Abstract
Background
Angina and hypertension are common in patients with coronary artery disease (CAD); however, the effect on mortality is unclear. We conducted this prespecified analysis of the International Verapamil/Trandolapril Study (INVEST) to assess relationships between angina, blood pressure (BP), and mortality among elderly, hypertensive CAD patients.
Hypothesis
Angina and elevated BP will be associated with higher mortality.
Methods
Extended follow‐up was performed using the National Death Index for INVEST patients in the United States (n = 16 951). Based on angina history at enrollment and during follow‐up visits, patients were divided into groups: persistent angina (n = 7184), new‐onset angina (n = 899), resolved angina (n = 4070), and never angina (n = 4798). Blood pressure was evaluated at baseline, during drug titration, and during follow‐up on‐treatment. On‐treatment systolic BP was classified as tightly controlled (<130 mm Hg), controlled (130–139 mm Hg), or uncontrolled (≥140 mm Hg). A Cox proportional hazards model was created adjusting for age, heart failure, diabetes, renal impairment, myocardial infarction, stroke, and smoking. The angina groups and BP control groups were compared using the never‐angina group as the reference.
Results
Only in the persistent‐angina group was a significant association with mortality observed, with an apparent protective effect (hazard ratio: 0.82, 95% confidence interval: 0.75‐0.89, P < 0.0001). Uncontrolled BP was associated with increased mortality risk (hazard ratio: 1.29, 95% confidence interval: 1.20‐1.40, P < 0.0001), as were several other known cardiovascular risk factors.
Conclusions
In hypertensive CAD patients, persistent angina was associated with lower mortality. The observed effect was small compared with other cardiovascular risk factors, such as BP, which were associated with increased mortality.
Introduction
The high prevalence of angina and hypertension among patients with coronary artery disease (CAD) is well documented; however, the relationships between angina and blood pressure (BP), as well as its treatment, on adverse outcomes are complex.1, 2 Because angina typically implies myocardial ischemia, patients with angina are assumed to have greater risk of cardiovascular events compared with similar patients without angina. Yet, though some have reported increased cardiovascular risk in patients with angina,3, 4, 5, 6, 7 others have not observed angina to be predictive of increased risk beyond that associated with other cardiovascular risk factors.8, 9, 10
Furthermore, although most CAD patients have hypertension, evidence‐based data to guide hypertension management are limited. For patients with angina, the optimal BP level for patients with angina is not known. Recent treatment recommendations, based largely on consensus opinion, advocate treating chronic stable angina patients to a goal BP <130/80 mm Hg, or <120/80 mm Hg if left ventricular dysfunction is present.11
In a prospective, randomized trial of hypertension‐management strategies in patients with CAD, the International Verapamil/Trandolapril Study (INVEST) found that a β‐blocker + thiazide diuretic strategy was equivalent to a calcium antagonist + angiotensin‐converting enzyme inhibitor strategy for reducing adverse outcomes (death, myocardial infarction [MI], or stroke).12 On‐treatment BP and BP control comparing the 2 treatment strategies were not different and both treatment strategies were led by drugs (atenolol and verapamil SR) with heart rate–lowering and antiangina effects. A prior analysis indicated that angina presence predicts poorer quality of life,13 but prognostic implications of angina and the influence of BP were not analyzed. Accordingly, to assess the impact of angina and BP on mortality, we hypothesized that angina would be associated with increased mortality risk and that lower systolic BP (SBP) would be associated with a decreased mortality risk.
Methods
Study Design
Details about the rationale, design, selection criteria, and treatment strategies for INVEST have been previously described.12 Briefly, participants with clinically stable CAD and hypertension were randomly assigned to structured hypertension treatment with a β‐blocker–based strategy (atenolol and hydrochlorothiazide) or a calcium antagonist–based strategy (verapamil SR and trandolapril). An assessment of the relationship between angina and antihypertensive strategy was prespecified as a secondary analysis. Institutional review boards and ethics committees at each study site approved the protocol, which was conducted in accordance with the Declaration of Helsinki. Written informed consent was provided by all patients. Information on presence of angina was collected at baseline and at each visit by the study physician. Because of the recognized high prevalence of angina among hypertensive CAD patients, both of the INVEST antihypertensive‐drug strategies contained an antianginal (verapamil SR or atenolol). Furthermore, the protocol provided specific instructions about angina management: (1) maximization of BP control; (2) optimization of other CAD risk factors (eg, weight loss, smoking cessation, lipid reduction, reduced sodium and alcohol intake); and (3) addition and/or uptitration of long‐acting nitrates and uptitration of antianginal drugs in the assigned treatment strategy. If these measures failed, patients assigned to the calcium‐antagonist strategy could be prescribed a β‐blocker, and those assigned to the β‐blocker strategy could be prescribed a calcium antagonist. Lastly, if necessary, coronary revascularization was recommended. To avoid international differences in angina management and revascularization, and also to utilize the extended follow‐up mortality data, this analysis was limited to participants in the United States cohort. Specific instructions were also provided for secondary prevention of atherosclerosis, including encouraging aspirin (80–170 mg daily), weight loss if overweight, daily physical activity, tobacco cessation, limitation of dietary cholesterol, treatment of hyperlipidemia, dietary sodium restriction to <2400 mg daily, limited daily alcohol ingestion, and adequate dietary potassium, calcium, and magnesium.
Group Assignment
Using standardized online forms, patients were asked by the study physician about symptoms of angina at each study visit, which by protocol were to occur every 6 weeks for the first 6 months and then every 6 months for ≥2 years. For this analysis, patient‐reported presence or absence of angina at the enrollment visit was used to categorize patients into one of 4 groups (Figure 1). At each study visit, the investigator was asked to determine if the participant had “Heberden's classical angina.” This epidemiologic‐type definition was used due to the international scope (14 countries) of the trial. Because our groups were defined based on symptoms present at study enrollment, subjects with a remote history of angina but with no symptoms at enrollment were eligible to be included in the never‐angina group. We also prospectively defined 3 groups of SBP control: tightly controlled (<130 mm Hg), controlled (130–139 mm Hg), and uncontrolled (≥140 mm Hg).
Figure 1.
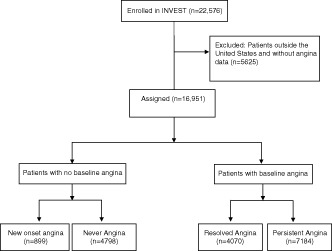
Strategy for assigning INVEST patients to one of 4 groups based on the presence or absence of angina at baseline and during the study. Abbreviations: INVEST, International Verapamil/Trandolapril Study.
Study Outcomes
The primary outcome of this analysis was all‐cause mortality assessed by searching the National Death Index for patients enrolled from sites in the United States up to 5 years after study follow‐up ended in 2003. To be considered a confirmed death, we used a previously described method, requiring 4 of 5 matches among name, Social Security number, date of birth, city, and state.14 We determined the rate of coronary revascularization (either percutaneous coronary intervention or coronary artery bypass grafting) during follow‐up, the influence of BP‐lowering using the mean on‐treatment BP, and percentage of patients in each of 3 BP‐control groups at SBP <140 mm Hg after 24 months of treatment.
Statistical Methods
Continuous variables are reported as mean values with SD unless otherwise indicated and compared using analysis of variance (ANOVA). Categorical data are presented as frequencies and compared using χ2 tests. Kaplan‐Meier curves were generated for each group, and differences in time to all‐cause mortality were tested by the log‐rank method. Patients who did not appear in the National Death Index were censored on the day the search was completed. To determine the independent prognostic value of baseline characteristics, stepwise selection of Cox proportional hazards models were performed with univariate P < 0.2 to enter and P < 0.05 to be retained in the multivariate model, as reported in prior INVEST risk‐prediction models (see Supporting Table 1 in the online version of this article for a complete list of variables included in the model). Multivariate Cox regression analysis was constructed to control for confounding factors, and hazard ratios (HRs) and 95% confidence intervals (CIs) were presented. An age‐ (by decade) and gender‐matched cohort was constructed to further control for these variables and conduct secondary analyses of the regression model. Statistical analyses were performed using SAS version 9.2 (SAS Institute Inc, Cary, NC). Statistical significance was defined as a 2‐sided P value <0.05, and CIs were calculated at the 95% level.
Table 1.
Pertinent Baseline Characteristics
| Never, n = 4798 | Resolved, n = 4070 | New Onset, n = 899 | Persistent, n = 7184 | P Value | |
|---|---|---|---|---|---|
| Age, y, mean (SD) | 68.2 (9.5) | 66.5 (10.3) | 67.6 (9.7) | 65.5 (9.9) | <0.0001 |
| BMI, kg/m2 (SD) | 29.2 (5.6) | 29.6 (6.1) | 29.4 (5.6) | 29.7 (5.9) | <0.0001 |
| Age >70 years | 43.1 | 36.0 | 39.6 | 30.7 | <0.0001 |
| Female | 38.4 | 57.1 | 43.8 | 63.7 | <0.0001 |
| Prior MI | 52.7 | 24.0 | 49.3 | 13.6 | <0.0001 |
| Prior CABG or PCI | 57.2 | 21.7 | 58.0 | 11.3 | <0.0001 |
| History of TIA/stroke | 9.1 | 7.7 | 8.3 | 6.1 | <0.0001 |
| LVH | 15.4 | 16.8 | 19.1 | 15.4 | 0.009 |
| HF (class I–III) | 5.9 | 6.1 | 7.3 | 4.1 | <0.0001 |
| PVD | 13.0 | 12.7 | 12.3 | 13.3 | 0.7 |
| Ever smoker | 55.3 | 40.6 | 55.6 | 38.3 | <0.0001 |
| DM | 28.1 | 28.4 | 34.9 | 30.6 | <0.0001 |
| CKD | 3.3 | 2.3 | 2.6 | 1.0 | <0.0001 |
| Hyperlipidemia | 68.5 | 56.2 | 66.9 | 45.7 | <0.0001 |
| Nitrate use | 17.2 | 28.0 | 30.4 | 40.1 | <0.0001 |
Abbreviations: BMI, body mass index; CABG, coronary artery bypass grafting; CKD, chronic kidney disease; DM, diabetes mellitus; HF, heart failure; LVH, left ventricular hypertrophy; MI, myocardial infarction; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; SD, standard deviation; TIA, transient ischemic attack.
Data are presented as % unless otherwise indicated.
Results
Baseline Characteristics and Treatment
The US‐based cohort with data on angina included 16 951 participants (Figure 1). In this cohort, 4798 patients never had angina, 4070 had resolved angina, 899 had new‐onset angina, and 7184 had persistent angina. Pertinent characteristics at baseline, based on these angina groups, are summarized in Table 1. Patients in the new‐onset and never‐angina groups were significantly older and more frequently male. The new‐onset and never‐angina groups had higher rates of history of prior MI, prior coronary revascularization, stroke/transient ischemic attack, hyperlipidemia, renal impairment, and smoking.
After 24 months of study antihypertensive treatment, systolic BP was higher in the never‐angina and new‐onset angina groups (ANOVA P < 0.0005) (Table 2). The new‐onset group also had a nonsignificantly lower rate of achieving goal BP of <140/90 mm Hg (never = 67%, new onset = 65%, persistent = 68%, resolved = 69%) (χ2 test P = 0.17). Median daily atenolol dose was 50 mg in the never and resolved groups, 75 mg in the new‐onset group, and 100 mg in the persistent group (ANOVA P < 0.0001). Median daily verapamil SR dose was 240 mg in all but the persistent‐angina group (360 mg; ANOVA P < 0.0001) (Table 2). Crossover (eg, β‐blocker use in the verapamil SR strategy or calcium‐antagonist use in the atenolol strategy) was infrequent. Coronary revascularization, percutaneous coronary intervention or coronary artery bypass grafting, was relatively infrequent during follow‐up (2.86% for the never‐angina group, 1.74% for the resolved‐angina group, 2.35% for the persistent‐angina group) except for the new‐onset angina group (12.79%).
Table 2.
Study‐Drug Use and BP Achieved After 24 Months of Treatment
| Never, n = 4798 | Resolved, n = 4070 | New Onset, n = 899 | Persistent, n = 7184 | P Value | |
|---|---|---|---|---|---|
| Mean (median) study‐drug dose, mg | |||||
| Verapamil | 297 (240) | 282 (240) | 305 (240) | 311 (360) | <0.0001 |
| Atenolol | 72 (50) | 76 (50) | 80 (75) | 82 (100) | <0.0001 |
| Trandolapril | 4.1 (4) | 3.6 (4) | 4.1 (4) | 4.1 (4) | <0.0001 |
| HCTZ | 29 (25) | 29.7 (25) | 29.5 (25) | 29.9 (25) | 0.0008 |
| Mean SBP at 24 months, mm Hg (SD) | 134.1 (16.0) | 133.0 (15.9) | 133.9 (18.2) | 132.5 (6.3) | <0.0005 |
| Mean DBP at 24 months, mm Hg (SD) | 75.6 (9.8) | 77.9 (9.2) | 75.8 (10.5) | 77.6 (9.2) | <0.0001 |
| BP control at 24 months, %a | 67 | 69 | 65 | 68 | 0.17 |
Abbreviations: BP, blood pressure; DBP, diastolic blood pressure; HCTZ, hydrochlorothiazide; SBP, systolic blood pressure; SD, standard deviation.
<140/90 mm Hg.
Unadjusted Mortality Risk
Median follow‐up was 8.37 years (interquartile range, 7.78–8.82 years). Overall, 3868 of the 16 951 participants died (never = 1428, resolved = 941, new‐onset = 253, persistent = 1246). Unadjusted mortality (per 1000 patient‐years) was significantly different comparing angina groups (35.59 for new‐onset angina, 22.35 for persistent angina, 29.99 for resolved angina, and 39.28 for never angina; P < 0.0001). Kaplan‐Meier analysis indicates that these mortality‐risk differences slowly diverge throughout the extended follow‐up (Figure 2). Sensitivity analysis performed on the persistent‐angina group and stratified by number of angina episodes per 4 weeks demonstrated that mortality risk was lowest among subjects with the most frequent angina (1 episode, 24.9% mortality; 2 episodes, 26.5% mortality; ≥3 episodes, 14.2% mortality). As a sensitivity analysis of mortality derived from the National Death Index, we also analyzed mortality data from only the original study, which yielded similar results (see Supporting Figure 1 in the online version of this article). Unadjusted mortality rates for the tight, controlled, and uncontrolled SBP categories were also significantly different (27.03, 26.55, and 39.58 per 1000 patient‐years, respectively; P < 0.0001).
Figure 2.
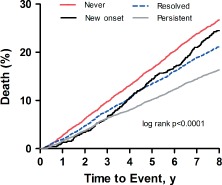
Kaplan‐Meier mortality curves. Each curve demonstrates the survival for patients in each of the 4 angina groups.
Adjusted Mortality Risk
A Cox proportional hazard model was created adjusting for numerous study variables (see online version of this article). The angina groups were compared using the never‐angina group as the reference. No significant differences were observed in mortality risk for the resolved (HR: 0.98, 95% CI: 0.90‐1.07, P = 0.61) and new‐onset (HR: 0.89, 95% CI: 0.77‐1.01, P = 0.075) angina groups. Whereas persistent angina was associated with reduced mortality (HR: 0.82, 95% CI: 0.75‐0.89, P < 0.0001), uncontrolled BP (systolic ≥140 mm Hg) was associated with increased mortality (HR: 1.29, 95% CI: 1.20‐1.40). Other known cardiovascular risk factors were also more robustly associated with mortality: heart failure (HR: 1.88, 95% CI: 1.69‐2.09), age (by decade; HR: 1.81, 95% CI: 1.75‐1.88), renal insufficiency (HR: 1.71, 95% CI: 1.47‐1.98), diabetes (HR: 1.63, 95% CI: 1.52‐1.74), smoking (HR: 1.41, 95% CI: 1.32‐1.51), stroke/transient ischemic attack (HR: 1.39, 95% CI: 1.26‐1.53), and history of MI (HR: 1.30, 95% CI: 1.21‐1.39) (Figure 3).
Figure 3.
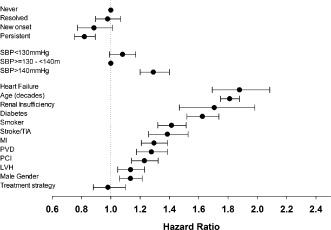
Hazard ratio plots demonstrating the hazard ratios and confidence intervals for the variables included in our multivariate Cox regression analysis model. Variables which did not contribute to the model were omitted from the figure. Abbreviations: LVH, left ventricular hypertrophy; MI, myocardial infarction; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; SBP, systolic blood pressure; TIA, transient ischemic attack.
Age‐ and Gender‐Matched Cohort
Given that the persistent‐angina group was younger and included more female subjects, we constructed an age‐ and gender‐matched cohort to conduct further comparisons between the persistent‐angina (n = 4277) and never‐angina groups (n = 4277). Using these matched subjects, we again conducted a proportional hazards model but also included recent tobacco use, remote tobacco use, and study‐drug dose above the median (atenolol dose >100 mg daily and verapamil SR dose >240 mg daily). In this analysis, gender was not significantly associated with mortality; however, recent tobacco use (HR: 2.00, 95% CI: 1.76‐2.28, P < 0.0001), former tobacco use (HR: 1.27, 95% CI: 1.15‐1.39, P < 0.0001), atenolol dose above the median (HR: 0.531, 95% CI: 0.29‐0.96, P = 0.04), and verapamil SR dose above median (HR: 0.66, 95% CI: 0.56‐0.77, P < 0.0001) were significant. Despite the age/gender matching and these additional variables in the model, the lower hazard of mortality in the persistent‐angina group was still observed (HR: 0.86, 95% CI: 0.78‐0.95, P = 0.0021).
Discussion
In older CAD patients with hypertension, we observed that persistent angina was associated with lower mortality compared with patients with no angina, new‐onset angina, or angina that resolved during follow‐up. Because some prior studies have noted a detrimental effect with angina,3, 4, 5, 6, 7 a protective effect of angina may be counterintuitive; however, this effect has been noted previously.8, 9, 10 In our model, traditional cardiovascular risk factors, such as uncontrolled BP, were more robust predictors of mortality risk. This finding is consistent with evidence that hypertension is strongly predictive of cardiovascular events, including new‐onset angina,15 and that in patients with angina, hypertension further increases the risk for cardiovascular events.16, 17, 18 Although persistently elevated BP was associated with increased mortality, we observed a trend toward higher mortality in patients with tight BP control (SBP < 120 mm Hg), as has been described previously.19
In evaluating the unadjusted mortality rates among the 4 angina groups, we noted some important differences in baseline characteristics, specifically when comparing the persistent‐ and resolved‐angina groups to the new‐onset and never‐angina groups. The persistent and resolved groups were younger and had better BP control. Clearly, aging is associated with increased risk,20 as is poor BP control.15 The persistent and resolved angina groups also had more women and less prior MI. We know that women have differences in their vasculature, which creates a phenotype different from men,21 including less frequent flow‐limiting coronary obstructions22, 23 and more frequent uncomplicated angina (ie, chest pain without ischemia or prior MI).24 Prior MI, which was less frequent in the persistent and resolved groups, is a stronger predictor of future events than angina.7, 8
In our proportional hazards model using the never‐angina group as reference, we observed that persistent angina was associated with lower mortality, whereas new‐onset angina and resolved angina were not significantly different. This association between symptoms and lower risk is similar to the 3031 patients enrolled in the Euro Heart Survey on Angina, in which angina of >6 months' duration was also associated with a lower rate of death or MI, although only 49% had CAD and 61% had hypertension.17 Those authors partially attribute their finding to antianginal medications, which were used in higher doses in the persistent‐angina group of our analysis. In the age‐ and gender‐matched cohort we conducted, we found that higher medication doses were protective; however, mortality was still reduced in the persistent‐angina cohort after adjusting for this effect. Another process that could contribute to the observed protective effect of angina is ischemic preconditioning. Brief periods of mild ischemia that manifest as angina may be protective against more severe ischemic insults.25, 26 Among patients with MI, preinfarction angina is associated with smaller infarct size as compared with those without preinfarction angina.27, 28, 29, 30
Development of new‐onset angina suggests progression or instability of atherosclerosis with an increased risk for adverse outcomes; however, we observed no significant difference in risk comparing new‐onset angina and never‐angina groups. Other lines of evidence show that objective test results for myocardial ischemia are a stronger predictor of risk than the subjective complaint of angina.6, 10, 31 Exercise capacity is another inverse predictor of risk; however, the causal relationship is complicated, as some have reported that patients with persistent angina may self‐limit their activity.32 We did observe that patients with new‐onset angina had a significantly higher rate of coronary revascularization procedures (3.54 per 100 patient‐years vs 0.55–0.84 per 100 patient‐years in all other groups; P < 0.0001). However, despite a higher rate of coronary interventions, mortality risk was not reduced, in concordance with others.33
Because all patients enrolled in INVEST had both CAD and hypertension, their risk of cardiovascular events was higher than that observed from similarly aged population‐based angina cohort studies. We observed that the prognostic implications of angina, although statistically significant, are modest relative to the hazard associated with other traditional CV risk factors: Diabetes, smoking, prior MI, and increasing age were associated with considerably greater mortality risk, which was similar to the findings of others.8, 34, 35 A recent analysis from the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial noted that the presence or absence of angina or angina‐equivalent symptoms was not a significant predictor of adverse cardiac events, including death.36 In a population with CAD and angina symptoms, similar to our persistent angina group, there was no outcome benefit with nifedipine.2 Their risk‐prediction analysis identified tobacco use, insulin‐dependent diabetes, prior stroke, and age as more robust risk predictors than angina; however, their angina variable (>1 episode of angina weekly) was different from that used in our analysis.16
Study Limitations
Data on ischemia testing and adherence to CAD treatment after the conclusion of the formal study were not collected. Further, data on angina were collected on standardized forms at each follow‐up visit, but such reports are subject to recall bias and were not adjudicated by a senior cardiologist. Our findings are limited to older (age ≥50 years) CAD patients with hypertension. As expected from the dose‐escalation instructions provided for angina management in the protocol, patients reporting more angina (persistent‐angina group) were prescribed a higher β‐blocker dose (100 mg daily) than the other groups (75 mg daily for the new‐onset angina group, and 50 mg daily for the never‐angina and resolved‐angina groups). When we included study‐drug dose above the median in our model, the reduced mortality hazard in the persistent‐angina group was still observed.
Conclusion
In hypertensive CAD patients, after accounting for control of BP and other risk factors, new‐onset angina is not associated with higher mortality, whereas persistent angina is associated with a modest but significantly lower mortality risk. Other conditions, such as uncontrolled BP, prior MI, diabetes, and increasing age have stronger, independent associations with increased mortality.
Supporting information
Fig S1. Xxxxxxx
Table S1. Variables included in multivariate Cox regression model
INVEST (http://www.clinicaltrials.gov identifier: NCT00133692) was funded by a grant from BASF Pharma, Ludwigshafen, Germany; Abbott Laboratories, Abbott Park, IL; and the University of Florida Research Foundation and Opportunity Fund. Dr. Cooper‐DeHoff's effort is funded by National Heart, Lung, and Blood Institute (NHLBI) K23HL086558. Dr. Pepine receives support in part from the National Institutes of Health (NIH)/National Center for Research Resources Clinical and Translational Science Award to the University of Florida UL1 TR000064. BASF Pharma and Abbott Laboratories had no role in the design or conduct of the study, collection or analysis of data, or preparation or approval of the manuscript.
Dr. Cooper‐DeHoff reported receiving research funding from Abbott Laboratories during the conduct of INVEST. Dr. Handberg reported receiving grant support from the NHLBI, Abbott Laboratories, Fujisawa, Pfizer, and GlaxoSmithKline and educational grants from the Vascular Biology Working Group (AstraZeneca, Sanofi Aventis, Schering‐Plough, Daiichi Sankyo Lilly, AtCor Medical, XOMA). Dr. Pepine reported receiving research grants from the NHLBI, Abbott Laboratories, Baxter, Pfizer, GlaxoSmithKline, and Bioheart Inc; serving as consultant for Abbott Laboratories, Forest Laboratories, Novartis/Cleveland Clinic, NicOx, Angioblast, Sanofi‐Aventis, NHLBI, NIH, Medtelligence, and SLACK Inc; and receiving unrestricted educational grants from AstraZeneca, AtCor Medical Inc, Daiichi Sankyo Inc, Eli Lilly, Pfizer Inc, Sanofi‐Aventis, and Schering‐Plough. Drs. Winchester and Gong reported that they have no financial disclosures.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Bavry AA, Anderson RD, Gong Y, et al. Outcomes among hypertensive patients with concomitant peripheral and coronary artery disease: findings from the International Verapamil‐SR/Trandolapril Study. Hypertension. 2010;55:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Poole‐Wilson PA, Lubsen J, Kirwan BA, et al. Effect of long‐acting nifedipine on mortality and cardiovascular morbidity in patients with stable angina requiring treatment (ACTION trial): randomised controlled trial. Lancet. 2004;364:849–857. [DOI] [PubMed] [Google Scholar]
- 3. Carpiuc KT, Wingard DL, Kritz‐Silverstein D, et al. The association of angina pectoris with heart disease mortality among men and women by diabetes status: the Rancho Bernardo Study. J Womens Health (Larchmt). 2010;19:1433–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hemingway H, McCallum A, Shipley M, et al. Incidence and prognostic implications of stable angina pectoris among women and men. JAMA. 2006;295:1404–1411. [DOI] [PubMed] [Google Scholar]
- 5. Kannel WB, Feinleib M. Natural history of angina pectoris in the Framingham study: prognosis and survival. Am J Cardiol. 1972;29:154–163. [DOI] [PubMed] [Google Scholar]
- 6. Murphy NF, Stewart S, Hart CL, et al. A population study of the long‐term consequences of Rose angina: 20‐year follow‐up of the Renfrew‐Paisley study. Heart. 2006;92:1739–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rosengren A, Wilhelmsen L, Hagman M, et al. Natural history of myocardial infarction and angina pectoris in a general population sample of middle‐aged men: a 16‐year follow‐up of the Primary Prevention Study, Goteborg, Sweden. J Intern Med. 1998;244:495–505. [DOI] [PubMed] [Google Scholar]
- 8. Buckley B, Murphy AW. Do patients with angina alone have a more benign prognosis than patients with a history of acute myocardial infarction, revascularisation or both? Findings from a community cohort study. Heart. 2009;95:461–467. [DOI] [PubMed] [Google Scholar]
- 9. Hjemdahl P, Eriksson SV, Held C, et al. Favourable long term prognosis in stable angina pectoris: an extended follow‐up of the angina prognosis study in Stockholm (APSIS). Heart. 2006;92:177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sigurdsson E, Thorgeirsson G, Sigvaldason H, et al. Unrecognized myocardial infarction: epidemiology, clinical characteristics, and the prognostic role of angina pectoris. The Reykjavik Study. Ann Intern Med. 1995;122:96–102. [DOI] [PubMed] [Google Scholar]
- 11. Rosendorff C, Black HR, Cannon CP, et al. Treatment of hypertension in the prevention and management of ischemic heart disease: a scientific statement from the American Heart Association Council for High Blood Pressure Research and the councils on Clinical Cardiology and Epidemiology and Prevention. Circulation. 2007;115:2761–2788. [DOI] [PubMed] [Google Scholar]
- 12. Pepine CJ, Handberg EM, Cooper‐DeHoff RM, et al. A calcium antagonist vs a non‐calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil‐Trandolapril Study (INVEST): a randomized controlled trial. JAMA. 2003;290:2805–2816. [DOI] [PubMed] [Google Scholar]
- 13. Gong Y, Handberg EM, Gerhard T, et al. Systolic blood pressure and subjective well‐being in patients with coronary artery disease. Clin Cardiol. 2009;32:627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cooper‐DeHoff RM, Gong Y, Handberg EM, et al. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA. 2010;304:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lewington S, Clarke R, Qizilbash N, et al. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 16. Clayton TC, Lubsen J, Pocock SJ, et al. Risk score for predicting death, myocardial infarction, and stroke in patients with stable angina, based on a large randomised trial cohort of patients. BMJ. 2005;331:869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Daly CA, De Stavola B, Sendon JL, et al. Predicting prognosis in stable angina—results from the Euro heart survey of stable angina: prospective observational study. BMJ. 2006;332:262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tenenbaum A, Fisman EZ, Boyko V, et al. Prevalence and prognostic significance of unrecognized systemic hypertension in patients with diabetes mellitus and healed myocardial infarction and/or stable angina pectoris. Am J Cardiol. 1999;84:294–298. [DOI] [PubMed] [Google Scholar]
- 19. Messerli FH, Mancia G, Conti CR, et al. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med. 2006;144:884–893. [DOI] [PubMed] [Google Scholar]
- 20. Heron M, Hoyert DL, Murphy SL, et al. Deaths: final data for 2006. Natl Vital Stat Rep. 2009;57:1–134. [PubMed] [Google Scholar]
- 21. Pepine CJ, Kerensky RA, Lambert CR, et al. Some thoughts on the vasculopathy of women with ischemic heart disease. J Am Coll Cardiol. 2006;47:S30–S35. [DOI] [PubMed] [Google Scholar]
- 22. Bugiardini R, Bairey Merz CN. Angina with “normal” coronary arteries: a changing philosophy. JAMA. 2005;293:477–484. [DOI] [PubMed] [Google Scholar]
- 23. Anderson RD, Pepine CJ. How to manage angina with normal coronary arteries (update). In: Fauci AS, Braunwald E, Kasper DL, et al, eds. Harrison's Principles of Internal Medicine. New York, NY: McGraw‐Hill; 2010. http://www.accessmedicine.com/updatesContent.aspx?aid=1001522.
- 24. Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26‐year follow‐up of the Framingham population. Am Heart J. 1986;111:383–390. [DOI] [PubMed] [Google Scholar]
- 25. Jennings RB, Murry CE, Steenbergen C Jr, et al. Development of cell injury in sustained acute ischemia. Circulation. 1990;82:II2–II12. [PubMed] [Google Scholar]
- 26. Kloner RA, Jennings RB. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 2. Circulation. 2001;104:3158–3167. [DOI] [PubMed] [Google Scholar]
- 27. Evrengul H, Celek T, Tanriverdi H, et al. The effect of preinfarction angina on clinical reperfusion time in patients with acute myocardial infarction receiving successful thrombolytic therapy. Can J Cardiol. 2005;21:915–920. [PubMed] [Google Scholar]
- 28. Mladenovic ZT, Angelkov‐Ristic A, Tavciovski D, et al. The cardioprotective role of preinfarction angina as shown in outcomes of patients after first myocardial infarction. Tex Heart Inst J. 2008;35:413–418. [PMC free article] [PubMed] [Google Scholar]
- 29. Ottani F, Galli M, Zerboni S, et al. Prodromal angina limits infarct size in the setting of acute anterior myocardial infarction treated with primary percutaneous intervention. J Am Coll Cardiol. 2005;45:1545–1547. [DOI] [PubMed] [Google Scholar]
- 30. Solomon SD, Anavekar NS, Greaves S, et al. Angina pectoris prior to myocardial infarction protects against subsequent left ventricular remodeling. J Am Coll Cardiol. 2004;43:1511–1514. [DOI] [PubMed] [Google Scholar]
- 31. Gehi AK, Ali S, Na B, et al. Inducible ischemia and the risk of recurrent cardiovascular events in outpatients with stable coronary heart disease: the heart and soul study. Arch Intern Med. 2008;168:1423–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brorsson B, Bernstein SJ, Brook RH, et al. Quality of life of patients with chronic stable angina before and four years after coronary revascularisation compared with a normal population. Heart. 2002;87:140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boden WE, O'Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. [DOI] [PubMed] [Google Scholar]
- 34. Buckley BS, Simpson CR, McLernon DJ, et al. Five year prognosis in patients with angina identified in primary care: incident cohort study. BMJ. 2009;339:b3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. LaCroix AZ, Guralnik JM, Curb JD, et al. Chest pain and coronary heart disease mortality among older men and women in three communities. Circulation. 1990;81:437–446. [DOI] [PubMed] [Google Scholar]
- 36. Dagenais GR, Lu J, Faxon DP, et al. Prognostic impact of the presence and absence of angina on mortality and cardiovascular outcomes in patients with type 2 diabetes and stable coronary artery disease: results from the BARI 2D (Bypass Angioplasty Revascularization Investigation 2 Diabetes) trial. J Am Coll Cardiol. 2013;61:702–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Xxxxxxx
Table S1. Variables included in multivariate Cox regression model


