Abstract
The purpose of this study was to examine whether exercise training reduced inflammation and symptomology in a mouse model of colitis. We hypothesized that moderate forced treadmill running (FTR) or voluntary wheel running (VWR) would reduce colitis symptoms and colon inflammation in response to dextran sodium sulfate (DSS). Male C57Bl/6J mice were randomized to sedentary, moderate intensity FTR (8–12 m/min, 40 min, 6 weeks, 5x/week), or VWR (30 days access to wheels). DSS was given at 2% (w/v) in drinking water over 5 days. Mice discontinued exercise 24 h prior to and during DSS treatment. Colons were harvested on Days 6, 8 and 12 in FTR and Day 8 post-DSS in VWR experiments. Contrary to our hypothesis, we found that moderate FTR exacerbated colitis symptomology and inflammation as measured by significant (p≤0.05) increases in diarrhea and IL-6, IL-1β, IL-17 colon gene expression. We also observed higher mortality (3/10 died vs. 0/10, p = 0.07) in the FTR/DSS group. In contrast, VWR alleviated colitis symptoms and reduced inflammatory gene expression in the colons of DSS-treated mice (p≤0.05). While DSS treatment reduced food/fluid intake and body weight, there was a tendency for FTR to exacerbate, and for VWR to attenuate, this effect. FTR (in the absence of DSS) increased gene expression of the chemokine and antibacterial protein CCL6 suggesting that FTR altered gut homeostasis that may be related to the exaggerated response to DSS. In conclusion, we found that FTR exacerbated, whereas VWR attenuated, symptoms and inflammation in response to DSS.
Keywords: exercise, inflammation, colitis, stress, treadmill running, voluntary wheel running, serum amyloid A, dextran sodium sulphate, inflammatory bowel disease
Introduction
Inflammatory bowel diseases (IBD), such as ulcerative colitis (UC), cause chronic morbidity that significantly reduce physical functioning and quality of life in afflicted patients. While UC is an idiopathic disorder, it is clear that environmental (e.g. infection, diet, psychological stress) and host genetic factors, in combination with disturbances in the intestinal microbiome, trigger barrier disruption leading to dysregulated inflammation in response to resident gut bacteria (Sanchez-Munoz et al., 2008). Immune cell infiltration stimulates the production of pro-inflammatory cytokines, which promote a chronic inflammatory state, instigating clinical symptoms including colon ulcers, rectal bleeding, diarrhea, abdominal pain, fatigue, and an overall altered emotional well-being (Sanchez-Munoz et al., 2008). Furthermore, UC significantly increases the risk of developing colorectal cancer later in life (Rizzo et al. 2011).
It is well established that anti-inflammatory therapies, designed to decrease colon inflammation, attenuate the symptoms of UC in humans and mouse models of the disease (Bi and Triadafilopoulos, 2003). Anti-inflammatory pharmaceutical treatments, such as 5-aminosalicylic acid and corticosteroids, are beneficial in reducing symptoms, but their efficacy is not complete and they have significant side-effects. Therefore, in addition to more efficacious and safer drug development, investigation of adjunct anti-inflammatory therapies that could attenuate colitis and counteract side effects of conventional treatment is of prime importance from a public health perspective. Such strategies could assist in improving the quality of life by reducing symptoms, hospitalizations, and the risk of colorectal cancer in UC patients.
Exercise has been suggested as an adjunct anti-inflammatory therapy for chronic diseases associated with inflammation (Astrom et al., 2010). Indeed, our laboratory as well as others, have provided evidence that moderate treadmill exercise acts in an anti-inflammatory fashion and bolsters immunity in humans (Vieira et al., 2009a; Packer et al., 2010; Walsh et al., 2011) and in animals where exaggerated or chronic inflammation exists (Lowder et al., 2006; Keylock et al., 2008; Vieira et al., 2009b). With respect to the gut, while there is significant evidence that regular moderate exercise can reduce risk for colon cancer, there are very few studies on whether it can exert positive effects in people with IBD. However, there is evidence that prolonged, intense exercise bouts can cause debilitating gastrointestinal complaints including cramps, diarrhea and gut inflammation most likely due to gut ischemia and/or mechanical trauma (Bi and Triadafilopoulos, 2003; Packer et al., 2010). Results from the few observational studies of exercise and IBD reveal that regularly performed moderate exercise neither protects from nor initiates IBD (Narula et al., 2008). However, definitive randomized exercise trials are lacking.
When given in the drinking water to rodents, DSS induces reproducible lesions in the distal colon by interfering with barrier function and stimulating local inflammation mimicking the pathophysiology of human colitis (Laroui et al., 2012). Although models such as DSS-induced colitis do not represent the complexity of the human disease, investigating intestinal inflammation in animal models has produced valuable data that have been useful in uncovering signaling pathways during colon inflammation (Fukuta et al., 2005), the cell types and cytokines responsible for disease activity (Elrod et al., 2005), and the local and systemic pathophysiology of acute and chronic colitis (Mizoguchi et al., 2003; Wirtz et al., 2007; Hamandi et al., 2008). Additionally, these models are valuable and indispensable tools for manipulating factors that affect pathogenesis and evaluating potential therapeutic strategies (Wirtz et al., 2007). Therefore, given the lack of knowledge in this area, the purpose of our study was to investigate the effect of moderate forced treadmill and voluntary wheel running on DSS-induced colitis mortality, morbidity, colon inflammation and colon histopathology in mice. We hypothesized that exercise would reduce symptoms and inflammatory burden in the colon in response to DSS.
Methods
Animals
Eight to ten week old male C57Bl/6J mice (forced treadmill run [FTR], n=132; and voluntary wheel run [VWR], n=26) were purchased from Jackson Laboratories (Bar Harbor, ME) and singly housed in an accredited specific pathogen-free (SPF) barrier facility. Animals were housed under a reverse light-dark cycle (lights on at 9 PM, lights off at 9 AM) in a low stress environment and given ad libitum access to sterile water (unless on study) and autoclaved rodent chow. Mice were acclimated to the facility for 1 week prior to the experiments. All corncob bedding and cages were sterilized before use and were changed weekly. All experiments were approved by the University of Illinois Urbana-Champaign IACUC. Three separate FTR experiments were performed with euthanasia occurring at Day 6 (n=10/group), Day 8 (n=13/group) or Day 12 (n=10/group) post-DSS treatment. The VWR experiment was performed with euthanasia occurring at Day 8 post-DSS at the time of peak symptoms (control: n=5/group; DSS: n=8/group).
Exercise Protocol
For the FTR experiments, mice were paired on body weight prior to training or sedentary control conditions and then randomized into one of four groups; sedentary water (SED/H2O), sedentary DSS (SED/DSS), exercised water (FTR/H2O) and exercised DSS (FTR/DSS). Mice (including exercise trained mice) did not run during or after DSS treatment to minimize distress. Using this design, we were interested in testing the effects of prior exercise training on response to DSS-induced colitis. FTR mice performed 6 weeks (5 days/week: total 30 sessions) of forced moderate treadmill running (8–12 m/min; 5% grade; equating to ~480m of running per session) for 40 minutes per day at the beginning of their dark cycle prior to DSS treatment. For the VWR experiment, mice were housed in cages with free access to telemetered running wheels (Respironics, Bend, OR) for 30 days. Thus, we controlled for the number of days of exposure to exercise, but not exercise intensity or volume between the two different exercise paradigms. The last exercise session occurred 24 hours prior to DSS administration. All sedentary mice were handled similarly and housed in close proximity to the treadmill and wheel cages during the exercise training to control for incidental stress associated with handling, noise and novel environmental exposure.
DSS Treatment
Regular drinking water was replaced by 2% DSS in sterile water (w/v, MP Biochemical 36,000–50,000 MW) for a period of 5 days after completion of the exercise intervention period. The DSS solution was monitored daily, refilled appropriately and replaced on Day 3.
Morbidity
Daily measurements of body weight and food and fluid intake were made during and after DSS administration. In addition, mouse feces were evaluated in an observer blinded manner for consistency/diarrhea (e.g. formed pellet vs. semi-formed/soft) and were tested daily for the presence of blood (Hemoccult®). Lastly, we assessed the physical activity behavior (e.g. 1 = ‘normal, 2 = ‘slight reduction’, 3 = ‘limited’, 4 = ‘immobile’) in the home cage and response to capture (e.g. 1 = ‘normal evasion’, 2 = ‘some evasion’, 3 = ‘no evasion’) as indicators of sickness behavior in a blinded manner.
Tissue Collection
Mice were euthanized by rapid CO2 asphyxiation and cervical dislocation. Blood was drawn from the inferior vena cava and stored on ice. Plasma was separated and stored at −80°C. Mice were then perfused via intra-cardial injection of sterile saline. The colon was removed (distal to the caecum to the anus) and colon length was assessed using digital calipers (Tresna) to the nearest mm. Mesentery tissue was removed from the colon which was flushed with PBS and separated into proximal and distal ends. A 2 cm piece was cut from the top portion of the distal half and fixed in 10% buffered formalin until sectioning for histological analysis.
Proximal and distal ends were stored at −80°C until analysis. Both adrenals and the thymus were excised and weighed to screen for adrenal hypertrophy and thymic involution.
Colon Histological Analysis
The 2 cm long 10% formalin fixed colon samples were paraffin embedded and 3 μm cross sections were stained with hematoxylin and eosin. Colon damage (necrosis) and immune cell infiltrate was scored by a blinded observer based on a published scoring system that considers architectural derangements, goblet cell depletion, edema/ulceration, and degree of inflammatory cell infiltrate (Wirtz et al., 2007). Each sample was ranked twice, once for necrosis and once for inflammation. Scoring for inflammatory cell infiltration was as follows: 0 = ‘normal’, 1 = ‘mild’, 2 = ‘moderate’, 3 = ‘severe’. Necrosis was scored as: 0 = ‘normal’, 1 = ‘mild’ (~1–25% necrotic), 2 = ‘moderate’ (~26–75% necrotic), 3 = ‘severe’ (>75% necrotic).
Analysis of Gene Expression
Quantitative real-time polymerase chain reaction (qRT-PCR) was used to determine gene expression in colon tissue samples. Approximately 30 mg of each colon sample was homogenized using a tissue homogenizer (PRO Scientific PRO-250 rotary). RNA was extracted using the Applied Bioscience protocol (Applied Bioscience, Carlsbad, CA) and stored at −80°C. Later, the reverse transcriptase reaction was performed using commercial first strand kits (Applied Biosciences, CA) and random hexamer primers, as described in the manufacturer’s protocol. The RNA sample concentrations and purities were analyzed spectrophotometrically. Gene expression was analyzed using a qRT-PCR machine (7900HT Fast Real-Time PCR system and SDS Enterprise Database). We assessed both pro- (IL-1β, TNF-α, IL-6,, IL-17A) and anti-inflammatory (IL-10) cytokines in the colon as markers of classic immune response (Applied Biosciences primers: IL-1β, Mm00434228; TNF-α, Mm00443258; IL-6, Mm00446190; Mm00518984; IL-17A, Mm00439618; IL-10, Mm00439616). Inflammatory cytokines such as IL-1β, TNF-α, and IL-17A have been implicated in the pathogenesis of UC and correlate with disease activity (Papadakis and Targan, 2000; Sanchez-Munoz et al., 2008; Malik and Mannon, 2012). IL-17A was measured because it is a potent cytokine involved in gut defense against bacteria that recruits neutrophils and stimulates epithelial defensin production, but also drives a T cell response that causes tissue damage in the gut (Monteleone et al., 2009).. Chemokines direct immune cell traffic (Kotarsky et al., 2010), exhibit anti-microbial properties (Coelho et al., 2007), and are attractive targets for the management of IBD because they are highly expressed chemokine in the inflamed colon of IBD patients, thus we measured the chemokine CCL6 (Atreya and Neurath, 2010) (Applied Biosciences primer: Mm01302419) and the neutrophil chemotactic CXCL1 (Puleston et al., 2005) (Applied Biosciences primer: Mm04207460). To perform the PCR reactions, Taqman® gene expression Q-PCR master mix was used (Applied Bioscience, CA). As an internal control, mRNA levels of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Su et al., 2007) were measured and all data are represented relative to its expression (i.e., using the ΔΔ-Ct method) as fold change from SED/H2O group (Livak and Schmittgen, 2001). SED/H2O values from Day 6 are considered referent to allow for comparison between the different days of the FTR studies.
Serum Amyloid A (SAA)
The acute phase protein serum amyloid A (SAA) concentration was measured in plasma by enzyme-linked immunosorbent assay (Alpco Diagnostics; Salem, NH) as an indicator of systemic inflammation.
Statistical Analysis
Analysis of variance (ANOVA) with repeated measures was used to determine between and within-group differences in body weight, and food and fluid intake as a result of intervention (exercise) and treatment (DSS). In the Day 12 experiment, 3 of 10 mice in the FTR/DSS group were lost to mortality (n = 2 on Day 8 and n = 1 on Day 10). We utilized the last observation carried forward (LOCF) procedure to be able to compute repeated measures analyses for these variables so as not to omit data from the sickest animals. The Log-rank (Mantel-Cox) test was used to test differences in survival. At a given time point, two-way (intervention x treatment) ANOVA were used to determine between-group differences in colon gene expression (e.g. IL-1β, IL-6, TNF-α, CCL6, CXCL1, and IL-10), and serum amyloid A. When a significant main effect was detected at a significance level p<0.05, post hoc analyses were performed using Bonferroni corrected Student’s t test. Data that were not normally distributed (as tested by the Kolmogorov-Smirnov test) were log transformed. Nonparametric analyses (Kruskal-Wallis & Mann-Whitney rank sum) were used to detect between-group differences in histological assessment of necrosis and inflammation, IL-6, IL-1, TNF-α in VWR mice, IL-17 gene expression on Days 6 and 8 in FTR mice. All gene expression data are represented relative to the expression of GAPDH (i.e., using the ΔΔ-Ct method) and reported as fold change from the Day 6 SED/H2O referent group in the FTR experiments. Data were analyzed using SPSS software (v 20.0, Chicago, IL) and for all statistical tests, α was set at ≤ 0.05.
Results
Effects of FTR on DSS-induced changes in mortality and morbidity
Mortality
No animals died due to DSS-treatment in the Day 6 or Day 8 experiments. However, in the Day 12 experiment, 3/10 mice died (two on Day 9 and one on Day 11) in the FTR/DSS group while none (0/10) died in the SED/DSS group. The Log-rank (Mantel-Cox) test revealed a tendency (χ2 = 3.3, p = 0.069) for higher mortality in the FTR/DSS group.
Body weight loss, food and fluid intake
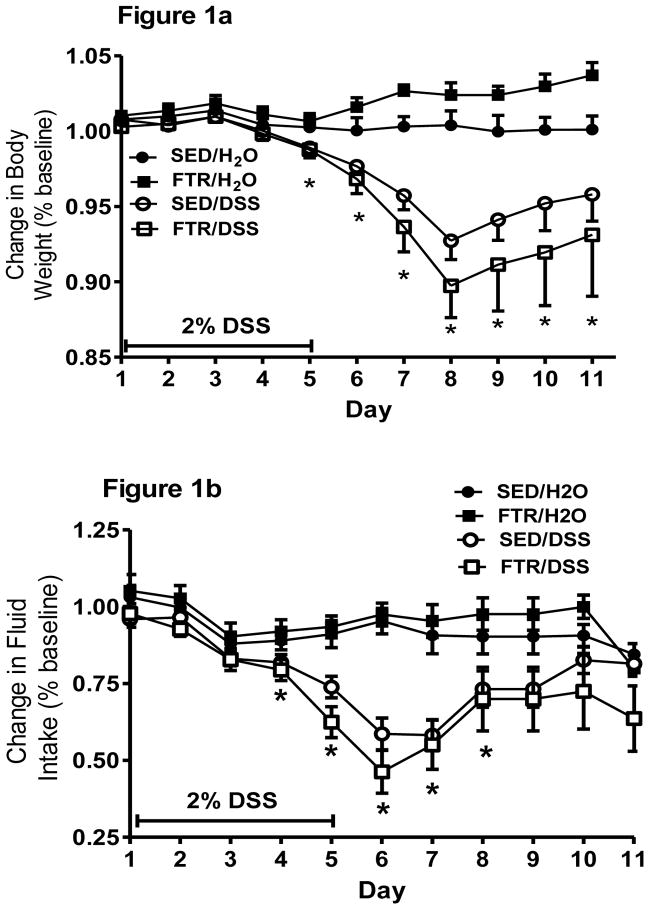
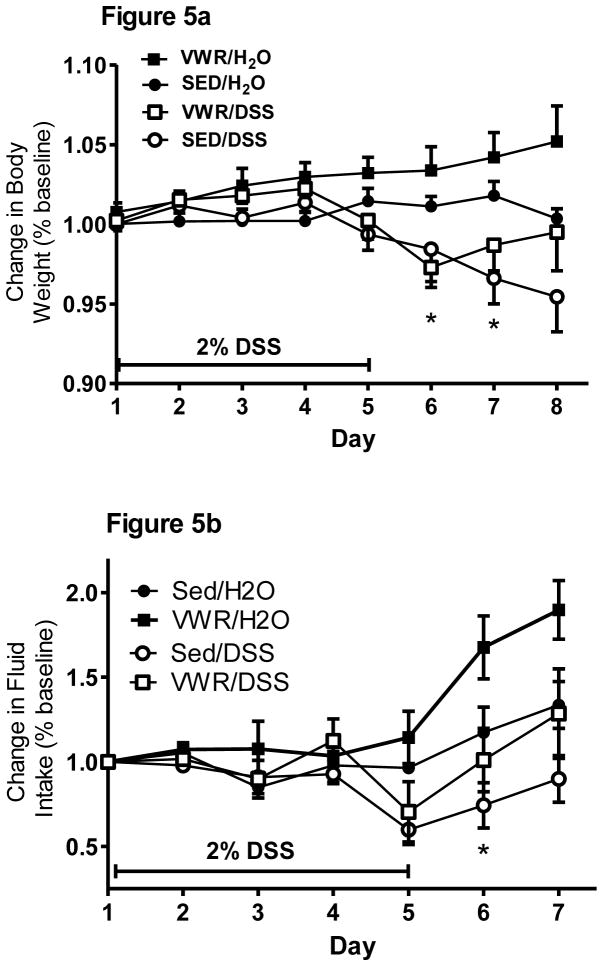
Indicative of the moderate intensity of the FTR, there were no significant differences in body weight (29.65 ± 0.47, 28.88 ± 0.40 g for SED and FTR, respectively) after the 6 wk FTR intervention, but there was both a time (F5,185 = 1901, p = 0.000) and a time x intervention interaction (F5,185 = 4.8, p = 0.000) suggesting that the FTR mice gained weight at a slower rate than the sedentary mice over the course of the 6 week FTR intervention (data not shown). To best represent the changes in body weight over time in response to DSS, we present data from the Day 12 experiment only (Figure 1a). DSS treatment resulted in significant weight loss beginning at Day 6 onward in both treated groups (Figure 1a). While FTR/DSS lost more body weight than SED/DSS, there was only a tendency (p = 0.14) for a time x treatment x intervention interaction. For fluid intake in the Day 12 experiment, DSS reduced fluid intake on Days 4–8 post-DSS, but there was no significant time x treatment x intervention interaction even though fluid intake was lower in FTR/DSS on Days 5 and 6 post-DSS (Figure 1b). It is important to note that there were no significant differences in fluid intake between SED/DSS and FTR/DSS indicating similar exposure to DSS. Food intake was significantly reduced on Days 5–7 post-DSS, but there was no time x treatment x intervention indicating no differences in food intake between the FTR/DSS and SED/DSS groups (data not shown).
Figure 1. Effects of prior FTR training on a) body weight loss and b) reduced fluid intake induced by DSS.
While DSS induced significant weight loss beginning at Day 5 (signified by *), there were no time x intervention x treatment (p = 0.14) or intervention x treatment (p = 0.09) interactions. DSS reduced fluid intake beginning at Day 4, but there was no difference between FTR and SED (p = 0.88 for interaction). N’s = 9, 10, 10 and 10 for SED/H2O, FTR/H2O, SED/DSS, FTR/DSS, respectively.
FTR Colitis Symptoms
While DSS reduced physical activity behavior in the home cage, response to capture and blood in the feces (Table 1), we observed no significant differences in these measures when comparing SED and FTR (other data not shown). However, FTR resulted in significant increase in the incidence of diarrhea on Days 7–9 post-DSS when compared to SED (Day 7 χ2 = 10.3, p = 0.001; Day 8 χ2 = 7.1, p = 0.008; Day 9 χ2 = 10.8, p = 0.001) (Table 1). There was no diarrhea detected in any of the H2O control groups.
Table 1. Morbidity Outcomes.
Effects of prior FTR on diarrhea incidence and fecal blood in DSS-treated mice.
| Diarrhea
|
Diarrhea
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Day | SED/H2O | VWR/H2O | SED/DSS | VWR/DSS | SED/H2O | FTR/H2O | SED/DSS | FTR/DSS |
|
|
|
|||||||
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6.1 |
| 5 | 0 | 0 | 100 | 87.5 | 0 | 0 | 39 | 30 |
| 6 | 0 | 0 | 100 | 50* | 0 | 0 | 57 | 63.6 |
| 7 | 0 | 0 | 100 | 37.5* | 0 | 0 | 47.8 | 91.3* |
| 8 | 0 | 0 | 75 | 37.5 | 0 | 0 | 34.8 | 73.9* |
| 9 | 0 | 0 | 30 | 100* | ||||
| 10 | 0 | 0 | 30 | 12.5 | ||||
| 11 | 0 | 0 | 10 | 12.5 | ||||
| 12 | 0 | 0 | 0 | 14 | ||||
|
Fecal Hemoccult
|
Fecal Hemoccult
|
|||||||
| SED/H2O | VWR/H2O | SED/DSS | VWR/DSS | SED/H2O | FTR/H2O | SED/DSS | FTR/DSS | |
|
|
|
|||||||
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 0 | 0 | 50 | 25 | 0 | 0 | 12.1 | 8.7 |
| 7 | 0 | 0 | 37.5 | 25 | 0 | 0 | 17.4 | 21.7 |
| 8 | 0 | 0 | 12.5 | 12.5 | 0 | 0 | 21.7 | 34.8 |
| 9 | 0 | 0 | 10 | 12.5 | ||||
| 10 | 0 | 0 | 0 | 12.5 | ||||
| 11 | 0 | 0 | 0 | 12.5 | ||||
| 12 | 0 | 0 | 0 | 12.5 | ||||
Data are reported as percentage. There was no diarrhea or fecal blood in water treated groups. There was a significant (*) increase in diarrhea incidence between FTR/DSS and SED/DSS on Days 7–9 (χ2 p ≤ 0.05) post-DSS administration. There was a significant (*) reduction in diarrhea incidence between VWR and SED on Days 6–7 (χ2 p ≤ 0.05) post-DSS administration. N = 8–13 mice/group for FTR and n = 8–10 mice/group for VWR.
Effects of FTR colon cytokine gene expression
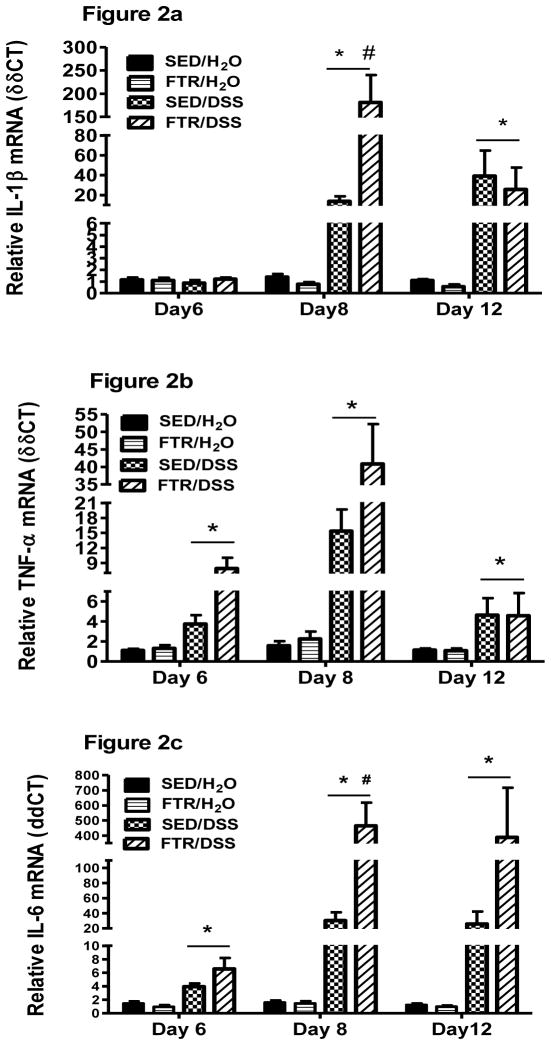
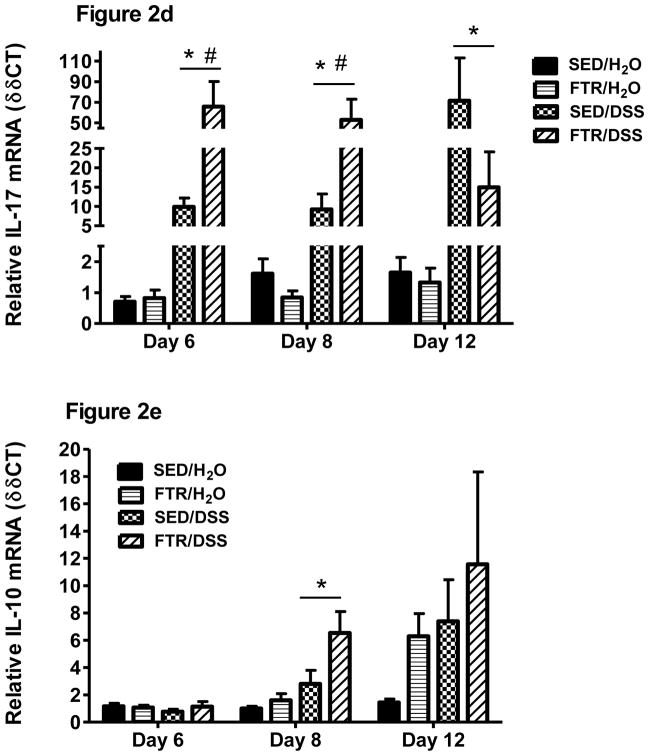
As expected, DSS increased pro-inflammatory cytokine gene expression in the colon (Figure 2). Interestingly, and in contrast to our hypothesis, moderate FTR led to an exacerbated pro-inflammatory cytokine gene response to DSS including significant increases in IL-1β (F1,45 = 15.2, p = 0.000 for interaction) and IL-6 (F1,45 = 5.4, p = 0.02 for interaction) (Figure 2 a and c) at Day 8 post-DSS. While there were no statistically significant interactions, TNF-α mRNA expression was higher in the FTR mice on Days 6 and 8 (Figure 2b). IL-17 gene expression was also exacerbated by FTR at Day 8 post-DSS (p = 0.001) (Figure 2d). Although not significant, by Day 12, the FTR group demonstrated lower Il-1β and IL-17 gene expression when compared to sedentary controls. This could be due to the death of 3 mice in the FTR group who likely expressed IL-1β and IL-17 at high levels.. IL-10, an anti- inflammatory cytokine, was also significantly (F1,45 = 4.9, p=0.03) higher in colons of the FTR group at Day 8 and tended to be higher at Day 12 (Figure 2e). IL-10 mRNA tended to be higher in the FTR groups at Day 12..
Figure 2. Colon cytokine gene expression in response to DSS in SED and FTR mice.
a) IL-1β, b) TNF-α, c) IL-6, d) IL-17, and e) IL-10. *denotes significant DSS-induced difference vs. SED/H2O controls; # denotes significant difference between FTR/DSS and SED/DSS groups. N = 10 and 13/group for Days 6 and 8, respectively and n = 9, 10, 10 and 7 for SED/H2O, FTR/H2O, SED/DSS and FTR/DSS, respectively for Day 12.
Effects of FTR on CCL-6 and CXCL1 gene expression in the colon
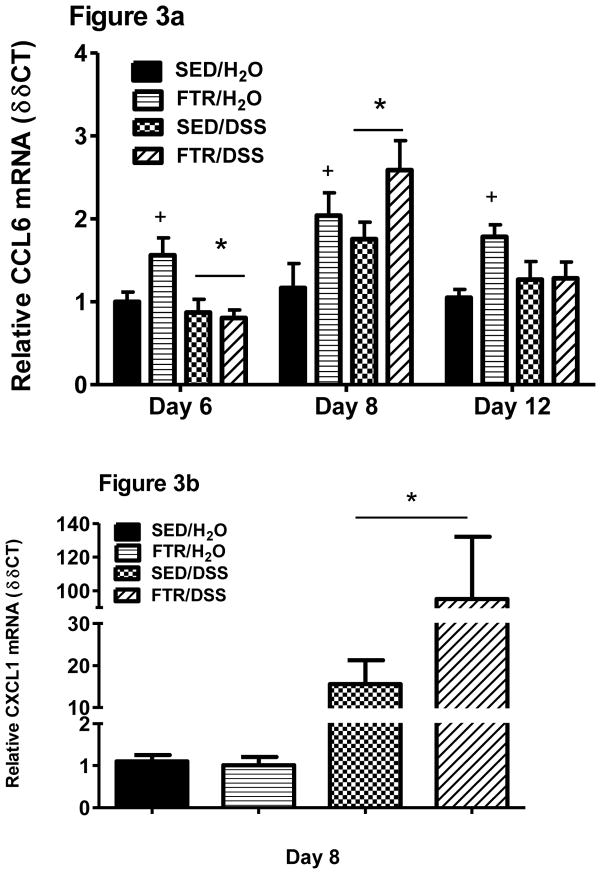
DSS tended (p = 0.07) to reduce CCL6 mRNA expression on Day 6 and significantly increased it on Day 8 (F1,45 = 5.85, p = 0.02) (Figure 3a). The most striking finding was that moderate intensity FTR significantly increased colonic CCL6 gene expression across all experiments (e.g. significant FTR main effects seen at each day; Day 6: F1,36 = 5.5, p = 0.025; Day 8: F1,45 = 12.0, p = 0.001; Day 12: F1,32 = 4.6, p = 0.04) when compared to SED/H2O. CXCL1 mRNA expression was only measured at Day 8 revealing a significant (F1,48 = 71.0, p=0.000) DSS treatment effect and a tendency (p=0.07) for a difference between SED/DSS and FTR/DSS (Figure 3b).
Figure 3. Effects of FTR and DSS on colonic CCL6 (a) and CXCL1 (b) gene expression.
*denotes significant DSS-induced difference; # indicates a significant intervention x treatment interaction; + indicates a significant difference between FTR/H2O and SED/H2O groups. FTR: n =10 and 13/group for Days 6 and 8, respectively and n = 9, 10, 10 and 7 for SED/H2O, FTR/H2O, SED/DSS and FTR/DSS, respectively for Day 12.
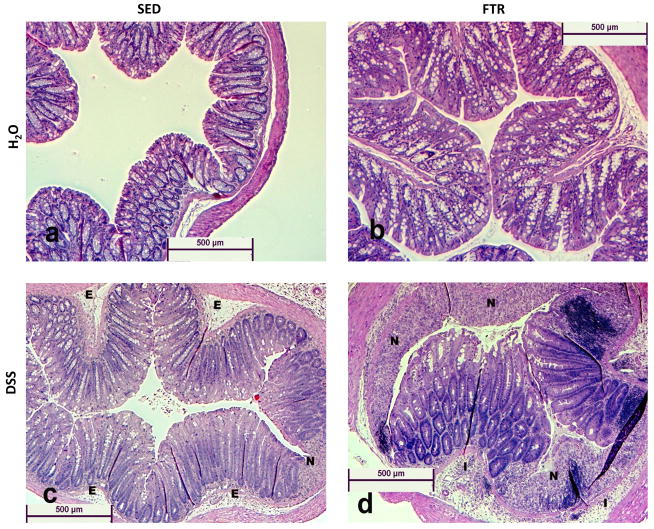
Effects of FTR on DSS-induced histopathology
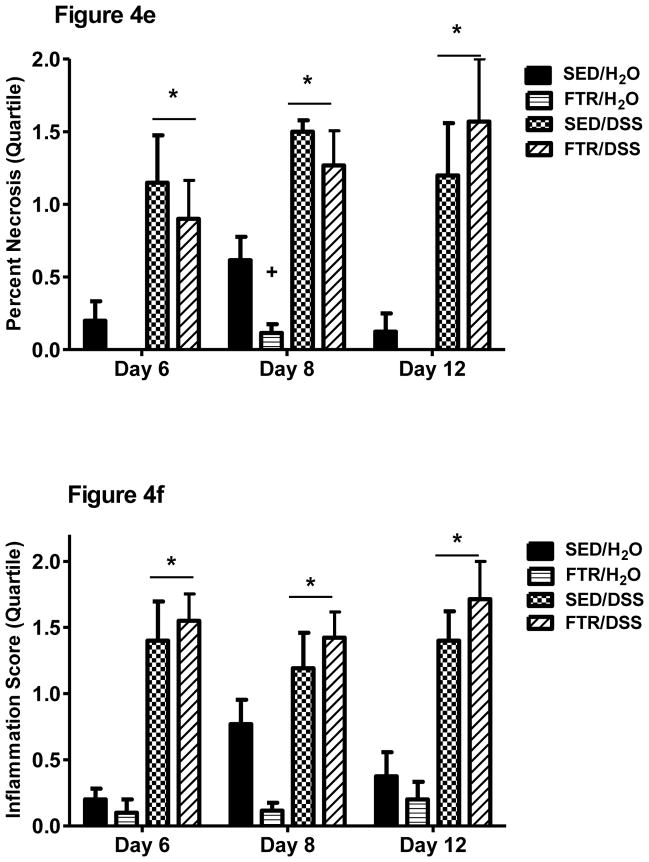
We examined hematoxylin and eosin stained colon cross-sections and scored them for necrosis and inflammation (see methods). Representative images (5X magnification) can be found in Figure 4a–d. Control water groups (SED/H2O and FTR/H2O) are represented and show normal histology. However, DSS-treated groups (SED/DSS and FTR/DSS) show signs of edema (E), inflammation (I), and necrosis (N). Quantification revealed that DSS increased necrosis (Figure 4e) and inflammation (Figure 4f) scores across all days; however, we found no intervention x treatment interactions. Necrosis and inflammation scores tended to be lower in non-DSS treated FTR mice and significantly so at Day 8 for necrosis (Figure 4e).
Figure 4. Effects of FTR and DSS on colon histology.
Representative (at 5× magnification) hematoxylin and eosin stained images (4a–4d, for SED/H2O, FTR/H2O, SED/DSS and FTR/DSS at disease peak Day 8, respectively). E, I and N, signify edema, inflammation, and necrosis, respectively. Quantification of images for percent necrotic tissue (4e) and inflammation (4f) scores (see methods for details). *denotes significant DSS-induced difference vs. SED/H2O; + indicates a significant difference between FTR/H2O and SED/H2O groups. n = 10 and 13/group for Days 6 and 8, respectively and n = 9, 10, 10 and 7 for SED/H2O, FTR/H2O, SED/DSS and FTR/DSS, respectively for Day 12.
Effects of DSS and FTR on Serum Amyloid A (SAA)
In the FTR experiments, SAA was only measured on Days 8 and 12 post-DSS in a subset of mice. SAA increased significantly (F1,26 = 18.5, p < 0.001) in response to DSS on Day 8 (112 ± 15, 84 ± 7, 183 ± 35, and 356 ± 54 μg/mL for SED/H2O, n=5; FTR/H2O, n=5; SED/DSS, n=7 and FTR/DSS, n=13, respectively) and was still significantly (F1,20 = 14.2, p = 0.001) elevated at Day 12 (112 ± 15, 79 ± 7, 129 ± 5, and 273 ± 62 μg/mL for SED/H2O, n=5; FTR/H2O, n=5; SED/DSS, n=7 and FTR/DSS, n=7, respectively). FTR significantly (F1,26 = 4.9, p = 0.036) exacerbated this effect on Day 8.
Effects of VWR training on DSS-induced changes in mortality and morbidity
As we found differences in the FTR experiment, we chose to examine the effects of VWR on DSS-induced colitis in a separate experiment where animals were sacrificed at Day 8 post-DSS at the time of peak symptoms and colon inflammation.
Mortality
No animals in the VWR experiment died through Day 8 post-DSS, the time at which the experiment was terminated for euthanasia and tissue collection.
Body weight loss, food and fluid intake
After the 30 day VWR intervention in which mice ran 6.32 ± 0.25 Km/day on average, VWR mice (23.7 ± 0.3 g) weighed significantly (t24 = 2.9, p = 0.007) less than sedentary (25 ± 0.3 g) mice indicative of a VWR training effect on body mass. DSS treatment significantly (time x treatment F7,154 = 6.3, p = 0.001) reduced body weight on Days 6 and 7, but there was no intervention x treatment x time interaction (Figure 5a). However, close inspection revealed that VWR/DSS mice began to regain body weight on Day 7 whereas SED/DSS mice continued to lose weight. This, along with the fact that VWR/H2O gained weight, was reflected in a tendency for a time x intervention (p = 0.13) and an intervention main effect (p = 0.06). Fluid intake (Figure 5b) was also reduced by DSS treatment (F6,132 = 4.8, p = 0.000) at Day 6 without a time x treatment x intervention interaction indicating that both VWR and SED groups responded similarly to DSS-induced fluid intake reduction. However, there was an intervention and time x intervention interaction, indicating that VWR groups had greater fluid intake in particular at Days 6 and 7. Food intake tended to be reduced by DSS, but there was no time x treatment x intervention indicating no differences in food intake between the FTR/DSS and SED/DSS groups (data not shown).
Figure 5. Effects of prior VWR training on body weight loss (a) and reduction in fluid intake (b) induced by DSS treatment.
While DSS induced significant weight loss (*) on Days 6 and 7, there was no time x intervention x treatment interaction. Fluid intake was also depressed by DSS at Day 6, but there was no time x intervention x treatment interaction. N = 5, 5, 8 and 8 for SED/H2O, VWR/H2O, SED/DSS, VWR/DSS, respectively.
VWR Colitis Symptoms
As was the case in the FTR experiment, DSS reduced physical activity behavior in the home cage, response to capture, and bloody stools (Table 1) but there were no significant differences in these measures when comparing SED and VWR (other data not shown). Diarrhea was present in DSS treated mice starting on Day 5 and, in contrast to FTR, was significantly less in the VWR mice (χ2 p values ≤ 0.05) on Days 6–7 when compared to their sedentary counterparts (Table 1).
Effects of VWR training on DSS-induced colon cytokine gene expression
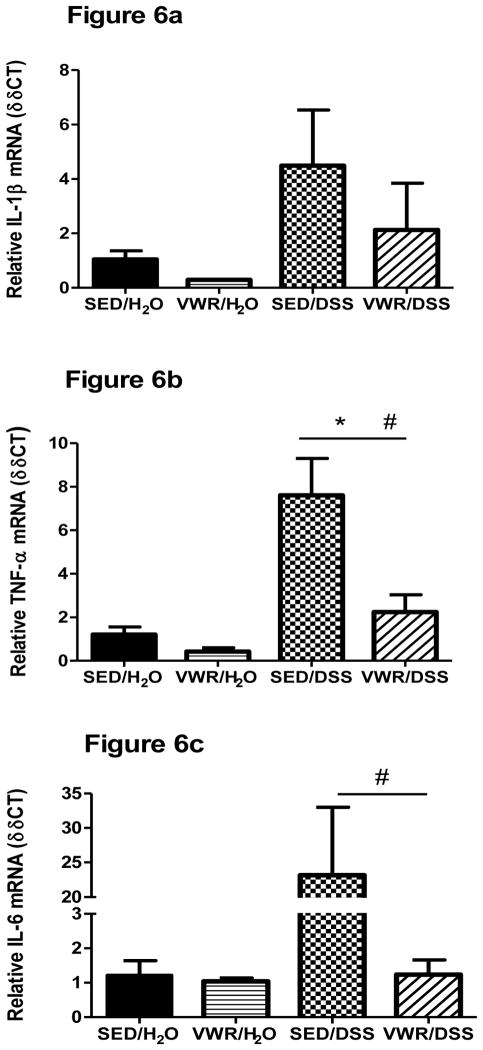
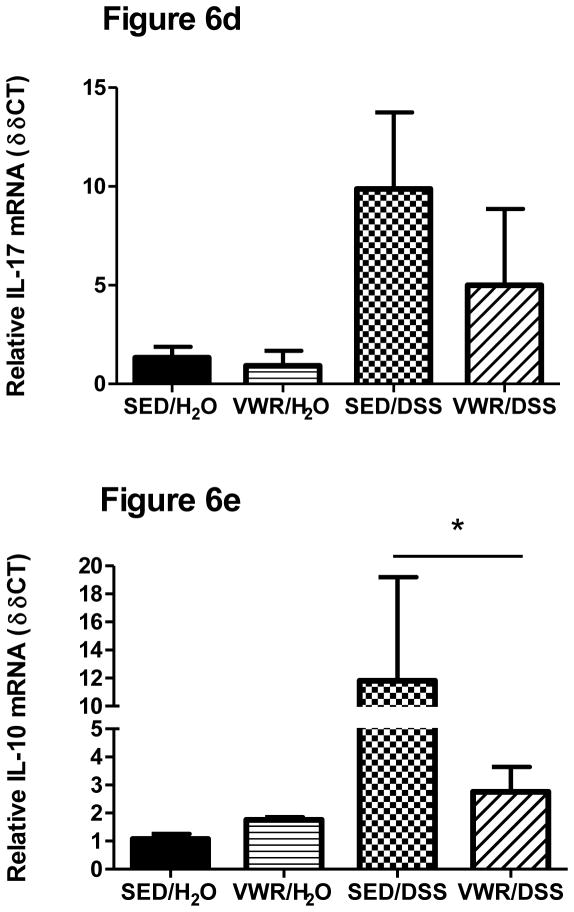
IL-1β mRNA, while elevated, was not statistically different (p = 0.11) when compared to H2O-treated groups (Figure 6a). There were no differences in IL-1β DSS responses between SED and VWR groups, but there was a tendency (p = 0.09) for IL-1β to be lower between VWR/H2O and SED/H2O. TNF- α cytokine gene expression was significantly increased in DSS-treated mice (Figure 6b). In contrast to FTR, VWR led to a significantly lower colonic TNF-α cytokine gene response to DSS (p = 0.005). There was a tendency (p = 0.10) for IL-6 mRNA to be increased in response to DSS and there was a significant (p = 0.001) difference between SED/DSS and VWR/DSS groups (Figure 6c). DSS tended to increase IL-17 (p = 0.06) and significantly increased IL-10 (F1,22 = 4.58, p= 0.044) gene expression (Figure 6d–e). While IL-17 and IL-10 gene expression were lower in VWR/DSS compared to SED/DSS, there were no statistical differences.
Figure 6. Colon cytokine gene expression in response to DSS in sedentary and VWR trained mice.
a) IL-1β, b) TNF-α, c) IL-6, d) IL-17, and e) IL-10). *denotes significant DSS main effect; + indicates an intervention main effect; # denotes significant intervention x treatment interaction. N = 5, 5, 8 and 8 for SED/H2O, VWR/H2O, SED/DSS and VWR/DSS, respectively.
Effects of VWR on DSS-induced CCL-6 and CXCL1 gene expression in the colon
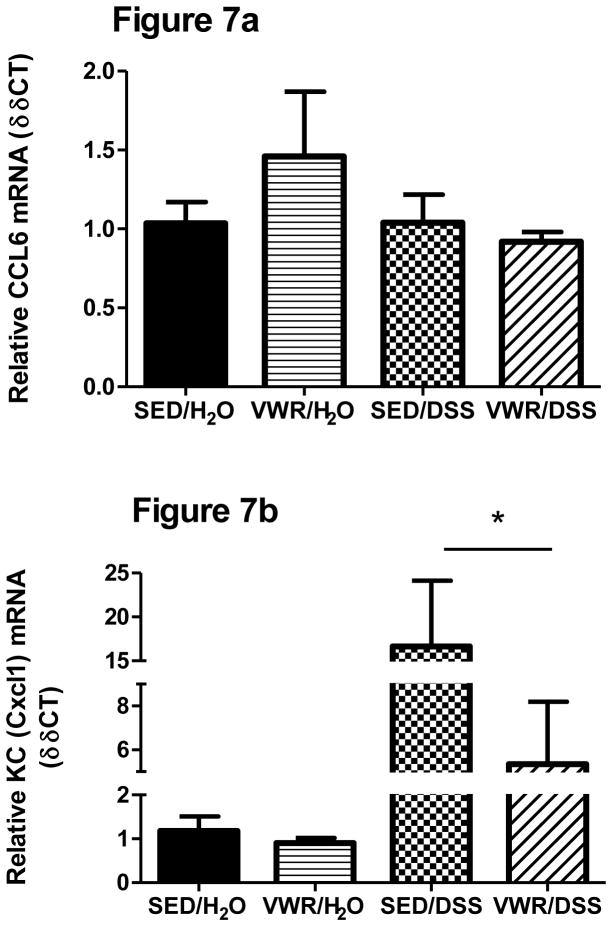
In contrast to FTR, CCL6 gene expression was not significantly altered by VWR independent of DSS treatment (Figure 7a) nor was it increased by DSS when measured at Day 8. Gene expression of the neutrophil chemokine CXCL1 was significantly increased with DSS (F1,22 = 13.2, p = 0.000) and, while lower in VWR/DSS, was not significantly different (p = 0.23) from SED/DSS (Figure 7b).
Figure 7. Effects of VWR and DSS-induced colonic CCL6 and CXCL1 gene expression.
a) CCL6 b) CXCL1. *denotes significant treatment effect. N = 5, 5, 8 and 8 for SED/H2O, VWR/H2O, SED/DSS and VWR/DSS, respectively.
Effects of VWR training on DSS-induced histopathology
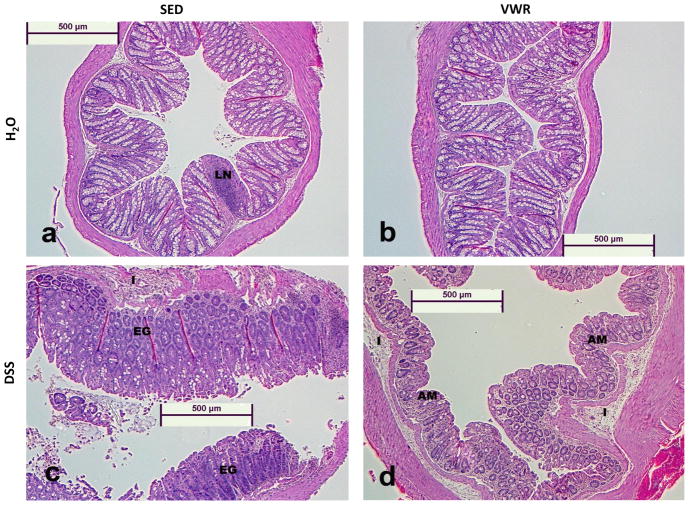
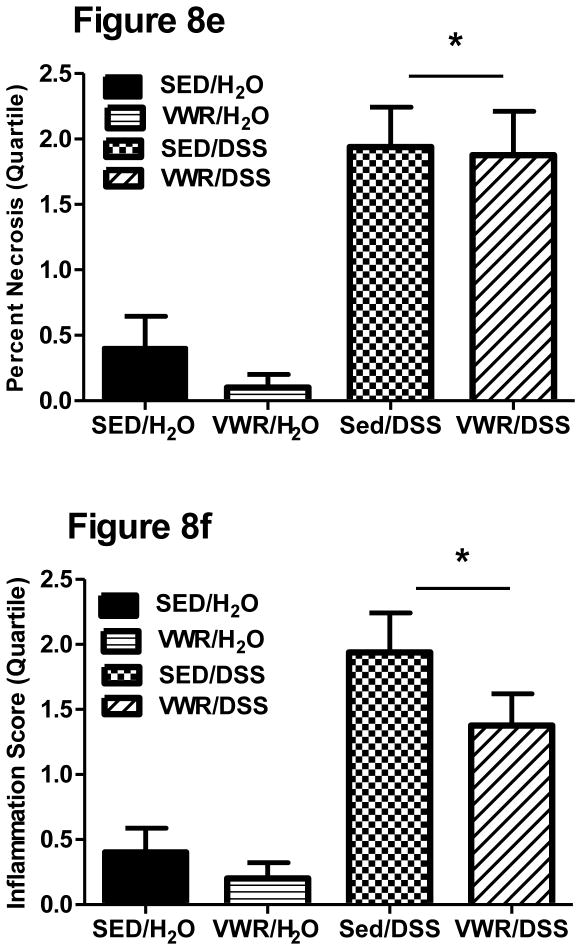
Representative hematoxylin and eosin stained images (5X magnification) can be found in Figure 8a–d. Control water groups (SED/H2O and VWR/H2O) are represented and show normal histology (LN = normal lymphoid nodule). Colitis groups (SED/DSS and VWR/DSS) show signs of glandular elongation (GE) and atrophied mucosa (AM) due to loss of goblet cells, inflammation (I), and necrosis (N). Mann-Whitney Rank-Sum analysis revealed significant colon necrosis (Figure 8e) and inflammation (Figure 8f) on Day 8 post-DSS (treatment p < 0.001 for both), however, we found no differences between sedentary and VWR DSS-treated groups.
Figure 8. Effects of VWR on DSS-induced colon histopathology.
Representative (at 5× magnification) hematoxylin and eosin stained images (8a–8d, for SED/H2O, FTR/H2O, SED/DSS and FTR/DSS at disease peak Day 8, respectively). LN, GE and AM signify normal lymphoid nodule, glandular elongation and atrophied mucosa due to loss of goblet cells, respectively. Quantification of images for percent necrotic tissue (8e) and inflammation (8f) scores (see methods for details). *denotes significant DSS-induced difference vs. SED/H2O. N = 5, 5, 8 and 8 for SED/H2O, VWR/H2O, SED/DSS and VWR/DSS, respectively.
Effects of DSS and VWR on Serum Amyloid A (SAA)
SAA increased significantly (F1,19 = 40.7; p = 0.000) in response to DSS (112 ± 15, 109 ± 6, 237 ± 10, and 324 ± 42 μg/mL for SED/H2O, n=5; VWR/H2O, n=5; SED/DSS, n=5 and VWR/DSS, n=8, respectively), however there were no differences between SED/DSS and VWR/DSS groups.
Effects of FTR and VWR on indices of chronic stress
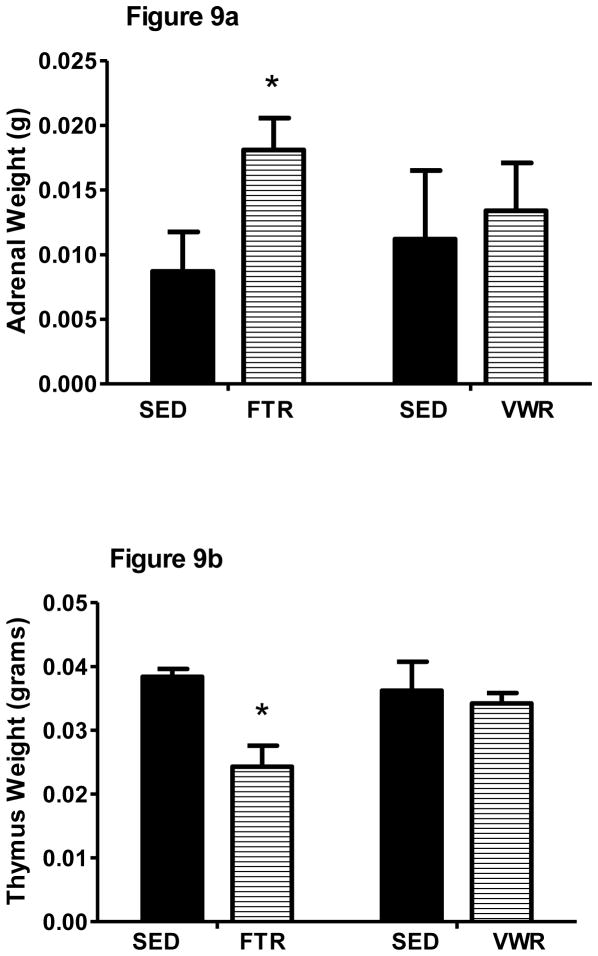
In a separate experiment in water only-treated FTR and VWR mice, we measured thymic and adrenal weights 24 hrs after the last training session to determine whether these different exercise paradigms affected stress-sensitive measures. Chronic stressors lead to thymic involution and adrenal hypertrophy in mice (Moraska et al., 2000). FTR mice had significantly larger adrenal (t18 = 2.4, p = 0.03) and lower thymic gland (t18 = 4.0, p = 0.001) weights when compared to SED mice (Figure 9). However, there were no differences when comparing sedentary and VWR mice in thymic or adrenal gland weights.
Figure 9. Effects of FTR and VWR on adrenal and thymic weight.
a) adrenal weight and b) thymic weight. *denotes significant difference in exercise vs. sedentary. n = 10/group for FTR cohort and n = 5/group in VWR cohort.
Discussion
Contrary to our hypothesis, we found that 6 weeks of prior forced moderate treadmill running (FTR) exacerbated inflammatory gene expression in the distal colon and increased hepatic acute phase SAA in the systemic circulation in response to DSS. This was accompanied by increased morbidity (e.g. diarrhea) and mortality. These negative findings led us to investigate whether voluntary wheel running (VWR) elicited similar effects. In contrast, we found that 30 days (e.g. same number of days of exposure as FTR) of VWR significantly attenuated inflammatory gene expression in the distal colon and protected mice from DSS-induced morbidity in that VWR/DSS-treated mice experienced reduced diarrhea.
Similar to our FTR results, chronic psychosocial stress has been shown to exacerbate colitis in the DSS mouse model. For example, Reber et al exposed mice to repeated social defeat and overcrowding stress for 19 consecutive days and found more severe symptoms and colon inflammation in response to 1% DSS (Reber et al., 2006). Therefore, it is likely that the FTR we applied in this study was perceived by mice as a chronic stressor even though the duration was short and the intensity of exercise was moderate. In support of this, we measured adrenal and thymic gland weights in response to FTR and VWR and found that only FTR resulted in adrenal hypertrophy and thymic atrophy. Our findings are similar to that of Moraska et al. who found that 8 wk of FTR (for longer durations and higher intensities than we applied), while inducing beneficial changes in body weight and muscle metabolism, caused potentially detrimental changes in the adrenal and thymic glands of rats (Moraska et al., 2000).
It is interesting to note that, in our hands, FTR is detrimental in terms of exacerbating inflammation in the DSS-induced mouse model of colitis, but exerts beneficial anti-inflammatory effects in other models of inflammation-associated morbidity. Indeed, we have published that similar FTR protocols in mice can reduce adipose and systemic inflammation in response to high-fat diet feeding (Vieira et al., 2009b, 2009c) and Kohut’s group has demonstrated that 14 wks of FTR reduced lung inflammation and improved symptoms in response to influenza virus infection (Sim et al., 2009) in mice. Moreover, 12 wks of FTR has been shown to repress neuroinflammation in the brains of mice transgenic for human tau23; a model of Alzheimer’s disease (Leem et al., 2011). The reasons for the differential effects of FTR on inflammation in these models are unclear; however it may have to do with the tissue being analyzed. The gut is highly innervated with both sympathetic and parasympathetic nerves, has its own enteric nervous system, contains a vast array of immune cells, and is at the interface of the ‘outside world’; a location where significant numbers of bacteria exist. This anatomy may make it different from other tissues as it relates to effector responses to stress. Indeed, psychological stress in humans is associated with perturbations in gut physiology and related to conditions such as irritable bowel syndrome and ulcerative colitis (O’Malley et al., 2011; Reber et al., 2006).
Aside from the forced or voluntary nature of the exercise, there were large differences in the volumes and perhaps intensities of exercise between the two paradigms. Given that mice in our VWR group ran ~4–8Km during the typical 8–10 h period of their dark cycle each day, it is clear that experiments are needed to titrate this wheel running effect. That is to say, ‘can lower volumes of VWR result in beneficial effects in this DSS mouse model of colitis?’ In an attempt to indirectly address this, we correlated VWR distance with DSS colitis outcomes including inflammatory gene expression and morbidity and we failed to document any statistically significant correlations (data not shown). However, it should be noted that the animal that ran the least on average still ran ~4km/day. This may be well above a threshold volume to see VWR effects. It is interesting that such a low volume (e.g. 40min/day) and intensity (8–12 m/min or ~ 60–75% VO2max) (Schefer and Talan, 1996) of FTR had negative effects while high volumes of exercise associated with VWR had beneficial effects. This contrasts the human literature demonstrating the prolonged, intense exercise can lead to gut dysfunction but moderate exercise may be beneficial (Martin, 2011). As mentioned above, a critical factor is likely the forced versus voluntary nature of the exercise paradigms. Future experiments will need to equate the dose (e.g. intensity and volume) of exercise between the two paradigms in order to tease out whether the nature of the exercise (e.g. forced vs. voluntary) or the exercise dose is the important factor.
There are several possible mechanisms for this detrimental effect of uncontrollable FTR on DSS-induced colitis. First, recent studies in animals examining the effects of chronic stress have demonstrated that it can cause spontaneous colitis and exacerbate chemically-induced colitis (Reber et al., 2006). In these studies, colitis development or exacerbation was associated with loss of anti-inflammatory glucocorticoid signaling and tissue sensitivity (Reber, 2012). Data such as these point to a critical role of the HPA axis in regulating UC. Although untested in the present experiment, FTR may induce a state of glucocorticoid resistance allowing for exaggerated and chronic inflammation in response to DSS. Second, we found that FTR caused a significant and long-lasting (at least 12 days after the last exercise session) elevation in CCL6 mRNA expression in non-DSS treated mice. This chemokine is chemotactic for innate immune effector cells such as dendritic cells and macrophages (Coelho et al., 2007) and is secreted by intestinal epithelial cells where it possesses antibacterial functions (Kotarsky et al., 2010). Thus, it may be that FTR, but not VWR, increases bacterial translocation from the gut lumen into the mucosal layers initiating anti-bacterial defense mechanisms such as increased CCL6 expression. One hypothesis would be that FTR, by increasing CCL6 chemokine expression, increased migration of dendritic cells and macrophages to the colon. This elevation in colonic innate immune cells could be responsible for the exaggerated inflammatory response to DSS seen in these FTR mice. We are currently exploring whether FTR (or VWR for that matter) in the absence of DSS exposure alters the number of innate immune cells in the colon or translocation of bacteria to mesenteric lymph nodes.
In addition to demonstrating a negative effect of moderate amounts of FTR, what is novel about our study is that 30 days of VWR resulted in beneficial effects, to the extent that VWR almost completely abrogated DSS-induced pro-inflammatory gene expression in the distal colon. This finding is important in that it supports a role for exercise in the adjunct treatment of ulcerative colitis in humans. While VWR has been shown to be anti-inflammatory in other models, there is less evidence regarding its role in the gut. In support of lower volumes of VWR acting beneficially in a colitis model, Kasimay et al. demonstrated that 200m of VWR, 3 times per week, for 6 wk reduced macro- and microscopic damage scores, myeloperoxidase activity (a marker of inflammation) and malondialdehyde (a marker of oxidative stress) in rats given intracolonic acetic acid (Kasimay et al., 2006). The authors noted that these effects were associated with a decrease in anxiety-like behavior. Clinical symptoms or more direct measures of inflammation were not reported. Recently, Saxena et al. (Saxena et al., 2012) reported that FTR did not affect clinical scores or ex vivo IL-1β, IL-6 or IL-10 protein production and reduced TNF-α protein production in response to DSS-induced colitis; findings that contrast our results. This was surprising given the many similarities (e.g. mouse strain, DSS treatment) between experiments. One difference was that their FTR protocol involved ~1080–1320m of running per day compared to ~480m in our study (> 2-fold difference) and their training duration was 4 wk vs. our 6 wk long paradigm. Whether or not the higher dose of exercise somehow protected their mice from the detrimental stress effect or their training duration was too short to see a detrimental effect remains to be determined. Another difference was that they analyzed ex vivo cytokine production from tissue obtained from the transverse colon whereas we measured gene expression in the descending colon. It is the descending (distal) colon that is most affected by DSS in mice (Perse and Cerar, 2012) and UC in humans (Danese and Fiocchi, 2011). Studies from Hoffman-Goetz et al have found that 16 wk of VWR reduces intestinal lymphocyte production of pro-inflammatory and increases production of anti-inflammatory cytokines in the absence of any inflammatory stimulus (Hoffman-Goetz et al., 2010). The mechanisms for these anti-inflammatory effects of exercise in the gut are unknown, but may involve activation of the parasympathetic cholinergic anti-inflammatory reflex (Van Der Zanden et al., 2009). In some instances (e.g. IL-1β and TNF-α) we observed trends towards anti-inflammatory effects of VWR in non-DSS treated mice consistent with this hypothesis (Fig. 6a & 6b).
There exist some limitations to the current study. It is interesting to note that while we found significant effects in pro-inflammatory gene expression, we failed to document corollary changes in inflammation using cross-sectional histological techniques despite our best efforts to perform these in a systematic fashion. We also measured colon length as a proxy for inflammation, and while DSS resulted in colon shortening, there were no differences between SED and either FTR or VWR groups (data not shown). Tissue necrosis (also measured histologically), was increased by DSS, but not altered by FTR or VWR. Laroui et al. (Laroui et al., 2012) described the mechanism of action of DSS as forming complexes with medium chain fatty acids (MCFA) produced by bacteria present in the microflora. Thus, lesions occur where these MCFA producing bacteria are colonized making it difficult, using cross-sectional histological analysis, to consistently capture areas where more damage has occurred. Therefore, longitudinal histological analysis may be necessary to overcome this limitation. Nevertheless, our current histological evaluation verified colitis in DSS treated animals. A potential confound existed in that differences existed in SED/DSS control diarrhea incidence rates between the FTR and VWR experiments. This variable was the only variable that differed statistically amongst the control groups of the two separate experiments. This diarrhea incidence difference was not due to differences in DSS administration (e.g. lot or dose) or to the age of the mice in the different experiments. However, two different research assistants who were trained identically in diarrhea scoring and blinded to treatment performed the diarrhea assessments in the different experiments. Whether this control group difference was due to different raters or to true inter-experimental biological variability is unknown. However, we our confident in our diarrhea outcome data because within any given experiment (e.g. FTR or VWR) this rating of diarrhea score was consistent and performed by the same ‘blinded to treatment’ rater. Lastly, we examined the effects of VWR only at Day 8. We reasoned that this would be the best time to compare the effects of VWR because this was the time of peak symptoms and colonic inflammation. Based upon our FTR experiment we would expect VWR effects to be smaller when measured at other time points.
UC affects up to 0.25% of the US population or ~750,000 people (Kappelman et al., 2007) and thus is a significant problem. While pharmaceuticals such as 5-aminosalicylic acid and corticosteroids can be of benefit, they are associated with negative side-effects and 10–30% of patients still require colon surgery to treat their disease. Unfortunately, there is very little information of the role that regular exercise may play in alleviating UC burden. Demonstration of proper exercise dosages that could alleviate UC symptoms, reduce colon inflammation, and counteract negative side-effects of pharmaceutical therapy would go a long way in convincing gastroenterologists and UC patients to adopt this lifestyle strategy as an adjunct therapy in their UC treatment. Unfortunately, to date, there is little information regarding the effects of regular exercise on IBD’s such as UC. This is surprising given that UC increases risk for subsequent colon cancer and that exercise has been found to be protective against colon cancer. To our knowledge, there is only one published randomized clinical trial that has examined the influence of exercise on UC symptoms (Elsenbruch et al., 2005). Unfortunately, exercise (1 time/week) was but one of several components (e.g. Mediterranean diet, stress management, other behavioral and self-care strategies) of a mind-body intervention. In this 10 wk intervention, Elsenbruch et al. (Elsenbruch et al., 2005) found significantly higher mental and physical health scores and significantly greater improvements on the Inflammatory Bowel Disease Questionnaire (IBDQ) than controls post-intervention. Unfortunately, no disease-specific objective markers (e.g. # of UC flares, stool lactoferrin, blood CRP) were measured. This study, and our pre-clinical data demonstrating a beneficial effect of VWR on DSS-induced colitis in mice, makes a case for exploring the use of exercise as an adjunct treatment for UC. In summary, we report that 6 wk of FTR exacerbates, while VWR attenuates inflammation and symptoms associated with DSS-induced colitis.
Research Highlight.
Forced exercise exacerbates, while voluntary exercise attenuates, colitis in mice; these findings are important for identifying proper exercise for treatment of colitis.
Acknowledgments
This work supported by American College of Sports Medicine Foundation Doctoral Student grants to M.D. Cook and NIH grant (AG029573-S1) to KW Kelley.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Astrom MB, Feigh M, Pedersen BK. Persistent low-grade inflammation and regular exercise. Front Biosci. 2010;2:96–105. doi: 10.2741/s48. [DOI] [PubMed] [Google Scholar]
- Atreya R, Neurath MF. Chemokines in inflammatory bowel diseases. Dig Dis. 2010;28(3):386–394. doi: 10.1159/000320392. [DOI] [PubMed] [Google Scholar]
- Bi L, Triadafilopoulos G. Exercise and gastrointestinal function and disease: an evidence-based review of risks and benefits. Clin Gastroenterol Hepatol. 2003;1 (5):345–355. doi: 10.1053/s1542-3565(03)00178-2. [DOI] [PubMed] [Google Scholar]
- Coelho AL, Schaller MA, Benjamim CF, Orlofsky AZ, Hogaboam CM, Kunkel SL. The chemokine CCL6 promotes innate immunity via immune cell activation and recruitment. J Immunol. 2007;179 (8):5474–5482. doi: 10.4049/jimmunol.179.8.5474. [DOI] [PubMed] [Google Scholar]
- Danese S, Fiocchi C. Ulcerative colitis. NEJM. 2011;365 (18):1713–1725. doi: 10.1056/NEJMra1102942. [DOI] [PubMed] [Google Scholar]
- Elrod JW, Laroux FS, Houghton J, Carpenter A, Ando T, Jennings MH, Grisham M, Walker N, Alexander JS. DSS-induced colitis is exacerbated in STAT-6 knockout mice. Inflamm Bowel Dis. 2005;11 (10):883–889. doi: 10.1097/01.mib.0000182871.76434.57. [DOI] [PubMed] [Google Scholar]
- Elsenbruch S, Langhorst J, Popkirowa K, Muller T, Luedtke R, Franken U, Paul A, Spahn G, Michalsen A, Janssen OE, Schedlowski M, Dobos GJ. Effects of mind-body therapy on quality of life and neuroendocrine and cellular immune functions in patients with ulcerative colitis. Psychother Psychosom. 2005;74 (5):277–287. doi: 10.1159/000086318. [DOI] [PubMed] [Google Scholar]
- Fukata M, Michelsen KS, Eri R, Thomas LS, Hu B, Lukasek K, Nast CC, Lechago J, Xu R, Naiki Y, Soliman A, Arditi M, Abreu MT. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am J Physiol Gastrointest Liver Physiol. 2005;288 (5):G1055–1065. doi: 10.1152/ajpgi.00328.2004. [DOI] [PubMed] [Google Scholar]
- Hamdani G, Gabet Y, Rachmilewitz D, Karmeli F, Bab I, Dresner-Pollak R. Dextran sodium sulfate-induced colitis causes rapid bone loss in mice. Bone. 2008;43 (5):945–950. doi: 10.1016/j.bone.2008.06.018. [DOI] [PubMed] [Google Scholar]
- Hoffman-Goetz L, Pervaiz N, Packer N, Guan J. Freewheel Training Decreases Pro- and Increases Anti-Inflammatory Cytokine Expression in Mouse Intestinal Lymphocytes. Brain Behav Immun. 2010;24(7):1105–15. doi: 10.1016/j.bbi.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Kappelman MD, Rifas-Shiman SL, Kleinman K, Ollendorf D, Bousvaros A, Grand RJ, Finkelstein JA. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5 (12):1424–1429. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Kasimay O, Guzel E, Gemici A, Abdyli A, Sulovari A, Ercan F, Yeğen BC. Colitis-induced oxidative damage of the colon and skeletal muscle is ameliorated by regular exercise in rats: the anxiolytic role of exercise. Exp Physiol. 2006;91 (5):897–906. doi: 10.1113/expphysiol.2006.034439. [DOI] [PubMed] [Google Scholar]
- Keylock KT, Vieira VJ, Wallig MA, DiPietro LA, Schrementi M, Woods JA. Exercise accelerates cutaneous wound healing and decreases wound inflammation in aged mice. Am J Physiol Regul Integr Comp Physiol. 2008;294(1):R179–184. doi: 10.1152/ajpregu.00177.2007. [DOI] [PubMed] [Google Scholar]
- Kotarsky K, Sitnik KM, Stenstad H, Kotarsky H, Schmidtchen A, Koslowski M, Wehkamp J, Agace WW. A novel role for constitutively expressed epithelial-derived chemokines as antibacterial peptides in the intestinal mucosa. Mucosal Immunol. 2010;3 (1):40–48. doi: 10.1038/mi.2009.115. [DOI] [PubMed] [Google Scholar]
- Laroui H, Ingersoll SA, Liu HC, Baker MT, Ayyadurai S, Charania MA, Laroui F, Yutao Y, Sitaraman SV, Merlin D. Dextran sodium sulfate (DSS) induces colitis in mice by forming nano-lipocomplexes with medium-chain-length fatty acids in the colon. PLoS One. 2012;7 (3):e32084. doi: 10.1371/journal.pone.0032084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leem YH, Lee YI, Son HJ, Lee SH. Chronic exercise ameliorates the neuroinflammation in mice carrying NSE/htau23. Biochem Biophys Res Commun. 2011;406(3):359–365. doi: 10.1016/j.bbrc.2011.02.046. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lowder T, Padgett DA, Woods JA. Moderate exercise early after influenza virus infection reduces the Th1 inflammatory response in lungs of mice. Exerc Immunol Rev. 2006;12:97–111. [PubMed] [Google Scholar]
- Malik T, Mannon P. Inflammatory bowel diseases: emerging therapies and promising molecular targets. Front Biosci. 2012;4:1172–1189. doi: 10.2741/s324. [DOI] [PubMed] [Google Scholar]
- Martin D. Physical Activity Benefits and Risks on the Gastrointestinal System. South Med J. 2011;104 (12):831–837. doi: 10.1097/SMJ.0b013e318236c263. [DOI] [PubMed] [Google Scholar]
- Mizoguchi E, Xavier RJ, Reinecker HC, Uchino H, Bhan AK, Podolsky DK, Mizoguchi A. Colonic epithelial functional phenotype varies with type and phase of experimental colitis. Gastroenterology. 2003;125 (1):148–161. doi: 10.1016/s0016-5085(03)00665-6. [DOI] [PubMed] [Google Scholar]
- Monteleone I, Pallone F, Monteleone G. Interleukin-23 and Th17 cells in the control of gut inflammation. Mediators Inflamm. 2009;2009:297645. doi: 10.1155/2009/297645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraska A, Deak T, Spencer RL, Roth D, Fleshner M. Treadmill running produces both positive and negative physiological adaptations in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol. 2000;279(4):R1321–1329. doi: 10.1152/ajpregu.2000.279.4.R1321. [DOI] [PubMed] [Google Scholar]
- Narula N, Fedorak RN. Exercise and inflammatory bowel disease. Can J Gastroenterol. 2008;22(5):497–504. doi: 10.1155/2008/785953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley D, Quigley EM, Dinan TG, Cryan JF. Do interactions between stress and immune responses lead to symptom exacerbations in irritable bowel syndrome? Brain Behav Immun. 2011;25 (7):1333–1341. doi: 10.1016/j.bbi.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Packer N, Hoffman-Goetz L, Ward G. Does physical activity affect quality of life, disease symptoms and immune measures in patients with inflammatory bowel disease? A systematic review. J Sports Med Phys Fitness. 2010;50 (1):1–18. [PubMed] [Google Scholar]
- Papadakis KA, Targan SR. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annual review of medicine. 2000;51:289–298. doi: 10.1146/annurev.med.51.1.289. [DOI] [PubMed] [Google Scholar]
- Perse M, Cerar A. Dextran sodium sulphate colitis mouse model: traps and tricks. J Biomed Biotechnol. 2012;718617 doi: 10.1155/2012/718617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puleston J, Cooper M, Murch S, Bid K, Makh S, Ashwood P, Bingham AH, Green H, Moss P, Dhillon A, Morris R, Strobel S, Gelinas R, Pounder RE, Platt A. A distinct subset of chemokines dominates the mucosal chemokine response in inflammatory bowel disease. Aliment Pharmacol Ther. 2005;21 (2):109–120. doi: 10.1111/j.1365-2036.2004.02262.x. [DOI] [PubMed] [Google Scholar]
- Reber SO. Stress and animal models of inflammatory bowel disease--an update on the role of the hypothalamo-pituitary-adrenal axis. Psychoneuroendocrinology. 2012;37 (1):1–19. doi: 10.1016/j.psyneuen.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Reber SO, Obermeier F, Straub RH, Falk W, Neumann ID. Chronic intermittent psychosocial stress (social defeat/overcrowding) in mice increases the severity of an acute DSS-induced colitis and impairs regeneration. Endocrinology. 2006;147 (10):4968–4976. doi: 10.1210/en.2006-0347. [DOI] [PubMed] [Google Scholar]
- Rizzo A, Pallone F, Monteleone G, Fantini MC. Intestinal inflammation and colorectal cancer: A double-edged sword? World J Gastroenterol. 2011;17 (26):3092–3100. doi: 10.3748/wjg.v17.i26.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Munoz F, Dominguez-Lopez A, Yamamoto-Furusho JK. Role of cytokines in inflammatory bowel disease. World J Gastroenterol. 2008;14 (27):4280–4288. doi: 10.3748/wjg.14.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena A, Fletcher E, Larsen B, Baliga MS, Durstine JL, Fayad R. Effect of exercise on chemically-induced colitis in adiponectin deficient mice. Journal Inflamm. 2012;9(1):30. doi: 10.1186/1476-9255-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schefer V, Talan MI. Oxygen consumption in adult and AGED C57BL/6JJ mice during acute treadmill exercise of different intensity. Exp Gerontol. 1996;31(3):387–392. doi: 10.1016/0531-5565(95)02032-2. [DOI] [PubMed] [Google Scholar]
- Sim YJ, Yu S, Yoon KJ, Loiacono CM, Kohut ML. Chronic exercise reduces illness severity, decreases viral load, and results in greater anti-inflammatory effects than acute exercise during influenza infection. J Infect Dis. 2009;200 (9):1434–1442. doi: 10.1086/606014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su W, Bush CR, Necela BM, Calcagno SR, Murray NR, Fields AP, Thompson EA. Differential expression, distribution, and function of PPAR- gamma in the proximal and distal colon. Physiol Genomics. 2007;30:342–353. doi: 10.1152/physiolgenomics.00042.2007. [DOI] [PubMed] [Google Scholar]
- Van Der Zanden EP, Boeckxstaens GE, de Jonge WJ. The vagus nerve as a modulator of intestinal inflammation. Neurogastroenterol Motil. 2009;21(1):6–17. doi: 10.1111/j.1365-2982.2008.01252.x. [DOI] [PubMed] [Google Scholar]
- Vieira VJ, Hu L, Valentine RJ, McAuley E, Evans EM, Baynard T, Woods JA. Reduction in trunk fat predicts cardiovascular exercise training-related reductions in C-reactive protein. Brain, behavior, and immunity. 2009a;23(4):485–491. doi: 10.1016/j.bbi.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Vieira VJ, Valentine RJ, Wilund KR, Antao N, Baynard T, Woods JA. Effects of exercise and low-fat diet on adipose tissue inflammation and metabolic complications in obese mice. Am J Physiol Endocrinol Metab. 2009b;296(5):E1164–1171. doi: 10.1152/ajpendo.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira VJ, Valentine RJ, Wilund KR, Woods JA. Effects of diet and exercise on metabolic disturbances in high-fat diet-fed mice. Cytokine. 2009c;46 (3):339–345. doi: 10.1016/j.cyto.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh NP, Gleeson M, Shephard RJ, Woods JA, Bishop NC, Fleshner M, Green C, Pedersen BK, Hoffman-Goetz L, Rogers CJ, Northoff H, Abbasi A, Simon P. Position statement. Part one: Immune function and exercise. Exercise Immunol Rev. 2011;17:6–63. [PubMed] [Google Scholar]
- Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2 (3):541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]