Abstract
Understanding disease vector composition is of priority in designing effective disease control programs. In integrated vector control management, understanding of disease vector species among species complexes simplifies priorities for effective control tools selection. This study identified members of the Anopheles funestus complex sampled in western Kenya from 2002 to 2011 from different breeding sites. Larval sampling was carried out using the standard dipper (350ml) in larval habitats in western Kenya highlands from January 2002 to December 2012. The morphologically identified Anopheles funestus larvae were preserved in absolute ethanol for molecular identification using polymerase chain reaction (PCR). Among the 184 identified specimens of Anopheles funestus sampled, only 76 specimens were clearly identified after DNA amplification and PCR. Among these, 25 (32.9%) were An. funestus s.s, 22 (28.9%) An. leesoni, 9 (11.8%) An. rivulorum and 20 (26.3%) were An. vaneedeni. None was identified as An. parensis. This study has demonstrated the existence of the siblings species of An. funestus complex in western Kenya highlands. However, there is need for further studies to evaluate the dynamics of the adults and sporozoite infectivity rates throughout the region based on these findings.
1.0. Introduction
In sub Saharan Africa malaria transmission is potentially vectored by Anopheles gambiae s.s. Giles, An. arabiensis Patton (Coetzee, et al., 2000) and An. funestus Giles (Gillies and Coetzee, 1987). Members of each complex group are difficult to distinguish morphologically. Both An. gambiae and An. funestus complex groups have been found to be potential vectors of malaria parasites in western Kenya highlands (Zhou, et al., 2004), with An. gambiae s.s. being the primary vector while An.funestus s.l is considered a secondary vector (Atieli, et al., 2011, Kweka, et al., 2011, Zhou, et al., 2004).
Anopheles funestus Giles complex consist of nine species that are distributed throughout Africa, these are An. parensis Gillies, An. aruni Sobti, An. confusus Evans and Leeson, An. funestus, An. vaneedeni Gillies and Coetzee, An. rivulorum Leeson, An. fuscivenosus Leeson, An.leesoni Evans, and An. brucei Service (Gillies and Coetzee, 1987). Apart from the morphological similarities among these sibling species their biology and vectorial competency is different. Except An. funestus s.s., other sibling species are zoophilic. An. funestus is closely associated with human dwellings hence it plays a critical role in malaria transmission. Both in Tanzania and Bukina Faso, An. funestus population have been found to increase at the end of the rainy season (Costantini, et al., 1999, Dabire, et al., 2007, Kweka, et al., 2008) hence are suggested to extend malaria transmission during the dry season. An. rivulorum has been found in Kenya to be an important malaria vector (Kawada, et al., 2012) and in Tanzania by Wilkes and others (Wilkes, et al., 1996). An. rivulorum has been found to have close association with human feeding preference of 40% in Nigeria (Awolola, et al., 2005) while in Kenya, incredibly higher biting and sporozoites rates in this species have been recorded (Kawada, et al., 2012). An. vaneedeni feeds readily on human outdoors but have been found to be infected with P. falciparum only under laboratory conditions and hence its efficiency in malaria transmission is questionable in natural settings (De Meillon, et al., 1977). Kamau and others in Kenya demonstrated that An. parensis resting indoors in human houses had low human blood index and were not infected with circumsporozoite protein (Kamau, et al., 2003, Kamau, et al., 2003). Other studies conducted elsewhere in Africa have not found An. parensis infected with sporozoites (Awolola, et al., 2005, Hargreaves, et al., 2000). In ecological studies in sub Saharan Africa, An. funestus occurs with other species and overlap with other three members of the group; An. rivulorum; An. leesoni and An. parensis (Awolola, et al., 2005, Kamau, et al., 2003, Temu, et al., 2007). In designing a cost-effective vector control tool it is important to understand the available vector species composition, biology and insecticide resistance status (Coetzee, et al., 2000, Hunt, et al., 2010, Hunt, et al., 2011).
In most studies conducted in western Kenya, the identification of An. funestus has been done based on morphological features (Zhou, et al., 2004). However, the modern molecular identification technique for An. funestus sibling species developed by Koekemoer and others (Koekemoer, et al., 2002) have made it possible for molecular differentiation of An. funestus complex members. This method identifies five species of An. funestus (An. vaneeden, An. parensis, An. leesoni An. rivulorum and An. funestus) which are most common with a minimum amount of DNA from any part and life stage of the mosquito. This reliable species identification method has increased the precision of effective control method selection and implementation for An. funestus sibling species. It has given better understanding of feeding, resting and host seeking behaviors which have given the best insight in vector control tool selection.
In order to clearly understand malaria vector ecology in western Kenya highlands, this study investigated the composition of An. funestus sibling species for larvae specimens collected from January, 2002 to December, 2011 to determine their species abundance.
2.0. Material and Method
2.1. Sampling and morphological identification
Mosquito larval specimens were collected from January 2002 to December, 2011 using standard 350 mL dipper (BioQuip Products, Inc. California, USA) from larval habitats. This study sites was conducted at Emakhanga, Iguhu, Emutete and Mbale (Figure 1). Four habitat types were considered during this study: drainage ditches, swamps, abandoned goldmines and hoof prints. All larvae were first identified morphologically as An. funestus complex using morphological keys by Gillies and Coetzee (Gillies and Coetzee, 1987) and preserved in absolute ethanol (98.7% purity) for species identification using polymerase chain reaction (PCR).
Figure 1.
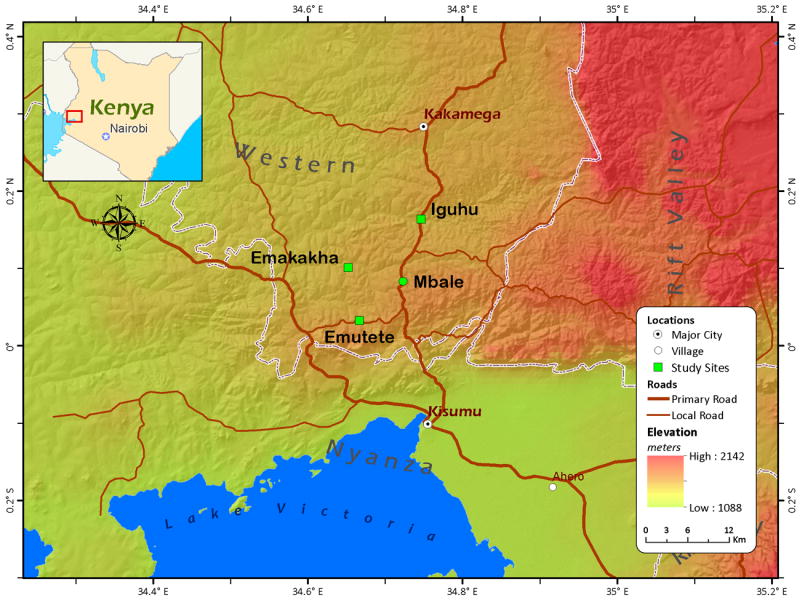
A map showing study sites in western Kenya highlands
2.2. Molecular identification of sibling species of An. funestus complex
Mosquito specimens belonging to the An. funestus group were analysed by a multiplex PCR assay protocol developed by Koekemoer and others (Koekemoer, et al., 2002). In each case, DNA was extracted from whole larvae body, amplified and the PCR product of unknown/known??? specimens together with positive controls (An. funestus, An. rivulorum and An. leesoni) were separated on a 2.5% agarose gel stained with ethidium bromide and visualized on a UV transilluminator.
3.0. Results
A total of 52,043 An. funestus larvae were sampled throughout the study duration. Among these; 31,812 (61.1%) were sampled from swamps, 12,319 (23.7%) in drainage ditches, 7912(15.2%) in abandoned goldmines and no An.funestus were sampled from hoof prints. Due to constraints of time and resources only 184 specimen were selected for PCR identification from the preserved specimens. These specimens were picked from different habitats singly during the study period. Among the 184 specimens of Anopheles funestus sibling species identified by polymerase chain, 76 had clear PCR results, 2 with unclear results and 106 had no DNA amplification. There are two plausible reasons for the PCR amplification failure in the 106 specimens. 1) Degradation of DNA in the larval samples occasioned by long storage period. 2) Poor storage since the samples which did not give the DNA amplification were those collected before 2008. The DNA might have degraded due to storage techniques not been good enough. Among the 76 specimens with clear DNA amplification and PCR results, 25 (32.9%) An. funestus s.s, 22 (28.9%) An. leesoni, 9(11.8%) An. rivulorum and 20 (26.3%) were An. vaneedeni. None was identified as An. parensis (Figure 2).
Figure 2.
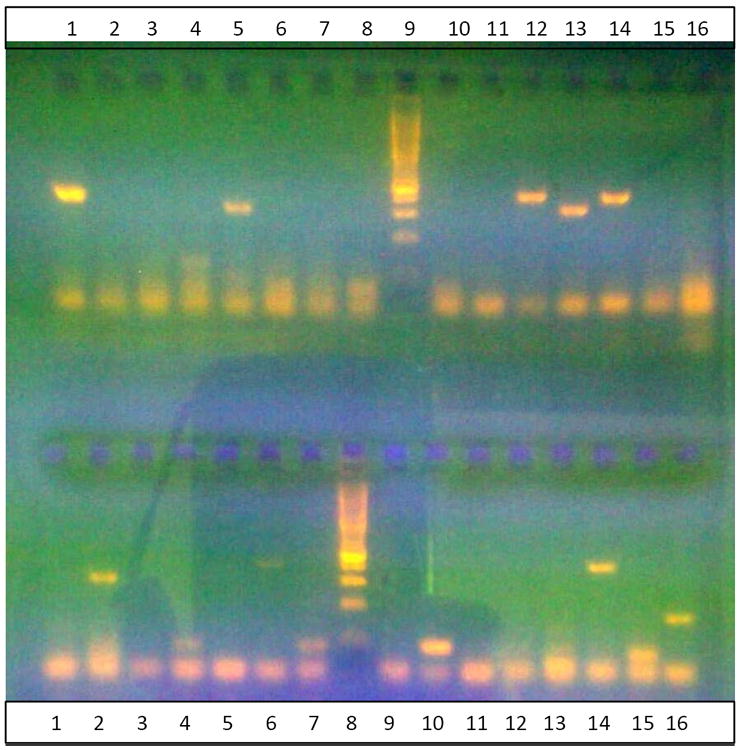
The PCR results for identified sibling species of Anopheles funestus results
Gel showing amplified fragments using the species-specific PCR method of Koekemoer et al. 2002. The DNA size ladder is in Lane 9 in the top row and Lane 8 in the bottom row; the bright band is 600bp. Top row; An. vaneedeni in Lane 1: An. Rivulorum in Lanes 5 & 13: An. funestus in Lanes 12 & 14. Bottom row; An. rivulorum in Lane 2; An. leesoni in Lanes 4, 7, 10,15&16; An. funestus in Lane 14.
4.0. Discussion
This study has documented the presence of An. funestus s.s, An. leesoni, An. rivulorum and An. vaneedeni in western Kenya highlands. However, absence of An. parensis was noted. Among the specimens tested, 106 (56.7%) of the specimens identified morphologically as An. funestus could not be identified by PCR possibly due to poor preservation method and hence protein degradation.
In western Kenya highlands, malaria control programmes thus needs to consider the biology of all species of Anopheles vectors involved in malaria transmission. Most of the information available currently at this study site is on An. gambiae s.l. (Kweka, et al., 2011, Zhou, et al., 2004). Due to availability of molecular tools identification of An. funestus has been made easier for choice of effective control tool and implementation. The four species of An. funestus identified in the current study site have been reported at the coastal regions of Kenya and Tanzania (Kamau, et al., 2003, Temu, et al., 2007) and west Africa (Awolola, et al., 2005, Coetzee and Fontenille, 2004) suggesting that, these species share the same ecological niche. The presence of these four species in aquatic stages revealed that, the adults of these species are available and might contribute to malaria transmission as it has been observed with An. rivulorum and An. funestus s.s. in western Kenya (Kawada, et al., 2012, Zhou, et al., 2004).
Among those four identified An. funestus sibling species, An. rivulorum has been shown to have malaria parasites circumsporozoite protein in lowland areas of western Kenya (Kawada, et al., 2012). With the current findings, there is need to further investigate and ascertain the biology of each species in western Kenya and any relevant active role in malaria transmission. These results should be taken as a basis for further studies to enhance the design of effective control programme of malaria vectors in this malaria epidemic prone area.
5.0. Conclusion
The findings of this study indicate that malaria vectors other than An. gambiae s.l. are found in the study area. Because of the presence of An. funestus s.s. and An. rivulorum the role played by each of these species in malaria transmission should be investigated further in western Kenya highlands.
Supplementary Material
Highlights.
Anopheles funestus sibling species characterization was conducted for specimen from western
The present members were An. funestus s.s, An. leesoni, An. rivulorum and An. vaneedeni.
An. funestus s.s (32.9%) was the dominant member among others in identified samples.
Acknowledgments
Authors wish to thank Amos Wabwile, Wilberforce Muyeso, Josephine Shikholwa (RIP), Geofrey Bulemi and Caroline Okoth for larvae sampling throughout. Ms. Lucy Wachira is highly appreciated for specimen preparation and identification for An. funestus complex species. This work received financial support from American National Institute of Health (D43 TW01505 and R01 AI-50243 to GY). This paper is published with the permission of the Director, Kenya Medical Research Institute, (KEMRI).
Footnotes
Authors’ contribution
EJK conceived and designed the study, carried out data analysis and results interpretation. EJK, LK and SM drafted the manuscript. LK, MCL, SM, AKG, GY and EJK revised the manuscript. All authors approved the final version of the manuscript before submission.
Competing interest
Authors declare to have no competing interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atieli HE, Zhou G, Lee MC, Kweka EJ, Afrane Y, Mwanzo I, Githeko AK, Yan G. Topography as a modifier of breeding habitats and concurrent vulnerability to malaria risk in the western kenya highlands. Parasites & vectors. 2011;4:241. doi: 10.1186/1756-3305-4-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awolola TS, Oyewole IO, Koekemoer LL, Coetzee M. Identification of three members of the anopheles funestus (diptera: Culicidae) group and their role in malaria transmission in two ecological zones in nigeria. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2005;99:525–531. doi: 10.1016/j.trstmh.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Coetzee M, Craig M, le Sueur D. Distribution of african malaria mosquitoes belonging to the anopheles gambiae complex. Parasitology today (Personal ed) 2000;16:74–77. doi: 10.1016/s0169-4758(99)01563-x. [DOI] [PubMed] [Google Scholar]
- Coetzee M, Fontenille D. Advances in the study of anopheles funestus, a major vector of malaria in africa. Insect biochemistry and molecular biology. 2004;34:599–605. doi: 10.1016/j.ibmb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Costantini C, Sagnon N, Ilboudo-Sanogo E, Coluzzi M, Boccolini D. Chromosomal and bionomic heterogeneities suggest incipient speciation in anopheles funestus from burkina faso. Parassitologia. 1999;41:595–611. [PubMed] [Google Scholar]
- Dabire KR, Baldet T, Diabate A, Dia I, Costantini C, Cohuet A, Guiguemde TR, Fontenille D. Anopheles funestus (diptera: Culicidae) in a humid savannah area of western burkina faso: Bionomics, insecticide resistance status, and role in malaria transmission. Journal of medical entomology. 2007;44:990–997. doi: 10.1603/0022-2585(2007)44[990:afdcia]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- De Meillon B, Van Eeden G, Coetzee L, Coetze M, Meiswinkel R, du Toit CLN, Hansford CF. Observations on a species of the anopheles funestus subgroup, a suspected exophilic vector ofmalaria parasites in north-eastern transvaal, south africa. Mosq News. 1977;37:657–661. [Google Scholar]
- Gillies T, Coetzee M. Supplement of the anopheles of africa south of sahara (afrotropical region) Johannesburg: Republic of South Africa Publication of The S. Afr. Insti. Med Research; 1987. [Google Scholar]
- Hargreaves K, Koekemoer LL, Brooke BD, Hunt RH, Mthembu J, Coetzee M. Anopheles funestus resistant to pyrethroid insecticides in south africa. Medical and veterinary entomology. 2000;14:181–189. doi: 10.1046/j.1365-2915.2000.00234.x. [DOI] [PubMed] [Google Scholar]
- Hunt R, Edwardes M, Coetzee M. Pyrethroid resistance in southern african anopheles funestus extends to likoma island in lake malawi. Parasites & vectors. 2010;3:122. doi: 10.1186/1756-3305-3-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt RH, Fuseini G, Knowles S, Stiles-Ocran J, Verster R, Kaiser ML, Choi KS, Koekemoer LL, Coetzee M. Insecticide resistance in malaria vector mosquitoes at four localities in ghana, west africa. Parasites & vectors. 2011;4:107. doi: 10.1186/1756-3305-4-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamau L, Koekemoer LL, Hunt RH, Coetzee M. Anopheles parensis: The main member of the anopheles funestus species group found resting inside human dwellings in mwea area of central kenya toward the end of the rainy season. Journal of the American Mosquito Control Association. 2003;19:130–133. [PubMed] [Google Scholar]
- Kamau L, Munyekenye GO, Koekemoer LL, Hunt RH, Coetzee M. A survey of the anopheles funestus (diptera: Culicidae) group of mosquitoes from 10 sites in kenya with special emphasis on population genetic structure based on chromosomal inversion karyotypes. Journal of medical entomology. 2003;40:664–671. doi: 10.1603/0022-2585-40.5.664. [DOI] [PubMed] [Google Scholar]
- Kawada H, Dida GO, Sonye G, Njenga SM, Mwandawiro C, Minakawa N. Reconsideration of anopheles rivulorum as a vector of plasmodium falciparum in western kenya: Some evidence from biting time, blood preference, sporozoite positive rate, and pyrethroid resistance. Parasites & vectors. 2012;5:230. doi: 10.1186/1756-3305-5-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koekemoer LL, Kamau L, Hunt RH, Coetzee M. A cocktail polymerase chain reaction assay to identify members of the anopheles funestus (diptera: Culicidae) group. The American journal of tropical medicine and hygiene. 2002;66:804–811. doi: 10.4269/ajtmh.2002.66.804. [DOI] [PubMed] [Google Scholar]
- Kweka EJ, Mahande AM, Nkya WM, Assenga C, Lyatuu EE, Nyale E, Mosha FW, Mwakalinga SB, Temu EA. Vector species composition and malaria infectivity rates in mkuzi, muheza district, north-eastern tanzania. Tanzania journal of health research. 2008;10:46–49. doi: 10.4314/thrb.v10i1.14341. [DOI] [PubMed] [Google Scholar]
- Kweka EJ, Zhou G, Lee MC, Gilbreath TM, 3rd, Mosha F, Munga S, Githeko AK, Yan G. Evaluation of two methods of estimating larval habitat productivity in western kenya highlands. Parasites & vectors. 2011;4:110. doi: 10.1186/1756-3305-4-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kweka EJ, Zhou G, Munga S, Lee MC, Atieli HE, Nyindo M, Githeko AK, Yan G. Anopheline larval habitats seasonality and species distribution: A prerequisite for effective targeted larval habitats control programmes. PloS one. 2012;7:e52084. doi: 10.1371/journal.pone.0052084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temu EA, Minjas JN, Tuno N, Kawada H, Takagi M. Identification of four members of the anopheles funestus (diptera: Culicidae) group and their role in plasmodium falciparum transmission in bagamoyo coastal Tanzania. Acta tropica. 2007;102:119–125. doi: 10.1016/j.actatropica.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Wilkes TJ, Matola YG, Charlwood JD. Anopheles rivulorum, a vector of human malaria in africa. Medical and veterinary entomology. 1996;10:108–110. doi: 10.1111/j.1365-2915.1996.tb00092.x. [DOI] [PubMed] [Google Scholar]
- Zhou G, Minakawa N, Githeko A, Yan G. Spatial distribution patterns of malaria vectors and sample size determination in spatially heterogeneous environments: A case study in the west kenyan highland. Journal of medical entomology. 2004;41:1001–1009. doi: 10.1603/0022-2585-41.6.1001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


