Abstract
BACKGROUND
Sleep disturbance is a common clinical complaint of oncology patients and contributes to substantial morbidity. However, because most sleep studies have been cross-sectional, associations between sleep quality and distress in ovarian cancer patients over time remain unclear. This prospective longitudinal study examined rates of sleep disturbance; contributions of depression, anxiety, and medication use in sleep disturbance; and associations between sleep quality and quality of life (QOL) during the first year post-diagnosis among women with ovarian cancer.
METHODS
Women with a pelvic mass completed measures of sleep quality, depression, anxiety, and QOL before surgery. Those diagnosed with primary epithelial ovarian, fallopian tube, or peritoneal cancer repeated surveys at six months and one-year post-diagnosis. Mixed modeling was used to examine trajectories of psychosocial measures over time, as well as associations between changes in distress and sleep quality. Relationships between changes in sleep and QOL were also examined.
RESULTS
The majority of patients reported disturbed global sleep (PSQI > 5) at all three time-points. Medications for sleep and pain were associated with worse sleep at all time-points. Greater increases in depression were associated with increased disturbances in sleep quality over time (p < .04). Worsening sleep was also associated with declines in QOL over time (p <.001).
CONCLUSION
Sleep disturbance is common and persistent in women with ovarian cancer, and is linked to depressive symptoms and QOL. Pharmacologic treatment does not appear to adequately address this problem. Results highlight the need for ongoing screening and intervention for sleep disturbance in this population.
Keywords: ovarian cancer, insomnia, depression, anxiety, quality of life
INTRODUCTION
Sleep disturbance is common and contributes to substantial morbidity among cancer patients.1–4 Rates of sleep disturbance in cancer patients are higher than in the general population1 and have been documented by polysomnography.2 Among heterogeneous advanced cancer patients, 72% reported sleep disturbance, with common complaints including difficulties with sleep onset and maintenance, not feeling rested in the morning, and daytime fatigue.3 Chemotherapy has been associated with significantly poorer sleep quality4 and daytime sleepiness.5 Disturbed sleep has functional consequences as it has been associated with poorer quality of life (QOL) in women with breast cancer, including impaired ability to perform work and daily tasks,6 although not all studies have yielded consistent results.7 A recent study of ovarian cancer patients within 5 years of diagnosis reported that sleep disturbance contributes to poorer QOL regardless of disease stage and chemotherapy status.8
Sleep disturbance often persists over extensive time periods in cancer patients. Disturbed sleep and fatigue have been reported in women with breast cancer even before initiation of treatment.9 Generally, sleep disturbance in breast and prostate cancer patients declines over the 6 months following treatment.10 However, at least one study has found that 23.3% of women treated for early stage breast cancer still have symptoms of insomnia for up to 2–5 years after treatment completion.11
Due to poor prognosis and rigorous treatment protocols,12 ovarian cancer patients often suffer high distress at the time of diagnosis and during treatment,13 potentially placing them at elevated risk for sleep disturbance. However, little is known about the trajectory of sleep disturbance following diagnosis and what factors confer increased risk for poor sleep in ovarian cancer patients over time. To address these issues, the first objective of the present study was to characterize prevalence of self-reported sleep disturbance in women with ovarian cancer prior to surgery, at six months and one year post-diagnosis. The second objective was to examine risk factors for poor sleep trajectories, including depression, anxiety, and menopausal status at the time of surgery, and to examine the extent to which antidepressants, pain, and sleep medications alleviate sleep disturbance over time. As ovarian cancer surgery initiates menopause in pre-menopausal patients, we hypothesized that sleep disturbance would be worse in pre-menopausal patients.14 To date, no studies have followed ovarian cancer patients prospectively to assess how depression, anxiety, and quality of life are related to sleep disturbance over time. Depression is common among individuals with cancer15 and has been associated with decreased sleep duration, daytime sleepiness, and nocturnal awakening among breast cancer patients.16 Anxiety is also commonly experienced by ovarian cancer patients17,18 and has been cross-sectionally associated with insomnia in this population.19 Thus, we hypothesized that worsening depression and anxiety over time would contribute to poorer sleep over time, and that poorer sleep trajectories would contribute to poorer QOL.
METHODS
Participants
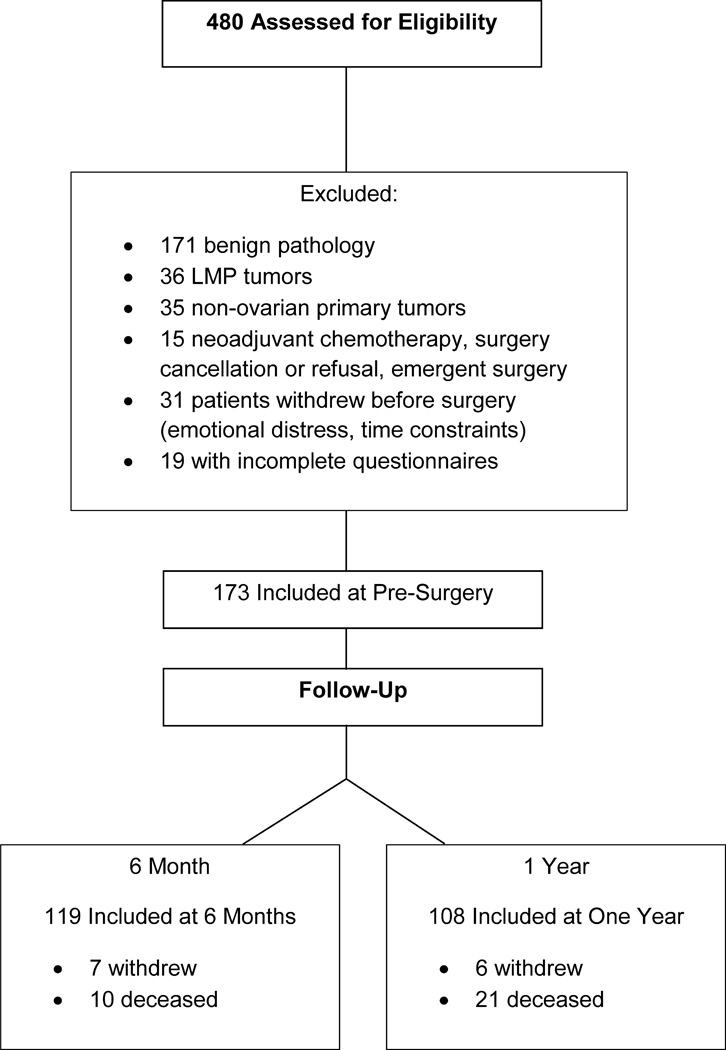
Following institutional review board approval, women over 18 years with a pelvic mass suspicious for ovarian cancer were recruited pre-surgically as part of a larger prospective study examining psychosocial factors and cancer progression. Exclusion criteria included corticosteroid use in the previous month, history of previous cancer, current pregnancy, inability to accurately answer questions, comorbid condition with known effects on immune function, and non-ovarian primary site. Inclusion was confirmed following surgical diagnosis of epithelial ovarian, fallopian tube or primary peritoneal cancer. The final sample is described in Figure 1. At pre-surgery, 173 women had histological confirmation of diagnosis with epithelial ovarian cancer and had complete data on relevant measures; at six months, 119 of these patients were available for assessment; at one year, 108 were available for assessment. Women completed psychosocial questionnaires prior to surgery and again at follow ups.
Figure 1.
Patient inclusion chart for participants at pre-surgery and follow-ups.
Measures
Sleep Quality
The Pittsburgh Sleep Quality Index (PSQI) is a 19-item self-report measure assessing types and frequency of sleep disturbances experienced over the last month.20 Seven subscale scores are summed to yield a global score; global scores > 5 indicate poor sleep;20 scores > 10 are considered to indicate clinically impaired sleep (personal communication, M. Hall, 2011). The scale has a diagnostic sensitivity of 89.6% and specificity of 86.5%.21 The scale is psychometrically sound in cancer patients22 and correlates with sleep log data in primary insomnia.23
Depressive Symptoms
The Center for Epidemiological Studies Depression Scale (CES-D) is a 20-item self-report measure rating mood during the previous week. Higher scores indicate greater depressive symptomatology, with scores ≥16 consistent with clinical depression.24 The CES-D is considered a valid and reliable assessment for depressive symptoms in cancer patients.25,26
Anxiety
The Profile of Mood States short-form (POMS-SF) is a 37-item inventory assessing six dimensions of mood27 often used in research with cancer patients.28,29 This study used the anxiety subscale as the indicator of anxiety.
Quality of Life (QOL)
The Functional Assessment of Cancer Therapy (for ovarian patients: FACT-O) assesses QOL in individuals with cancer. The FACT provides a total QOL score as well as subscales for physical, functional, social, spiritual, and emotional well-being from 51 items.30 Statements are rated on a scale from 0 (not at all) to 4 (very much) and cued to well-being during the previous week.
Performance Status
Participants rated physical functioning in the past seven days from 0 (fully active) to 3 (completely disabled) using the Gynecologic Oncology Group (GOG) Performance Status measure.31 This was used as a covariate in regression analyses.
Demographic and Clinical Information
Self-reported information on age, education, race, ethnicity, and marital status was collected prior to surgery. Clinical information including tumor stage and grade, menopausal status, and use of medications was extracted from medical records.
Statistical Analysis
Analyses were conducted using Statistical Package for the Social Sciences v. 19.0 (SPSS, Chicago, IL). Data distributions were examined for violations of normality and outliers. Descriptive statistics were used to examine the presence of poor sleep at baseline and one year. One way analyses of variance (ANOVA) were used to examine differences in sleep disturbance between users and non-users of specific medications at each time point as well as between patients receiving versus not receiving chemotherapy at follow-up. As sleep disturbance, depression, and anxiety were unrelated to chemotherapy status, this variable was not included in subsequent analyses (all p’s>.13).
Longitudinal analyses used repeated measures mixed models (SPSS MIXED procedure). All models included disease stage, pre-surgical menopausal status, and the use of pain medications, anxiolytics/antidepressants, and sleep medications prior to surgery as a priori covariates. Moreover, we tested models with additional potential psychosocial, medical, and demographic covariates to determine if they exerted a significant influence on model estimations. Covariates exerting a significant influence on model estimations were retained in final models. Models examining the change in sleep quality as a function of change in depression and anxiety included change in self-reported disability as a covariate. Changes in depression and anxiety were both included in models examining the effects of distress on sleep quality to disaggregate the effects of depression versus anxiety on changes in sleep.
Covariance structure was evaluated with Wald Z tests of rho covariance parameters and comparison of Schwarz’s Bayesian Criterion (BIC) between models with different covariance structures as suggested by existing literature.32,33 First order auto-regressive covariance structures were supported for all models by significance of rho covariance parameters (all p’s<.001) and lower BIC compared to diagonal and unstructured covariance structures. Pairwise comparisons of estimated marginal means, using a Sidak adjustment, were conducted to examine changes from baseline to 6 months and from 6 months to one year.
Covariates
Income, race, ethnicity, relationship status, body mass index, histopathology, presence/absence of residual tumor, use of caffeine, alcohol and nicotine, presence/absence of chemotherapy at 6 months and one year, and presence of comorbid conditions such as chronic obstructive pulmonary disease, diabetes, sleep apnea, heart disease or hypertension were non-significant covariates in all models (all p’s>0.112). Disease stage was not a significant predictor of change in QOL, global sleep score, depression or anxiety (all p’s>.32). Significant interactions of covariates with outcomes in longitudinal models will be addressed in the results section.
RESULTS
Participant Characteristics
Participants were primarily non-Hispanic Caucasians with a mean age of 56 years. The majority of participants had advanced stage and high grade disease. The association between sleep disturbance and presence of ascites at the time of surgery was non-significant (r = .13, p = .09). (Table 1)
Table 1.
Patient Characteristics
| Characteristic | Ovarian Cancer Patients |
|---|---|
| Age, years | |
| Mean (S.D) | 59.39 (12.46) |
| Ethnicity | |
| Non-Hispanic | 90.9% |
| Hispanic | 9.1% |
| Race | |
| American Indian/Alaska Native | 1.1% |
| Asian | 0.5% |
| Pacific Islander | 0.0% |
| Black/African American | 2.7% |
| White | 95.7% |
| Education | |
| Less than high school graduate | 5.2% |
| High school graduate | 29.7% |
| Trade school/some college | 31.8% |
| College graduate | 15.6% |
| Postgraduate | 7.3% |
| Marital status | |
| Single | 10.4% |
| Divorced/Separated | 12.2% |
| Widowed | 11.6% |
| Married/Living with Partner | 65.9% |
| Stage | |
| I | 20.7% |
| II | 6.5% |
| III | 62.5% |
| IV | 10.3% |
| Grade | |
| 1 | 10.3% |
| 2 | 12.4% |
| 3 | 77.3% |
| Tumor histology | |
| Serous | 75.4% |
| Endometrioid | 11.2% |
| Mucinous | 4.8% |
| Clearcell | 3.7% |
| Other/Unknown | 4.8% |
| Surgical Debulking | |
| Optimal | 73.1% |
| Suboptimal | 26.9% |
| Medication Use Pre-Surgery | |
| Hypnotics | 11.8% |
| Antidepressants | 22.6% |
| Anxiolytics | 12.4% |
| Pain Medications | 23.1% |
| Medication Use at 6 Month | |
| Hypnotics | 11.3% |
| Antidepressants | 12.9% |
| Anxiolytics | 8.6% |
| Pain Medications | 17.7% |
| Medication Use at One Year | |
| Hypnotics | 9.3% |
| Antidepressants | 25.8% |
| Anxiolytics | 13.4% |
| Pain Medications | 28.9% |
Medication use and sleep quality
Medication use was largely similar at pre-surgery, six months, and one year (Table 1). At all time-points, patients using prescribed sleep or analgesic medications reported significantly worse sleep quality than those not using these medications (all p’s > .025). There were generally no differences in global sleep quality for users and non-users of antidepressant and anxiolytic medications (all p’s > .10), although at 6 months, patients taking antidepressants had significantly worse sleep quality than non-users (p = .03).
Additionally, specific patterns of medication use were associated with psychosocial recovery over time. For example, use of pain medication pre-surgically was associated with less improvement in sleep quality, depression, anxiety, and QOL over time (all p’s < .038). In contrast, sleep medication and anxiolytics/antidepressants generally had no associations with changes in these measures over time (all p’s > .15).
Menopausal Status
At baseline, mean PSQI scores for pre-menopausal women were slightly worse (PSQI=8.98, SD=3.91) than scores for post-menopausal women (PSQI=7.25, SD=3.48), but this difference was non-significant. Women who were pre-menopausal at study entry had less improvement in sleep quality, depression, and anxiety over time than their post-menopausal counterparts (all p’s < .025). In contrast, menopausal status was not associated with changes in QOL over time (p = .41).
Sleep, Depression, Anxiety and QOL over Time
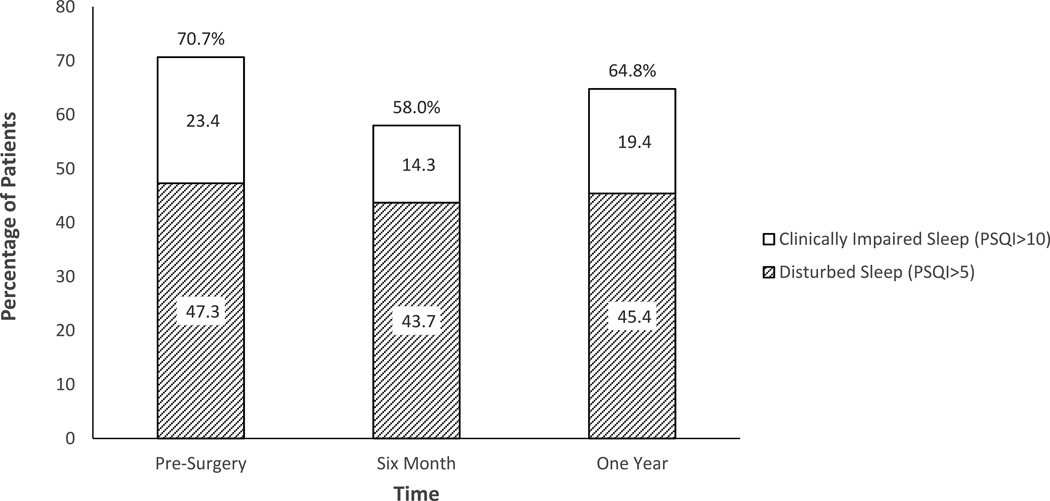
Prior to surgery, 70.7% of patients reported disturbed global sleep (PSQI>5); of these patients, 24.3% reported global sleep poor enough to be rated “clinically impaired” (PSQI≥10). During the year following surgery, significant improvements were seen in global sleep quality (p = .035), depression (p < .001), anxiety (p < .001) and QOL (p < .001). Pairwise comparisons of sleep, depression, anxiety, and QOL scores revealed significant improvement from surgery to six months (p’s < .033), but between six months and one year these factors were relatively stable (all p’s > .88). Examination of the clinical meaning of these scores revealed that although global sleep quality had improved from surgery to six months, more than half of the sample (58%) still reported disturbed sleep and 14.3% remained in the clinically impaired range. By one year, almost two thirds of patients (64.8%) still reported disturbed sleep, and 19.4% reported clinical impairment (Figure 2). In contrast, a majority of patients reported improvement to non-clinical levels of depression over time. Specifically, at pre-surgery, 45% of patients reported depression scores above the CESD clinical depression cutoff (≥ 16); percentages of patients above the cutoff improved to 20.7% at six months and 20.9% at one year. (Table 2)
Figure 2.
Proportion of patients reporting sleep disturbance on the PSQI at pre-surgery, six months, and one year.
Table 2.
Estimated Marginal Means of Psychosocial Measures.
| Measure | Pre-surgery - M(SE) | 6 Month – M(SE) | One Year – M(SE) |
|---|---|---|---|
| Global Sleep | 7.26 (1.26) | 6.38 (1.29) | 6.52 (1.30) |
| CESD | 22.16 (3.42) | 16.10 (3.46) | 16.68 (3.49) |
| POMS Anxiety | 11.01 (1.99) | 7.42 (2.05) | 7.67 (2.05) |
| FACT-O Total | 69.95 (5.03) | 78.03 (5.10) | 78.93 (5.15) |
Associations between Changes in Distress and Changes in Sleep Quality
Adjusting for cancer stage, menopause, performance status, and medications, increased depression from surgery to one year was associated with less improvement in overall sleep quality (p < .001). Changes in anxiety were not significantly associated with changes in sleep quality (p = .09). However, those patients using pain (p = .017) or sleep medications (p = .001) pre-surgery or who had been pre-menopausal before surgery (p = 0.027) showed less improvement in sleep quality as their depression improved. Improved self-reported performance status was also significantly associated with improved sleep (p = .005).
Change in QOL as a Function of Change in Sleep
Between surgery and one-year, improvements in global sleep quality were associated with improvements in QOL scores, adjusting for stage, menopausal status, and medication use (p < .001). Women with earlier stage disease shower more QOL improvement with improved sleep than did those with advanced stage disease (p = .03).
DISCUSSION
The present data demonstrate that a high proportion of women with ovarian cancer reported sleep disturbance before surgery, and these disruptions in sleep tended to persist over the first year after diagnosis, independent of whether patients were receiving chemotherapy. The proportion of ovarian cancer patients reporting sleep disturbances at each of the measured time-points was similar to rates in other oncology populations.8,9 At all time-points, patients using either sleep or pain medication reported worse global sleep than those not taking these medications; in contrast, antidepressant and anxiolytic medication use was generally unrelated to sleep quality. Longitudinal improvements in depression were associated with improvements in global sleep, though improvements over time in anxiety were not independently associated with better sleep. Despite statistically significant improvement in global sleep between pre-surgery and 6 months, mean sleep quality remained in the disturbed range over the course of the study. Furthermore, as predicted, sleep disturbance was a risk factor for diminished QOL over time, while improvements in sleep improved QOL.
The current study is the first to longitudinally assess sleep disturbance in ovarian cancer survivors and to demonstrate that sleep disturbance is common and persistent during the first year post-diagnosis. These data thus extend previous cross sectional descriptions of sleep problems in ovarian cancer.18,19 These data highlight risk factors associated with disturbed sleep trajectories and reveal that sustained sleep disruptions have a significant impact on patients’ overall QOL. Risk factors for sustained sleep disturbance include depression, performance status, use of pain medication, and pre-menopausal status. The associations between depressive mood and sleep disturbance are consistent with findings in metastatic breast cancer patients, where depressive symptoms predict worsening of sleep over time.16
Potential Mechanisms
Several mechanisms may underlie disrupted sleep in cancer patients. Dysfunctions in the gamma-aminobutyric (GABA) system as well as in serotonin receptors have been associated with sleep disturbance and depression.34,35 Depression has been related to heterogeneous difficulties including sleep maintenance and early morning awakening.36 As difficulties with sleep onset and maintenance both contribute to the global sleep disturbance score, it is possible that multiple mechanisms are operating here. Depression may additionally contribute to worse sleep; thus, a bidirectional mechanism may underlie the current findings.
Tumor-related processes may also contribute to both depressive symptoms and discomfort, thereby causing impaired sleep. For example, pre-surgery, abdominal bloating and ascites may contribute to discomfort37 with concomitant sleep impairment. This may be reflected in the association of poor performance status with impaired sleep; however, presence of ascites per se was not associated with sleep disturbance. Additionally, tumor derived-inflammatory cytokines may play a role in both sleep and depression. For example, we and others have previously reported elevations in the pro-inflammatory cytokine interleukin-6 (IL-6),38,39 and links between poorer sleep quality and elevated IL-6 in ovarian cancer patients.40 High levels of circulating pro-inflammatory cytokines such as IL-6 have in turn been associated with central vegetative symptoms including fatigue, malaise, and sleep alterations, as well as depressive symptoms.41 Thus, it is possible that tumor-derived inflammation may contribute to both depressive symptoms and sleep disturbance. Alternatively, sleep and cytokines may have a bi-directional relationship whereby alterations in sleep influence cytokine expression, and cytokines in turn have a regulatory influence on sleep.42
Bidirectional relationships may exist between chronic pain and sleep disturbance.43 Specifically, chronic pain is thought to contribute to poorer sleep which in turn may increase pain sensitivity. Though untreated pain may contribute to discomfort and inability to initiate and maintain sleep, analgesic medications may produce daytime side effects which also interfere with nighttime sleep.44 Although there was not a direct measure of pain in the current study, the present findings support an association between pain medications and poorer sleep.
Women who were pre-menopausal prior to surgery reported worse sleep trajectories over time. Surgical oophorectomy induces an immediate menopause and thus may initiate the onset of classic menopausal symptoms such as nocturnal temperature fluctuations and hot flashes, both of which interfere with sleep.14 Additionally, facing a life-threatening diagnosis at a younger age and/or loss of childbearing status may give rise to sleep disturbances.
Limitations
The absence of data on premorbid sleep patterns limits our ability to determine how the sleep patterns reported here may relate to long-term disturbance. We also do not have data regarding contextual issues such as insomnia arising from sleeping with a partner who snores. Additionally, study data rely on self-report measures, which are subject to reporting biases and retrospective recall. Although polysomnography and actigraphy provide objective assessments of sleep disturbances, these technologies were deemed too intrusive for this patient population, particularly when facing surgery for a life-threatening disease. As study design was correlational, definitive causal relationships cannot be determined, and it is possible that sleep disturbance may lead to depression as well.45 Due to the minimal numbers of women taking hormone replacement therapy in this sample, influences of hormonal replacement on sleep could not be examined. Additionally, potential third variables such as inflammation, cortisol dysregulation, and stressful life events could also be contributing to poor sleep, affective disorders, and poor QOL.
Clinical Implications
The extent of initial and sustained sleep disturbance in this population, and its impact on QOL, highlights the clinical relevance of this problem in ovarian cancer patients. These findings suggest the importance of screening for possible sleep disturbance, both at time of diagnosis, as well as during treatment and follow-up. The extent of sleep disturbance, combined with findings that hypnotics, antidepressants, anxiolytics, and pain medications don’t appear to facilitate improved sleep, suggest that other effective interventions for sleep disturbance may need to be explored. For example, behavioral interventions such as cognitive behavioral therapy (CBT) and mindfulness based stress reduction (MBSR) have known effectiveness for insomnia46,47 and have shown positive benefits in oncology patients.48,49
Conclusions
Women with ovarian cancer report sustained sleep quality disturbance up to one year post-diagnosis, and this disturbance is associated with impaired QOL. Furthermore, depression confers increased risk for poor sleep quality in this population. These findings highlight the importance of ongoing screening for sleep disturbance in clinical cancer care. Further, as pharmacological interventions appear to have limited effectiveness in this population, behavioral interventions to improve depression and sleep quality may contribute to improved QOL and decreased morbidity in this population.
Acknowledgments
This research was supported in part by NIH grants CA88293, CA104825, and CA140933 to SL.
Footnotes
There are no financial disclosures from any of the authors.
REFERENCES
- 1.Anderson KO, Getto CJ, Mendoza TR, et al. Fatigue and sleep disturbance in patients with cancer, patients with clinical depression, and community-dwelling adults. J Pain Symptom Manage. 2003;25:307–318. doi: 10.1016/s0885-3924(02)00682-6. [DOI] [PubMed] [Google Scholar]
- 2.Parker KP, Bliwise DL, Ribeiro M, et al. Sleep/wake patterns of individuals with advanced cancer measured by ambulatory polysomnography. J Clin Oncol. 2008;26:2464–2472. doi: 10.1200/JCO.2007.12.2135. [DOI] [PubMed] [Google Scholar]
- 3.Sela RA, Watanabe S, Nekolaichuk CL. Sleep disturbances in palliative cancer patients attending a pain and symptom control clinic. Palliat Support Care. 2005;3:23–31. doi: 10.1017/s1478951505050042. 2005. [DOI] [PubMed] [Google Scholar]
- 4.Owen DC, Parker KP, McGuire DB. Comparison of subjective sleep quality in patients with cancer and healthy subjects. Oncol Nurs Forum. 1999;26:1649–1651. [PubMed] [Google Scholar]
- 5.Kuo H, Chiu M, Liao W, Hwang SL. Quality of sleep and related factors during chemotherapy in patients with stage I/II breast cancer. J Formos Med Assoc. 2009;105:64–69. doi: 10.1016/S0929-6646(09)60110-8. [DOI] [PubMed] [Google Scholar]
- 6.Fortner BV, Stepanski EJ, Wang SC, Kasprowicz S, Durrence HH. Sleep and quality of life in breast cancer patients. J Pain Symptom Manage. 2002;24:471–480. doi: 10.1016/s0885-3924(02)00500-6. [DOI] [PubMed] [Google Scholar]
- 7.Redeker NS, Lev EL, Ruggiero J. Insomnia, fatigue, anxiety, depression and quality of life of cancer patients undergoing chemotherapy. Sch Inq Nurs Pract. 2000;14:275–290. [PubMed] [Google Scholar]
- 8.Sandadi S, Frasure H, Broderick MJ, Waggoner SE, Miller JA, von Gruenigen VE. The effect of sleep disturbance on quality of life in women with ovarian cancer. Gynecol Oncol. 2011;123:351–355. doi: 10.1016/j.ygyno.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 9.Ancoli-Israel S, Liu L, Marler MR, et al. Fatigue, sleep and circadian rhythms prior to chemotherapy for breast cancer. Support Care Cancer. 2006;14:201–209. doi: 10.1007/s00520-005-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas K, Bower J, Hoyt MA, Sepah S. Disrupted sleep in breast and prostate cancer patients undergoing radiation therapy: the role of coping processes. Psychooncology. 2010;19:767–776. doi: 10.1002/pon.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindley C, Vasa S, Sawyer WT, Winer EP. Quality of life and preferences for treatment following systemic adjuvant therapy for early-stage breast cancer. J Clin Oncol. 1998;16:1380–1387. doi: 10.1200/JCO.1998.16.4.1380. [DOI] [PubMed] [Google Scholar]
- 12.American Cancer Society. Cancer Facts and Figures 2010. Atlanta: American Cancer Society; 2010. [Google Scholar]
- 13.Costanzo ES, Lutgendorf SK, Rothrock NE, Anderson B. Coping and quality of life among women extensively treated for gynecologic cancer. Psychooncology. 2006;15:132–142. doi: 10.1002/pon.930. [DOI] [PubMed] [Google Scholar]
- 14.Lin EM, Aikin JL, Good BC. Premature menopause after cancer treatment. Cancer Pract. 1999;7:114–121. doi: 10.1046/j.1523-5394.1999.07306.x. [DOI] [PubMed] [Google Scholar]
- 15.Spiegel D. Cancer and depression. Br J Psychiatry. 1996;168:109–116. [PubMed] [Google Scholar]
- 16.Palesh OG, Collie K, Batichok D, et al. A longitudinal study of depression, pain, and stress as predictors of sleep disturbance among women with metastatic breast cancer. Biol Psychol. 2007;75:37–44. doi: 10.1016/j.biopsycho.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bodourka-Bevers D, Basen-Engquist K, Carmack CL, et al. Depression, anxiety, and quality of life in patients with epithelial ovarian cancer. Gynecol Oncol. 2000;78:302–308. doi: 10.1006/gyno.2000.5908. [DOI] [PubMed] [Google Scholar]
- 18.Hipkins J, Whitworth M, Tarrier N, Jayson G. Social support, anxiety, and depression after chemotherapy for ovarian cancer: a prospective study. Br J Health Psychol. 2004;9:569–581. doi: 10.1348/1359107042304542. [DOI] [PubMed] [Google Scholar]
- 19.Price MA, Zachariae R, Butow PN, et al. Prevalence and predictors of insomnia in women with invasive ovarian cancer: anxiety a major factor. Eur J Cancer. 2009;45:3262–3270. doi: 10.1016/j.ejca.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 20.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 21.Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res. 1998;45:5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- 22.Beck SL, Schwartz AL, Towsley G, Dudley W, Barsevick A. Psychometric evaluation of the Pittsburgh Sleep Quality Index in cancer patients. J Pain Symptom Manage. 2004;27:140–148. doi: 10.1016/j.jpainsymman.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53:737–740. doi: 10.1016/s0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- 24.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 25.Hann D, Winter K, Jacobsen P. Measurement of depressive symptoms in cancer patients: evaluation of the center for epidemiological studies depression scale (CES-D) J Psychosom Res. 1999;46:437–443. doi: 10.1016/s0022-3999(99)00004-5. [DOI] [PubMed] [Google Scholar]
- 26.Schroevers MJ, Sanderman R, van Sonderen E, Ronchor AV. The evaluation of the Center for Epidemiologic Studies Depression (CES-D) scale: depress and positive affect in cancer patients and healthy reference subjects. Qual Life Res. 2000;9:1015–1029. doi: 10.1023/a:1016673003237. [DOI] [PubMed] [Google Scholar]
- 27.Shacham S. A shortened version of the Profile of Mood States. J Pers Assess. 1983;47:305–306. doi: 10.1207/s15327752jpa4703_14. [DOI] [PubMed] [Google Scholar]
- 28.Lutgendorf SK, Mullen-Houser E, Russell D, et al. Preservation of immune function in cervical cancer patients during chemotherapy using a novel integrative approach. Brain Behav Immun. 2010;24:1231–1240. doi: 10.1016/j.bbi.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bradley S, Rose S, Lutgendorf S. Quality of Life and mental health in cervical and endometrial cancer survivors. Gynecol Oncol. 2006;100:479–486. doi: 10.1016/j.ygyno.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 30.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 31.Rubin S. Society of Gynecologic Oncologists: Chemotherapy of Gynecologic Cancers. 2nd ed. Philadelphia: Lippincott Williams and Wilkins; 2004. [Google Scholar]
- 32.Chou C, Bentler PM. Model modification in covariance structure modeling: A comparison among likelihood ratio, lagrange multiplier, and wald tests. Multivariate Behav Res. 1990;25:115–136. doi: 10.1207/s15327906mbr2501_13. [DOI] [PubMed] [Google Scholar]
- 33.Littell RC, Pendergast J, Natarajan R. Modelling covariance structure in the analysis of repeated measures data. Stat Med. 2000;19:1793–1819. doi: 10.1002/1097-0258(20000715)19:13<1793::aid-sim482>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 34.Kalueff AV, Nutt DJ. Role of GABA in anxiety and depression. Depress Anxiety. 2007;24:495–517. doi: 10.1002/da.20262. [DOI] [PubMed] [Google Scholar]
- 35.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 36.Berk M. Sleep and depression: theory and practice. Aust Fam Physician. 2009;38:302–304. [PubMed] [Google Scholar]
- 37.Goff BA, Mandel LS, Melacon CH, Muntz HG. Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. JAMA. 2004;291:2705–2712. doi: 10.1001/jama.291.22.2705. [DOI] [PubMed] [Google Scholar]
- 38.Lutgendorf SK, Weinrib AZ, Penedo F, et al. Interleukin-6, cortisol, and depressive symptoms in ovarian cancer patients. J Clin Oncol. 2008;26:4820–4827. doi: 10.1200/JCO.2007.14.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tempfer C, Zeisler H, Sliutz G, Haeusler G, Hanzal E, Kainz C. Serum evaluation of interleukin 6 in ovarian cancer patients. Gyncol Oncol. 1997;66:27–30. doi: 10.1006/gyno.1997.4726. [DOI] [PubMed] [Google Scholar]
- 40.Clevenger L, Schrepf A, Christensen D, et al. Sleep disturbance, cytokines, and fatigue in women with ovarian cancer. Brain Behav. Immun. 2012;26:1037–1044. doi: 10.1016/j.bbi.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-to-brain communications for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- 42.Irwin M. (2002). Effects of sleep and sleep loss on immunity and cytokines. Brain Behav Immun. 2002;16:503–512. doi: 10.1016/s0889-1591(02)00003-x. [DOI] [PubMed] [Google Scholar]
- 43.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8:119–132. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 44.Theobald DE. Cancer pain, fatigue, distress, and insomnia in cancer patients. Clin Cornerstone. 2004;6(suppl 1D):S15–S21. doi: 10.1016/s1098-3597(05)80003-1. [DOI] [PubMed] [Google Scholar]
- 45.Cho HJ, Lavretsky H, Olmstead R, Levin MJ, Oxman MN, Irwin MR. Sleep disturbance and depression recurrence in community-dwelling older adults: A prospective study. Am J Psychiatry. 2008;165:1543–1550. doi: 10.1176/appi.ajp.2008.07121882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychol. 2006;25:3–14. doi: 10.1037/0278-6133.25.1.3. [DOI] [PubMed] [Google Scholar]
- 47.Manber R, Edinger JD, Gress JL, San Pedro-Salcedo MG, Kuo TF, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31:489–495. doi: 10.1093/sleep/31.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carlson LE, Garland SN. Impact of Mindfulness-Based Stress Reduction (MBSR) on sleep, mood, stress, and fatigue symptoms in cancer outpatients. Int J Behav Med. 2005;12:278–285. doi: 10.1207/s15327558ijbm1204_9. [DOI] [PubMed] [Google Scholar]
- 49.Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of cognitive behavioral therapy for insomnia secondary to breast cancer, part I: sleep and psychological effects. J Clin Oncol. 2005;23:6083–9096. doi: 10.1200/JCO.2005.09.548. [DOI] [PubMed] [Google Scholar]