Abstract
Evidence that the delta opioid receptor (DOR) is an attractive target for the treatment of brain disorders has strengthened in recent years. This receptor is broadly expressed in the brain, binds endogenous opioid peptides, and shows as functional profile highly distinct from those of mu and kappa opioid receptors. Our knowledge of DOR function has enormously progressed from in vivo studies using pharmacological tools and genetic approaches. The important role of this receptor in reducing chronic pain has been extensively overviewed; therefore this review focuses on facets of delta receptor activity relevant to psychiatric and other neurological disorders. Beneficial effects of DOR agonists are now well established in the context of emotional responses and mood disorders. DOR activation also regulates drug reward, inhibitory controls and learning processes, but whether delta compounds may represent useful drugs in the treatment of drug abuse remains open. Epileptogenic and locomotor-stimulating effects of delta agonists appear drug-dependent, and the possibility of biased agonism at DOR for these effects is worthwhile further investigations to increase benefit/risk ratio of delta therapies. Neuroprotective effects of DOR activity represent a forthcoming research area. Future developments in DOR research will benefit from in-depth investigations of DOR function at cellular and circuit levels.
Keywords: Delta opioid receptor, Knockout, Pharmacology, in vivo, pathology
1. Introduction
Mu, delta and kappa opioid receptors are G protein coupled receptors, which play a central role in pain control, and are key players in hedonic homeostasis, mood and well-being. The three receptors and their endogenous opioid peptides also regulate responses to stress, and a number of peripheral physiological functions including respiratory, gastrointestinal, endocrine and immune processes. Opioid receptors are highly homologous in sequence, and their crystal structure has been recently elucidated at high-resolution by X-Ray crystallography (Granier et al., 2012; Manglik et al., 2012; Wu et al., 2012). All three receptors inhibit neuronal activity, via reduced neuronal firing or lower transmitter release, and a main goal in opioid research is the identification of receptor-mediated signaling pathways that operate in vivo, to regulate physiology and behavior (Pradhan et al., 2012).
In the past two decades, refinement of pharmacological tools and availability of genetic approaches have clarified the specific role of each opioid receptor in many aspects of opioid-related responses (Shippenberg et al., 2008; Gianoulakis, 2009; Sauriyal et al., 2011; Lutz & Kieffer, 2012; Gaveriaux-Ruff, 2013). Mu opioid receptors mediate both analgesic and addictive properties of clinically useful and abused opiates. Mu opioid receptor activation strongly inhibits severe pain, and is a major target for post-operative and cancer pain management (Zollner & Stein, 2007). Mu receptors are also central for reward processing (Le Merrer et al., 2009), representing a main factor in the initiation of addictive behaviors. Kappa opioid receptors also release pain (Chavkin, 2011) but oppose mu receptors in the regulation of hedonic homeostasis. The notion that kappa receptor blockade alleviates stress responses and depressive states is raising increasing interest (Shippenberg, 2009; Knoll & Carlezon, 2010).
Delta opioid receptors (also known as δ receptors, DORs or DOP receptors in the IUPHAR nomenclature) have emerged as an attractive target in many respects. In accordance with the rodent mRNA distribution, DOR in the human central nervous system is expressed in cortical regions and limbic structures such as hippocampus and amygdala, as well as basal ganglia and hypothalamus (Simonin et al., 1994; Peckys & Landwehrmeyer, 1999; Smith et al., 1999; Peng et al., 2012).
The development of highly selective delta opioid agonists and rapid progress in mouse mutagenesis approaches targeting the Oprd1 gene (Filliol et al., 2000; Scherrer et al., 2006; Scherrer et al., 2009; Gaveriaux-Ruff et al., 2011) have set delta receptors as a model system for the analysis of G protein coupled receptor (GPCR) trafficking and biased signaling in vivo, and established this receptor as a promising target to treat chronic pain and mood disorders (Pradhan et al., 2011). The stimulation of delta opioid receptors strongly reduces pain, specifically under situations of persistent pain, and mechanisms of delta agonist analgesia have been extensively overviewed recently (Gaveriaux-Ruff & Kieffer, 2011). Here we will focus on non-nociceptive facets of delta receptor function, and summarize accumulating preclinical data supporting the key role of delta receptors in emotional processes (Tables 1 and 2), drug reward and addiction (Table 3), and other aspects of potential therapeutic relevance (Table 4). Both genetic approaches and behavioral pharmacology concur to support an implication of delta receptors in psychiatric and neurological disorders, and delta agonists have entered clinical trials (Table 5).
Table 1.
Delta opioid receptor function in anxiety-related behavior control
| Approach | Model / compound | Test | Delta compound administration (route/dose) | Anxiety level (vs control) | References |
|---|---|---|---|---|---|
| Genetic | |||||
| DOR KO mice | Elevated plus maze | ↑ | (Filliol et al., 2000) | ||
| Light-dark box | ↑ | (Filliol et al., 2000) | |||
| Open field | ↔ | (Filliol et al., 2000) | |||
| Enk KO mice | Open field | ↑ | (Konig et al., 1996; Ragnauth et al., 2001) | ||
| Elevated O- maze | ↑ | (Konig et al., 1996) | |||
| Resident- intruder test | ↑ | (Konig et al., 1996) | |||
| Light-Dark Box | ↑ | (Ragnauth et al., 2001) | |||
| Fear conditioning | ↑ | (Ragnauth et al., 2001) | |||
| Pharmacologic | |||||
| Rats / NTI | Elevated plus maze | s.c. (1, 3 or 5 mg/kg) | ↑ | (Saitoh et al., 2004; Saitoh et al., 2005; Perrine et al., 2006) | |
| Elevated plus maze | local into Hipp (0.5, 1 or 2 μg/rat) | ↑ | (Solati et al., 2010) | ||
| Light-dark box | local into BLA (10 pmol/rat) | ↑ | (Narita et al., 2006a) | ||
| Mice / NTI | Light-dark box | i.c.v. (1 nmol/mouse) | ↑ | (Narita et al., 2006a) | |
| Light-dark box | s.c. (1 mg/kg) | ↑ | (Narita et al., 2006b) | ||
| Light-dark box | local into cingulate Cx (1 pmol/mouse) | ↑ | (Narita et al., 2006b) | ||
| Elevated plus maze | s.c. (1 mg/kg) | ↑ | (Narita et al., 2006b) | ||
| Elevated plus maze | local into cingulate Cx (1 pmol/mouse) | ↑ | (Narita et al., 2006b) | ||
| Rats / SNC80 | Fear conditioning | s.c. (1 or 3 mg/kg) | ↓ | (Saitoh et al., 2004) | |
| Elevated plus maze | s.c. (1–20 mg/kg) | ↓ | (Saitoh et al., 2004; Perrine et al., 2006) | ||
| Open field | s.c. (1 or 3 mg/kg) | ↔ | (Saitoh et al., 2004) | ||
| Defensive burying paradigm | s.c. (5 mg/kg) | ↓ | (Perrine et al., 2006) | ||
| Elevated O- maze | s.c. (5 mg/kg) | ↓ | (Ambrose-Lanci et al., 2010) | ||
| Rats / DPDPE | Elevated plus maze | local into CeA (0.5 or 1.5 μg/μl; 1 μl/CeA) | ↓ | (Randall-Thompson et al., 2010) | |
| Mice / UFP-512 | Light-dark box | i.p. (1 mg/kg) | ↓ | (Vergura et al., 2008) | |
| Elevated plus maze | i.p. (0.1 or 1 mg/kg) | ↓ | (Vergura et al., 2008) | ||
| Open field | i.p. (0.1 or 1 mg/kg) | ↔ | (Vergura et al., 2008) | ||
| Rats / Enkephalin | Elevated plus maze | local into Hipp (1, 2 or 5 μg/rat) | ↓ | (Solati et al., 2010) | |
| Mice / RB101 | Elevated O- maze | i.p. (80 mg/kg) | ↓ | (Mas Nieto et al., 2005) | |
| Rats / Opiorphin | Defensive burying paradigm | i.v. (1 mg/kg) | ↔ | (Javelot et al., 2010) | |
| Rats / AZD2327 | Modified geller-seifter conflict test | p.o. (0.5, 1 or 5 mg/kg) | ↓ | (Hudzik et al., 2011) |
Table 2.
Delta opioid receptor function in depressive-like behavior control
| Approach | Model / compound | Test | Delta compound administration (dose/route) | Despair level (vs control) | References |
|---|---|---|---|---|---|
| Genetic | |||||
| DOR KO mice | Forced swim test | ↑ | (Filliol et al., 2000) | ||
| Motility conditioned suppression test | ↔ | (Filliol et al., 2000) | |||
| Enk KO mice | Forced swim test | ↔ | (Bilkei-Gorzo et al., 2007) | ||
| Tail suspension test | ↔ | (Bilkei-Gorzo et al., 2007) | |||
| Pharmacologic | |||||
| Mice / NTI | Forced swim test | s.c. (1 or 3 mg/kg) | ↔ | (Saitoh et al., 2004) | |
| Rats / SNC80 | Forced swim test | s.c. (3.2, 10 or 32 mg/kg) | ↓ | (Jutkiewicz et al., 2005a; Jutkiewicz et al., 2005b) | |
| Mice / SNC80 | Forced swim test | s.c. (1 or 3 mg/kg) | ↓ | (Saitoh et al., 2004) | |
| Rats / DPDPE | Forced swim test | i.c.v. (155 nmol/rat) | ↓ | (Torregrossa et al., 2006) | |
| Rats / Deltorphin II | Forced swim test | i.c.v. (0.03 or 0.1 nmol/rat) | ↓ | (Torregrossa et al., 2006) | |
| Rats / JOM-13 | Forced swim test | i.v. (32 mg/kg) | ↓ | (Torregrossa et al., 2006) | |
| Mice / NIH 11082 | Tail suspension test | i.p. (16 or 32 mg/kg) | ↓ | (Naidu et al., 2007) | |
| Mice / UFP-512 | Forced swim test | i.p. (0.1 or 0.3 mg/kg) | ↓ | (Vergura et al., 2008) | |
| Rats / RB101 | Forced swim test | i.v. (32 mg/kg) | ↓ | (Jutkiewicz et al., 2006) | |
| Mice / RB101 | Forced swim test | i.p. (80 mg/kg) | ↓ | (Mas Nieto et al., 2005) | |
| Motility conditioned suppression test | i.p. (80 mg/kg) | ↓ | (Mas Nieto et al., 2005) | ||
| Rats / Opiorphin | Forced swim test | i.v. (1 mg/kg) | ↓ | (Javelot et al., 2010) | |
| Rats / AZD2327 | Learned helplessness | p.o. (1 or 10 mg/kg) | ↓ | (Hudzik et al., 2011) | |
| Mice / KNT-127 | Forced swim test | s.c. (0.1, 0.3 or 1 mg/kg) | ↓ | (Saitoh et al., 2011) |
Table 3.
Delta opioid receptor function in reward and addiction
| Drug of abuse / approach | Model / compound | Test | Delta compound administration (dose/route) | Behavioral level (vs control) | References |
|---|---|---|---|---|---|
| Morphine | |||||
| Genetic | DOR KO mice | CPP | ↓ | (Chefer & Shippenberg, 2009; Le Merrer et al., 2011) | |
| CPA (Lithium) | ↓ | (Le Merrer et al., 2011) | |||
| SA | ↔ | (Le Merrer et al., 2011) | |||
| SA | ↔ | (David et al., 2008) | |||
| DOR antagonist | Mice / NTI | CPP | s.c. (0.3 mg/kg) | ↓ | (Chefer & Shippenberg, 2009) |
| Rats / Naltriben | CPP | i.p. (1 mg/kg) | ↓ | (Billa et al., 2010) | |
| DOR agonist | Mice / TAN-67 | CPP | s.c. (10 or 20 mg/kg) | ↑ | (Suzuki et al., 1996) |
| Ethanol | |||||
| Genetic | DOR KO mice | SA (Two bottle choice CA) | ↑ | (Roberts et al., 2001) | |
| Operant SA | ↑ | (Roberts et al., 2001) | |||
| Enk KO mice | SA (Two bottle choice CA) | ↔ | (Racz et al., 2008) | ||
| DOR antagonist | Rats / NTI | Cue or context induced drug-seeking | i.p. (1, 5, 7.5 or 15 mg/kg) | ↓ | (Marinelli et al., 2009) |
| SA (Two bottle choice CA) | i.p. (5 or 10 mg/kg) | ↓ | (Nielsen et al., 2008) | ||
| CPP | intra-CeA (2 nM) | ↓ | (Bie et al., 2009) | ||
| SA (Two bottle choice IA) | intra-striatal (1 or 2 μg) | ↓ | (Nielsen et al., 2012) | ||
| Mice / Naltriben | SA (Two bottle choice IA) | s.c. (6 or 10 mg/kg) | ↓ | (van Rijn & Whistler, 2009) | |
| Rats / TIPPΨ | SA (Two bottle choice CA) | intra-VTA (5 μM) | ↓ | (Margolis et al., 2008) | |
| Rats / S0RI-9409 | SA (Two bottle choice CA and IA) | i.p. (5, 15 or 30 mg/kg) | ↓ | (Nielsen et al., 2008) | |
| DOR agonist | Rats / SNC80 | SA (Two bottle choice IA) | i.p. (20 mg/kg) | ↑ | (van Rijn et al., 2010) |
| intra-striatal (5 ng) | ↑ | (Nielsen et al., 2012) | |||
| Rats / DPDPE | SA (Two bottle choice CA) | intra-VTA (10 mM) | ↓ | (Margolis et al., 2008) | |
| Rats / DALA | SA (Two bottle choice IA) | intra-PVN (7.1 or 14.2 nM) | ↑ | (Barson et al., 2010) | |
| Cannabinoids | |||||
| Genetic | DOR KO mice | CPP | ↔ | (Ghozland et al., 2002) | |
| Nicotine | |||||
| Genetic | DOR KO mice | Nicotine CPP | ↓ | (Berrendero et al., 2012) | |
| Nicotine SA | ↓ | (Berrendero et al., 2012) | |||
| DOR antagonist | Rats / NTI | Nicotine SA (0.03 mg/kg/infusion) | s.c. (0.3, 1 or 3 mg/kg) | Trend ↓ | (Ismayilova & Shoaib, 2010) |
| Mice / NTI | Nicotine SA (30 μg/kg/infusion) | i.p. (5 mg/kg) | ↓ | (Berrendero et al., 2012) | |
| Psychostimulant | |||||
| DOR antagonist | Mice / NTI | Amphetamine- induced CPP | s.c. (5 mg/kg) | ↓ | (Belkai et al., 2009) |
| Rats / NTI | Cocaine SA (PR) (1.5 mg/kg/infusion) | intra-NAcc (5 nM/side) | ↓ | (Ward & Roberts, 2007) | |
| intra-VTA (5 nM/side) | ↑ | (Ward & Roberts, 2007) | |||
| intra-amygdala (5 nM/side) | ↔ | (Ward & Roberts, 2007) | |||
| Rats / NTI | Cocaine reinstatement | intra-NAcc (300, 1000 or 3000 ng/side) | ↔ | (Simmons & Self, 2009) |
Table 4.
elta opioid receptor role in epileptic seizures, hypoxia/ischemia and parkinson disease
| Condition / pathology | Model / compound | Test / measures | Delta compound administration (dose/route) | Results | References |
|---|---|---|---|---|---|
| Epileptic seizures | |||||
| DOR KO mice / | Ethological observations | s.c. (10–100 mg/kg) | DOR agonist- mediated seizures abolished | (Broom et al., 2002) | |
| Mice / SNC80 | Ethological observations | s.c. (10–100 mg/kg) | Seizures↑ | (Broom et al., 2002) | |
| Mice / BW373U86 | Ethological observations | s.c. (1–32 mg/kg) | Seizures↑ | (Broom et al., 2002) | |
| Rats / SNC80 | Ethological observations / EEG recording | s.c. or i.v. (1–100 mg/kg) | Seizures↑ | (Jutkiewicz et al., 2005a; Jutkiewicz et al., 2005b; Jutkiewicz et al., 2006a) | |
| Rats / NTI | Ethological observations of SNC80-induced convulsions | s.c. (0.1–10 mg/kg) | Seizures↑ | (Jutkiewicz et al., 2005b) | |
| Mice / KNT-127 | Ethological observations | s.c. (30 or 100 mg/kg) | No seizures | (Saitoh et al., 2011) | |
| Mice / RB101 | Ethological observations / EEG recording | i.v. (32 mg/kg) | No seizures | (Jutkiewicz et al., 2006b) | |
| Rats / ADL5859 | EEG recording | i.v. (10 or 30 mg/kg) | No seizures | (Le Bourdonnec et al., 2008) | |
| Rats / ADL5747 | EEG recording | i.v. (10 or 30 mg/kg) | No seizures | (Le Bourdonnec et al., 2009) | |
| Motor control | |||||
| Mice / SNC80 | Spontaneous locomotor activity | s.c. (1, 5 or 10 mg/kg) | ↑ | (Saitoh et al., 2011; Nozaki et al., 2012) | |
| Rats / SNC80 | Spontaneous locomotor activity | s.c. (3.2, 10 or 32 mg/kg) | ↑ | (Jutkiewicz et al., 2005a) | |
| Rats / RB101 | Spontaneous locomotor activity | i.v. (32 mg/kg) | ↑ | (Jutkiewicz et al., 2006b) | |
| Rats / DVL2DAL5La nEnk | Ethological observations | i.t. (0.1–30 μg) i.p. (0.1, 1 or 3 mg/kg) |
↔ | (Svensson et al., 2003) | |
| Mice / KNT-127 | Spontaneous locomotor activity | s.c. (1 or 10 mg/kg) | ↔ | (Saitoh et al., 2011) | |
| Mice / ADL5747 and ADL 5859 | Spontaneous locomotor activity | p.o. (10–300 mg/kg) | ↔ | (Nozaki et al., 2012) | |
| Rats / ADL5859 | Spontaneous locomotor activity | p.o. (up to 1000 mg/kg) | ↔ | (Le Bourdonnec et al., 2008) | |
| Rats / ADL5747 | Spontaneous locomotor activity | p.o. (30, 100 or 300 mg/kg) | ↔ | (Le Bourdonnec et al., 2009) | |
| Parkinson’s disease | |||||
| Rats / UFP-512 | Hemiparkinsonian 6- OHDA-induced unilateral lesions / drag test-rotarod | i.p. (0.1–1000 μg/kg) | Low dose UFP-512 Motor coordination ↑ High dose UFP-512 Motor coordination ↑ |
(Mabrouk et al., 2009) | |
| Rats / DPDPE | Hemiparkinsonian 6- OHDA-induced unilateral lesions / ethological observation | i.c.v. (10 μg/5μl/rat) | Abnormal movements ↑ | (Billet et al., 2012) | |
| Rats / NTI | Hemiparkinsonian 6- OHDA-induced unilateral lesions / ethological observation | i.c.v. (10 μg/5μl/rat) | Abnormal movements ↓ | (Billet et al., 2012) |
Table 5.
Clinical trials targeting the delta opioid receptor
| Sponsor | Drug | Condition | Clinical Phase | References (ID) |
|---|---|---|---|---|
| AstraZeneca | AZD2327 | Anxious Major Depressive Disorder | 2 | NCT00759395 |
| Cubist Pharmaceuticals | ADL5859 | Acute Pain | 2 | NCT00993863 |
| Cubist Pharmaceuticals; Pfizer | ADL5859 | Osteoarthritis of the Knee | 2 | NCT00979953 |
| ADL5747 | Osteoarthritis of the Knee | 2 | NCT00979953 | |
| Cubist Pharmaceuticals; Pfizer | ADL5747 | Postherpetic Neuralgia | 2 | NCT01058642 |
| Cubist Pharmaceuticals | ADL5945 | Opioid-Induced Constipation | 2 | NCT01207427 |
| Diamyd Inc | NP2 | Intractable Pain | 2 | NCT01291901 |
| Penn State University | NP2 | Hepatocellular Cancer | 1 | NCT00706576 |
| Head and Neck Squamous Cell Carcinoma | 2 | NCT00905099 |
2. Delta opioid receptor and the control of emotional processes
Genetic studies have revealed a prominent role for DORs in emotional processing more than a decade ago. Knockout of the Oprd1 gene, encoding DOR, led to higher anxiety-related responses and depressive-like behaviors (Filliol et al., 2000). This activity was clearly DOR-selective, since neither mu receptor knockout mice nor kappa receptor knockout mice showed a similar phenotype (Filliol et al., 2000). Mice deficient for Penk gene, encoding the pre-proenkephalin precursor, also showed increased levels of anxiety using a large number of experimental testing conditions (Konig et al., 1996; Ragnauth et al., 2001), suggesting that DOR/enkephalinergic systems exert control over anxiety-related behaviors. This was later supported by experiments performed in wild-type and mu receptor mutant mice, which both showed similar decreased levels of anxiety upon systemic administration of RB101, an enkephalinase inhibitor (Mas Nieto et al., 2005). Interestingly, over-expression of enkephalin by a virus approach in the amygdala potentiates the anxiolytic effect of benzodiazepines and this effect is abolished by systemic naltrindole (NTI) administration (Primeaux et al., 2006). Altogether therefore, genetic approaches have opened the way to explore DOR function in the areas of anxiety (Table 1) and depression (Table 2).
Pharmacological studies using both delta agonists and antagonists in rodents confirmed anxiolytic activity of the opioid tone mediated by DOR. As observed for knockout mice, receptor blockade by NTI administration, a selective DOR antagonist, increased anxiety-related behaviors in mice (Narita et al., 2006b) and rats (Saitoh et al., 2004; Saitoh et al., 2005; Perrine et al., 2006). DOR activation by selective agonists such as SNC80 (Saitoh et al., 2004; Perrine et al., 2006; Ambrose-Lanci et al., 2008), UFP-512 (Vergura et al., 2008) and ARM390 (Pradhan et al., 2010) decreased anxiety-related behaviors in most classical experimental paradigms (Table 1).
Regarding depressive states, and as predicted from knockout mice data, most currently existing DOR agonists (Pradhan et al., 2011) consistently decreased despair-like behaviors in a large number of tests (summarized in Table 2) in both mice (Saitoh et al., 2004; Naidu et al., 2007; Vergura et al., 2008) and rats (Jutkiewicz et al., 2005a; Jutkiewicz et al., 2005b; Torregrossa et al., 2006; Le Bourdonnec et al., 2008). Although no depression-related phenotype could be detected in animals lacking preproenkephalin (Bilkei-Gorzo et al., 2007), systemic administration of enkephalinase inhibitors had an antidepressant effect (Jutkiewicz et al., 2006b; Javelot et al., 2010). These studies suggest that the DOR/enkephalinergic system plays an important role in the control of depressive-like behaviors.
The circuitry of emotional processing has been extensively studied (LeDoux, 2000; Price & Drevets, 2012). Sensory information reaches cortical regions mostly through the thalamus and is integrated in limbic structures such as prefrontal cortex, hippocampus and amygdala. These brain areas, which attribute emotional value to internal and external stimuli show high DOR densities (Figure 1). Stereotaxic microinjection of several DOR agonists in the hippocampus (Solati et al., 2010), amygdala (Narita et al., 2006a; Randall-Thompson et al., 2010) and cingulate cortex (Narita et al., 2006b) reduced anxiety, and conversely, NTI administration at these brain sites increased levels of anxiety (Table 1). These data together suggest that DOR acting at the level of amygdala-cortico-hippocampal circuitry regulates emotional responses. Gene conditional approaches may be instrumental in the future to elucidate neural processes underlying DOR-controlled emotional responses at the cellular level.
Figure 1.
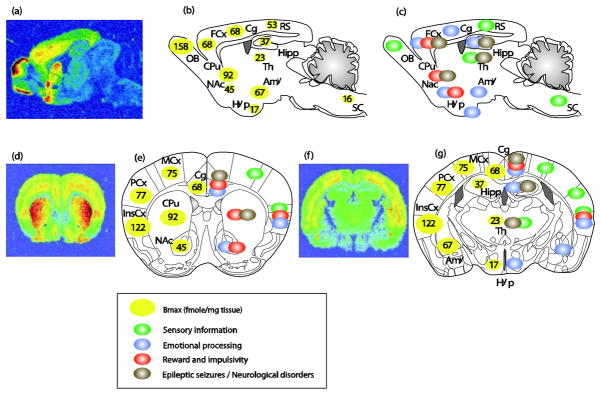
Anatomical distribution of delta opioid receptors and relevant brain functions. Top panels, sagittal sections; bottom panels, coronal sections at 2 different anterio-posterior positions ((e) bregma 0.98mm; (g) bregma −1.46mm). (a, d and f) ([3H]deltorphin ligand autoradiography reveals delta opioid receptor binding sites (courtesy of Ian Kitchen). (b, e left part and g left part) Quantification of DOR expression levels in fmole/mg of tissue (means from (Kitchen et al., 1997; Simonin et al., 1998; Slowe et al., 1999; Goody et al., 2002). DORs are particularly abundant in the OB, cortical regions (FCx, Cg, MCx, PCx and InsCx), amygdala and striatum (CPu and NAc). DORs are also expressed at moderate levels in the Hipp, RS, and at much lower level in Hyp, Th and SC. (c, e right part and g right part) Schematic representation of potential neural sites for DOR function. DORs are expressed in sensory regions (green circles), brain areas important for the regulation of anxiety and depression (blue circles adapted from (File et al., 2000; LeDoux, 2000; Cardinal et al., 2002; B. J. Everitt et al., 2003; Paulus & Stein, 2006; Rodrigues et al., 2009; Etkin et al., 2011; Gross & Canteras, 2012; Steenland et al., 2012)), brain sites for reward processing and inhibitory controls (red circles adapted from (Robbins & Everitt, 1996; Balleine & Dickinson, 1998; Kesner & Gilbert, 2007; Paton & Louie, 2012; Richard et al., 2012)) and areas relevant to epileptic seizures (grey circles adapted from (Andre et al., 1998; Brevard et al., 2006)). Abbreviations: Amy, Amygdala; Cg, Cingulate cortex; Cpu, Caudate Putamen; FCx, Frontal cortex; Hipp, Hippocampus; Hyp, Hypothalamus; InsCx, Insular cortex; MCx, Motor cortex; NAc, Nucleus Accumbens; OB, Olfactory Bulb; PCx, Parietal cortex; RS, Retrosplenial; SC, Spinal Cord; Th, Thalamus.
3. Delta opioid receptor, reward and addiction
Drugs of abuse activate brain reward systems, and initially produce pleasurable effects. Repeated drug exposure may lead to loss of control over drug intake, and drug dependence. A well-accepted view describes drug abuse as a three-stage vicious circle involving intoxication/withdrawal/craving episodes (Koob & Volkow, 2010). Animal studies have demonstrated the development of altered reward processes and enhanced stress responses (Koob & Le Moal, 2008), the setting of aberrant learning mechanisms (Belin et al., 2009) and habitual behaviors (Barry J. Everitt et al., 2008), the disruption of self-control (Baler & Volkow, 2006) and the engagement of cue-induced relapse mechanisms (Pickens et al., 2011), which all contribute to maintaining drug use. All three opioid receptors are largely expressed in reward and associated neural circuits (Le Merrer et al., 2009; Koob & Volkow, 2010), which adapt to chronic drug exposure, and are involved in both recreational drug use (reward) and the many aspects of addictive behaviors.
Animal and human studies have clearly established that mu opioid receptors are essential to mediate rewarding properties of both natural stimuli and drugs of abuse, and that kappa receptors mediate dysphoria, particularly under stressful conditions (Lutz & Kieffer, 2012). The implication of DOR in drug reward is more complex and differs across drugs of abuse. Data from conditioned place preference (CPP) and self-administration (SA) experiments for four distinct classes of drugs of abuse are compiled in Table 3. Beyond drug reward, delta receptors also contribute to the development of adaptations upon chronic drug exposure, mainly examined for morphine.
3.1 Morphine
DOR knockout mice showed decreased morphine-induced CPP in two studies (Chefer & Shippenberg, 2009; Le Merrer et al., 2011). However this effect was independent from rewarding properties of the drug, since mutant mice also exhibited decreased conditioned place aversion to lithium, as well as normal motivation to obtain morphine in a SA paradigm (David et al., 2008; Le Merrer et al., 2011). The association of stimuli that predict morphine administration was able to restore full expression of morphine CPP in these KO animals (Le Merrer et al., 2012). This set of experiments strongly suggests that DOR does not mediate morphine reward per se, but rather modulates learning processes in a place conditioning setting. Pharmacological studies using CPP experiments in rodents also support a role for DOR involvement in place conditioning paradigms (Suzuki et al., 1996; Shippenberg et al., 2009; Billa et al., 2010). A potential implication from all these data is that DOR may facilitate opiate-context association, which may be critical clinically in situations of context-induced relapse. A recent study, combining gene knockout and pharmacology, suggests that DOR is required to assign hedonic value to a reward-associated stimulus, a process that might influence motivation to get a reward (Laurent et al., 2012). The latter study, involving sucrose reward provides another indication for DOR-mediated associative processes.
Regarding chronic morphine effects, DOR knockout mice showed enhanced sensitization to locomotor effects of morphine (Chefer & Shippenberg, 2009), and pharmacological blockade of DOR by NTI (Chefer & Shippenberg, 2009) or naltriben (Billa et al., 2010) increased morphine-induced locomotor sensitization. Notably, morphine acts at mu opioid receptors in vivo (Contet et al., 2004) and does not directly activate DORs, as suggested by intact morphine analgesia (Y. Zhu et al., 1999; Scherrer et al., 2009) and reward (Table 3) in DOR knockout mice. Therefore the exact nature of delta-mu opioid receptor interactions in vivo and mechanisms underlying DOR-regulated chronic morphine effects remain to be clarified.
3.2 Ethanol
Pharmacological blockade of DOR systemically by NTI, naltriben or SORI-9409 decreased voluntary ethanol consumption (Nielsen et al., 2008; van Rijn & Whistler, 2009) and also cue-mediated drug seeking (Marinelli et al., 2009). Those studies suggested that DOR are likely involved in both rewarding properties of alcohol and learning processes responsible for the context-drug consumption association. Local administration of DOR antagonists into the ventral tegmental area (VTA) (Margolis et al., 2008), the dorsal striatum (Nielsen et al., 2012) or the central nucleus of the amygdala (Bie et al., 2009) also disrupted ethanol self-administration or ethanol-induced CPP. In accordance, systemic or local administration (dorsal striatum and paraventricular nucleus of the hypothalamus) of DOR agonists stimulated ethanol SA (Barson et al., 2010; van Rijn et al., 2010a; Nielsen et al., 2012). Therefore, pharmacology approaches concur to indicate that DOR activation at several brain sites, and overall, facilitates ethanol drinking in rodents.
Paradoxically, DOR knockout mice showed increased ethanol consumption in a two bottle choice test (SA paradigm) (Roberts et al., 2001). Because these mutant mice exhibit high levels of anxiety (Filliol et al., 2000), and ethanol SA reduced their innate high anxiety levels (Roberts et al., 2001), high voluntary ethanol intake in mutant mice may reflect a self-medication approach. No alcohol phenotype could be detected in animals lacking the Penk gene in two-bottle-choice and ethanol-induced conditioned place preference paradigms (Racz et al., 2008).
3.3 Psychostimulants
DOR knockout mice showed decreased nicotine-induced CPP and SA (Berrendero et al., 2012). Systemic DOR blockade by NTI produced a similar effect in rats and mice (Ismayilova & Shoaib, 2010; Berrendero et al., 2012), and also abolished amphetamine-induced CPP (Belkai et al., 2009). Endogenous DOR activity therefore seems to contribute to reinforcing properties of these two drugs, as for alcohol. NTI infused locally in the nucleus accumbens, VTA and amygdala had contrasting effects on cocaine SA (Ward & Roberts, 2007; Simmons & Self, 2009), suggesting differing roles of DORs at distinct brain sites of reward processing (Figure 1). Finally, a recent SNP study showed association between an Oprd1 variant and cocaine addiction in the African American population (Crist et al., 2013), providing support for a role of DOR in psychostimulant dependence in humans.
In sum, both genetic and pharmacologic approaches suggest a regulatory role for DOR in drug intake, seeking and dependence, which vary depending on the drug and testing paradigm. DOR activity seems to facilitate alcohol and psychostimulant reward, but does not contribute to rewarding properties of morphine. Examination of reinforcing effects of cannabinoids showed no difference between DOR knockout and their control mice (Ghozland et al., 2002), and a contribution of DOR to cannabinoid reward has not been established. DORs are also involved in other aspects contributing to the development of drug abuse, including context learning and the development of tolerance (morphine), or the regulation of emotional responses (alcohol). The latter aspects may be critical in the development of therapeutic strategies. Indeed, targeting aspects of DOR function other than reward, which contribute to maintaining drug dependence, to the negative mood of protracted abstinence or to context-induced relapse, might be of particular interest. Finally, DOR was shown to regulate inhibitory controls in mice (Olmstead et al., 2009) and rats (Befort et al., 2011), revealing yet another facet of DOR function in cognitive processes with potential implication in substance abuse disorders.
4. Delta opioid receptor and epileptic seizures
Early studies showed that the first developed non-peptidic DOR agonists, BW373U86 and SNC80 exhibit convulsive properties (Broom et al., 2002; Jutkiewicz et al., 2005b) and data are overviewed in Table 4. Convulsions induced by the agonists SNC80 are abolished both in DOR knockout mice and after pharmacological blockade of DOR with NTI (Jutkiewicz et al., 2005b). Notably, electroencephalographic and behavioral changes elicited by acute SNC80 administration remain brief and non-lethal as compared to those obtained with the reference seizurogenic GABA antagonist pentylenetetrazole (Jutkiewicz et al., 2006a). Mechanisms underlying DOR-mediated convulsions remain poorly understood, but likely relate to the neural circuitry involved in absence epilepsy (Jutkiewicz et al., 2006a).
SNC80-induced convulsions, but not anti-depressant effects, were greatly diminished when slowing the rate of administration (Jutkiewicz et al., 2005b), indicating a possible dissociation between proconvulsant and antidepressant activities of SNC80. Importantly also, recently developed delta agonists showed no detectable convulsing effects. ADL5859 in both rats and mice at doses up to 1000 mg/kg (p. o.) induced no seizures and no EEG disturbances (Le Bourdonnec et al., 2008), and a similar result was found for ADL5747 (Le Bourdonnec et al., 2009). Therefore the pro-epileptic activity of DOR seems agonist-dependent and opens the way to developing therapeutic compounds with a better benefit/risk profile. Whether this is a pharmacokinetics issue or another indication of biased-agonism at DOR in vivo (Pradhan et al., 2011) remains to be determined.
5. Delta opioid receptor and motor control
The DOR receptor is strongly expressed in the striatum (Figure 1) and the agonist SNC80 shows locomotor-stimulating properties (Fraser et al., 2000; Jutkiewicz et al., 2005a; Saitoh et al., 2011; Nozaki et al., 2012). On the other hand, DOR knockout mice showed hyperactivity in actimetry boxes (Filliol et al., 2000), and deficient striatal-dependent responses in a cross-maze assessing the hippocampal/striatal balance (Le Merrer et al., 2013). These data suggest a significant but complex implication of DOR in the regulation of motor activity and this facet of DOR function is of potential interest in diseases involving impaired motor control such as Parkinson ’s disease (PD). Indeed, DOR activation by the agonist UFP-512 at low dose increased locomotor coordination in a hemiparkinsonian rat model (Mabrouk et al., 2009), and had opposing effects at a high dose (Mabrouk et al., 2009). The antagonist NTI diminished abnormal movements classically described in the 6-OHDA model (Billet et al., 2012). More studies are necessary to understand DOR-mediated mechanisms regulating direct and indirect striatal output pathways.
Notably, recently developed DOR agonists do not show locomotor-activating properties (Svensson et al., 2003; Le Bourdonnec et al., 2008; Le Bourdonnec et al., 2009; Saitoh et al., 2011; Nozaki et al., 2012). Therefore, as for epileptic seizures, DOR-mediated locomotor effects appear agonist-dependent. Further investigations are required to define whether DOR agonist-mediated epileptic seizures and locomotor activity may share common neural circuitry and signaling pathway mechanisms.
6. Delta opioid receptor in hypoxia/ischemia
Hypoxic/ischemic conditions are characterized by reduced oxygen availability and trigger broad physiological alterations leading to cell death. The neuroprotective function of DOR activation has emerged recently, and offers interesting clinical perspectives for hypoxic/ischemic stress (Chao & Xia, 2010; Johnson & Turner, 2010). Beneficial effects of DOR activity deduced from in vivo models of hypoxia and ischemia are summarized in Table 4. Pharmacological studies showed that DOR activation by DADLE, a specific agonist, significantly increased neuronal survival in a model of asphyxia cardiac arrest, and that NTI opposed neuroprotective effects of hypoxic preconditioning in this model (Gao et al., 2010; Gao et al., 2012). DADLE also showed significant protective effects on astrocyte death in the hippocampus in another model of global ischemia (Duan et al., 2011). Studies in cell cultures suggested a critical role in ionic homeostasis in DOR-mediated neuroprotection (Chao et al., 2008; Chao et al., 2009). In a mitochondrial respiratory chain injury model, DOR activation protected neurons by decreasing pro-apoptotic factor expression levels like cytochrome c and caspase-3 (M. Zhu et al., 2009; M. Zhu et al., 2011). Altogether, these data strongly support a role for DOR to maintain cellular metabolic homeostasis and counteract detrimental effects of hypoxic/ischemic injury.
DOR may also minimize consequences of hypoxia on autonomic neural responses. In models of panic attack, CO2 exposure produces acute dyspnea. This response is alleviated by diazepam in wild-type but not DOR knockout mice, suggesting a role for DOR in diazepam-regulated respiratory responses (Borkowski et al., 2011). Also, low oxygen-evoked decrease in body temperature returned to normal levels more slowly upon DOR blockade by NTI (Scarpellini Cda et al., 2009). Altogether these data indicate that DOR agonists may be beneficial under ischemic conditions via multiple, direct and indirect, mechanisms.
7. Clinical perspectives
The pain-reducing (Gaveriaux-Ruff & Kieffer, 2011) and mood-enhancing (Tables 1 and 2) properties of delta opioid agonists in animal models have attracted lots of interest, and efforts are being developed to bring delta drugs to the clinic (Table 5). Several agonists are being tested for pain, including a number of indications in chronic pain patients. The AstraZeneca compound ADZ2327 went successfully through Phase II trials in patients with anxiety-associated major depressive disorder (NCT00759395) (Hudzik et al., 2011). Clinical trials with delta agonists are only at their beginning. Potential convulsant effects need to be carefully controlled, and whether delta agonists could be useful for neuroprotection or to treat Parkinson’s disease will require additional validation from animal research.
With regard to drug design, the notion that DOR may heterodimerize with MOR, KOR, or another GPCR in vivo has fostered the development of dimer-specific drugs endowed with pharmacological properties distinct from agonists acting at DOR homomers (Panetta & Greenwood, 2008; van Rijn et al., 2010b; Costantino et al., 2012; Kleczkowska et al., 2013). Also, the recent demonstration of biased agonism at DOR in vivo may have clinical implications. The “biased agonism” concept (Galandrin et al., 2007; Kenakin, 2011), also referred to as functional selectivity, stems from the observation that distinct agonists acting at the same GPCR can engage different active receptor conformations and/or complexes with other GPCRs or intracellular effectors, leading to agonist-specific signaling responses. Opioid receptors were among the first GPCRs for which agonist-biased responses in vivo were demonstrated (Pradhan et al., 2012). The observation that delta opioid receptor agonists causing high (SNC80) or low (ARM00390) receptor internalization lead to distinct forms of tolerance (Pradhan et al., 2010) opens novel avenues towards drug design for therapeutic effects with limited side effects.
8. Concluding remarks
Delta opioid receptors and opioid peptides are broadly expressed across the brain. Our understanding of DOR function has tremendously progressed from in vivo studies using pharmacological tools and genetic approaches. Beneficial effects of DOR agonists are of a particular interest in the case of emotional responses and mood disorders. DOR regulates drug reward, and also plays a significant role in inhibitory controls and learning processes whose dysfunction contributes to the development of addiction. Whether delta compounds will represent useful drugs in addiction treatment remains open. DOR control over epileptic seizure mechanisms deserves further studies to enable the development of delta drugs with limited side effects. The neuroprotective role of DOR represents an emerging research field, with potential new opportunities for delta opioid drugs in the clinic. In the future, the development of improved delta drugs will also benefit from a better understanding of DOR function at distinct brain sites within neural circuits of emotion and cognition.
Acknowledgments
This work was supported by Centre de la Recherche National Scientifique, Institut National de la Santé et de la Recherche Médicale, Université de Strasbourg. We would like to thank the Fondation pour la Recherche Médicale (FRM FDT20120925269), the US National Institutes of Health (National Institute of Drug Addiction, grant #05010 and National Institute on Alcohol Abuse and Alcoholism, grant #16658) for financial support. We are grateful to Claire Gavériaux-Ruff for helpful discussion of the manuscript.
Abbreviations
- Amy
amygdala
- CA
continuous access
- CeA
central nucleus of the amygdala
- Cg
cingulate cortex
- CPA
conditioned place aversion
- CPP
conditioned place preference
- Cpu
caudate putamen nucleus
- Cx
cortex
- DOR
delta opioid receptor
- EEG
electroencephalography
- Enk
enkephalin
- FCx
frontal cortex
- GPCR
G protein coupled receptor
- Hipp
hippocampus
- Hyp
hypothalamus
- i.c.v
intracerebroventricular
- i.p
intraperitoneal
- i.v
intraveneous
- IA
intermittent access
- KO
knockout
- Nacc
nucleus accumbens
- NTI
naltrindole
- OB
olfactory bulb
- p.o
pers os
- PR
progressive ratio
- PVN
paraventricular nucleus
- RS
retrosplenial cortex
- s.c
subcutaneous
- SA
self-administration
- SC
spinal cord
- th
thalamus
- VTA
ventral tegmental area
Footnotes
Conflict of Interest : The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambrose-Lanci LM, Peiris NB, Unterwald EM, Van Bockstaele EJ. Cocaine withdrawal-induced trafficking of delta-opioid receptors in rat nucleus accumbens. Brain Res. 2008;1210:92–102. doi: 10.1016/j.brainres.2008.02.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre V, Pineau N, Motte JE, Marescaux C, Nehlig A. Mapping of neuronal networks underlying generalized seizures induced by increasing doses of pentylenetetrazol in the immature and adult rat: a c-Fos immunohistochemical study. Eur J Neurosci. 1998;10:2094–2106. doi: 10.1046/j.1460-9568.1998.00223.x. [DOI] [PubMed] [Google Scholar]
- Baler RD, Volkow ND. Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med. 2006;12:559–566. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37:407–419. doi: 10.1016/s0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Barson JR, Carr AJ, Soun JE, Sobhani NC, Rada P, Leibowitz SF, Hoebel BG. Opioids in the hypothalamic paraventricular nucleus stimulate ethanol intake. Alcohol Clin Exp Res. 2010;34:214–222. doi: 10.1111/j.1530-0277.2009.01084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Befort K, Mahoney MK, Chow C, Hayton SJ, Kieffer BL, Olmstead MC. Effects of delta opioid receptors activation on a response inhibition task in rats. Psychopharmacology (Berl) 2011;214:967–976. doi: 10.1007/s00213-010-2108-0. [DOI] [PubMed] [Google Scholar]
- Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. Behav Brain Res. 2009;199:89–102. doi: 10.1016/j.bbr.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Belkai E, Scherrmann JM, Noble F, Marie-Claire C. Modulation of MDMA-induced behavioral and transcriptional effects by the delta opioid antagonist naltrindole in mice. Addict Biol. 2009;14:245–252. doi: 10.1111/j.1369-1600.2009.00156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrendero F, Plaza-Zabala A, Galeote L, Flores A, Bura SA, Kieffer BL, Maldonado R. Influence of delta-opioid receptors in the behavioral effects of nicotine. Neuropsychopharmacology. 2012;37:2332–2344. doi: 10.1038/npp.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bie B, Zhu W, Pan ZZ. Ethanol-induced delta-opioid receptor modulation of glutamate synaptic transmission and conditioned place preference in central amygdala. Neuroscience. 2009;160:348–358. doi: 10.1016/j.neuroscience.2009.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilkei-Gorzo A, Michel K, Noble F, Roques BP, Zimmer A. Preproenkephalin knockout mice show no depression-related phenotype. Neuropsychopharmacology. 2007;32:2330–2337. doi: 10.1038/sj.npp.1301370. [DOI] [PubMed] [Google Scholar]
- Billa SK, Xia Y, Moron JA. Disruption of morphine-conditioned place preference by a delta2-opioid receptor antagonist: study of mu-opioid and delta-opioid receptor expression at the synapse. Eur J Neurosci. 2010;32:625–631. doi: 10.1111/j.1460-9568.2010.07314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billet F, Costentin J, Dourmap N. Influence of corticostriatal delta-opioid receptors on abnormal involuntary movements induced by L-DOPA in hemiparkinsonian rats. Exp Neurol. 2012;236:339–350. doi: 10.1016/j.expneurol.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Borkowski AH, Barnes DC, Blanchette DR, Castellanos FX, Klein DF, Wilson DA. Interaction between delta opioid receptors and benzodiazepines in CO(2)-induced respiratory responses in mice. Brain Res. 2011;1396:54–59. doi: 10.1016/j.brainres.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brevard ME, Kulkarni P, King JA, Ferris CF. Imaging the neural substrates involved in the genesis of pentylenetetrazol-induced seizures. Epilepsia. 2006;47:745–754. doi: 10.1111/j.1528-1167.2006.00502.x. [DOI] [PubMed] [Google Scholar]
- Broom DC, Nitsche JF, Pintar JE, Rice KC, Woods JH, Traynor JR. Comparison of receptor mechanisms and efficacy requirements for delta-agonist-induced convulsive activity and antinociception in mice. J Pharmacol Exp Ther. 2002;303:723–729. doi: 10.1124/jpet.102.036525. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Chao D, Balboni G, Lazarus LH, Salvadori S, Xia Y. Na+ mechanism of delta-opioid receptor induced protection from anoxic K+ leakage in the cortex. Cell Mol Life Sci. 2009;66:1105–1115. doi: 10.1007/s00018-009-8759-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao D, Bazzy-Asaad A, Balboni G, Salvadori S, Xia Y. Activation of DOR attenuates anoxic K+ derangement via inhibition of Na+ entry in mouse cortex. Cereb Cortex. 2008;18:2217–2227. doi: 10.1093/cercor/bhm247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao D, Xia Y. Ionic storm in hypoxic/ischemic stress: can opioid receptors subside it? Prog Neurobiol. 2010;90:439–470. doi: 10.1016/j.pneurobio.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C. The therapeutic potential of kappa-opioids for treatment of pain and addiction. Neuropsychopharmacology. 2011;36:369–370. doi: 10.1038/npp.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer VI, Shippenberg TS. Augmentation of morphine-induced sensitization but reduction in morphine tolerance and reward in delta-opioid receptor knockout mice. Neuropsychopharmacology. 2009;34:887–898. doi: 10.1038/npp.2008.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contet C, Kieffer BL, Befort K. Mu opioid receptor: a gateway to drug addiction. Curr Opin Neurobiol. 2004;14:370–378. doi: 10.1016/j.conb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Costantino CM, Gomes I, Stockton SD, Lim MP, Devi LA. Opioid receptor heteromers in analgesia. Expert Rev Mol Med. 2012;14:e9. doi: 10.1017/erm.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist RC, Ambrose-Lanci LM, Vaswani M, Clarke TK, Zeng A, Yuan C, Ferraro TN, Hakonarson H, Kampman KM, Dackis CA, Pettinati HM, O’Brien CP, Oslin DW, Doyle GA, Lohoff FW, Berrettini WH. Case-control association analysis of polymorphisms in the delta-opioid receptor, OPRD1, with cocaine and opioid addicted populations. Drug Alcohol Depend. 2013;127:122–128. doi: 10.1016/j.drugalcdep.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David V, Matifas A, Gavello-Baudy S, Decorte L, Kieffer BL, Cazala P. Brain regional Fos expression elicited by the activation of mu- but not delta-opioid receptors of the ventral tegmental area: evidence for an implication of the ventral thalamus in opiate reward. Neuropsychopharmacology. 2008;33:1746–1759. doi: 10.1038/sj.npp.1301529. [DOI] [PubMed] [Google Scholar]
- Duan YL, Wang SY, Zeng QW, Su DS, Li W, Wang XR, Zhao Z. Astroglial reaction to delta opioid peptide [D-Ala2, D-Leu5] enkephalin confers neuroprotection against global ischemia in the adult rat hippocampus. Neuroscience. 2011;192:81–90. doi: 10.1016/j.neuroscience.2011.06.067. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW. Appetitive behavior: impact of amygdala-dependent mechanisms of emotional learning. Ann N Y Acad Sci. 2003;985:233–250. [PubMed] [Google Scholar]
- File SE, Kenny PJ, Cheeta S. The role of the dorsal hippocampal serotonergic and cholinergic systems in the modulation of anxiety. Pharmacol Biochem Behav. 2000;66:65–72. doi: 10.1016/s0091-3057(00)00198-2. [DOI] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gaveriaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer BL. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- Fraser GL, Parenteau H, Tu TM, Ducharme J, Perkins MN, Clarke PB. The effects of delta agonists on locomotor activity in habituated and non-habituated rats. Life Sci. 2000;67:913–922. doi: 10.1016/s0024-3205(00)00690-1. [DOI] [PubMed] [Google Scholar]
- Galandrin S, Oligny-Longpre G, Bouvier M. The evasive nature of drug efficacy: implications for drug discovery. Trends Pharmacol Sci. 2007;28:423–430. doi: 10.1016/j.tips.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Gao CJ, Li JP, Wang W, Lu BC, Niu L, Zhu C, Wei YY, Zhang T, Wu SX, Chai W, Li YQ. Effects of intracerebroventricular application of the delta opioid receptor agonist [D-Ala2, D-Leu5] enkephalin on neurological recovery following asphyxial cardiac arrest in rats. Neuroscience. 2010;168:531–542. doi: 10.1016/j.neuroscience.2010.02.025. [DOI] [PubMed] [Google Scholar]
- Gao CJ, Niu L, Ren PC, Wang W, Zhu C, Li YQ, Chai W, Sun XD. Hypoxic preconditioning attenuates global cerebral ischemic injury following asphyxial cardiac arrest through regulation of delta opioid receptor system. Neuroscience. 2012;202:352–362. doi: 10.1016/j.neuroscience.2011.11.060. [DOI] [PubMed] [Google Scholar]
- Gaveriaux-Ruff C. Opiate-Induced Analgesia: Contributions from Mu, Delta and Kappa Opioid Receptors Mouse Mutants. Curr Pharm Des. 2013 doi: 10.2174/138161281942140105163727. [DOI] [PubMed] [Google Scholar]
- Gaveriaux-Ruff C, Kieffer BL. Delta opioid receptor analgesia: recent contributions from pharmacology and molecular approaches. Behav Pharmacol. 2011;22:405–414. doi: 10.1097/FBP.0b013e32834a1f2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaveriaux-Ruff C, Nozaki C, Nadal X, Hever XC, Weibel R, Matifas A, Reiss D, Filliol D, Nassar MA, Wood JN, Maldonado R, Kieffer BL. Genetic ablation of delta opioid receptors in nociceptive sensory neurons increases chronic pain and abolishes opioid analgesia. Pain. 2011;152:1238–1248. doi: 10.1016/j.pain.2010.12.031. [DOI] [PubMed] [Google Scholar]
- Ghozland S, Matthes HW, Simonin F, Filliol D, Kieffer BL, Maldonado R. Motivational effects of cannabinoids are mediated by mu-opioid and kappa-opioid receptors. J Neurosci. 2002;22:1146–1154. doi: 10.1523/JNEUROSCI.22-03-01146.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianoulakis C. Endogenous opioids and addiction to alcohol and other drugs of abuse. Curr Top Med Chem. 2009;9:999–1015. doi: 10.2174/156802609789630956. [DOI] [PubMed] [Google Scholar]
- Goody RJ, Oakley SM, Filliol D, Kieffer BL, Kitchen I. Quantitative autoradiographic mapping of opioid receptors in the brain of delta-opioid receptor gene knockout mice. Brain Res. 2002;945:9–19. doi: 10.1016/s0006-8993(02)02452-6. [DOI] [PubMed] [Google Scholar]
- Granier S, Manglik A, Kruse AC, Kobilka TS, Thian FS, Weis WI, Kobilka BK. Structure of the delta-opioid receptor bound to naltrindole. Nature. 2012;485:400–404. doi: 10.1038/nature11111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross CT, Canteras NS. The many paths to fear. Nat Rev Neurosci. 2012;13:651–658. doi: 10.1038/nrn3301. [DOI] [PubMed] [Google Scholar]
- Hudzik TJ, Maciag C, Smith MA, Caccese R, Pietras MR, Bui KH, Coupal M, Adam L, Payza K, Griffin A, Smagin G, Song D, Swedberg MD, Brown W. Preclinical pharmacology of AZD2327: a highly selective agonist of the delta-opioid receptor. J Pharmacol Exp Ther. 2011;338:195–204. doi: 10.1124/jpet.111.179432. [DOI] [PubMed] [Google Scholar]
- Ismayilova N, Shoaib M. Alteration of intravenous nicotine self-administration by opioid receptor agonist and antagonists in rats. Psychopharmacology (Berl) 2010;210:211–220. doi: 10.1007/s00213-010-1845-4. [DOI] [PubMed] [Google Scholar]
- Javelot H, Messaoudi M, Garnier S, Rougeot C. Human opiorphin is a naturally occurring antidepressant acting selectively on enkephalin-dependent delta-opioid pathways. J Physiol Pharmacol. 2010;61:355–362. [PubMed] [Google Scholar]
- Johnson SM, Turner SM. Protecting motor networks during perinatal ischemia: the case for delta-opioid receptors. Ann N Y Acad Sci. 2010;1198:260–270. doi: 10.1111/j.1749-6632.2010.05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutkiewicz EM, Baladi MG, Folk JE, Rice KC, Woods JH. The convulsive and electroencephalographic changes produced by nonpeptidic delta-opioid agonists in rats: comparison with pentylenetetrazol. J Pharmacol Exp Ther. 2006a;317:1337–1348. doi: 10.1124/jpet.105.095810. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Kaminsky ST, Rice KC, Traynor JR, Woods JH. Differential behavioral tolerance to the delta-opioid agonist SNC80 ([(+)-4-[(alphaR)-alpha-[(2S,5R)-2,5-dimethyl-4-(2-propenyl)-1-piperazinyl ]-(3-methoxyphenyl)methyl]-N,N-diethylbenzamide) in Sprague-Dawley rats. J Pharmacol Exp Ther. 2005a;315:414–422. doi: 10.1124/jpet.105.088831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutkiewicz EM, Rice KC, Traynor JR, Woods JH. Separation of the convulsions and antidepressant-like effects produced by the delta-opioid agonist SNC80 in rats. Psychopharmacology (Berl) 2005b;182:588–596. doi: 10.1007/s00213-005-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutkiewicz EM, Torregrossa MM, Sobczyk-Kojiro K, Mosberg HI, Folk JE, Rice KC, Watson SJ, Woods JH. Behavioral and neurobiological effects of the enkephalinase inhibitor RB101 relative to its antidepressant effects. Eur J Pharmacol. 2006b;531:151–159. doi: 10.1016/j.ejphar.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T. Functional selectivity and biased receptor signaling. J Pharmacol Exp Ther. 2011;336:296–302. doi: 10.1124/jpet.110.173948. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Gilbert PE. The role of the agranular insular cortex in anticipation of reward contrast. Neurobiol Learn Mem. 2007;88:82–86. doi: 10.1016/j.nlm.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchen I, Slowe SJ, Matthes HW, Kieffer B. Quantitative autoradiographic mapping of mu-, delta- and kappa-opioid receptors in knockout mice lacking the mu-opioid receptor gene. Brain Res. 1997;778:73–88. doi: 10.1016/s0006-8993(97)00988-8. [DOI] [PubMed] [Google Scholar]
- Kleczkowska P, Lipkowski AW, Tourwe D, Ballet S. Hybrid Opioid/Non-Opioid Ligands in Pain Research. Curr Pharm Des. 2013 doi: 10.2174/138161281942140105165646. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA., Jr Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig M, Zimmer AM, Steiner H, Holmes PV, Crawley JN, Brownstein MJ, Zimmer A. Pain responses, anxiety and aggression in mice deficient in pre-proenkephalin. Nature. 1996;383:535–538. doi: 10.1038/383535a0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3113–3123. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent V, Leung B, Maidment N, Balleine BW. mu- and delta-opioid-related processes in the accumbens core and shell differentially mediate the influence of reward-guided and stimulus-guided decisions on choice. J Neurosci. 2012;32:1875–1883. doi: 10.1523/JNEUROSCI.4688-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bourdonnec B, Windh RT, Ajello CW, Leister LK, Gu M, Chu GH, Tuthill PA, Barker WM, Koblish M, Wiant DD, Graczyk TM, Belanger S, Cassel JA, Feschenko MS, Brogdon BL, Smith SA, Christ DD, Derelanko MJ, Kutz S, Little PJ, DeHaven RN, DeHaven-Hudkins DL, Dolle RE. Potent, orally bioavailable delta opioid receptor agonists for the treatment of pain: discovery of N,N-diethyl-4-(5-hydroxyspiro[chromene-2,4′-piperidine]-4-yl)benzamide (ADL5859) J Med Chem. 2008;51:5893–5896. doi: 10.1021/jm8008986. [DOI] [PubMed] [Google Scholar]
- Le Bourdonnec B, Windh RT, Leister LK, Zhou QJ, Ajello CW, Gu M, Chu GH, Tuthill PA, Barker WM, Koblish M, Wiant DD, Graczyk TM, Belanger S, Cassel JA, Feschenko MS, Brogdon BL, Smith SA, Derelanko MJ, Kutz S, Little PJ, DeHaven RN, DeHaven-Hudkins DL, Dolle RE. Spirocyclic delta opioid receptor agonists for the treatment of pain: discovery of N,N-diethyl-3-hydroxy-4-(spiro[chromene-2,4′-piperidine]-4-yl) benzamide (ADL5747) J Med Chem. 2009;52:5685–5702. doi: 10.1021/jm900773n. [DOI] [PubMed] [Google Scholar]
- Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89:1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Merrer J, Faget L, Matifas A, Kieffer BL. Cues predicting drug or food reward restore morphine-induced place conditioning in mice lacking delta opioid receptors. Psychopharmacology (Berl) 2012;223:99–106. doi: 10.1007/s00213-012-2693-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Merrer J, Plaza-Zabala A, Del Boca C, Matifas A, Maldonado R, Kieffer BL. Deletion of the delta opioid receptor gene impairs place conditioning but preserves morphine reinforcement. Biol Psychiatry. 2011;69:700–703. doi: 10.1016/j.biopsych.2010.10.021. [DOI] [PubMed] [Google Scholar]
- Le Merrer J, Rezai X, Scherrer G, Becker JA, Kieffer BL. Impaired Hippocampus-Dependent and Facilitated Striatum-Dependent Behaviors in Mice Lacking the Delta Opioid Receptor. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lutz PE, Kieffer BL. Opioid receptors: distinct roles in mood disorders. Trends Neurosci. 2012 doi: 10.1016/j.tins.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabrouk OS, Marti M, Salvadori S, Morari M. The novel delta opioid receptor agonist UFP-512 dually modulates motor activity in hemiparkinsonian rats via control of the nigro-thalamic pathway. Neuroscience. 2009;164:360–369. doi: 10.1016/j.neuroscience.2009.08.058. [DOI] [PubMed] [Google Scholar]
- Manglik A, Kruse AC, Kobilka TS, Thian FS, Mathiesen JM, Sunahara RK, Pardo L, Weis WI, Kobilka BK, Granier S. Crystal structure of the micro-opioid receptor bound to a morphinan antagonist. Nature. 2012;485:321–326. doi: 10.1038/nature10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Fields HL, Hjelmstad GO, Mitchell JM. Delta-opioid receptor expression in the ventral tegmental area protects against elevated alcohol consumption. J Neurosci. 2008;28:12672–12681. doi: 10.1523/JNEUROSCI.4569-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Harding S, Li Z, Juzytsch W, Le AD. Roles of opioid receptor subtypes in mediating alcohol-seeking induced by discrete cues and context. Eur J Neurosci. 2009;30:671–678. doi: 10.1111/j.1460-9568.2009.06851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mas Nieto MM, Guen SL, Kieffer BL, Roques BP, Noble F. Physiological control of emotion-related behaviors by endogenous enkephalins involves essentially the delta opioid receptors. Neuroscience. 2005;135:305–313. doi: 10.1016/j.neuroscience.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Naidu PS, Lichtman AH, Archer CC, May EL, Harris LS, Aceto MD. NIH 11082 produces anti-depressant-like activity in the mouse tail-suspension test through a delta-opioid receptor mechanism of action. Eur J Pharmacol. 2007;566:132–136. doi: 10.1016/j.ejphar.2007.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Kaneko C, Miyoshi K, Nagumo Y, Kuzumaki N, Nakajima M, Nanjo K, Matsuzawa K, Yamazaki M, Suzuki T. Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology. 2006a;31:739–750. doi: 10.1038/sj.npp.1300858. [DOI] [PubMed] [Google Scholar]
- Narita M, Kuzumaki N, Kaneko C, Hareyama N, Miyatake M, Shindo K, Miyoshi K, Nakajima M, Nagumo Y, Sato F, Wachi H, Seyama Y, Suzuki T. Chronic pain-induced emotional dysfunction is associated with astrogliosis due to cortical delta-opioid receptor dysfunction. J Neurochem. 2006b;97:1369–1378. doi: 10.1111/j.1471-4159.2006.03824.x. [DOI] [PubMed] [Google Scholar]
- Nielsen CK, Simms JA, Li R, Mill D, Yi H, Feduccia AA, Santos N, Bartlett SE. delta-opioid receptor function in the dorsal striatum plays a role in high levels of ethanol consumption in rats. J Neurosci. 2012;32:4540–4552. doi: 10.1523/JNEUROSCI.5345-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen CK, Simms JA, Pierson HB, Li R, Saini SK, Ananthan S, Bartlett SE. A novel delta opioid receptor antagonist, SoRI-9409, produces a selective and long-lasting decrease in ethanol consumption in heavy-drinking rats. Biol Psychiatry. 2008;64:974–981. doi: 10.1016/j.biopsych.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki C, Le Bourdonnec B, Reiss D, Windh RT, Little PJ, Dolle RE, Kieffer BL, Gaveriaux-Ruff C. delta-Opioid mechanisms for ADL5747 and ADL5859 effects in mice: analgesia, locomotion, and receptor internalization. J Pharmacol Exp Ther. 2012;342:799–807. doi: 10.1124/jpet.111.188987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead MC, Ouagazzal AM, Kieffer BL. Mu and delta opioid receptors oppositely regulate motor impulsivity in the signaled nose poke task. PLoS One. 2009;4:e4410. doi: 10.1371/journal.pone.0004410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panetta R, Greenwood MT. Physiological relevance of GPCR oligomerization and its impact on drug discovery. Drug Discov Today. 2008;13:1059–1066. doi: 10.1016/j.drudis.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Paton JJ, Louie K. Reward and punishment illuminated. Nat Neurosci. 2012;15:807–809. doi: 10.1038/nn.3122. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Peckys D, Landwehrmeyer GB. Expression of mu, kappa, and delta opioid receptor messenger RNA in the human CNS: a 33P in situ hybridization study. Neuroscience. 1999;88:1093–1135. doi: 10.1016/s0306-4522(98)00251-6. [DOI] [PubMed] [Google Scholar]
- Peng J, Sarkar S, Chang SL. Opioid receptor expression in human brain and peripheral tissues using absolute quantitative real-time RT-PCR. Drug Alcohol Depend. 2012;124:223–228. doi: 10.1016/j.drugalcdep.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrine SA, Hoshaw BA, Unterwald EM. Delta opioid receptor ligands modulate anxiety-like behaviors in the rat. Br J Pharmacol. 2006;147:864–872. doi: 10.1038/sj.bjp.0706686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Befort K, Nozaki C, Gaveriaux-Ruff C, Kieffer BL. The delta opioid receptor: an evolving target for the treatment of brain disorders. Trends Pharmacol Sci. 2011;32:581–590. doi: 10.1016/j.tips.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Smith ML, Kieffer BL, Evans CJ. Ligand-directed signalling within the opioid receptor family. Br J Pharmacol. 2012;167:960–969. doi: 10.1111/j.1476-5381.2012.02075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Walwyn W, Nozaki C, Filliol D, Erbs E, Matifas A, Evans C, Kieffer BL. Ligand-directed trafficking of the delta-opioid receptor in vivo: two paths toward analgesic tolerance. J Neurosci. 2010;30:16459–16468. doi: 10.1523/JNEUROSCI.3748-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Primeaux SD, Wilson SP, McDonald AJ, Mascagni F, Wilson MA. The role of delta opioid receptors in the anxiolytic actions of benzodiazepines. Pharmacol Biochem Behav. 2006;85:545–554. doi: 10.1016/j.pbb.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racz I, Schurmann B, Karpushova A, Reuter M, Cichon S, Montag C, Furst R, Schutz C, Franke PE, Strohmaier J, Wienker TF, Terenius L, Osby U, Gunnar A, Maier W, Bilkei-Gorzo A, Nothen M, Zimmer A. The opioid peptides enkephalin and beta-endorphin in alcohol dependence. Biol Psychiatry. 2008;64:989–997. doi: 10.1016/j.biopsych.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragnauth A, Schuller A, Morgan M, Chan J, Ogawa S, Pintar J, Bodnar RJ, Pfaff DW. Female preproenkephalin-knockout mice display altered emotional responses. Proc Natl Acad Sci U S A. 2001;98:1958–1963. doi: 10.1073/pnas.041598498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall-Thompson JF, Pescatore KA, Unterwald EM. A role for delta opioid receptors in the central nucleus of the amygdala in anxiety-like behaviors. Psychopharmacology (Berl) 2010;212:585–595. doi: 10.1007/s00213-010-1980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JM, Castro DC, Difeliceantonio AG, Robinson MJ, Berridge KC. Mapping brain circuits of reward and motivation: In the footsteps of Ann Kelley. Neurosci Biobehav Rev. 2012 doi: 10.1016/j.neubiorev.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol. 1996;6:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Gold LH, Polis I, McDonald JS, Filliol D, Kieffer BL, Koob GF. Increased ethanol self-administration in delta-opioid receptor knockout mice. Alcohol Clin Exp Res. 2001;25:1249–1256. [PubMed] [Google Scholar]
- Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- Saitoh A, Kimura Y, Suzuki T, Kawai K, Nagase H, Kamei J. Potential anxiolytic and antidepressant-like activities of SNC80, a selective delta-opioid agonist, in behavioral models in rodents. J Pharmacol Sci. 2004;95:374–380. doi: 10.1254/jphs.fpj04014x. [DOI] [PubMed] [Google Scholar]
- Saitoh A, Sugiyama A, Nemoto T, Fujii H, Wada K, Oka J, Nagase H, Yamada M. The novel delta opioid receptor agonist KNT-127 produces antidepressant-like and antinociceptive effects in mice without producing convulsions. Behav Brain Res. 2011;223:271–279. doi: 10.1016/j.bbr.2011.04.041. [DOI] [PubMed] [Google Scholar]
- Saitoh A, Yoshikawa Y, Onodera K, Kamei J. Role of delta-opioid receptor subtypes in anxiety-related behaviors in the elevated plus-maze in rats. Psychopharmacology (Berl) 2005;182:327–334. doi: 10.1007/s00213-005-0112-6. [DOI] [PubMed] [Google Scholar]
- Sauriyal DS, Jaggi AS, Singh N. Extending pharmacological spectrum of opioids beyond analgesia: multifunctional aspects in different pathophysiological states. Neuropeptides. 2011;45:175–188. doi: 10.1016/j.npep.2010.12.004. [DOI] [PubMed] [Google Scholar]
- da Scarpellini CS, Gargaglioni LH, Branco LG, Bicego KC. Role of preoptic opioid receptors in the body temperature reduction during hypoxia. Brain Res. 2009;1286:66–74. doi: 10.1016/j.brainres.2009.06.039. [DOI] [PubMed] [Google Scholar]
- Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O’Donnell D, Kieffer BL, Basbaum AI. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–1159. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Laustriat D, Cao YQ, Basbaum AI, Dierich A, Vonesh JL, Gaveriaux-Ruff C, Kieffer BL. Knockin mice expressing fluorescent delta-opioid receptors uncover G protein-coupled receptor dynamics in vivo. Proc Natl Acad Sci U S A. 2006;103:9691–9696. doi: 10.1073/pnas.0603359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS. The dynorphin/kappa opioid receptor system: a new target for the treatment of addiction and affective disorders? Neuropsychopharmacology. 2009;34:247. doi: 10.1038/npp.2008.165. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Chefer VI, Thompson AC. Delta-opioid receptor antagonists prevent sensitization to the conditioned rewarding effects of morphine. Biol Psychiatry. 2009;65:169–174. doi: 10.1016/j.biopsych.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, LeFevour A, Chefer VI. Targeting endogenous mu- and delta-opioid receptor systems for the treatment of drug addiction. CNS Neurol Disord Drug Targets. 2008;7:442–453. doi: 10.2174/187152708786927813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D, Self DW. Role of mu- and delta-opioid receptors in the nucleus accumbens in cocaine-seeking behavior. Neuropsychopharmacology. 2009;34:1946–1957. doi: 10.1038/npp.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonin F, Befort K, Gaveriaux-Ruff C, Matthes H, Nappey V, Lannes B, Micheletti G, Kieffer B. The human delta-opioid receptor: genomic organization, cDNA cloning, functional expression, and distribution in human brain. Mol Pharmacol. 1994;46:1015–1021. [PubMed] [Google Scholar]
- Simonin F, Valverde O, Smadja C, Slowe S, Kitchen I, Dierich A, Le Meur M, Roques BP, Maldonado R, Kieffer BL. Disruption of the kappa-opioid receptor gene in mice enhances sensitivity to chemical visceral pain, impairs pharmacological actions of the selective kappa-agonist U-50,488H and attenuates morphine withdrawal. EMBO J. 1998;17:886–897. doi: 10.1093/emboj/17.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slowe SJ, Simonin F, Kieffer B, Kitchen I. Quantitative autoradiography of mu-,delta- and kappa1 opioid receptors in kappa-opioid receptor knockout mice. Brain Res. 1999;818:335–345. doi: 10.1016/s0006-8993(98)01201-3. [DOI] [PubMed] [Google Scholar]
- Smith JS, Zubieta JK, Price JC, Flesher JE, Madar I, Lever JR, Kinter CM, Dannals RF, Frost JJ. Quantification of delta-opioid receptors in human brain with N1′-([11C]methyl) naltrindole and positron emission tomography. J Cereb Blood Flow Metab. 1999;19:956–966. doi: 10.1097/00004647-199909000-00003. [DOI] [PubMed] [Google Scholar]
- Solati J, Zarrindast MR, Salari AA. Dorsal hippocampal opioidergic system modulates anxiety-like behaviors in adult male Wistar rats. Psychiatry Clin Neurosci. 2010;64:634–641. doi: 10.1111/j.1440-1819.2010.02143.x. [DOI] [PubMed] [Google Scholar]
- Steenland HW, Li XY, Zhuo M. Predicting aversive events and terminating fear in the mouse anterior cingulate cortex during trace fear conditioning. J Neurosci. 2012;32:1082–1095. doi: 10.1523/JNEUROSCI.5566-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Tsuji M, Mori T, Misawa M, Endoh T, Nagase H. Effect of the highly selective and nonpeptide delta opioid receptor agonist TAN-67 on the morphine-induced place preference in mice. J Pharmacol Exp Ther. 1996;279:177–185. [PubMed] [Google Scholar]
- Svensson CI, Rew Y, Malkmus S, Schiller PW, Taulane JP, Goodman M, Yaksh TL. Systemic and spinal analgesic activity of a delta-opioid-selective lanthionine enkephalin analog. J Pharmacol Exp Ther. 2003;304:827–832. doi: 10.1124/jpet.102.039750. [DOI] [PubMed] [Google Scholar]
- Torregrossa MM, Jutkiewicz EM, Mosberg HI, Balboni G, Watson SJ, Woods JH. Peptidic delta opioid receptor agonists produce antidepressant-like effects in the forced swim test and regulate BDNF mRNA expression in rats. Brain Res. 2006;1069:172–181. doi: 10.1016/j.brainres.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn RM, Brissett DI, Whistler JL. Dual efficacy of delta opioid receptor selective ligands for ethanol drinking and anxiety. J Pharmacol Exp Ther. 2010a doi: 10.1124/jpet.110.170969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn RM, Whistler JL. The delta(1) opioid receptor is a heterodimer that opposes the actions of the delta(2) receptor on alcohol intake. Biol Psychiatry. 2009;66:777–784. doi: 10.1016/j.biopsych.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn RM, Whistler JL, Waldhoer M. Opioid-receptor-heteromer-specific trafficking and pharmacology. Curr Opin Pharmacol. 2010b;10:73–79. doi: 10.1016/j.coph.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergura R, Balboni G, Spagnolo B, Gavioli E, Lambert DG, McDonald J, Trapella C, Lazarus LH, Regoli D, Guerrini R, Salvadori S, Calo G. Anxiolytic- and antidepressant-like activities of H-Dmt-Tic-NH-CH(CH2-COOH)-Bid (UFP-512), a novel selective delta opioid receptor agonist. Peptides. 2008;29:93–103. doi: 10.1016/j.peptides.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Roberts DC. Microinjection of the delta-opioid receptor selective antagonist naltrindole 5′-isothiocyanate site specifically affects cocaine self-administration in rats responding under a progressive ratio schedule of reinforcement. Behav Brain Res. 2007;182:140–144. doi: 10.1016/j.bbr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Wacker D, Mileni M, Katritch V, Han GW, Vardy E, Liu W, Thompson AA, Huang XP, Carroll FI, Mascarella SW, Westkaemper RB, Mosier PD, Roth BL, Cherezov V, Stevens RC. Structure of the human kappa-opioid receptor in complex with JDTic. Nature. 2012;485:327–332. doi: 10.1038/nature10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Li M, Yang F, Ou X, Ren Q, Gao H, Zhu C, Guo J. Mitochondrial ERK plays a key role in delta-opioid receptor neuroprotection against acute mitochondrial dysfunction. Neurochem Int. 2011;59:739–748. doi: 10.1016/j.neuint.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Zhu M, Li MW, Tian XS, Ou XM, Zhu CQ, Guo JC. Neuroprotective role of delta-opioid receptors against mitochondrial respiratory chain injury. Brain Res. 2009;1252:183–191. doi: 10.1016/j.brainres.2008.11.030. [DOI] [PubMed] [Google Scholar]
- Zhu Y, King MA, Schuller AG, Nitsche JF, Reidl M, Elde RP, Unterwald E, Pasternak GW, Pintar JE. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in delta opioid receptor knockout mice. Neuron. 1999;24:243–252. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]
- Zollner C, Stein C. Opioids. Handb Exp Pharmacol. 2007:31–63. doi: 10.1007/978-3-540-33823-9_2. [DOI] [PubMed] [Google Scholar]


