self-administration, extinction, and reinstatement data for meth self-administration in males and females. Figure 3A shows the average active lever responding over the last 5 days of self-administration and the last 2 days of extinction. No differences emerged between males and females in active or inactive lever presses (see Table 1 for inactive lever data). However, females (14.50±1.19) took significantly more days to meet extinction criterion than males (10.93±0.62) [t(27)=2.7, p<0.05].
Figure 3.
Active lever responding during meth self-administration and subsequentreinstatement tests. A) Active lever responding during the last 5 days of self-administration and the last 2 days of extinction in males and females. B) Active lever responding during cue-induced reinstatement in males and females after oxytocin or vehicle. Females reinstated to conditioned cues to a greater extent than males. Oxytocin decreased cue-induced reinstatement in females, but not males. C) Active lever responding during meth-primed reinstatement. Females responded more during the meth prime test than males. Oxytocin decreased meth-primed reinstatement in both males and females. D) Active lever responding during stress-induced reinstatement by yohimbine. Oxytocin decreased yohimbine-induced reinstatement in males and females. Significant differences from same sex control (*p<0.05) or between males and females treated with vehicle (†p<0.05) are indicated.
After extinction, the rats were first tested on cue-induced reinstatement tests (Figure 3B). Active lever presses for males and females varied according to treatment. Overall, females responded more during cue-induced reinstatement than males [sex main effect, F(1,24)=11.55, p<0.05] and oxytocin attenuated active lever responding only in females [sex × treatment interaction, F(1,24)=7.35, p<0.05, and post hoc, p<0.05]. Oxytocin also decreased inactive lever responding [Table 1, treatment main effect, F(1,24)=1.56, p<0.05]. During meth-primed reinstatement (Figure 3C) females had more active lever responses [sex main effect, F(1,24)=4.52, p<0.05] and oxytocin decreased active [treatment main effect, [F(1,24)=51.14, p<0.0001] and inactive [Table 1, treatment main effect, [F(1,24)=8.6, p<0.01] lever responding in both females and males. Rats were then tested on a yohimbine stress induced reinstatement test (Figure 3D). During yohimbine-induced reinstatement, females reinstated to a greater extent than males [sex main effect, F(1,20)=5.73, p<0.05] and oxytocin attenuated active [treatment main effect, F(1,20)=16.87, p<0.001] and inactive [Table 1, treatment main effect, [F(1,22)=4.35, p<0.05] lever responding in both sexes.
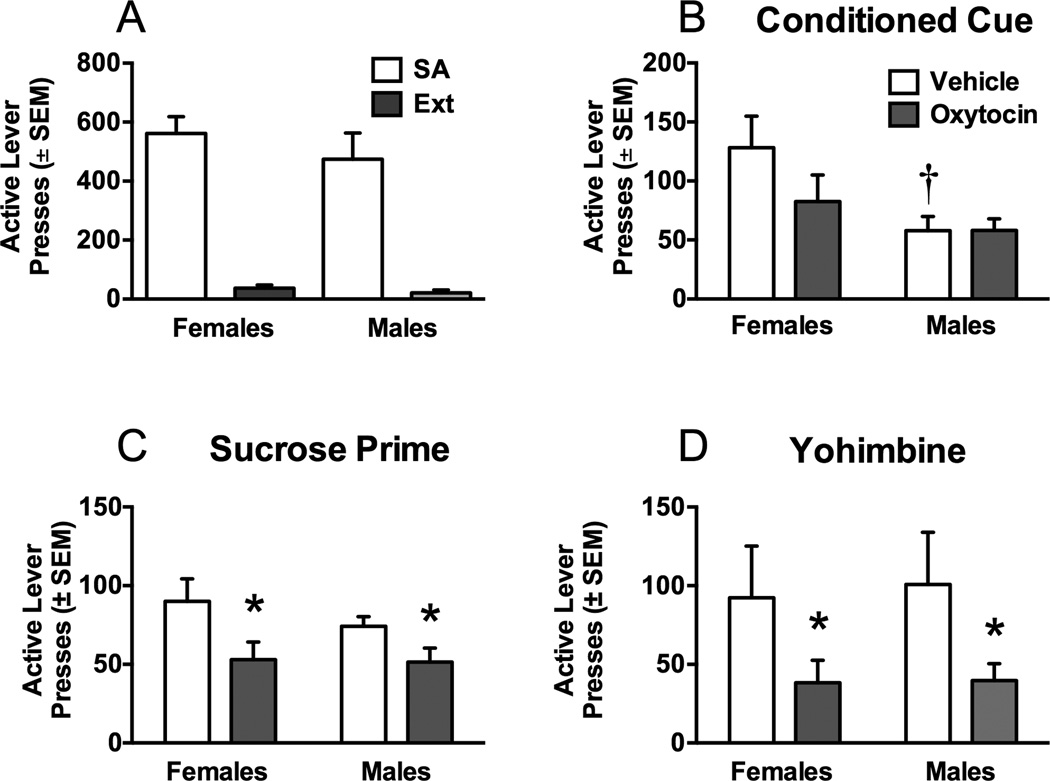
3.3. Experiment 3: Oxytocin effects on sucrose self-administration and reinstatement
Figure 4 depicts the impact of oxytocin on lever presses, infusions, and break points during the PR tests for sucrose self-administration in females and males. There were no sex differences or oxytocin effects on the number of active (Figure 4A) or inactive (Table 2) lever presses, break point (Figure 4B), or pellets earned (Figure 4C).
Figure 4.
Progressive ratio (PR) responding for males and females during sucrose self-administration. There were no differences in A) active lever responding, B) breakpoints, or C) pellets earned during PR tests in males and females after oxytocin or vehicle.
Table 2.
Mean (± SEM) inactive lever responding during sucrose self-administration, progressive ratio, and reinstatement tests.
| Females | Males | |||
|---|---|---|---|---|
| Self-administration | 34.5 (± 5.9) | 28.4 (± 2.5) | ||
| Extinction | 17.1 (± 2.7) | 22.1 (± 0.6) | ||
| Vehicle | Oxytocin | Vehicle | Oxytocin | |
| Progressive ratio | 70.3 (± 28.2) | 37.4 (± 24.2) | 36.6 (± 8.8) | 28.9 (± 10.4) |
| Reinstatement Test | ||||
| Cue | 16.4 (± 7.4) | 9.3 (± 5.0) | 26.0 (± 7.6) | 15.1 (± 5.4) |
| Prime | 11.5 (± 5.2) | 8.8 (± 3.0) | 14.7 (± 5.0) | 13.4 (± 3.7) |
| Yohimbine | 77.4 (± 37.1) | 19.4 (± 16.3) | 79.7 (± 36.4) | 27.9 (± 13.2) |
Figure 5A shows the average active lever responding over the last 5 days of sucrose self-administration (when self-administration behavior had stabilized), and no differences were seen between males and females. Extinction responding did not differ during the last 2 days between sexes. Following extinction, the rats were first tested on a cue-induced reinstatement test (Figure 5B). Females responded more on the active lever than males [sex main effect, F(1,13)=6.97 p<0.05]. Oxytocin did not affect responding in either sex. On the sucrose prime test (Figure 5C), oxytocin decreased active lever responding in both females and males [treatment main effect, [F(1,13)=8.61, p<0.05]. In addition, during the yohimbine-induced reinstatement test, oxytocin attenuated active lever responding in both sexes [Figure 5D, F(1,13)=8.01, p<0.005]. Responding on the inactive lever did not differ on any measure for sucrose-trained rats (Table 2.)
Figure 5.
Active lever responding during sucrose self-administration and subsequent reinstatement tests in males and females treated with oxytocin or vehicle. A) Active lever responding during that last 5 days of self-administration and the last 2 days of extinction in males and females. B) Active lever responding during cue-induced reinstatement in males and females after oxytocin or vehicle. Females reinstated to conditioned cues to a greater extent than males. Oxytocin decreased cue-induced reinstatement in females, but not males. C) Active lever responding during sucroseprimed reinstatement. Females responded more during the sucrose prime test than males. Oxytocin decreased sucrose-primed reinstatement in both males and females. D) Active lever responding during stress-induced reinstatement by yohimbine. Oxytocin decreased yohimbine-induced reinstatement in males and females. Significant differences from same sex control (*p<0.05) or between males and females treated with vehicle (†p<0.05) are indicated.
4. Discussion
Here, we have established that the ability of systemic oxytocin to reduce reinstatement of meth seeking may be dependent on sex and the specific type of relapse trigger. Also, we found sex specific effects of oxytocin in reducing motivation to take meth, as well as reward seeking in response to conditioned reinforcers. The similarities between sexes were evident by the ability of oxytocin to decrease meth seeking in both males and females in response to a drug prime and a pharmacological stressor. To date, only one study exists (Holtz et al., 2012) that directly compared the ability of a treatment compound (allopregnanolone or modafinil) to block reinstatement to meth seeking in both males and females. However, prior studies with rats have clearly demonstrated pronounced differences between male and female meth intake (Reichel et al., 2012), motivation to self-administer meth (current report, (Roth and Carroll, 2004), and reinstatement of meth seeking (Holtz et al., 2012; Reichel et al., 2012).
In the first experiment, estrous cycle did not interact with oxytocin during meth-primed reinstatement testing and females reinstated to a meth prime regardless of cycle phase. As such, neither meth-primed reinstatement nor attenuation with oxytocin appeared to be influenced by circulating ovarian hormones. However, PR responding and reinstatement tests were conducted based on response criteria, rather than estrous cycle for the females. Therefore, the possibility remains that the reported sex differences may be due, in part, to differential regulation of oxytocin receptor affinity, surface expression, and/or coupling mechanisms by estrogen and progesterone. For example, estrogen increased oxytocin receptor affinity in the medial preoptic area of the hypothalamus (Caldwell et al., 1994) and receptor density in the ventromedial nucleus (Coirini et al., 1991). Additionally, progesterone increased oxytocin receptor density in limbic structures (Patchev et al., 1993), which could subsequently increase the effects of oxytocin in females relative to males. Fluctuations in gonadal hormones may account for the sex differences in motivation to take meth during the PR tests and meth seeking during cue-induced reinstatement.
Oxytocin reduced responding for meth along the PR schedule of reinforcement in females down to a level comparable to males. However, motivation for sucrose was similar in males and females regardless of pretreatment condition. In males, oxytocin did not have an effect on either meth or sucrose taking during the PR. A distinction between drug and natural reward occurred in females, as oxytocin only decreased meth taking, but not sucrose taking, during the PR. Interestingly, females in general demonstrated greater motivated meth taking, which is in line with previous self-administration studies with meth (Roth and Carroll, 2004), cocaine (Roberts et al., 1989), heroin (Cicero et al., 2002), and alcohol (Peters et al., 2013). While we found the ability of oxytocin to reduce motivation to obtain meth was specific to females, a previous study showed that oxytocin (1 mg/kg) decreased responding on a similar PR task in male rats (Carson et al., 2010a). Although strain differences may exist between studies, these contrasting findings in males likely arise from several methodological differences. For example, in our study, rats were maintained on a FR schedule of reinforcement and two PR ratio tests were given in a counterbalanced manner with a minimum of two days for intervening stabilization. In contrast, rats in the earlier study transitioned to and maintained their responding along a PR schedule of reinforcement and oxytocin administration occurred on five consecutive days with ascending doses. As such, these repeated daily doses of oxytocin might have resulted in a sensitized response to oxytocin. Also, rats maintained along a PR schedule received fewer daily meth infusions on average than rats in our study that were maintained along a FR. The increased meth intake for males may have rendered them less susceptible to lower doses of oxytocin (i.e., oxytocin’s efficacy may be related to total meth intake).
Oxytocin only reduced cue-induced reinstatement in meth females, indicating some specificity for sex and conditioning of drug-cue associations. For example, in females, oxytocin decreased responding for meth-conditioned cues, but not sucrose. This difference indicates that oxytocin has some degree of specificity to reduce relapse to drug related cues, rather than those associated with natural reward. Alternatively, different response rates between the meth and sucrose studies provide a potential explanation for this difference. Specifically, the sucrose females received on average 561.8 (±25.4) primary and secondary reinforcers (i.e., sucrose pellets and light + tone stimulus complex) during the last 5 days of self-administration as compared to the meth females that received 162.9 (±10.5) reinforcers. Consequently, this difference in response rate and reinforcement history may have rendered sucrose females less susceptible to oxytocin’s ability to attenuate cue-induced reward seeking.
In the current study, females responded more to meth and sucrose conditioned cues in comparison to males. Previous studies have shown that females responded equally to males in response to conditioned cues during reinstatement of cocaine (Feltenstein et al., 2011; Zhou et al., 2012) or nicotine (Feltenstein et al., 2012) seeking. Importantly, these effects were evident when rats were trained and tested along an FR1 schedule of reinforcement. Meth rats in our study were trained and tested on an FR5 schedule of reinforcement, whereby the light + tone stimulus complex occurred after every 5 active lever presses during the reinstatement test. Male meth rats had low levels of reinstatement on this test schedule relative to our experiences typically observed with FR1 cue-induced reinstatement (Reichel and See, 2010; Reichel et al., 2012), suggesting a possible floor effect. In spite of the low reinstatement in males, females demonstrated markedly more robust reinstatement in the identical situation. Surprisingly, females were impervious to the lean reinforcement schedule during cue-induced reinstatement. This enhanced responding to conditioned reinforcers suggests that females may entrain the original associations to a greater degree than males, indicating differences in interpretation of the associative strength of the reward. Associative strength is defined as the degree of strength of learning that occurs between a conditioned stimulus (CS, i.e., light + tone) and the unconditioned stimulus (US, i.e., physiological effects of meth or sucrose). Increased meth-cue and sucrose-cue reinstatement in females may be due to increased salience or value of the US, which imbues the associative strength of the CS. Support for this notion comes from a demonstration that estrogen enhanced conditioned learning in delay and trace conditioning paradigms (Shors et al., 2000). Since we utilized freely cycling females with an intact hormonal system, fluctuations in estrogen and progesterone levels during self-administration may have influenced the original associative processes.
Oxytocin was equally effective at reducing reinforcer-primed reinstatement of meth and sucrose seeking, although females reinstated to a greater extent to a meth prime than males. This sex difference has been seen before and demonstrates that, regardless of session length, females exhibit enhanced meth seeking to a priming injection [current report, (Holtz et al., 2012; Reichel et al., 2012)]. Importantly, meth-primed reinstatement did not depend upon estrous cycle phase for females, nor did cycle interact with the ability of oxytocin to reduce meth seeking. In fact, oxytocin reduced prime-induced meth seeking during all cycle phases for females. Further, oxytocin decreased sucrose seeking during sucrose primed reinstatement tests. Taken together, oxytocin decreased seeking for both meth and sucrose during the reinstatement tests in both sexes. This effect suggests that oxytocin decreased seeking for both drug and natural reward, perhaps through similar mechanisms. Although the underlying mechanisms are beyond the scope of this study, one suggestion relies on the interaction of oxytocin with the dopamine system (Baskerville and Douglas, 2010). Oxytocin may decrease the saliency of reward via interactions with dopamine activity in regions known to be involved in drug seeking. For example, in mice, oxytocin attenuated meth-induced dopamine efflux in the nucleus accumbens (Qi et al., 2009), and regulated meth-induced changes in extracellular glutamate and γ-aminobutyric acid (GABA) in mouse brain (Qi et al., 2012). In rats, oxytocin decreased meth-induced Fos expression in the nucleus accumbens core (Carson et al., 2010b). Future studies will need to directly elucidate the mechanism by which oxytocin attenuates drug seeking.
The route of systemic oxytocin administration results in a more homologous comparison for therapeutic use, rather than site specific brain infusions. However, peripheral administration of a neuropeptide raises the issue of the degree of transfer across the blood brain barrier to exert a centrally mediated effect (Landgraf et al., 1979). We suspect that decreased meth seeking was primarily mediated through actions in the brain and not via peripheral effects for several reasons. First, peripherally administered oxytocin attenuated cocaine induced sniffing behavior, which was subsequently blocked by .intracerebroventricular administration of an oxytocin antagonist (Sarnyai et al., 1991). Second, peripherally administered oxytocin increased fos (a marker of neuronal activation) expression in oxytocin cells of the supraoptic and paraventricular nuclei of the hypothalamus (Carson et al., 2010b). Finally, methamphetamine-induced fos expression was decreased in the nucleus accumbens core and subthalamic nucleus following peripheral oxytocin treatment. Combined, these data demonstrate that peripherally administered oxytocin can exert central actions.
In all subjects, oxytocin attenuated yohimbine-induced reinstatement, suggesting a common mechanism for reductions in stress-induced reinstatement. Oxytocin produces anxiolytic effects in humans (Macdonald and Macdonald, 2010) and animals (Neumann et al., 2000) through inhibition of the hypothalamic-pituitary-adrenal axis, which is a sex independent effect in rats (Neumann et al., 2000). The pharmacological stressor, yohimbine, produced similar reinstatement in both sexes of rats with a meth history. This finding was somewhat surprising, given that female cocaine addicts are purportedly more likely to relapse to stressful life events (McKay et al., 1996; Back et al., 2005). Although, results have been somewhat mixed in animal models of relapse with cocaine. While one study showed greater yohimbine-induced reinstatement in females relative to males (Anker and Carroll, 2010), our laboratory previously reported that sex differences in reinstatement only emerged when cues were simultaneously presented in combination with yohimbine (Feltenstein et al., 2011). In the current study, we also found no sex differences in yohimbine-induced reinstatement of sucrose seeking. Earlier reports showed that yohimbine reinstated sucrose seeking in males (Ghitza et al., 2006; Richards et al., 2008), but to our knowledge, this effect has not been previously shown in females.
Although inactive lever presses were uniformly lower than active lever presses, oxytocin reduced inactive lever presses in rats with a history of meth self-administration, but not sucrose. Changes in inactive lever responding could indicate alternative strategies in exploratory drive for reward and/or non-specific motor behavior. These options are unlikely, however, as pilot data from our laboratory has found that oxytocin (1 mg/kg, 30 min before test) does not reduce meth-induced or basal motor activity beyond baseline values (data not shown). Furthermore, oxytocin did not decrease responding for sucrose during the PR test, suggesting an effect specific to methexperienced rats.
In conclusion, we report: 1) oxytocin reduced motivation to self-administer meth in females, but not males; 2) females responded more to conditioned reinforcers during cue-induced reinstatement tests; and 3) sex similarities in the ability of oxytocin to decrease meth and sucrose seeking in response to either a meth prime or a pharmacological stressor. Oxytocin appears to have the greatest influence on meth-experienced females, decreasing motivation and relapse in response to cue, meth prime, and yohimbine-induced reinstatement. In contrast, oxytocin only impacted males in response to meth and yohimbine-primed reinstatement. Taken together, these results indicate that oxytocin or oxytocin receptor agonists may be a promising pharmacotherapeutic treatment strategy for meth addiction in both males and females in response to multiple relapse triggers.
Acknowledgements
This research was supported by NIDA grants DA016511 (RES), HD055885 (CMR), Specialized Center on Research-Pilot Research Grant (CMR), and NIH grant C06 RR015455. The authors thank Shannon M. Ghee, Clifford Chan, and Jennifer Hergatt for technical assistance.
Role of the Funding Source
The National Institutes of Health (NIH) provided funding for this study. The NIH had no involvement in study design, data collection, analysis, and interpretation. Further the funding source did not have a role in writing the research report or the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflicts of interest to report.
Contributors
Author BMC conducted the methamphetamine experiments, assisted in experimental design, and wrote the first manuscript draft. Author ABY assisted in running the sucrose experiment, contributed to data analysis, and the first manuscript draft. Author RES consulted on experimental design, analysis, and edited the final manuscript. Author CMR designed the experiments, ran the statistical analysis, and assisted in writing and editing the final manuscript. All authors contributed to and have approved the final manuscript.
References
- Anker JJ, Carroll ME. Sex differences in the effects of allopregnanolone on yohimbine-induced reinstatement of cocaine seeking in rats. Drug Alcohol Depend. 2010;107:264–267. doi: 10.1016/j.drugalcdep.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SE, Brady KT, Jackson JL, Salstrom S, Zinzow H. Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology (Berl) 2005;180:169–176. doi: 10.1007/s00213-004-2129-7. [DOI] [PubMed] [Google Scholar]
- Baracz SJ, Rourke PI, Pardey MC, Hunt GE, McGregor IS, Cornish JL. Oxytocin directly administered into the nucleus accumbens core or subthalamic nucleus attenuates methamphetamine-induced conditioned place preference. Behav Brain Res. 2012;228:185–193. doi: 10.1016/j.bbr.2011.11.038. [DOI] [PubMed] [Google Scholar]
- Baskerville TA, Allard J, Wayman C, Douglas AJ. Dopamine-oxytocin interactions in penile erection. Eur J Neurosci. 2009;30:2151–2164. doi: 10.1111/j.1460-9568.2009.06999.x. [DOI] [PubMed] [Google Scholar]
- Baskerville TA, Douglas AJ. Dopamine and oxytocin interactions underlying behaviors: potential contributions to behavioral disorders. CNS Neurosci Ther. 2010;16:e92–e123. doi: 10.1111/j.1755-5949.2010.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: II. Clinical studies. Synapse. 1996;23:39–51. doi: 10.1002/(SICI)1098-2396(199605)23:1<39::AID-SYN5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Burkett JP, Young LJ. The behavioral, anatomical and pharmacological parallels between social attachment, love and addiction. Psychopharmacology (Berl) 2012;224:1–26. doi: 10.1007/s00213-012-2794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell JD, Walker CH, Pedersen CA, Barakat AS, Mason GA. Estrogen increases affinity of oxytocin receptors in the medial preoptic area-anterior hypothalamus. Peptides. 1994;15:1079–1084. doi: 10.1016/0196-9781(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Carson DS, Cornish JL, Guastella AJ, Hunt GE, McGregor IS. Oxytocin decreases methamphetamine self-administration, methamphetamine hyperactivity, and relapse to methamphetamine-seeking behaviour in rats. Neuropharmacology. 2010a;58:38–43. doi: 10.1016/j.neuropharm.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Carson DS, Hunt GE, Guastella AJ, Barber L, Cornish JL, Arnold JC, Boucher AA, McGregor IS. Systemically administered oxytocin decreases methamphetamine activation of the subthalamic nucleus and accumbens core and stimulates oxytocinergic neurons in the hypothalamus. Addict Biol. 2010b;15:448–463. doi: 10.1111/j.1369-1600.2010.00247.x. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Davis LA, LaRegina MC, Meyer ER, Schlegel MS. Chronic opiate exposure in the male rat adversely affects fertility. Pharmacol Biochem Behav. 2002;72:157–163. doi: 10.1016/s0091-3057(01)00751-1. [DOI] [PubMed] [Google Scholar]
- Coirini H, Schumacher M, Flanagan LM, McEwen BS. Transport of estrogen-induced oxytocin receptors in the ventromedial hypothalamus. J Neurosci. 1991;11:3317–3324. doi: 10.1523/JNEUROSCI.11-11-03317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dluzen DE, Liu B. Gender differences in methamphetamine use and responses: a review. Gend Med. 2008;5:24–35. doi: 10.1016/s1550-8579(08)80005-8. [DOI] [PubMed] [Google Scholar]
- Dumont GJ, Sweep FC, van der Steen R, Hermsen R, Donders AR, Touw DJ, van Gerven JM, Buitelaar JK, Verkes RJ. Increased oxytocin concentrations and prosocial feelings in humans after ecstasy (3,4-methylenedioxymethamphetamine) administration. Soc Neurosci. 2009;4:359–366. doi: 10.1080/17470910802649470. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Ghee SM, See RE. Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug Alcohol Depend. 2012;121:240–246. doi: 10.1016/j.drugalcdep.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, Henderson AR, See RE. Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: sex differences and the role of the estrous cycle. Psychopharmacology (Berl) 2011;216:53–62. doi: 10.1007/s00213-011-2187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Gray SM, Epstein DH, Rice KC, Shaham Y. The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: a role of CRF1 receptors. Neuropsychopharmacology. 2006;31:2188–2196. doi: 10.1038/sj.npp.1300964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Lundahl LH, Steinmiller CL. Yohimbine increases opioid-seeking behavior in heroin-dependent, buprenorphine-maintained individuals. Psychopharmacology (Berl) 2013;225:811–824. doi: 10.1007/s00213-012-2868-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtz NA, Lozama A, Prisinzano TE, Carroll ME. Reinstatement of methamphetamine seeking in male and female rats treated with modafinil and allopregnanolone. Drug Alcohol Depend. 2012;120:233–237. doi: 10.1016/j.drugalcdep.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI, Evans E, Huang YC. Treatment outcomes among women and men methamphetamine abusers in California. J Subst Abuse Treat. 2005;28:77–85. doi: 10.1016/j.jsat.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Kovacs GL, Sarnyai Z, Barbarczi E, Szabo G, Telegdy G. The role of oxytocin-dopamine interactions in cocaine-induced locomotor hyperactivity. Neuropharmacology. 1990;29:365–368. doi: 10.1016/0028-3908(90)90095-9. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Ermisch A, Hess J. Indications for a brain uptake of labelled vasopressin and ocytocin and the problem of the blood-brain barrier. Endokrinologie. 1979;73:77–81. [PubMed] [Google Scholar]
- Macdonald K, Macdonald TM. The peptide that binds: a systematic review of oxytocin and its prosocial effects in humans. Harv Rev Psychiatry. 2010;18:1–21. doi: 10.3109/10673220903523615. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62:609–614. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Bowen MT. Breaking the loop: oxytocin as a potential treatment for drug addiction. Horm Behav. 2012;61:331–339. doi: 10.1016/j.yhbeh.2011.12.001. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Callaghan PD, Hunt GE. From ultrasocial to antisocial: a role for oxytocin in the acute reinforcing effects and long-term adverse consequences of drug use? Br J Pharmacol. 2008;154:358–368. doi: 10.1038/bjp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JR, Rutherford MJ, Cacciola JS, Kabasakalian-McKay R, Alterman AI. Gender differences in the relapse experiences of cocaine patients. J Nerv Ment Dis. 1996;184:616–622. doi: 10.1097/00005053-199610000-00006. [DOI] [PubMed] [Google Scholar]
- Milesi-Halle A, McMillan DE, Laurenzana EM, Byrnes-Blake KA, Owens SM. Sex differences in (+)-amphetamine- and (+)-methamphetamine-induced behavioral response in male and female Sprague-Dawley rats. Pharmacol Biochem Behav. 2007;86:140–149. doi: 10.1016/j.pbb.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Wigger A, Torner L, Holsboer F, Landgraf R. Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: partial action within the paraventricular nucleus. J Neuroendocrinol. 2000;12:235–243. doi: 10.1046/j.1365-2826.2000.00442.x. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Schlosser SF, Hassan AH, Almeida OF. Oxytocin binding sites in rat limbic and hypothalamic structures: site-specific modulation by adrenal and gonadal steroids. Neuroscience. 1993;57:537–543. doi: 10.1016/0306-4522(93)90003-x. [DOI] [PubMed] [Google Scholar]
- Peters S, Slattery DA, Flor PJ, Neumann ID, Reber SO. Differential effects of baclofen and oxytocin on the increased ethanol consumption following chronic psychosocial stress in mice. Addict Biol. 2013;18:66–77. doi: 10.1111/adb.12001. [DOI] [PubMed] [Google Scholar]
- Qi J, Han WY, Yang JY, Wang LH, Dong YX, Wang F, Song M, Wu CF. Oxytocin regulates changes of extracellular glutamate and GABA levels induced by methamphetamine in the mouse brain. Addict Biol. 2012;17:758–769. doi: 10.1111/j.1369-1600.2012.00439.x. [DOI] [PubMed] [Google Scholar]
- Qi J, Yang JY, Wang F, Zhao YN, Song M, Wu CF. Effects of oxytocin on methamphetamine-induced conditioned place preference and the possible role of glutamatergic neurotransmission in the medial prefrontal cortex of mice in reinstatement. Neuropharmacology. 2009;56:856–865. doi: 10.1016/j.neuropharm.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Gonzales R, Obert JL, McCann MJ, Brethen P. Methamphetamine use among treatment-seeking adolescents in Southern California: participant characteristics and treatment response. J Subst Abuse Treat. 2005;29:67–74. doi: 10.1016/j.jsat.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Chan CH, Ghee SM, See RE. Sex differences in escalation of methamphetamine self-administration: cognitive and motivational consequences in rats. Psychopharmacology (Berl) 2012;223:371–380. doi: 10.1007/s00213-012-2727-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, See RE. Modafinil effects on reinstatement of methamphetamine seeking in a rat model of relapse. Psychopharmacology (Berl) 2010;210:337–346. doi: 10.1007/s00213-010-1828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, Bartlett SE. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology (Berl) 2008;199:109–117. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology (Berl) 1989;98:408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology (Berl) 2004;172:443–449. doi: 10.1007/s00213-003-1670-0. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Babarczy E, Krivan M, Szabo G, Kovacs GL, Barth T, Telegdy G. Selective attenuation of cocaine-induced stereotyped behaviour by oxytocin: putative role of basal forebrain target sites. Neuropeptides. 1991;19:51–56. doi: 10.1016/0143-4179(91)90073-r. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Kovacs GL. Role of oxytocin in the neuroadaptation to drugs of abuse. Psychoneuroendocrinology. 1994;19:85–117. doi: 10.1016/0306-4530(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Bross JG, Thorndike EB. Gender differences in the behavioral effects of methamphetamine. Eur J Pharmacol. 2002;442:231–235. doi: 10.1016/s0014-2999(02)01550-9. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Coirini H, Pfaff DW, McEwen BS. Behavioral effects of progesterone associated with rapid modulation of oxytocin receptors. Science. 1990;250:691–694. doi: 10.1126/science.2173139. [DOI] [PubMed] [Google Scholar]
- See RE, Waters RP. Pharmacologically-induced stress: a cross-species probe for translational research in drug addiction and relapse. Am J Transl Res. 2010;3:81–89. [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Beylin AV, Wood GE, Gould E. The modulation of Pavlovian memory. Behav Brain Res. 2000;110:39–52. doi: 10.1016/s0166-4328(99)00183-7. [DOI] [PubMed] [Google Scholar]
- Yahyavi-Firouz-Abadi N, See RE. Anti-relapse medications: preclinical models for drug addiction treatment. Pharmacol Ther. 2009;124:235–247. doi: 10.1016/j.pharmthera.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen CF, Chong MY. Comorbid psychiatric disorders, sex, and methamphetamine use in adolescents: a case-control study. Compr Psychiatry. 2006;47:215–220. doi: 10.1016/j.comppsych.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Zhou L, Ghee SM, Chan C, Lin L, Cameron MD, Kenny PJ, See RE. Orexin-1 receptor mediation of cocaine seeking in male and female rats. J Pharmacol Exp Ther. 2012;340:801–809. doi: 10.1124/jpet.111.187567. [DOI] [PMC free article] [PubMed] [Google Scholar]