Abstract
Background
Oxidative stress is elevated in obese youth, but less is known regarding racial disparities in the relationship of oxidative stress with metabolic risk factors.
Objectives
To determine the relationship between oxidative stress and metabolic risk factors, adiposity, leptin, adiponectin, and cardiovascular fitness (VO2PEAK) in healthy African American and White American youth.
Methods
A marker of oxidative stress (F2-isoprostane), validated markers of metabolic risk factors, fitness and body composition were measured in African American (n=82) and White American (n=76) youth (8–17 years old) recruited over a range of body mass index (BMI) percentiles (4th to 99th).
Results
F2-isoprostane concentration was positively correlated with percentage body fat (r=0.198) and percentage truncal fat (r=0.173), but was not different between African American and White American males and females (p =0.208). African American youth had significantly higher mean systolic and diastolic blood pressure (p =0.023 and p =0.011, respectively). After adjusting for gender, age, BMI, and Tanner stage, African American youth varied from White Americans in the association of F2-isoprostane with diastolic blood pressure (p =0.047), but not with systolic blood pressure, triglycerides, VO2PEAK, or HOMA-IR (all p>0.05).
Conclusions
Oxidative stress, as measured by urinary F2-isoprostane concentrations, was positively associated with percent body fat and percent truncal fat in youth. Oxidative stress levels were similar among African American and White American youth. Among markers of the metabolic syndrome, a significant difference between African American and White American youth was demonstrated only in the association of oxidative stress with diastolic blood pressure.
Keywords: obesity, metabolic syndrome, isoprostane, adiposity, adipokine
Introduction
African Americans are disproportionately affected by obesity compared to White Americans, and these disparities begin in childhood.1 Recent rapid increase in childhood obesity has been accompanied by concomitant increases in hypertension, dyslipidemia, and type 2 diabetes.2,3 It has been established that compared to White American adults, African Americans are at higher risk for cardiovascular disease and type 2 diabetes.4,5 Because of the increased metabolic risk for African American youth and consequent obesity-related pathophysiologies, which track into adulthood, it is imperative to determine clinically relevant racial disparities in early markers of metabolic dysfunction.
Oxidative stress, defined as an imbalance between production of reactive oxygen species and antioxidant defenses, is an early marker of metabolic dysfunction and has been implicated in atherosclerosis, microvascular complications of diabetes, beta cell failure in type 2 diabetes, and insulin resistance, making it a unifying mechanism of metabolic dysfunction.6,7 Previous studies have shown that oxidative stress is increased in obese adults8,9 and children.10–16 In addition, oxidative stress was elevated in individuals who displayed metabolic risk factors associated with metabolic syndrome compared to individuals with no metabolic dysfunction.9–11,17
While these studies support the role of oxidative stress as an early marker of metabolic derangements, more data are needed regarding racial differences between the relationship of oxidative stress and individual metabolic risk factors. Despite an increased risk of developing obesity, diabetes type 2, and hypertension in African American compared to White American adults and children, certain cardiometabolic risk factors tend to be more favorable in African Americans (higher HDL, lower triglycerides)18,19. No study, to the best of our knowledge, has evaluated and compared racial disparities in the relationship of oxidative stress with metabolic risk factors, adipokine concentration, or cardiorespiratory fitness. Therefore, the objectives of the current study were to determine the relationship between F2-isoprostane, an established marker of oxidative stress, and metabolic risk factors, adiposity, adipokine concentrations, and cardiorespiratory fitness in African American and White American youth.
Methods
Participants
A group of 158 healthy African American and White American youth (8 to 17 years old) were recruited over a range of BMI percentiles from the Nashville general population using flyers, e-mail distribution lists, and personal contacts. Participants or their parents classified their own ethnicity according to investigator-defined options (African American, White American, or other). All volunteers were healthy as determined by a physical exam performed by a board-certified pediatrician. Participants were not involved in a weight loss program or in an intensive exercise program in 6 months before the study. Exclusion criteria included smoking or using tobacco products, diabetes, cardiovascular disease, significant recent weight change, chronic pulmonary conditions (asthma, sleep apnea), or other health issues that would preclude participation in physical activities as assessed by a pediatrician. All applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this study, in accordance with the ethical principles of the Helsinki-II Declaration. All participants and their parents or legal guardians signed an informed consent or assent document approved by the university-affiliated Institutional Review Board.
Protocol
The details of the protocol were discussed, and all questions answered by the study staff before scheduling a study visit. Participants were asked to maintain normal daily routine but avoid any unusual patterns of physical activity (PA) such as strenuous exercise and stress, on the day before the study. No dietary restrictions were stipulated before the study visit. Participants reported to the Clinical Research Center (CRC) after an overnight fast for baseline measurements for a study on the role of physical activity in adolescent obesity.
Anthropometric and body composition measurements
The National Health and Nutrition Examination Survey (NHANES) protocols were followed for all anthropometrical measurements20. Stature (height) was measured within 0.5 cm using a calibrated wall-mounted stadiometer (Perspective Enterprises, Portage, MI). Body weight was measured within 0.1 kg using a calibrated beam platform scale (Detecto-Medic, Detecto Scales, Inc, Northbrook, IL) with participants wearing light clothing and no shoes. Body mass index (BMI), BMI percentiles, and BMI z-scores were calculated from height, weight, and age using Centers of Disease Control (CDC) growth charts21. Total body fat mass and truncal fat mass were measured using dual energy x-ray absorptiometry (DXA) (GE Medical Systems, Madison WI, enCORE 2007 software version 11.40.004). For quality assurance and equilibration, a calibration block was scanned each morning and a spine phantom was scanned on a weekly basis. The coefficient of variation in for DXA measurements in our laboratory for youth is 0.7%.
Blood pressure
Systolic and diastolic blood pressure (SBP and DBP, respectively) were measured after 10 minutes of resting in a supine position using an automatic inflating blood pressure cuff (DINAMAP, GE Healthcare). SBP and DBP percentiles, corrected for age, sex, and height, were calculated according to 2004 National High Blood Pressure Education Working Group guidelines22.
Cardiorespiratory fitness
Peak oxygen uptake (VO2PEAK) was measured using a modified Bruce treadmill exercise test protocol23. Breath-by-breath oxygen consumption and carbon dioxide production were measured using a MedGraphics Ultima Series system, and processed and analyzed with the BreezeSuite software Version 6.4.023 (St. Paul, MN).
Urine collection and isoprostane analysis
Urine was collected and pooled from a 24 hr period and stored at −80°C until analysis. The major urinary metabolite of 15-F2t-IsoP, 2,3-dinor-5,6- dihydro-15-F2t-IsoP (2,3-dinor-5,6-dihydro-8-iso-PGF2α), was used as a marker of oxidative stress. The metabolite (F2-isoprostane) was measured by gas chromatography/negative ion chemical ionization mass spectrometry, as previously reported in detail24. Precision of the assay is ±4%, accuracy is 97%, and the lower limit of sensitivity is approximately 20 pg24. F2- isoprostane concentrations were normalized to urinary creatinine measured using Sirrus Clinical Chemistry analyzer (Stanbio Laboratory, Boerne, TX).
Plasma collection and measurements
Fasting blood samples were collected and plasma was separated by centrifugation and stored at −80°C. Plasma triglycerides, total cholesterol, low- density lipoprotein (LDL), and high-density lipoprotein (HDL) concentrations were measured using enzymatic kits from Cliniqa Corp. (San Marcos, CA). Free fatty acids were measured using the NEFA-C kit by Wako (Nneuss, Germany) and by gas chromatography. Glucose was measured using the Vitros Chemistry analyzer. Insulin and leptin measurements were performed using RIAs. Adiponectin analysis was done using a kit from Millipore (Billerica, MA) and Luminex multiplexing technology. Homeostatic model assessment for insulin resistance (HOMA-IR) was calculated using fasting glucose and insulin measures (HOMA IR = fasting glucose (mmol/L) × fasting insulin (μU/mL)/22.5)25.
Statistical analysis
Descriptive statistics were calculated as the mean with standard deviation for continuous variables. For categorical variables, frequency and percentage were presented. ANOVA and t-test were used to compare the continuous variables between groups. Categorical variables were tested using Pearson chi-square test. Partial correlations between F2-isoprostane and metabolic and anthropometric parameters were adjusted for age, gender, race, Tanner stage, and BMI (metabolic parameters only). Separate linear models, adjusted for age, gender, race, BMI and Tanner stage, were performed to assess the association between the F2-isoprostane data and the percent body fat, glucose, insulin, HOMA-IR, mean systolic and diastolic blood pressure, triglycerides, VO2PEAK, and leptin. Interaction terms of the clinical factor and race were included in all models. All continuous variables were modeled as linear trend due to the limited sample size. All tests were two-tailed, with a significance level of 5%. All statistical analyses were performed using open source R statistical software (version 2.13.0. Vienna, Austria).
Results
Personal characteristics
Seventy-six White American (55% male) and 82 African American (48% male) youth between the ages of 8 and 17 years participated in our study. BMI percentile distribution of the entire study group was 41% normal (<85th percentile), 17% overweight (≥85th percentile to <95th percentile), and 42% obese (≥95th percentile). There were no significant differences in the median height, weight, BMI percentile, BMI z-score, or percent truncal fat between White American and African American males and females (Table 1). Significant differences were seen in Tanner stage (p=0.020) and percent body fat (p=0.021) between White American and African American males and females (Table 1).
Table 1.
Comparison of baseline characteristics and anthropometric measurements.
| Female
|
Male
|
P-value | |||
|---|---|---|---|---|---|
| African American (n=43) | White American (n=34) | African American (n=39) | White American (n=42) | ||
| Age (years) | 13.6 ± 2.0 | 13.2 ± 2.4 | 13.4 ± 2.3 | 12.7 ± 2.4 | 0.415 a |
| Tanner Stage | 0.020 b | ||||
| 1 | 0% (0/43) | 6% (2/33) | 0% (0/37) | 5% (2/40) | |
| 2 | 14% (6/43) | 18% (6/33) | 35% (13/37) | 48% (19/40) | |
| 3 | 26% (11/43) | 30% (10/33) | 22% (8/37) | 10% (4/40) | |
| 4 | 26% (11/43) | 21% (7/33) | 27% (10/37) | 25% (10/40) | |
| 5 | 35% (15/43) | 24% (8/33) | 16% (6/37) | 12% (5/40) | |
| Height (cm) | 160.8 ± 7.1 | 157.5 ± 7.3 | 162.9 ± 13.3 | 160.0 ± 11.9 | 0.178 a |
| Weight (kg) | 67.3 ± 16.9 | 60.6 ± 19.0 | 68.7 ± 23.4 | 61.7 ± 20.0 | 0.200 a |
| BMI percentile | 81.2 ± 23.7 | 73.2 ± 30.6 | 82.1 ± 22.4 | 77.1 ± 25.8 | 0.419 a |
| BMI z-score | 1.2 ± 1.0 | 0.9 ± 1.2 | 1.3 ± 1.0 | 1.1 ± 1.0 | 0.383 a |
| Body fat (% total mass) | 33.8 ± 10.4 | 34.9 ± 10.6 | 28.4 ± 12.4 | 28.2 ± 12.9 | 0.021 a |
| Truncal fat (% total mass) | 14.6 ± 5.8 | 15.2 ± 7.1 | 12.4 ± 6.6 | 12.2 ± 7.0 | 0.117 a |
Data are presented as mean and standard deviation or percentage and number.
Differences between values measured by ANOVA for continuous variables (a) or Pearson chi-square test for categorical variables (b).
Oxidative stress
Urinary F2-isoprostane concentrations were used as a biomarker of oxidative stress. There was a significant correlation between F2-isoprostane concentrations and age (r= − 0.232, p=0.005). There was no significant difference in the median F2-isoprostane concentrations between White American and African American groups (35.2 ± 18.3 and 32.0 ± 16.4 ng/mg creatinine, respectively; p=0.265) (Table 2). There were also no significant differences in the median F2-isoprostane concentrations of male and female participants (male=33.0 ± 18.9 ng/mg creatinine vs. female=34.2 ± 15.5 ng/mg creatinine, p=0.660; Supplementary Table 1).
Table 2.
Comparison of baseline measures of metabolic risk in Black and White youth by race.
| African American | White American | P-value | |
|---|---|---|---|
| Triglycerides (mg/dL) | 64.5 ± 36.2 | 75.8 ± 38.1 | 0.071 |
| FFA (mg/dL) | 11.6 ± 6.9 | 10.2 ± 4.1 | 0.163 |
| LDL (mg/dL) | 83.3 ± 25.8 | 86.0 ± 30.3 | 0.560 |
| HDL (mg/dL) | 52.8 ± 14.2 | 48.4 ± 14.1 | 0.066 |
| SBP (mmHg) | 115.4 ± 11.0 | 111.2 ± 10.2 | 0.022 |
| DBP (mmHg) | 66.2 ± 5.8 | 63.3 ± 5.4 | 0.003 |
| Glucose (mg/dL) | 99.9 ± 17.8 | 99.1 ± 16.5 | 0.774 |
| Insulin (μU/mL) | 31.6 ± 25.9 | 25.4 ± 15.3 | 0.097 |
| HOMA-IR | 8.1 ± 7.9 | 6.3 ± 4.1 | 0.124 |
| VO2PEAK (mL/kg/min) | 30.5 ± 7.3 | 37.2 ± 11.1 | <0.001 |
| Leptin (ng/mL) | 17.6 ± 14.3 | 15.4 ± 14.8 | 0.375 |
| Adiponectin (mcg/mL) | 23.9 ± 21.3 | 23.4 ± 15.3 | 0.888 |
| F2-isoprostane (ng/mg creatinine) | 32.0 ± 16.1 | 35.2 ± 18.3 | 0.265 |
Data are presented as mean and standard deviation.
Abbreviations: FFA, free fatty acids; LDL, low-density lipoprotein; HDL, high-density lipoprotein; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Differences between values in African Americans and White Americans as measured by t-test.
Metabolic risk factors and oxidative stress
Lipid profile
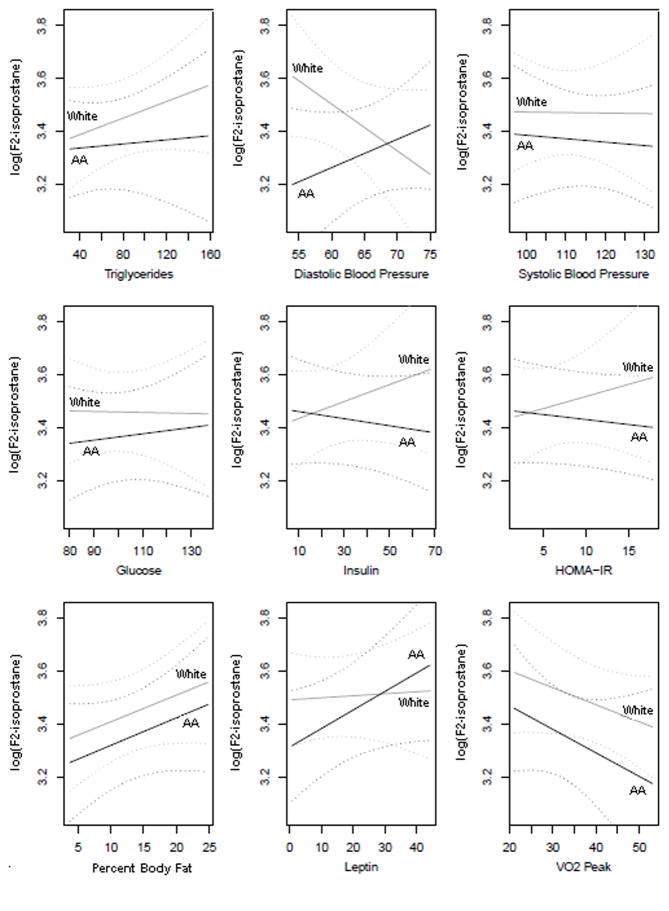
The median triglyceride concentration was lower in African American (64.5 ± 36.4 mg/dl) compared to White American (75.8 ± 38.1 mg/dl) youth, though not significantly (p=0.071, Table 2). There was no significant difference between African American and White American youth in LDL, HDL, or free fatty acid measures (Table 2). There were no significant correlations between triglyceride and F2- isoprostane measures (Table 3). When adjusted for gender, age, BMI and Tanner stage, there was no significant racial difference in the association between F2-isoprostane and triglyceride concentrations (p=0.548) (Figure 1-a).
Table 3.
Partial correlation of metabolic risk factors with oxidative stress (F2-isoprostane), adjusted for age, gender, race, Tanner stage, and BMI.
| Correlation (r) | P-value | |
|---|---|---|
| Triglycerides (mg/dL) (n=137) | 0.056 | 0.525 |
| FFA (mg/dL) (n=124) | −0.091 | 0.338 |
| HDL (mg/dL) (n=138) | −0.016 | 0.857 |
| SBP (mmHg) (n=132) | −0.034 | 0.708 |
| DBP (mmHg) (n=132) | −0.057 | 0.525 |
| Glucose (mg/dL) (n=136) | 0.015 | 0.891 |
| Insulin(μU/mL) (n=129) | −0.103 | 0.252 |
| HOMA-IR (n=126) | −0.127 | 0.161 |
| Leptin (n=127) | 0.002 | 0.985 |
| Adiponectin (mcg/mL) (n=127) | 0.013 | 0.889 |
| VO2PEAK(mL/kg/min) (n=140) | −0.163 | 0.059 |
Significant at p<0.05.
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure.
Figure 1.
Figure 1a–i. Linear regression of F2-isoprostane as a function of risk factors for metabolic syndrome (x-axis), adjusted for age, gender, and Tanner stage in African American (black solid line, labeled “AA”) and White American (gray solid line, labeled “White”) youth. Dashed lines represent 95% confidence intervals for African American (black dashed lines) and White American (grey dashed lines) youth.
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; HOMA-IR, homeostasis model assessment-insulin resistance; VO2PEAK, peak oxygen uptake.
* p<0.05
Blood pressure
Median systolic BP was significantly higher in African American (115.4 ± 11.0 mmHg) compared to White American (111.2 ± 10.2 mmHg)(p=0.022). Similarly, diastolic BP was also significantly higher in African American (66.2 ± 5.8 mmHg) compared to White American (63.3 ± 5.4 mmHg) (p=0.003) (Table 2). Neither systolic nor diastolic BP were significantly correlated with F2-isoprostane concentrations (Table 3). However, when adjusted for gender, age, BMI and Tanner stage, there was a significant racial difference in the association between F2-isoprostane concentrations and mean diastolic BP (p=0.047) (Figure 1-b). No racial difference was seen in the association between F2-isoprostane concentrations and mean systolic BP (p=0.884) (Figure 1-c).
Insulin sensitivity
There were no significant differences between White American and African American males and females in glucose, insulin, or HOMA-IR measures (Table 2). All insulin and HOMA-IR values were included in analyses, including outliers. Additionally, there were no significant correlations between F2-isoprostane and glucose, insulin, or HOMA-IR measures (Table 3). However, insulin significantly correlated with HOMA-IR (r=0.976, p<0.001) and leptin (r=0.265, p=0.002). When adjusted for gender, age, BMI and Tanner stage, no racial differences were seen in the association of F2-isoprostane concentrations with glucose (p=0.754), insulin (p=0.245), or HOMA-IR (p=0.341) (Figure 1-d, e, and f).
Measures of adiposity and oxidative stress
Percent body fat (r=0.175, p=0.041) and percent truncal fat (r=0.173, p=0.045) were significantly and positively correlated with F2-isoprostane concentrations. Correlation between BMI (r=0.090, p=0.288) or BMI z-score (r=0.120, p=0.153) with F2-isoprostane concentrations were not significant (Table 4). When adjusted for gender, age, and Tanner stage, no racial differences were seen in the association between F2-isoprostane concentrations and either percent truncal fat (p=0.976) or percent body fat (p=0.974) (Figure 1-g).
Table 4.
Partial correlation of body composition measures with oxidative stress (F2-isoprostane), adjusted for age, gender, race, and Tanner stage.
| Correlation (r) | P-value | |
|---|---|---|
| BMI (n=147) | 0.090 | 0.288 |
| BMI z-score (n=147) | 0.120 | 0.153 |
| Body fat (% total mass) (n=141) | 0.175 | 0.041* |
| Truncal fat (% total mass) (n=140) | 0.173 | 0.045* |
Significant at p<0.05.
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure.
Plasma leptin and adiponectin concentrations and oxidative stress
There were no significant differences in the median plasma leptin (p=0.375) or adiponectin (p=0.888) concentrations between White American and African American groups (Table 2). Plasma leptin in females was higher than in males (p <0.001, Supplementary Table 2). Unadjusted leptin concentrations demonstrated a significant, positive relationship with F2-isoprostane measures (r=0.231, p=0.009). However, correlation between F2-isoprostane and leptin adjusted for age, race, gender, Tanner stage, and BMI was non-significant (r=0.002, p=0.985) (Table 3). No racial differences were seen in the association between F2-isoprostane concentrations and leptin (p=0.222) (Figure 1-h).
There was no significant correlation between adiponectin and F2-isoprostane concentrations (r=0.013, p=0.889) When adjusted for gender, age, BMI and Tanner stage, no racial differences were seen in the association between F2-isoprostane concentrations and leptin (p=0.222) (Figure 1-h).
Cardiorespiratory fitness and oxidative stress
Average VO2PEAK was significantly lower in African Americans (30.5 ± 7.3 ml/kg/min) compared to White Americans (37.2 ± 11.1 ml/kg/min) (p<0.001, Table 2) and in females (30.6 ± 8.6 ml/kg/min) compared to males (37.0 ± 10.3 ml/kg/min) (p <0.001, Supplementary Table 1). VO2PEAK was not significantly correlated with F2-isoprostane concentrations (r= −0.163, p=0.059) (Table 3). When adjusted for gender, age, BMI and Tanner stage, no racial differences were seen in the association between F2-isoprostane concentrations and VO2PEAK (p=0.781) (Figure 1-i).
Discussion
In this study, we explored racial differences in oxidative stress and potential associations between oxidative stress and specific metabolic risk factors in healthy African American and White American youth. Our data reveal novel findings and confirm previous reports of a close relationship between oxidative stress and obesity in youth10–16. First, we found that F2-isoprostane concentrations were significantly and positively associated with total body fat and truncal fat in both African American and White American youth. Second, we did not observe significant differences in F2-isoprostane concentrations between African American and White American males and females when controlled for body fat content. Third, F2-isoprostane concentrations were associated significantly and positively with diastolic blood pressure in the African American but not in the White American youth. Fourth, triglycerides, systolic blood pressure, VO2PEAK, HOMA-IR, and BMI content were not significantly associated with F2-isoprostane concentration.
Cardiovascular disease, the leading cause of death in the US, is highly prevalent in African Americans 5. Plasma lipids are well known risk factors for cardiovascular disease, but there are considerable racial differences in lipid profiles between African Americans and White Americans. Multiple previous reports in adults26,27 and children28,29 have demonstrated significantly lower plasma triglycerides in African American compared to White American youth. While this difference did not reach significant in our study, the racial disparity may explain, at least in part, the lower than expected prevalence of the metabolic syndrome in African Americans 19,30. Clinical consequences of the more favorable plasma lipid profile in African American compared with White American youth found in our study are unclear and cannot be overstated. For example, it has been postulated that African Americans have a different lipid profile threshold for cardiovascular disease than White Americans, explaining, at least in part, well-documented ethnic disparity in cardiovascular disease prevalence in the US31.
In addition to the impact of lipid profiles, oxidative stress has also been linked with important cardiovascular risk factors, in particular hypertension32–34. Although it has been shown that increased oxidative stress is associated with overt hypertension in obese children, there is minimal evidence of its association with early, pre-clinical abnormalities of blood pressure. It has been documented that African American adults have a higher prevalence of hypertension compared to White American adults 5. The reason for the racial differences in our study is unclear, although the African American group had higher median body weight in the than White American group. However, previous research has demonstrated that elevations in SBP and DBP seen in African American compared to White American children were not explained by body composition35. Additionally, our small study population might have limited the detection of a racial difference in association of oxidative stress concentrations with other cardiometabolic risk factors. Other recent studies with homogenous study populations suggest the potential for such racial differences. For example, Kelly et al.11 found higher oxidative stress in children with metabolic syndrome. In a study of predominantly African American and Hispanic youth, Ostrow et al.17 found a significant association between oxidative stress and mean 24-hour systolic blood pressure, but not with other markers of metabolic syndrome. These results are in agreement with the results for African American youth found in present study. Although trends in racial differences in the association between oxidative stress and other cardiometabolic risk factors could be conceived from trends seen in our statistical models (Figure 1), studies with larger, heterogenous study populations are needed to further clarify the relationships.
Despite the lack of a clear link between body composition and blood pressure in children and adolescents, the association of adiposity with oxidative stress has been well documented. Previous studies in both youth and adults have demonstrated that obese individuals had elevated oxidative stress level when compared to normal weight persons and this association was further augmented by the presence of other risk factors associated with metabolic syndrome9–12. In present study we evaluated several measures of obesity and found that all measures obtained through DXA (percent whole body and truncal fat) were significantly and positively associated with F2-isoprostane concentration. This finding demonstrates that overall adiposity is associated with of oxidative stress more than either general measures of obesity based upon height and weight (i.e. BMI) in youth.
A major present clinical concern is that obesity-related risk factors appearing early in childhood are tracking into adulthood. Previous studies in lean and obese children have shown significant associations of urinary isoprostanes with carotid intima media thickness36, with isoprostanes considered an independent risk factor for coronary heart disease37. We did not measure the intima-media thickness and thus, could not speculate about the relationship of adiposity-induced increased oxidative stress and cardiovascular risk in our study population.
The study has several strengths. The collection of the urine for F2-isoprostane concentration, metabolic and cardiorespiratory measures, took place within the highly controlled environment of the indirect room calorimeter. Second, we used reference standard DXA measurements for estimations of adiposity. While waist circumference is often used as a proxy measure of truncal adiposity, DXA measures of truncal/abdominal fat mass are highly correlative with abdominal and visceral fat estimates obtained from CT scans 38. Third, we have a study sample with a wide range of age and BMI percentiles (from 4th to 99th percentiles for age and gender).
The study also has some limitations. First, it is a cross-sectional study limited to White American and African American youth, which does not enable us to determine whether there is a causative link among oxidative stress, adiposity, and metabolic risk factors. Second, the study would have benefited from measurement of water-soluble markers of early oxidation such as thiobarbituric acid reactive substances 39 or total antioxidant capacity 40,41. However, F2-isoprostane concentrations, which assess oxidation of lipids 42, have been shown to provide one of the most accurate assessments of oxidative stress status, and in turn, a more likely occurrence of endothelial dysfunction43,44 and increased risk of cardiovascular disease. Additionally, the F2-isoprostane data is reported per mg creatinine, which is linked to lean body mass, as opposed to fat mass, which is the key correlate of F2-isoprostane. Finally, we used HOMA-IR as a surrogate marker of insulin resistance in our population45. A reference standard hyperinsulinemic-euglycemic clamp method might have provided results that are more reliable, but the method is invasive, less practical, and costly in large studies. In addition, several previous studies have demonstrated acceptable correlation between HOMA-IR and the hyperinsulinemic-euglycemic clamp or IV glucose tolerance test46,47. In our study, HOMA-IR results were on average higher than reported in other studies in healthy youth48–50. A plausible explanation is that with higher than expected average insulin and HOMA-IR values, and with large standard deviations, it is likely some study participants did not comply fully with the prescribed overnight fast before the study visit.
In summary, oxidative stress, as measured by urinary F2-isoprostane concentrations, was positively associated with percent body fat and leptin in youth. Oxidative stress levels were similar among African American and White American youth. Among markers of the metabolic syndrome, a significant difference between African American and White American youth was demonstrated only in the association of oxidative stress with diastolic blood pressure.
Supplementary Material
What is already known about this subject
African Americans are disproportionately affected by obesity and other metabolic risk factors in comparison to White Americans.
Increasing prevalence of obesity has been associated with concomitant increases in childhood hypertension, dyslipidemia, and type 2 diabetes.
Oxidative stress is associated with obesity in both adults and children.
What this study adds
Oxidative stress is positively associated with total body fat and truncal fat, but not with BMI or BMI z-score in healthy youth.
Oxidative stress is associated with diastolic blood pressure in African American but not in White American healthy youth.
Acknowledgments
This work was supported by grants from the NIH (RO1HL082988) and the National Center for Research Resources, Grant UL1 RR024975-01, and is now at the National Center for Advancing Translational Sciences, Grant 2 UL1 TR000445-06. KC was supported by a Vanderbilt Research Training in Diabetes and Endocrinology grant (NIH T32 DK07061-35) and JW by a NIDDK training grant (NIH T32 DK007673-17). Additional support came from an NIH MERIT Award (GM42056) awarded to LJR. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health. Hormone, lipid, and isoprostane assays were performed in the core laboratories of the Vanderbilt Diabetes Research and Training Center supported by NIH grant DK20593. We would like to thank Stephane Daphnis, Denise Dunlap, Cindy Dorminy, Natalie Meade, Elizabeth Provenzano, and Daniel Short for their contributions to the acquisition of the data for this study.
Footnotes
Conflicts of interest
The authors have no conflicts of interest to disclose.
KC and JK contributed to study conception, design, and conduct of the experiments. LEW contributed to design and conduct of the experiments. LW contributed to data analysis and interpretation. JW contributed to data analysis and interpretation and writing of the article. SA and LJR contributed to design and data analysis and interpretation. MB contributed to the study conception, design, data analysis and interpretation, and revision of the article.
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006 Apr 5;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Freedman DS, Dietz WH, Srinivasan SR, Berenson GS. The relation of overweight to cardiovascular risk factors among children and adolescents: the Bogalusa Heart Study. Pediatrics. 1999 Jun;103(6 Pt 1):1175–1182. doi: 10.1542/peds.103.6.1175. [DOI] [PubMed] [Google Scholar]
- 3.Hannon TS, Rao G, Arslanian SA. Childhood obesity and type 2 diabetes mellitus. Pediatrics. 2005 Aug;116(2):473–480. doi: 10.1542/peds.2004-2536. [DOI] [PubMed] [Google Scholar]
- 4.Clark LT, El-Atat F. Metabolic syndrome in African Americans: implications for preventing coronary heart disease. Clin Cardiol. 2007 Apr;30(4):161–164. doi: 10.1002/clc.20003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cossrow N, Falkner B. Race/ethnic issues in obesity and obesity-related comorbidities. J Clin Endocrinol Metab. 2004 Jun;89(6):2590–2594. doi: 10.1210/jc.2004-0339. [DOI] [PubMed] [Google Scholar]
- 6.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005 Jun;54(6):1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 7.Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem. 2004 Oct 8;279(41):42351–42354. doi: 10.1074/jbc.R400019200. [DOI] [PubMed] [Google Scholar]
- 8.Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004 Dec;114(12):1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Guilder GP, Hoetzer GL, Greiner JJ, Stauffer BL, Desouza CA. Influence of metabolic syndrome on biomarkers of oxidative stress and inflammation in obese adults. Obesity. 2006 Dec;14(12):2127–2131. doi: 10.1038/oby.2006.248. [DOI] [PubMed] [Google Scholar]
- 10.Araki S, Dobashi K, Yamamoto Y, Asayama K, Kusuhara K. Increased plasma isoprostane is associated with visceral fat, high molecular weight adiponectin, and metabolic complications in obese children. Eur J Pediatr. 2010 Aug;169(8):965–970. doi: 10.1007/s00431-010-1157-z. [DOI] [PubMed] [Google Scholar]
- 11.Kelly AS, Steinberger J, Kaiser DR, Olson TP, Bank AJ, Dengel DR. Oxidative stress and adverse adipokine profile characterize the metabolic syndrome in children. J Cardiometab Syndr. 2006 Summer;1(4):248–252. doi: 10.1111/j.1559-4564.2006.05758.x. [DOI] [PubMed] [Google Scholar]
- 12.Oliver SR, Rosa JS, Milne GL, et al. Increased oxidative stress and altered substrate metabolism in obese children. Int J Pediatr Obes. 2010 Mar 17;5(5):436–444. doi: 10.3109/17477160903545163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sinaiko AR, Steinberger J, Moran A, et al. Relation of body mass index and insulin resistance to cardiovascular risk factors, inflammatory factors, and oxidative stress during adolescence. Circulation. 2005 Apr 19;111(15):1985–1991. doi: 10.1161/01.CIR.0000161837.23846.57. [DOI] [PubMed] [Google Scholar]
- 14.Stringer DM, Sellers EA, Burr LL, Taylor CG. Altered plasma adipokines and markers of oxidative stress suggest increased risk of cardiovascular disease in First Nation youth with obesity or type 2 diabetes mellitus. Pediatr Diabetes. 2009 Jun;10(4):269–277. doi: 10.1111/j.1399-5448.2008.00473.x. [DOI] [PubMed] [Google Scholar]
- 15.Ustundag B, Gungor S, Aygun AD, Turgut M, Yilmaz E. Oxidative status and serum leptin levels in obese prepubertal children. Cell Biochem Funct. 2007 Sep-Oct;25(5):479–483. doi: 10.1002/cbf.1334. [DOI] [PubMed] [Google Scholar]
- 16.Codoñer-Franch P, Boix-García L, Simó-Jordá R, del Castillo-Villaescusa C, Maset-Maldonado J, Valls-Bellés V. Is obesity associated with oxidative stress in children? Int J Pediatr Obes. 2010;5(1):56–63. doi: 10.3109/17477160903055945. [DOI] [PubMed] [Google Scholar]
- 17.Ostrow V, Wu S, Aguilar A, Bonner R, Jr, Suarez E, De Luca F. Association between oxidative stress and masked hypertension in a multi-ethnic population of obese children and adolescents. J Pediatr. 2011 Apr;158(4):628–633. e621. doi: 10.1016/j.jpeds.2010.09.081. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman RP. Metabolic syndrome racial differences in adolescents. Curr Diabetes Rev. 2009 Nov;5(4):259–265. doi: 10.2174/157339909789804332. [DOI] [PubMed] [Google Scholar]
- 19.Sumner AE. Ethnic differences in triglyceride levels and high-density lipoprotein lead to underdiagnosis of the metabolic syndrome in black children and adults. J Pediatr. 2009 Sep;155(3):S7, e7–11. doi: 10.1016/j.jpeds.2009.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CDC. National Health and Nutrition Examination: Anthropometry Procedures Manual. US Department of Health and Human Services, Centers for Disease Control and Prevention; Hyattsville, MD, USA: Aug, 2009. [Google Scholar]
- 21.Centers for Disease Control and Prevention, National Center for Health Statistics. CDC Growth Charts: United States. 2000 May 30; http://www.cdc.gov/growthcharts/
- 22.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004 Aug;114(2 Suppl):555–576. 4th Report. [PubMed] [Google Scholar]
- 23.Bruce RA. Exercise testing of patients with coronary heart disease. Principles and normal standards for evaluation. Ann Clin Res. 1971 Dec;3(6):323–332. [PubMed] [Google Scholar]
- 24.Morrow JD, Zackert WE, Yang JP, et al. Quantification of the Major Urinary Metabolite of 15-F2t-Isoprostane (8-iso-PGF2α) by a Stable Isotope Dilution Mass Spectrometric Assay. Anal Biochem. 1999;269(2):326–331. doi: 10.1006/abio.1999.4008. [DOI] [PubMed] [Google Scholar]
- 25.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985 Jul;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 26.Donahue R, Jacobs DR, Jr, Sidney S, Wagenkneckt L, Albers J, Hunley S. Distribution of lipoproteins and apolipoproteins in young adults: The CARDIA Study. Arteriosclerosis. 1989;9(5):656–664. doi: 10.1161/01.atv.9.5.656. [DOI] [PubMed] [Google Scholar]
- 27.Sumner A, Cowie CC. Ethnic differences in the ability of triglyceride levels to identify insulin resistance. Atherosclerosis. 2008;196(2):696–703. doi: 10.1016/j.atherosclerosis.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 28.Srinivasan SR, Frerichs R, Webber L, Berenson GS. Serum lipoprotein profile in children from a biracial commnity: the Bogalusa Heart Study. Circulation. 1976;54(2):309–318. doi: 10.1161/01.cir.54.2.309. [DOI] [PubMed] [Google Scholar]
- 29.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a Metabolic Syndrome Phenotype in Adolescents: Findings From the Third National Health and Nutrition Examination Survery, 1988–1994. Arch Pediatr Adolesc Med. 2003;157(8):821–827. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 30.Yu SSK, Castillo DC, Courville AB, Sumner AE. The Triglyceride Paradox in People of African Descent. Metab Syndr Relat D. 2012 Apr;10(2):77–82. doi: 10.1089/met.2011.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stein E, Kushner H, Gidding S, Falkner B. Plasma lipid concentrations in nondiabetic African American adults: associations with insulin resistance and the metabolic syndrome. Metabolism. 2007 Jul;56(7):954–960. doi: 10.1016/j.metabol.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodrigo R, Prat H, Passalacqua W, Araya J, Guichard C, Bachler J. Relationship between Oxidative Stress and Essential Hypertension. Hypertens Res. 2007;30:1159–1167. doi: 10.1291/hypres.30.1159. [DOI] [PubMed] [Google Scholar]
- 33.Grossman E. Does increased oxidative stress cause hypertension? Diabetes Care. 2008;31(Suppl 2):S185–189. doi: 10.2337/dc08-s246. [DOI] [PubMed] [Google Scholar]
- 34.Ceriello A. Possible role of oxidative stress in the pathogenesis of hypertension. Diabetes Care. 2008;31(Suppl 2):S181–184. doi: 10.2337/dc08-s245. [DOI] [PubMed] [Google Scholar]
- 35.Cruz ML, Huang TT, Johnson MS, Gower BA, Goran MI. Insulin sensitivity and blood pressure in black and white children. Hypertension. 2002 Jul;40(1):18–22. doi: 10.1161/01.hyp.0000019972.37690.ef. [DOI] [PubMed] [Google Scholar]
- 36.Giannini C, de Giorgis T, Scarinci A, et al. Increased carotid intima-media thickness in pre-pubertal children with constitutional leanness and severe obesity: the speculative role of insulin sensitivity, oxidant status, and chronic inflammation. Eur J Endocrinol. 2009;161(1):73–80. doi: 10.1530/EJE-09-0042. [DOI] [PubMed] [Google Scholar]
- 37.Schwedhelm E, Bartling A, Lenzen H, et al. Urinary 8-iso-prostaglandin F2alpha as a risk marker in patients with coronary heart disease: a matched case-control study. Circulation. 2004;109(7):843–848. doi: 10.1161/01.CIR.0000116761.93647.30. [DOI] [PubMed] [Google Scholar]
- 38.Clasey JL, Bouchard C, Teates CD, et al. The use of anthropometric and dual-energy X-ray absorptiometry (DXA) measures to estimate total abdominal and abdominal visceral fat in men and women. Obes Res. 1999 May;7(3):256–264. doi: 10.1002/j.1550-8528.1999.tb00404.x. [DOI] [PubMed] [Google Scholar]
- 39.Yagi K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem Med. 1976 Apr;15(2):212–216. doi: 10.1016/0006-2944(76)90049-1. [DOI] [PubMed] [Google Scholar]
- 40.Davalos A, Gomez-Cordoves C, Bartolome B. Extending applicability of the oxygen radical absorbance capacity (ORAC-fluorescein) assay. J Agric Food Chem. 2004 Jan 14;52(1):48–54. doi: 10.1021/jf0305231. [DOI] [PubMed] [Google Scholar]
- 41.Apak R, Guclu K, Ozyurek M, Bektasoglu B, Bener M. Cupric ion reducing antioxidant capacity assay for antioxidants in human serum and for hydroxyl radical scavengers. Methods Mol Biol. 2010;594:215–239. doi: 10.1007/978-1-60761-411-1_15. [DOI] [PubMed] [Google Scholar]
- 42.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts Lj., II A Series of Prostaglandin F2-Like Compounds are Produced in vivo in Humans by a Non-Cyclooxygenase, Free Radical-Catalyzed Mechanism. PNAS. 1990;87(23):9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milne GL, Yin H, Hardy KD, Davies SS, Roberts LJ. Isoprostane Generation and Function. Chem Rev. 2011;111(10):5973–5996. doi: 10.1021/cr200160h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kadiiska MB, Gladen BC, Baird DD, et al. Biomarkers of Oxidative Stress Study II: Are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic Biol Med. 2005;38(6):698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 45.Shaibi GQ, Davis JN, Weigensberg MJ, Goran MI. Improving insulin resistance in obese youth: choose your measures wisely. Int J Pediatr Obes. 2011 Jun;6(2–2):e290–296. doi: 10.3109/17477166.2010.528766. [DOI] [PubMed] [Google Scholar]
- 46.Gungor N, Saad R, Janosky J, Arslanian S. Validation of surrogate estimates of insulin sensitivity and insulin secretion in children and adolescents. J Pediatr. 2004 Jan;144(1):47–55. doi: 10.1016/j.jpeds.2003.09.045. [DOI] [PubMed] [Google Scholar]
- 47.Keskin M, Kurtoglu S, Kendirci M, Atabek ME, Yazici C. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics. 2005 Apr;115(4):e500–503. doi: 10.1542/peds.2004-1921. [DOI] [PubMed] [Google Scholar]
- 48.d’Annunzio G, Vanelli M, Pistorio A, et al. Insulin resistance and secretion indexes in healthy Italian children and adolescents: a multicentre study. Acta Biomed. 2009 Apr;80(1):21–28. [PubMed] [Google Scholar]
- 49.Conwell LS, Trost SG, Brown WJ, Batch JA. Indexes of insulin resistance and secretion in obese children and adolescents: a validation study. Diabetes Care. 2004 Feb;27(2):314–319. doi: 10.2337/diacare.27.2.314. [DOI] [PubMed] [Google Scholar]
- 50.Adam TC, Hasson RE, Lane CJ, et al. Fasting indicators of insulin sensitivity: effects of ethnicity and pubertal status. Diabetes Care. 2011 Apr;34(4):994–999. doi: 10.2337/dc10-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.