Abstract
Objective
Maternal immune activation (MIA) triggered by infections, has been identified as a cause of autism in the offspring. Considering the involvement of perturbations in innate immunity in epilepsy, we examined whether MIA represents a risk factor for epilepsy as well. The role of specific MIA components– interleukin-6 and interleukin-1β was also addressed.
Methods
MIA was induced in C57BL/6 mice by polyinosinic–polycytidylic acid (PIC) injected during embryonic days 12–16. Beginning from postnatal day 40, the propensity of the offspring to epilepsy was examined using hippocampal kindling; autism-like behavior was studied using the sociability test. The involvement of interleukin-6 and interleukin-1β in PIC-induced effects was studied by the co-administration of the cytokine antibodies with PIC, and by delivering recombinant cytokines in lieu of PIC.
Results
The offspring of PIC-exposed mice exhibited increased hippocampal excitability, accelerated kindling rate, prolonged increase of seizure susceptibility after kindling, and diminished sociability. Epileptic impairments were abolished by antibodies to interleukin-6 or interleukin-1β. Neither of the recombinant cytokines alone increased the propensity to seizures; however when combined, they produced effects similar to the ones induced by PIC. PIC- induced behavioral deficits were abolished by interleukin-6 antibodies and were mimicked by recombinant interleukin-6; interleukin-1β was not involved.
Interpretation
In addition to confirming previously established critical role of interleukin-6 in the development of autism-like behavior following MIA, the present study shows that concurrent involvement of interleukin-6 and interleukin-1β is required for priming the offspring for epilepsy. These data shed light on mechanisms of comorbidity between autism and epilepsy.
INTRODUCTION
There has been growing evidence supporting reciprocal connection between epilepsy and brain inflammation. On the one hand, chronic epilepsy is accompanied by the activation of inflammatory pathways in the brain 1, 2. On the other hand, perturbations in innate immunity resulting from both infections and autoimmune conditions can precipitate seizures 3, 4. Mechanisms, via which brain inflammation facilitates seizures are manifold and involve both the enhanced excitation and the compromised inhibition5, 6.
Maternal immune activation (MIA) which is triggered by either viral or bacterial infection during pregnancy, has been receiving an increasing attention due to potential detrimental effects on the offspring. Pathophysiologically, MIA represents a “cytokine storm” whereby the infection-induced activation of various inflammatory factors interferes with proper development of fetal brain 7–11. The resulting morbidities in the offspring are predominantly psychiatric, specifically schizophrenia and autism12, 13. Maternal infection, mimicked in laboratory conditions by injecting pregnant rats or mice with lipopolysaccharide (mimicking Gram negative bacterial infection via binding to toll-like receptor 410, 14), or polyinosinic–polycytidylic acid (PIC, mimicking viral infection via binding to toll-like receptor 310, 15) affects the offspring in various ways, including impaired social behavior, cognition, memory, mood and motor abilities 10, 16, 17. As for the connection between components of innate immunity involved in the MIA and the resulting pathology, an inflammatory cytokine interleukin-6 (IL-6) has been identified as a factor primarily responsible for autism identified 18.
Considering pathophysiological connection between inflammatory cytokines and epilepsy, it is highly plausible that MIA, among other chronic sequelae, would produce increased propensity to seizures in the offspring. Indeed, following MIA, the brain shows various abnormalities, such as the increased hippocampal pyramidal cell excitability, glia activation, and the increased expression of inflammatory cytokines that may last into the adulthood 10, 11, 19 and may prime the offspring for epilepsy. However, there is no direct evidence that MIA represents a risk factor for the development of epilepsy in the offspring.
In the present study, by employing the PIC model of viral infection in pregnant mice, we examined whether MIA increases seizure susceptibility long-term, using the kindling model of epilepsy in the adult offspring. Given the importance of IL-1β in epilepsy 20, we examined its possible involvement in the MIA-induced seizure phenotype. Furthermore, as the impaired social interaction (i.e. an experimental equivalent of autism) represents an established behavioral deficit in the offspring of PIC-exposed mice, and is mediated by IL-618, we studied whether and how the susceptibility to seizures correlates with social behavior following MIA, and a possible role of IL-6 in this correlation.
MATERIALS AND METHODS
Experimental subjects
The experiments were performed in C57BL/6 J mice; breeding pairs were from the Jackson Laboratory (Sacramento, CA). Breeding was performed at the UCLA Department of Laboratory Animal Medicine. All experimental procedures followed the policies of the National Institutes of Health.
Treatments
On the embryonic days 12 through 16 (E12-E16), mice received one of the treatments described in Table 1. PIC (Sigma, St. Louis, MO), recombinant IL-6 (rIL-6) and recombinant IL-1β (rIL-1β, both cytokines from R&D systems, Minneapolis, MN) were injected intraperitoneally, daily. When administered together, each of the cytokines was injected at half of the dose used for the single cytokine administration, so that to avoid possible non-specific additive effect of the cytokines. Mouse monoclonal IL-6 and IL-1β antibodies (both from R&D systems) were administered subcutaneously, at the time of PIC injection, followed by an additional injection on E17. The initial doses of PIC, the cytokines and the antibodies were based on published data 11, 18 and were further optimized in pilot studies (Supplementary data, Table 1S). There were no differences among the offspring of different groups in terms of general behavior, or body weight.
Table 1.
Experimental groups and treatments.
| Treatment | Dose | Total number of offspring mice used for the experiments |
|---|---|---|
| Saline (control for PIC) | - | 10 |
| PIC | 2.5 mg/kg | 10 |
| PIC + IL-6 AB | 2.5 mg/kg + 250 μg/kg respectively | 8 |
| PIC + IL-1β AB | 2.5 mg/kg + 250 μg/kg respectively | 7 |
| IL-6 AB + IL-1β AB (to account for effects of antibodies proper). | 250 μg/kg + 250 μg/kg respectively | 6 |
| Saline (control for recombinant cytokines) | - | 10 |
| rIL-6 | 20 μg/kg | 9 |
| rIL-1β | 20μg/kg | 7 |
| rIL-6 + rIL-1β | 10 μg/kg + 10μg/kg respectively | 10 |
Core temperature and serum cytokine assay
Separate female adult mice were used for measuring plasma cytokine levels and core temperature after PIC and recombinant cytokine administration (Supplementary data).
Sociability test
At postnatal day 40 (P40), sociability was examined as described 21, 22. The apparatus (Noldus, Leesburg, VA) was a 60 × 40 cm Plexiglas box divided into three connected chambers. Each of the end compartments contained wired cylindrical enclosures (11 cm high, 10 cm diameter, bar space 1 cm apart). Mice were placed into the chamber one at a time, were allowed to accommodate for 10 min, and were then removed. An unfamiliar age- and sex-matched mouse was placed inside one of the enclosures, and an unfamiliar object (cube) –inside another enclosure. Test mice were re-introduced into the chamber and were allowed to explore it for 10 minutes. Behavior was recorded and analyzed off-line. Cumulative time of direct contact between the test mouse and the enclosure containing the stranger mouse (tstranger) and between the test mouse and the enclosure containing the object (tobject) were measured. The sociability index was expressed as [tstranger / tstranger +tobject] X 100 – 5017, 18, 23. On the resulting scale, animals’ sociability spans from +50 (full preference for a stranger) - to 0 (social indifference) - to −50 (complete avoidance of the stranger).
Rapid kindling
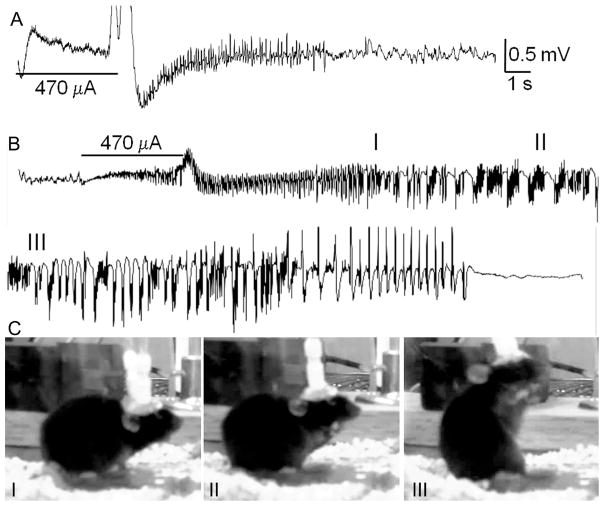
Rapid kindling is a modification of kindling model of epileptogenesis, whereby the process takes several hours rather than 10 or more days24 . The experiments involved same animals which had been used in behavioral tests. Between P41 and P45, under Isoflurane anesthesia, animals were stereotaxically implanted with the stimulating/recording bipolar electrode into the left hippocampus (coordinates from Bregma- posterior 2.9 mm, lateral 2.8 mm, down – 4.0 mm). After 72 hrs recovery, the animals were placed into Plexiglas chambers with free access to food and water. The electrodes were connected to low torque swivels (PlasticsOne, Roanoke, VA), which in turn were connected to the DS8000 electrical stimulator (World Precision Instruments, Sarasota, Florida) and MP100/EEG100C acquisition system (Biopac, Santa Barbara, CA). Electrographic responses and animals’ behavior was recorded throughout the procedure. Hippocampal afterdischarge was induced by applying the train of constant current consisting of 200 bipolar square wave stimuli, width 1 ms, inter-stimulus interval 20 ms. The initial intensity was set at 100 μA; stimulations were repeated every 15 min with 50 μA increments until the occurrence of the afterdischarge (Fig. 1A). Kindling started after the detection of afterdischarge threshold (ADT) and consisted of 60 stimulations delivered at the afterdischarge intensity every 15 minutes (i.e. the procedure lasted for 15 hours). Behavioral seizures were classified using the following scale: 1: facial clonus; 2: head nodding; 3: forelimb clonus; 4: rearing and forelimb clonus; 5: rearing and falling (Fig. 1C). The number of stimulations needed to develop first stage 4–5 seizure and total number of stage 4–5 seizures were calculated to express kindling progression. Pre-kindling and post-kindling (1 day and 2 weeks) ADT, afterdischarge duration (ADD), as well as seizure severity in response to respective threshold stimulation were detected as measures of hippocampal excitability and kindling retention.
Fig. 1. Rapid kindling in a P40 mouse.
A. Baseline afterdischarge. Horizontal bar indicates the applied electrical stimulus and the number below- the afterdischarge threshold. B. Electrographic seizures in response to the stimulation during kindling (stimulation number 28 out of 60). C. Snapshot photographs taken during behavioral seizure corresponding to the electrographic activity presented on B. Shown is the progression from stages 2 (I) to 3 (II) to 4–5 (III). Roman numerals on B and C indicate corresponding electrographic and behavioral activities.
Histology and immunostaining
Separate groups of animals were exposed at E12-E16 to saline, PIC or rIL-6+rIL-1β as described; at P40 animals underwent sociability test, after which they were euthanized. Hippocampi were processed for evaluating cell count (using cresyl violet staining), expression of astrocyte marker glial fibrillary acidic protein (GFAP), microglial marker CD11b 1 and a marker of neurogenesis doublecortin 25(Supplementary data).
Statistical analysis
Data were analyzed using Prizm 5 software (GraphPad, San Diego, CA). Sample sizes are indicated in Table 1. Statistical methods used are described in corresponding figure legends.
RESULTS
Serum cytokine levels and core temperature
Administration of PIC, as well as of recombinant cytokines produced significant increase of both IL-6 and IL-1β in plasma but did not affect core temperature (Supplementary data),
Sociability test (Fig. 2)
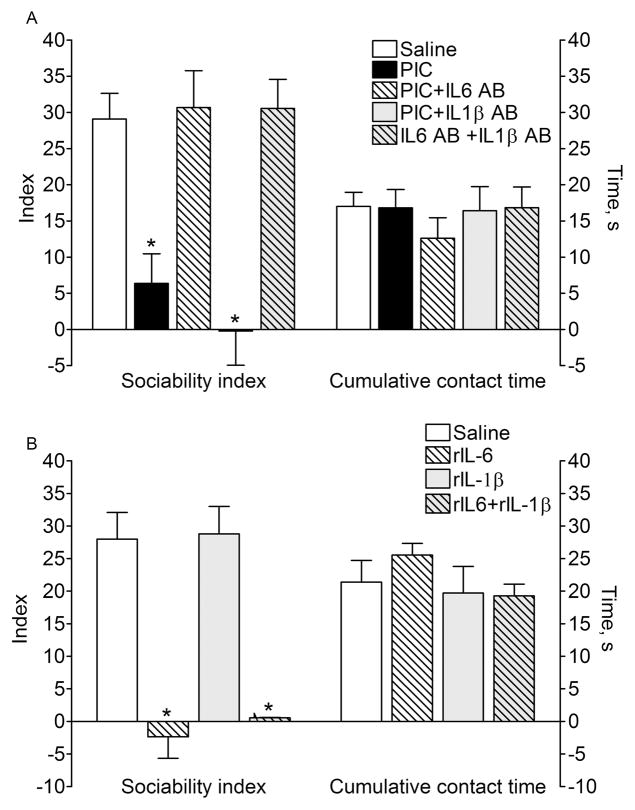
Fig. 2. Social interaction.
A. P40 offspring of PIC-exposed mice showed impaired sociability. The effect of PIC was abolished by the co-administration of IL-6 antibody, but not of IL-1β antibody. Cytokine antibodies themselves had no effects on behavior. B. P40 offspring of rIL-6 –treated mice developed impairments in sociability similar to the one observed after PIC treatment (compare with effects of PIC on A). The administration of rIL-1β had no effects on social behavior in the offspring. On A and B: total engagement time between the test mice and strangers + non-animated objects was not affected by any of treatments. Data are presented as Mean±SEM. *- p<0.05 vs. Saline (One way ANOVA+Bonferroni posttest).
The offspring of saline-treated mice exhibited strong preference towards an unfamiliar mouse vis-à-vis an indifferent object. In agreement with previous findings17, 18, the offspring of PIC-exposed mice showed significant impairment in sociability, which was evident as dividing interaction time nearly equally between the stranger mouse and the object (Fig. 2A). Total interaction time (i.e. stranger mouse plus the object) was similar between the animals of control and PIC groups, thus pointing towards the specific reduction of social engagement. The offspring of PIC-exposed mice, which were also treated with IL-6 antibodies displayed normal (i.e. similar to naïve animals) social interaction behavior. At the same time, the administration of IL-1β antibodies did not improve the detrimental effects of PIC.
The offspring of mice treated with rIL-6 developed impairments in sociability similar to those observed after PIC administration (Fig. 2B). However, treatment with rIL-1β had no effect on social behavior in the adult offspring.
Baseline hippocampal excitability (Fig. 3)
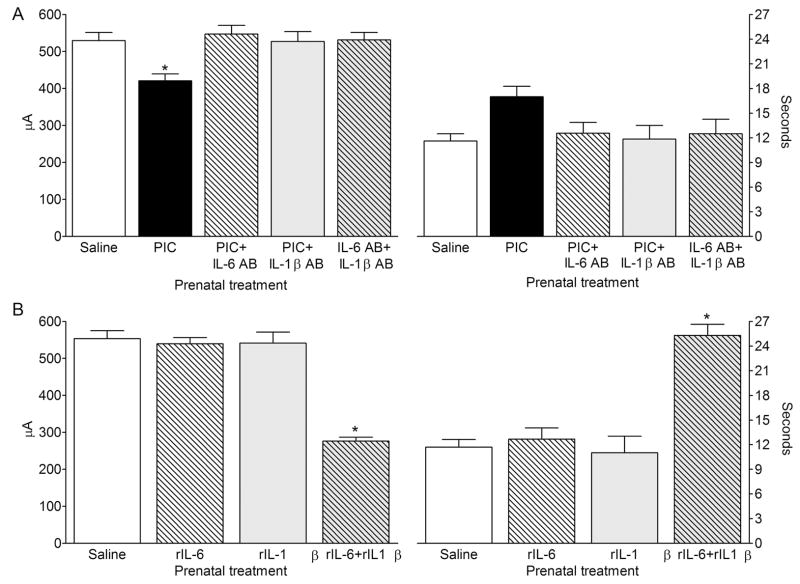
Fig. 3. Baseline afterdischarge properties.
A. P40 offspring of PIC-treated mice showed lower hippocampal ADT than the offspring of saline-treated animals. The PIC-induced increase of hippocampal excitability was abolished by the co-administration of either IL-6 antibody, or IL-1β antibody. Antibodies themselves had no effects on afterdischarge properties. B. Prenatal treatment with neither rIL6, not rIL-1β administered alone had any effects on the afterdischarge properties. However, the offspring of mice that had received rIL-6+rIL-1β injections displayed the decreased ADT and prolonged ADD. Data are presented as Mean±SEM. *- P<0.05 vs. Saline (One way ANOVA + Bonferroni posttest).
Compared with control animals, the offspring of PIC-treated mice showed significant increase of hippocampal excitability, which was evident as the decrease of ADT (Fig. 3A). This effect of PIC was abolished by the co-administration with either IL-6 antibody, or IL-1β antibody.
The administration of neither of the recombinant cytokines alone modified afterdischarge properties in the offspring. However, when the two cytokines were given together, mice displayed the increased hippocampal excitability, which was evident as both the decrease of the ADT and the increase of the ADD (Fig. 3B).
None of animals in any group exhibited any behavioral seizures in response to the afterdischarge stimulation.
Kindling progression (Fig. 4)
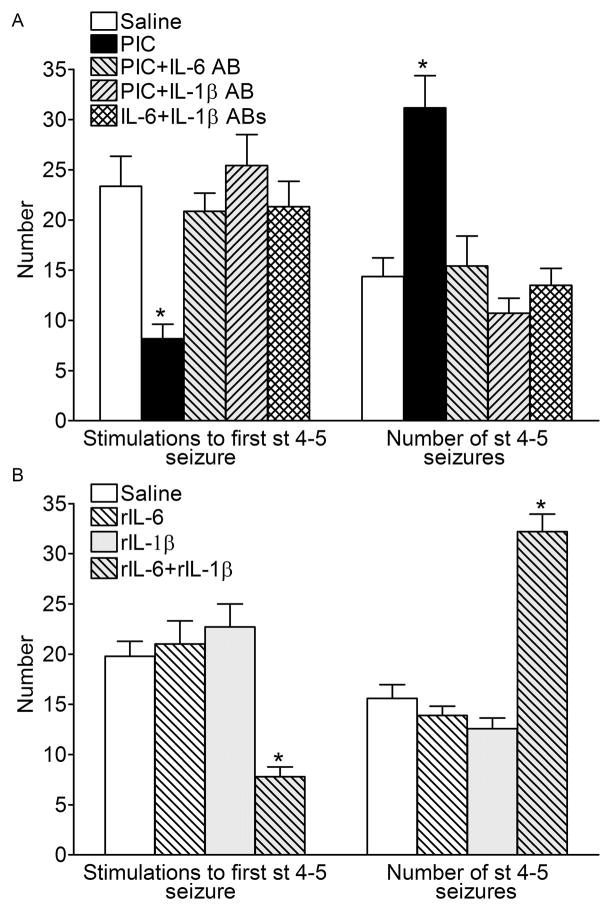
Fig. 4. Kindling progression.
A. Mice, which had been prenatally exposed to PIC required fewer electrical stimulations to develop the first stage 4–5 convulsion, and exhibited more of such seizures during the course of kindling procedure, as compared with the offspring of saline-treated animals. Kindling-facilitating effects of PIC were abolished by the co-administration of either IL-6 antibody, or IL-1β antibody. B. Treatment of pregnant mice with neither of the recombinant cytokine alone affected the progression of kindling in the offspring. However, the administration of cytokine combination accelerated the occurrence and increased the number of stage 4–5 seizures. Data are presented as Mean±SEM. *- P<0.05 vs. Saline (One way ANOVA + Bonferroni posttest).
During kindling procedure, in the offspring of PIC-exposed mice stage 4–5 convulsions occurred significantly earlier, and in higher numbers than in the offspring of saline-treated animals (Fig. 4A). The administration of either IL-6 antibody, or of IL-1β antibody abolished this effect of PIC. Similar to the effects of PIC, treatment of pregnant mice with the combination of rIL6 and rIL-1β produced the offspring showing the accelerated kindling rate, and more stage 4–5 seizures than controls (Fig. 4B). However, neither rIL-6, nor IL-1β affected kindling progression in the offspring, when the cytokines were administered alone.
Kindling retention (Fig. 5)
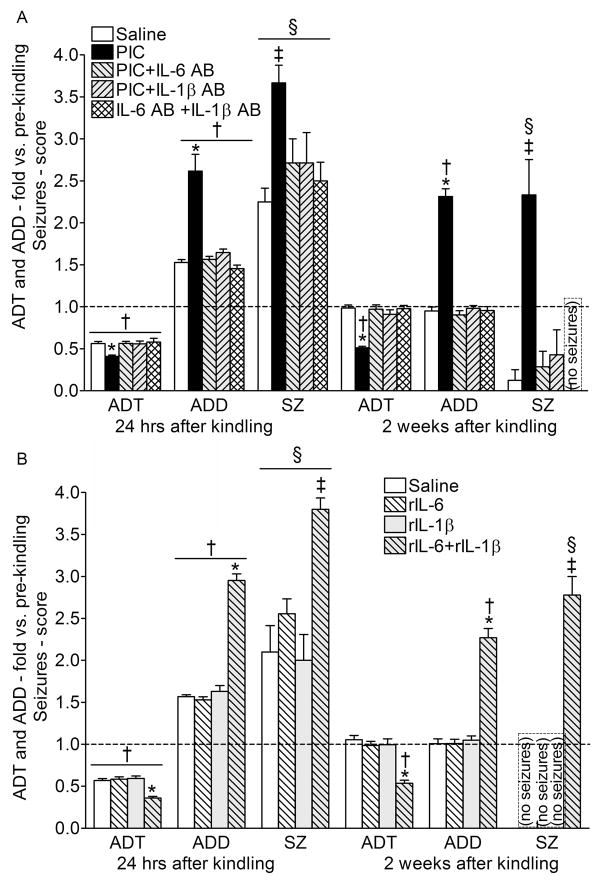
Fig. 5. Kindling retention.
A. Twenty fur hours after the completion of kindling procedure, mice of all groups showed the increased hippocampal excitability as compared with the baseline values: the decreased ADT, the prolonged ADD, and behavioral seizures in response to the threshold stimulation. However, in the offspring of PIC-treated animals, these responses were augmented as compared with the animals of saline group. Two weeks after kindling in mice of the saline group, all the examined parameters returned to pre-kindling values; at the same time, in the animals of PIC group, the increased hippocampal excitability and susceptibility to seizures persisted. B. Prenatal exposure to neither rIL-6 alone, nor IL-1β alone affected the extent and the duration of the kindling-induced increase hippocampal excitability and susceptibility to seizures. Prenatal treatment with the cytokine combination resulted in a more profound and longer-lasting kindling-induced changes, similar to the ones observed after PIC exposure. Data are presented as Mean±SEM and are expressed as fold-changes vs. pre-kindling values. For statistical comparisons, absolute values were used. *- p<0.05 vs. Saline (One way ANOVA+ Bonferroni posttest); ‡- p<0.05 vs. Saline, (Fisher’s exact test for the number of animals with stage 4–5 seizures); †- p<0.05 vs. respective values before kindling (the latter are shown on Fig. 4; repeated measures ANOVA+Bonferroni posttest). §- p<0.05 vs. values before kindling (Fisher’s exact test for the number of animals with seizures of any behavioral score).
Twenty four hours after the completion of the kindling procedure, all animals exhibited augmented responses to hippocampal stimulations, as compared with the pre-kindling tests. These included lowered ADT, prolonged ADD, as well the development of motor seizures in response to the threshold stimulation. However, all these indices were significantly more amplified in the offspring of PIC-treated, than in the offspring of saline-treated mice (Fig. 5A).
In contrast to conventional kindling, rapid kindling does not create the life-long increase of seizure susceptibility; instead the latter dissipates within several weeks24, 26. Indeed, in the offspring of the saline-treated animals all the examined parameters returned to pre-kindling values two weeks after kindling. However, at two weeks animals of the PIC group were still showing the decrease of the ADT and the increase of the ADD, and the threshold stimulation was still triggering motor convulsions. The PIC-induced augmentation and persistence of all post-kindling responses was disrupted by the co-administration of either IL-6, or IL-1β antibodies.
Compared with control animals, the offspring of mice which had received the combination of rIL-6 and rIL-1β, showed more exacerbated and prolonged increase of hippocampal excitability and propensity to seizures after kindling; the responses in these mice were similar to the ones observed in the animals of the PIC group (Fig. 5B). Once again, prenatal exposure to either rIL-6 alone or r-IL-1β alone had no effects on post-kindling increase of hippocampal excitability and seizure responses both at 24 hours and 2 weeks.
Histology and immunostaining
Prenatal exposure to neither PIC, nor rIL-6+rIL-1β produced measurable cell loss, activation of astroglia or microglia in the hippocampus, or changes in neurogenesis in the subgranular zone of dentate gyrus (Supplementary data, Fig. 2S).
DISCUSSION
The main findings of this study are: (i) The offspring of mice which have been administered PIC during pregnancy shows increased hippocampal excitability, faster progression of kindled seizures and prolonged persistence of the kindling state, along with the impaired social interaction; (ii) Behavioral impairments depend on the PIC-induced activation of IL-6; (iii) The increased propensity to seizures requires the activation of both IL-6 and IL1-β.
The involvement of inflammatory cytokines in the pathophysiology of epilepsy has been well established 1, 2. However, ours is the first report documenting long-term increase of seizure susceptibility in the offspring following MIA, using the kindling model of epilepsy. Following MIA, complex lasting changes in levels of inflammatory cytokines (including IL-6 and IL-1β) in the neocortex, the hippocampus and serum have been reported 11, 27, as well as abnormal regulation of multiple genes involved in cell proliferation and survival 19, 28. Morphological and functional perturbations include activation of micro- and astroglia, neuronal degeneration, impairments in dendritic arborization and neurogenesis in the hippocampus 10, 19, 29. Many of the observed abnormalities are held as factors contributing to epilepsy. However, our experiments did not reveal any measurable histopathological changes in the hippocampus of offspring following MIA (although it cannot be excluded that such changes had appeared transiently at earlier time and contributed to long-term alterations in behavior and propensity to seizures). The difference between our and others’ findings may be attributed to different treatment regimens whereby the amount of both PIC and rIL-6 used by us were lower than those employed by others (e.g. PIC was administered as three 5 mg/kg, or single 20 mg/kg injection, and rIL-6 – at 250μg/kg)11, 17, 18. Therefore, our findings show that even subtle MIA- associated activation of inflammatory cytokines, which does not result in profound histopathology, is sufficient for producing specific behavioral and seizure impairments in the offspring. While the scope of our assays was limited, it seems more plausible that causes underlying the reported impairments should be sought a on more profound level, for example perturbations in translation, transcription or function of glutamate and GABAA receptors, as the latter have been demonstrated following MIA 30–32. Among several inflammatory pathways associated with epilepsy, IL-1β appears to be particularly important 2. The expression of both IL-1β and its receptor IL-1RI is increased in the hippocampus of patients with temporal lobe epilepsy, as well as in experimental animals with chronic epilepsy 1. Blockade of interleukin-1 receptor 33, or of IL-1β synthesis34 exert antiepileptic effects. The administration of PIC to neonatal rats increases their seizure susceptibility in the adulthood via early-life transient activation of IL1-β signaling 35. IL-1β – induced phosphorylation of NR2B subunit of N-methyl-D-aspartate (NMDA) receptor has been identified as a major factor determining proconvulsant effects of this cytokine 6.
The involvement of IL-6 in long-term outcomes of MIA has been well documented. Both PIC and lipopolysaccharide administration are accompanied by the elevated level of IL-6 in serum, as well as in the placenta of pregnant animals and brains of their embryos10. Direct prenatal exposure to IL-6 leads to neurodegeneration, astrogliosis and increased expression of NR1 subunit of NMDA receptor in the hippocampus 32. With regard to epilepsy, elevated levels of IL-6 have been reported in plasma and cerebro-spinal fluid of patients after generalized tonic clonic seizures and febrile seizures 36. Furthermore, seizures induced in experimental animals transiently induce IL-6 and its receptor, in several brain areas, including hippocampus 20, 37–39. However, pathophysiological significance of IL-6 in epilepsy is not clear as both proconvulsant and anticonvulsant effects of the cytokine have been reported 37.
Overall, the discussed data demonstrate that MIA-associated activation of IL-1β and IL-6 each contributes to the long-term increase of seizure susceptibility in the offspring. Indeed, immunological blockade of either of the cytokines during pregnancy in PIC-treated mice abolished all proepileptic effects of MIA in the offspring, thus showing that both IL-6 and IL-1β were necessary for promoting kindling. At the same time, neither of the cytokines alone increased baseline excitability or epileptogenicity in the offspring, suggesting that neither of them by itself was sufficient for promoting kindling. Finally, when combined, the two cytokines fully mimicked effects of PIC on kindling, thereby suggesting that their combined action was both sufficient and necessary for mediating effects of prenatal PIC exposure on kindling in the adulthood. Since total amount of recombinant cytokines was same across all groups (i.e. the amount of rIL-6 alone and the amount of rIL-1β alone was equal to the amount of rIL-6+rIL-1β), the observed effects on seizures cannot be ascribed merely to a higher cytokine plasma content. Instead, the two cytokines appear to interact on some level, although the exact nature of this interaction remains to be established.
An important implication of our findings pertains to comorbidity between epilepsy and autism. Among environmental risk factors for the development of autism, MIA has been receiving increasing attention. Recent epidemiological studies established an association between infections in pregnant women and autism in their children 40, 41. Experimental studies have confirmed that autism-like behavioral abnormalities develop in the offspring of mice exposed to PIC or lipopolysaccharide in the absence of any genetic trait 16, 17. IL-6 has been established as the key factor of MIA determining the development of autism in the offspring. The presence of IL-6 in the amniotic fluid of pregnant women is associated with the increased risk of white matter lesion in the offspring 42, and such lesions are known to contribute to autism43. Under conditions of the mouse model of MIA, PIC- induced behavioral abnormalities in the offspring were abolished by IL-6 antibodies, and in turn PIC failed to produce such changes when administered to IL-6 knockout mice; conversely, rIL-6 administration to pregnant mice produced autism-like behavior in the offspring 18.
Epidemiologically, reciprocal connection between autism and epilepsy has been widely accepted44–46. The presented data outline one possible scenario of such comorbidity, whereby the development of epilepsy and/or autism in the offspring depends on individual variations of IL-6 and/or IL-1β activation which is triggered by infections in pregnant women.
It should be clarified that our data do not allow concluding whether MIA alone is sufficient for inducing epilepsy in the offspring, as long-term EEG and video monitoring was not performed. Rather, we propose that the MIA – induced activation of IL-6 and IL1-β makes the offspring more prone to a subsequent second hit which in our experiments has been mimicked by kindling.
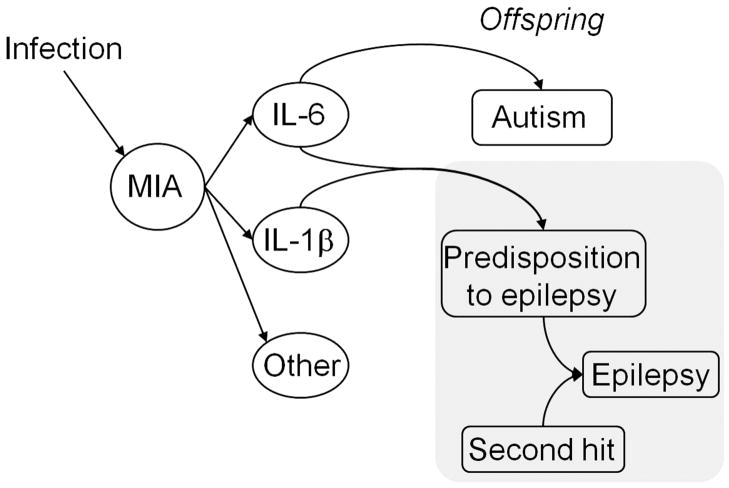
In conclusion, we propose a mechanism via which infections in pregnant women and subsequent immune response lead to the development of epilepsy in the offspring (Fig. 6): during MIA, the activation of IL-6 and IL1-β primes the offspring to epilepsy, and the latter evolves following a secondary event which is inconsequential under normal circumstances.
Fig. 6. The proposed involvement of IL-6 and IL-1β as components of maternal immune activation (MIA) in the development of epilepsy and autism-like impairments in the offspring.
Among various components of the MIA triggered by infection (mimicked by PIC in the present studies), IL-6 is necessary and sufficient for producing autism in the offspring in the adulthood. At the same time, the combined induction of IL-6 and IL-1β is both sufficient and necessary for increasing propensity to epilepsy in the offspring. The exposure to a second hit (mimicked by the kindling procedure in these studies) then may produce full epileptic phenotype. Hence, according to the proposed model, the autism-epilepsy comorbidity following an infection during pregnancy depends on the extent of the exposure of fetal brain to IL-6 and IL-1β.
Supplementary Material
Acknowledgments
The study was supported by the NIH research grant R01 NS065783 (Andrey Mazarati). The authors wish to thank Mr. Steven Hasday for technical help with immunohistochemistry.
References
- 1.Ravizza T, Gagliardi B, Noe F, Boer K, Aronica E, Vezzani A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis. 2008;29:142–160. doi: 10.1016/j.nbd.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 2.Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7:31–40. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bien CG, Urbach H, Schramm J, et al. Limbic encephalitis as a precipitating event in adult-onset temporal lobe epilepsy. Neurology. 2007;69:1236–1244. doi: 10.1212/01.wnl.0000276946.08412.ef. [DOI] [PubMed] [Google Scholar]
- 4.Solbrig MV, Adrian R, Baratta J, Lauterborn JC, Koob GF. Kappa opioid control of seizures produced by a virus in an animal model. Brain. 2006;129:642–654. doi: 10.1093/brain/awl008. [DOI] [PubMed] [Google Scholar]
- 5.Stuck ED, Christensen RN, Huie JR, et al. Tumor necrosis factor alpha mediates GABA(A) receptor trafficking to the plasma membrane of spinal cord neurons in vivo. Neural Plast. 2012;2012:261345. doi: 10.1155/2012/261345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viviani B, Bartesaghi S, Gardoni F, et al. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buehler MR. A proposed mechanism for autism: an aberrant neuroimmune response manifested as a psychiatric disorder. Med Hypotheses. 2011;76:863–870. doi: 10.1016/j.mehy.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 8.Jonakait GM. The effects of maternal inflammation on neuronal development: possible mechanisms. Int J Dev Neurosci. 2007;25:415–425. doi: 10.1016/j.ijdevneu.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Ponzio NM, Servatius R, Beck K, Marzouk A, Kreider T. Cytokine levels during pregnancy influence immunological profiles and neurobehavioral patterns of the offspring. Ann N Y Acad Sci. 2007;1107:118–128. doi: 10.1196/annals.1381.013. [DOI] [PubMed] [Google Scholar]
- 10.Boksa P. Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav Immun. 2010;24:881–897. doi: 10.1016/j.bbi.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Garay PA, Hsiao EY, Patterson PH, McAllister AK. Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown AS, Susser ES. In utero infection and adult schizophrenia. Ment Retard Dev Disabil Res Rev. 2002;8:51–57. doi: 10.1002/mrdd.10004. [DOI] [PubMed] [Google Scholar]
- 13.Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 15.Fortier ME, Kent S, Ashdown H, Poole S, Boksa P, Luheshi GN. The viral mimic, polyinosinic:polycytidylic acid, induces fever in rats via an interleukin-1-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2004;287:R759–766. doi: 10.1152/ajpregu.00293.2004. [DOI] [PubMed] [Google Scholar]
- 16.Patterson PH. Maternal infection and immune involvement in autism. Trends Mol Med. 2011;17:389–394. doi: 10.1016/j.molmed.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun. 2012;26:607–616. doi: 10.1016/j.bbi.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.SH, Reutiman TJ, Folsom TD, et al. Maternal infection leads to abnormal gene regulation and brain atrophy in mouse offspring: implications for genesis of neurodevelopmental disorders. Schizophr Res. 2008;99:56–70. doi: 10.1016/j.schres.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vezzani A, Moneta D, Richichi C, et al. Functional role of inflammatory cytokines and antiinflammatory molecules in seizures and epileptogenesis. Epilepsia. 2002;43 (Suppl 5):30–35. doi: 10.1046/j.1528-1157.43.s.5.14.x. [DOI] [PubMed] [Google Scholar]
- 21.Crawley JN. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007;17:448–459. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nadler JJ, Moy SS, Dold G, et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 23.Jamain S, Radyushkin K, Hammerschmidt K, et al. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc Natl Acad Sci U S A. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lothman EW, Williamson JM. Closely spaced recurrent hippocampal seizures elicit two types of heightened epileptogenesis: a rapidly developing, transient kindling and a slowly developing, enduring kindling. Brain Res. 1994;649:71–84. doi: 10.1016/0006-8993(94)91050-2. [DOI] [PubMed] [Google Scholar]
- 25.Parent JM, von dem Bussche N, Lowenstein DH. Prolonged seizures recruit caudal subventricular zone glial progenitors into the injured hippocampus. Hippocampus. 2006;16:321–328. doi: 10.1002/hipo.20166. [DOI] [PubMed] [Google Scholar]
- 26.Mazarati A, Shin D, Auvin S, Caplan R, Sankar R. Kindling epileptogenesis in immature rats leads to persistent depressive behavior. Epilepsy Behav. 2007;10:377–383. doi: 10.1016/j.yebeh.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arrode-Bruses G, Bruses JL. Maternal immune activation by poly(I:C) induces expression of cytokines IL-1beta and IL-13, chemokine MCP-1 and colony stimulating factor VEGF in fetal mouse brain. J Neuroinflammation. 2012;9:83. doi: 10.1186/1742-2094-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garbett KA, Hsiao EY, Kalman S, Patterson PH, Mirnics K. Effects of maternal immune activation on gene expression patterns in the fetal brain. Transl Psychiatry. 2012;2:e98. doi: 10.1038/tp.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsiao EY, McBride SW, Chow J, Mazmanian SK, Patterson PH. Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc Natl Acad Sci U S A. 2012;109:12776–12781. doi: 10.1073/pnas.1202556109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forrest CM, Khalil OS, Pisar M, Smith RA, Darlington LG, Stone TW. Prenatal activation of Toll-like receptors-3 by administration of the viral mimetic poly(I:C) changes synaptic proteins, N-methyl-D-aspartate receptors and neurogenesis markers in offspring. Mol Brain. 2012;5:22. doi: 10.1186/1756-6606-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roumier A, Pascual O, Bechade C, et al. Prenatal activation of microglia induces delayed impairment of glutamatergic synaptic function. PLoS One. 2008;3:e2595. doi: 10.1371/journal.pone.0002595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samuelsson AM, Jennische E, Hansson HA, Holmang A. Prenatal exposure to interleukin-6 results in inflammatory neurodegeneration in hippocampus with NMDA/GABA(A) dysregulation and impaired spatial learning. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1345–1356. doi: 10.1152/ajpregu.00268.2005. [DOI] [PubMed] [Google Scholar]
- 33.Vezzani A, Moneta D, Conti M, et al. Powerful anticonvulsant action of IL-1 receptor antagonist on intracerebral injection and astrocytic overexpression in mice. Proc Natl Acad Sci U S A. 2000;97:11534–11539. doi: 10.1073/pnas.190206797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maroso M, Balosso S, Ravizza T, et al. Interleukin-1beta biosynthesis inhibition reduces acute seizures and drug resistant chronic epileptic activity in mice. Neurotherapeutics. 2011;8:304–315. doi: 10.1007/s13311-011-0039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galic MA, Riazi K, Henderson AK, Tsutsui S, Pittman QJ. Viral-like brain inflammation during development causes increased seizure susceptibility in adult rats. Neurobiol Dis. 2009;36:343–351. doi: 10.1016/j.nbd.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lehtimaki KA, Keranen T, Palmio J, et al. Increased plasma levels of cytokines after seizures in localization-related epilepsy. Acta Neurol Scand. 2007;116:226–230. doi: 10.1111/j.1600-0404.2007.00882.x. [DOI] [PubMed] [Google Scholar]
- 37.Li G, Bauer S, Nowak M, et al. Cytokines and epilepsy. Seizure. 2011;20:249–256. doi: 10.1016/j.seizure.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Lehtimaki KA, Peltola J, Koskikallio E, Keranen T, Honkaniemi J. Expression of cytokines and cytokine receptors in the rat brain after kainic acid-induced seizures. Brain Res Mol Brain Res. 2003;110:253–260. doi: 10.1016/s0169-328x(02)00654-x. [DOI] [PubMed] [Google Scholar]
- 39.Rosell DR, Nacher J, Akama KT, McEwen BS. Spatiotemporal distribution of gp130 cytokines and their receptors after status epilepticus: comparison with neuronal degeneration and microglial activation. Neuroscience. 2003;122:329–348. doi: 10.1016/s0306-4522(03)00593-1. [DOI] [PubMed] [Google Scholar]
- 40.Atladottir HO, Thorsen P, Ostergaard L, et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2010;40:1423–1430. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- 41.Atladottir HO, Henriksen TB, Schendel DE, Parner ET. Autism After Infection, Febrile Episodes, and Antibiotic Use During Pregnancy: An Exploratory Study. Pediatrics. 2012;130:e1447–e1454. doi: 10.1542/peds.2012-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoon BH, Jun JK, Romero R, et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 1997;177:19–26. doi: 10.1016/s0002-9378(97)70432-0. [DOI] [PubMed] [Google Scholar]
- 43.Booth R, Wallace GL, Happe F. Connectivity and the corpus callosum in autism spectrum conditions: insights from comparison of autism and callosal agenesis. Prog Brain Res. 2011;189:303–317. doi: 10.1016/B978-0-444-53884-0.00031-2. [DOI] [PubMed] [Google Scholar]
- 44.Clarke DF, Roberts W, Daraksan M, et al. The prevalence of autistic spectrum disorder in children surveyed in a tertiary care epilepsy clinic. Epilepsia. 2005;46:1970–1977. doi: 10.1111/j.1528-1167.2005.00343.x. [DOI] [PubMed] [Google Scholar]
- 45.Steffenburg S, Gillberg C, Steffenburg U. Psychiatric disorders in children and adolescents with mental retardation and active epilepsy. Arch Neurol. 1996;53:904–912. doi: 10.1001/archneur.1996.00550090114017. [DOI] [PubMed] [Google Scholar]
- 46.Tuchman R, Rapin I. Epilepsy in autism. Lancet Neurol. 2002;1:352–358. doi: 10.1016/s1474-4422(02)00160-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.