Abstract
The adeno-associated virus (AAV) is one of the most useful viral vectors for gene delivery for both in vivo and in vitro applications. A variety of methods have been established to produce and characterize recombinant AAV (rAAV) vectors; however most methods are quite cumbersome and obtaining consistently high titer can be problematic. This protocol describes a triple-plasmid co-transfection approach with 25 kDa linear polyethylenimine (PEI) in 293T cells for the production of AAV serotype 2. Seventy-two hours post-transfection, supernatant and cells were harvested and purified by a discontinuous iodixanol density gradient ultracentrifugation, then dialyzed and concentrated with an Amicon 15 100,000MWCO concentration unit. To optimize the protocol for AAV2 production using PEI, various N/P ratios and DNA amounts were compared. We found that an N/P ratio of 40 coupled with 1.05ug DNA per ml of media (21ug DNA/15cm dish) was found to produce the highest yields for viral replication and assembly measured multiple ways. The infectious units, as determined by serial dilution, were between 1×108 to 2×109 IU/ml. The genomic titer of the viral stock was determined by qPCR and ranged from 2×1012 to 6×1013 vg/ml. These viral vectors showed high expression both in vivo within the brain and in vitro in cell culture. The use of linear 25kDa polyethylenamine PEI as a transfection reagent is a simple, more cost-effective, and stable means of high-throughput production of high-titer AAV serotype 2. The use of PEI also eliminates the need to change cell medium post-transfection, lowering cost and workload, while producing high-titer, efficacious AAV2 vectors for routine gene transfer.
Keywords: recombinant virus, in vivo, infection, genetic, gene expression, optimization, AAV, AAV2-GFP, brain, cortex, hippocampus, cerebellum, polyethylenimine (PEI), N/P ratio
1. Introduction
Recombinant adeno-associated viruses (rAAV), a 22nm naked, single-stranded DNA member of parvoviridae family (Hoggan et al., 1966), has emerged as one of the preferred gene-delivery viral vectors in the past decade. Use of rAAV has lead to promising leaps in gene therapy (Bainbridge et al., 2008; Bowles et al., 2012; Jayandharan et al., 2011; Kaplitt et al., 2007; Niemeyer et al., 2009). It has become such a powerful tool in the laboratory and clinic because of its ability to infect a large range of tissues. It exhibits long-term gene transduction, offers a wide variety of cell and tissue tropism due to availability of multiple serotypes, exhibits low immunogenicity, and has an overall good safety profile (Lock et al., 2010; Wright, 2009). High-throughput production of rAAV serotype 2 (rAAV2) is the goal of our viral vector core, and it requires the subsequent implementation and optimization of a production protocol that is adaptable to multiple inserted transgenes, in order to obtain a pure and high titer virus without extensive workload and cost.
In this protocol, rAAV2 was produced by a three-plasmid co-transfection system in HEK293T cells, wherein (1) a helper plasmid (pHelper) contained the adenovirus genes E2a, E4 and viral associated RNAs necessary for AAV reproduction, (2) a vector plasmid carries the transgene flanked by inverted terminal repeats (ITRs), and (3) the packaging plasmid provides the AAV serotype 2 replication (rep2) and encapsidation (cap2) genes. The HEK293T cell line provides trans-acting adenovirus E1 genes. This protocol reports an optimization of rAAV2 production by reducing the amount of DNA used for transfection (to 1.05ug/ml), which decreased the amount of work and cost in DNA purification while observing no decrease in viral titers.
Established calcium-phosphate transfection (Ca-P) has been found to be more laborious, expensive, and yield inconsistent and unstable results because of narrow optimal conditions, especially temperature and pH (Wright, 2009). Comparatively, using linear 25 kDa polyethylenimine (PEI) as a transfection reagent is cost-effective, has improved stability, and has the ability to function at a wider pH range (Lock et al., 2010; Reed et al., 2006). The use of PEI also eliminates the need to change cell medium post-transfection (Durocher et al., 2007), drastically lowering cost and workload.
PEI is a polymer in which a secondary amine occupies the third atom per monomer unit. It is the amino group of PEI that causes the polymer to display a high cationic charge density potential and buffer capacity (Boussif et al., 1995b; Clamme et al., 2003a; Clamme et al., 2003b). This allows the PEI to form ionic interactions with the phosphate backbones of DNA to form a condensed PEI-DNA complex that can be transported into the cell via endocytosis (Godbey et al., 1999). Behr (Behr, 1997) observed that DNA-PEI complexes escape endosomes via a proton sponge effect that promotes osmotic swelling and disrupting of the endosomal membrane. However, how DNA later enters the nucleus is still unknown. Within the complex, the particular ratio between molar units of PEI nitrogen atoms to units of DNA phosphate atoms, known as the N/P ratio, has been found to correlate with enhanced transfection efficiencies (Guo et al., 2012; Ogris et al., 1998; Reed et al., 2006). However, the N/P ratio can differ between protocols, and most ratios utilize an excess of PEI for transfection. One reason that these ratios sometimes include excessive PEI is centered on PEI’s ability to enhance the proton sponge effect and disrupt endosomes (Boeckle et al., 2004)
In the described experiments, it was observed that using a linear 25kDa PEI to transfect HEK293T cells with a three plasmid system using N/P ratio of 40, with an excess of PEI, yields high titer rAAV2, without observable cell death after 72 hours transfection. In addition to harvesting viral particles in lysed whole cell homogenate, the supernatant was also harvested using a 40% PEG8000/2.5N NaCl precipitation. While some consider this to be trivial to final viral titers (Reed et al., 2006; Zolotukhin et al., 1999), others have found supernatant to contain up to 50% of total viral particles (Ayuso et al., 2010) and suggest that it is a pure (no cellular debris) source of rAAV vectors (Lock et al., 2010). To further isolate virions, a discontinuous iodixanol gradient centrifugation followed by dialysis in an Amicon 15 100,000 MWCO concentration unit was employed. Optimization of this PEI protocol resulted in the use of less DNA, decreased workload, and lower cost for reagents. A simple, effective, and adaptable approach for producing high titer recombinant AAV2 using 25 kDa linear PEI is described. This further demonstrates the utility of AAV2 in vitro in cell culture and in vivo in the rodent brain.
2. Materials and Methods
2.1 AAV vectors
Three vectors were used in each experiment: (1) An AAV vector containing GFP or GOI (gene of interest) flanked by the ITRs; (2) a packaging vector containing the AAV serotype rep2 and cap2 genes; (3) a helper vector containing the adenovirus helper functions. (All plasmids purchased from Cell biolabs Inc).
2.2 PEI preparation and calculation
PEI (Polyethylenimine, linear, MW 25,000 from Polysciences, cat # 23966) was prepared to a final concentration of 7.5mM (based on monomer units). pH was adjusted to 8.0 and filtered through 0.22um filter. 20ml-aliquots were frozen and thawed three times, and stored at −20°C. The amount (ul) of PEI was calculated based on the following equation (Reed et al., 2006): PEI (ul)=3 × D X R/S
Where D = total amount of plasmid DNA used (ug), R = N/P ratio (ratio of nitrogen content in PEI to phosphorous content in DNA), S = concentration of the PEI stock (mM, monomer unit).
2.3 Preparation of AAV-293T cells for transfection
HEK 293T cells (gift from Dr. Thomas Kukar; also available from ATTC) were maintained in complete medium (4.5g/L Glucose and L- Glutamine containing DMEM supplemented with 10% FBS and 1% Pen-Strep) and incubated at 37°C, 5% CO2. One day before transfection, HEK 293T cells were seeded onto 20 150mm plates at a density of 1×107 cells per plate in 18ml of complete medium. The cells were approximately 70% confluent on the day of transfection.
2.4 Co-transfection of three plasmids
All plasmid concentrations were adjusted to 1ug/ul in TE buffer before mixing the following: 105ug of pAAV vector, 105ug of pAAV-R2C2 and 210ug of pHelper (total of 420 ug DNA for 20 15cm plates; equal to 1.05ug per ml of media).
The transfection mixture, which included a total of 420ug DNA, 4ml of 1.5M NaCl, 6.72ml of PEI and 29.28ml of sterile dd H2O in a 50ml sterile tube, was vortexed a few seconds, and incubated at room temperature for 20 min. 2ml of the mixture was then added to each plate in a dropwise manner. The plates were returned to the incubator for 72 hours at 37°C, 5%CO2.
2.5 Virus Harvest
Cell culture media and transfected cells were harvested separately. 40% PEG (cat# P2139, Sigma) in 2.5N NaCl was added to the supernatant to a final concentration of 8%, and incubated on ice for 2 hours. The cell pellet was suspended in 14 ml of lysis buffer (50mM Tris-Cl, 150mM NaCl and 2mM MgCl2) and stored at 4°C. Following the two-hour incubation, the supernatant was centrifuged at 2500g for 30min at 4°C to pellet the PEG-precipitated virus. The cell lysate and pelleted supernatant precipitate were combined and then treated with 750ul of 10% sodium deoxycholate (cat# BP349-100, Fisher) to final concentration 0.5%, and benzonase (cat# E8263-25KU, Sigma) to a final concentration of 50ug/ml. After 30mins incubation at 37°C, 3 ml of 5M NaCl were added to the sample to decrease aggregation of the virus followed by a 30-min 50°C incubation, and three freeze-thaw cycles between −80°C and 37°C. Debris was pelleted by spinning at 12,000g for 30mins at 4°C, and separated from the lysate with the use of a popper pipette needle attached to a 20ml syringe. The lysate was then pipetted into a Beckman 25×89 quick seal tube and immediately underlaid with 9ml of 15%, 6ml of 25%, 5ml of 40% and 5ml of 60% iodixanol (cat# D1556-250, sigma) using a pipetting needle attached to a 10ml syringe. If necessary, 1X PBS was added to fill the tube completely.
2.6 Centrifugation and virus retrieval
The quick seal tube containing the prepared iodixanol gradient and lysate was centrifuged at 500,000 × g using rotor 70Ti for 1.5 hours at 18°C. To retrieve the virus, the tube was then secured in a clamp stand set to eye-level, after which an 18G needle was inserted into the top of the tube to allow air to enter. Another 18G needle attached to a 10ml syringe was inserted just below the interface of the 40% and 60% iodixanol layers with the bevel of the needle up. Approximately 4 ml of sample were extracted containing the AAV in the 40% iodixanol layer.
2.7 Dialysis and concentration
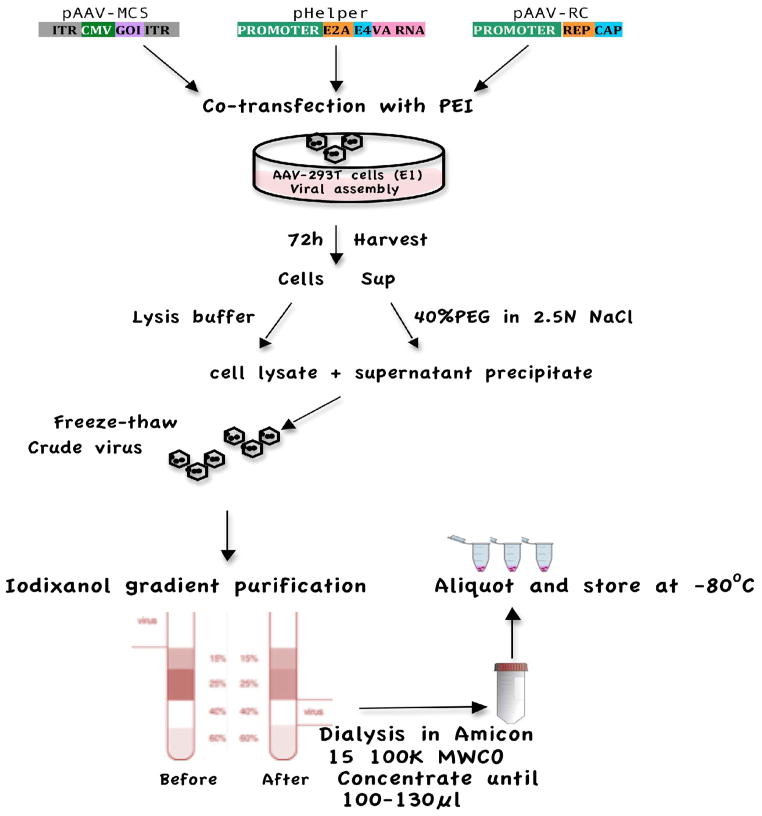
The extracted virus was then added to 7ml of PBS previously dispensed into the top portion of an Amicon 15 100,000MWCO (cat# UFC910024, Fisher) concentration unit followed by a 10-min centrifugation at 3000g. When most of the solution had sufficiently passed through the filter, 10ml of PBS were added followed by 4 rounds of repeated washing and spinning. After the final wash, the remnant solution was spun repeatedly until 150ul remained before the virus was subsequently aliquoted and stored at −80°C for future use. Figure 1 shows the summarizing process of AAV2 production.
Figure 1.
Schematic of AAV2 production using PEI –mediated co-transfection with three plasmids in HEK 293T cells.
2.8 Determination of virus titer
The infectious units (IU) of rAAV were determined by a 10-fold serial dilution. GFP expressing HEK293T cells were counted by Fluorescence Microscopy 72 hours post-infection. The vector genome copy number (VG) was determined by qPCR using Brilliant III Ultra Fast SYBRgreen qPCR Master Mix (cat# 600882, Stratagene). The viral DNA was extracted from 1ul of purified virus and was treated with 0.5U DNase I (cat# 18068-015, Invitrogen) to digest any contaminating unpackaged DNA, followed by an additional 10ug proteinase K (25530-049, Invitrogen) treatment to assist in breaking capsids and releasing viral DNA. qPCR was run in Applied Biosystems Mx3000P with primers for the ITRs common to AAV transfer vector plasmids: forward primer 5′-GGA ACC CCT AGT GAT GGA GTT-3′ and reverse primer 5′-CGG CCT CAG TGA GCG A-3′; set with a program: 95°C 10 min, then cycled 40 times at 95°C for 15 sec, 60°C for 30 sec and 72°C for 30 sec. To generate a standard curve, a pAAV-GFP plasmid was used in serial dilutions from 1×107 to 1×103 genome copies, performed in triplicate. A no-template negative control was also performed in triplicate. The positive control was 5×105 genome copies of pAAV-GFP, performed in duplicate.
2.9 Cell survival assay
To quantify cell death following transfection, a tetrozolium-based technique, the WST-1 (Water soluble Tetrazolium salts) assay was performed. HEK 293 T cells were seeded into each 24-well plate at a density of 1×105 in 500ul of complete medium. The cells were approximately 70% confluent on the day of transfection. Three plasmids were co-transfected as described previously. 0.35ug of total DNA per well was used. Cell survival was ascertained for N/P ratios 20, 40, 60 and 80. A blank control of media only was used while cells were used as a negative control. 72 hours after transfection, 50ul of WST1 (cat# 5015944001, Roche) were added to each well and incubated for 30min at 37%, 5% CO2. The absorbance was measured in a microplate reader (BioTek instrument Inc.) at 450 nm and the percent of cell survival was calculated using this formula: Survival rate (%) =Asample − Ab/Ac−Ab ×100; Ab = absorbance of blank; Ac = absorbance of negative control.
2.10 P0 and stereotaxic injections of mice
For P0 injections, newborn (P0) C57BL/6 mice were injected with AAV2-GFP (2 uL of 1.5 × 1012 vg/ml) in both hemispheres approximately 1–1.5 mm deep according to published protocols (Li et al., 2002; Passini et al., 2003). For stererotaxic injections, two month old C57BL/6 mice were anesthetized with 95 mg/kg ketamine and 5 mg/kg xylazene and placed in stereotaxic frame. Eyes were covered in ophthalmic ointment, and the scalp was sterilized before the skull was exposed. A unilateral injection of AAV2-GFP (2 uL of 1.5 × 109 vg/ml) was given at a rate of 1 μl/2.5 min into the hippocampus of the right hemisphere (anteroposterior (AP), −2.1 mm from the bregma; mediolateral (ML), 1.8 mm from the bregma; and dorsoventral (DV), −1.8 mm from below the surface of the dura) according to published protocols (Kiyota et al., 2009). Four weeks after P0 or stereotaxic injection, animals were sacrificed and tissue was processed for fluorescence immunohistochemistry as described below.
2.11 Perfusion and tissue processing for fluorescence immunohistochemistry
Four weeks post-injection, animals were deeply anesthetized with isofluorane and intracardially perfused with 4% paraformaldehyde in PBS (pH 7.4). Brains were fixed for 48 h in the same paraformaldehyde solution and cryoprotected in 10% sucrose for 24 h followed by 30% sucrose for 24 h. Serial coronal sections (40 μm thick) through the cortex and cerebellum were collected and stored in anti-freeze solution for further analyses. Tissue sections were washed and mounted using a standard immunofluorescence protocol (Pranski et al., 2012). Images were captured using a Zeiss LSM 510 laser scanning confocal microscope.
3. Results
3.1 Optimization of N/P ratios for PEI-mediated AAV2-GFP production
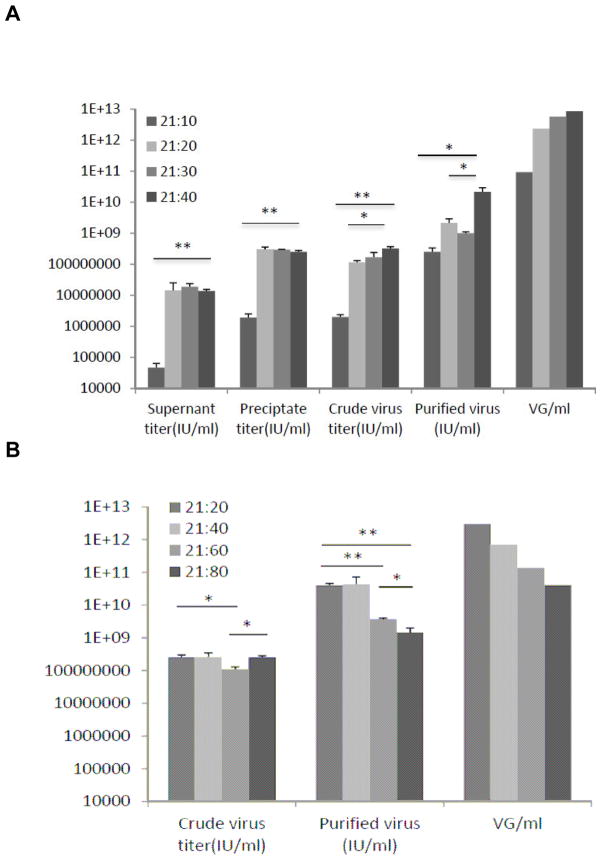
It has been indicated that the N/P ratio is of paramount influence on the formation of PEI complexes, which in turn affects optimum transfection efficiency (Thomas et al., 2005b). We initially examined N/P ratios of 10 – 40, while maintaining the transfected DNA amount at 21ug/15cm plate (Figure 2A). We found that the lowest functional virus titers (IU/ml) were observed for the supernatant, crude and purified virus for N/P ratio of 10 (2.5 × 108 IU/ml). The N/P ratio of 10 also had the lowest vector genome titers while the highest titers corresponded to an N/P ratio of 40 (7.7 × 109 IU/ml). All experiments were conducted in triplicate while maintaining a DNA amount of 21ug/15cm plate (1.05ug/ml) for each trial.
Figure 2. Differential effects on AAV2 titer of various N/P ratios.
(A): Effect of N/P ratios 10, 20, 30 and 40 on supernatant, precipitate, crude, purified mean Infectious unit titers (IU/ml) and the mean vector genome titers (VG/ml) of purified virus. The same DNA amount of 21 ug/15cm dish (or 1.05 ug/ml of media) was employed throughout. (B) Effect of N/P ratios 20, 40, 60 and 80 on crude and purified mean Infectious unit and mean vector genome titers of purified virus. The same DNA amount of 21 ug/15cm dish (or 1.05 ug/ml of media) was employed throughout. Crude and purified infectious unit titers were performed in triplicate, whereas genomic titers (VG/ml) was performed once per group. Bars show mean value ± sem.
Given these experiments were conducted with ratios 40 and below, the effect of higher N/P ratios on titer was further tested by employing a broader range of ratios under similar experimental conditions (N/P 20, 40, 60, and 80). The lowest averaged titer of 1.4 × 109 IU/ml was obtained for N/P ratio 80 while the highest was observed for N/P ratio 40 at 4.3 × 1010 IU/ml (Figure 2B).
These findings demonstrated that optimal IU/ml and VG/ml titers were obtained for N/P ratio 40 with very little cell death observed by fluorescence (Figure 3) and WST-1 assays (see below, Figure 5) 72 hours post-transfection.
Figure 3. Efficacy and Viability of AAV2 transfection and infection in vitro in 293T cells.
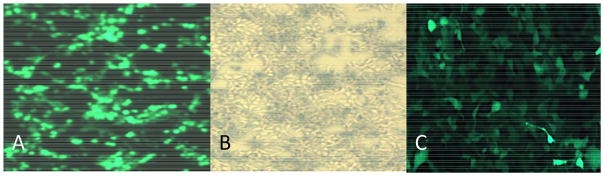
(A) 72-hours post-transfection using protocol above with pHelper, pAAV-GFP and pAAV-R2C2, showing high efficiency. (B) Same as Figure A in brightfield, without GFP filter. Approximately 20 percent cell death observed. (C) 293T cells infected with purified AAV2-GFP virus, MOI=3.
Figure 5. WST1 based cell survival.
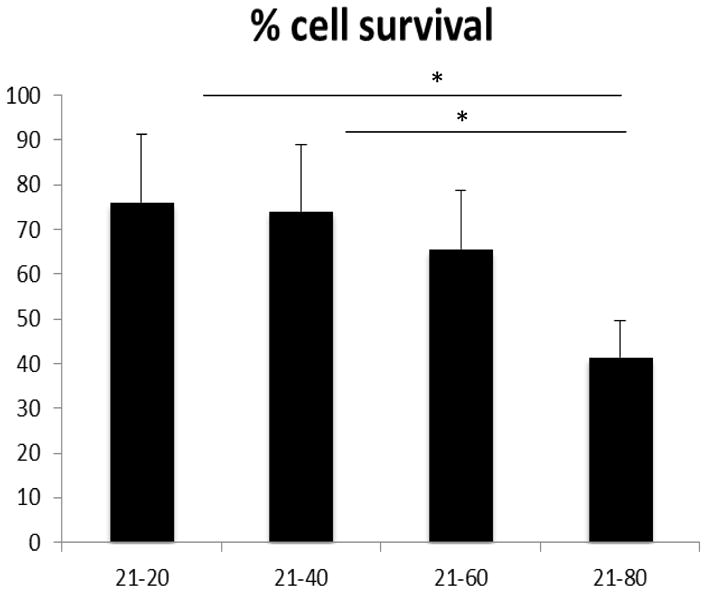
Comparison of N/P ratios 20, 40, 60 and 80 post-transfection cell survival using WST1 assay. No significant differences observed for N/P 20 and 40. Significant loss in cell viability observed for N/P 60 and 80. Bars show mean % survival ± sem.
3.2 Optimization of DNA amount for PEI-mediated AAV2-GFP production
After optimizing the N/P ratio, comparable or higher titers were further sought by comparing the DNA amount of 2.25ug/ml to 1.05ug/ml. For this experiment, DNA amounts of 0.525ug/ml, 1.05ug/ml and 2.25ug/ml were compared. A ratio of N/P 40 was maintained for all trials. The highest titers were observed with DNA 1.05ug/ml of total plasmid DNA with functional titers just over 109 IU/ml which corresponds to a genome copy titer in excess of 1012 VG/ml as determined by qPCR. An N/P ratio of 40 coupled with DNA amount of 1.05ug of total plasmid DNA per ml of culture media was subsequently employed for all other AAV2 productions (5.2ug of PEI per ug of DNA). These titers ranged from 108 – 6 × 109 IU/ml and 4 × 1010 – 6 × 1013 VG/ml.
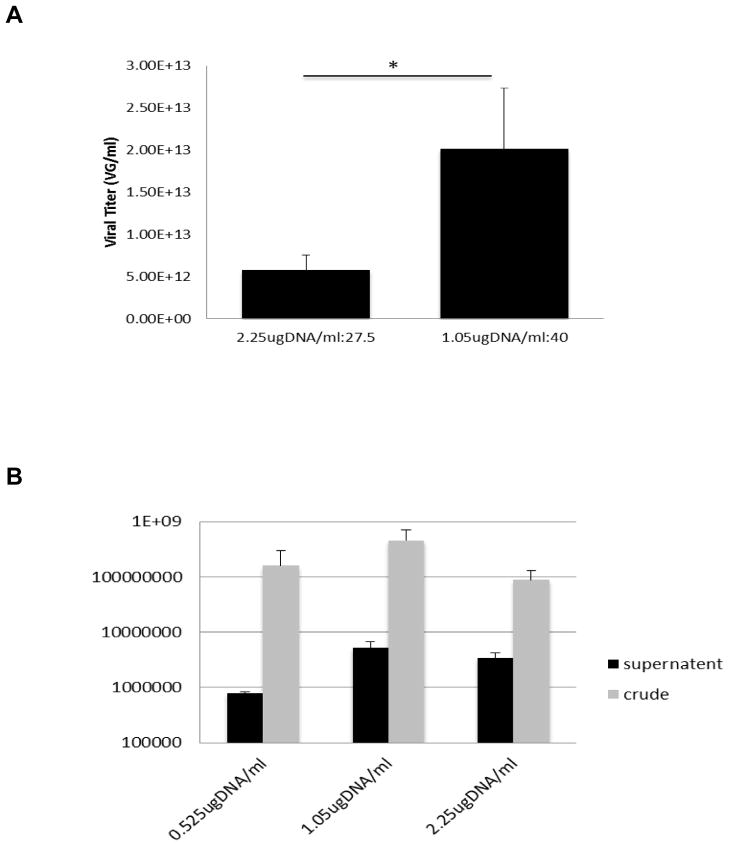
A total of 17 complete viral productions were then examined across numerous different packaging plasmids. The 1.05ug/ml/40 N/P ratio (9 productions, mean titer 2.0×1013 VG/ml) consistently produced superior titers to the 2.25ug/ml/27.5 N/P ratio (8 productions, mean titer 5.8×1012 VG/ml) combination, (Figure 4A, p<0.05). The utility and reproducibility of this particular N/P ratio and DNA amount for the production of rAAV2 with diverse transgene sizes and structures was thus further demonstrated.
Figure 4. Differential effects on AAV2 titer of DNA amounts and N/P ratios and inclusion of Cellular Supernatant in Virus Production.
(A) Comparison of mean VG titers of DNA amount: 2.25ug DNA/ml coupled with N/P ratio 27.5 and optimized DNA amount of 1.05ug DNA/ml coupled with N/P ratio 40. The 1.05ug/40 N/P ratio was significantly more effective at producing high-titer virus (p<0.05). (B) Comparing titers of crude and purified virus harvested with and without supernatant (sup virus was precipitated by 40% PEG/2.5N NaCl). Bars show mean value ± sem.
3.3 Influence of the supernatant on final AAV2 vector titers
Current methodology for AAV production and harvesting focuses on the heparin-binding mechanism of the virus to cells, and hence the commonly held view that most of the virus (80–90%) is strongly cell-associated (Reed et. al 2006; Zolotukhin et. al 1999). However, it has been reported that AAV heparin affinity is serotype specific (Guo et. al 2012), and thus it was important to determine whether harvesting the supernatant along with the lysate of HEK293T cells affected overall titer for serotype 2. A 60 fold increase in purified virus was observed when the supernatant was treated with 40%PEG8000/2.5N NaCl and added to the cell lysate (Titers were 1.4×107 IU/ml without supernatant and 8.3×108 IU/ml with supernatant). The data also showed that virus released to the supernatant constitutes 43–62% of the total AAV2-containing clarified cell homogenate (Figure 4B). Thus, for all subsequent experiments, the supernatant was harvested and treated according to protocol.
3.4 Effect of N/P ratio on WST-1 based cell survival
As a secondary means of testing post-transfection cell survival for various N/P ratios, the WST-1 assay was employed. The WST-1 assay provided a sensitive and accurate method of measuring cell viability and proliferation by the reduction of tetrazolium salts to colored formazan compounds by succinate-tetrazolium reductase in live cells. N/P ratios 20, 40, 60 and 80 were used in this assay. N/P 20 and N/P 40 had the highest percent cell survival (76% and 74%, respectively, which were not significantly different). The lowest percent survival was obtained for N/P ratios 60 and 80 at 65% and 41%, respectively (Figure 5).
3.5 Variability of the RT-PCR method for quantification of AAV2 vector genome particles
Infectious unit and genome copy viral titers were respectively ascertained by serial dilution replication assays and real-time PCR. Quantification of rAAV2 particles by qPCR has been established as one of the most practical and time-efficient methods for obtaining vector genome copy titers (Rohr et al., 2002). However, the limitations of standardizing this procedure as a principal means of viral particle quantification was also recognized as there yet remained a significant degree of inter-experimental variability. Results showed ratios of the genomic copies (VG) to infectious rAAV2 particles (IU) ranging from 333 (VG:IU) to 6000 (VG:IU), with a mean ratio of 1138 (data not shown). Rohr and others quantified the mean ratio of the same sample as 253. Still others have reported ratios ranging from 60–120 (Atkinson et al., 1998) and mean ratios as low as 8.3 (VG:IU) (Zhen et al., 2004). This discrepancy was attributed to the difference in assay sensitivity, which may result in underestimated infectious unit titers and subsequently overestimated VG:IU ratios. Possible high multiplicity of infection of a single cell as well as the presence of infectious-defective particles in the rAAV2 sample population has been another school of thought (Rohr et al., 2002). For the experiments discussed herein, the high sensitivity of the qPCR method and failure to achieve identical experimental conditions consistently may have further confounded titers. For this reason, it was posited that the functional titers (IU) provided a more reliable representation of the presence of infective competent viral particles in a given rAAV sample albeit the number of genome copies could not have been known by this method alone.
3.6 P0 injection of neonatal mice and stereotaxic injection of adult mouse brain
Injection of AAV2 in neonatal (P0) murine pups as well as stereotaxic injection of adult mice was employed to test virus efficacy in vivo. Brains of P0 pups were injected with AAV2 harboring the chicken β-actin (CBA) promoter to drive expression of green fluorescent protein (GFP, 2 uL of 1.5 × 1012 vg/ml). Four weeks post-injection, brains were harvested and prepared for immunohistochemistry. Free-floating tissue sections revealed extensive GFP fluorescence in nearly all brain regions, including but not limited to, the cortex, hippocampus, and cerebellum (Figure 6A). In parallel, stereotaxic injection of 8 week old mice was performed to target AAV2 to the hippocampus. A single stereotaxic injection of AAV2 expressing GFP (2 uL of 1.5 × 109 vg/ml) was delivered into the right hemisphere targeting the dentate gyrus. Four weeks later, brains were sectioned for analysis by confocal microscopy. Free-floating tissue samples revealed intense GFP fluorescence in the dentate gyrus and other hippocampal areas in all mice (Figure 6B).
Figure 6. Efficacy of AAV2 in vivo across different brain areas.
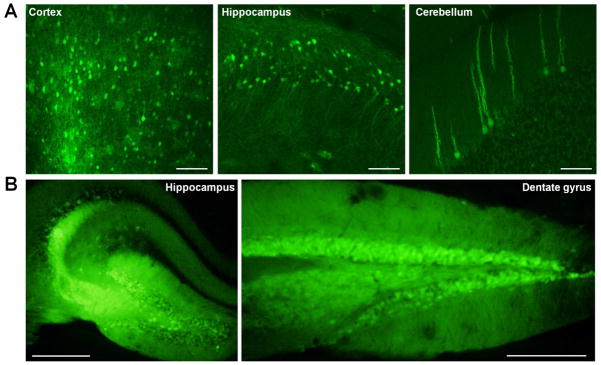
To test efficacy of AAV2 in vivo, P0 or stereotaxic injection of mice was performed and expression of GFP was qualitatively evaluated four weeks post-injection. (A) Representative images of P0 injection of AAV2. Extensive spread of GFP fluorescence was observed in multiple brain regions, including (left to right) cortex, hippocampus, and cerebellum. Scale bars = 100 μM. (B) Representative images of stereotaxic injection of AAV2. GFP fluorescence was observed in all hippocampal regions. Image on right is more posterior. Scale bars = 500 μM. Data representative of three mice.
4. Discussion
In previously described protocols, adeno-associated virus was chiefly produced via co-infection with another helper virus such as Adenovirus or herpes-virus for effective viral replication. Unfortunately, with the use of a helper virus, there yet remained a possibility of contamination from the helper viruses in the final vector preparation. In the current experiments, a helper-free triple-plasmid transfection protocol was used, conferring greater purity, potency and safety (Wright, 2009) in the production of recombinant AAV. Of the three plasmids used for transfection, pHelper plasmid contained the required E2A, E4, and VA RNA adenoviral genes, which eliminated the need for a helper adenovirus (Xiao et al., 1998) (See Figure 1).
A variety of approaches have been reported for the production of rAAV using triple plasmid co-transfection. A co-precipitation method utilizing Calcium phosphate as the transfection reagent has long been described and was established as early as 1973 (Graham et al., 1973) as an efficient means of transferring exogenous DNA into mammalian cells. Under carefully stringent conditions, yields exceeding 105 VG/cell (Wright, 2009) have been obtained and consistently reproduced. However, failure to achieve and maintain highly specific transfection cocktail conditions with a very narrow window for pH variability, results in appreciably lower productivity overall (Wright 2009). With such exacting conditions as pH (7.05±0.01units), reagent concentrations, precipitate formation size, temperature, and the requirement for post-transfection media change, the calcium phosphate-mediated production of AAV proves time-consuming, laborious and ultimately more costly. Still others have employed cationic lipids such as lipofectamine (Reed et al., 2006), which can be extremely efficient but remain inhibitory to recurrent, practical use due to high costs.
The advent of the polycation PEI has proven to be very promising, practical and cost-efficient (127X less than CaCl2 for reagent cost only) for the production of rAAV. Titers up to ~1014 VG per 3.5-liter bioreactor have been reported using PEI-mediated transfection of suspension HEK-293 cells under serum-free conditions, and without the need for exchange of the cell culture medium (Durocher et al., 2007). While this approach appears quite reasonable for the large-scale, high-titer production required for clinical grade cGMP rAAV, it may not be as pragmatic for the pre-clinical research-grade, smaller-scale productions of only 400ml starting volume and concentrated volume of 130–140ul as employed in this study. By optimizing current PEI-mediated protocols for AAV2 production, it was demonstrated that high-titer, research-grade virus in excess of 1013 VG/ml was achieved with feasible starting volumes and less DNA amounts. Additionally, this PEI protocol for adherent cells was uninhibited by serum. AAV serotype 2 has efficiently transduced brain, bone, kidney, lung, liver and, numerous other tissue types (Grimm et al., 2003). All reported viral stocks were near-purified and infected with high transduction efficiency, displaying broad tropism both in vivo in rodent brain (Figure 6) and, in vitro cell culture assays (see Figure 3).
Consistent with prior reports, PEI-mediated transfection efficiency and AAV vector assembly varied according to the ratio of nitrogen to phosphorus in the PEI-DNA polyplexes (Zhang et. al 2004). While the lowest efficiency (5–20%) and crude virus titer (2.3 × 106 IU/ml) were observed with N/P=10, the titer increased with higher ratios in a tenable manner, with N/P=40 producing the highest crude titers of up to 3 × 108 IU/ml (corresponding to 8.5 × 1012 VG/ml). To investigate whether further increasing the N/P ratios would yield correspondingly higher titers, with little to no exacerbated cell death, functional and vector genome titers were determined for N/P ratios 20, 40, 60, and 80 using the previously described protocol. An approximate ten to thirty-fold reduction in titer was observed for N/P ratios 60 and 80, which could be partially attributed to the post-transfection cytotoxicity observed for these ratios (Figure 5). These findings suggested that there might be a threshold for the N/P ratio beyond which PEI becomes unfavorably toxic to the cell, resulting in inefficient viral vector assembly. Reduced viral assembly and thus reduced titers for N/P ratios 60 and 80 may have also been due to the exclusion of excessively large aggregates from endocytotic pathways as reported by Boussif and others (Boussif et al., 1995a).
Although PEI has been proven to be one of the most efficient gene delivery vectors, its efficiency and cytoxicity has been thought to be dose dependent (Thomas et al., 2005a). In this study, we employed a WST-1 based assay to measure cell viability and proliferation in live cells for a relatively broad range of N/P ratios. In this case, as the N/P ratio increased, cell viability decreased (Figure 5). Fischer and others have reported that increasing cytotoxicity of high molecular weight PEI such as 25-kD could be caused by the high affinity binding of the PEI to the outer surface of the plasma membrane. This particular study has demonstrated that, as the overall PEI concentration increased above N/P 40, a commensurate cytopathic effect was observed morphologically by fluorescence and, metabolically, by WST-1 assays.
For approximately 1/5th of AAV2 batches, a total DNA amount of 2.25ug per ml of media and a ratio of N/P=27.5 were employed before the attempt was first made to optimize. To further investigate the utility of an optimal N/P ratio of 40, experiments were performed to test whether approximately halving the DNA amount from 2.25ug/ml to 1.05ug/ml while maintaining this ratio would yield similar or disparate results. An almost 7-fold increase in crude virus titers was observed when the DNA amount was halved, while an approximate 75% reduction in the DNA amount (0.525ug/ml of media) was analogous to a 10-fold decrease in productivity.
These results were congruent with previous findings, demonstrating that AAV2 triple plasmid transfection with the optimal DNA amount and N/P ratio (1.05ug/ml and 40 respectively), crucially affected the size, weight and dynamic geometry of the PEI-DNA complex formation (HOU et al., 2011) and ultimately, overall efficiency. In this case, reasonably decreasing the DNA amount while maintaining a high N/P ratio, increased the amount of excess PEI relative to DNA in solution (5.2ug PEI: 1ug DNA), which in turn accelerated both the settling of the complexes on the cell surface as well as the escape of the DNA from the endosomes via the proton sponge effect (Behr, 1997) as soon as 4hrs post-transfection (Reed et al., 2006).
Based on findings in the literature, it was anticipated that 10–20% of total purified virus would be recovered from the supernatant (Zolotukhin et al., 1999). Reed and colleagues also reported that an approximate 73% of total virus was found in cell lysates of HeLa and XDC293 cells and hence, did not deem the supernatant harvest a worthwhile endeavor (Reed et al., 2006). Surprisingly however, this study has shown that an average of 43–62% of the total virus was contributed by the media harvest for AAV2, a yield well worth the effort of harvesting (Figure 4B). While this phenomenon remains incompletely understood, it could be partially attributed to a possible active virion export pathway (Guo et al., 2012), cell lineage, as well as incubation time post-transfection. The latter is consistent with data revealing that culture media titers were higher with increased incubation time post transfection, with as much as 86% of virus in the supernatant being reported for varied serotypes (Lock et al., 2010; Vandenberghe et al., 2010).
In conclusion, the importance of DNA amount and N/P ratio in optimization of the PEI-mediated AAV2 vector production protocol was elucidated. This optimized protocol for increasing overall titers by harvesting the supernatant along with crude cell lysate, was also demonstrated as being efficacious. With reasonable starting volumes, less plasmid DNA amounts, and by eliminating the need for media change, overall production cost and workload were lowered substantially compared to Calcium phosphate-based protocols. The described method was simple, highly reproducible, and extremely efficient in yielding high-titer functional AAV2 vector particles. It is expected that the utility of this protocol proves applicable to all AAV serotypes with relevance for the production of other viral vectors such as lentivirus. Thus far, this protocol has been successfully implemented in this laboratory for serotypes 1, 5, and 9. Future optimization approaches should focus on the influence of serum on vector productivity as well as the role of differential effects of media choice on PEI-mediated transfection efficiency.
Highlights.
We describe a triple-plasmid co-transfection approach with PEI to produce AAV2.
The genomic titer of the viral stock was determined by qPCR to be >1013 VG/ml.
High expression occurred both in vivo within the brain and in vitro in cell culture.
PEI increases efficiency, lowers cost, while producing high-titer, efficacious AAV2.
Acknowledgments
We would like to thank Dr. Thomas Kukar for gifting the HEK293T cells and Dr. Nancy Ciliax for administrative support. This research project was supported by Emory Neuroscience NINDS Core Facility grant for the Viral Vector Core, P30NS055077.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atkinson EM, Debelak DJ, Hart LA, Reynolds TC. A high-throughput hybridization method for titer determination of viruses and gene therapy vectors. Nucleic Acids Res. 1998;26:2821–3. doi: 10.1093/nar/26.11.2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayuso E, Mingozzi F, Montane J, Leon X, Anguela XM, Haurigot V, Edmonson SA, Africa L, Zhou S, High KA, Bosch F, Wright JF. High AAV vector purity results in serotype- and tissue-independent enhancement of transduction efficiency. Gene Ther. 2010;17:503–510. doi: 10.1038/gt.2009.157. [DOI] [PubMed] [Google Scholar]
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, Petersen-Jones S, Bhattacharya SS, Thrasher AJ, Fitzke FW, Carter BJ, Rubin GS, Moore AT, Ali RR. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358:2231–9. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- Behr JP. The proton sponge: A trick to enter cells the viruses did not exploit. Chimia. 1997;51:34–36. [Google Scholar]
- Boeckle S, von Gersdorff K, van der Piepen S, Culmsee C, Wagner E, Ogris M. Purification of polyethylenimine polyplexes highlights the role of free polycations in gene transfer. J Gene Med. 2004;6:1102–1111. doi: 10.1002/jgm.598. [DOI] [PubMed] [Google Scholar]
- Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proceedings of the National Academy of Sciences of the United States of America. 1995a;92:7297–301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussif O, Lezoualch F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A Versatile Vector for Gene and Oligonucleotide Transfer into Cells in Culture and in-Vivo -Polyethylenimine. Proceedings of the National Academy of Sciences of the United States of America. 1995b;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles DE, McPhee SWJ, Li CW, Gray SJ, Samulski JJ, Camp AS, Li J, Wang B, Monahan PE, Rabinowitz JE, Grieger JC, Govindasamy L, Agbandje-McKenna M, Xiao X, Samulski RJ. Phase 1 Gene Therapy for Duchenne Muscular Dystrophy Using a Translational Optimized AAV Vector. Mol Ther. 2012;20:443–455. doi: 10.1038/mt.2011.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clamme JP, Azoulay J, Mely Y. Monitoring of the formation and dissociation of polyethylenimine/DNA complexes by two photon fluorescence correlation spectroscopy. Biophysical Journal. 2003a;84:1960–1968. doi: 10.1016/S0006-3495(03)75004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clamme JP, Krishnamoorthy G, Mely Y. Intracellular dynamics of the gene delivery vehicle polyethylenimine during transfection: investigation by two-photon fluorescence correlation spectroscopy. Biochimica Et Biophysica Acta-Biomembranes. 2003b;1617:52–61. doi: 10.1016/j.bbamem.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Durocher Y, Pham PL, St-Laurent G, Jacob D, Cass B, Chahal P, Lau CJ, Nalbantoglu J, Kamen A. Scalable serum-free production of recombinant adeno-associated virus type 2 by transfection of 293 suspension cells. Journal of Virological Methods. 2007;144:32–40. doi: 10.1016/j.jviromet.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Godbey WT, Wu KK, Mikos AG. Tracking the intracellular path of poly(ethylenimine)/DNA complexes for gene delivery. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:5177–5181. doi: 10.1073/pnas.96.9.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham FL, Vandereb AJ. New Technique for Assay of Infectivity of Human Adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Grimm D, Kay MA, Kleinschmidt JA. Helper virus-free, optically controllable, and two-plasmid-based production of adeno-associated virus vectors of serotypes 1 to 6. Mol Ther. 2003;7:839–850. doi: 10.1016/s1525-0016(03)00095-9. [DOI] [PubMed] [Google Scholar]
- Guo P, El-Gohary Y, Prasadan K, Shiota C, Xiao XW, Wiersch J, Paredes J, Tulachan S, Gittes GK. Rapid and simplified purification of recombinant adeno-associated virus. Journal of Virological Methods. 2012;183:139–146. doi: 10.1016/j.jviromet.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggan MD, Blacklow NR, Rowe WP. Studies of Small DNA Viruses Found in Various Adenovirus Preparations - Physical Biological and Immunological Characteristics. Proceedings of the National Academy of Sciences of the United States of America. 1966;55:1467. doi: 10.1073/pnas.55.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayandharan GR, Aslanidi G, Martino AT, Jahn SC, Perrin GQ, Herzog RW, Srivastava A. Activation of the NF-kappaB pathway by adeno-associated virus (AAV) vectors and its implications in immune response and gene therapy. Proc Natl Acad Sci U S A. 2011;108:3743–8. doi: 10.1073/pnas.1012753108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, Bland RJ, Young D, Strybing K, Eidelberg D, During MJ. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet. 2007;369:2097–105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- Kiyota T, Yamamoto M, Schroder B, Jacobsen MT, Swan RJ, Lambert MP, Klein WL, Gendelman HE, Ransohoff RM, Ikezu T. AAV1/2-mediated CNS Gene Delivery of Dominant-negative CCL2 Mutant Suppresses Gliosis, beta-amyloidosis, and Learning Impairment of APP/PS1 Mice. Mol Ther. 2009;17:803–809. doi: 10.1038/mt.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Daly TM. Adeno-associated virus-mediated gene transfer to the neonatal brain. Methods. 2002;28:203–207. doi: 10.1016/s1046-2023(02)00224-4. [DOI] [PubMed] [Google Scholar]
- Lock M, Alvira M, Vandenberghe LH, Samanta A, Toelen J, Debyser Z, Wilson JM. Rapid, Simple, and Versatile Manufacturing of Recombinant Adeno-Associated Viral Vectors at Scale. Human Gene Therapy. 2010;21:1259–1271. doi: 10.1089/hum.2010.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer GP, Herzog RW, Mount J, Arruda VR, Tillson DM, Hathcock J, van Ginkel FW, High KA, Lothrop CD., Jr Long-term correction of inhibitor-prone hemophilia B dogs treated with liver-directed AAV2-mediated factor IX gene therapy. Blood. 2009;113:797–806. doi: 10.1182/blood-2008-10-181479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogris M, Steinlein P, Kursa M, Mechtler K, Kircheis R, Wagner E. The size of DNA/transferrin-PEI complexes is an important factor for gene expression in cultured cells. Gene Therapy. 1998;5:1425–1433. doi: 10.1038/sj.gt.3300745. [DOI] [PubMed] [Google Scholar]
- Passini MA, Watson DJ, Vite CH, Landsburg DJ, Feigenbaum AL, Wolfe JH. Intraventricular brain injection of adeno-associated virus type 1 (AAV1) in neonatal mice results in complementary patterns of neuronal transduction to AAV2 and total long-term correction of storage lesions in the brains of beta-glucuronidase-deficient mice. Journal of Virology. 2003;77:7034–7040. doi: 10.1128/JVI.77.12.7034-7040.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranski EL, Van Sanford CD, Dalal NV, Orr AL, Karmali D, Cooper DS, Costa N, Heilman CJ, Gearing M, Lah JJ, Levey AI, Betarbet RS. Comparative distribution of protein components of the A20 ubiquitin-editing complex in normal human brain. Neuroscience Letters. 2012;520:104–109. doi: 10.1016/j.neulet.2012.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed SE, Staley EM, Mayginnes JP, Pintel DJ, Tullis GE. Transfection of mammalian cells using linear polyethylenimine is a simple and effective means of producing recombinant adeno-associated virus vectors. Journal of Virological Methods. 2006;138:85–98. doi: 10.1016/j.jviromet.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Rohr UP, Wulf MA, Stahn S, Steidl U, Haas R, Kronenwett R. Fast and reliable titration of recombinant adeno-associated virus type-2 using quantitative real-time PCR. Journal of Virological Methods. 2002;106:81–88. doi: 10.1016/s0166-0934(02)00138-6. [DOI] [PubMed] [Google Scholar]
- Thomas M, Ge Q, Lu JJ, Chen J, Klibanov AM. Cross-linked small polyethylenimines: while still nontoxic, deliver DNA efficiently to mammalian cells in vitro and in vivo. Pharmaceutical research. 2005a;22:373–80. doi: 10.1007/s11095-004-1874-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Ge Q, Lu JJ, Chen JZ, Klibanov AM. Cross-linked small polyethylenimines: While still nontoxic, deliver DNA efficiently to mammalian cells in vitro and in vivo. Pharmaceutical Research. 2005b;22:373–380. doi: 10.1007/s11095-004-1874-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe LH, Xiao R, Lock M, Lin JP, Korn M, Wilson JM. Efficient Serotype-Dependent Release of Functional Vector into the Culture Medium During Adeno-Associated Virus Manufacturing. Human Gene Therapy. 2010;21:1251–1257. doi: 10.1089/hum.2010.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JF. Transient Transfection Methods for Clinical Adeno-Associated Viral Vector Production. Human Gene Therapy. 2009;20:698–706. doi: 10.1089/hum.2009.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. Journal of Virology. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen Z, Espinoza Y, Bleu T, Sommer JM, Wright JF. Infectious titer assay for adeno-associated virus vectors with sensitivity sufficient to detect single infectious events. Human Gene Therapy. 2004;15:709–715. doi: 10.1089/1043034041361262. [DOI] [PubMed] [Google Scholar]
- Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K, Summerford C, Samulski RJ, Muzyczka N. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Therapy. 1999;6:973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]