Abstract
Pediatric pharmacotherapy is often challenging due to the paucity of available clinical data on the safety and efficacy of medications that are commonly used in children. This quandary is even more prevalent in children with rare diseases. Although extrapolations for dosing and administration are often made from available adult data with similar disease states, this translation becomes even more problematic in rare pediatric diseases. Understanding of disease pathophysiology is typically poor, and few, if any, effective therapies have been studied and identified. One condition that illustrates these issues is plastic bronchitis, a rare, most often pediatric disease that is characterized by the production of obstructive bronchial airway casts. This illness primarily occurs in children with congenital heart disease, often after palliative surgery. Plastic bronchitis is a highly clinically relevant and therapeutically challenging problem with a high mortality rate, and, to date, a generally accepted effective pharmacotherapy regimen has not been identified. Furthermore, the disease is ill defined, which makes timely identification and treatment of children with plastic bronchitis difficult. The pharmacotherapies currently used to manage this disease are largely anecdotal and vary between the use of macrolide antibiotics, mucolytics, bronchodilators, and inhaled fibrinolytics in a myriad of combinations. The purpose of this review is twofold: first, to highlight the dilemma of treating plastic bronchitis, and second, to bring attention to the continuing need for studies of drug therapies used in children so that safe and effective drug regimens can be established, particularly for rare diseases, which often have no treatment options.
Keywords: congenital heart disease, Fontan procedure, adverse effects, pulmonary disease, fibrinolysis, antiinflammatory agents
In 1963, Dr. Harry Shirkey described children as “therapeutic orphans” with regard to the pharmacologic treatments tested and available for this population.1 Numerous factors have been identified that contribute to this problem, ranging from a lack of financial incentives for pharmaceutical companies to conduct such studies to concerns over the ethics of carrying out nontherapeutic trials in this age demographic.2 Multiple legislative measures, notably the Orphan Drug Act in 1983, the Food and Drug Administration (FDA) Modernization Act in 1997, and the Best Pharmaceutical for Children Act in 2002, sought to address some of these shortcomings by providing incentives for pharmaceutical companies to conduct studies in the pediatric population.1–3 More extensive rules to safeguard this orphan population were established by the Pediatric Research Equity Act in 2003, which required pediatric clinical studies for all new biologics and drugs entering the market unless a waiver or deferral is granted by the FDA.3 Although increases in industry-sponsored pediatric studies have resulted from these acts, nearly 80% of commercially available medications are not labeled for use in children, and only one third of the medications used in pediatric patients have been sufficiently studied in the disease states for which they are being used.1–3 Many of the dosage regimens used in children have been extrapolated from available adult pharmacokinetic and pharmacodynamic data.4 Based on these statistics alone, it is not surprising that the practice of off-label use of medications has become widespread among healthcare professionals who care for these pediatric patients. Reluctance to initiate a therapy due to adverse events or the alternative of starting treatments due to fears over withholding a potentially beneficial therapy contribute additional dilemmas to the therapeutic decision making of clinicians providing care to this population.2
These issues are further complicated in pediatric patients with rare diseases for which few treatments have been studied and successfully used. Approximately 8,000 diseases have been categorized as rare, and it is estimated that 6–10% of the global population is living with one of these conditions.5 Only 200 of the several thousand rare diseases have approved therapies, and only about one fourth of available orphan drugs have been designated for use in rare pediatric diseases.3, 6 Many of these illnesses develop in childhood and can be progressively debilitating, presenting significant challenges for patients, families, and healthcare providers.5 The limited knowledge of disease pathologies further complicates the ability to identify and utilize safe and efficacious therapies.5
Plastic bronchitis (PB) is a rare disease in which these shortcomings in pediatric pharmacotherapy can be aptly illustrated. The pathophysiology of this condition remains poorly understood, treatment regimens are mostly anecdotal, and morbidity and mortality are significant.7–10 The goal of this review is to highlight the therapies that are used in this population in the absence of clinical efficacy and safety data. Select patient cases are included to illustrate key concepts in the diagnosis and management of PB. This review also seeks to bring attention to the need for continued work in the field of pediatric pharmacotherapy so that safe and effective therapies can be identified for children.
Plastic Bronchitis
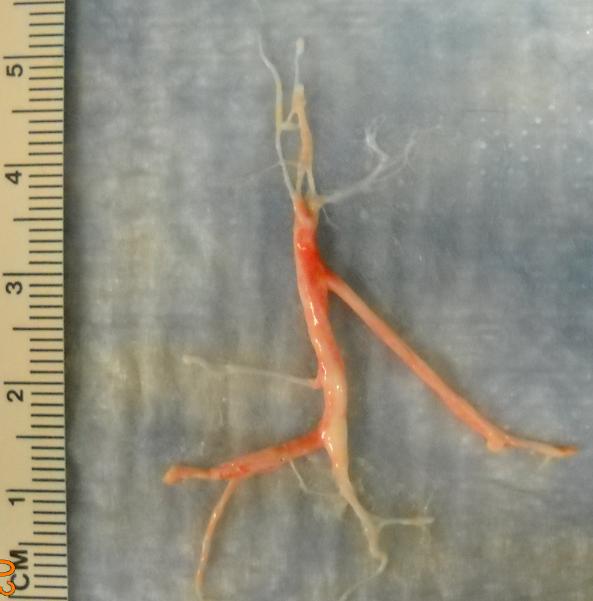
Plastic bronchitis is a predominantly pediatric disease characterized by the formation of obstructive bronchiolar casts in the lung airways.7–10 Its etiology and overall prevalence remain unknown, but it most often occurs in children with congenital heart disease (CHD) surgically palliated by the Fontan procedure.11–13 PB has also been reported as a secondary complication in a variety of pulmonary conditions, including asthma, cystic fibrosis (CF), and acute chest syndrome.9, 10, 14, 15 Casts have been described as “rubbery” in consistency and have been reported to be composed of fibrin, mucin, or both, depending on the underlying disease state.10, 13, 16 However, in children with CHD, casts are primarily composed of fibrin (Figure 1).10, 17
Figure 1.
A representative spontaneously expectorated plastic bronchitis cast (~10 cm in length) from a pediatric patient with Fontan-associated plastic bronchitis. Pathology showed that it was primarily composed of fibrin.
Although PB can develop in association with a variety of conditions, it is particularly problematic in the pediatric CHD population. This subgroup experiences both the highest frequency of life-threatening events and the highest mortality rate of any disease associated with PB.9 The clinical presentation of PB generally manifests in episodes of dyspnea, tachycardia, hypoxemia, fever, and cough due to airway obstruction.8, 13, 14, 18, 19 The chest x-ray may show opacification of the affected lung field due to partial or full atelectasis.13, 14, 18, 20 However, this observation is not useful as a definitive diagnostic tool.20 Presently, the diagnosis of PB hinges on visualization of bronchial casts that are either expectorated or removed by bronchoscopy.7, 9 There are currently no phenotypic biomarkers that assist in the diagnosis or differentiation of Fontan patients who have or are prone to develop the disease.
Classification systems of airway cast composition have been proposed to bring insight into the etiology of cast formation. These schemes seek to provide a formative guide for treatments used in this population based on the cast type and underlying disease state. The most widely used scheme delineates casts into one of two main types: type I (inflammatory) and type II (acellular).21 Inflammatory, or type I, airway casts were defined by the presence of fibrin, eosinophilic infiltrates, and small amounts of mucin.12, 21 Acellular, or type II, casts were distinguished by the presence of mucin, little to no fibrin, and a lack of cellular content other than mononuclear cells in some cases.21 In the case reports examined by the authors of this classification scheme, type I casts were produced by patients with histories of pulmonary conditions, such as asthma or diffuse mucositis, and type II casts were primarily reported in patients with surgically corrected CHD.12, 21 A revised classification system was proposed in 2002 based on the underlying disease state of the patient, which included the categories of allergic or asthmatic, cardiac, and idiopathic origins.9 Another classification scheme was introduced in 2005, which proposed diagnosis first according to the primary disease state of the patient, and then by cast histology if the underlying disease state was not readily defined.16 Although these classification schemes have been widely applied for differentiating cast types, they were primarily developed by using published case reports that retrospectively examined available pathology data of cast composition. Use of existing pathology reports for this purpose could result in incomplete information due to variations in histopathological assessment between institutions and the degree of completeness of the reports.10
More recently, a prospective, longitudinal approach was used to assess cast composition, and it determined that airway casts produced by children with CHD were primarily composed of fibrin, with very little mucin.10 This finding was recently confirmed by using a global protein profiling approach to more completely define airway cast composition.17 This study also noted that these casts had cellular infiltrates, further challenging the longstanding belief that casts from this PB population are primarily acellular. Knowledge of bronchial cast composition in the CHD population is essential for elucidating the mechanisms of airway cast formation, which in turn is imperative for the identification of drug target opportunities aimed at preventing and reducing airway cast burden in children with PB. This finding also shifts the approach of PB cast characterization from one that is disease associated to one that is focused on prospective histopathological assessment.
Insight into the Clinical Dilemma
The pharmacotherapy for PB is largely anecdotal and varies widely according to the underlying disease state and the severity of symptoms.10, 13, 14, 18, 20 Treatments initiated in response to acute exacerbations of PB typically involve the initiation of inhaled mucolytics and inhaled and systemic corticosteroids, and, if necessary, bronchoscopic lavage and extraction of obstructing airway casts.14, 15, 18, 20 Other pharmacotherapies that have been used in this population include aerosolized fibrinolytics,13, 14, 18, 20 macrolides,13, 18 bronchodilators,13 anticoagulants,14, 20 and hypertonic saline (Tables 1 and 2).13 The degree of efficacy of each of these interventions remains widely unknown, as use of these agents has primarily been highlighted in case reports. As such, we present the clinical cases of two patients with recurrent episodes of PB to illustrate treatment approaches used at our institution for acute and chronic management of PB. Case no. 1 highlights the complex initial presentation of a patient who ultimately was diagnosed with PB and also exemplifies the sporadic and unpredictable nature of airway cast formation and expectoration. Case no. 2, which occurred 10 years after the initial presentation of case no. 1, demonstrates the continued challenges of diagnosing PB. In particular, this case shows how the absence of phenotypic markers exclusive of airway cast visualization impairs the timely identification and treatment of children with PB.
Table 1.
Representative Therapies for the Acute Management of a Plastic Bronchitis Exacerbation*
| Medication | Indication | Drug Class | Route of Administration | Typical Doses |
|---|---|---|---|---|
| Albuterol | Airway Clearance | Bronchodilator | Inhaled | 2.5 mg q4-6h prn |
| Levalbuterol | Inhaled | 0.63–1.25 mg q4-6hrs prn | ||
| Prednisone | Antiinflammat ory | Corticosteroid | Oral | 1–2 mg/kg/day divided qd-bid |
| Methylprednisone | Intravenous | 1–2 mg/kg/day divided qd-bid | ||
| N-Acetylcysteine | Airway Cast Reduction | Mucolytic | Inhaled | Infants: 10% solution: 1–2 mLtid-qid |
| 20% solution: 2–4 mL tid-qid | ||||
| Children: 10% solution: 3–5 mL tid-qid | ||||
| 20% solution: 6–10 mL tid-qid | ||||
| Adolescents: 10% or 20% | ||||
| solution: 5–10 mL tid-qid | ||||
| Dornase alfa | Inhaled | 2.5 mg qd-bid | ||
| Alteplase (tPA) | Airway Cast Reduction | Fibrinolytic | Inhaled | Optional dose of 10–12 mg followed by 5 mg q4-6hrs |
| UFH | Anticoagulant | Inhaled | Various regimens: 5000 U q4-8hrs | |
| Subcutaneous | Variable Dosing |
UFH: unfractionated heparin
tPA: tissue plasminogen activator
in addition to supplemental oxygen and chest physiotherapy, these medications may be used in a variety of combinations to facilitate airway cast expectoration and reduce airway cast burden.
Table 2.
Representative Therapies for the Chronic Management of a Plastic Bronchitis*
| Medications | Indication | Drug Class | Route of Administration | Typical Doses |
|---|---|---|---|---|
| Albuterol | Airway Clearance | Bronchodilator | Inhaled | 2.5 mg q4-6h prn |
| Levalbuterol | Inhaled | 0.63–1.25 mg q4-6h prn | ||
| Budesonide | Antiinflammatory | Corticosteroid | Inhaled | 0.25–1 mg bid |
| Budesonide-formoterol | Airway Clearance | Corticosteroid-bronchodilator | Inhaled | 2 puffs bid (strength dependent on age of patient) |
| Fluticosone-Salmeterol | Inhaled | 1 puff bid (strength dependent on age of patient) | ||
| Hypertonic Saline (3% and 7%) | Airway Clearance | Inhaled | 4 mL bid | |
| Azithromycin | Antiinflammatory | Macrolide | Oral | 250–500 mg three times per week |
| Montelukast | Antiinflammatory | Leukotriene Pathway | Oral | 6 months to 5 years: 4 mg qd |
| Inhibitor | 6–14 years: 5 mg qd | |||
| ≥ 15 years: 10mg qd | ||||
| N-Acetylcysteine | Airway Cast Reduction | Mucolytic | Inhaled | Infants: 10% solution: 1–2 mL tid-qid |
| 20% solution: 2–4 mL tid-qid | ||||
| Children: 10% solution: 3–5 mL tid-qid | ||||
| 20%: 6–10 mL tid-qid | ||||
| Adolescents: 10% or 20% | ||||
| solution: 5–10 mL tid-qid | ||||
| Dornase alfa | Inhaled | 2.5 mg qd-bid | ||
| Alteplase (tPA) | Airway Cast Reduction | Fibrinolytic | Inhaled | Optional dose of 10–12 mg followed by 5 mg q4-6h |
| UFH | Anticoagulant | Inhaled | Various regimens: 5000 U q8h | |
| Subcutaneous | Variable Dosing |
UFH: unfractionated heparin
tPA: tissue plasminogen activator
some of these medications may be used in a variety of combinations with the intent to reduce cast formation and facilitate cast expectoration.
Case No. 1
A child with hypoplastic left heart syndrome diagnosed prior to birth underwent a series of cardiac palliative procedures consisting of the Norwood, hemi-Fontan, and fenestrated Fontan procedures by the age of 17 months. His medical history became significant for respiratory illness after completion of his final palliative surgery. He was hospitalized for episodes of pneumonia, parainfluenza bronchiolitis, and respiratory distress secondary to the formation of presumed chylous plugs in the airway. After multiple hospitalizations, the goal of treatment began to focus on the resolution of recurrent chylous plug formation, and, as a result, the patient underwent a right upper lobectomy without complications. Despite this intervention, the patient continued to produce airway casts that required extraction by bronchoscopy.
The diagnosis of chylopoeisis respiratory failure was made two years after his Fontan surgery following another hospitalization for recurrent cast formation and the development of chylous effusions. Bilateral chest tubes were placed for drainage of chylous effusions, and bronchoscopies were performed to aid in cast removal. A lymphatic nuclear scan showed no signs of thoracic drainage, which dismissed the need for a thoracic duct ligation. Airway clearance therapy was initiated following improved oxygenation secondary to cast removal by bronchoscopy. This regimen consisted of intermittent positive-negative pressure breathing, nebulized albuterol, and N-acetylcysteine (Table 1). The patient did well with these therapies, and his clinical status continued to improve. He was discharged from the hospital on a regimen of subcutaneous unfractionated heparin (UFH) and albuterol as needed.
The patient attended routine follow-up visits for the management of his chyloptysis, which was later reclassified as PB. He experienced intermittent flares of the condition concurrently with the development of upper respiratory viral infections. He was able to expectorate casts without medical intervention, and he did not have any significant worsening of PB that warranted hospitalization again until the age of thirteen.
As an adolescent, the patient has been hospitalized multiple times for PB exacerbations. Most recently, he presented to the emergency department with symptoms consistent with an exacerbation of PB, including increased cough, difficulties with breathing, and active cast production. He was found to be afebrile, normotensive, tachycardic, and hypoxic (83% on room air), with the latter necessitating the use of oxygen. The chest x-ray showed prominent bilateral perihilar bronchovascular markings, with no evidence of pleural effusion or pneumothorax. Given the clinical presentation, there was a suspicion of pneumonia, and antibiotic and antiviral therapies were empirically started. The patient's maintenance PB regimen (Table 2) was continued throughout the course of his hospital stay, which included inhaled budesonide, albuterol, azithromycin, prednisone, subcutaneous heparin, inhaled tissue plasminogen activator (tPA) and chest physiotherapy. Increases in the dosing frequencies of inhaled tPA, budesonide, and albuterol were implemented for the acute management of the patient's PB flare (Table 1).
Following the expectoration of several casts over the next 24 hours, the patient's oxygen saturation increased to 89%, and his clinical status significantly improved. As a result, he was discharged home from the hospital the following day.
Case No. 2
A patient with a double-inlet left ventricle, transposition of the great arteries, and mild aortic arch hypoplasia diagnosed prior to birth underwent a series of palliative surgeries by the age of 18 months, including a modified Norwood, a hemi-Fontan, and a lateral tunnel fenestrated Fontan. Four years after the Fontan procedure, the patient developed a chronic cough with intermittent chest congestion. After two weeks of progressively worsening symptoms, the patient was hospitalized and diagnosed with pneumonia. Treatment with azithromycin and cefotaxime was initiated, and the patient was discharged two days later on a 14-day course of amoxicillin-clavulanate and supplemental oxygen. Despite these treatments, the patient continued to experience intermittent cough and episodes of shallow breathing, and, unbeknown to the medical team, was actively expectorating airway casts at home.
Subsequently, the patient produced a cast during hospitalization and was diagnosed with PB. Histologic examination of cast showed fibrin and inflammatory cells. Based on these findings, a medication regimen focused on maintenance of airway clearance using levalbuterol and hypertonic saline was prescribed by the pulmonologist (Table 2). The regimen also included montelukast and dornase alfa as needed for PB exacerbations. Despite long-term use of these therapies, the patient continued to experience recurrent severe PB exacerbations that required hospitalization and more intensive pharmacologic treatment with inhaled tPA and dornase alfa.
Plastic Bronchitis Pharmacotherapy
The management of PB can be divided into two types of therapeutic regimens, chronic and acute, depending on the clinical status of the patient. All therapies are symptomatic, and no known curative therapy exists other than heart transplantation for the condition. Presently, chronic therapy is aimed at maintaining airway clearance and targeting potential underlying causes of cast formation. The primary focus of acute treatment is to facilitate cast expectoration and reduce airway cast mass. However, as illustrated by the two patients' cases and the evidence available in published case reports, there are currently no accepted standard therapeutic regimens for either the prevention or treatment of PB. The regimens presented for the acute (Table 1) and chronic (Table 2) management of this disease are representative of those that are employed at the University of Michigan and at other centers that treat children with PB (see Acknowledgments section).
Airway Clearance Regimens
Agents traditionally used for the maintenance of airway clearance are used in the management of PB to facilitate airway cast expectoration. Airway clearance regimens are similar to those used in patients with CF and generally consist of bronchodilator administration followed by chest physiotherapy and nebulized hypertonic saline. Chest physiotherapies have demonstrated efficacy in prolonging mucociliary clearance in CF22 and chronic bronchitis23 without adversely affecting lung function. Although the clinical phenotypes of CF and PB are distinct, the commonality of airway obstruction observed in each of the disease states warrants the consideration of using airway clearance strategies in patients with PB.
Bronchodilators
Both short- and long-acting bronchodilators are used in the acute and chronic management of PB. The potential benefits of bronchodilators include increased mucociliary clearance,24, 25 in vitro inhibition of eosinophil chemotaxis,26 and the inhibition of mediator release from mast cells in the airway.27 However, unlike the reactive airways of CF and asthma in which mucociliary clearance is impaired,28 there is presently no evidence that this mechanism is involved in PB. In fact, it has been our observation that PB patients have normal pulmonary function in the absence of an obstructing airway cast. Nevertheless, use of a short-acting bronchodilator like albuterol or levalbuterol in advance of chest physiotherapy may aid in the relaxation of bronchial smooth muscle and ease the expectoration of airway casts (Tables 1 and 2). Although the rationale for the use of a long-acting bronchodilator may be the same, there is no evidence that either short- or long-acting agents are effective for this purpose.
Recent evidence showing that cast pathology consists of inflammatory cells, including eosinophils,17 may substantiate the use of a long-acting bronchodilator for its antiinflammatory activity in the treatment of PB. However, the role of eosinophils in cast formation is not fully understood, and there is no evidence to date that the use of a long-acting bronchodilator has any impact on airway cast formation or disease progression.
Hypertonic Saline
Administration of aerosolized hypertonic saline is intended to prolong hydration of airway surfaces and promote airway mucociliary clearance.29 These effects have been attributed to an increase in the osmotic gradient between airway surface liquid (ASL) and the epithelial cells of the lungs after its administration, which in turn draws water back into the lung airway and increases the volume of the ASL. The induction of cough has also been noted as a result of the changes in osmolality and the ionic composition of the airway.30 In addition to these effects, hypertonic saline also demonstrates mucolytic properties through its ability to dissociate extracellular DNA from mucoproteins, in turn permitting proteolysis by native enzymes.31 Antiinflammatory and immunomodulatory effects, including inhibition of tumor necrosis factor-α-induced nuclear factor-κ B activation in pulmonary epithelium,32 the ability to modify the airway cytokine profile in CF,33 and a reduction in superoxide anion production by neutrophils in vitro34 have also been demonstrated.
Collectively, these actions could be beneficial in the treatment of PB. However, like bronchodilators, the use of hypertonic saline in PB is currently aimed at facilitating cast expectoration. This is highlighted in case no. 2, in which nebulized hypertonic saline was initiated in conjunction with other airway clearance therapies. Whether hypertonic saline is efficacious in improving overall clinical outcomes or cast formation remains to be ascertained. Administration of hypertonic saline has been associated with bronchoconstrictive responses, an effect that could counter effective cast expectoration.35 Although the use of bronchodilators prior to this therapy can alleviate this response, the implication of this effect in the management of PB should be considered.
Antiinflammatory Therapies
Antiinflammatory therapies are routinely used in the management of acute and chronic PB. These therapies include inhaled and systemic corticosteroids, macrolides, and leukotriene pathway inhibitors. Although the etiology of this rare disease is poorly understood, the fibrin and cellular composition of casts produced in PB implicate the involvement of an inflammatory response in cast formation. However, there is presently no evidence that any of the following antiinflammatory agents have disease-modifying activity.
Leukotriene Pathway Inhibitors
Use of cysteinyl-leukotriene receptor (Cys-LTR) antagonists like montelukast for the treatment of PB has arisen due to the antiinflammatory effects and efficacy of these drugs in the treatment of patients with asthma.36, 37 These agents inhibit the actions of leukotriene D4 by directly binding to Cys-LTRs present on smooth muscle cells and airway macrophages, subsequently attenuating leukotriene-mediated inflammatory effects.38, 39 This therapy has been associated with decreases in airway eosinophilic inflammation,40 reductions in the use of bronchodilator therapy, and improvements in asthma symptoms and overall control.41 There is no evidence to substantiate that PB is an eosinophil-mediated airway disease, but eosinophilic infiltrates have been noted in PB cast samples.17 Whether the addition of a Cys-LTR inhibitor to the chronic medication regimen of PB patients could aid in attenuating this response is not presently known. The patient in case no. 2 received and tolerated this therapy well for nearly two years. However, its use did not prevent subsequent PB exacerbations.
Macrolides
Long-term use of macrolide antibiotics, namely azithromycin, has been shown to convey a number of actions including increased mucosal clearance, prevention of the formation of bacterial biofilm, and reductions in bronchoconstriction through the inhibition of cholinergic responses in the smooth muscles of the airway.42–44 In addition, azithromycin has antiinflammatory activity that is evidenced by its ability to decrease the synthesis of reactive oxygen species, inhibit neutrophil activation and mobilization, and stimulate neutrophil apoptosis. However, these effects often take several weeks of treatment before they are fully tangible and are only exhibited by 14- and 15-member macrolides.43 For these reasons, in addition to its more favorable adverse-effect profile when compared with other agents, azithromycin is most often the macrolide of choice.
Evidence for the efficacy of macrolide therapy in PB is limited to a few case reports. Schultz and Oermann reported complete resolution of cast production after the addition of low-dose azithromycin therapy to a patient's pharmacologic regimen and also noted improvement in lung volume and expiratory flow rates.45 Another case report attributed the ability to reduce the frequency of inhaled tPA administration to the addition of long-term azithromycin therapy.14 The antibacterial activity of azithromycin likely does not contribute to the potential benefit of its use in PB as there is no evidence of an infectious etiology or that bacterial byproducts contribute to cast formation in the airways.
Corticosteroids
Both inhaled and systemic corticosteroids are used in the acute and chronic management of PB (Tables 1 and 2). Corticosteroids exert their mechanism of action primarily by suppressing the expression of numerous mediators and inhibiting both the innate and adaptive immune cell responses typically involved with allergic and nonallergic inflammatory responses.46, 47 Immunomodulatory effects are also observed with these agents.47 Typically, an inhaled corticosteroid therapy is coupled with the administration of a long-acting bronchodilator, as the use of both agents has been shown to be more efficacious than monotherapy with either agent.27 The combined use of budesonide and formoterol yields elevated expression of glucocorticoid receptor (GR) binding to glucocorticoid response elements and subsequent induction of GR-dependent mitogen-activated protein kinase phosphatase 1 in macrophages.48 This combination therapy also permits the administration of a lower dose of budesonide (200 μg) as it demonstrates similar efficacy to that of a higher dose of budesonide (800 μg) alone,48, 49 potentially minimizing the risk for adverse effects. Beyond the observed pharmacologic benefits, this therapy has also demonstrated improvements in lung function and symptoms and reductions in the frequency of exacerbations in asthma patients receiving chronic treatment.49 However, the wealth of evidence for the use of glucocorticoids in the treatment of asthma is not met with a similar level of support for their use in the treatment of PB. Interestingly, the most abundant cytokine in a recent sample of PB airway casts was macrophage migration inhibitory factor (MIF).17 MIF is an inflammatory cytokine that is known to have the ability to sustain inflammatory responses in the presence of endogenous or exogenous glucocorticoids.50 As such, MIF may represent a potential drug target for PB.
In an effort to minimize toxicity, the use of systemic corticosteroids is generally limited to acute PB exacerbations, as was demonstrated in case no. 1 (Table 1). Patients are generally started on a short-term course of steroids with either prednisone or methylprednisolone, the doses of which are tailored to the individual patient. Although adverse side effects are a concern with this therapy, the systemic administration of corticosteroids has demonstrated efficacy in minimizing hospitalizations and improving pulmonary function in asthma patients with acute exacerbations.51 Reductions in unscheduled medical visits, symptoms of dyspnea, and the use of inhaled bronchodilators in asthma patients after an eight-day tapered dosing regimen have been reported as well.52 However, prospective assessments confirming the efficacy of inhaled and systemic corticosteroids in this patient population are lacking. One case report described the complete resolution of cast production after the initiation of oral prednisone 2 mg/kg/day in combination with inhaled beclomethasone dipropionate 600 μg/day.53 The dose of systemic corticosteroid was eventually tapered, whereas the inhaled corticosteroid therapy was continued. Although the patient did not have any additional PB flares during the three-year follow up period, “tenacious secretions” were found by bronchoscopy, suggesting that corticosteroid therapy alone may not lead to complete resolution of PB. In addition, given the abundance of MIF in PB airway casts, the utility of corticosteroids for PB treatment warrants more thorough investigation.17
Therapies Targeted at Airway Cast Reduction
Mucolytics
Mucolytic therapy in PB has widely stemmed from the belief that airway casts are primarily composed of mucin secondary to mucosal hypersecretion.16, 21 This can result in the obstruction of lung airways due to excessive mucosal accumulation, the occurrence of which has been observed in other respiratory diseases such as CF, asthma, and chronic obstructive pulmonary disease.28, 54N-Acetylcysteine and dornase alfa are mucolytic agents commonly used in respiratory conditions to reduce mucosal viscoelasticity, with dornase alfa being solely indicated for CF. Both of these agents have been used in the management of PB; however, the evidence for the efficacy of these therapies for the treatment of PB is limited to case reports.
Brogan, et. al., completed an in vitro comparison of N-acetylcysteine, dornase alfa, urokinase plasminogen activator (uPA), and tPA, which showed that N-acetylcysteine possessed the greatest ability to dissolve PB bronchial casts.9 Other case reports have detailed success with the direct instillation of dornase alfa onto casts in the airway to facilitate removal by bronchoscopy.55–57 Improvements in pulmonary function after treatment with dornase alfa have also been detailed.57 In contrast, another case reported no change in cast size after the direct instillation of dornase alfa onto a cast fragment in the airway of a PB patient.15
Although these case reports varied with regard to the efficacy observed with mucolytic therapy, this class of agents could be useful in the management of PB. Extracellular DNA can contribute to airway mucosal thickening, and this effect is commonly observed in CF patients due to the degradation of neutrophils in the airways.58 Airway casts from Fontan patients contain inflammatory cells, extracellular DNA, and histones that could contribute to airway cast formation.17 It is therefore plausible that dornase alfa may be useful in reducing airway cast burden because it hydrolyzes long, extracellular DNA molecules into smaller fragments.59 As described in case no. 2, dornase alfa was used for an acute PB exacerbation to facilitate cast expectoration. However, mucolytic therapy alone is likely not a reliable strategy to effectively manage PB, as casts are predominantly composed of fibrin and cast formation most likely arises from a number of complex processes. The efficacy of dornase alfa alone has been further brought into question in a more recent case report in which topical application of the agent failed to reduced airway cast mass.15
The evidence supporting the hypothesis that mucosal hypersecretion in CHD patients with PB is primarily based on retrospective reviews of case reports, some of which do not detail cast histology.9, 16, 21 In addition, some of the CHD cases noted the presence of either inflammatory cells or fibrin in addition to mucin, which are features that are not typical of mucosal hypersecretion. Histological staining of cast samples obtained from a longitudinal study of PB airway cast pathology revealed that they were primarily composed of fibrin and lymphocytes, and that composition did not change over time.10 These findings were substantiated by protein profiling using a sophisticated proteomics approach.17 In both studies, mucin was either evident in small amounts or not found. Although these observations may seem extraneous, clarification of cast histology is useful for determining the utility of therapies aimed at reducing cast mass as well as for providing insight into the etiology of cast formation. As such, patients who produce fibrin casts would more likely benefit from the use a fibrinolytic agent rather than mucolytics to reduce airway cast burden.15
Unfractionated Heparin
Unfractionated heparin is a heavily sulfated glycosaminoglycan that is primarily used for its anticoagulant properties.60 This agent exerts its main mechanism of action by inactivating thrombin and factor Xa following its binding to antithrombin, which prevents the conversion of fibrinogen to fibrin. Heparin also possesses antiinflammatory and immunoregulatory properties that have been demonstrated in a variety of processes, including inhibition of inflammatory mediators and lymphocyte activation, neutrophil chemotaxis, smooth muscle growth, and complement activation.61, 62 As a result, the use of UFH in an aerosolized dosage form for the management of inflammatory lung diseases has recently become an active area of research.61, 62
Reductions in bronchoconstrictive response have been observed in patients with exercise-induced asthma after the administration of inhaled UFH.63 In addition, attenuation of early and reduced late allergic responses in patients with asthma and house dust mite allergies has also been noted with the use of this intervention. In pediatric patients with smoke-inhalation injuries, inhaled therapies of aerosolized UFH (5,000 U) and N-acetylcysteine (3 ml of 20% solution) alternated every 2 hours over a seven-day period yielded significant improvements in a variety of clinical outcomes.64 These were evidenced by overall decreases in the rates of atelectasis, reintubation attributed to respiratory failure, number of ventilator days, and overall mortality. Of note, smoke-inhalation injuries are characterized in part by the formation of fibrinous, cellular casts in the airway. Twice daily administration of inhaled UFH in patients with idiopathic pulmonary fibrosis has also been found to be safe and well tolerated.65 However, the efficacy of this intervention in idiopathic pulmonary fibrosis has yet to be determined. Like inhaled tPA, there is the potential for adverse bleeding events, but none have been observed in any studies conducted to date.
Inhaled UFH use in PB has been documented in two case reports, each of which reported success with regimens of 5,000 units every 8 hours66 and 5,000 units every 4 hours during acute exacerbations.67 One of the patients was maintained on an inhaled regimen of 5,000 units every 8 hours for an additional twelve months until she was tapered off of this therapy.67 Intravenous or subcutaneous administration of UFH in PB patients has been documented in multiple case reports with variable success.14, 20, 68 However, one report claimed nearly complete resolution of fibrin cast formation using a regimen of UFH 5,000 units daily administered subcutaneously in a PB patient with CHD.68 Attempts at discontinuing the therapy were associated with a recurrence of active cast production. As outlined in case no. 1, subcutaneous UFH has been used as a chronic therapy for several years with no adverse events. However, the contribution this medication makes toward curtailing airway cast formation is not readily apparent.
The improvements in clinical status that have been documented in studies and select case reports to date encourage the continued evaluation of inhaled UFH therapy for the management of PB. However, attention must be paid to the potential for serious adverse effects such as hemorrhage and thrombocytopenia that can occur with this agent.68 Furthermore, the optimal dosage regimen and the mechanism by which UFH may modulate airway cast formation require further study.
Fibrinolytics
Inhaled fibrinolytics, and more specifically recombinant tPA, constitute a more recent addition to the wide range of therapeutic interventions used to manage acute exacerbations of PB. As described in each of the patient cases, inhaled tPA is typically administered every four hours for the management of acute exacerbations of PB (Table 1). The use of inhaled fibrinolytic therapy for PB was first outlined by Quasney, et. al., who used aerosolized uPA every four hours at a dose of 40,000 U to acutely manage a patient with PB.69 A reduction in cast size and tenacity was noted. Following this observation, expectorated casts were treated in vitro with normal saline, uPA 300 U/ml or tPA 1,000 U/mL. The results of these experiments showed that casts were unchanged following incubation in normal saline and became “soft and friable” following incubation with uPA. In contrast, the cast samples exposed to tPA were completely degraded, a finding that has been substantiated by another study.10 Based on this observation, Costello, et. al., administered inhaled tPA to a PB patient with underlying CHD after five days of failed treatment with bronchodilators, corticosteroids, antibiotics, and mucolytics.18 Marked clearance of the airways was observed by bronchoscopy following the initiation of this therapy, and significant improvements in oxygen saturation levels were noted as well. Multiple, unrelated case reports detailing experiences with inhaled tPA followed, each of which reported varying degrees of success in resolving airway cast burden.13–15, 18, 20 Importantly, no adverse events in association with the use of this intervention were noted in any of these cases. Experience with inhaled tPA at the University of Michigan has been consistent with these observations.
Although both uPA and tPA have been utilized to reduce cast burden, the extent of cast degradation observed with the administration of tPA in both the clinical setting and experimental models has encouraged the continued use of tPA over other fibrinolytic agents.10, 15, 69 Tissue plasminogen activator is a serine protease that catalyzes fibrinolysis through direct conversion of plasminogen to plasmin.70 Although all fibrinolytic agents exhibit this mechanism of action, tPA demonstrates a higher affinity for fibrin compared with other plasminogen activators. This property in the context of fibrin airway cast production, along with observations of a favorable response in clinical and experimental settings, makes targeted delivery of this agent a logical strategy to reduce airway cast burden. However, to our knowledge, no clinical studies have been conducted to assess the safety and efficacy of inhaled tPA in PB patients. Subsequently, there is a scarcity of clinical data on appropriate dosage regimens and potential toxicities that may arise from repeated dosing by inhalation for a drug that is typically given as a single intravenous dose.71, 72
The doses of inhaled tPA utilized in PB case reports have been extrapolated from adult systemic dose recommendations for acute myocardial infarction or acute ischemic stroke.18 Although this is an acceptable framework to use when initially selecting doses of medications for off-label use, the regimen implemented in the clinical setting of PB stands in great contrast to those used for the FDA-approved indications and route of administration.73, 74 Tissue plasminogen activator is typically given intravenously in a continuous, tapered dosing scheme over the course of a few hours. However, repeated dosing of inhaled tPA is required to target fibrin airway casts, and this regimen can potentially span several days in duration depending on the severity of the exacerbation. Presently, the response to inhaled tPA is monitored by pulse oximetry and the frequency of cast expectoration. There are no biomarkers of drug efficacy or safety to date.
One of the main concerns associated the use of inhaled tPA is the potential for adverse bleeding events, the likelihood of which could potentially increase with prolonged and repeated administration of this medication. Preclinical studies examining the safety of accelerated and prolonged dosing regimens of a pulmonary formulation of tPA have been conducted in healthy murine models in the absence of airway fibrin.71, 72 Pulmonary-delivered tPA doses of 3 mg/kg every two hours for up to 12 hours and 0.3 mg/kg/day divided twice daily over a 28-day period were well tolerated. However, prolonged administration of doses exceeding 1 mg/kg/day were associated with acute and fatal pulmonary hemorrhage. Albeit no adverse events have been reported to date with inhaled tPA in humans, this could be due to the rarity of PB and the overall infrequent use of inhaled tPA for its treatment and not necessarily due to the safety of currently used doses. This makes it all the more important for continued work toward establishing a safe and efficacious dosing regimen of inhaled tPA for children with PB.
Conclusion
Despite numerous well-intended legislative initiatives, there is still an unmet need for medication testing in children. This urgency is even greater in children with rare diseases. The clinically challenging and perplexing problem of PB is an example of this because it is associated with high morbidity and mortality, and there is no known effective pharmacotherapy. Certainly, improving knowledge of disease pathogenesis is likely to unveil drug target opportunities; however, in the meantime, in our opinion, the most promising agents are those that are directed at airway cast reduction. Given that PB casts are primarily fibrin, agents aimed at preventing fibrin formation, such as inhaled UFH, and dissociating fibrin, such as inhaled tPA, could be especially useful.
Recently, more details about the inflammatory nature of PB airway casts have emerged. Notably, the most abundant cytokine in casts was MIF. This finding raises the possibility that MIF could be a potential drug target for PB and could make glucocorticoid therapy less effective. Undoubtedly, much more work needs to be done before definitive conclusions about the role of MIF in airway cast formation can be made. Nevertheless, this illustrates how advancing understanding about disease pathogenesis can be of value for developing and optimizing therapeutic strategies. Collectively, this review illustrates the importance of continued work in the field of pediatric pharmacotherapy so that safe and effective therapies can be identified for all children.
Acknowledgments
We would like to acknowledge the following centers for providing information about the therapeutic regimens they use to treat children with plastic bronchitis: the University of California, San Francisco Benioff Children's Hospital (San Francisco, CA); the James Whitcomb Riley Hospital for Children (Indianapolis, IN); the Children's Hospital of Philadelphia (Philadelphia, PA); the Arnold Palmer Hospital for Children (Orlando, FL); Children's Hospital of Orange County (Orange, CA); the Monroe Carell Jr. Children's Hospital at Vanderbilt (Nashville, TN); and Texas Children's Hospital (Houston, TX).
This work was supported in part by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD; grant no. HD065594 [KAS]) and the University of Michigan's Clinical Translational Science Award (UL1RR024986). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the National Institutes of Health.
References
- 1.Roberts R, Rodriguez W, Murphy D, Crescenzi T. Pediatric drug labeling: improving the safety and efficacy of pediatric therapies. JAMA. 2003;7:905–11. doi: 10.1001/jama.290.7.905. [DOI] [PubMed] [Google Scholar]
- 2.Osuntokun B. Clinical trials in pediatrics: The drug delivery dimension. Adv Drug Deliv Rev. 2006;1:90–105. doi: 10.1016/j.addr.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Connor E, Cure P. “Creating hope” and other incentives for drug development for children. Sci Transl Med. 2011;66:66cm1. doi: 10.1126/scitranslmed.3001707. [DOI] [PubMed] [Google Scholar]
- 4.Budetti PP. Ensuring safe and effective medications for children. JAMA. 2003;7:950–1. doi: 10.1001/jama.290.7.950. [DOI] [PubMed] [Google Scholar]
- 5.Zurynski Y, Frith K, Leonard H, Elliott E. Rare childhood diseases: how should we respond? Arch Dis Child. 2008;12:1071–4. doi: 10.1136/adc.2007.134940. [DOI] [PubMed] [Google Scholar]
- 6.Thorat C, Xu K, Freeman SN, et al. What the Orphan Drug Act has done lately for children with rare diseases: a 10-year analysis. Pediatrics. 2012;3:516–21. doi: 10.1542/peds.2011-1798. [DOI] [PubMed] [Google Scholar]
- 7.Caruthers RL, Kempa M, Loo A, et al. Demographic Characteristics and Estimated Prevalence of Fontan-Associated Plastic Bronchitis. Pediatr Cardiol. 2012 doi: 10.1007/s00246-012-0430-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cajaiba MM, Borralho P, Reyes-Mugica M. The potentially lethal nature of bronchial casts: plastic bronchitis. Int J Surg Pathol. 2008;2:230–2. doi: 10.1177/1066896907307234. [DOI] [PubMed] [Google Scholar]
- 9.Brogan TV, Finn LS, Pyskaty DJ, Jr., et al. Plastic bronchitis in children: a case series and review of the medical literature. Pediatr Pulmonol. 2002;6:482–7. doi: 10.1002/ppul.10179. [DOI] [PubMed] [Google Scholar]
- 10.Heath L, Ling S, Racz J, et al. Prospective, longitudinal study of plastic bronchitis cast pathology and responsiveness to tissue plasminogen activator. Pediatr Cardiol. 2011;8:1182–9. doi: 10.1007/s00246-011-0058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Healy F, Hanna BD, Zinman R. Pulmonary complications of congenital heart disease. Paediatr Respir Rev. 2012;1:10–5. doi: 10.1016/j.prrv.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg DJ, Dodds K, Rychik J. Rare problems associated with the Fontan circulation. Cardiol Young. 2010:113–9. doi: 10.1017/S1047951110001162. [DOI] [PubMed] [Google Scholar]
- 13.Do P, Randhawa I, Chin T, Parsapour K, Nussbaum E. Successful Management of Plastic Bronchitis in a Child Post Fontan: Case Report and Literature Review. Lung. 2012;190:463–8. doi: 10.1007/s00408-012-9384-x. [DOI] [PubMed] [Google Scholar]
- 14.Wakeham MK, Van Bergen AH, Torero LE, Akhter J. Long-term treatment of plastic bronchitis with aerosolized tissue plasminogen activator in a Fontan patient. Pediatr Crit Care Med. 2005;1:76–8. doi: 10.1097/01.PCC.0000149320.06424.1D. [DOI] [PubMed] [Google Scholar]
- 15.Gibb E, Blount R, Lewis N, et al. Management of Plastic Bronchitis With Topical Tissue-type Plasminogen Activator. Pediatrics. 2012;2:e446–50. doi: 10.1542/peds.2011-2883. [DOI] [PubMed] [Google Scholar]
- 16.Madsen P, Shah SA, Rubin BK. Plastic bronchitis: new insights and a classification scheme. Paediatr Respir Rev. 2005;4:292–300. doi: 10.1016/j.prrv.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Racz J, Mane G, Ford M, et al. Immunophenotyping and protein profiling of Fontan-associated plastic bronchitis airway casts. Ann Am Thorac Soc. doi: 10.1513/AnnalsATS.201209-080OC. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costello JM, Steinhorn D, McColley S, Gerber ME, Kumar SP. Treatment of plastic bronchitis in a Fontan patient with tissue plasminogen activator: a case report and review of the literature. Pediatrics. 2002;4:e67. doi: 10.1542/peds.109.4.e67. [DOI] [PubMed] [Google Scholar]
- 19.Werkhaven J, Holinger LD. Bronchial casts in children. Ann Otol Rhinol Laryngol. 1987;96:86–92. doi: 10.1177/000348948709600121. [DOI] [PubMed] [Google Scholar]
- 20.Do TB, Chu JM, Berdjis F, Anas NG. Fontan patient with plastic bronchitis treated successfully using aerosolized tissue plasminogen activator: a case report and review of the literature. Pediatr Cardiol. 2009;3:352–5. doi: 10.1007/s00246-008-9312-2. [DOI] [PubMed] [Google Scholar]
- 21.Seear M, Hui H, Magee F, Bohn D, Cutz E. Bronchial casts in children: a proposed classification based on nine cases and a review of the literature. Am J Respir Crit Care Med. 1997;1:364–70. doi: 10.1164/ajrccm.155.1.9001337. [DOI] [PubMed] [Google Scholar]
- 22.Braggion C, Cappelletti LM, Cornacchia M, Zanolla L, Mastella G. Short-term effects of three chest physiotherapy regimens in patients hospitalized for pulmonary exacerbations of cystic fibrosis: a cross-over randomized study. Pediatr Pulmonol. 1995;1:16–22. doi: 10.1002/ppul.1950190104. [DOI] [PubMed] [Google Scholar]
- 23.Bellone A, Lascioli R, Raschi S, Guzzi L, Adone R. Chest physical therapy in patients with acute exacerbation of chronic bronchitis: effectiveness of three methods. Arch Phys Med Rehabil. 2000;5:558–60. doi: 10.1016/s0003-9993(00)90034-0. [DOI] [PubMed] [Google Scholar]
- 24.Lindberg S, Khan R, Runer T. The effects of formoterol, a long-acting beta 2-adrenoceptor agonist, on mucociliary activity. Eur J Pharmacol. 1995;3:275–80. doi: 10.1016/0014-2999(95)00416-i. [DOI] [PubMed] [Google Scholar]
- 25.van As A. The role of selective beta2-adrenoceptor stimulants in the control of ciliary activity. Respiration. 1974;2:146–51. doi: 10.1159/000193106. [DOI] [PubMed] [Google Scholar]
- 26.Eda R, Sugiyama H, Hopp RJ, Okada C, Bewtra AK, Townley RG. Inhibitory effects of formoterol on platelet-activating factor induced eosinophil chemotaxis and degranulation. Int Arch Allergy Immunol. 1993;4:391–8. doi: 10.1159/000236588. [DOI] [PubMed] [Google Scholar]
- 27.Black JL, Oliver BG, Roth M. Molecular mechanisms of combination therapy with inhaled corticosteroids and long-acting beta-agonists. Chest. 2009;4:1095–100. doi: 10.1378/chest.09-0354. [DOI] [PubMed] [Google Scholar]
- 28.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;23:2233–47. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donaldson SH, Bennett WD, Zeman KL, Knowles MR, Tarran R, Boucher RC. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med. 2006;3:241–50. doi: 10.1056/NEJMoa043891. [DOI] [PubMed] [Google Scholar]
- 30.Eschenbacher WL, Boushey HA, Sheppard D. Alteration in osmolarity of inhaled aerosols cause bronchoconstriction and cough, but absence of a permeant anion causes cough alone. Am Rev Respir Dis. 1984;2:211–5. [PubMed] [Google Scholar]
- 31.Lieberman J, Kurnick NB. Influence of deoxyribonucleic acid content on the proteolysis of sputum and pus. Nature. 1962:988–90. doi: 10.1038/196988a0. [DOI] [PubMed] [Google Scholar]
- 32.Nydam TL, Moore EE, McIntyre RC, Jr., et al. Hypertonic saline attenuates TNF-alpha-induced NF-kappaB activation in pulmonary epithelial cells. Shock. 2009;5:466–72. doi: 10.1097/SHK.0b013e31818ec47d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reeves EP, Williamson M, O'Neill SJ, Greally P, McElvaney NG. Nebulized hypertonic saline decreases IL-8 in sputum of patients with cystic fibrosis. Am J Respir Crit Care Med. 2011;11:1517–23. doi: 10.1164/rccm.201101-0072OC. [DOI] [PubMed] [Google Scholar]
- 34.Lee L, Kelher MR, Moore EE, Banerjee A, Silliman CC. Hypertonic saline inhibits arachidonic acid priming of the human neutrophil oxidase. J Surg Res. 2012;1:24–8. doi: 10.1016/j.jss.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elkins MR, Bye PT. Mechanisms and applications of hypertonic saline. J R Soc Med. 2011:S2–5. doi: 10.1258/jrsm.2011.s11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wasfi YS, Kemp JP, Villaran C, et al. Onset and duration of attenuation of exercise-induced bronchoconstriction in children by single-dose of montelukast. Allergy Asthma Proc. 2011;6:453–9. doi: 10.2500/aap.2011.32.3482. [DOI] [PubMed] [Google Scholar]
- 37.Lemanske RF, Jr., Mauger DT, Sorkness CA, et al. Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. N Engl J Med. 2010;11:975–85. doi: 10.1056/NEJMoa1001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peters-Golden M, Sampson AP. Cysteinyl leukotriene interactions with other mediators and with glucocorticosteroids during airway inflammation. J Allergy Clin Immunol. 2003;1(Suppl):S37–42. doi: 10.1067/mai.2003.23. discussion S43-8. [DOI] [PubMed] [Google Scholar]
- 39.Spinozzi F, Russano AM, Piattoni S, et al. Biological effects of montelukast, a cysteinyl-leukotriene receptor-antagonist, on T lymphocytes. Clin Exp Allergy. 2004;12:1876–82. doi: 10.1111/j.1365-2222.2004.02119.x. [DOI] [PubMed] [Google Scholar]
- 40.Pizzichini E, Leff JA, Reiss TF, et al. Montelukast reduces airway eosinophilic inflammation in asthma: a randomized, controlled trial. Eur Respir J. 1999;1:12–8. doi: 10.1034/j.1399-3003.1999.14a04.x. [DOI] [PubMed] [Google Scholar]
- 41.Reiss TF, Chervinsky P, Dockhorn RJ, Shingo S, Seidenberg B, Edwards TB. Montelukast, a once-daily leukotriene receptor antagonist, in the treatment of chronic asthma: a multicenter, randomized, double-blind trial. Montelukast Clinical Research Study Group. Arch Intern Med. 1998;11:1213–20. doi: 10.1001/archinte.158.11.1213. [DOI] [PubMed] [Google Scholar]
- 42.Jaffe A, Bush A. Anti-inflammatory effects of macrolides in lung disease. Pediatr Pulmonol. 2001;6:464–73. doi: 10.1002/ppul.1076. [DOI] [PubMed] [Google Scholar]
- 43.Shinkai M, Rubin BK. Macrolides and airway inflammation in children. Paediatr Respir Rev. 2005;3:227–35. doi: 10.1016/j.prrv.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 44.Kanoh S, Rubin BK. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev. 2010;3:590–615. doi: 10.1128/CMR.00078-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schultz KD, Oermann CM. Treatment of cast bronchitis with low-dose oral azithromycin. Pediatr Pulmonol. 2003;2:139–43. doi: 10.1002/ppul.10196. [DOI] [PubMed] [Google Scholar]
- 46.Koh MS, Tee A, Lasserson TJ, Irving LB. Inhaled corticosteroids compared to placebo for prevention of exercise induced bronchoconstriction. Cochrane Database Syst Rev. 2007;3:CD002739. doi: 10.1002/14651858.CD002739.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Franchimont D, Kino T, Galon J, Meduri GU, Chrousos G. Glucocorticoids and inflammation revisited: the state of the art. NIH clinical staff conference. Neuroimmunomodulation. 2002;5:247–60. doi: 10.1159/000069969. [DOI] [PubMed] [Google Scholar]
- 48.Essilfie-Quaye S, Ito K, Ito M, Kharitonov SA, Barnes PJ. Comparison of Symbicort(R) versus Pulmicort(R) on steroid pharmacodynamic markers in asthma patients. Respir Med. 2011;12:1784–9. doi: 10.1016/j.rmed.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 49.Pauwels RA, Lofdahl CG, Postma DS, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. N Engl J Med. 1997;20:1405–11. doi: 10.1056/NEJM199711133372001. [DOI] [PubMed] [Google Scholar]
- 50.Flaster H, Bernhagen J, Calandra T, Bucala R. The macrophage migration inhibitory factor-glucocorticoid dyad: regulation of inflammation and immunity. Mol Endocrinol. 2007;6:1267–80. doi: 10.1210/me.2007-0065. [DOI] [PubMed] [Google Scholar]
- 51.Scarfone RJ, Fuchs SM, Nager AL, Shane SA. Controlled trial of oral prednisone in the emergency department treatment of children with acute asthma. Pediatrics. 1993;4:513–8. [PubMed] [Google Scholar]
- 52.Chapman KR, Verbeek PR, White JG, Rebuck AS. Effect of a short course of prednisone in the prevention of early relapse after the emergency room treatment of acute asthma. N Engl J Med. 1991;12:788–94. doi: 10.1056/NEJM199103213241202. [DOI] [PubMed] [Google Scholar]
- 53.Onoue Y, Adachi Y, Ichida F, Miyawaki T. Effective use of corticosteroid in a child with life-threatening plastic bronchitis after Fontan operation. Pediatr Int. 2003;1:107–9. doi: 10.1046/j.1442-200x.2003.01659.x. [DOI] [PubMed] [Google Scholar]
- 54.Rogers DF, Barnes PJ. Treatment of airway mucus hypersecretion. Ann Med. 2006;2:116–25. doi: 10.1080/07853890600585795. [DOI] [PubMed] [Google Scholar]
- 55.Kamin W, Klar-Hlawatsch B, Truebel H. Easy removal of a large mucus plug with a flexible paediatric bronchoscope after administration of rhDNase (Pulmozyme) Klin Padiatr. 2006;2:88–91. doi: 10.1055/s-2005-836608. [DOI] [PubMed] [Google Scholar]
- 56.Manna SS, Shaw J, Tibby SM, Durward A. Treatment of plastic bronchitis in acute chest syndrome of sickle cell disease with intratracheal rhDNase. Arch Dis Child. 2003;7:626–7. doi: 10.1136/adc.88.7.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tzifa A, Robards M, Simpson JM. Plastic bronchitis; a serious complication of the Fontan operation. Int J Cardiol. 2005;3:513–4. doi: 10.1016/j.ijcard.2004.03.085. [DOI] [PubMed] [Google Scholar]
- 58.Akong-Moore K, Chow OA, von Kockritz-Blickwede M, Nizet V. Influences of chloride and hypochlorite on neutrophil extracellular trap formation. PLoS One. 2012;8:e42984. doi: 10.1371/journal.pone.0042984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones AP, Wallis C. Dornase alfa for cystic fibrosis. Cochrane Database Syst Rev. 2010;3:CD001127. doi: 10.1002/14651858.CD001127.pub2. [DOI] [PubMed] [Google Scholar]
- 60.Hirsh J, Anand SS, Halperin JL, Fuster V. Mechanism of action and pharmacology of unfractionated heparin. Arterioscler Thromb Vasc Biol. 2001;7:1094–6. doi: 10.1161/hq0701.093686. [DOI] [PubMed] [Google Scholar]
- 61.Tuinman PR, Dixon B, Levi M, Juffermans NP, Schultz MJ. Nebulized anticoagulants for acute lung injury - a systematic review of preclinical and clinical investigations. Crit Care. 2012;2:R70. doi: 10.1186/cc11325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Glas GJ, van der Sluijs KF, Schultz MJ, Hofstra JJ, van der Poll T, Levi M. Bronchoalveolar hemostasis in lung injury and acute respiratory distress syndrome. J Thromb Haemost. 2013;11:17–25. doi: 10.1111/jth.12047. [DOI] [PubMed] [Google Scholar]
- 63.de Boer JD, Majoor CJ, van 't Veer C, Bel EH, van der Poll T. Asthma and coagulation. Blood. 2012;14:3236–44. doi: 10.1182/blood-2011-11-391532. [DOI] [PubMed] [Google Scholar]
- 64.Desai MH, Mlcak R, Richardson J, Nichols R, Herndon DN. Reduction in mortality in pediatric patients with inhalation injury with aerosolized heparin/N-acetylcystine [correction of acetylcystine] therapy. J Burn Care Rehabil. 1998;3:210–2. doi: 10.1097/00004630-199805000-00004. [DOI] [PubMed] [Google Scholar]
- 65.Markart P, Nass R, Ruppert C, et al. Safety and tolerability of inhaled heparin in idiopathic pulmonary fibrosis. J Aerosol Med Pulm Drug Deliv. 2010;3:161–72. doi: 10.1089/jamp.2009.0780. [DOI] [PubMed] [Google Scholar]
- 66.Walker PA, Shah SK, Letourneau PA, Allison ND, Cox CS. Treatment of Plastic Bronchitis Using Serial Flexible Bronchoscopy and Aerosolized Heparin Therapy. Eur J Pediatr Surg. 2012 Jul 10; doi: 10.1055/s-0032-1315803. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 67.Eason DE, Cox K, Moskowitz WB. Aerosolised heparin in the treatment of Fontan-related plastic bronchitis. Cardiol Young. 2013 Jan 18; doi: 10.1017/S1047951112002089. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 68.Stiller B, Riedel F, Paul K, van Landeghem FK. Plastic bronchitis in children with Fontan palliation: analogue to protein losing enteropathy? Pediatr Cardiol. 2002;1:90–4. doi: 10.1007/s00246-001-0024-0. [DOI] [PubMed] [Google Scholar]
- 69.Quasney MW, Orman K, Thompson J, et al. Plastic bronchitis occurring late after the Fontan procedure: treatment with aerosolized urokinase. Crit Care Med. 2000;6:2107–11. doi: 10.1097/00003246-200006000-00074. [DOI] [PubMed] [Google Scholar]
- 70.Collen D, Lijnen HR, Todd PA, Goa KL. Tissue-type Plasminogen Activator - A Review of its Pharmacology and Therapeutic Use as a Thrombolytic Agent. Drugs. 1989;3:346–88. doi: 10.2165/00003495-198938030-00003. [DOI] [PubMed] [Google Scholar]
- 71.Lackowski NP, Pitzer JE, Tobias M, et al. Safety of prolonged, repeated administration of a pulmonary formulation of tissue plasminogen activator in mice. Pulmonary pharmacology & therapeutics. 2010;2:107–14. doi: 10.1016/j.pupt.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stringer KA, Tobias M, Dunn JS, et al. Accelerated dosing frequency of a pulmonary formulation of tissue plasminogen activator is well-tolerated in mice. Clinical and experimental pharmacology & physiology. 2008;12:1454–60. doi: 10.1111/j.1440-1681.2008.05011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meister FL, Stringer KA, Spinler SA, et al. Thrombolytic therapy for acute myocardial infarction. Pharmacotherapy. 1998;4:686–98. [PubMed] [Google Scholar]
- 74.Hess DC, Fagan SC. Repurposing an old drug to improve the use and safety of tissue plasminogen activator for acute ischemic stroke: minocycline. Pharmacotherapy. 2010;7(Pt 2):55S–61S. doi: 10.1592/phco.30.pt2.55S. [DOI] [PMC free article] [PubMed] [Google Scholar]