Abstract
BACKGROUND
This study examined whether prazosin reduces alcohol drinking over the course of prolonged treatment and whether it blocks initiation of alcohol drinking in rats with a genetic predisposition toward high alcohol drinking, i.e, alcohol-preferring (P) rats.
METHODS
In study one, alcohol-experienced P rats that had been drinking alcohol 2 hrs/day for several months were treated daily with prazosin (0, 0.5, 1.0 or 2.0 mg/kg BW) for 7 weeks. In study two, alcohol-naïve P rats were treated daily with prazosin (0, 1.0 or 2.0 mg/kg BW) for two weeks prior to, or concomitantly with, initiation of alcohol access and throughout 3 weeks of alcohol availability. Prazosin treatment and alcohol access were then discontinued for 2 weeks followed by reinstatement of alcohol access without prazosin treatment for 4 weeks, followed by resumption of daily prazosin treatment (2.0 mg/kg BW) for 3 weeks.
RESULTS
Prazosin reduced alcohol drinking throughout 7 weeks of treatment in P rats accustomed to drinking alcohol. Following termination of prazosin treatment, alcohol drinking slowly returned to pretreatment baseline. Reduced alcohol intake was accompanied by increased water intake. In alcohol-naïve P rats, prazosin administration prior to the first opportunity to drink alcohol and throughout 3 weeks of alcohol access retarded acquisition of alcohol drinking and reduced the amount of alcohol consumed. When prazosin was administered concomitantly with the first opportunity to drink alcohol, it abolished acquisition of alcohol drinking. Discontinuation of prazosin treatment allowed expression of a genetic predisposition toward high alcohol drinking to gradually emerge. Prazosin retained the ability to reduce alcohol intake with repeated treatments.
CONCLUSIONS
Prazosin decreased alcohol drinking during prolonged treatment and may be useful for treating alcoholism and alcohol use disorders. Prazosin may also be useful for deterring initiation of drinking in individuals with a family history of alcoholism.
Keywords: prazosin, alcohol, alcohol-preferring (P) rats, noradrenergic system, alcohol relapse, alcoholism pharmacotherapies
INTRODUCTION
Agents that reduce noradrenergic signaling in the brain may prove useful for treating alcohol abuse and alcoholism. Central noradrenergic activation, which accompanies anxiety and hyperexcitability (Aston-Jones et al, 1994), is associated with a genetic predisposition toward alcoholism and contributes to alcohol abuse (Begleiter and Porjesz, 1999; Spanagel et al., 1995). Noradrenergic activation thus may lead to excessive alcohol drinking in an effort to self-medicate, since alcohol is both anxiolytic and sympatho-suppressive (Kushner et al., 2000; Shirao et al., 1988). Several lines of evidence support this view: a) alcoholics commonly report that relief of anxiety is an important reason for drinking (e.g., Kushner et al., 2000); b) alcoholism co-occurs at high rates with anxiety disorders (Merikangas et al., 1998); c) patients with co-morbid anxiety and alcoholism use alcohol to control anxiety symptoms (Sinha et al., 1998); d) blocking norepinephrine biosynthesis decreases alcohol self-administration by rodents (Amit et al., 1977), and e) sympathetic activation and anxiety are common in humans and rodents risk for high alcohol drinking (Aston-Jones et al., 1994, Kushner et al., 2000; Rasmussen et al., 2006; Southwick et al., 1997; Stewart et al., 1993). It can be inferred from these findings that a reduction in central noradrenergic signaling should, under some conditions, decrease alcohol drinking.
We recently initiated a preclinical research program to identify which agents that decrease CNS noradrenergic activity are effective in decreasing alcohol drinking in a rodent model of alcoholism (Froehlich, 2010). One promising agent is prazosin, an α1-adrenergic receptor antagonist. First introduced in 1973 under the trade name “Minipress,” it has been used chronically by millions of people for reduction of arterial blood pressure, control of hypertension, and treatment of urinary symptoms associated with benign prostatic hypertrophy (Hieble and Ruffolo, 1996; Koshy et al., 1977; Bauer et. al 1984). Prazosin has good safety and clinical compliance records and is without significant adverse side effects (Graham, 1984). When given peripherally at clinically relevant doses, prazosin blocks brain α1-adrenergic receptors that mediate central noradrenergic signaling (Menkes et al., 1981; Rogawski and Aghajanian, 1982). In short-term studies, prazosin reduces alcohol drinking in a rodent model of alcoholism (Rasmussen et al., 2009a). This effect is robust since prazosin decreases alcohol intake even when alcohol craving is high, such as during withdrawal (Walker et al., 2008) or when alcohol is reintroduced following periods of deprivation (Rasmussen et al., 2009b,c). However for a drug to be valuable for treating alcoholism and alcohol use disorders, it must continue to be effective during long-term treatment. The current study addressed the following clinically relevant questions using a rodent model of alcoholism: 1) Does prazosin maintain its ability to reduce alcohol drinking during prolonged administration? 2) Is this reduction sustained following termination of treatment? and 3) Can prazosin block acquisition of alcohol drinking in subjects with a genetic predisposition toward high voluntary alcohol intake?
MATERIALS AND METHODS
Subjects
In Study 1, 40 alcohol-naïve male rats from the 64th and 65th generations of selective breeding for alcohol preference (alcohol preferring or “P” line) served as subjects. In study 2, 48 alcohol-naïve male rats from the 71st generation of the P line served as subjects. All rats were 64–71 days of age and weighed 271–393 g at study onset. The rats were individually housed in stainless steel hanging cages in an isolated vivarium with controlled temperature (21±1°C) and reversed 12 hour light/dark cycle (lights off at 1000 hrs). Rodent chow (Laboratory Rodent Diet #7001; Harlan Teklad, Madison, WI) and water were available ad libitum throughout the study. All subjects were acclimated to individual housing for two weeks prior to onset of alcohol access. All experimental procedures were approved by the Indiana University Institutional Animal Care and Use Committee and conducted in strict compliance with the NIH Guide for the Care and Use of Laboratory Animals.
Alcohol
A 15% (v/v) alcohol solution was prepared by diluting 95% alcohol (ethanol) with distilled, deionized water. The alcohol solution and water were presented in separate calibrated glass drinking tubes and daily intakes were recorded to the nearest ml. Alcohol intake was converted to g alcohol/kg BW.
Drug
Prazosin, expressed as free base mass (Fluka Chemical Corp., Milwaukee, WI), was dissolved in deionized, distilled water containing 3 g dextrose (Sigma-Aldrich, St. Louis, MO) and 125 mg sodium saccharin (Sigma-Aldrich, St. Louis, MO) per 100 ml water. Berry-flavored Jell-O (21.0 g/100 ml) and Knox gelatin (18.0 g/100 ml) were added to the solution, heated, and the solution containing drug or no drug (vehicle) was aliquoted into molds to form star-shaped pieces of gelatin, one per rat per day, with the volume of each aliquot determined by body weight of the rat to produce doses of 0, 0.5, 1.0, or 2.0 mg prazosin/3.0 ml solution/kg BW. The gelatin (approximately 1.8 g), with or without prazosin, was fed to the rats once each day at 45 minutes prior to the 2hr alcohol access period. The rats grabbed the gelatin and consumed it within 1 minute. Cages were checked to ensure that no pieces were dropped, which they seldom were. In an earlier study (data not shown), consumption of gelatin without drug (vehicle) at 45 min prior to 2 hr alcohol access did not alter alcohol intake. Specifically, average daily 2 hr alcohol intake during the 5 days prior to consumption of vehicle gelatin pieces was 1.8 g/kg BW (N=64 adult male P rats) and average daily 2 hr alcohol intake in these same rats during 5 days of vehicle gelatin consumption was 1.7 g/kg BW. To familiarize the rats in the current study with the gelatin, vehicle gelatin pieces were fed to the rats once/day for one week prior to initiation of drug treatment. Feeding drug in flavored gelatin is an approach we previously used successfully for the prolonged administration of prazosin in rats (Froehlich et al., 2010). This method of drug delivery is optimal for long-term treatment and can be used with drugs that are orally active such as naltrexone, prazosin, or buprenorphine (Froehlich et al., 2010; Liles et al., 1997). Prazosin is well-absorbed after oral administration and its bioavailability is high (about 70%) (Goodman and Gilman, 2006), so doses that are effective when administered via non-oral routes are also effective orally. The doses of prazosin used in the current study are those that we previously found to be effective in reducing alcohol drinking in P rats when administered intraperitoneally (Rasmussen et al., 2009a).
Prazosin Effects on Blood Alcohol Concentration (BAC)
Rats received alcohol by gastric gavage, in a dose of 2.0 g alcohol/10.1 mls of a 25% v/v alcohol solution/kg BW. This dose is similar to the amount of alcohol consumed by P rats during a daily 2 hr alcohol access period (Rasmussen et al., 2009a). This concentration of alcohol is far below the threshold that produces damage to gastric mucosa (Gillespie and Lucas, 1961) or paralysis of smooth muscle (Bernard et al., 1964) and the volume is far below the gastric capacity of an adult rat (Bull and Pitts., 1971). On the day before IG alcohol infusion, rats received half the amount of food normally consumed (determined as average weight of food consumed daily by the group over the previous 4 days) in order to limit and equate the amount of food in the stomach at the time of alcohol infusion. Tail blood was collected at 15, 30, 60, 90 and 120 minutes after onset of alcohol infusion. A razor blade was used to cut the tip (<1 mm) of the tail and 0.075 ml of blood was “milked” into a heparinized capillary tube, dispensed into an ice cold 0.5 ml microcentrifuge tube, sealed and centrifuged at 4o C. Spontaneous bleeding stopped immediately after sample collection and subsequent samples were collected by removing the coagulate, without additional cuts. Recovered plasma was sealed and refrigerated until assayed for alcohol content by NAD ADH enzymatic assay (Diagnostic Chemicals Ltd., Oxford, CT).
Scheduling Access to Alcohol
To maximize alcohol intake during 2-hr alcohol access periods, daily access to alcohol was reduced using a “step down” procedure as previously described (Rasmussen et al., 2009a). Except in Study 2 which required alcohol-naïve rats, rats had ad libitum access to food, water, and 15 % (v/v) alcohol solution for 13 weeks (5 days a week for weeks 1–7 and 6 days a week for weeks 8–13). Daily alcohol access was then shortened from 24 hrs/day to 8 hrs/day, 5 days a week for 1 week, then to 4 hrs/day, 5 days a week for 1 week, and then to 2 hrs/day, 5 days a week for 3 weeks. Throughout the study, rats were maintained with free access to food and water and scheduled access to 15% (v/v) alcohol solution for 2 hr/day (1000–1200 hrs). Food was removed during the daily alcohol access period. This procedure produces stable alcohol intake of approximately 2.0 g alcohol/kg BW/2 hrs in P rats (Rasmussen et al., 2009a).
Assigning Rats to Drug Groups
Effects of prazosin on alcohol drinking were assessed in four groups of rats that received either vehicle (no drug) or one of 3 doses of prazosin (0.5, 1.0 or 2.0 mg/kg BW). During the week prior to onset of prazosin treatment, baseline alcohol intake was calculated for each rat over 3 consecutive days. The rats were ranked in descending order of average daily alcohol consumption and were assigned to treatment groups in a manner that ensured that the groups did not differ in baseline alcohol intake. Specifically, the top alcohol drinkers were randomly assigned to the vehicle group or to one of the prazosin treatment groups, followed by randomly assigning the next highest alcohol drinkers, etc.
Study One assessed the effect of 7 weeks of daily prazosin treatment on alcohol and water intake in P rats for whom voluntary alcohol drinking was an established daily behavior. All rats were fed one of 3 doses of prazosin (0.5, 1.0, or 2.0 mg/kg) or vehicle in gelatin at 45 minutes prior to onset of each daily 2-hr alcohol access period for 5 days/week for 7 weeks.
Study Two assessed the effects prazosin on the initiation of alcohol drinking and the development of alcohol preference in alcohol-naïve P rats. All rats were fed one of two doses of prazosin (1.0, or 2.0 mg/kg BW) or vehicle in gelatin at 45 minutes before the daily 2 hr alcohol access period for 7 days/week, starting either 2 weeks prior to, or concomitantly with, initiation of alcohol access. We previously found that oral prazosin does not become effective in decreasing established alcohol drinking until the second to third week of treatment (Froehlich et al., 2010; 2012). Therefore, two groups of rats were pretreated daily with prazosin for 2 weeks prior to onset of daily alcohol access while three other groups were treated with either vehicle or prazosin concomitantly with the first opportunity to drink alcohol. Daily prazosin treatment continued throughout 3 weeks of daily alcohol access (2 hrs/day, 7 days/wk). Prazosin treatments and daily alcohol access were then discontinued for 2 weeks before daily 2 hr alcohol access was reintroducted, without prazosin treatment, for 29 days. Prazosin treatment (2.0 mg/kg BW) was then administered to a subset of rats for an additional 20 days.
BAC
To determine whether prazosin alters BAC produced by IG alcohol, four new groups of adult male P rats (N=9/group) were treated with oral prazosin (2.0 mg/kg BW) or gelatin vehicle for 2 weeks prior to, or concomitantly with, onset of daily 2 hr access to alcohol. Prazosin or vehicle treatment and daily alcohol access continued for 9 days in the two concomitant onset groups and for 20 days in the two 2-week pretreated groups prior to determination of BAC.
Data Analysis
Alcohol and water intake were analyzed using separate two-way repeated measures ANOVA (treatment X day or week) followed, when justified, by pairwise multiple comparisons using Fisher’s least significant difference (LSD) test. Significance was accepted at p <0.05 and data are presented as means +SE. BAC was analyzed using a 2-way repeated measures ANOVA (dose X time, with repeated measures on time).
RESULTS
Alcohol Intake
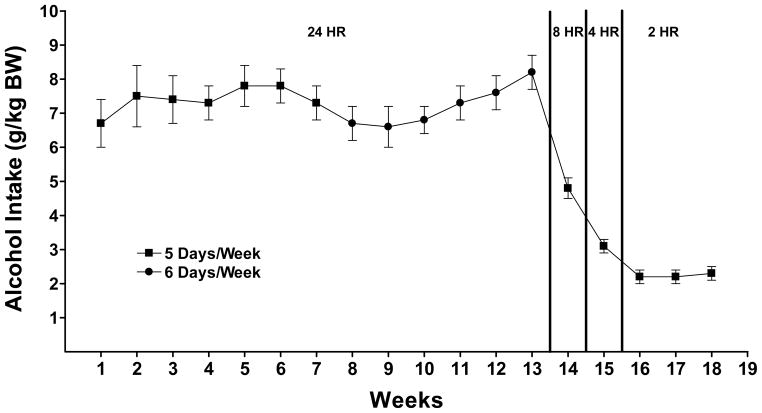
P rats consumed about 7 grams alcohol/kg BW per day when given 24 hour access to an alcohol solution (15% v/v) and water (Figure 1) which corresponds to a 80 kg person (176 lbs) drinking 10.0 standard drinks/day. The alcohol elimination rate (AER) in humans is approximately 0.11 grams/kg BW/hr (Parlesak et al., 2004; von Wartburg, 1980) while the AER in rats is approximately 0.44 grams/kg BW/hr (Ardies et al., 1989; Forsander and Sinclair 1992) or 4 times greater than that in humans. Hence, consumption of 7 g/kg BW per day in rats is equivalent to consumption of 1.75 g/kg BW per day in humans or 140g for an 80kg person. A standard drink contains approximately 14 grams of alcohol (NIAAA, 2008). When alcohol access was reduced to 2 hours a day, alcohol intake in P rats was approximately 2 grams/kg BW which is equivalent to a human drinking approximately 3 drinks in 2 hours. This level of intake results in physiologically relevant BACs in P rats (Murphy et al., 1986; Rasmussen et al., 2009a).
Figure 1.
Alcohol intake in P rats given alcohol (15 %v/v) ad libitum, 5 days a week for 13 weeks followed by scheduled access to alcohol for 8 hrs a day for 1 week, 4 hours a day for 1 week, and 2 hours a day for 3 weeks prior to initiation of prazosin treatment. Each point represents the mean ± S.E.
Effects of Prolonged Prazosin Treatment on Established Alcohol Drinking
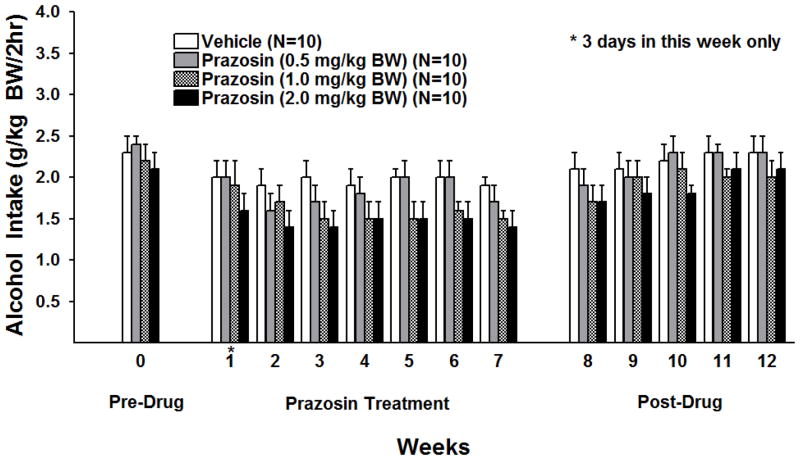
Weekly alcohol intake was averaged for each rat and analyzed over 7 weeks of prazosin treatment using a two-way ANOVA (treatment X week with repeated measures on week). There were significant main effects of treatment [F (3, 36) = 2.97, p < 0.05] and week [F (6, 216) = 3.91, p < 0.01], but no treatment X week interaction. Fishers least significant difference (LSD) test revealed that, when compared with vehicle, prazosin in a dose of 0.5 mg/kg BW did not decrease alcohol intake but both 1.0 mg/kg and 2.0 mg/kg BW prazosin significantly reduced alcohol intake (p<0.05 and p<0.01 vs vehicle, respectively) (Figure 2).
Figure 2.
Effect of oral prazosin (0, 0.5, 1.0, or 2.0 mg/kg BW) on alcohol intake in P rats given access to alcohol (15%v/v) for 2 hrs a day, 5 days a week for 7 consecutive weeks. Weekly alcohol intake was averaged for each rat. Each bar represents the mean ± S.E.
To determine when prazosin treatment began to effectively decrease alcohol drinking, alcohol intakes during each of the first 3 weeks of treatment were analyzed using separate one-way ANOVAs (Figure 2). There were no significant effects of treatment in weeks one or two but there was a significant effect of treatment in week three [F (3, 39 = 3.61, p <0.05]. Fisher’s LSD revealed that prazosin, in a dose of 0.5 mg/kg BW, compared with vehicle, did not decrease alcohol intake but both 1.0 mg/kg BW and 2.0 mg/kg BW prazosin significantly reduced alcohol intake (p<0.05 and p<0.01 respectively).
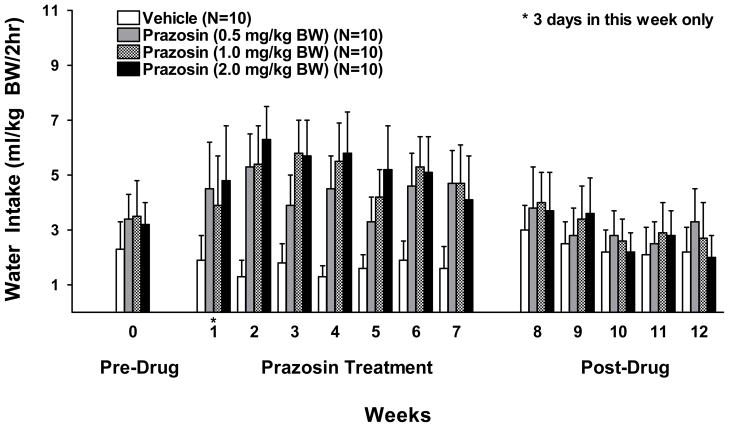
Water intake was likewise averaged weekly for each rat and analyzed over 7 weeks of prazosin treatment using a two-way ANOVA (treatment X week with repeated measures on week) (Figure 3). There was a significant effect of treatment [F (3, 36) = 6.55, p<0.01] and week [F (6, 216) = 2.33, p<0.05] but no treatment X week interaction. Fisher’s LSD revealed that water intake in all three prazosin treated groups was greater than in the vehicle treated group during the 7 weeks of prazosin treatment (vehicle vs. 0.5 mg prazosin, p<0.01; vehicle vs 1.0 mg prazosin, p<0.01; and vehicle vs 2.0 mg prazosin, p<0.001).
Figure 3.
Effect of oral prazosin (0, 0.5, 1.0, or 2.0 mg/kg BW) on water intake in P rats during the daily 2 hr alcohol access period, 5 days a week for 7 consecutive weeks. Weekly water intake was averaged for each rat. Each bar represents the mean ± S.E.
The effect of prolonged prazosin treatment on alcohol drinking following cessation of treatment was dose-dependent (Figure 2). One-way ANOVAs for each dose in each week revealed that a dose of 2.0 mg/kg BW of prazosin, compared with vehicle, reduced alcohol intake for 3 weeks following cessation of treatment (week 8 F (1,19) = 5.8, p<0.05; week 9 F (1,19 = 8.87, p<0.01; week 10 F(1,19), =4.59; p<0.05) while a dose of 1.0 mg/kg BW of prazosin reduced alcohol intake only during the first week after cessation of treatment (week 8 F (1,19) =4.56, p<0.05) and a 0.5 mg/kg BW dose was ineffective after cessation of treatment. Water intake during the 5 weeks following cessation of chronic prazosin treatment was analyzed using separate one-way ANOVAs, one for each dose in each week. None of the prazosin doses (0.5, 1.0 or 2.0 mg/kg BW) significantly altered water intake.
Effect of prolonged prazosin treatment on initiation of alcohol drinking and acquisition of alcohol preference
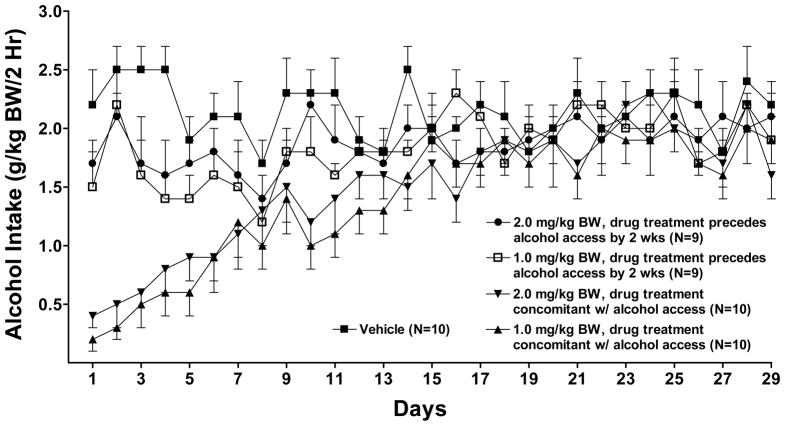
Daily alcohol intake and water intake were analyzed using two-way repeated measures ANOVAs (treatment X day with repeated measures on day) followed by pairwise multiple comparisons using Fisher’s least significant difference (LSD) test or one-way ANOVAs one for each day. Effect of prazosin on alcohol intake was assessed during a) 21 days of prazosin treatment (0, 1.0 or 2.0 mg/kg BW prior to or concomitant with onset of alcohol access) (Figure 4); b) the subsequent 29 days of reaccess to alcohol without prazosin treatment after 2 weeks of alcohol deprivation (Figure 5); and c) 21 days of reinitiation of prazosin treatment (2.0 mg/kg BW) (Figure 6). Daily water intake was analyzed similarly.
Figure 4.
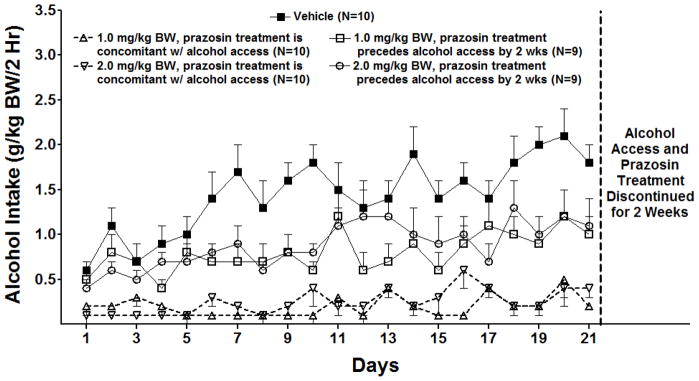
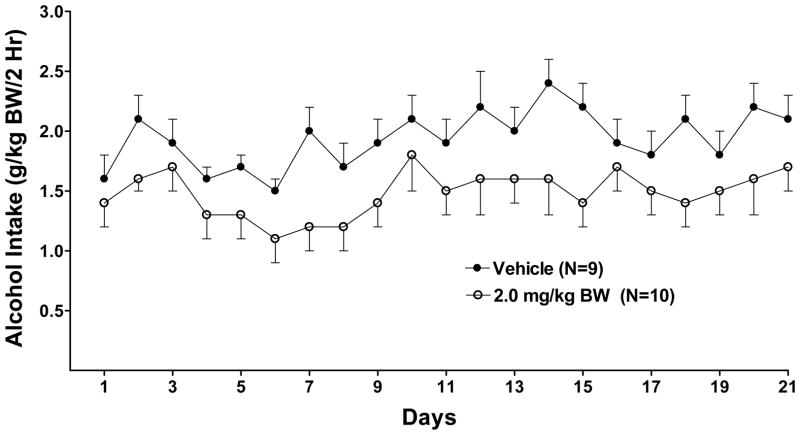
Effect of oral prazosin (0, 1.0 or 2.0 mg/kg BW) on acquisition of alcohol drinking in alcohol-naïve P rats introduced to a daily 2-hr alcohol access period which continued for 3 weeks. Each point represents the mean ± S.E.
Figure 5.
Alcohol intake during the month following termination of prazosin treatment in P rats given daily access to alcohol for 2 hrs/day. Each point represents the mean ± S.E.
Figure 6.
Effect of reinstatement of prazosin treatment (0, 2.0 mg/kg BW) on alcohol intake in P rats given daily access to alcohol for 2 hrs/day for 3 weeks. Each point represents the mean ± S.E.
With regard to effect of prazosin on alcohol intake during initiation of alcohol drinking in alcohol-naïve rats (Figure 4), there were significant effects of treatment [F (4,43) = 26.3, p < 0.001] and day [F (20,860) = 7.0, p < 0.001] and a treatment X day interaction [F (80,860) = 1.6, p = 0.001]. Further analyses using one-way ANOVAs, one for each day, revealed that prazosin, when delivered concomitantly with onset of alcohol access, significantly decreased alcohol intake compared with vehicle on all 21 days (p<0.001 for all days except day 3 (p<.01 for 2.0 mg/kg; p <0.05 for 1.0 mg/kg). When prazosin was delivered 2 weeks prior to onset of alcohol access, 1.0 mg/kg prazosin decreased alcohol intake on 15 of 21 treatment days when compared with vehicle (p <0.05 or 0.01 or 0.001, depending on the day) and 2.0 mg/kg prazosin decreased alcohol intake on 13 of 21 days (p <0.05 or 0.01 or 0.001, depending on the day), when compared with vehicle.
With regard to water intake during initiation of alcohol drinking in alcohol-naïve rats, there were significant effects of prazosin treatment [F (4, 42) = 18.8, p < 0.001] and day [F (20, 840) = 17.1, p < 0.001] but no treatment X day interaction. Fishers LSD post-hoc analyses revealed that prazosin (1.0 mg/kg or 2.0 mg/kg) compared to vehicle, delivered either 2 weeks prior to, or concomitantly with, onset of alcohol access, significantly increased water intake in all four treatment groups (p<0.01, respectively) (data not shown).
Following 3 weeks of alcohol access and prazosin treatment, both alcohol access and prazosin treatment were discontinued for 2 weeks. Then daily 2 hr alcohol access was reinstated for 29 days in the absence of prazosin treatment (Figure 5). Alcohol and water intake were analyzed using separate repeated measure ANOVAs (treatment X day with repeated measures on day). With regard to alcohol intake, there were significant effects of treatment [F (4, 43,) = 4.68 p<0.01] and day [F (28,1204) =10.53, p<0.001] and a treatment X day interaction [F (112,1204) = 2.76, p<0.001]. Post-hoc multiple comparisons with Fishers LSD for each individual day revealed that, with three exceptions (one dose on day 21, 26, and 29; p<0.05 respectively) the last day on which there was a significant effect of prior prazosin treatment on alcohol intake was day 14 (Figure 5). The two groups treated concomitantly with prazosin began with low alcohol intake and acquired alcohol drinking at a rate similar to vehicle-treated alcohol-naïve P rats (Figure 4). By day 14 there were no consistent differences in alcohol drinking between any of the groups indicating that the rats previously treated concomitantly with prazosin had acquired the same drinking level as vehicle-treated controls.
Second Prazosin Treatment
Following 29 days of daily access to alcohol without prazosin treatment (Figure 5), a subset of rats, randomly selected from prior treatment groups, were again treated with prazosin (2.0 mg/kg BW), or vehicle, prior to daily 2 hr alcohol access for 3 weeks (Figure 6). Daily alcohol intakes were analyzed by repeated measures ANOVA (dose X day with repeated measures on day). There were significant effects of prazosin treatment [F (1,17) = 4.95, p<0.05] and day [F (20, 340) = 3.08, p<0.001], but no treatment X day interaction. Prazosin reduced alcohol intake throughout 3 weeks of daily treatment. Water intakes during the 3 weeks of prazosin treatment were likewise analyzed, revealing no significant effect of prazosin on water intake (data not shown).
Effect of Prazosin on BAC
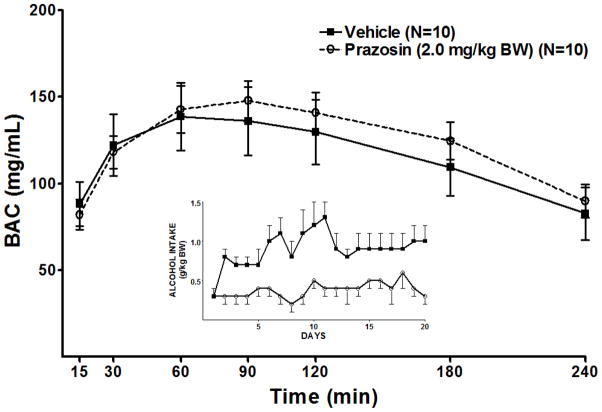
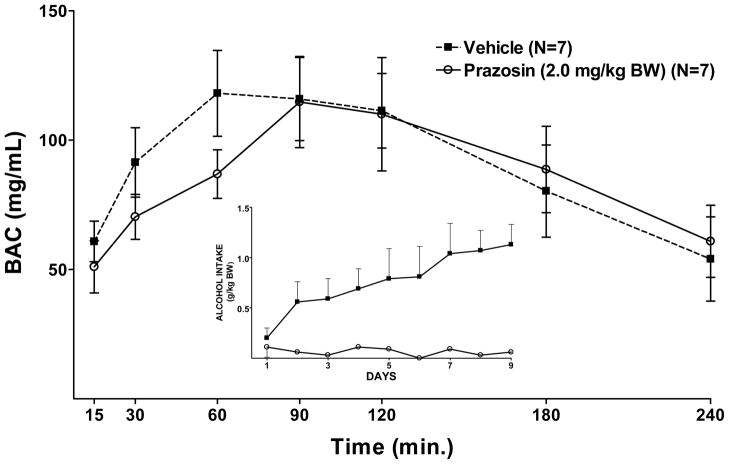
Repeated measure ANOVAs revealed that treatment with the highest dose of prazosin (2.0 mg/kg) for 2 weeks prior to (Figure 8), or concomitantly with (Figure 7), onset of daily 2 hr alcohol access, in separate groups of rats, did not alter the BAC resulting from an IG infusion of alcohol.
Figure 8.
Effect of prazosin (2.0 mg/kg BW), given for 2 weeks prior to onset of 9 days of alcohol access, on BAC following an IG infusion of alcohol (2.0 g/kg BW) given on day 10. The inset illustrates alcohol intake during the daily 2 hr alcohol access period on days 1–9. Each point represents the mean ± S.E.
Figure 7.
Effect of 9 days of prazosin (2.0 mg/kg BW) treatment, begun concomitant with onset of 9 days of alcohol access, on BAC following an IG infusion of alcohol (2.0 g/kg BW) given on day 10. The inset illustrates alcohol intake during the daily 2 hr alcohol access period on days 1–9. Each point represents the mean ± S.E.
DISCUSSION
New drugs to treat alcoholism and alcohol abuse are needed (Froehlich et al., 2003; O’Malley and Froehlich, 2003; Anton et al 2006), but in order for a drug to be useful it must be effective during prolonged treatment. Many drugs become ineffective with prolonged use due to adaptive changes such as decreases in receptor binding or receptor number. Duration of drug action is difficult to investigate in rodents because most routes used for drug administration cannot be employed for prolonged periods without increasing stress and health risks. To circumvent this problem, we developed a voluntary oral drug self-administration approach for rodents (Froehlich et al., 2010; 2013) that eliminates the stress associated with parenteral administration (Brown et al., 2000; Rosen et al., 1992). This approach eliminates the need for handling the animal, minimizes potential harm, duplicates the route most often used in humans, and is optimal for prolonged drug treatment.
Prazosin reduced alcohol intake throughout seven weeks of daily treatment. Onset of reduction was gradual, which agrees with our prior findings (Froehlich et al., 2010; 2013) and those of a clinical pilot study in which prazosin evidenced a gradual onset in reducing alcohol drinking in treatment-seeking alcohol-dependent men (Simpson et al., 2009). The prolonged efficacy of prazosin is clinically relevant. The average life span of a rat is 36 months (Farris and Griffith, 1949), so the 7 weeks of prazosin treatment in the current study represents 4.9% of a rat’s lifespan, analogous to 3.8 years of the human life span which currently averages 78.5 years in the United States (Centers for Disease Control and Prevention, 2012). Hence, the duration of prazosin treatment in the current study can reasonably be considered to far exceed the length of time that drugs are normally prescribed to deter alcohol relapse in alcoholics or to reduce drinking in individuals with alcohol use disorders (6–12 months; personal communication, Dr. Tim Kelly, Medical Director, Fairbanks Alcohol and Drug Treatment Center, Indianapolis, Indiana).
The effect of prazosin persisted after termination of treatment in a dose-dependent manner. The highest dose (2.0 mg/kg BW) reduced alcohol intake for 3 weeks, the 1.0 mg/kg BW dose reduced intake for 1 week, and the lowest dose (0.5 mg/kg BW) was ineffective. The oral doses used in the current study (0.5, 1.0 or 2.0 mg/kg BW) also decreased alcohol intake in P rats when prazosin was administered IP (Rasmussen et al., 2009a). Bioavailability of drugs is generally greater when administered parenterally compared to orally, so a higher dose is commonly required with oral administration. However, prazosin is well absorbed after oral administration and bioavailability is high (Goodman and Gilman, 2006).
Prazosin reduced alcohol intake but did not eliminate it. Rats do not receive counseling, behavioral interventions, or social pressure to stop drinking and they lack the support networks available to alcoholics and heavy drinkers. The fact that prazosin reduces alcohol drinking without the significant contribution of these factors suggests that prazosin may be even more effective as part of a comprehensive program of treatment for alcohol addiction.
Prazosin increased water intake, in agreement with our prior results (Froehlich et al., 2013; Rasmussen et al., 2009a,b) and those of others (Oryan et al., 2003). This demonstrates that the prazosin-induced reduction in alcohol intake was not due to motor impairment, inability to drink, or induction of nausea or another aversive state. Since alcohol access was limited to 2 hrs/day, the question arises whether the prazosin-induced reduction in alcohol intake is secondary to the moderately increased water intake. This is unlikely. Time does not appear to be a limiting factor because rats do not drink alcohol or water continuously throughout a 2 hour alcohol access period. Instead, they drink alcohol and water in bouts, grooming and eating food between bouts of alcohol and water drinking. They have adequate time to drink more alcohol in 2 hrs. Volume of fluid in the stomach does not appear to be a limiting factor either because the rats in this study were drinking a total of 10–11 mls (alcohol plus water) during the daily 2-hr alcohol access period and the gastric capacity of a 500 gram rat is approximately 18 mls (Bull and Pitts, 1970). The increase in water intake could be due to prazosin-induced activation of the renin-angiotensin system. Prazosin can induce a small transient rise in plasma renin and angiotensin in both humans and rats, regardless of whether a change in blood pressure occurs (Atkinson et al., 1986; McAreavey et al., 1981), although a rise is not always seen (Koshy et al., 1977; Webb et al 1987). Activation of the renin-angiotensin system results in thirst and leads to increased water intake (for review see Evered, 1983).
The effect of prazosin on alcohol intake appears to be relatively specific. In rats, prazosin does not alter operant responding for food (Le et al., 2011) and has no effect on total caloric intake, meal size or number of meals eaten (Janhunen et al., 2011). In humans, there do not appear to be any reports of prazosin altering appetite, caloric intake or body weight in individuals undergoing prolonged prazosin treatment for post traumatic stress disorder (PTSD) or hypertension. With regard to sucrose intake, we have previously reported that prazosin, in the highest dose tested, decreases both alcohol and sucrose seeking and drinking in P rats (Verplaetse et al., 2012). However, prazosin has a larger effect on alcohol than on sucrose intake. Prazosin decreased alcohol seeking and drinking by 76% and 67% respectively, while the same dose decreased sucrose seeking and drinking by only 44% and 39% respectively. Prazosin also increased response latency for alcohol, but not for sucrose (Verplaetse et al., 2012).
When prazosin treatment was begun 2 weeks prior to alcohol access in alcohol-naïve P rats, it retarded acquisition of alcohol drinking and reduced the amount of alcohol consumed, similar to the effect of naloxone (Badia-Elder et al., 1999) on initiation of alcohol drinking in P rats. When prazosin treatment began concomitantly with onset of alcohol access, it abolished acquisition of alcohol drinking. Very little alcohol was sampled; not enough to produce significant pharmacological effects. The reason for this is not clear. The reduction in alcohol intake is not due to prazosin-induced changes in alcohol absorption or clearance as evidenced by the similar magnitude and time course of BAC produced by IG alcohol regardless of whether they were treated with prazosin for 2 weeks prior to, or concomitantly with, the first opportunity to drink alcohol. The low intake of alcohol in these groups is more compatible with a neophobia than with a conditioned taste aversion to alcohol. The adaptive response of a rat to a novel taste is to consume relatively little on initial encounter and to wait until the post-ingestive effects are experienced. This reluctance to consume a novel taste is called taste or gustatory neophobia (Garcia et al., 1955). In the absence of aversive post-ingestive effects, the avoidance of the novel taste normally disappears with repeated trials. However, exaggerated gustatory neophobia is seen in rats with decreased noradrenergic (NE) activity in discrete brain regions as a result aging (Collier et al., 2004) or lesions of NE pathways (Martin-Iverson, 1982; Tombaugh et al., 1983). It may be that prazosin-induced reduction in central NE signaling results in an increased gustatory neophobia and avoidance of alcohol since alcohol is a novel taste for alcohol-naïve rats. Treating the rats with prazosin for two weeks prior to onset of alcohol access could have allowed for habituation of the neophobic response. More work is needed to resolve this issue.
When prazosin treatment was discontinued, expression of the P rat’s genetic predisposition toward high alcohol drinking emerged and alcohol drinking gradually increased to the level of vehicle-treated rats. A second period of prazosin treatment again reduced alcohol intake, illustrating that the effect is reliable.
There may be a benefit to introducing prazosin during early drinking experiences. Prazosin slowed the acquisition of alcohol drinking and reduced the amount of alcohol consumed when administered prior to, or concomitantly with, the first opportunity to drink in rats genetically predisposed toward high alcohol intake. In all species, a genetic predisposition toward alcohol drinking is probabilistic rather than deterministic. Prazosin treatment may reduce the elevated probability of drinking that accompanies certain genetic backgrounds and may thus allow alcohol-inexperienced individuals with a genetic predisposition toward alcohol abuse (family history positive) to resist the impulse to drink heavily, averting early onset of alcohol abuse.
Prazosin, a safe, well-characterized, well-tolerated, inexpensive, FDA-approved α1-adrenergic receptor antagonist may be beneficial in treating alcoholism and alcohol use disorders.
Acknowledgments
We thank Dr. Ting-Kai Li and the Indiana Alcohol Research Center for supplying the selectively bred rats used in this study. This work was supported by NIH Grants AA018604 (JCF and DDR), AA07611 (JCF) and AA13881 (DDR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism or the National Institutes of Health.
References
- Amit Z, Brown ZW, Levitan DE, Ogren SO. Noradrenergic mediation of the positive reinforcing properties of ethanol. I. Suppression of ethanol consumption in laboratory rats following dopamine-beta-hydroxylase inhibition. Arch Int Pharmacodyn Ther. 1977;230:65–75. [PubMed] [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A. Combined pharmacotherapies and behavioral interventions for alcohol dependence. The COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Ardies CM, Morris GS, Erickson CK, Farrar RP. Both acute and chronic exercise enhance in vivo ethanol clearance in rats. J Appl Physiol. 1989;66:550–560. doi: 10.1152/jappl.1989.66.2.555. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Valentino RJ, Van Bockstaele EJ, Meyerson AT. Locus coeruleus, stress, and PTSD: Neurobiological and clinical parallels. In: Murburg MM, editor. Catecholamine Function in Posttraumatic Stress Disorder: Emerging Concepts. American Psychiatric Press, Inc; Washington, DC: 1994. pp. 17–62. [Google Scholar]
- Atkinson J, Luthi P, Sonnay M, Boillat N. Effect of acute administration of prazosin on blood pressure, heart rate and plasma renin level in the conscious normotensive rat. Clin Exp Pharmacol Physiol. 1986;13:535–541. doi: 10.1111/j.1440-1681.1986.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Badia-Elder NE, Mosemiller AK, Elder RL, Froehlich JC. Naloxone retards the expression of a genetic predisposition toward alcohol drinking. Psychopharmacology. 1999;144:205–212. doi: 10.1007/s002130050995. [DOI] [PubMed] [Google Scholar]
- Bauer JH, Jones LB, Gaddy P. Effects of prazosin therapy on BP, renal function, and body fluid composition. Arch Intern Med. 1984;144:1196–1200. [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. What is inherited in the predisposition toward alcoholism? A proposed model. Alcohol Clin Exp Res. 1999;23:1125–1135. doi: 10.1111/j.1530-0277.1999.tb04269.x. [DOI] [PubMed] [Google Scholar]
- Bernard A, Delattre A, Lamelin P. The mechanism of action of alcohol on the gastric mucosa: its hygroscopic and dehydrating properties. Acta Gasteroenterol Belg. 1964;27:129–147. [PubMed] [Google Scholar]
- Brown AP, Dinger N, Levine BS. Stress produced by gavage administration in the rat. Contemp Top Lab Anim Sci. 2000;39:17–21. [PubMed] [Google Scholar]
- Bull LS, Pitts GC. Gastric Capacity and energy absorption in the force-fed Rat. J Nutr. 1971;101:593–596. doi: 10.1093/jn/101.5.593. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention: Division of Vital Statistics. 2009 Mortality Multiple Cause Vital Statistics. 2012 < http://www.cdc.gov/nchs/data/dvs/deaths_2009_release.pdf>.
- Collier TJ, Greene JG, Felten DL, Stevens SY, Collier KS. Reduced cortical noradrenergic neurotransmission is associated with increased neophobia and impaired spatial memory in aged rats. Neurobiol Aging. 2004;25:209–221. doi: 10.1016/s0197-4580(03)00042-3. [DOI] [PubMed] [Google Scholar]
- Evered MD. Neuropeptides and thirst. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7:469–476. doi: 10.1016/0278-5846(83)90013-1. [DOI] [PubMed] [Google Scholar]
- Farris E, Griffith J. The Rat in Laboratory Investigation. 2. Hafner Publishing Company; New York: 1949. [Google Scholar]
- Forsander OA, Sinclair JD. Alcohol elimination and the regulation of alcohol consumption in AA and ANA rats. Alcohol. 1992;27:411–416. [PubMed] [Google Scholar]
- Froehlich JC. Medications development: Relevance and advantage of rodent models of alcohol drinking. Alcoholism: Clinical and Experimental Research. 2010;34:68. [Google Scholar]
- Froehlich JC, Federoff D, Rasmussen DD. Prazosin, an α1-adrenergic receptor antagonist, maintains continued suppression of alcohol drinking during prolonged administration. Alcoholism: Clinical and Experimental Research. 2010;34:19. [Google Scholar]
- Froehlich JC, Hausauer BJ, Rasmussen DD. Combining naltrexone and prazosin in a single oral medication decreases alcohol drinking more effectively than does either drug alone. Alcohol: Clin Exp Res. 2013 doi: 10.1111/acer.12148. (in-press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich J, O’Malley S, Hyytiä P, Davidson D, Farren C. Preclinical and clinical studies on naltrexone: what have they taught each other? Alcohol Clin Exp Res. 2003;27:533–539. doi: 10.1097/01.ALC.0000057943.57330.AB. [DOI] [PubMed] [Google Scholar]
- Garcia T, Kimeldorf DJ, Koelling RA. Conditioned aversion to saccharin resulting from exposure to gamma radiation. AAAS. 1955;122:157–158. [PubMed] [Google Scholar]
- Gillespie RJ, Lucas CC. Effect of single intoxicating doses of ethanol on the gastric and intestinal mucosa of rats. Can J Biochem Physiol. 1961;39:237–241. doi: 10.1139/o61-021. [DOI] [PubMed] [Google Scholar]
- Goodman LS, Gilman A. The Pharmacological Basis of Therapeutics. 11. McGraw-Hill; Chicago: 2006. [Google Scholar]
- Graham RM. Selective alpha 1-adrenergic antagonists: Therapeutically relevant antihypertensives. Am J Cardiol. 1984;53:16A–20A. doi: 10.1016/0002-9149(84)90829-4. [DOI] [PubMed] [Google Scholar]
- Hieble JP, Ruffolo RRJ. The use of alpha adrenoceptor antagonists in the pharmacological management of benign prostatic hypertrophy: An overview. Pharmacol Res. 1996;33:145–160. doi: 10.1006/phrs.1996.0022. [DOI] [PubMed] [Google Scholar]
- Janhunen SK, van der Zwaal EM, la Fleur SE, Adan RAH. Inverse agonism at α2A adrenoceptors augments the hypophagic effect of sibutramine in rats. Obesity. 2011;19:1979–1986. doi: 10.1038/oby.2011.51. [DOI] [PubMed] [Google Scholar]
- Koshy MC, Mickley D, Bourgiognie J, Blaufox MD. Physiologic evaluation of a new antihypertensive agent: prazosin HCl. Circulation. 1977;55:533–537. doi: 10.1161/01.cir.55.3.533. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Abrams K, Borchardt C. The relationship between anxiety disorders and alcohol use disorders: A review of major perspectives and findings. Clin Psychol Rev. 2000;20:149–71. doi: 10.1016/s0272-7358(99)00027-6. [DOI] [PubMed] [Google Scholar]
- Le AD, Funk D, Juzytsch W, Coen K, Navarre BM, Cifani C, Shaham Y. Effect of prazosin and guanifacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology. 2011;218:89–99. doi: 10.1007/s00213-011-2178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liles JH, Flecknell PA, Roughan J, Cruz-Madorran I. Influence of oral buprenorphine, oral naltrexone or morphine on the effects of laparotomy in the rat. Lab Anim. 1998;32:149–61. doi: 10.1258/002367798780600025. [DOI] [PubMed] [Google Scholar]
- Martin-Iverson MT, Pisa M, Chan E, Fibiger HC. Enhanced neophobia but normal plasma corticosterone levels in rats with dorsal noradrenergic bundle lesions. Pharmacol Biochem Behav. 1982;17:639–643. doi: 10.1016/0091-3057(82)90337-9. [DOI] [PubMed] [Google Scholar]
- McAreavey D, Cumming AM, Sood VP, Leckie BJ, Morton JJ, Murray GD, Robertson JI. The effect of oral prazosin on blood pressure and plasma concentrations of renin and angiotensin II in man. Clin Sci (Lond) 1981;61:457S–460S. doi: 10.1042/cs061457s. [DOI] [PubMed] [Google Scholar]
- Menkes DB, Baraban JM, Aghajanian GK. Prazosin selectively antagonizes neuronal responses mediated by α1-adrenoceptors in brain. Naunyn-Schmiedeberg’s Arc Pharmacol. 1981;317:273–275. doi: 10.1007/BF00503830. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Stevens D, Fenton B, Stolar M, O’Malley SS, Woods SW, Risch N. Comorbidity and familial aggregation of alcoholism and anxiety disorders. Psychol Med. 1998;28:773–788. doi: 10.1017/s0033291798006941. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Gatto GJ, Waller MB, McBride WJ, Lumeng L, Li TK. Effects of scheduled access on ethanol intake by the alcohol-preferring (P) lines of rats. Alcohol. 1986;3(5):331–6. doi: 10.1016/0741-8329(86)90010-8. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol and Alcohol Abuse. [Accessed on December 13th, 2012];What is a standard drink? [NIAAA Website] 2008 Apr; Available at: http://onlinelibrary.wiley.com/journal/10.1111/%28ISSN%291530-0277/homepage/ForAuthors.html.
- O’Malley SS, Froehlich JC. Advances in the use of naltrexone: An integration of preclinical and clinical findings. In: Galanter Marc., editor. Recent Developments in Alcoholism XVI: Research on Alcoholism Treatment. Kluwer Academic/Plenum Publishers; New York: 2003. pp. 217–245. [PubMed] [Google Scholar]
- Oryan S, Eidi M, Eidi A, Kohanrooz B. Effects of α1-adrenoceptors and muscarinic cholinoceptors on water intake in rats. European J Pharmacol. 2003;477:123–127. doi: 10.1016/j.ejphar.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Parlesak A, Billinger MHU, Schafer C, Wehner HD, Bode C, Bode JC. First-pass metabolism of ethanol in human beings: effect of intravenous infusion of fructose. Alcohol. 2004;34:121–125. doi: 10.1016/j.alcohol.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Alexander LL, Raskind MA, Froehlich JC. The α1-adrenergic receptor antagonist, prazosin, reduces alcohol drinking in alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2009a;33:264–272. doi: 10.1111/j.1530-0277.2008.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Federoff D, Froehlich JC. Prazosin reduces alcohol drinking in an animal model of alcohol relapse. Alcohol Clin Exp Res. 2009b;33:146. doi: 10.1111/acer.12789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Federoff D, Froehlich JC. Prazosin reduces voluntary alcohol drinking in both non-deprived and alcohol-deprived rats selectively bred for alcohol preference. Alcohol Clin Exp Res. 2009c;33:312. doi: 10.1111/acer.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Wilkinson CW, Raskind MA. Chronic daily ethanol and withdrawal: 6. Effects on rat sympathoadrenal activity during “abstinence”. Alcohol. 2006;38:173–177. doi: 10.1016/j.alcohol.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski MA, Aghajanian GK. Activation of lateral geniculate neurons by locus coeruleus or dorsal noradrenergic bundle stimulation: Selective blockade by the α1-adrenoceptor antagonist prazosin. Brain Res. 1982;250:31–39. doi: 10.1016/0006-8993(82)90950-7. [DOI] [PubMed] [Google Scholar]
- Rosen A, Brodin K, Eneroth P, Brodin E. Short-term restraint stress and s.c. saline injection alter the tissue levels of substance P and cholecystokinen in the peri-aqueductal grey and limbic regions of rat brain. Acta Physiol Scand. 1992;146:341–348. doi: 10.1111/j.1748-1716.1992.tb09428.x. [DOI] [PubMed] [Google Scholar]
- Shirao I, Tsuda A, Ida Y, Tsujimaru S, Satoh H, Oguchi M, Tanaka M, Inanaga K. Effect of acute ethanol administration on noradrenaline metabolism in brain regions of stressed and nonstressed rats. Pharmacol Biochem Behav. 1988;30:769–773. doi: 10.1016/0091-3057(88)90097-4. [DOI] [PubMed] [Google Scholar]
- Simpson TL, Saxon AJ, Meredith CW, Malte CA, McBride B, Ferguson LC, Gross CA, Hart KL, Raskind M. A pilot trial of the alpha-1 adrenergic antagonist, prazosin, for alcohol dependence. Alcohol Clin Exp Res. 2009;33:255–263. doi: 10.1111/j.1530-0277.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- Sinha R, Robinson J, O’Malley S. Stress response dampening: Effects of gender and family history of alcoholism and anxiety disorders. Psychopharmacology. 1998;137:311–320. doi: 10.1007/s002130050624. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Morgan CA, III, Bremner AD, Grillon CG, Krystal JH, Nagy LM, Charney DS. Noradrenergic alteration in posttraumatic stress disorder. Ann NY Acad Sci. 1997;821:125–141. doi: 10.1111/j.1749-6632.1997.tb48274.x. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Montkowski A, Allingham K, Stöhr T, Shoaib M, Holsboer F, Landgraf R. Anxiety: A potential predictor of vulnerability to the initiation of ethanol self-administration in rats. Psychopharmacology. 1995;122:369–373. doi: 10.1007/BF02246268. [DOI] [PubMed] [Google Scholar]
- Stewart RB, Gatto GJ, Lumeng L, Li TK, Murphy JM. Comparison of alcohol-preferring (P) and nonpreferring (NP) rats on tests of anxiety and for the anxiolytic effects of ethanol. Alcohol. 1993;10:1–10. doi: 10.1016/0741-8329(93)90046-q. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, Pappas BA, Roberts CS, Vickers GJ, Szostak C. Failure to replicate the dorsal bundle extinction effect: telencephalic norepinephrine depletion does not reliably increase resistance to extinction but does augment gustatory neophobia. Brain Res. 1983;261:231–242. doi: 10.1016/0006-8993(83)90626-1. [DOI] [PubMed] [Google Scholar]
- Verplaetse TL, Rasmussen DD, Froehlich JC, Czachowski CL. Effect of prazosin, an α1-adrenergic receptor antagonist, on the seeking and intake of alcohol and sucrose in alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2012;36:881–886. doi: 10.1111/j.1530-0277.2011.01653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Wartburg . Comparison of alcohol metabolism in human and anima. In: Eriksson K, Sinclair JD, Kiianmaa K, editors. Animal Models in Alcohol Research. Academic Press; New York: 1980. pp. 427–443. [Google Scholar]
- Walker BM, Rasmussen DD, Raskind MA, Koob GF. The effects of α1-noradrenergic receptor antagonism on dependence-induced increases in responding for ethanol. Alcohol. 2008;42:91–97. doi: 10.1016/j.alcohol.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]