Abstract
Introduction
Barasertib is the pro-drug of barasertib-hQPA, a selective Aurora B kinase inhibitor that has demonstrated preliminary anti-acute myeloid leukemia (AML) activity in the clinical setting.
Methods
This Phase I dose-escalation study evaluated the safety and tolerability of barasertib, combined with low-dose cytosine arabinoside (LDAC), in patients aged ≥60 years with de novo or secondary AML. Barasertib (7-day continuous intravenous infusion) plus LDAC 20 mg (subcutaneous injection twice daily for 10 days) was administered in 28-day cycles. The maximum tolerated dose (MTD) was defined as the highest dose at which ≤1 patient within a cohort of six experienced a dose-limiting toxicity (DLT) (clinically significant adverse event [AE] or laboratory abnormality considered related to barasertib). The MTD cohort was expanded to 12 patients.
Results
Twenty-two patients (median age, 71 years) received ≥1 treatment cycle (n=6, 800 mg; n=13, 1000 mg; n=3, 1200 mg). DLTs were reported in two patients (both, CTCAE grade 3 stomatitis/mucositis; 1200 mg cohort). The most common AEs were infection (73%), febrile neutropenia (59%), nausea (50%), and diarrhea (46%). Barasertib plus LDAC resulted in an overall response rate (International Working Group criteria) of 45% (n=10/22; by investigator opinion).
Conclusion
The MTD of 1000 mg barasertib in combination with LDAC in older patients with AML was associated with acceptable tolerability and preliminary anti-AML activity. Clinicaltrials.gov, number NCT00926731.
Keywords: Acute myeloid leukemia, barasertib-hQPA, dose-escalation study
Introduction
The incidence of acute myeloid leukemia (AML) increases with age, with most patients aged older than 65 years at initial diagnosis.1 From data collated from 2000–2004 for the Surveillance, Epidemiology, and End Results (SEER) Program, the 5-year relative survival rates in elderly AML patient populations were reported to be 9.2% and 2.5% in those aged 65–74 years, and >75 years, respectively. In comparison, the 5-year relative survival rates observed during the same timeframe in younger patients were 19.9% (55–64 years) and 36.6% (35–54 years).2
The standard treatment for AML involves a regimen of intensive induction chemotherapy (usually 3 days of anthracycline and 7 days of cytarabine).3 While median survival with induction chemotherapy in younger adults is 16–24 months and early mortality is <10%,4 median survival in older adults is less than 6 months and 8-week mortality rates are in excess of 30%.5 Low-dose cytosine arabinoside (LDAC) is the only agent to have demonstrated clinical benefit prospectively in randomized clinical trials of AML patients aged ≥60 years who were deemed unfit for intensive chemotherapy.6 However, there has been little improvement in survival rates in elderly patients over the past three decades2 and treatment options remain limited and largely palliative in elderly AML patients.
The Aurora kinase family of proteins (Aurora A, B, and C) regulates mitosis and chromosome segregation.7 Aurora B kinase is involved in the spindle assembly checkpoint component of the mitotic process and is specifically over expressed in AML.8,9 Barasertib (AZD1152), is a prodrug that rapidly undergoes phosphatase-mediated cleavage in human serum to release the more active drug, barasertib hydroxy-QPA (barasertib-hQPA), a highly potent and selective Aurora B kinase inhibitor. In preclinical studies, barasertib has been shown to inhibit the growth and survival of AML cell lines and human tumor xenograft models.10–13 Barasertib has also demonstrated preliminary efficacy in the clinical setting, including elderly patients with advanced AML.14–16
This is the first study to assess the safety, tolerability and pharmacokinetics (PK) of barasertib and LDAC combination treatment in elderly patients with AML. The study was conducted with the principal aim of providing information on feasibility and dosing for future Phase II studies evaluating the efficacy of barasertib/LDAC combination treatment.
Patients and Methods
Patients
Eligible patients had newly diagnosed de novo or secondary AML, or chronic myelomonocytic leukemia (CMML), according to World Health Organization (WHO) pathologic classification;17 were aged ≥60 years, judged unsuitable for intensive induction chemotherapy, and were considered likely to be able to complete 12 weeks (three cycles) of treatment. Cytogenetic risk groups were assigned according to Medical Research Council criteria.18 Patients were required to have a WHO performance status (PS) of 0–3 (PS of 3 was acceptable if solely attributed to the underlying AML) and considered likely to complete three cycles (12 weeks) of treatment. Patients with asymptomatic central nervous system (CNS) disease were eligible if symptom free for >10 days. Exclusion criteria included: diagnosis of acute promyelocytic leukemia (APL) or blast crisis of chronic myeloid leukemia; chemotherapy, radiotherapy or an investigational anticancer agent within 2 weeks of the start of study treatment; persistent clinically significant toxicities from any prior anticancer therapy of National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) grade >1 (except alopecia); serum creatinine >1.5×the upper limit of normal (ULN) or 24-hour creatinine clearance <50 mL/min (Cockcroft-Gault); serum bilirubin >1.5 × ULN; aspartate aminotransferase (AST) or alanine aminotransferase (ALT) >2.5 × ULN (unless considered due to leukemic organ involvement); and QTc interval ≥470 ms. All patients provided written informed consent prior to study entry.
Study design
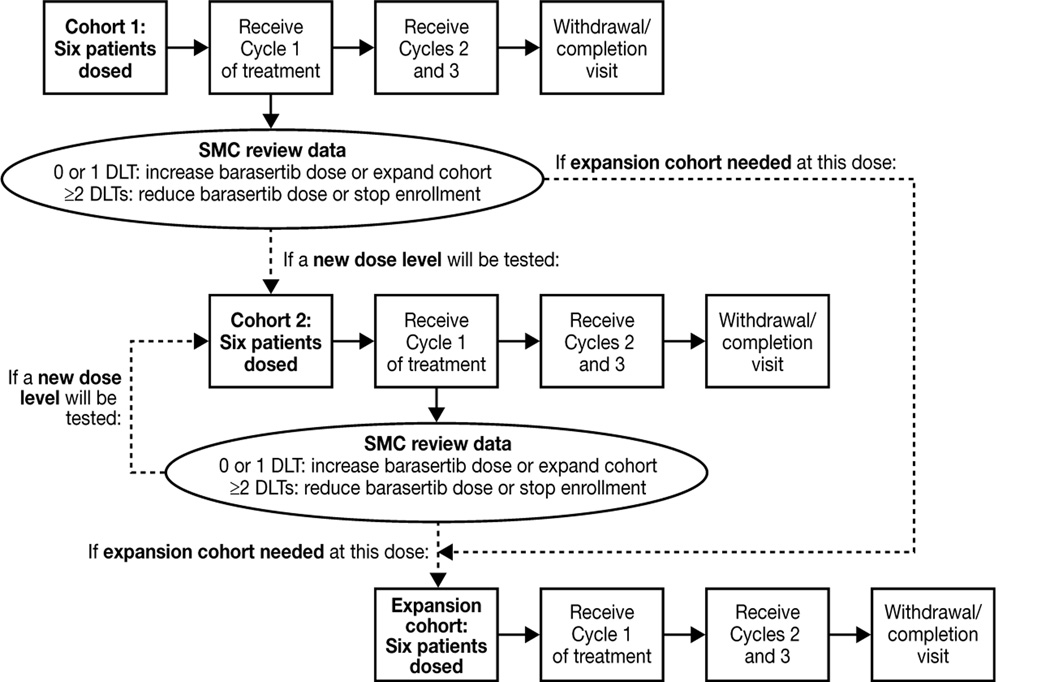
This was a multicenter, open-label, Phase I, dose-escalation study to assess the safety, tolerability and PK of barasertib in combination with LDAC in elderly patients with newly diagnosed AML. Patients received barasertib as a 7-day continuous intravenous infusion from Day 1 to Day 7, and LDAC (20 mg; twice daily as subcutaneous injections) from Day 1 to Day 10, of 28-day treatment cycles (clinicaltrials.gov identifier NCT00926731). In the first dose cohort, six patients received a starting dose of 800 mg barasertib (Figure 1). On completion of the first treatment cycle, the clinical and laboratory data from all patients were reviewed by the Safety Monitoring Committee (SMC) to determine either progress to the next dose level (escalation or reduction) or additional patient recruitment to this initial cohort. In each subsequent dose cohort, the first three patients recruited were to complete the first treatment cycle before additional patients were dosed. If ≥2 patients experienced DLTs at a given dose level, this dose was considered nontolerated and dose escalation was stopped. Consequently, the maximum tolerated dose (MTD) of barasertib in combination with LDAC was defined as the highest dose of barasertib with ≤1 patient reporting DLTs within treatment cycle 1. Once established, the MTD cohort was expanded to a maximum of 12 evaluable patients. No intra-patient dose escalation was permitted. Barasertib doses selected by the SMC did not exceed the previously identified MTD for monotherapy (1200 mg)15 or, in cases of poor tolerability, decrease below 400 mg (the lowest dose associated with clinical activity). DLTs were defined as a clinically significant adverse event (AE) or laboratory abnormality considered to be related to barasertib and occurring within the first treatment cycle; specifically, NCI CTCAE (version 3.0) grade 3, 4 or 5 non-hematological (including biochemical) toxicity despite adequate supportive care or severe bone marrow suppression for ≥56 days. The study was approved by the local ethics committee or independent review board for each participating site. The trial complied with the principles of the Declaration of Helsinki and was conducted in accordance with Good Clinical Practice and the AstraZeneca policy on bioethics.19
Figure 1. Study design.
Footnote: SMC, safety monitoring committee
Endpoints and assessments
The primary objective of this study was to evaluate the safety and tolerability of barasertib in combination with LDAC to identify a recommended dose for future Phase II trials. Secondary objectives were to evaluate the PK profiles of barasertib, barasertib-hQPA and LDAC when in combination and alone. Assessment of efficacy was an exploratory objective.
Safety and tolerability were evaluated throughout the study by the incidence and severity of AEs (graded according to NCI CTCAE version 3.0) and changes in physical examination, vital signs, hematology, ECG and clinical laboratory parameters. For patients who completed three cycles of treatment (irrespective of the decision to continue treatment), completion/withdrawal assessments were performed at Day 28 of Cycle 3 and at follow-up (28 days after the last dose of study medication). Patients who discontinued treatment before Cycle 3 underwent assessment at Day 28 of the last cycle that was initiated. Blood samples (3 mL) were collected for determination of barasertib and barasertib-hQPA PK profiles according to the following schedule: pre-dose on Day 1; 24, 48 and 144 hours during infusion; 5 minutes before end of infusion (EOI; 167.92 hours) and post-EOI at 0.25, 1, 2, 6 hours (Day 8), 24 hours (Day 9), 48 hours (Day 10), 168 hours (Day 15) and 336 hours (Day 22). For analysis of LDAC PK, blood samples (2 mL) were collected pre-dose, and post-dose on Days 7 and 10 at 0.25, 1, 1.5, 2, 3, 4, 6, 8 hours and 12 hours (or immediately prior to next injection if earlier). Plasma concentrations of barasertib, barasertib-hQPA and LDAC were determined using liquid chromatography with mass spectrometry by an independent laboratory (PRA, The Netherlands). Lower limits of quantification (LLOQ) for barasertib/barasertibhQPA and LDAC were 0.25 and 0.5 ng/mL, respectively. AML response was assessed by investigators according to the International Working Group response criteria,20 by means of bone marrow aspirate, trephine biopsy and peripheral blood analysis, according to local clinical practice.
Statistical analysis
Sample size for this study was determined using standard 3+3 Phase I dose escalation guidelines, with a cohort expansion of an additional six patients to obtain additional safety, tolerability, and efficacy data. Demographic and baseline disease characteristics of all patients were summarized descriptively, using means, medians, and ranges where appropriate. AEs and efficacy were summarized for the overall treated population and within dosing cohorts. The safety of the combination was evaluated by the frequency and severity of AEs according to NCI CTCAE (version 3.0). The incidence and percentage of AEs were summarized overall and within each dosing cohort. All patients who received ≥1 dose of study treatment and for whom post-dose data were available were included in the assessment of safety, tolerability and efficacy. Patients with sufficient PK blood sampling comprised the PK analysis population. To assess potential PK interactions between barasertib/barasertib-hQPA and LDAC, individual and summary statistics for PK variables (determined using standard non-compartmental methods) during combination treatment were compared with PK profiles determined for barasertib monotherapy obtained from a previous study15 and with LDAC alone (Day 10 of current study).
Results
Patients
Between June 2009 and June 2010, 22 patients with AML across four study sites in the US and France were assigned to treatment (barasertib 800 mg [n=6], 1000 mg [n=13], 1200 mg [n=3]). The median age (range) was 71 (61–82) years, and patients were mainly male (64%) and Caucasian (96%) with de novo AML (36%) or AML secondary to MDS (41%) (Table 1). At data cut-off (DCO; 17 December 2010), 20 patients (barasertib 800 mg, n=6; 1000 mg, n=11; 1200 mg, n=3) had discontinued study treatment. Discontinuation was due to disease progression (n=9), lack of response (n=5), voluntary discontinuation (n=2), AEs (n=2), change in therapy (n=1) and improvement in condition (n=1). Two patients, both receiving 1000 mg barasertib, were ongoing at DCO. These patients subsequently discontinued due to improvement (n=1; duration of treatment, 6.7 months) or worsening (n=1; duration of treatment, 13.8 months) of their condition.
Table 1.
Patient demographics and baseline characteristics
| Barasertib dose + LDAC 400 mg | ||||
|---|---|---|---|---|
| 800 mg (n=6) |
1000 mg (n=13) |
1200 mg (n=3) |
Total (n=22) |
|
| Male/female | 3/3 | 9/4 | 2/1 | 14/8 |
| Median age (range), years | 75 (70–82) | 71 (61–79) | 69 (64–70) | 71 (61–82) |
| Age group, n (%) | ||||
| ≥60 to 69 y | 0 | 5 | 2 | 7 (32) |
| ≥70 to 74 y | 3 | 4 | 1 | 8 (36) |
| ≥75 y | 3 | 4 | 0 | 7 (32) |
| Race, n (%) | ||||
| Caucasian | 6 | 12 | 3 | 21 (96) |
| African American | 0 | 1 | 0 | 1 (5) |
| Performance status, n (%) | ||||
| 0 | 2 | 5 | 3 | 10 (46) |
| 1 | 3 | 4 | 0 | 7 (32) |
| 2 | 1 | 4 | 0 | 5 (23) |
| AML type, n (%) | ||||
| Secondary to myelodysplastic syndrome | 2 | 5 | 2 | 9 (41) |
| De novo | 4 | 4 | 0 | 8 (36) |
| Secondary to myeloproliferative disorder | 0 | 1 | 1 | 2 (9) |
| Chronic myelomonocytic leukemia | 0 | 2 | 0 | 2 (9) |
| Secondary to chemotherapy | 0 | 1 | 0 | 1 (5) |
| WBC count at screening, 109/L, n (%) | ||||
| 0–9.9 | 4 | 11 | 2 | 17 (77) |
| ≥10–49.9 | 1 | 2 | 1 | 4 (18) |
| ≥50 | 1 | 0 | 0 | 1 (5) |
| Bone marrow cytogenetics, n (%)* | ||||
| Intermediate risk, no abnormality | 1 | 6 | 2 | 9 (41) |
| Adverse risk, +8 | 0 | 1 | 0 | 1 (5) |
| del(7q) | 1 | 3 | 0 | 4 (18) |
| other | 1 | 0 | 1 | 2 (9) |
| Sample not available | 3 | 3 | 0 | 6 (27) |
Medical Research Council prognostic groups18
WBC, white blood cell
Safety and tolerability
All patients received ≥1 treatment cycle, 11 patients received two cycles and five patients received ≥3 cycles. During the dose-escalation phase, one DLT (grade 3 pancytopenia) that was considered related to study treatment was reported in the initial cohort (barasertib 800 mg). Two DLTs (both grade 3 stomatitis/mucositis) were subsequently reported in the barasertib 1200 mg cohort. Thus, this dose level was considered non-tolerated. An intermediate dose (barasertib 1000 mg) was identified by the SMC for further investigation. No DLTs were reported at this dose level which was designated as the MTD and investigated further in the dose expansion phase.
The most common AEs were febrile neutropenia (n=13; 59%), nausea (n=11; 50%), diarrhea (n=10; 46%), peripheral edema (n=9; 41%), and stomatitis (n=9; 41%; Table 2). Grade ≥3 AEs were reported in 21 patients (Table 3); the most common of which was febrile neutropenia, occurring in 13 (59%) patients overall and nine (69%) patients who received the MTD of barasertib 1000 mg. Thirty-six infection events were experienced by 16 (73%) patients while on study treatment, although only two of these events were considered related to study treatment (barasertib 800 mg, oral herpes; barasertib 1000 mg, anorectal infection). Grade ≥3 infection was reported in seven (32%) patients. Two grade 4 AEs were reported, both of thrombocytopenia observed in one patient each in the barasertib 800 mg and 1000 mg cohorts. Serious AEs (SAEs) were reported in 15 (68%) patients overall; all were single events except for febrile neutropenia (n=8, 36%) and stomatitis (n=2, 9%). Five patients experienced SAEs considered related to study treatment (n=1, grade 3 pancytopenia; n=1, grade 3 febrile neutropenia and anorectal infection; n=1, grade 3 febrile neutropenia; n=2, stomatitis). Three patients had dose interruptions due to AEs (n=1, grade 3 pancytopenia; n=1, grade 3 febrile neutropenia and acute myocardial infarction; n=1, grade 3 febrile neutropenia and urinary tract infection). Dose reduction occurred in one patient (barasertib 1000 mg reduced to 800 mg in cycle 3) due to grade 3 stomatits. One patient discontinued study treatment due to an AE of hypoxia; this event was not considered by the investigator to be related to barasertib or LDAC. The observed AE, in conjunction with disease progression resulted in the death of this patient. A further two deaths occurred during the study, with the causes reported as disease progression and ‘sudden death’ (cause unknown). ECG assessment showed no clinically significant changes as a result of combination treatment in the study. Three patients had AEs of atrial fibrillation, one of which became a SAE on day 15 of treatment. All three patients had concurrent cardiac morbidities and none of these AEs of atrial fibrillation were considered treatment related by the investigators. One patient suffered a myocardial infarction on day 48 (cycle 2), CTC grade 4, which was also considered unrelated to study treatment. There were no clinically relevant adverse changes in vital signs, hematology, or clinical chemistry parameters as a result of combination treatment with barasertib and LDAC.
Table 2.
Adverse events (any cause; all grades) occurring in ≥15% of patients, by MedDRA preferred term
| Barasertib dose + LDAC 400 mg | ||||
|---|---|---|---|---|
| Adverse event (AE), n (%) | 800 mg (n=6) |
1000 mg (n=13) |
1200 mg (n=3) |
Total (n=22) |
| Any AE | 6 | 13 | 3 | 22 (100) |
| Febrile neutropenia | 4 | 9 | 0 | 13 (59) |
| Nausea | 4 | 6 | 1 | 11 (50) |
| Diarrhea | 5 | 5 | 0 | 10 (46) |
| Peripheral edema | 2 | 7 | 0 | 9 (41) |
| Stomatitis | 3 | 4 | 2 | 9 (41) |
| Alopecia | 2 | 4 | 0 | 6 (27) |
| Constipation | 3 | 3 | 0 | 6 (27) |
| Petechiae | 3 | 2 | 1 | 6 (27) |
| Pneumonia | 3 | 3 | 0 | 6 (27) |
| Vomiting | 3 | 2 | 1 | 6 (27) |
| Confusional state | 2 | 3 | 0 | 5 (23) |
| Headache | 3 | 2 | 0 | 5 (23) |
| Oropharyngeal pain | 1 | 4 | 0 | 5 (23) |
| Pyrexia | 3 | 2 | 0 | 5 (23) |
| Cough | 1 | 3 | 0 | 4 (18) |
| Decreased appetite | 2 | 1 | 1 | 4 (18) |
| Hemorrhoids | 1 | 3 | 0 | 4 (18) |
| Hypertension | 0 | 3 | 1 | 4 (18) |
| Insomnia | 0 | 3 | 1 | 4 (18) |
| Musculoskeletal pain | 2 | 2 | 0 | 4 (18) |
| Neutropenia | 1 | 2 | 1 | 4 (18) |
| Rash | 2 | 2 | 0 | 4 (18) |
Table 3.
Grade ≥3 adverse events occurring in ≥2 patients, by MedDRA preferred term
| Barasertib dose + LDAC 400 mg | ||||
|---|---|---|---|---|
| Adverse event, n (%) | 800 mg (n=6) |
1000 mg (n=13) |
1200 mg (n=3) |
Total (n=22) |
| Any grade ≥3 AE* | 6 | 12 | 3 | 21 (96) |
| Febrile neutropenia | 4 | 9 | 0 | 13 (59) |
| Neutropenia | 1 | 2 | 1 | 4 (18) |
| Stomatitis | 0 | 2 | 2 | 4 (18) |
| Pneumonia | 0 | 3 | 0 | 3 (14) |
| Anemia | 2 | 0 | 0 | 2 (9) |
| Diarrhea | 1 | 1 | 0 | 2 (9) |
| Thrombocytopenia | 1 | 1 | 0 | 2 (9) |
All events are grade 3 except for thrombocytopenia (both grade 4)
Pharmacokinetics
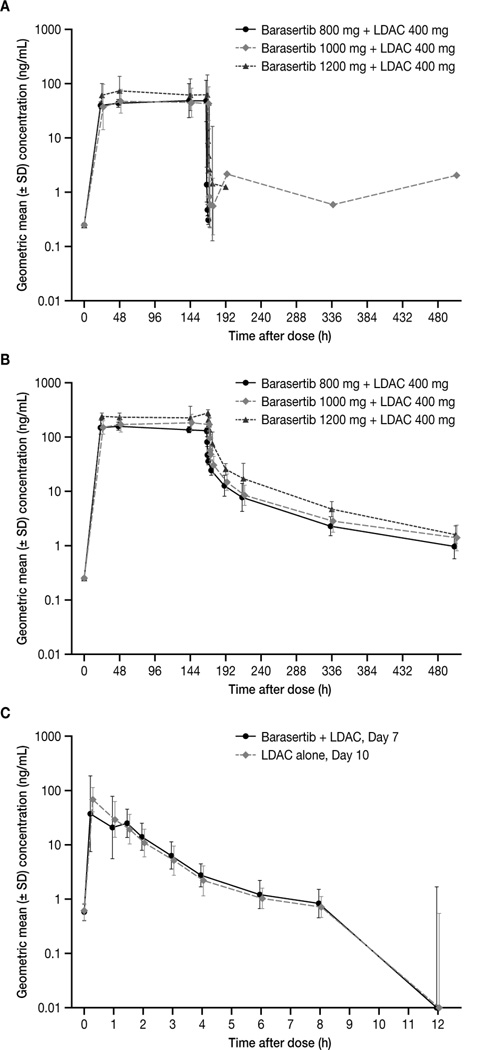
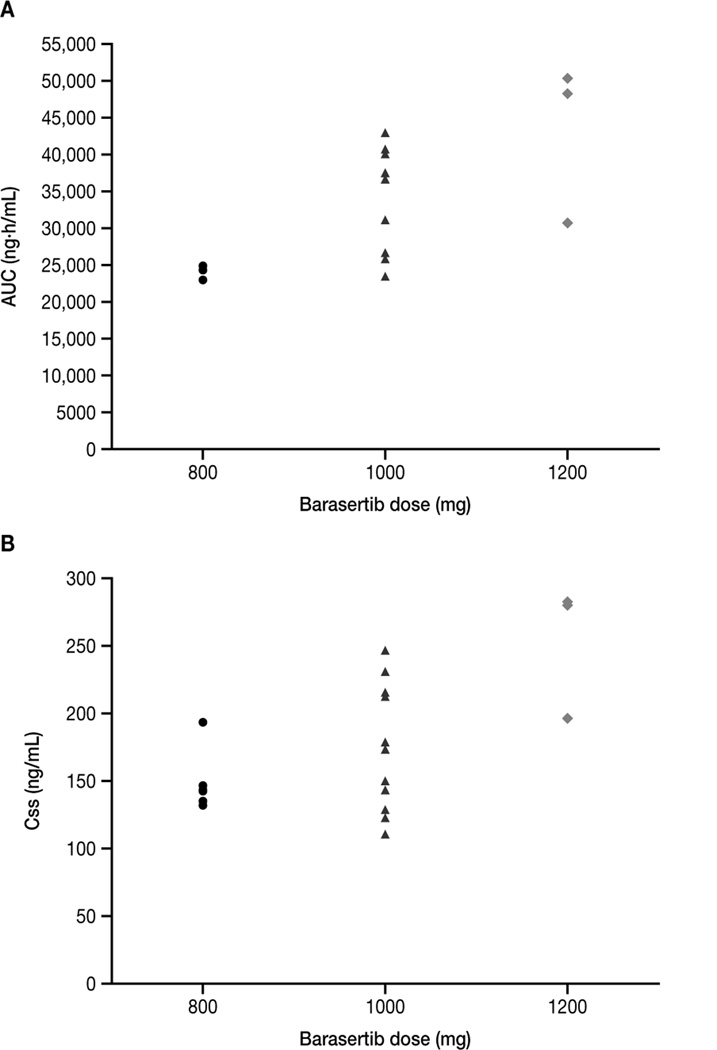
Steady-state plasma concentrations of barasertib and barasertib-hQPA were observed from 24 hours after the start of the barasertib infusion on Day 1, and were maintained until EOI (Figure 2). Consistent with metabolism to its more active form, barasertib levels were generally undetectable 2 hours following EOI whereas barasertib-hQPA was detected in samples up to and including 336 hours post EOI. Exposure to barasertib-hQPA during the infusion was approximately four-fold higher than that of barasertib. A trend for barasertib-hQPA exposure to increase more than proportionally with barasertib dose was apparent, although the low patient numbers preclude definitive conclusions (Figure 3). Due to its rapid conversion to barasertib-hQPA, it was not possible to determine the terminal elimination half-life (t½), clearance or apparent volume of distribution at steady state (VSS) for barasertib. Following EOI, barasertib-hQPA was eliminated in a biphasic manner, with an initial rapid decline followed by a longer terminal phase (t½, 3.5–4.5 days). For the barasertib 1000 mg cohort, the geometric mean clearance rate was 30.5 L/h (range, 23.4–43.3); the highest geometric mean volume of distribution at steady state was achieved in this cohort (713 L [range, 509–1050]) (Table 4). Minimal accumulation of barasertib-hQPA was observed. LDAC was rapidly absorbed, with peak plasma concentrations (Cmax) generally observed from 0.25–1 hour post administration (Figure 2). Subsequent elimination of LDAC occurred in a monophasic manner and was rapid (t½, 1–2 hours). Overall, and irrespective of the barasertib dose level administered, exposure to LDAC, clearance and distribution at steady state did not differ between Day 7 (in combination with barasertib) and 10 (without barasertib) (Table 4).
Figure 2. Plasma concentration–time curves for (A) barasertib; (B) barasertib-hQPA; and (C) LDAC.
Figure 3. Exposure (AUC and Css) to barasertib-hQPA by barasertib dose.
Table 4.
Pharmacokinetic parameters for barasertib, barasertib-hQPA and LDAC
| Barasertib dose + LDAC 400 mg | ||||||
|---|---|---|---|---|---|---|
| Parameter* | 800 mg (n=6) |
1000 mg (n=13) |
1200 mg (n=3) |
|||
| Barasertib | ||||||
| Css (ng/mL) | 46.7 (55.8) | 44.4 (55.5) | 64.9 (67.4) | |||
| AUC0–t (ng.h/mL) | 7299 (48.8) | 7046 (54.2) | 10400 (66.3) | |||
| AUC0–EOI (ng.h/mL) | 7265 (49.1) | 6985 (54.8) | 10330 (66.7) | |||
| Barasertib-hQPA | ||||||
| Css (ng/mL) | 147.3 (13.9) | 170.2 (27.4) | 249.2 (21.0) | |||
| AUC0–t (ng.h/mL) | 24040 (3.4) | 32840 (23.1) | 41850 (28.2) | |||
| AUC0–EOI (ng.h/mL) | 23240 (14.9) | 26860 (29.8) | 37510 (26.6) | |||
| Day | ||||||
| LDAC | 7 | 10 | 7 | 10 | 7 | 10 |
| Cmax (ng/mL) | 54.3 (62.4) | 62.1 (65.1) | 47.5 (88.1) | 72.3 (52.0) | 76.1 (120.6) | 64.2 (130.4) |
| t½ (h)† | 1.5 (1.0–1.8) | 1.5 (1.0–1.9) | 1.2 (0.6–2.1) | 1.3 (0.9–2.1) | NC | NC |
| AUC (ng.h/mL) | 94.1 (40.0) | 96.8 (57.2) | 71.9 (55.7) | 96.6 (29.1) | NC | NC |
| AUC0–8 (ng.h/mL) | 93.0 (35.8) | 98.4 (36.3) | 76.1 (60.5) | 90.4 (43.5) | 115.4 (57.4) | 90.9 (54.9) |
| AUC0–8 ratio (ng.h/mL), Day 7:Day10 | 1.1 (14.6) | 1.2 (24.2) | 0.79 (105.7) | |||
| CL (L/h) | 212.6 (40.0) | 206.7 (57.2) | 278.1 (55.7) | 207.0 (29.1) | NC | NC |
| VSS (L) | 447.2 (51.3) | 361.6 (92.1) | 448.2 (85.8) | 279.5 (47.0) | NC | NC |
AUC indicates area under the plasma–concentration time curve from zero to infinity; Css, plasma concentration at steady-state; AUC0–t, AUC from zero to time t; AUC0–EOI, AUC from zero to end of infusion; Cmax, maximum plasma concentration; t½, terminal elimination half-life; AUC0–8, AUC from zero to 8 hours; CL, total body clearance; VSS, apparent volume of distribution at steady state; NC, not calculable
All values are geometric mean (CV%) unless otherwise stated;
Arithmetic mean (range).
Efficacy
Ten (45%) patients had a response to treatment (Table 5). Complete responses were reported in six (27%) patients, with an additional two (9%) having a complete response with incomplete recovery of neutrophils and/or platelets, and two (9%) patients with a partial response. The response rate was 46% (6/13 patients) in the barasertib 1000 mg cohort. Bone marrow cytogenetic data were available for 16/22 patients (n=9, intermediate risk; n=7, adverse risk). Four (44%) of the patients with intermediate risk cytogenetics experienced complete responses (n=3, complete response; n=1, complete response with incomplete recovery of neutrophils and/or platelets). Further, one (14%) patient with adverse cytogenetic risk also experienced a complete response.
Table 5.
Best clinical response (as assessed by study investigators)
| Barasertib dose + LDAC 400 mg | |||
|---|---|---|---|
| Response, n (%) | 800 mg (n=6) |
1000 mg (n=13) |
1200 mg (n=3) |
| CR | 2 | 3 | 1 |
| CRi | 0 | 2 | 0 |
| PR | 1 | 1 | 0 |
| ORR | 3 (50) | 6 (46) | 1 (33) |
CR, complete response; CRi, complete response with incomplete recovery of neutrophils and/or platelets; PR, partial response; ORR, overall response rate
Discussion
In this Phase I study, barasertib 1000 mg in combination with LDAC 400 mg was established as the MTD in elderly patients with newly diagnosed AML. No new safety concerns were reported in this older patient population following this combination treatment.
The AE profile of barasertib from monotherapy studies has been shown to be predictable and manageable at doses up to and including 1200 mg, and typically includes oral mucositis, alopecia and myelosuppressive AEs. The safety profile of LDAC is well established and is similar to that characterized for barasertib monotherapy, with myelosuppression being the most common AE reported in a large randomized study.6 Encouragingly, the observed AEs in these previous monotherapy studies of AML patients were effectively treated in most patients, highlighting the potential for successful management of these anticipated side effects in clinical practice.6,15,16 Thus, management strategies implemented here for the treatment of myelosuppression and for stomatitis would be expected to address any potential overlapping toxicity between the LDAC regimen and barasertib that may be anticipated from this combination, particularly with regard to myelosuppression.
The safety profiles determined here are consistent with previous studies,14–16 although the patient numbers are small and definitive conclusions cannot be drawn. The AEs encountered were as expected for these agents in this setting, with a slightly higher degree of myelosuppression than might be anticipated for either agent alone, although many patients were reported to have experienced these events at study entry. For example, the incidence of febrile neutropenia (59%), was higher than observed in a similarly designed Phase I study that established barasertib 1200 mg as the monotherapy MTD in an elderly (aged ≥60 years) Western patient population (47%).15 However, as with our study, the small patient numbers in the Phase I trial mean that definitive conclusions cannot be made. Infection, a major cause of morbidity and mortality in patients with AML, occurred at a higher frequency (73%) than shown with LDAC monotherapy (45%),6 possibly as a consequence of the increased myelosuppressive effects of the combination with barasertib.
The PK profiles of barasertib and the more active drug barasertib-hQPA in this study were generally consistent with those observed in previous studies of barasertib monotherapy in patients with malignant solid tumors and AML,14–16 indicating that the combination with LDAC does not affect barasertib PK parameters. The trend for greater than dose-proportional exposure to barasertib-hQPA observed in this study has not been reported in previous monotherapy studies although the small patient numbers available for analysis in this study prevents definitive conclusions. Results also indicated that the exposure of this patient population to subcutaneous LDAC was not affected by the addition of intravenous barasertib.
The overall response rate of 45% at the MTD provides preliminary evidence of the efficacy of combination treatment with barasertib and LDAC, particularly in this difficult-to-treat population of older patients with AML. These are promising findings, which may lead to better rates of response than for LDAC or decitabine alone, with rates approximating 20% for both.6,21 Recent studies of other agents with activity versus Aurora kinases have also demonstrated preliminary evidence of anticancer activity. These include AMG900,22 a pan-Aurora kinase inhibitor, that is currently being investigated in a Phase I trial in adult patients with acute leukemias (NCT01380756). However, since AMG900 is not selective for Aurora B, it remains to be established whether the anticancer activity observed with this agent is due to an effect on Aurora B and/or other target(s). The Aurora A kinase inhibitor MLN8237 has demonstrated preclinical anti-AML activity,23 and is currently in a Phase II trial in patients with AML or myelodysplastic syndrome (NCT00830518).
In conclusion, the combination of barasertib with LDAC showed acceptable tolerability at doses up to and including 1000 mg of barasertib in patients aged ≥60 years with newly diagnosed AML who were unfit for intensive induction chemotherapy. Results suggested no evidence of PK interaction between barasertib and LDAC while preliminary efficacy observations were promising. This combination therapy is being investigated further in a Phase II trial in this patient setting (NCT00952588).
Clinical Practice Points.
-
▪
The incidence of acute myeloid leukemia (AML) increases with advancing age. With standard intensive induction chemotherapy, 5-year survival rates in patients aged <60 years approach 50%; however in older patients, prognosis remains poor. Currently, low dose cytosine arabinoside (LDAC) is the only agent to have demonstrated clinical benefit in randomized studies in patients aged ≥60 years with AML who are considered unsuitable to receive intensive induction chemotherapy; however, improvement in survival rates among elderly patients remains elusive.
-
▪
Twenty-two patients (median age, 71 years) received barasertib (n=6, 800 mg; n=13, 1000 mg; n=3, 1200 mg; 7-day intravenous infusion) in combination with LDAC 400 mg (10 days of 20 mg bid subcutaneous injections), in 28-day treatment cycles. Dose-limiting toxicities were reported in two patients (both, grade 3 stomatitis/mucositis; 1200 mg cohort); this dose was therefore considered non-tolerated and the barasertib 1000 mg dose level was identified as the maximum tolerated dose. Adverse event (AE) profiles were as expected for these agents in this setting, and the most common AEs were infection (73%), febrile neutropenia (59%), nausea (50%), and diarrhea (46%). Exploratory assessment of efficacy demonstrated an overall response rate (by investigator opinion) of 45% (n=10/22).
-
▪
The preliminary efficacy data reported in this study indicate a promising response rate for barasertib in combination with LDAC in this patient setting, and was associated with acceptable tolerability. Therefore, barasertib plus LDAC represents a potential treatment option in elderly patients with AML and is being investigated further in a Phase II study.
Acknowledgments
We wish to thank the patients who participated in this study, and the study staff.
This study was sponsored by AstraZeneca. We thank Zoё van Helmond PhD from Mudskipper Bioscience who provided medical writing support funded by AstraZeneca.
Funding source: AstraZeneca
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
Dr Kantarjian has received research grants from AstraZeneca; Dr Sekeres has attended advisory boards for Celgene and Amgen; Dr Ribrag has received research support from Servier, Bayer and Sanofi, and has served as a consultant for Takeda, Servier and AstraZeneca; Drs Owen, Stockman and Oliver are employees of and own stock in AstraZeneca. All other authors state that they have no conflicts of interest.
References
- 1.Löwenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341:1051–1062. doi: 10.1056/NEJM199909303411407. [DOI] [PubMed] [Google Scholar]
- 2.Pulte D, Gondos A, Brenner H. Improvements in survival of adults diagnosed with acute myeloblastic leukemia in the early 21st century. Haematologica. 2008;93:594–600. doi: 10.3324/haematol.12304. [DOI] [PubMed] [Google Scholar]
- 3.Rowe JM, Tallman MS. Intensifying induction therapy in acute myeloid leukemia: has a new standard of care emerged? Blood. 1997;90:2121–2126. [PubMed] [Google Scholar]
- 4.Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009;361:1249–1259. doi: 10.1056/NEJMoa0904544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kantarjian H, Ravandi F, O'Brien S, et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood. 2010;116:4422–4429. doi: 10.1182/blood-2010-03-276485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnett AK, Milligan D, Prentice AG, et al. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007;109:1114–1124. doi: 10.1002/cncr.22496. [DOI] [PubMed] [Google Scholar]
- 7.Meraldi P, Honda R, Nigg EA. Aurora kinases link chromosome segregation and cell division to cancer susceptibility. Curr Opin Genet Dev. 2004;14:29–36. doi: 10.1016/j.gde.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Ikezoe T, Yang J, Nishioka C, et al. A novel treatment strategy targeting Aurora kinases in acute myelogenous leukemia. Mol Cancer Ther. 2007;6:1851–1857. doi: 10.1158/1535-7163.MCT-07-0067. [DOI] [PubMed] [Google Scholar]
- 9.Keen N, Taylor S. Mitotic drivers--inhibitors of the Aurora B Kinase. Cancer Metastasis Rev. 2009;28:185–195. doi: 10.1007/s10555-009-9184-9. [DOI] [PubMed] [Google Scholar]
- 10.Joel SP, Oke A, Foot N, et al. The activity of the novel Aurora Kinase B inhibitor AZD1152 in acute myeloid leukaemia cells. Blood. 2005;106:3374. [Google Scholar]
- 11.Oke A, Pearce D, Wilkinson RW, et al. AZD1152 rapidly and negatively affects the growth and survival of human acute myeloid leukemia cells in vitro and in vivo. Cancer Res. 2009;69:4150–4158. doi: 10.1158/0008-5472.CAN-08-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsby E, Walsh V, Pepper C, et al. Effects of the aurora kinase inhibitors AZD1152-HQPA and ZM447439 on growth arrest and polyploidy in acute myeloid leukemia cell lines and primary blasts. Haematologica. 2008;93:662–669. doi: 10.3324/haematol.12148. [DOI] [PubMed] [Google Scholar]
- 13.Wilkinson RW, Odedra R, Heaton SP, et al. AZD1152, a selective inhibitor of aurora B kinase, inhibits human tumor xenograft growth by inducing apoptosis. Clin Cancer Res. 2007;13:3682–3688. doi: 10.1158/1078-0432.CCR-06-2979. [DOI] [PubMed] [Google Scholar]
- 14.Boss DS, Witteveen PO, van der Sar J, et al. Clinical evaluation of AZD1152, an i.v. inhibitor of Aurora B kinase, in patients with solid malignant tumors. Ann Oncol. 2011;22:431–437. doi: 10.1093/annonc/mdq344. [DOI] [PubMed] [Google Scholar]
- 15.Löwenberg B, Muus P, Ossenkoppele G, et al. Phase I/II study to assess the safety, efficacy, and pharmacokinetics of barasertib (AZD1152) in patients with advanced acute myeloid leukemia. Blood. 2011;118:6030–6036. doi: 10.1182/blood-2011-07-366930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuboi K, Yokozawa T, Sakura T, et al. A Phase I study to assess the safety, pharmacokinetics and efficacy of barasertib (AZD1152), an Aurora B kinase inhibitor, in Japanese patients with advanced acute myeloid leukemia. Leuk Res. 2011;35:1384–1349. doi: 10.1016/j.leukres.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 18.Grimwade D, Hills RK. Independent prognostic factors for AML outcome. Hematology Am Soc Hematol Educ Program. 2009:385–395. doi: 10.1182/asheducation-2009.1.385. [DOI] [PubMed] [Google Scholar]
- 19.AstraZeneca. Global Policy: Bioethics. 2011 Available at: http://www.astrazeneca.com/Responsibility/Code-policies-standards/Our-global-policies.
- 20.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 21.Thomas XG, Dmoszynska A, Wierzbowska A, et al. Results from a randomized phase III trial of decitabine versus supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed AML. J Clin Oncol. 2011;29((15S)) doi: 10.1200/JCO.2011.38.9429. abst 6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Payton M, Bush TL, Chung G, et al. Preclinical evaluation of AMG 900, a novel potent and highly selective pan-aurora kinase inhibitor with activity in taxane-resistant tumor cell lines. Cancer Res. 2010;70:9846–9854. doi: 10.1158/0008-5472.CAN-10-3001. [DOI] [PubMed] [Google Scholar]
- 23.Kelly KR, Nawrocki ST, Espitia CM, et al. Targeting Aurora A kinase activity with the investigational agent alisertib increases the efficacy of cytarabine through a FOXO-dependent mechanism. Int J Cancer. 2012;131:2693–2703. doi: 10.1002/ijc.27579. [DOI] [PMC free article] [PubMed] [Google Scholar]