Abstract
To characterize the magnitude of clearance changes during pregnancy for multiple antiepileptic drugs (AEDs) and to assess seizure frequency and factors increasing seizure risk in pregnant women with epilepsy. A retrospective analysis was performed for 115 pregnancies in 95 women with epilepsy followed at the Emory Epilepsy Center between 1999 and 2012. AED blood levels (ABLs) obtained during routine clinical practice were used to calculate AED clearance at multiple points during pregnancy. AED doses and seizure activity were also recorded. The data were analyzed for changes in clearance and dose across pregnancy and for an association between ABL and changes in seizure frequency. Significant changes in clearance during pregnancy were observed for lamotrigine (p < 0.001) and levetiracetam (p<0.006). Average peak clearance increased 191% for lamotrigine and 207% for levetiracetam from non-pregnant baseline. Marked variance was present across individual women and also across repeat pregnancies in individual women. Despite increased AED dose across most AEDs, seizures increased in 38.4% of patients during pregnancy. Seizure deterioration was significantly more likely in patients with seizures in the 12 months prior to conception (p < 0.001) and those with localization related epilepsy (p = 0.005). When ABL fell >35% from preconception baseline, seizures worsened significantly during the second trimester when controlling for seizure occurrence in the year prior to conception. Substantial pharmacokinetic changes during pregnancy occur with multiple AEDs and may increase seizure risk. Monitoring of AED serum concentrations with dose adjustment is recommended in pregnant women with epilepsy. Further studies are needed for many AEDs.
Keywords: Antiepileptic drugs, pregnancy, epilepsy, pharmacokinetics, clearance
1. Introduction
The management of epilepsy during pregnancy presents substantial challenges. Fetal antiepileptic drug (AED) exposure is associated with a dose-dependent increase in the risk of congenital malformations [1] and neurocognitive deficits [2,3]. These risks must be balanced against the adverse health effects of seizures for both mother and fetus [4,5].
Maintaining seizure control is complicated by pharmacokinetic alterations during pregnancy, including increased volume of distribution, elevated renal clearance, and induction of hepatic metabolism [4,6]. These changes may result in decreased serum AED concentrations, although the degree of decline differs across medications and individuals [4,6,7].
Reductions in AED concentrations are associated with increased seizure frequency during pregnancy. This relationship has been shown most clearly for lamotrigine [8], with a similar trend observed for oxcarbazepine [9]. Accordingly, the American Academy of Neurology has recommended therapeutic drug monitoring for several AEDs, with the goal of maintaining serum levels near pre-conception baseline [10]. However, insufficient data are available to justify a recommendation for many AEDs currently in use [10, 11].
In this study, we aim to further characterize clearance changes across pregnancy for multiple AEDs by presenting a large series of pregnancies managed with periodic measurements of AED serum concentrations. Additionally, we present information on seizure control in women whose AED levels were used as a guide for dose adjustment.
2. Methods
2.1. Study population and design
This is a retrospective study of 135 women with epilepsy on AED therapy during pregnancy seen at the Emory Epilepsy Center (February 1999–February 2012). The Institutional Review Board of Emory University School of Medicine approved the study. Charts were reviewed for AED blood levels (ABLs) obtained through routine clinical practice, and patients were selected if they had at least one ABL for each trimester of pregnancy. Patients were excluded if any AEDs were added or removed during the pregnancy. Based on these criteria, 115 pregnancies in 95 women were selected for analysis.
During pregnancy, patients typically underwent monthly ABL measurements, with ABLs obtained more frequently if seizures occurred. Blood draws were performed at the Emory Clinic or by the patient’s obstetrician. Plasma AED concentrations were measured via routine methods in clinical laboratories, and total concentrations were used as free concentrations were not consistently available. For oxcarbazepine, the active metabolite 10-monohydroxy derivate (MHD) was measured. The timing of the blood draws relative to last dose was not standardized. Dose adjustments were made by the clinician with the goal of maintaining ABL within the individualized therapeutic range based on the patient’s history and previous ABLs. Dose may have also been adjusted if seizure frequency increased, regardless of ABL. In cases of polytherapy, all medications were adjusted based on the above criteria.
During data collection, gestational age (GA) for the date of each ABL was determined based on last menstrual period (LMP) or as determined by ultrasound. The ABL was then classified as preconception, first trimester (<14 weeks GA), second trimester (14–28 weeks GA), third trimester (>28 weeks GA-delivery), or postpartum. Baseline ABL was defined as an ABL obtained during the preconception period or, if unavailable, as a postpartum value from >6 weeks following delivery.
2.2. Data Analysis
Statistical analyses were performed using SPSS (Version 20).
2.2.1. AED Clearance
For comparison despite changes in dose and patient weight, apparent oral clearance (Cl) was calculated for each ABL, where AED Cl = daily dose (mg/kg)/serum AED concentration (μg/mL). Patient weight for each calculation was obtained from the most recent clinic visit prior to the date the ABL was drawn.
To investigate the magnitude of Cl change during pregnancy, we compared baseline Cl to maximum Cl obtained during pregnancy. These differences were tested using a general linear model. Fifteen patients did not have a baseline Cl available and were not included in the analysis. Analyses were done for all AEDs combined and for lamotrigine (LTG) and levetiracetam (LEV) monotherapy alone, as these groups had the largest sample sizes. Data for AEDs with smaller sample sizes were pooled for analysis, and analyses were also performed for polytherapy with and without LTG.
To test for differences between monotherapy and polytherapy groups, a two-way analysis of variance (ANOVA) was used to compare maximum LTG Cl in patients on LTG monotherapy and patients on combination therapy that included LTG. We also assessed racial differences in maximum clearance change during pregnancy using a two-way ANOVA.
2.2.2. AED dose
For each ABL, the dose of the associated AED was recorded. Baseline non-pregnant dose for each AED was compared to maximum pregnancy dose using a general linear model. AED groupings in the analyses were the same as for clearance, described above. Patients without a known baseline dose were not included in the analysis.
2.2.3. Seizure Frequency
Seizure frequency during each stage of pregnancy was abstracted from patient charts. Average seizure frequency over the twelve months prior to conception was also recorded, both for all seizure types and for convulsive seizures alone. For each trimester, the relative frequencies of all seizure types and of generalized tonic clonic seizures (GTCS) were coded as 1 if the frequency was greater than the preconception frequency and 0 if it was decreased or unchanged. The ratio of mean ABL to non-pregnant ABL was calculated for each trimester. T-test was used to compare this value for each trimester across all seizure types in patients who had an increase in seizure frequency versus patients who did not. The same analysis was performed for the ratio of minimum trimester ABL to non-pregnant ABL, as the minimum ABL would correspond to the highest risk for seizure breakthrough. Fifteen patients without baseline ABL were not included. This analysis was performed for all drugs combined and for LTG and LEV monotherapies alone.
Since a fall in ABL >35% has previously been associated with increased seizures in patients taking LTG [8], t-tests were also used to compare the rate of seizure worsening between patients whose minimum trimester ABL fell >35% from preconception baseline with those who did not. The analysis was performed for each trimester while controlling for occurrence of seizures in the 12 months prior to conception.
We compared (via t-tests) increased frequency of all seizure types at any point during pregnancy between patients diagnosed with primary generalized epilepsy and those with localization related epilepsy. Increased seizure frequency was also compared via t-test between patients who were seizure free in the year prior to conception and patients who were not.
3. Results
One hundred fifteen pregnancies (113 singleton and two sets of twins) in 95 women were included in the study. In women with multiple pregnancies, each pregnancy was analyzed separately. AED monotherapy was employed in 100 pregnancies, while 15 required two AEDs and one required three (Table 1). Nine different AEDs were represented, with LTG used most frequently. Data from 22 pregnancies on LTG monotherapy have previously been included in a report of a prospective study analyzing LTG concentrations from batched assays in a research laboratory [8].
Table 1.
Study population characteristics and details of antiepileptic drug therapy
| Total pregnancies, n | 115 |
| Mean patient age (range), years | 30.3 (15–43) |
| Mean patient gravida (range) | 1.85 (1–6) |
| Racial distribution, n (% of total) | |
| Caucasian | 89 (77) |
| African-American | 19 (17) |
| Asian | 7 (6) |
| Epilepsy diagnosis, n (% of total) | |
| Localization-related epilepsy | 72 (63) |
| Generalized epilepsy | 37 (32) |
| Juvenile myoclonic epilepsy | 17 (15) |
| Childhood absence epilepsy | 4 (3) |
| Juvenile absence epilepsy | 4 (3) |
| Generalized epilepsy, unspecified | 12 (10) |
| Epilepsy, type unknown | 6 (5) |
| Antiepileptic drug, n | |
| Total | Monotherapy | Polytherapy | |
|---|---|---|---|
| Lamotrigine | 80 | 69 | 11 |
| Levetiracetam | 27 | 15 | 12 |
| Carbamazepine | 7 | 6 | 1 |
| Topiramate | 7 | 3 | 4 |
| Oxcarbazepine | 4 | 2 | 2 |
| Phenytoin | 2 | 1 | 1 |
| Valproate | 2 | 1 | 1 |
| Zonisamide | 2 | 1 | 1 |
| Ethosuximide | 1 | 1 | 0 |
3.1. AED clearance in pregnancy
Apparent oral clearance was calculated from each available ABL. Seventy-six of the 115 pregnancies had a preconception baseline Cl, and postpartum samples (>6 weeks following delivery) were used as baseline for an additional 24 women. Application of the general linear model to patients with an available non-pregnant Cl showed that maximum Cl during pregnancy was significantly different from non-pregnant baseline for LTG monotherapy (increased 191%, p < 0.001), and LEV monotherapy (increased 207%, p = 0.006). Although the mean maximum Cl change for all other monotherapies was much smaller than for LTG or LEV monotherapy, the change was significant (p = 0.048). However, Cl changes are variable across the other monotherapies, and the sample sizes for these individual AEDs are small (see Table 2). Of note, the AED with the largest sample in other monotherapies was carbamazepine (CBZ, n=6), which exhibited virtually no change in Cl during pregnancy. There was no difference between Caucasian, African American, and Asian patients in Cl change for all drugs combined (p = 0.872) or for LTG monotherapy (p = 0.404). The sample size of LEV (n=15) and other AEDs in monotherapy were inadequate to assess racial differences.
Table 2.
Antiepileptic drug clearance during pregnancy
| AED | N | Non-Pregnant
|
1st Trimester
|
2nd Trimester
|
3rd Trimester
|
|||
|---|---|---|---|---|---|---|---|---|
| Mean Clearance (SD) (range) | Mean Peak Clearance (SD) (range) | % Increasea | Mean Peak Clearance (SD) (range) | % Increasea | Mean Peak Clearance (SD) (range) | % Increasea | ||
| Monotherapy | ||||||||
| LTG | 69 | 0.87 (0.42) (0.23–2.70) | 1.64 (0.82) (0.39–4.18) | 89 | 2.53 (1.47) (0.57–9.23) | 191 | 2.09 (1.01) (0.41–5.66) | 140 |
| LEV | 15 | 1.09 (0.30) (0.68–1.63) | 2.16 (1.72) (1.10–8.15) | 98 | 3.35 (2.60) (1.18–9.51) | 207 | 2.15 (1.11) (0.71–4.32) | 97 |
| All Other Monotherapyb | 15 | 0.95 (0.64) (0.12–2.06) | 1.05 (0.82) (0.18–3.46) | 11 | 1.06 (0.72) (0.18–2.89) | 12 | 1.13 (0.81) (0.20–2.64) | 19 |
| CBZ | 6 | 1.57 (0.33) (1.21–2.06) | 1.32 (0.34) (1.00–1.88) | −16 | 1.49 (0.41) (1.09–2.26) | −5 | 1.76 (0.48) (1.29–2.54) | 12 |
| TPM | 3 | 0.59 (0.06) (0.54–0.63) | 1.58 (1.64) (0.49–3.46) | 168 | 1.36 (1.33) (0.57–2.89) | 131 | 0.65 (0.10) (0.55–0.75) | 10 |
| OXC | 2 | 0.91 (0.23) (0.74–1.07) | 1.11 (0.45) (0.79–1.42) | 22 | 2.33 (0.37) (2.07–2.59) | 156 | 2.16 (0.68) (1.68–2.64) | 137 |
| PHT | 1 | N/A | 0.52 | N/A | 0.35 | N/A | 0.40 | N/A |
| VPA | 1 | 0.15 | 0.18 | 20 | 0.18 | 20 | 0.20 | 33 |
| ZNS | 1 | 0.12 | 0.25 | 108 | 0.29 | 142 | 0.26 | 117 |
| ESM | 1 | 0.31 | 0.50 | 61 | 0.34 | 10 | 0.44 | 42 |
|
| ||||||||
| Polytherapy | ||||||||
| Allc | 33 | 1.09 (1.08) (0.18–5.19) | 2.45 (3.52) (0.23–16.79) | 125 | 2.90 (4.12) (0.26–24.26) | 166 | 1.86 (1.22) (0.35–7.02) | 71 |
| Polytherapy with LTG or LEV | 23 | 1.32 (1.19) (0.34–5.19) | 2.78 (3.74) (0.70–16.79) | 112 | 3.68 (4.71) (0.91–24.26) | 180 | 2.20 (1.23) (0.91–7.02) | 676 |
| Polytherapy without LTG or LEVd | 10 | 1.01 (0.39) (0.18–1.34) | 1.99 (2.99) (0.23–10.08) | 96 | 2.75 (0.99) (0.26–3.40) | 171 | 1.93 (0.80) (0.35–2.71) | 90 |
Compared to non-pregnant baseline.
Other AEDs used in monotherapy were carbamazepine (n=6), topiramate (n=3), oxcarbazepine (n=3), phenytoin (n=1), valproate (n=1), zonisamide (n=1), and ethosuximide (n=1). Data are separated by individual AED below.
Average of all AEDs used by each patient on polytherapy treatment. The mean number of AEDs per patient was 2.1 (range = 2–3).
These polytherapies included the following AEDs: topiramate (n=4), oxcarbazepine (n=2), valproate (n=1), phenytoin (n=1), zonisamide (n=1), and carbamazepine (n=1).
AED = antiepileptic drug; LTG = lamotrigine, LEV = levetiracetam; CBZ = carbamazepine; TPM = topiramate; OXC = oxcarbazepine; PHT = phenytoin; VPA = valproate; ZNS = zonisamide; ESM = ethosuximide; N/A = none available.
Maximum Cl change was also significant for all polytherapies combined (p < 0.001), polytherapies with LTG or LEV (p = 0.005), and polytherapy without LTG or LEV (p = 0.007). Because of common interactions of LTG with other AEDs, differences between monotherapy and polytherapy were examined for LTG. While no AEDs were added or removed during pregnancy, the dose of the concomitant AED was not kept constant. However, no difference in Cl change for LTG was seen between monotherapy and polytherapy across baseline and three trimesters (p = 0.154). AEDs combined with LTG in these patients were levetiracetam (n=7), topiramate (n=3), zonisamide (n=1), and oxcarbazepine (n=1).
Our sample included two sets of twins and 19 women who had a second pregnancy (one with a 3rd and 4th pregnancy). Due to concerns that twin gestations may impact pharmacokinetics differentially than singleton pregnancies and that the inclusion of multiple pregnancies in some women might unduly affect the results, we repeated the primary analyses with these pregnancies deleted. Maximum Cl during pregnancy remained significantly different from non-pregnant baseline for LTG monotherapy (increased 185%, p < 0.001) and LEV monotherapy (increased 224%, p = 0.014). Both twin pregnancies were treated with lamotrigine monotherapy, and their peak CL increased 360% above the non-pregnant baseline compared to an average increase of 191% for other patients on LTG monotherapy (range −9% to 652%). With the twin pregnancies excluded, the mean peak Cl for LTG was 2.89 (SD 1.47), an increase of 189% above baseline. Maxium CL levels are listed in Table 3 for the 15 women who had a second pregnancy on monotherapy LTG (one woman with 4 pregnancies). Maximum CL changes from the 1st to the 2nd pregnancy varied widely on an individual basis ranging from a 69% reduction to a 76% increase across the two pregnancies.
Table 3.
Lamotrigine monotherapy exposures across two pregnancies.
| Subject | CL 1st Pregnancy | CL 2nd Pregnancy | % CL change |
|---|---|---|---|
| 1 | 9.23 | 2.86 | −69% |
| 2 | 3.03 | 2.47 | −18% |
| 3 | 1.61 | 2.83 | +76% |
| 4 | 3.42 | 3.43 | 0% |
| 5 | 4.93 | 2.04 | −59% |
| 6 | 4.04 | 2.53 | −37% |
| 7 | 1.86 | 1.44 | −23% |
| 8 | 3.45 | 3.86 | +12% |
| 9 | 1.98 | 1.88 | −05% |
| 10 | 3.78 | 3.07 | −19% |
| 11 | 4.28 | 2.29 | −46% |
| 12 | 2.02 | 2.06 | +02% |
| 13 | 5.45 | 3.86 | −29% |
| 14 | 4.43 | 3.53 | −20% |
| 15 | 1.04 | 1.26 | +21% |
| Mean | 3.64 | 2.63 | −28% |
3.2. AED dose and ABL changes during pregnancy
Dose was adjusted on an individual basis throughout pregnancy to maintain the patient’s individualized target concentration. Dose tended to increase for all AEDs as pregnancy progressed, although some AEDs were decreased in the first trimester from baseline before trending upward during the remainder of pregnancy (Figure 1, Table e-1). Only four patients had no dose adjustment during gestation (one on LTG, two on LEV, and one on carbamazepine therapy).
Figure 1.
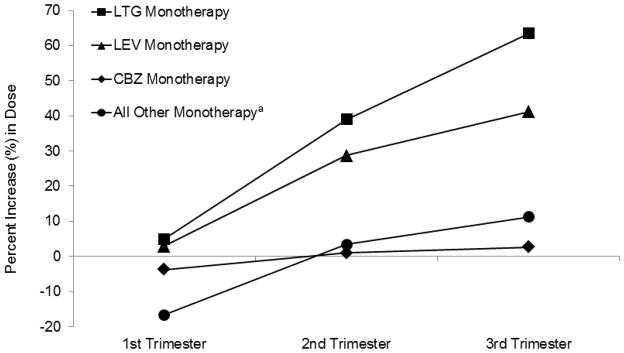
Percent change in monotherapy antiepileptic drug dosage each trimester of pregnancy.
aOther AED monotherapy included topiramate (n=3), oxcarbazepine (n=2), valproate (n=1), zonisamide (n=1), and ethosuximide (n=1). Phenytoin was not included because no non-pregnant baseline dose was available. Dosage data for all individual AEDs and for polytherapy regimens is provided in Table e-2. LTG = lamotrigine; LEV = levetiracetam; CBZ = carbamazepine.
Application of the general linear model to patients with an available preconception baseline revealed a significant difference between baseline dose and maximum pregnant dose LTG monotherapy (p < 0.001), LEV monotherapy (p < 0.001), all other monotherapies (p < 0.001), and for all polytherapies (p = 0.004), polytherapies with LTG or LEV (p = 0.005), and polytherapies without LTG or LEV (p = 0.007). Note that the mean dose for CBZ was virtually unchanged, as were several other monotherapies with very small sample sizes (see Table e-1)
Despite significant changes in dose for most AEDs, mean ABL decreased to some degree during pregnancy for all AEDs (Table e-2), reaching a nadir during the second trimester for most AEDs. The smallest declines in ABL were seen for carbamazepine, ethosuximide, and valproate monotherapies.
3.3. Analysis of seizure frequency
Overall, 49% of patients were seizure-free in the 12 months prior to conception. Fifty-two (78.8%) of these patients remained seizure free during pregnancy. In contrast, only 20% of women with seizures in the year preceding conception had no seizures during pregnancy. Of the total 115 pregnancies, 44 (38.3%) had an increase in any seizure type during any trimester of pregnancy, while 51 (44.3%) had no change and 20 (17.4%) experienced a decrease in all trimesters compared to preconception baseline.
Figure 2 shows seizure deterioration for different AED categories, with the highest rate of increased seizure frequency in polytherapy patients. Change in seizure frequency for each AED is presented in Table e-3. Of note, none of the 6 patients on CBZ had an increase in seizures. Seizure deterioration was significantly more common in patients with any type of seizure in the 12 months prior to conception (p < 0.001) and in patients with localization related epilepsy (p = 0.005). Fifteen (13%) patients had at least one GTCS during pregnancy, of which only one had no GTCS in the year prior to conception, and none of these women were completely seizure free in the prior year.
Figure 2.
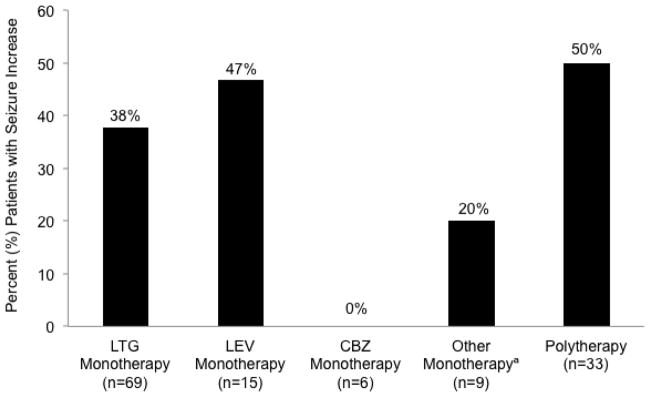
Percent patients with increased seizure frequency during pregnancy compared to the twelve months prior to conception.
aOther monotherapy includes topiramate (n=3), oxcarbazepine (n=2), phenytoin (n=1), valproate (n=1), zonisamide (n=1), and ethosuximide (n=1). LTG = lamotrigine; LEV = levetiracetam; CBZ = carbamazepine.
To examine the relation of seizure frequency to ABL changes, the ratio of each trimester ABL to baseline ABL were compared (t-tests) for patients with an increase in any type of seizure versus those without seizure increase. No significant effects were found between ABL ratio and increased seizures in any trimester for either mean or minimum ABL.
The occurrence of seizure worsening was compared between patients with >35% decrease in ABL below preconception versus patients with less ABL change during each trimester separately for patients who were seizure-free and those who were not in the 12-months preconception. A reduction of ABL >35% below preconception baseline was associated with significantly higher rates of increased seizures during the second trimester, both for patients with seizures in the 12 months prior to conception (p<0.001) and for patients who were seizure free during the preconception year (p=0.030). The results were not significant for the first or third trimesters.
4. Discussion
This study demonstrates that changes in AED pharmacokinetics are common during pregnancy. We used apparent oral clearance as a dose- and weight-corrected measure of plasma AED concentrations and found significant increases in Cl that would produce large drops in AED serum concentration if not counteracted by dose adjustments, particularly for LTG and LEV monotherapy. We also observed significant variability across AEDs and individuals.
The most common AED examined in this study was LTG, which has emerged as a major treatment for reproductive-aged women with epilepsy, given its low teratogenic risk relative to other AEDs [1, 12–14]. The observed increase in Cl for LTG monotherapy in this study is in the range of previous work. One prospective study of 53 pregnancies reported an increase of 94% above baseline [8], while Cl increases in smaller studies have ranged from 65–230%, peaking in the second or third trimesters [15–17]. Larger dose adjustments were also used in patients on LTG monotherapy than for any other AED in our study. This finding is consistent with a large prospective study in pregnant women with epilepsy that found LTG treatment was associated with higher dosage or number of AEDs needed [18]. In addition to marked individual variability in LTG CL, women with repeat singleton pregnancies on monotherapy LTG exhibited exhibited substantial variance in maximum CL from the 1st to 2nd pregnancy. This suggests that at least for LTG, CL in the 1st pregnancy is not predictative of CL in subsequent pregnancies.
Our analysis of LTG monotherapy and polytherapy demonstrated similar increases in LTG Cl for both groups. LTG metabolism is known to be influenced by co-administration with other AEDs with enzyme inducing or inhibiting properties [19, 20]; however, medications used in combination with LTG in our sample would not be expected to affect its levels, with the exception of oxcarbazepine and topiramate at high doses. The most common AED used with LTG was LEV, which does not alter LTG metabolism [21] and would not be expected to affect Cl changes.
Previous data on LEV Cl during pregnancy are more limited, though suggestive of substantial pharmacokinetic change. One study of 21 pregnancies on LEV therapy (five on monotherapy) showed a 54% increase over baseline Cl [22], while a separate analysis of 15 pregnancies (six on monotherapy) demonstrated a 243% increase [23]. Our study included a larger group of monotherapy patients than previously reported, with a peak increase closer to the upper range of prior reports.
Other AEDs investigated tended to show substantially more modest increases in Cl, though the analysis was limited by small sample sizes. Although we were unable to assess free levels, minor changes in carbamazepine Cl are consistent with previous work reporting carbamazepine Cl remains relatively constant during pregnancy [4,24]. Few reports exist on several AEDs we examined, including ethosuximide, which has shown inconsistent pharmacokinetic changes during pregnancy [25]; and zonisamide, which has only been presented in a case report [11, 26]. Our results in one subject for each of these AEDs showed increases in Cl, but further research is needed to better characterize changes that should be anticipated during pregnancy.
We found no difference in LTG Cl change across racial groups. A larger Cl increase has previously been shown for white patients on LTG, although the difference was only significant for free LTG [8], which is not readily available clinically and was not used in our study. The relatively small number of African American and Asian patients in the study also limited our ability to assess racial differences.
Increased seizures during pregnancy compared to the prior year occurred in over a third of our entire cohort. LTG is the most common AED in our population, and 38% of LTG monotherapy pregnancies exhibited increased seizures during any trimester, which is similar to previous reports for patients on LTG [8], the. Rates of increased seizures were also high for LEV (47%) and for AED polytherapy (50%), but low for the small number of women on CBZ (0%). Of particular interest, however, are convulsive seizures, which pose significant risk to maternal and fetal health [4,5]. These seizures were less common in our population, with only eight patients (6.9%) experiencing an increase in GTC frequency during gestation.
Several factors were associated with increased seizure frequency. The highest rate of seizure deterioration occurred in polytherapy patients, which is not surprising since these individuals are likely to have more severe cases of epilepsy. Increased seizure frequency was also more common in patients with localization related epilepsy, which has been observed in previous work [18]. Current AAN guidelines note that seizure control in the preconception period is a significant predictor of seizure activity during pregnancy [27]. In patients with no seizures in the preconception year, 79% remained seizure free during pregnancy, which is a significant predictor but lower than previously reported [27]. Patients with poorer preconception control had significantly higher seizure rates while pregnant. These findings emphasize the importance of counseling and therapy optimization in the preconception period.
Previous work has noted increased seizure risk for patients on LTG when ABL fell below 65% of preconception ABL, especially in the second trimester [8]. When analyzing the ratio of trimester ABL to baseline ABL in each trimester, we did not observe an association with seizure worsening. However, women who had >35% decrease in ABL in the second trimester experienced increased seizures irrespective of whether they had seizures in the 12-months preconception, indicating the clinical significance of large reductions in AED concentration. The lack of a linear relationship between ABL decline and seizure risk may indicate the importance of other pregnancy-related factors contributing to seizure breakthrough, including hormone fluctuations, sleep cycle disturbance, and heightened stress [4]. Additionally, the picture may be complicated by differential susceptibility to ABL changes, such that patients with fragile epilepsy are more likely to seize with small changes in serum levels, while other patients may tolerate larger drops in ABL.
Limitations of this retrospective study include several methodological restrictions, such as the inability to standardize the timing of blood draws relative to last dose and the lack of free drug level assessments (pertinent for some AEDs like CBZ, phenytoin and valproate). Additionally, while patients were repeatedly counseled on the importance of compliance for a healthy pregnancy, pill-counting or other methods of compliance monitoring were not performed. These factors may have influenced the blood levels we obtained. The study is also limited by sample size, with a total of 115 pregnancies and very small patient numbers for several of the examined AEDs. However, even these small numbers represent a substantial addition to the current body of literature. A recent review reported a total of only 80 reported pregnancies on LTG monotherapy and 45 pregnancies on LEV therapy currently published, with many AEDs having very limited available data [11].
Our study thus expands available data on AED pharmacokinetic changes in pregnancy. The 58 new LTG pregnancies increase the number of cases in the literature by 72%, and the 29 LEV pregnancies increase it by 60%. Our findings show that significant alterations in AED levels, which may increase seizure risk, should be anticipated by clinicians. We also demonstrate substantial heterogeneity between medications and individuals, making Cl changes difficult to predict in a particular patient. AEDs are also used extensively in non-epileptic women for psychiatric and pain indications, and the pregnancy-related changes we observed are likely to apply to this group as well.
Current AAN practice parameters state that monitoring should be considered for lamotrigine, carbamazepine, and phenytoin, while monitoring of levetiracetam and oxcarbazepine may be considered [10]. The importance of therapeutic drug monitoring does vary between AEDs, and is most important in medications undergoing dramatic pharmacokinetic alterations during pregnancy. In particular, the substantial Cl changes we demonstrate for relatively large groups of patients on LTG and LEV therapy emphasize the importance of regular monitoring for these medications during gestation, with dose adjustments to maintain levels near the patient’s non-pregnant baseline. Future work should include larger studies on newer AEDs to better inform the need for monitoring and dose adjustment in patients treated with these medications.
5. Conclusions
Significant changes in AED clearance during pregnancy in women with epilepsy were observed in this retrospective study. Considerable variability was seen across AEDs and across individual women. Seizures worsened when AED levels fell >35% from preconception levels. We recommend monitoring of AED levels during pregnancy with dose adjustment if indicated to maintain AED levels near preconception levels.
Supplementary Material
Highlights.
We studied antiepileptic drug (AED) clearance in pregnancy in women with epilepsy.
Significant changes in AED clearance during pregnancy were observed.
When AED levels fell >35% from preconception, seizures worsened significantly.
Monitoring of AED levels with dose adjustment is recommended during pregnancy.
Acknowledgments
This work was supported by the National Institutes of Health (NS038455-11 to KJM and PBP).
Abbreviations
- AED
Antiepileptic drug
- ABL
AED blood level
- Cl
clearance
- LTG
lamotrigine
- LEV
levetiracetam
- CBZ
carbamazepine
- TPM
topiramate
- OXC
oxcarbazepine
- PHT
phenytoin
- VPA
valproate
- ZNS
zonisamide
- ESM
ethosuximide
Footnotes
Conflicts of Interest
Dr. Meador reports receiving research support from the GlaxoSmithKline, EISAI Medical Research, Myriad Pharmaceuticals, Marinus Pharmaceuticals, NeuroPace, Pfizer, SAM Technology, Schwartz Biosciences, and UCB Pharma, the Epilepsy Foundation, and the NIH; received salary support to Emory University from the Epilepsy Consortium for research consultant work related for NeuroPace, Novartis, Upsher-Smith, and Vivus; served as a consultant for Eisai, GlaxoSmithKline, Johnson and Johnson (Ortho McNeil), Medtronics Spherics, and UCB Pharma, but the monies went to a charity of the company’s choice; received travel support from Sanofi Aventis; and also serves on the Professional Advisory Board for the Epilepsy Foundation and the editorial boards for Cognitive and Behavioral Neurology, Epilepsy and Behavior, Neurology, and Journal of Clinical Neurophysiology. Dr. Pennell has received grant support from NIH, CDC, Milken Family Foundation, UCB Pharma and Marinus Pharmaceuticals; and serves on the Professional Advisory Board for the Epilepsy Foundation, the Board of Directors for the American Epilepsy Society, and the editorial boards for Epilepsy Currents and Epilepsia. Dr. Loring reports receiving consulting fees from UCB, NeuroPace and Sanofi-Aventis and grant support from Myriad Pharm, Sam Technology, and Novartis. No other potential conflicts of interest are reported.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tomson T, Battino D, Bonizzoni E, et al. Dose-dependent risk of malformations with antiepileptic drugs: an analysis of data from the EURAP epilepsy and pregnancy registry. Lancet Neurol. 2011;10:609–617. doi: 10.1016/S1474-4422(11)70107-7. [DOI] [PubMed] [Google Scholar]
- 2.Meador K, Reynolds MW, Crean S, Fahrbach K, Probst C. Pregnancy outcomes in women with epilepsy: a systematic review and meta-analysis of published pregnancy registries and cohorts. Epilepsy Res. 2008;81:1–13. doi: 10.1016/j.eplepsyres.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banach R, Boskovic R, Einarson T, Koren G. Long-term developmental outcome of children of women with epilepsy, unexposed or exposed prenatally to antiepileptic drugs. Drug Saf. 2010;33:73–79. doi: 10.2165/11317640-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Burakgazi E, Pollard J, Harden C. The effect of pregnancy on seizure control and antiepileptic drugs in women with epilepsy. Rev Neurol Dis. 2011;8:16–22. [PubMed] [Google Scholar]
- 5.Pennell PB. Antiepileptic drug pharmacokinetics during pregnancy and lactation. Neurology. 2003;61(Suppl 2):S35–S42. doi: 10.1212/wnl.61.6_suppl_2.s35. [DOI] [PubMed] [Google Scholar]
- 6.De Santis M, De Luca C, Mappa I, et al. Antiepileptic drugs during pregnancy: pharmacokinetic and transplacental transfer. Curr Pharm Biotechnol. 2011;12:781–788. doi: 10.2174/138920111795470958. [DOI] [PubMed] [Google Scholar]
- 7.Sabers A, Tomson T. Managing antiepileptic drugs during pregnancy and lactation. Curr Opin Neurol. 2009;22:157–161. doi: 10.1097/WCO.0b013e32832923d7. [DOI] [PubMed] [Google Scholar]
- 8.Pennell PB, Peng L, Newport DJ, et al. Lamotrigine in pregnancy. Neurology. 2008;70:2130–2136. doi: 10.1212/01.wnl.0000289511.20864.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrenaite V, Sabers A, Hansen-Schwartz J. Seizure deterioration in women treated with oxcarbazepine during pregnancy. Epilepsy Res. 2009;84:245–249. doi: 10.1016/j.eplepsyres.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Harden CL, Pennell PB, Koppel BS, et al. Practice parameter update: Management issues for women with epilepsy - focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breastfeeding. Neurology. 2009;73:142–149. doi: 10.1212/WNL.0b013e3181a6b325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomson T, Johannessen CL, Battino D. Antiepileptic drug treatment in pregnancy: Changes in drug disposition and their clinical implications. Epilepsia. 2013;54:405–414. doi: 10.1111/epi.12109. [DOI] [PubMed] [Google Scholar]
- 12.Morrow J, Russell A, Guthrie E, et al. Malformation risks of antiepileptic drugs in pregnancy: a prospective study from the UK Epilepsy and Pregnancy Registry. J Neurol Neurosurg Psychiatry. 2006;77:193–198. doi: 10.1136/jnnp.2005.074203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernandez-Diaz S, Smith CR, Shen A, et al. Comparative safety of antiepileptic drugs during pregnancy. Neurology. 2012;78:1692–1699. doi: 10.1212/WNL.0b013e3182574f39. [DOI] [PubMed] [Google Scholar]
- 14.Sabers A, Dam M, A-Rogvi-Hansen B, et al. Epilepsy and pregnancy: lamotrigine as main drug used. Acta Neurol Scand. 2004;109:9–13. doi: 10.1034/j.1600-0404.2003.00200.x. [DOI] [PubMed] [Google Scholar]
- 15.Pennell PB, Newport DJ, Stowe ZN, Helmers SL, Montgomery JQ, Henry TR. The impact of pregnancy and childbirth on the metabolism of lamotrigine. Neurology. 2004;62:292–295. doi: 10.1212/01.wnl.0000103286.47129.f8. [DOI] [PubMed] [Google Scholar]
- 16.Petrenaite V, Sabers A, Hansen-Schwartz J. Individual changes in lamotrigine plasma concentrations during pregnancy. Epilepsy Res. 205;65:185–188. doi: 10.1016/j.eplepsyres.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Tran TA, Leppik IE, Blesi K, Sathanandan ST, Remmel R. Lamotrigine clearance during pregnancy. Neurology. 2002;59:251–255. doi: 10.1212/wnl.59.2.251. [DOI] [PubMed] [Google Scholar]
- 18.The EURAP Study Group. Seizure control and treatment in pregnancy. Neurology. 2006;66:354–360. doi: 10.1212/01.wnl.0000195888.51845.80. [DOI] [PubMed] [Google Scholar]
- 19.Anderson G, Gidal B, Messenheimer G, Gilliam F. Time course of lamotrigine de-induction: impact of step-wise withdrawal of carbamazepine or phenytoin. Epilepsy Res. 2002;49:211–217. doi: 10.1016/s0920-1211(02)00033-5. [DOI] [PubMed] [Google Scholar]
- 20.Kanner AM, Frey M. Adding valproate to lamotrigine: a study of their pharmacokinetic interaction. Neurology. 2000;55:588–591. doi: 10.1212/wnl.55.4.588. [DOI] [PubMed] [Google Scholar]
- 21.Gidal BE, Baltes E, Otoul C, Perucca E. Effect of levetiracetam on the pharmacokinetics of antiepileptic drugs: a pooled analysis of data from randomized clinical trials. Epilepsy Res. 2005;64:1–11. doi: 10.1016/j.eplepsyres.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Westin AA, Reimers A, Helde G, Nakken KO, Brodtkorb E. Serum concentration/dose ratio of levetiracetam before, during and after pregnancy. Seizure. 2008;17:192–198. doi: 10.1016/j.seizure.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 23.Tomson T, Palm R, Kallen K, et al. Pharmacokinetics of levitiracetam during pregnancy, delivery, in the neonatal period, and lactation. Epilepsia. 2007;48:1111–1116. doi: 10.1111/j.1528-1167.2007.01032.x. [DOI] [PubMed] [Google Scholar]
- 24.Tomson T, Lindbom V, Ekvist B, Sundqvist A. Epilepsy and pregnancy: a prospective study of seizure control in relation to free and total plasma concentrations of carbamazepine and phenytoin. Epilepsia. 1994;35:122–130. doi: 10.1111/j.1528-1157.1994.tb02921.x. [DOI] [PubMed] [Google Scholar]
- 25.Kuhnz W, Koch S, Jakob S, Hartman A, Helge H, Nau H. Ethosuximide in epileptic women during pregnancy and lactation period. Placental transfer, serum concentrations in nursed infants and clinical status. Br J Clin Pharmac. 1984;18:671–677. doi: 10.1111/j.1365-2125.1984.tb02528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oles KS, Bell WL. Zonisamide concentrations during pregnancy. Ann Pharmacother. 2008;42:1139–1141. doi: 10.1345/aph.1L052. [DOI] [PubMed] [Google Scholar]
- 27.Harden CL, Hopp J, Ting TY, et al. Management issues for women with epilepsy - Focus on pregnancy (an evidence-based review): I. Obstetrical complications and change in seizure frequency. Epilepsia. 2009;50:1229–1236. doi: 10.1111/j.1528-1167.2009.02128.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


