Abstract
Objective
Seizures have been implicated as a cause of secondary brain injury, but the systemic and cerebral physiologic effects of seizures after acute brain injury are poorly understood.
Methods
We analyzed intracortical EEG and multimodality physiological recordings in 48 comatose subarachnoid hemorrhage patients to better characterize the physiological response to seizures after acute brain injury.
Results
Intracortical seizures were seen in 38% of patients and 8% had surface seizures. Intracortical seizures were accompanied by elevated heart rate (P=0.001), blood pressure (P<0.001), and respiratory rate (P<0.001). There were trends for rising cerebral perfusion pressure (P=0.03) and intracranial pressure (P =0.06) seen after seizure onset. Intracortical seizure associated increases in global brain metabolism, partial brain tissue oxygenation, and regional cerebral blood flow (rCBF) did not reach significance, but a trend for a pronounced delayed rCBF rise was seen for surface seizures (P=0.08). Functional outcome was very poor for patients with severe background attenuation without seizures and best for those without severe attenuation or seizures (77% vs. 0% dead or severely disabled, respectively). Outcome was intermediate for those with seizures independent of the background EEG and worse for those with intracortical only seizures when compared to those with intracortical and scalp seizures (50% and 25% death or severe disability, respectively).
Interpretation
We replicated in humans complex physiologic processes associated with seizures after acute brain injury previously described in laboratory experiments and illustrated differences such as the delayed increase in regional cerebral blood flow. These real-world physiologic observations may permit more successful translation of laboratory research to the bedside.
Keywords: electrographic seizures, nonconvulsive seizures, subarachnoid hemorrhage, multimodality monitoring, continuous EEG monitoring, brain metabolism
INTRODUCTION
Acute brain injuries are common and a significant public health issue. While more patients now survive the acute event due to advances in critical care and neurosurgical techniques, functional outcome is driven to a large extent by secondary complications such as brain swelling, inflammation, and seizures, most of which are potentially amenable to therapy.1,2 Nonconvulsive seizures (NCSz) are frequent3,4, associated with indicators of secondary brain injury4–7 and poor outcome, particularly after subarachnoid hemorrhage (SAH).4,5,7,8 Controversy about underlying mechanisms and consequences of NCSz prevails; although most believe they are potentially harmful in acute brain injury, some have suggested that NCSz are an epiphenomenon of deafferented cortex9 or a surrogate marker for the extent of brain damage.10,11 Treatment in form of antiepileptics is available but carries risks.12,13
Animal models of neocortical seizures have demonstrated a strain on metabolic resources of the cortex that may result in inadequate perfusion14–17 and lead to shunting of blood from surrounding brain regions to the seizure focus.16 It is unclear if similar mechanisms are at play in seizures following brain injury in humans as baseline metabolism is altered,18–20 waves of spreading depolarization, spreading ischemia, and spreading convulsions are frequent,21,22 and vasoreactivity is frequently abnormal.23 Further, impairment of the autonomic nervous system24 including tachycardia25 and tachypnea26 are common after acute brain injury, which may impair typically observed compensatory responses for seizures such as those seen in epilepsy patients.27
Studying systemic and cerebral physiologic effects of seizures after acute brain injury in humans has proven to be difficult due to notoriously poor signal to noise ratios in the intensive care unit (ICU).28,29 The purpose of the current study is to illustrate the potential of investigating real-world human physiology after acute brain injury obtained in an ICU. Here we test if intracortical seizures30 after SAH are associated with physiologic changes seen in animal models and if isolated intracortical_seizures are associated with similar physiologic responses as scalp seizures. We will investigate this by applying computational techniques to systemic and invasive brain monitoring data collected in patients with aneurysmal SAH. These insights may allow better understanding of mechanisms underlying secondary brain injury from seizures in humans, potentially help identify subjects that would benefit from prophylactic interventions (i.e. choice of anesthetic or seizure prophylaxis), and estimate differences between bench and bedside pathophysiology leading to more realistic and ultimately successful clinical trials.
MATERIALS AND METHODS
Study Population
We studied all poor-grade aneurysmal SAH-patients admitted to the neurological ICU at Columbia University Medical Center between June 2006 and May 2011 that underwent invasive brain multimodality monitoring including minidepth EEG as part of their routine clinical care following our institutional protocol.30,31 Multimodality monitoring was initiated in comatose patients with a Glasgow Coma Scale of ≤8 if (1) patients were unlikely to regain consciousness within the following 48 hours, and (2) had a high probability to survive for the next 48 hours. This decision was made by the attending neurointensivist and head neurosurgeon. The diagnosis of SAH was established by computed tomography (CT) or xanthochromia of the cerebrospinal fluid if the CT was negative.32 Patients were not enrolled in this observational cohort study if any of the following were met (1) age <18 years old, (2) pregnant, or (3) patients or families did not want to participate in the study. Patients with clinical seizures or NCSz prior to or at the start of invasive monitoring were excluded from the analysis. Data were collected as part of an ongoing prospective database approved by the local Institutional Review Board and following recently published recommendations for core data element collection.33
Multimodality monitoring
According to our protocol invasive neuromonitoring includes measurements of intracranial pressure (Integra Neurosciences Inc, Plainsborough, NJ), interstitial cerebral microdialysis (CMA-70 microdialysis catheter™ [20 kDa pores], analyzed for lactate, pyruvate, and glucose using the CMA-600™, CMA Inc, Stockholm, Sweden; metabolic crisis was defined as lactate pyruvate ratio [LPR] > 40 and brain glucose < 0.7mmol/L), partial brain tissue oxygenation (PbtO2) and brain temperature (using a flexible polarographic Licox Clark-type probe; LICOX™, Integra Neurosciences Inc, Kiel, Germany), regional cerebral blood flow (Bowman Perfusion Monitor™, Hemedex Inc, Cambridge, MA). Together with these invasive monitoring probes we placed an EEG minidepth electrode (eight-contact Spencer depth electrode™, ADTech Inc, Racine, WI; with 2.2 mm center-to-center intercontact spacing, contact width 1.32 mm, 0.9 mm spacing between electrodes).30,31 This commercially available electrode is designed for clinical intracranial EEG recording and is placed at the bedside details of the placement have been described in detail in earlier publications.30,31
Monitoring probes were placed ipsilateral to the aneurysm in patients that underwent aneurysm coiling and those with focal structural lesions. In patients that underwent aneurysm clipping, probes were placed contralateral to the bone flap31 as soon as possible after securing the aneurysm, usually within 2 days of the bleed. All intracranial monitoring devices were placed at the bedside in the ICU and affixed with a bolt (for details on technical aspects of placement please refer to prior our publication.30 Minidepth electrodes were placed to span the cortical ribbon with the goal of having one electrode in the skull, 2–3 in the cortical grey matter, and the remaining 4–5 electrodes in the white matter. Location of monitoring probes was confirmed by CT-scan immediately after the procedure. After removal of the monitoring probes patients underwent CT scanning and a subset of them also received a brain MRI for clinical purposes.
Jugular venous bulb catheters (PediaSat Oximetry catheter™, Edwards Life Science Inc, Irvine, CA, USA) were generally placed into the right internal jugular vein to record jugular bulb oxygen saturation [SjvO2]. Cardiovascular parameters were obtained from the arterial and central venous line catheters and included blood pressure (systolic, diastolic, mean arterial pressure), and heart rate. Respiratory parameters such as respiratory rate and minute ventilation were obtained directly from the ventilator (840-Puritan Bennett™, Nellcor Puritan Bennett Inc, Boulder, CO, USA) and the endtidal CO2 (EtCO2) from an infrared capnometer (Respironics™, Koninklijke Philips Electronics Inc, NV, USA). Body temperature was measured using a bladder temperature probe (Bardex™, Bard Inc, Covington, GA, USA). Scalp EEG was recorded using 21 standard scalp disk electrodes placed according to the International 10–20 system, affixed with collodion.3
General Management
Medical and surgical treatment followed guidelines by the American Heart Association34 and existing management protocols at most large medical centers.1 Patients received mechanical ventilation, external ventricular drainage, daily interruption of sedation, prophylactic oral nimodipine, and intravenous hydration according to a standardized management protocol.1 All patients were on prophylactic phenytoin for one week after SAH which was then discontinued unless seizures had been recorded on scalp EEG. Isolated scalp seizures were initially treated with levetiracetam and status epilepticus with midazolam infusions.35 Periodic epileptiform discharges (PEDs) were not considered seizures and there was no attempt to eliminate them with medications; however, patients with PEDs were maintained on anticonvulsants to prevent seizures. Anticonvulsants were not altered for isolated minidepth electrode findings.30
Data collection
All digital physiologic data was acquired using a high-resolution acquisition system (BedmasterEX™; Excel Medical Electronics Inc) at a sampling frequency of every 5 seconds from General Electric Solar 8000i monitors and inserted into a Microsoft SQL database. EEG was recorded using a digital video EEG bedside monitoring system (XLTEK™, Oakville, ON; low-pass filter 70 Hz, high-pass 0.1 Hz, sampling rate 200 Hz). Additionally we collected a large number of clinical variables including demographics, disease specific variables (i.e., aneurysm size), monitoring probe location, laboratory values, neurological examination findings, medications, hospital complications, and three months functional outcome data as part of an ongoing, prospective observational SAH outcomes study.32
EEG classification
Each minute of EEG was classified into one of three categories after visual inspection by an experienced electroencephalographer:36,37 (1)“ictal” defined as any spikes, sharp-waves, or sharp-and-slow wave complexes lasting for 10 seconds or more at either a frequency of at least 3 per second or a frequency of at least 1 per second with clear evolution in frequency, morphology, or location, (2)“ictal-interictal continuum” (IIC) if repetitive generalized or focal spikes, sharp-waves, spike-and-wave or sharp-and-slow wave complexes lasted for 10 seconds or more with a frequency between 1 and 3 per second without clear evolution in frequency, morphology, or location, and (3) “non-ictal” if neither conditions for category (1) or category (2) were met. All scores were recorded separately for surface and minidepth EEG recordings. Each minute of EEG was evaluated by one of the investigators (JC) blinded to the clinical course of the patient. Any minute of EEG that was controversial was independently evaluated by a second experienced electroencephalographer (LJH) and scores were based on consensus.
Event definition
EEGs often fluctuate between ictal and non-ictal patterns after acute brain injury.36 To maximize the chance of detecting physiologic changes we aimed to identify new-onset seizures defined as lasting at least 5 minutes and preceded by 30 minutes without any seizure activity. We then determined the exact second that each one of these new-onset seizure events started and merged EEG measures with physiologic measurements.
Quantitative EEG parameters were calculated and visualized using commercially available software (MATLAB™, The Mathworks Inc and Magic Marker Insight™, Persyst Inc, Prescott AZ, USA). EEG clips between 30 minutes before and 30 minutes after intracortical seizure onset were generated (Supplementary Figure 1, Panel A and B). Spectrograms representing the power in each frequency bin between 0 and 20 Hz were calculated based on Fast Fourier Transform (FFT) analysis for every 4-second epoch of these 60-minute clips. The following procedure was then applied to transform the resulting spectrograms of individual intracortical seizures into grouped averages (Supplementary Figure 1, Panel C): (1) a ratio was calculated between the measurement in each individual frequency bin and the average for that particular frequency bin over the entire 60 minute time series to account for inter-patient and intra-event differences in overall power, (2) these normalized frequency change scores were then averaged in between events to generate group averages for all intracortical seizures (Supplementary Figure 1, Panel C).
Physiologic data preparation
Clinically recorded physiological data may have gaps due to device malfunction or loss of connectivity between the recording and data storage location. For analysis purposes we inferred inter-observation values with linear interpolation.38 Outlier removal can be challenging since brief deviations from the mean may be the very signal that needs to be detected. We first removed physiologically not plausible outliers (i.e., MAP of 0)28 and extracted the raw data for all monitoring values for a 60 minute time window surrounding intracortical seizure onset (Supplementary Figure 1, Panel D). We then normalized all monitoring parameters to individual patient averages for this window and represented the time series as deltas from individual patient means (Supplementary Figure 1, Panel E). We then employed an additional step for outlier removal based on Chauvenet’s criterion39 to account for the distribution of a physiologic variable at a specific time relative to seizure onset (Supplementary Figure 1, Panel F).
Statistical Analysis
Once filtered, time series windows for all measurements were aggregated and the mean and standard error were estimated based on a bootstrap procedure (Supplementary Figure 1, Panel G). To evaluate the significance of these changes from baseline, we constructed a permutation test where we resampled by patient and evaluated a Monte Carlo estimate of the significance level.40–42 The null distribution for this permutation test considers the time at which seizure onset occurs for all seizures to be random while preserving the number of seizures observed per patient. The test statistic was the area under the standard-deviation weighted mean time series for all time points in the window. This approach is a slight modification of lagged linear correlation.43 Building on animal experiments14–17,44, we tested the hypotheses that changes observed in each of the variables surrounding seizure onset are different from chance and specifically that NCSz are associated with increases in heart rate (HR), mean arterial pressure (MAP), respiratory rate (RR), minute ventilation (MV), intracranial pressure (ICP), cerebral perfusion pressure ([CPP] calculated by subtracting ICP from MAP), global brain metabolism (indicated by a drop in the jugular venous oxygen saturation [SjvO2]), increase in regional cerebral blood flow (rCBF), and brain tissue hypoxia (indicated by a drop in the partial brain tissue oxygenation [PbtO2]). To determine the significance of isolated intracortical seizures we compared events with and without IIC or seizures on scalp recordings. Comparisons between baseline and follow-up microdialysis measurements as well as between seizure subgroups were made using generalized-estimating-equations with an autoregressive (AR-1) working correlation matrix.
Interrater reliability testing of EEG coding was performed by calculating weighted Kappa scores45 on a random sample of 127 one-minute surface and minidepth EEG clips by comparing scores of two study physicians (JC, LJH).
All analyses were made using commercially available software (Python™, Python Software Foundation, Wolfeboro Falls, NH; R, Institute for Statistics and Mathematics, Vienna; SPSS 18, SPSS Inc., Chicago, IL; MATLAB, The MathWorks Inc, Natick, MA). After Bonferroni correction (13 experiments) only P-values <0.0038 were considered statistically significant resulting in a family wise error rate of 5%.
RESULTS
Between June 2006 and May 2011, 434 aneurysmal SAH patients were screened for possible enrollment and 344 did not meet inclusion criteria (238 with Glasgow Coma Scale score >8, 51 that were predicted to be dead and 37 to have woken up within 48 hours of admission, 18 had severe coagulopathy). From 90 poor grade SAH patients that were eligible, 48 received invasive brain monitoring including minidepth EEG (26 did not based on surrogate decisions, and 16 had invasive brain monitoring without minidepth EEG). Baseline characteristics of eligible patients that did and those that did not undergo monitoring were similar (Table 1).
Table 1.
Follow up bias analysis amongst patients eligible for invasive brain monitoring (N=90).
| Minidepth EEG (N=48) |
No minidepth EEG (N=42) |
|
|---|---|---|
| Demographics | ||
| Age [years] | 53+/−15 | 53+/−15 |
| Female | 31 (65) | 30 (71) |
| White | 17 (35) | 15 (36) |
| Admission findings | ||
| Hunt Hess score | 4 (3, 5) | 4 (2, 5) |
| APACHE 2 score | 21+/−7 | 22+/−8 |
| SAH sum score | 19+/−8 | 15+/−11 |
| IVH | 4+/−4 | 4+/−4 |
| Global cerebral edema | 38 (83) | 34 (74) |
| Aneurysm treatment | ||
| Aneurysm clipping | 32 (67) | 20 (49) |
| Aneurysm coiling | 10 (21) | 14 (33) |
| Not protected* | 6 (13) | 8 (19) |
| Hospital course | ||
| Delayed cerebral ischemia | 13 (28) | 12 (30) |
| Worst Hunt Hess | 5 (4, 5) | 5 (4, 5) |
| Functional outcome at 3 months | ||
| Modified Rankin score | 4.5 (1.5, 6.0) | 5.0 (2.0, 6.0) |
| Dead or severely disabled | 17 (50) | 18 (51) |
Data represented as N (%), mean +/− SD or medians (IQR);
did not undergo angiography, were angiographic negative, or were left unprotected. None of the comparisons were significantly different.
Data collection
Monitoring probes were placed on median post SAH day 2 (IQR 2, 3), in half of the patients into the right and the other half into the left frontal lobe (median distance between the tip of the minidepth electrode and the inner table of the skull was 14.5mm [IQR 12.5, 16.5]; monitoring probes were placed ipsilateral to the craniotomy site in 8% (N=4) and ipsilateral to the aneurysm site in 19% of cases (N=9). Seizure frequency did not differ by laterality of probe placement. We coded each minute of a total of 376 days of intracortical EEG into 3 categories: ictal, ictal interictal continuum (IIC), or non-ictal. Intracortical seizures were seen in 38% (N=18) of patients with 12,894 minutes of cumulative seizure duration. Scalp seizures were seen in 8% (N=4) with 3,444 minutes of cumulative seizure duration. New onset depth seizures lasted a median of 51 minutes (IQR 17, 125) with only 3% (N=2) lasting 5 minutes and 77% (N=59) lasting 15 minutes or more. Interrater reliability scores were very good for surface and minidepth EEG recordings (weighted kappa scores 0.90 and 0.80, respectively).
Physiologic profiles of intracortical seizures
We identified 77 new onset intracortical seizures (Figure 1, Panels A to C) and created physiologic profiles for recorded variables in a 60-minute time window surrounding intracortical seizure onset. Microdialysis measurements did not indicate pre seizure brain tissue hypoxia or metabolic crisis (Figure 1, Panel D). Baseline values for monitored variables at the time of seizure onset were (median, IQR): HR 74 beats/minute (69,80), MAP 105 mmHg (94,120), RR 17 breaths/minute (14,22), MV 7.7 l/minute (7.2,9.3), EtCO2 32 mmHg (28,36), ICP 5 mmHg (2,9), CPP 100 mmHg (91,113), SjvO2 84 % (82,89), rCBF 31 ml/g/minute (18,37), PbtO225 mmHg (13,33), body temperature 37.5 °C (37.1,37.8), and brain temperature 37.0 °C (36.5,37.7). Prior to intracortical seizures, 70% (N=54) of patients were on propofol, 48% on fentanyl (N=37), and 7% on dexmethomedine (N=5).
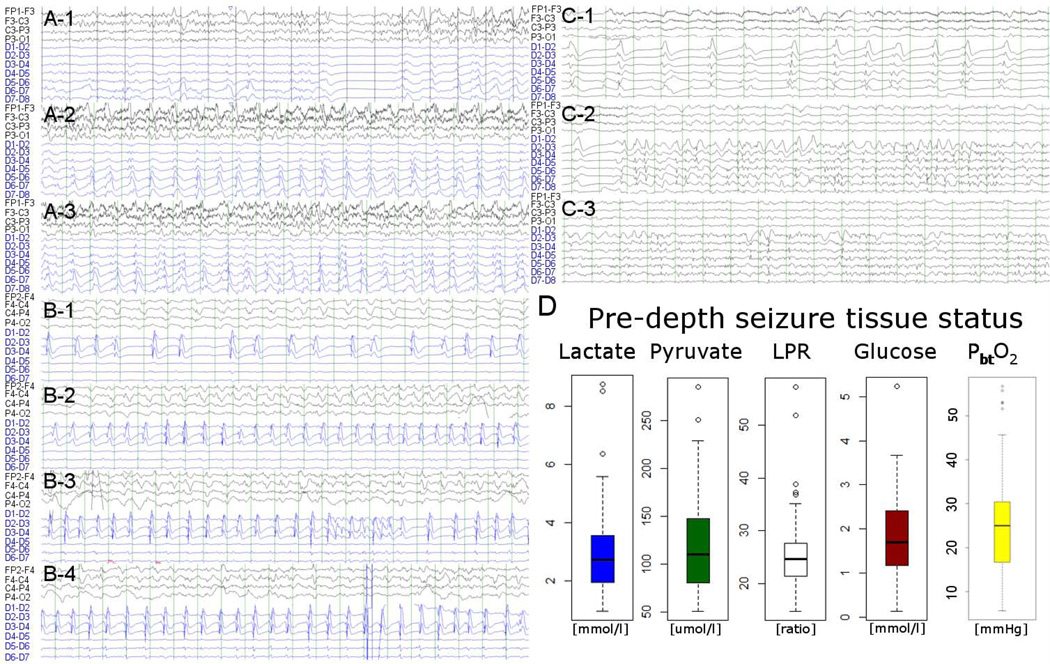
Figure 1.
Three illustrative intracortical seizure cases (A through C) are displayed (ipsilateral scalp EEG each in the top 4 and minidepth EEG in the bottom 6 to 7 channels; all bipolar montage). Case A intracortical seizure with surface seizure: 54 year old woman with poor grade SAH (Hunt Hess 5, APACHE-2 27), that underwent clipping of an anterior communicating artery aneurysm. Case B intracortical seizure with ictal-interictal continuum surface recording: 47 year old man with poor grade SAH (Hunt Hess 5, APACHE-2 14), that underwent clipping of a right anterior cerebral artery aneurysm. Case C intracortical seizure with non-ictal patterns on surface recordings: 57 year old woman with poor grade SAH (Hunt Hess 4, APACHE-2 17), that underwent clipping of an anterior communicating artery aneurysm. Right lower panel shows baseline tissue status based on microdialysis and partial brain tissue oxygenation averaged for all patients over 60 minutes prior to intracortical seizure onset indicating an overall non-ischemic state (median glucose 1.7mmol/L [IQR 1.2–2.4], pyruvate 110umol/L [IQR 80–148], lactate 2.8mmol/L [IQR 1.9–3.6], LPR 25 [IQR 21–28], PbtO2 24.4mmHg [IQR 4.7–63.0]). Only two events were preceded by an LPR >40 and 9 had a cerebral glucose of 0.7 of below, no event was preceded by metabolic crisis (LPR > 40 and brain glucose < 0.7mmol/L).
Intracortical seizures were associated with an increase in HR (P=0.001), MAP (P<0.001), RR (P<0.001), and MV (P=0.002; Figure 2). A trend for higher CPP (P=0.03) and ICP (P=0.06) was seen. Increases in global brain metabolism (SjvO2 drop), transient drops in PbtO2 (Figure 3), and delayed increases in rCBF did not reach significance. There was no change in hourly microdialysis measurements of lactate, pyruvate, glucose, or LPR in relation to seizure onset (Supplementary Figure 2). 32% of depth seizures were associated with increase in HR by more than 20 beats/minute when comparing the 30 minute median value prior to the depth seizure onset to the maximum value in the 30 minutes after seizure onset, 52% an increase in MAP of >20 mmHg, 72% an increase in RR of >10 breaths/minute, and 71% an increase in MV of >2.0 l/minute. Amongst the intracranial monitoring parameters ICP elevations >5 mmHg were seen in 64% and >10 mmHg in 28% of seizures, CPP elevations >10 mmHg were seen in 88% and >20 mmHg in 43%, SjvO2, dropped in 33% by >10% and none >20%, PbtO2 dropped in 28% by >5 mmHg and 13% by >10 mmHg, and rCBF increased by >10 ml/g/minute in 55% and >20 ml/g/minute in 36% of events.
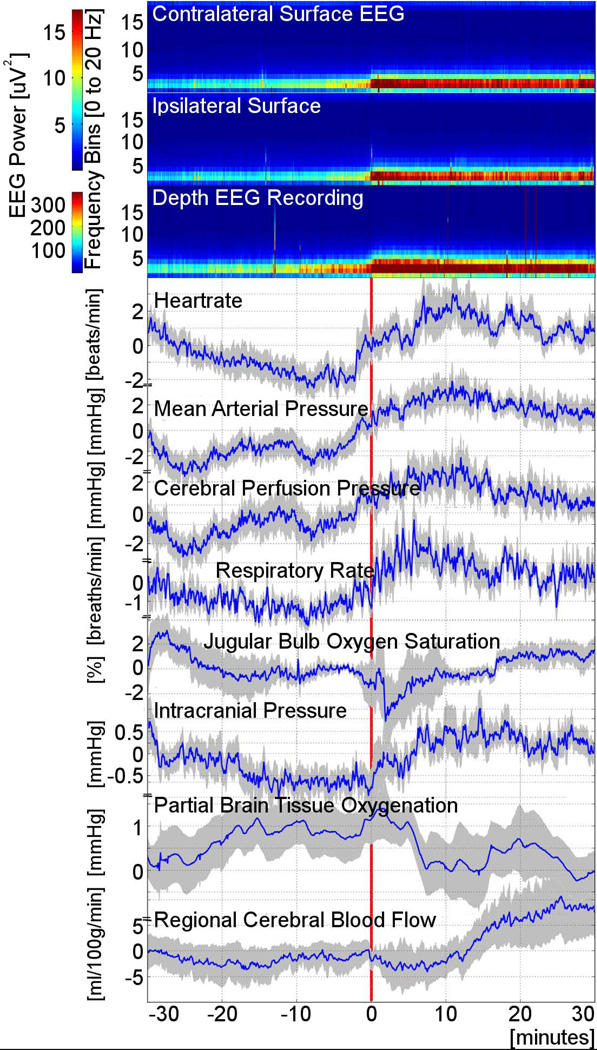
Figure 2.
Grouped data of physiologic changes associated with the onset of intracortical seizures. Spectrograms (upper three panels) displayed as relative changes on a group level, demonstrate increases in EEG power predominantly in the 2–5 Hz frequency range, first seen in the minidepth EEG recording (third panel from top) followed by contra- (top panel) and ipsilateral scalp (second panel from top) recordings. Spectrograms reveal clear changes in EEG power recorded both form the minidepth as well as the scalp, which precedes the seizure onset determined by visual inspection of raw EEG tracings (indicated as “0” on the x-axis and by the vertical red line). Physiology recordings: Timing of increases in cardiovascular (heart rate, mean arterial pressure) and respiratory (respiratory rate, minute ventilation [not shown]) parameters coincides with detection of first intracortical spectral power changes, while rising intracranial pressure is only detected later when seizures become recognizable on inspection of the raw EEG. Global brain metabolism increases sharply for a short time as suggested by the transient drop in jugular bulb oxygenation (approximately 2 minutes after seizure onset). This lasts for several minutes followed by gradual return to pre seizure baseline global metabolism (approximately 8 minutes after seizure onset). There is a small drop of partial brain tissue oxygenation starting 5 minutes after seizure onset. While cerebral perfusion pressure rises with increase in mean arterial pressure at the time the first spectrogram changes are recognizable on the minidepth recording, there is a very delayed increase in regional cerebral blood flow seen starting about 10 minutes after seizure onset. Physiology graphs are displayed as means (blue lines) with one standard error of the mean (shaded area).
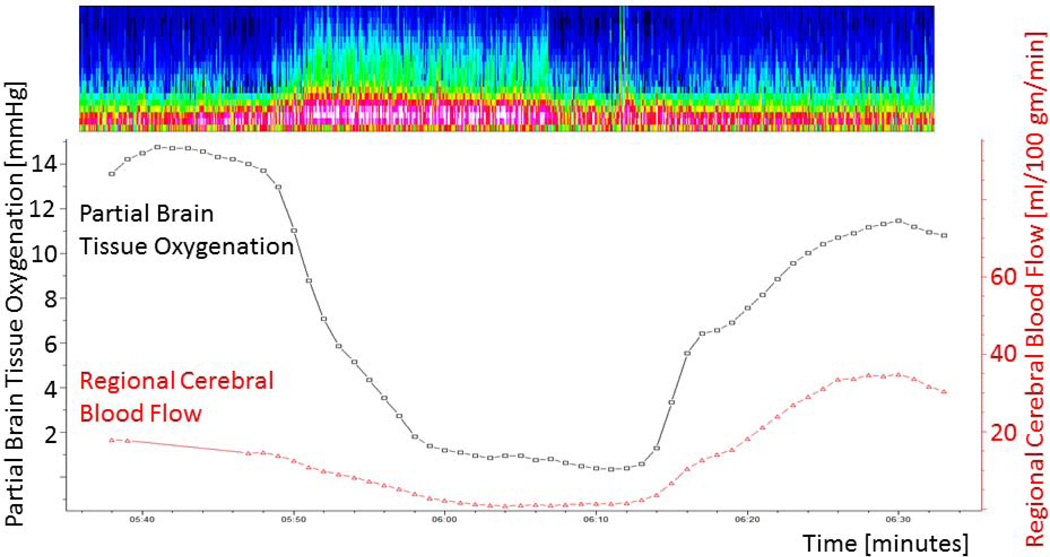
Figure 3.
46 year old man with SAH (HH grade 4, modified Fisher score 4) underwent clipping of an anterior communicating artery aneurysm who experienced several depth and surface seizures. Here displayed is a depth seizure (spectrogram at top of the Figure) on post bleed day 9 with a drop in PbtO2 from 15 to 1 mmHg following seizure onset and a drop in regional cerebral blood flow. There is a delayed rise of rCBF starting approximately 15 minutes after seizure onset accompanied by an improvement of PbtO2 to baseline values.
Surface EEG (Figure 4)
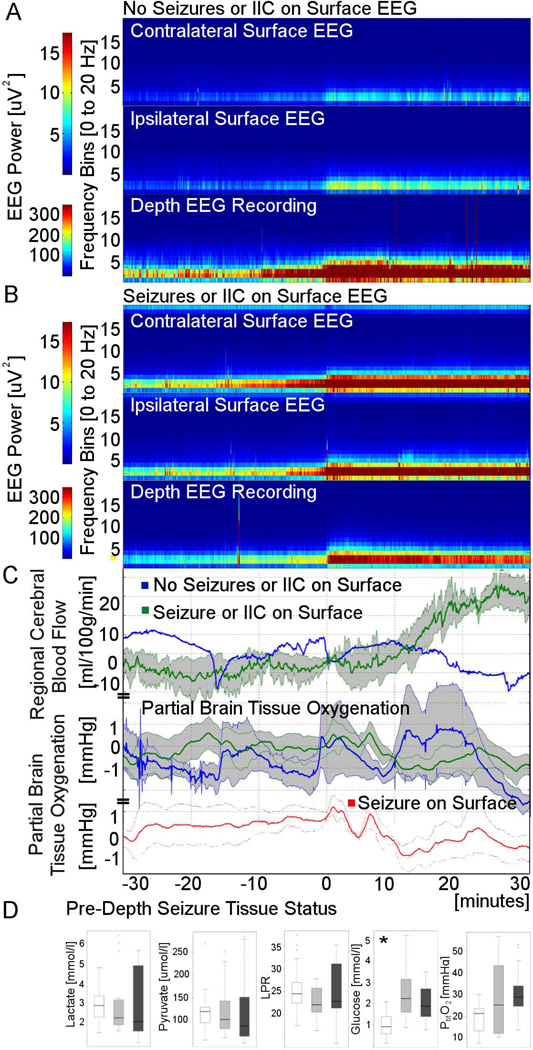
Figure 4.
Surface EEG findings. Spectrograms for intracortical seizure events stratified into those without and those with surface EEG correlate (panels A and B, respectively). rCBF did not change for those with isolated intracortical seizures while those with IIC or seizures on the surface showed a delayed rise starting approximately 10 minutes after onset (panel C). For those with seizures on the surface (red graph; right lower panel) a brief rise in PbtO2 was followed by a pronounced persistent drop, while a brief period of hyperoxia was not followed by a PbtO2 dip for those without concomitant surface seizures. Physiology graphs are displayed as means (blue lines minidepth only seizures, green lines ictal-interictal continuum or seizures on surface EEG, red line seizures on the surface) with one standard error of the mean (shaded area). Baseline cerebral tissue status characterized by pre-seizure microdialysis (interstitial lactate; pyruvate; LPR, lactate pyruvate ratio; and glucose) and partial brain tissue oxygenation (PbtO2) averaged over 60 minutes preceding intracortical seizure onset stratified by surface EEG findings (white box – no seizures or ictal interictal surface EEG findings, light grey box – ictal interictal surface EEG findings, dark grey box – seizures on surface EEG; * P=0.002).
43% (N=29) of intracortical seizures were restricted to the minidepth electrode, while simultaneously recorded scalp EEG showed patterns on the ictal interictal continuum (IIC) in 19% (N=13) and seizures in 37% (N=25). There was no adequate surface EEG available to categorize 10 of the intracortical seizure events. Overall lower baseline glucose was seen for those with seizures restricted to the minidepth electrode (median 2.2 [IQR 1.4;2.7] vs. 1.1 [IQR 0.6;1.6] for those with IIC or seizures on scalp vs. those without, respectively, P=0.002). While baseline PbtO2 also appeared lower for those with isolated intracortical seizures particularly when comparing this to those with seizures on the surface, these differences were not significant. rCBF did not change for those with isolated intracortical seizures while there was a trend for a delayed rise starting approximately 10 minutes after onset for those with IIC or seizures on surface EEG (P=0.08). Other parameters did not have significantly different responses when comparing the subgroups.
Delayed cerebral ischemia
Overall 28% (N=13) of SAH patients that underwent invasive brain monitoring including depth EEG developed delayed cerebral ischemia (DCI). Depth seizures were not more common amongst those with compared to those without DCI (31% of those with vs 41% of those without DCI, P=0.511).
Safety (Supplementary Figure 3)
At least one CT scan was obtained immediately following placement of the monitoring bundle. We carefully screened each CT scan performed in our patient cohort post probe placement and identified new intraparenchymal bleeds in four patients. Two of these appeared in the immediate proximity of the EVD distant to the monitoring site. One 2 ml left frontal bleed appeared after placement and one 5 ml bleed after removal of the monitoring probes, both in close proximity of the monitoring devices. None of the bleeds led to any detectable neurological worsening. 11 patients had MRI scans performed after removal of the monitoring probes. There was a small amount of signal change seen on gradient echo sequences in 10 and a very small area of increased T2 signal on FLAIR in 9 patients. On routine surveillance CSF studies we identified two positive CSF cultures (one each with candida and Kelbsiella oxytoca). Both patients also had EVDs placed. The former appeared 6 days after placing the EVD and monitoring bundle and was successfully treated with a 21 day course of fluconazole. The latter had CSF cultures positive for Klebsiella preceding the placement of the monitoring bundle and was successfully treated with a 21 day course of vancomycin and cefepime.
Outcome
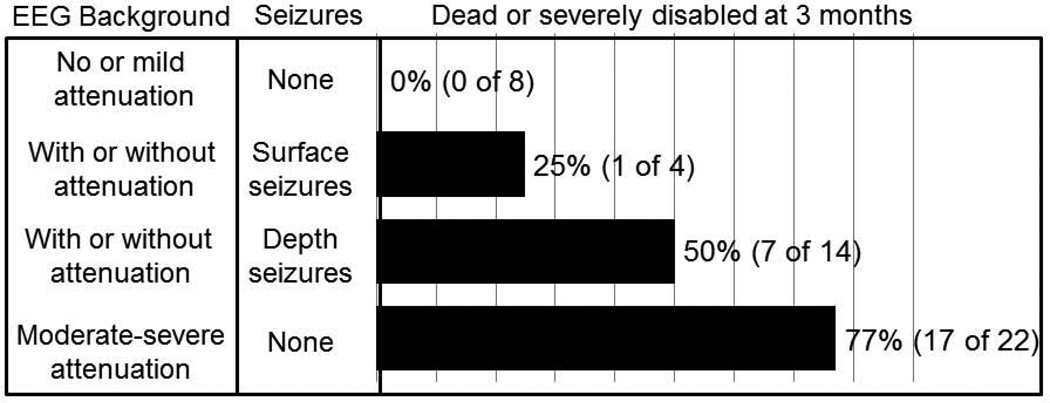
Median modified Rankin score was 5.0 (IQR 3.3, 6.0) three months after SAH and 52% (N=25) of patients were severely disabled or dead (modified Rankin score of 5 or 6). Patients without surface or intracortical seizures and baseline non-attenuated intracortical EEG had a 0% risk for severe disability or death at 3 months (0 of 8), while the risk for poor outcome was 25% with surface (1 of 4; all of these patients also had intracortical seizures) and 50% with intracortical seizures (7 of 14, with or without background attenuation but without surface seizures), and 77% with severe background attenuation without any seizures (17 of 22; Figure 5). Intracortical EEG features (seizures and background attenuation) remained significant predictors of functional outcome (OR 5.0, 95%-CI 1.7–14.2) after controlling for age, admission Hunt Hess, APACHE2 scores, and SAH sum score.
Figure 5.
Three month functional outcome after SAH stratified by EEG background activity and presence of intracortical or surface seizures.
DISCUSSION
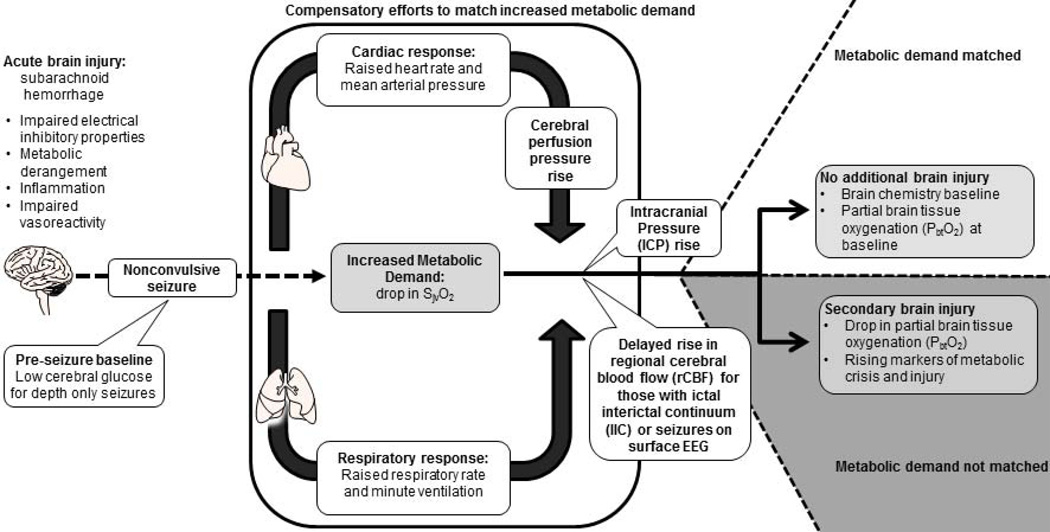
Despite promising laboratory data, randomized controlled trials in acute brain injury are often disappointing and equivocal at best.46–49 There are many reasons for this disconnect of translating research from the laboratory to the bedside but inadequate understanding of the underlying pathophysiology and a failure of existing animal models to adequately represent the complexity of acute human brain injury are overriding themes.50–53 We show that intracortical seizures following acute brain injury are often associated with a sympathetic response reflected in tachycardia, hypertension, and tachypnea as well as trends for elevated CPP and ICP, but failed to demonstrate global hypermetabolism or brain tissue hypoxia. Rising regional cerebral blood flow was only present for those with surface seizures and occurred ten minutes after seizure onset. Intracortical seizures may or may not be associated with scalp seizures, are more frequent in brain tissue with low glucose, and carry a worse prognosis than scalp seizures. We demonstrate the ability to replicate in the clinical setting findings previously made in highly controlled laboratory experiments, despite the high-complexity and poor signal to noise ratio of physiologic data collected in patients with acute brain injury (Figure 6). These observations made in human acute brain injury confirm some of the laboratory observations but maybe even more importantly highlight discrepancies, which may allow us to more successfully translate laboratory findings to the bedside.
Figure 6.
Model illustrating the relationship between acute brain injury complicated by seizures and secondary brain injury accounting for findings made in the current study. Physiologic observations supported by significant changes or trends are indicated by white boxes. Observations made in isolated cases or by visualization of grouped graphs but that were not found to be significant are kept in grey boxes.
Intracortical seizure model
Seizures recorded with the minidepth electrode30 are an ideal model to gain insights into seizure associated pathophysiology due to the spatial proximity between different monitoring probes, however, larger studies need to then correlate intracortical to scalp EEG observations. Identification of seizure onset based on minidepth EEG signals has limitations as some of the very early particularly contralateral scalp EEG changes likely represent seizures that started remotely from the minidepth electrode and are only secondarily detected at the electrode once they have spread. This may also explain in part the observation that some physiologic responses (i.e. heart rate) appear to precede intracortical seizure onset. Unfortunately measurements of cerebral microdialysis with currently available methodology have very poor temporal resolution and are therefore not ideally suited to study early metabolic effects of seizures. Our observations are not necessarily generalizable to all patients with SAH or other types of acute brain injury and apply only to comatose SAH patients with severe acute brain injury; however, these patients are the ones at highest risk of secondary brain injury. Safety data collected as part of the current study confirm the low risk of bleeding and infection as previously reported.31
Compensatory mechanisms
The current study demonstrates that the expected seizure related cardiac and respiratory sympathomimetic changes27 may also be seen after acute brain injury. Interestingly, a measurable increase in rCBF, one of the presumed major compensatory mechanisms for increased metabolic demand, was primarily seen in patients with scalp seizures suggesting that a critical volume of brain needs to be seizing to lead to a compensatory increase in rCBF. Animal work has demonstrated that increases in rCBF only occur in the seizure focus while a transient decrease of flow may be seen in the surrounding tissue.16 In our study, probes located in the seizure focus would be expected to show the most impressive increases in rCBF while those in the surround territory may initially even show a decrease.15,16 More importantly, the time course of rising rCBF was quite different to prior reports in animal models of neocortical epilepsy. While rCBF elevations were registered within seconds in these studies,15,16 our data suggest that after acute brain injury up to ten minutes elapse before a rise in rCBF is registered. Reasons for this discrepancy may in part be technique related as our approach, which was validated using Xenon computed tomography54, estimates blood flow based on a thermal dilution model while prior animal work employed intrinsic optical imaging. However, alterations in vasoreactivity may also play a role as small and large vessel responses are frequently abnormal after acute brain injury.55 The observed discrepancies between animal work and human acute brain injury if confirmed may be of clinical relevance as they may guide strategies to support compensatory efforts in an attempt to minimize secondary brain injury.
Metabolism and injury
We demonstrated a trend for rising in ICP with seizures corroborating prior studies that found an overall increase in ICP in patients with traumatic brain injury4 and a case of seizures after cardiac arrest56. It is unclear if ICP elevations with seizures are primarily related to increases in blood flow or metabolism, which may be clinical relevant as electrographic seizures after ICH have been linked to increasing midline shift and mass effect.4,7 We were not able to demonstrate significant changes in global brain metabolism (SjvO2) or brain tissue hypoxia (PbtO2) for the group, which have previously been reported in animal models of neocortical epilepsy14–17 and patients with temporal lobe epilepsy.44,57 However, isolated cases (Figure 3) and inspection of aggregated graphs (Figure 2) suggest that transient global hypermetabolism and brain tissue hypoxia may occur after acute brain injury. Interestingly, intracortical seizures were more likely to remain localized, i.e. these seizures were only detected at the minidepth electrode in hypoglycemic bran tissue. This observation implicates that adequate glucose supplies are required to allow spread of focal seizure activity. Larger sample sizes are required to determine if seizure duration, maximum frequency, or intactness of vasoreactivity impact metabolism and compensatory responses. More importantly, future studies using intrinsic optical or magnet resonance imaging and rapid-sampling microdialysis methods58 may more directly explore the link between seizures and regional metabolism as well as secondary brain injury.
Outcome
Patients without seizures and without moderate or severe EEG background changes had the best functional outcome, while those with severely abnormal EEG background activity and no seizures had the worst outcome (Figure 5). This observation is likely primarily a reflection of the extent of the underlying brain injury and may be of importance as a strong prognostic indicator for patients with acute brain injury. Diffuse background changes may be affected by sedative medications and potentially reversible complications such as elevated intracranial pressure or hydrocephalus. However, the potential importance as a prognostic indicator may be highlighted by the recently recognized importance of absence of EEG reactivity in predicting outcome after cardiac arrest.59
Interestingly, functional outcome of patients with seizures was in between these two groups of patients and those with seizures reflected on the surface EEG had a better outcome than those with isolated intracortical seizures. Reasons for this remain speculative at this point but the inability for seizures to become synchronous in a large enough region of cortex or to propagate and be reflected in the scalp EEG may be a sign of more extensive cortical and subcortical injury. As outlined above, alterations in baseline metabolism or substrate delivery (lower baseline cerebral glucose for those with intracortical only seizures, see Figure 4, Panel D) possibly as a direct or indirect result of brain injury may contribute to the brains’ ability to propagate seizures.
Supplementary Material
ACKNOWLEDGMENTS
We thank the attendings, fellows, and neurology and neurosurgery residents of the Neuroscience ICU and the Epilepsy Division for their overall support of this project.
FUNDING
This publication was made possible by a Grant entitled “Impact of electrographic seizures and periodic epileptiform patterns on neuronal function and outcome in brain injured patients” (UL1 RR024156 to JC) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Information on NCRR is available at the NCRR Website. Information on Re-engineering the Clinical Research Enterprise can be obtained from the NIH Roadmap website. Additional support for this work included grants from the National Library of Medicine, "Discovering and applying knowledge in clinical databases" (R01 LM006910 to GH and DA), "Training in Biomedical Informatics at Columbia University" (T15 LM007079 to GH and AP), the Charles A. Dana Foundation (to SAM), and the National Science Foundation, for the Computing Research Association CIFellows project (#1019343 to SK). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR, NIH, NSF, or the Dana Foundation.
ABREVIATIONS
- CPP
cerebral perfusion pressure
- CVP
central venous pressure
- EEG
electroencephalogram
- EtCO2
endtidal carbon dioxide
- HR
heart rate
- ICP
intracranial pressure
- IIC
ictal-interictal continuum
- ICU
intensive care unit
- MAP
mean arterial blood pressure
- MV
minute ventilation
- NCSE
nonconvulsive status epilepticus
- NCSz
nonconvulsive seizure
- PbtO2
partial brain tissue oxygenation
- PED
periodic epileptiform discharges
- PRX
pressure reactivity index
- rCBF
regional cerebral blood flow
- RR
respiratory rate
- SAH
subarachnoid hemorrhage
- SjvO2
jugular bulb oxygen saturation
References
- 1.Komotar RJ, Schmidt JM, Starke RM, et al. Resuscitation and critical care of poor-grade subarachnoid hemorrhage. Neurosurgery. 2009;64:397–410. doi: 10.1227/01.NEU.0000338946.42939.C7. discussion -1. [DOI] [PubMed] [Google Scholar]
- 2.Losiniecki A, Shutter L. Management of traumatic brain injury. Curr Treat Options Neurol. 2010;12:142–154. doi: 10.1007/s11940-010-0063-z. [DOI] [PubMed] [Google Scholar]
- 3.Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62:1743–1748. doi: 10.1212/01.wnl.0000125184.88797.62. [DOI] [PubMed] [Google Scholar]
- 4.Vespa PM, Miller C, McArthur D, et al. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit Care Med. 2007;35:2830–2836. [PMC free article] [PubMed] [Google Scholar]
- 5.Vespa PM, McArthur DL, Xu Y, et al. Nonconvulsive seizures after traumatic brain injury are associated with hippocampal atrophy. Neurology. 2010;75:792–798. doi: 10.1212/WNL.0b013e3181f07334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vespa P, Prins M, Ronne-Engstrom E, et al. Increase in extracellular glutamate caused by reduced cerebral perfusion pressure and seizures after human traumatic brain injury: a microdialysis study. J Neurosurg. 1998;89:971–982. doi: 10.3171/jns.1998.89.6.0971. [DOI] [PubMed] [Google Scholar]
- 7.Claassen J, Jette N, Chum F, et al. Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurology. 2007;69:1356–1365. doi: 10.1212/01.wnl.0000281664.02615.6c. [DOI] [PubMed] [Google Scholar]
- 8.Claassen J, Hirsch LJ, Frontera JA, et al. Prognostic significance of continuous EEG monitoring in patients with poor-grade subarachnoid hemorrhage. Neurocrit Care. 2006;4:103–112. doi: 10.1385/NCC:4:2:103. [DOI] [PubMed] [Google Scholar]
- 9.Topolnik L, Steriade M, Timofeev I. Partial cortical deafferentation promotes development of paroxysmal activity. Cereb Cortex. 2003;13:883–893. doi: 10.1093/cercor/13.8.883. [DOI] [PubMed] [Google Scholar]
- 10.Drislane FW. Evidence against permanent neurologic damage from nonconvulsive status epilepticus. J Clin Neurophysiol. 1999;16:323–331. doi: 10.1097/00004691-199907000-00004. discussion 53. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan PW. The clinical features, diagnosis, and prognosis of nonconvulsive status epilepticus. Neurologist. 2005;11:348–361. doi: 10.1097/01.nrl.0000162954.76053.d2. [DOI] [PubMed] [Google Scholar]
- 12.Litt B, Wityk RJ, Hertz SH, et al. Nonconvulsive status epilepticus in the critically ill elderly. Epilepsia. 1998;39:1194–1202. doi: 10.1111/j.1528-1157.1998.tb01311.x. [DOI] [PubMed] [Google Scholar]
- 13.Iyer VN, Hoel R, Rabinstein AA. Propofol infusion syndrome in patients with refractory status epilepticus: an 11-year clinical experience. Crit Care Med. 2009;37:3024–3030. doi: 10.1097/CCM.0b013e3181b08ac7. [DOI] [PubMed] [Google Scholar]
- 14.Bahar S, Suh M, Zhao M, Schwartz TH. Intrinsic optical signal imaging of neocortical seizures: the 'epileptic dip'. Neuroreport. 2006;17:499–503. doi: 10.1097/01.wnr.0000209010.78599.f5. [DOI] [PubMed] [Google Scholar]
- 15.Geneslaw AS, Zhao M, Ma H, Schwartz TH. Tissue hypoxia correlates with intensity of interictal spikes. J Cereb Blood Flow Metab. 2011;31:1394–1402. doi: 10.1038/jcbfm.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao M, Nguyen J, Ma H, Nishimura N, Schaffer CB, Schwartz TH. Preictal and ictal neurovascular and metabolic coupling surrounding a seizure focus. J Neurosci. 2011;31:13292–13300. doi: 10.1523/JNEUROSCI.2597-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suh M, Bahar S, Mehta AD, Schwartz TH. Temporal dependence in uncoupling of blood volume and oxygenation during interictal epileptiform events in rat neocortex. J Neurosci. 2005;25:68–77. doi: 10.1523/JNEUROSCI.2823-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helbok R, Ko SB, Schmidt JM, et al. Global Cerebral Edema and Brain Metabolism After Subarachnoid Hemorrhage. Stroke. 2011;42:1534–1539. doi: 10.1161/STROKEAHA.110.604488. [DOI] [PubMed] [Google Scholar]
- 19.Frykholm P, Andersson JL, Langstrom B, Persson L, Enblad P. Haemodynamic and metabolic disturbances in the acute stage of subarachnoid haemorrhage demonstrated by PET. Acta Neurol Scand. 2004;109:25–32. doi: 10.1034/j.1600-0404.2003.00174.x. [DOI] [PubMed] [Google Scholar]
- 20.Hillered L, Vespa PM, Hovda DA. Translational neurochemical research in acute human brain injury: the current status and potential future for cerebral microdialysis. J Neurotrauma. 2005;22:3–41. doi: 10.1089/neu.2005.22.3. [DOI] [PubMed] [Google Scholar]
- 21.Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med. 2011;17:439–447. doi: 10.1038/nm.2333. [DOI] [PubMed] [Google Scholar]
- 22.Dreier JP, Major S, Pannek HW, et al. Spreading convulsions, spreading depolarization and epileptogenesis in human cerebral cortex. Brain. 2012;135:259–275. doi: 10.1093/brain/awr303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Czosnyka M, Smielewski P, Czosnyka Z, et al. Continuous assessment of cerebral autoregulation: clinical and laboratory experience. Acta Neurochir Suppl. 2003;86:581–585. doi: 10.1007/978-3-7091-0651-8_118. [DOI] [PubMed] [Google Scholar]
- 24.Su IC, Li CH, Wang KC, et al. Prediction of early secondary complications in patients with spontaneous subarachnoid hemorrhage based on accelerated sympathovagal ratios. Acta Neurochir (Wien) 2009;151:1631–1637. doi: 10.1007/s00701-009-0517-9. [DOI] [PubMed] [Google Scholar]
- 25.van der Bilt IA, Hasan D, Vandertop WP, et al. Impact of cardiac complications on outcome after aneurysmal subarachnoid hemorrhage: a meta-analysis. Neurology. 2009;72:635–642. doi: 10.1212/01.wnl.0000342471.07290.07. [DOI] [PubMed] [Google Scholar]
- 26.Carrera E, Schmidt JM, Fernandez L, et al. Spontaneous hyperventilation and brain tissue hypoxia in patients with severe brain injury. J Neurol Neurosurg Psychiatry. 2010;81:793–797. doi: 10.1136/jnnp.2009.174425. [DOI] [PubMed] [Google Scholar]
- 27.Baumgartner C, Lurger S, Leutmezer F. Autonomic symptoms during epileptic seizures. Epileptic disorders : international epilepsy journal with videotape. 2001;3:103–116. [PubMed] [Google Scholar]
- 28.Sorani MD, Hemphill JC, 3rd, Morabito D, Rosenthal G, Manley GT. New approaches to physiological informatics in neurocritical care. Neurocrit Care. 2007;7:45–52. doi: 10.1007/s12028-007-0043-7. [DOI] [PubMed] [Google Scholar]
- 29.McIntosh N. Intensive care monitoring: past, present and future. Clin Med. 2002;2:349–355. doi: 10.7861/clinmedicine.2-4-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waziri A, Claassen J, Stuart RM, et al. Intracortical Electroencephalography in Acute Brain Injury. Ann Neurol. 2009;66:366–377. doi: 10.1002/ana.21721. [DOI] [PubMed] [Google Scholar]
- 31.Stuart RM, Schmidt M, Kurtz P, et al. Intracranial Multimodal Monitoring for Acute Brain Injury: A Single Institution Review of Current Practices. Neurocrit Care. 2010;12:188–198. doi: 10.1007/s12028-010-9330-9. [DOI] [PubMed] [Google Scholar]
- 32.Claassen J, Bernardini GL, Kreiter K, et al. Effect of cisternal and ventricular blood on risk of delayed cerebral ischemia after subarachnoid hemorrhage: the Fisher scale revisited. Stroke. 2001;32:2012–2020. doi: 10.1161/hs0901.095677. [DOI] [PubMed] [Google Scholar]
- 33.Manley GT, Diaz-Arrastia R, Brophy M, et al. Common data elements for traumatic brain injury: recommendations from the biospecimens and biomarkers working group. Arch Phys Med Rehabil. 2010;91:1667–1672. doi: 10.1016/j.apmr.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 34.Bederson JB, Connolly ES, Jr, Batjer HH, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40:994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- 35.Claassen J, Hirsch LJ, Emerson RG, Bates JE, Thompson TB, Mayer SA. Continuous EEG monitoring and midazolam infusion for refractory nonconvulsive status epilepticus. Neurology. 2001;57:1036–1042. doi: 10.1212/wnl.57.6.1036. [DOI] [PubMed] [Google Scholar]
- 36.Chong DJ, Hirsch LJ. Which EEG patterns warrant treatment in the critically ill? Reviewing the evidence for treatment of periodic epileptiform discharges and related patterns. J Clin Neurophysiol. 2005;22:79–91. doi: 10.1097/01.wnp.0000158699.78529.af. [DOI] [PubMed] [Google Scholar]
- 37.Hirsch LJ, Brenner RP, Drislane FW, et al. The ACNS subcommittee on research terminology for continuous EEG monitoring: proposed standardized terminology for rhythmic and periodic EEG patterns encountered in critically ill patients. J Clin Neurophysiol. 2005;22:128–135. doi: 10.1097/01.wnp.0000158701.89576.4c. [DOI] [PubMed] [Google Scholar]
- 38.Gornbein JA, Lazaro CG, Little RJ. Incomplete data in repeated measures analysis. Stat Methods Med Res. 1992;1:275–295. doi: 10.1177/096228029200100304. [DOI] [PubMed] [Google Scholar]
- 39.Zerbet ANM. A new statistics for detecting outliers in exponential case. Communications in Statistics: Theory and Methods. 2003;32:573–584. [Google Scholar]
- 40.Fried R, Gather U, Imhoff M, Bauer M. Some statistical methods in intensive care online monitoring - A review. Lect Notes Comput Sc. 2000;1933:67–77. [Google Scholar]
- 41.Imhoff M, Bauer M, Gather U, Lohlein D. Statistical pattern detection in univariate time series of intensive care on-line monitoring data. Intens Care Med. 1998;24:1305–1314. doi: 10.1007/s001340050767. [DOI] [PubMed] [Google Scholar]
- 42.Jones PA, Minns RA, Lo TY, Andrews PJ, Taylor GS, Ali S. Graphical display of variability and inter-relationships of pressure signals in children with traumatic brain injury. Physiol Meas. 2003;24:201–211. doi: 10.1088/0967-3334/24/1/315. [DOI] [PubMed] [Google Scholar]
- 43.Hripcsak G, Albers DJ, Perotte A. Exploiting time in electronic health record correlations. Journal of the American Medical Informatics Association : JAMIA. 2011;18(Suppl 1):i109–i115. doi: 10.1136/amiajnl-2011-000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suh M, Ma H, Zhao M, Sharif S, Schwartz TH. Neurovascular coupling and oximetry during epileptic events. Mol Neurobiol. 2006;33:181–197. doi: 10.1385/MN:33:3:181. [DOI] [PubMed] [Google Scholar]
- 45.Landis JR, Koch GG. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics. 1977;33:363–374. [PubMed] [Google Scholar]
- 46.O'Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–477. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- 47.Shuaib A, Lees KR, Lyden P, et al. NXY-059 for the treatment of acute ischemic stroke. N Engl J Med. 2007;357:562–571. doi: 10.1056/NEJMoa070240. [DOI] [PubMed] [Google Scholar]
- 48.Zhang S, Wang L, Liu M, Wu B. Tirilazad for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD006778.pub2. CD006778. [DOI] [PubMed] [Google Scholar]
- 49.Clifton GL, Valadka A, Zygun D, et al. Very early hypothermia induction in patients with severe brain injury (the National Acute Brain Injury Study: Hypothermia II): a randomised trial. Lancet neurology. 2011;10:131–139. doi: 10.1016/S1474-4422(10)70300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cernak I. Animal models of head trauma. NeuroRx. 2005;2:410–422. doi: 10.1602/neurorx.2.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kirkman MA, Allan SM, Parry-Jones AR. Experimental intracerebral hemorrhage: avoiding pitfalls in translational research. J Cereb Blood Flow Metab. 2011;31:2135–2151. doi: 10.1038/jcbfm.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iadecola C, Anrather J. Stroke research at a crossroad: asking the brain for directions. Nat Neurosci. 2011;14:1363–1368. doi: 10.1038/nn.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van der Worp HB, Howells DW, Sena ES, et al. Can animal models of disease reliably inform human studies? PLoS medicine. 2010;7 doi: 10.1371/journal.pmed.1000245. e1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vajkoczy P, Roth H, Horn P, et al. Continuous monitoring of regional cerebral blood flow: experimental and clinical validation of a novel thermal diffusion microprobe. J Neurosurg. 2000;93:265–274. doi: 10.3171/jns.2000.93.2.0265. [DOI] [PubMed] [Google Scholar]
- 55.Steiner LA, Coles JP, Johnston AJ, et al. Assessment of cerebrovascular autoregulation in head-injured patients: a validation study. Stroke. 2003;34:2404–2409. doi: 10.1161/01.STR.0000089014.59668.04. [DOI] [PubMed] [Google Scholar]
- 56.Ko SB, Ortega-Gutierrez S, Choi HA, et al. Status epilepticus-induced hyperemia and brain tissue hypoxia after cardiac arrest. Arch Neurol. 2011;68:1323–1326. doi: 10.1001/archneurol.2011.240. [DOI] [PubMed] [Google Scholar]
- 57.la Fougere C, Rominger A, Forster S, Geisler J, Bartenstein P. PET and SPECT in epilepsy: a critical review. Epilepsy Behav. 2009;15:50–55. doi: 10.1016/j.yebeh.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 58.Hopwood SE, Parkin MC, Bezzina EL, Boutelle MG, Strong AJ. Transient changes in cortical glucose and lactate levels associated with peri-infarct depolarisations, studied with rapid-sampling microdialysis. J Cereb Blood Flow Metab. 2005;25:391–401. doi: 10.1038/sj.jcbfm.9600050. [DOI] [PubMed] [Google Scholar]
- 59.Rossetti AO, Oddo M, Logroscino G, Kaplan PW. Prognostication after cardiac arrest and hypothermia: a prospective study. Ann Neurol. 2010;67:301–307. doi: 10.1002/ana.21984. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.