Abstract
Purpose
Resistance to cetuximab, a monoclonal antibody against the epithelial growth factor receptor (EGFR), in colorectal cancer (CRC) may result from compensatory signaling through ErbB receptors, ErbB2/neu/HER2 (HER2) and ErbB3/HER3 (HER3). Pertuzumab is a monoclonal antibody that blocks HER2 hetero-dimerization; thus the combination of pertuzumab and cetuximab could possibly overcome cetuximab resistance.
Patients and methods
This single-arm, open-label, multicenter phase I/II study was designed to assess the safety and efficacy of pertuzumab and cetuximab in patients with cetuximab-resistant KRAS wild type metastatic CRC. Thirteen patients were enrolled and received cetuximab in combination with pertuzumab at several dose levels in a 3+3 design. Patients were assessed for dose-limiting toxicity (DLT) during the first cycle. A phase II portion was planned, but not initiated due to toxicity.
Results
Six of the thirteen patients (46%) experienced DLTs, therefore the study was terminated early. Grade 3 or higher DLTs included dermatitis with desquamation and/or acneiform rash (n=6), mucositis or stomatitis (n=5), and diarrhea (n=2). There was one Grade 5 event (myocardial infarction) attributed to underlying disease. Among the 13 patients, seven (54%) were evaluable for response. The objective response rate was 14%: one patient had a partial response lasting 6 months. Two patients had stable disease (29%), and four had progressive disease (57%). Median progression free survival was 2.1 months (95% CI, 1.5–4.9) and median overall survival was 3.7 months (95% CI, 1.6–7.9).
Conclusion
Combination pertuzumab and cetuximab in refractory CRC was associated with potential antitumor activity; however, the combination was not tolerable due to overlapping toxicities.
Keywords: Pertuzumab, Cetuximab, Phase I, Phase II, Colorectal Cancer
Introduction
The use of cetuximab, a chimeric monoclonal antibody against the epidermal growth factor receptor (EGFR), in KRAS wild type, metastatic colorectal adenocarcinoma has expanded from single-agent use for chemorefractory disease to combination therapy in the first-, second- and third-line settings [1–5]. Initial investigation of EGFR inhibitors in unselected colorectal cancer patients showed them to be ineffective in a majority of patients. Investigation of this primary resistance to EGFR inhibition demonstrated that a subset of non-responders possessed activating mutations in the KRAS oncogene allowing EGFR-independent activation of mitogenic signaling pathways[4,6,7]. While application of EGFR inhibition only to patients with KRAS-wild type tumors has improved response rates, there remains a significant population of patients who fail to respond to anti-EGFR therapy. One subset (~5–10%) may reflect patients with the activating BRAF mutation V600E[8], but additional mechanisms of primary resistance account for the low observed response rates. Further, in patients that initially benefit from cetuximab, resistance emerges within months and few effective treatment options remain for refractory patients. One proposed hypothesis for both primary and acquired cetuximab resistance invokes activation of parallel signaling pathways mediated by the alternate ErbB family members, HER2, HER3, and HER4[9]. For example, HER2 upregulation and activity has been consistently demonstrated in a subset of cetuximab-resistant cell lines[10–12], providing a potential target for overcoming cetuximab resistance.
Pertuzumab is a monoclonal antibody that disrupts the dimerization domain of HER2 and prevents formation and signaling through HER2 homodimers and HER2/EGFR, HER2/HER3, and HER2/HER4 heterodimers. Unlike trastuzumab in HER2-positive breast cancer, pertuzumab activity does not require HER2 overexpression[13]. It has previously been studied as monotherapy[14] and combination therapy[15] in ovarian cancer. Most recently, in the first-line treatment of HER2-positive breast cancer, the addition of pertuzumab to trastuzumab and docetaxel was associated with a significant improvement in patient outcome. [16]. This additive benefit likely reflects the different binding site and mechanism of action of pertuzumab compared to trastuzumab.
Although a study of pertuzumab in colorectal cancer cell lines and xenografts failed to demonstrate activity as a single-agent or in combination with irinotecan, some antitumor activity was seen when pertuzumab was given in combination with the small-molecule EGFR inhibitor, erlotinib[17]. The combination of pertuzumab with erlotinib was subsequently explored in a phase Ib study of advanced non-small cell lung cancer which showed that the combination was well tolerated and demonstrated early signs of clinical efficacy[18]. Another study explored the combination of erlotinib and cetuximab for combined targeting of the EGFR receptor in colorectal cancer and showed some additive benefit[19]. However, no study has attempted to simultaneously target multiple ErbB receptors in colorectal cancer. We therefore conducted a phase I/II study to evaluate the tolerability and efficacy of the combination of pertuzumab and cetuximab in cetuximab-resistant, KRAS wild type, metastatic colorectal cancer patients.
Patients and Methods
Study design and treatment
We conducted a single-arm, open-label, multicenter phase I/II study of pertuzumab in combination with cetuximab under a Phase 2 Consortium contract with the Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute (NCI). The phase I portion utilized a 3+3 dose escalation design, and was originally written to evaluate two dose levels of pertuzumab in combination with standard doses of cetuximab and irinotecan in patients with mCRC previously treated with an oxaliplatin-based regimen, but who were cetuximab- and irinotecan-naïve. Between November 26, 2007 and May 7, 2008, three patients were enrolled to Dose Level 1 (pertuzumab 420 mg IV loading dose on Cycle 1 Day 1, then 210 mg IV maintenance dose every 21 days thereafter; plus cetuximab 400 mg/m2 IV loading dose on Cycle 1 Day 2, then 250 mg/m2 IV maintenance dose weekly, with irinotecan 350 mg/m2 IV every 21 days). One subject developed grade 3 diarrhea requiring hospitalization, and skin toxicity was seen among the three subjects. Due to these adverse events and difficulty with patient accrual, the study was amended by the investigators, in conjunction with CTEP, in November 2008 to test the combination of pertuzumab and cetuximab in cetuximab-refractory mCRC patients. The loading dose of cetuximab was eliminated to help ameliorate skin toxicity, and patients whose tumors harbored KRAS mutations were excluded. Herein we report the results of the subjects enrolled on this amended portion of the trial.
Each treatment cycle of pertuzumab and cetuximab was 21 days in duration. All patients received cetuximab at the maintenance dose of 250 mg/m2 IV, without a loading dose, every 7 days. For pertuzumab, patients enrolled on Dose Level 1 received a loading dose of 420 mg IV on Cycle 1 Day 1, followed by a maintenance dose of 210 mg on Day 1 of each subsequent cycle. Patients enrolled on Dose Level 1a received pertuzumab 210 mg IV on Day 1 of every cycle, whereas those on Dose Level −1 received 175 IV on Day 1 of every cycle, both without loading doses. A Dose Level 2 consisting of a load of pertuzumab 840 mg IV on Cycle 1 Day 1, followed by a maintenance dose of 420 mg IV, was planned but did not accrue subjects. Patients were treated until disease progression, intolerability, or withdrawal of consent.
A phase II portion was planned but also did not accrue subjects due to discontinuation of the study in the phase I portion for toxicity.
Eligibility
Eligible subjects were at least 18 years of age with histologically-confirmed adenocarcinoma of the colon or rectum who had failed at least one prior line of therapy for metastatic disease. All subjects must have previously received 5-fluorouracil (5-FU) or capecitabine, irinotecan or oxaliplatin, and cetuximab, and must have demonstrated radiographic progression of disease on a cetuximab-containing regimen. Eastern Cooperative Oncology Group (ECOG) performance status had to be 0–1, with adequate bone marrow and organ function and measurable disease per Response Evaluation Criteria in Solid Tumors, version 1.0 (RECIST)[20].
Exclusion criteria included subjects whose tumors harbored KRAS mutations; subjects who were pregnant or breastfeeding; brain metastases; or presence of serious medical conditions that would impose excessive risk to the patient. Because pertuzumab led to mild decreases in left ventricular ejection fraction (LVEF) in early-phase trials[21], subjects with LVEF <50%, wall motion abnormalities, or a history of congestive heart failure were also excluded. All subjects provided written informed consent, and the study was approved by the Institutional Review Boards of Dana-Farber/Harvard Cancer Center and all participating institutions.
Safety and Response Evaluation
Patients were seen at least once every 21 days throughout the study for safety assessment, physical examination, and laboratory studies. Echocardiograms or Multi Gated Acquisition Scans (MUGAs) were obtained at baseline and after every three cycles. Dose limiting toxicity (DLT) was graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. DLTs were assessed during the first cycle of treatment; subjects were replaced if they were not monitored for a minimum duration of one cycle. DLTs were defined as: a) any grade 4 neutropenia lasting for more than 7 days, or any grade 4 neutropenia with fever, b) any grade 4 thrombocytopenia, c) any grade 3 or higher non-hematologic toxicity that was possibly related to study treatment (except nausea or vomiting, skin toxicity, alopecia, and treatment-related allergic reaction/hypersensitivity), d) grade 4 rash/desquamation despite aggressive skin toxicity management, e) grade 3 diarrhea requiring hospitalization or lasting more than 24 hours despite aggressive anti-diarrheal therapy, f) grade 4 diarrhea despite aggressive anti-diarrheal therapy, g) grade 4 vomiting despite optimal anti-emetic therapy, or h) grade 3 or higher left ventricular dysfunction (i.e., symptomatic congestive heart failure). Toxicity-related treatment delays lasting more than three weeks, or requirement of more than two dose reductions of cetuximab due to treatment-related toxicity, were also considered DLTs.
Diagnostic imaging with computed tomography (CT) or magnetic resonance imaging (MRI) scans and tumor marker assessment with measurement of carcinoembryonic antigen (CEA) were performed after every two cycles to evaluate treatment response using RECIST guidelines[22]. In addition, positron emission tomography (PET)/CT scans were obtained at baseline and after 2 cycles for exploratory studies, and were reviewed and interpreted by the Dana-Farber/Harvard Cancer Center Tumor Imaging Metrics Core.
Statistical Analysis
The primary end point of the phase I study was the identification of the recommended phase II dose (RP2D) of the combination of pertuzumab and cetuximab, defined as the dose level at which no more than 1 out of 6 patients experienced a DLT. The primary end point of the phase II portion was objective tumor response rate (RR), defined as the proportion of patients with a best overall response of complete response (CR) or partial response (PR) according to RECIST. Secondary end points included PFS, defined as the duration of time from the start of treatment to objective disease progression or death, and OS, defined as the duration of time from the start of treatment to death. Subjects were considered evaluable for toxicity if they received at least one dose of study treatment, and evaluable for response if they received at least one dose of study treatment and had their disease re-evaluated with scans. Patients were followed for outcome and survival through May 31st, 2012, when the database was locked. Descriptive statistics were used to summarize patient characteristics, treatment received, and adverse events. PFS and OS curves were determined with the Kaplan Meier method, and median PFS and OS were calculated with 95% confidence intervals (CI). Statistical analyses were conducted using SAS software version 9.2 (SAS Institute Inc, Cary, NC).
Based on the dose escalation schema of the phase I study, there was a 91% probability of escalating given a 10% true DLT rate, 70% probability with a 20% rate, 17% probability with a 50% rate, and an 8% probability with a 60% rate.
Results
Patient Characteristics and Disposition
Between March 2009 to July 2010, 14 patients were enrolled on the phase I portion of the study and 13 received ≥1 dose of antibody therapy. One patient withdrew consent prior to receiving any study drug, and was excluded from safety and efficacy analyses. Table 1 shows the baseline demographics. Fifty-seven per cent of patients had received 4 or more prior lines of therapy), and the majority of patients (64%) had metastases to two or more organ sites. Among the 13 patients who received treatment, the median number of cycles received was 2 (range 1–8). Five patients discontinued treatment for radiographic disease progression per RECIST, two discontinued for symptomatic or clinical progression, and four terminated study therapy for unacceptable toxicity. One patient was taken off study at the treating physician’s discretion, and one patient withdrew consent.
Table I.
Baseline patient and disease characteristics
| Median age (range), years | 60 (46–73) |
| Sex, no. (%) | |
| Male | 6 (42.8) |
| Female | 8 (57.2) |
| Race | |
| White | 12 (85.7) |
| Black | 1 (7.1) |
| Other | 1 (7.1) |
| ECOG performance status, no. (%) | |
| 0 | 5 (35.7) |
| 1 | 9 (64.3) |
| Histologic grade, no. (%) | |
| Well differentiated | 1 (7.1) |
| Moderately differentiated | 10 (71.4) |
| Poorly differentiated | 2 (14.3) |
| Unknown | 1 (7.1) |
| Sites of metastatic disease, no. (%) | |
| Liver only | 2 (14.3) |
| Liver plus other sites | 9 (64.3) |
| Non-liver only | 3 (21.4) |
| Other | 0 |
| Previous radiation treatment, no. (%) | 2 (14.3) |
| Number of prior chemotherapy regimens, no. (%) | |
| 1 | 0 |
| 2 | 1 (7.1) |
| 3 | 5 (35.7) |
| 4+ | 8 (57.2) |
| Median baseline CEA (range), ng/mL | 42.2 (2.0–3,743.3) |
| Median baseline left ventricular ejection fraction (range), % | 67 (57–73) |
Safety
Unacceptable toxicity was observed at each dose level tested. Initially three patients were enrolled on Dose Level 1, and one experienced a DLT (grade 3 mucositis) after the first cycle. This cohort was then expanded, and an additional two subjects enrolled also experienced DLTs of grade 3 mucositis. On this basis, the trial was amended in September 2009 to eliminate the loading dose of pertuzumab (Dose Level 1a: pertuzumab 210 mg IV every 21 days; cetuximab 250mg/m2 IV weekly) and to add a lower dose of pertuzumab for evaluation if necessary (Dose Level −1: pertuzumab 175 mg IV every 21 days; cetuximab 250 mg/m2 IV weekly). Two patients were enrolled onto Dose Level 1a and both experienced DLTs: one patient developed grade 3 diarrhea requiring hospitalization while the second experienced grade 3 mucositis. Accrual was then opened to Dose Level −1 with three subjects enrolled. One of the three experienced a DLT (grade 3 mucositis), therefore the cohort was expanded with an additional three patients enrolled. Of these additional three patients, an additional patient experienced a DLT (grade 3 mucositis). At that point, on the basis of the pre-defined protocol criteria and the high unacceptable toxicity rate, in conjunction with the National Cancer Institute, the trial was permanently closed to accrual.
Table 2 summarizes all adverse events experienced at any time during study treatment regardless of attribution. The primary Grade 3 or higher toxicities related to this regimen were rash/desquamation (46% of patients), mucositis or stomatitis (38%), and diarrhea (15%). One patient experienced a Grade 5 event, suffering a fatal myocardial infarction 27 days following his last treatment. The patient had previously come off study after two cycles due to unacceptable toxicity with Grade 3 mucositis. The cardiac event occurred in the setting of clinical deterioration and was not felt to be related to study treatment. Four patients underwent reassessment of LVEF. Three patients had an increase in LVEF (61% to 65%, 64% to 67%, and 65% to 72%) and one patient had a decrease in LVEF (70% to 65%).
Table 2.
Adverse events, by worst grade per patient (n=13)
| TOXICITY | GRADE 1/2 (%) | GRADE 3/4 (%) | GRADE 5 (%) | TOTAL (%) |
|---|---|---|---|---|
|
| ||||
| Cardiovascular | ||||
| Cardiac ischemia | 0 | 0 | 1 (8) | 1 (8) |
| Troponin elevation | 0 | 1 (8) | 0 | 1 (8) |
|
| ||||
| Constitutional | ||||
| Fatigue | 11 (85) | 0 | 0 | 11 (85) |
| Fever without neutropenia | 2 (15) | 0 | 0 | 2 (15) |
| Insomnia | 2 (15) | 1 (8) | 0 | 3 (23) |
| Rigors/chills | 3 (23) | 0 | 0 | 3 (23) |
| Weight loss | 1 (8) | 0 | 0 | 1 (8) |
| Weakness (non-neuropathic) | 1 (8) | 0 | 0 | 1 (8) |
|
| ||||
| Dermatologic | ||||
| Acneiform rash | 3 (23) | 1 (8) | 0 | 4 (31) |
| Dry skin | 1 (8) | 1 (8) | 0 | 2 (15) |
| Edema of head and neck | 2 (15) | 0 | 0 | 2 (15) |
| Edema of limb | 3 (23) | 0 | 0 | 3 (23) |
| Edema trunk/genital | 1 (8) | 0 | 0 | 1 (8) |
| Erythema multiforme | 2 (15) | 0 | 0 | 2 (15) |
| Lymphedema | 1 (8) | 0 | 0 | 1 (8) |
| Nail changes | 3 (23) | 0 | 0 | 3 (23) |
| Other | 1 (8) | 0 | 0 | 1 (8) |
| Pruritis | 5 (38) | 0 | 0 | 5 (38) |
| Rash/desquamation | 4 (31) | 6 (46) | 0 | 10 (77) |
| Skin pain | 0 | 3 (23) | 0 | 3 (23) |
| Skin (other) | 1 (8) | 0 | 0 | 1 (8) |
|
| ||||
| Gastrointestinal | ||||
| Abdominal pain | 5 (38) | 0 | 0 | 5 (38) |
| Anorexia | 5 (38) | 0 | 0 | 5 (38) |
| Small bowel obstruction | 0 | 1 (8) | 0 | 1 (8) |
| Constipation | 1 (8) | 0 | 0 | 1 (8) |
| Dehydration | 1 (8) | 0 | 0 | 1 (8) |
| Diarrhea | 6 (46) | 2 (15) | 0 | 8 (61) |
| Distension/bloating | 1 (8) | 0 | 0 | 1 (8) |
| Dyspepsia | 1 (8) | 0 | 0 | 1 (8) |
| Esophagitis | 1 (8) | 0 | 0 | 1 (8) |
| Mucositis or stomatitis | 3 (23) | 5 (38) | 0 | 8 (61) |
| Nausea | 6 (46) | 0 | 0 | 6 (46) |
| Vomiting | 3 (23) | 0 | 0 | 3 (23) |
|
| ||||
| Genitourinary | ||||
| Incontinence – urinary | 1 (8) | 0 | 0 | 1 (8) |
| Proteinuria | 1 (8) | 0 | 0 | 1 (8) |
| Vaginal discharge | 1 (8) | 0 | 0 | 1 (8) |
|
| ||||
| Hematologic | ||||
| Anemia | 9 (69) | 0 | 0 | 9 (69) |
| Leukopenia | 1 (8) | 0 | 0 | 1 (8) |
| Lymphopenia | 1 (8) | 0 | 0 | 1 (8) |
| Thrombocytopenia | 1 (8) | 0 | 0 | 1 (8) |
|
| ||||
| Infection | ||||
| Urinary tract | 1 (8) | 0 | 0 | 1 (8) |
| Colitis – infectious | 1 (8) | 0 | 0 | 1 (8) |
|
| ||||
| Metabolic/Laboratory | ||||
| ALT elevation | 2 (15) | 0 | 0 | 2 (15) |
| AST elevation | 5 (38) | 1 (8) | 0 | 6 (46) |
| Alkaline phosphatase elevation | 6 (46) | 0 | 0 | 6 (46) |
| Hepatic (other) | 1 (8) | 0 | 0 | 1 (8) |
| Hyperbilirubinemia | 1(8) | 1 (8) | 0 | 2 (15) |
| Hyperglycemia | 5 (38) | 1 (8) | 0 | 6 (46) |
| Hyperkalemia | 1 (8) | 0 | 0 | 1 (8) |
| Hypoalbuminemia | 5 (38) | 1 (8) | 0 | 6 (46) |
| Hypocalcemia | 2 (15) | 0 | 0 | 2 (15) |
| Hypokalemia | 2 (15) | 1 (8) | 0 | 3 (23) |
| Hypomagnesemia | 5 (38) | 0 | 0 | 5 (38) |
| Hyponatremia | 2 (15) | 0 | 0 | 2 (15) |
| Hypophosphatemia | 4 (30) | 1 (8) | 0 | 5 (38) |
| INR elevation | 1 (8) | 0 | 0 | 1 (8) |
| PTT prolongation | 1 (8) | 0 | 0 | 1 (8) |
| Metabolic/lab (other) | 1 (8) | 0 | 0 | 1 (8) |
|
| ||||
| Musculoskeletal/Connective | ||||
| Back pain | 1 (8) | 0 | 0 | 1(8) |
|
| ||||
| Neurologic | ||||
| Neuropathy (sensory) | 2 (15) | 0 | 0 | 2 (15) |
|
| ||||
| Ocular | ||||
| Eyelid dysfunction | 1 (8) | 0 | 0 | 1 (8) |
| Blurry vision | 1 (8) | 0 | 0 | 1 (8) |
| Ocular (other) | 1 (8) | 0 | 0 | 1 (8) |
|
| ||||
| Other | ||||
| Epistaxis | 3 (23) | 0 | 0 | 3 (23) |
| Voice changes/ dysarthria | 1 (8) | 0 | 0 | 1 (8) |
|
| ||||
| Psychiatric | ||||
| Depression | 1 (8) | 0 | 0 | 1 (8) |
|
| ||||
| Respiratory | ||||
| Dyspnea | 1 (8) | 1 (8) | 0 | 2 (15) |
All patients required dose delays for toxicity (most commonly rash). Across all dose levels, all patients experienced grade ≥ 1 rash following their first cycle. In addition to the typical acneiform rash seen with EGFR inhibitors (Figure 1b), 10 of 13 patients also exhibited a desquamating or dermatitic rash (Grade 3 or higher in six patients) (see Figure 1a, c). The rash had a lichenoid appearance with a reddish-purple erythema and scaling and crusting of the skin.
Figure 1.
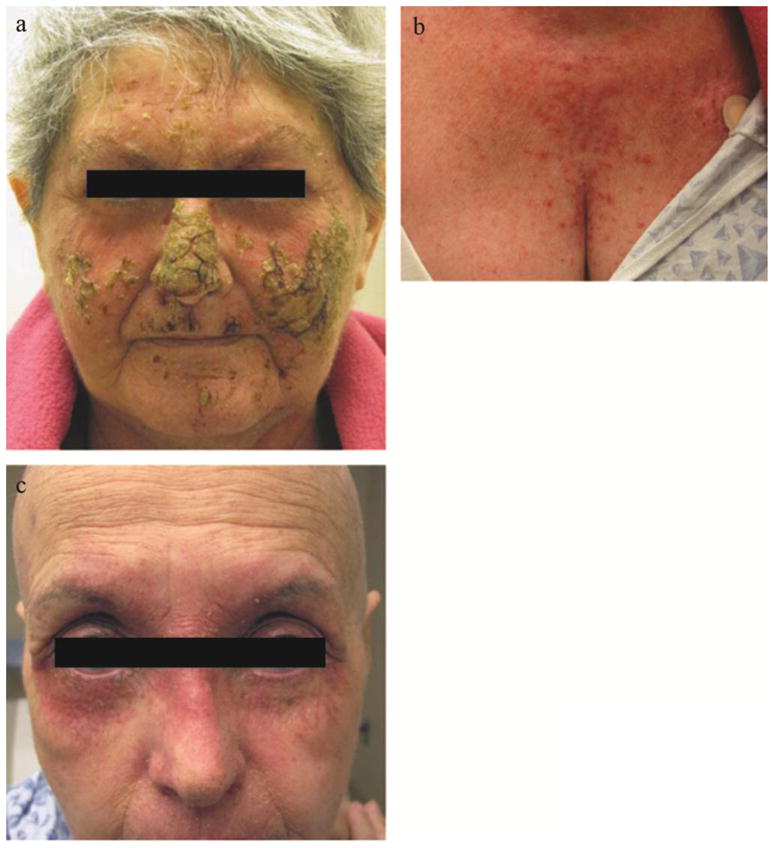
Dermatologic toxicity
Dematologic toxicity as observed in two patients on combination cetuximab and pertuzumab. a. Dermatitic and desquamating rash with underlying erythema. b. More typical actinic rash affecting the chest of the same patient as 1a. c. A second patient with a dermatitic reaction predominantly affecting the periocular skin with a reddish-purple erythema.
Efficacy
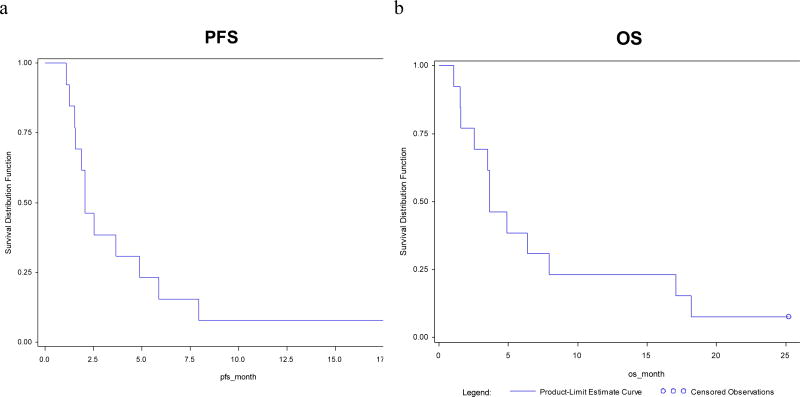
Seven of the 13 treated patients were evaluable for response; six patients were not rescanned due to study discontinuation for toxicity. One patient (14%) had a confirmed partial response after 40 days and did not experience disease progression until after 182 days (approximately 6 months) of treatment. This patient is still alive as of May 30, 3012 and continues to be followed on study for survival. Two patients (29%) had a best overall response of stable disease and four patients (57%) had progressive disease. The median PFS was 2.1 (95% CI, 1.5–4.9) months and median OS was 3.7 (95% CI, 1.6–7.9) months (Figure 2).
Figure 2.
PFS and OS in patients treated with cetuximab and pertuzumab
Survival curves showing progression free survival (a) and overall survival (b) among the seven patients evaluable for response.
The median plasma CEA at study entry was 42.2ng/mL (range 2.0 – 3,743.3). Of the ten patients who had at least one follow-up CEA obtained following initiation of therapy, four patients (40%) experienced a >50% reduction in plasma CEA, including the patient who experienced a radiologic partial response.
An exploratory analysis attempted to correlate PET/CT response after 2 cycles per European Organization for Research and Treatment of Cancer (EORTC) criteria[23] with CT scan findings and clinical outcomes. Five patients underwent a PET/CT following two cycles, with one patient (20%) showing a partial response and 4 patients (80%) with stable disease. No correlation with CT or clinical outcome could be assessed due to the small number of evaluable patients.
Discussion
This study sought to assess the safety and efficacy of the combination of pertuzumab and cetuximab in patients with cetuximab-resistant, metastatic colorectal cancer. Despite multiple dose modifications, the combination of cetuximab and pertuzumab could not be tolerated. Patients experienced significant mucositis and an unanticipated desquamating dermatitis distinct from the typical acneiform rash observed with either drug alone. Neither toxicity could be ameliorated by dose reduction, and this ultimately led to the discontinuation of the trial. Among the limited number of evaluable patients there was a suggestion of efficacy with both a radiologic partial response in one patient and a biochemical response in four patients – all among patients who were documented to be cetuximab-resistant.
While evidence suggests that activation of the RAS/BRAF cascade may be one mechanism for cetuximab resistance[9,24], a substantial subset of colorectal tumors employ alternative mechanisms. The hypothesis that underlies this study is that cetuximab resistance may occur through the activation of alternate ErbB receptors. Recent efforts have highlighted the role of HER2 signaling as a targetable cetuximab resistance pathway[10,25]. Lapatinib, a small molecule inhibitor of EGFR and HER2, was evaluated as monotherapy in chemo-refractory colorectal cancer[26,27]. This therapy was well-tolerated but had limited efficacy, possibly reflecting either the absence of concurrent cytotoxic chemotherapy and/or insufficient inhibition of the ErbB pathway. The use of trastuzumab to inhibit HER2 in colorectal cancer was explored in a Phase II study in which enrollment was limited to patients with HER2 overexpression. The low rate of HER2 overexpression in colorectal cancer (observed in 8% of tumors in that trial) [10] resulted in poor enrollment although partial responses were observed in 5 of 7 evaluable patients[28]. Similarly, a Cancer and Leukemia Group B (CALGB) phase II trial of trastuzumab in combination with 5-fluoruracil, leucovorin and oxaliplatin in refractory patients showed some efficacy but again was limited by poor enrollment due to the low rate of HER2-overexpressing colorectal tumors[29].
By interfering with HER2 homo- and heterodimerization independent of HER2 DNA amplification, pertuzumab may be better suited than trastuzumab for those tumors utilizing HER2 to evade cetuximab in colorectal cancer. Pertuzumab as a single-agent demonstrated minimal activity in preclinical models of colorectal cancer[17]. Thus, the addition of pertuzumab to cetuximab in cetuximab-refractory colorectal cancer directly tests the hypothesis that signaling through alternative ErbB receptors provides a mechanism for cetuximab resistance. Several drugs currently in early-phase studies in solid malignancies are also attempting to completely inhibit the ErbB pathway by simultaneously targeting multiple ErbB receptors[18,30]. These efforts have suggested that concurrent inhibition of ErbB receptors could be tolerated with acceptable side effects and that some combinations may have efficacy in refractory colorectal cancers.
In the current trial, the combination of cetuximab and pertuzumab caused skin toxicity in every patient, including an acneiform and exfoliative dermatitic rash. The dermatitic rash, not previously reported with either drug, demonstrated significant improvement within one week of holding the study drugs and initiating highly-potent topical corticosteroids. This contrasts with the characteristic acneiform rash shared among all agents targeting ErbB receptors - occurring in 88.2% of patients receiving cetuximab[31] and 24.6% of patients receiving pertuzumab [32] – and is believed to reflect ErbB receptor inhibition within the skin. In this trial 77% of patients had evidence of exfoliation or rash with desquamation. Among cetuximab-treated colorectal cancer patients, the characteristic acneiform rash has been associated with a greater likelihood of anti-tumor activity [33]. In the current trial, the limited number of evaluable subjects prevented an assessment of whether the presence or severity of rash was predictive of tumor response.
ErbB receptors are present within multiple layers of the epidermis and dermis, with EGFR being the primary ErbB receptor within keratinocytes and HER2 predominantly expressed within the proliferating basal cells of the dermis[32,34]. HER2 can also be found in the keratinocyte layer and HER2/EGFR heterodimerization has been proposed to contribute to signaling in this layer[32,35]. The unique dermatitic rash observed in our trial may reflect both the increased toxicity to the superficial keratinocyte layer due to inhibition of EGFR and EGFR/HER2 heterodimers as well as impaired repair due to inhibition of HER2 within the proliferating basal cell layer. Recent trials targeting multiple ErbB receptors with small molecules or other drug combinations have not recapitulated this phenotype[18,30], suggesting that the observed dermatitis may be unique to this drug combination and not a class effect. Further studies with ErbB-targeting agents will be needed to clarify the tolerability of broad ErbB receptor inhibition.
A tolerable dose of cetuximab and pertuzumab was unable to be identified despite the elimination of the loading dose and reductions in pertuzumab dose. Assessment of efficacy in this study was impeded by drug toxicity resulting in dose delays, patient withdrawal, and early termination of the trial. Within the handful of patients evaluable for response there was some evidence of biochemical and radiologic efficacy, including one patient who maintained a partial response for 6 months. This efficacy signal suggests that broad inhibition of ErbB receptors may be a therapeutic strategy worth pursing in colorectal cancer. Evaluation of alternative agents directed at ErbB receptors may identify a subset of cetuximab-resistant colorectal tumors that employ HER2-dependent mechanisms to overcome EGFR inhibition by cetuximab. Given the number of proposed mechanisms for cetuximab resistance it may prove useful to identify biomarkers that are instructive as to the mode of cetuximab resistance employed in a given tumor. Re-biopsy of metastatic tumors or collection of circulating tumor cells to re-assay for KRAS mutation would identify the subset of tumors that would be unlikely to respond to ErbB inhibition. Immunohistochemical assessment of ErbB receptor expression and phosphorylation status could identify those tumors employing a HER2-based mechanism for cetuximab resistance that would be amenable to therapy.
In conclusion, the combination of cetuximab and pertuzumab in cetuximab-refractory metastatic colorectal cancer was associated with some antitumor activity. However, the combination was intolerable due to overlapping toxicities of diarrhea, rash and mucositis. Additionally, a unique rash with desquamation was seen in most patients across all dose levels. Neither the elimination of the loading dose of pertuzumab, dose reductions of pertuzumab and cetuximab, nor dose delays of both drugs were sufficient to reduce toxicity to acceptable levels. Consequently, pertuzumab and cetuximab cannot be safely administered together and will not be studied further in metastatic colorectal cancer.
Acknowledgments
Pertuzumab was supplied by the Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute (NCI) in Bethesda, MD. The trial was conducted under a Phase 2 Consortium contract with the NCI. Genentech also provided supplementary funding for the study.
Footnotes
Conflicts of Interest
Dr. Hochster has received support from Genentech and Bristol-Myers Squibb. Dr. Wolpin has received support from Agensys/Astellas, Momenta Pharmaceuticals, Merrimack Pharmaceuticals, and Genentech. Dr. Lenz has served on Advisory Boards and received honoraria for lectures from Genentech. Dr. Bekaii-Saab has served as a consultant for Bristol Myers-Squibb and Genentech. Dr. Fuchs has received support from Genentech, Metamark Genetics, Sanofi, Amgen, Momenta Pharmaceuticals, Celgene, and Bayer.
References
- 1.Saltz LB, Meropol NJ, Loehrer PJ, Sr, Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22(7):1201–1208. doi: 10.1200/JCO.2004.10.182. [DOI] [PubMed] [Google Scholar]
- 2.Martin-Martorell P, Rosello S, Rodriguez-Braun E, Chirivella I, Bosch A, Cervantes A. Biweekly cetuximab and irinotecan in advanced colorectal cancer patients progressing after at least one previous line of chemotherapy: results of a phase II single institution trial. British journal of cancer. 2008;99(3):455–458. doi: 10.1038/sj.bjc.6604530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C, Loos AH, Zubel A, Koralewski P. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(5):663–671. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 4.Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pinter T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. The New England journal of medicine. 2009;360(14):1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 5.Van Cutsem E, Tejpar S, Vanbeckevoort D, Peeters M, Humblet Y, Gelderblom H, Vermorken JB, Viret F, Glimelius B, Gallerani E, Hendlisz A, Cats A, Moehler M, Sagaert X, Vlassak S, Schlichting M, Ciardiello F. Intrapatient Cetuximab Dose Escalation in Metastatic Colorectal Cancer According to the Grade of Early Skin Reactions: The Randomized EVEREST Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(23):2861–2868. doi: 10.1200/JCO.2011.40.9243. [DOI] [PubMed] [Google Scholar]
- 6.Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, Patterson SD, Chang DD. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(10):1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 7.Baselga J, Rosen N. Determinants of RASistance to anti-epidermal growth factor receptor agents. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(10):1582–1584. doi: 10.1200/JCO.2007.15.3700. [DOI] [PubMed] [Google Scholar]
- 8.Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, Idziaszczyk S, Harris R, Fisher D, Kenny SL, Kay E, Mitchell JK, Madi A, Jasani B, James MD, Bridgewater J, Kennedy MJ, Claes B, Lambrechts D, Kaplan R, Cheadle JP MCT Investigators. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377(9783):2103–2114. doi: 10.1016/S0140-6736(11)60613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diaz LA, Jr, Williams RT, Wu J, Kinde I, Hecht JR, Berlin J, Allen B, Bozic I, Reiter JG, Nowak MA, Kinzler KW, Oliner KS, Vogelstein B. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature. 2012;486(7404):537–540. doi: 10.1038/nature11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliveras-Ferraros C, Massaguer Vall-Llovera A, Vazquez-Martin A, Salip DC, Queralt B, Cufi S, Martin-Castillo B, Bosch-Barrera J, Brunet J, De Llorens R, Menendez JA. Transcriptional upregulation of HER2 expression in the absence of HER2 gene amplification results in cetuximab resistance that is reversed by trastuzumab treatment. Oncology reports. 2012;27(6):1887–1892. doi: 10.3892/or.2012.1732. [DOI] [PubMed] [Google Scholar]
- 11.Bertotti A, Migliardi G, Galimi F, Sassi F, Torti D, Isella C, Cora D, Di Nicolantonio F, Buscarino M, Petti C, Ribero D, Russolillo N, Muratore A, Massucco P, Pisacane A, Molinaro L, Valtorta E, Sartore-Bianchi A, Risio M, Capussotti L, Gambacorta M, Siena S, Medico E, Sapino A, Marsoni S, Comoglio PM, Bardelli A, Trusolino L. A molecularly annotated platform of patient-derived xenografts (“xenopatients”) identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer discovery. 2011;1(6):508–523. doi: 10.1158/2159-8290.CD-11-0109. [DOI] [PubMed] [Google Scholar]
- 12.Quesnelle KM, Grandis JR. Dual kinase inhibition of EGFR and HER2 overcomes resistance to cetuximab in a novel in vivo model of acquired cetuximab resistance. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17(18):5935–5944. doi: 10.1158/1078-0432.CCR-11-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agus DB, Akita RW, Fox WD, Lewis GD, Higgins B, Pisacane PI, Lofgren JA, Tindell C, Evans DP, Maiese K, Scher HI, Sliwkowski MX. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer cell. 2002;2 (2):127–137. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 14.Gordon MS, Matei D, Aghajanian C, Matulonis UA, Brewer M, Fleming GF, Hainsworth JD, Garcia AA, Pegram MD, Schilder RJ, Cohn DE, Roman L, Derynck MK, Ng K, Lyons B, Allison DE, Eberhard DA, Pham TQ, Dere RC, Karlan BY. Clinical activity of pertuzumab (rhuMAb 2C4), a HER dimerization inhibitor, in advanced ovarian cancer: potential predictive relationship with tumor HER2 activation status. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(26):4324–4332. doi: 10.1200/JCO.2005.05.4221. [DOI] [PubMed] [Google Scholar]
- 15.Makhija S, Amler LC, Glenn D, Ueland FR, Gold MA, Dizon DS, Paton V, Lin CY, Januario T, Ng K, Strauss A, Kelsey S, Sliwkowski MX, Matulonis U. Clinical activity of gemcitabine plus pertuzumab in platinum-resistant ovarian cancer, fallopian tube cancer, or primary peritoneal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(7):1215–1223. doi: 10.1200/JCO.2009.22.3354. [DOI] [PubMed] [Google Scholar]
- 16.Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, Clark E, Benyunes MC, Ross G, Swain SM CS Group. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. The New England journal of medicine. 2012;366(2):109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pohl M, Stricker I, Schoeneck A, Schulmann K, Klein-Scory S, Schwarte-Waldhoff I, Hasmann M, Tannapfel A, Schmiegel W, Reinacher-Schick A. Antitumor activity of the HER2 dimerization inhibitor pertuzumab on human colon cancer cells in vitro and in vivo. Journal of cancer research and clinical oncology. 2009;135(10):1377–1386. doi: 10.1007/s00432-009-0579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Felip E, Ranson M, Cedres S, Dean E, Brewster M, Martinez P, McNally V, Ross G, Galdermans D. A Phase Ib, Dose-Finding Study of Erlotinib in Combination With a Fixed Dose of Pertuzumab in Patients With Advanced Non-Small-Cell Lung Cancer. Clinical lung cancer. 2012 doi: 10.1016/j.cllc.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Weickhardt AJ, Price TJ, Chong G, Gebski V, Pavlakis N, Johns TG, Azad A, Skrinos E, Fluck K, Dobrovic A, Salemi R, Scott AM, Mariadason JM, Tebbutt NC. Dual targeting of the epidermal growth factor receptor using the combination of cetuximab and erlotinib: preclinical evaluation and results of the phase II DUX study in chemotherapy-refractory, advanced colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(13):1505–1512. doi: 10.1200/JCO.2011.38.6599. [DOI] [PubMed] [Google Scholar]
- 20.Tsuchida Y, Therasse P. Response evaluation criteria in solid tumors (RECIST): new guidelines. Medical and pediatric oncology. 2001;37(1):1–3. doi: 10.1002/mpo.1154. [DOI] [PubMed] [Google Scholar]
- 21.Agus DB, Gordon MS, Taylor C, Natale RB, Karlan B, Mendelson DS, Press MF, Allison DE, Sliwkowski MX, Lieberman G, Kelsey SM, Fyfe G. Phase I clinical study of pertuzumab, a novel HER dimerization inhibitor, in patients with advanced cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(11):2534–2543. doi: 10.1200/JCO.2005.03.184. [DOI] [PubMed] [Google Scholar]
- 22.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute. 2000;92 (3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 23.Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, Pruim J, Price P. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. European journal of cancer. 1999;35 (13):1773–1782. doi: 10.1016/s0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]
- 24.Misale S, Yaeger R, Hobor S, Scala E, Janakiraman M, Liska D, Valtorta E, Schiavo R, Buscarino M, Siravegna G, Bencardino K, Cercek A, Chen CT, Veronese S, Zanon C, Sartore-Bianchi A, Gambacorta M, Gallicchio M, Vakiani E, Boscaro V, Medico E, Weiser M, Siena S, Di Nicolantonio F, Solit D, Bardelli A. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012;486(7404):532–536. doi: 10.1038/nature11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yonesaka K, Zejnullahu K, Okamoto I, Satoh T, Cappuzzo F, Souglakos J, Ercan D, Rogers A, Roncalli M, Takeda M, Fujisaka Y, Philips J, Shimizu T, Maenishi O, Cho Y, Sun J, Destro A, Taira K, Takeda K, Okabe T, Swanson J, Itoh H, Takada M, Lifshits E, Okuno K, Engelman JA, Shivdasani RA, Nishio K, Fukuoka M, Varella-Garcia M, Nakagawa K, Janne PA. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Science translational medicine. 2011;3(99):99ra86. doi: 10.1126/scitranslmed.3002442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Y, Li S, Hu YP, Wang J, Hauser J, Conway AN, Vinci MA, Humphrey L, Zborowska E, Willson JK, Brattain MG. Blockade of EGFR and ErbB2 by the novel dual EGFR and ErbB2 tyrosine kinase inhibitor GW572016 sensitizes human colon carcinoma GEO cells to apoptosis. Cancer research. 2006;66(1):404–411. doi: 10.1158/0008-5472.CAN-05-2506. [DOI] [PubMed] [Google Scholar]
- 27.Fields AL, Rinaldi DA, Henderson CA, Germond CJ, Chu L, Brill KJ, Leopold LH, Berger MS. An Open-Label Multicenter Phase II Study of Oral Lapatinib (GW572016) as Single Agent, Second-Line Therapy in Patients with Metastatic Colorectal Cancer. Journal of Clinical Oncology. 2005;23 (16S):1. [Google Scholar]
- 28.Ramanathan RK, Hwang JJ, Zamboni WC, Sinicrope FA, Safran H, Wong MK, Earle M, Brufsky A, Evans T, Troetschel M, Walko C, Day R, Chen HX, Finkelstein S. Low overexpression of HER-2/neu in advanced colorectal cancer limits the usefulness of trastuzumab (Herceptin) and irinotecan as therapy. A phase II trial. Cancer investigation. 2004;22 (6):858–865. doi: 10.1081/cnv-200039645. [DOI] [PubMed] [Google Scholar]
- 29.Clark JW, Niedzwiecki D, Hollis D, Mayer R. Cancer and Leukemia Group B Phase II trial of 5-fluorouracil (5-FU), leucovorin (LV), oxaliplatin (Ox), and trastuzumab (T) for patients with metastatic colorectal cancer (CRC) refractory to initial therapy. 2003 ASCO Annual Meeting; Chicago, IL. ASCO; 2003. [Google Scholar]
- 30.Takahashi T, Boku N, Murakami H, Naito T, Tsuya A, Nakamura Y, Ono A, Machida N, Yamazaki K, Watanabe J, Ruiz-Garcia A, Imai K, Ohki E, Yamamoto N. Phase I and pharmacokinetic study of dacomitinib (PF-00299804), an oral irreversible, small molecule inhibitor of human epidermal growth factor receptor-1, -2, and -4 tyrosine kinases, in Japanese patients with advanced solid tumors. Investigational new drugs. 2012 doi: 10.1007/s10637-011-9789-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su X, Lacouture ME, Jia Y, Wu S. Risk of high-grade skin rash in cancer patients treated with cetuximab--an antibody against epidermal growth factor receptor: systemic review and meta-analysis. Oncology. 2009;77(2):124–133. doi: 10.1159/000229752. [DOI] [PubMed] [Google Scholar]
- 32.Drucker AM, Wu S, Dang CT, Lacouture ME. Risk of rash with the anti-HER2 dimerization antibody pertuzumab: a meta-analysis. Breast cancer research and treatment. 2012 doi: 10.1007/s10549-012-2157-7. [DOI] [PubMed] [Google Scholar]
- 33.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. The New England journal of medicine. 2004;351(4):337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 34.Laux I, Jain A, Singh S, Agus DB. Epidermal growth factor receptor dimerization status determines skin toxicity to HER-kinase targeted therapies. British journal of cancer. 2006;94(1):85–92. doi: 10.1038/sj.bjc.6602875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Potter IY, Poumay Y, Squillace KA, Pittelkow MR. Human EGF receptor (HER) family and heregulin members are differentially expressed in epidermal keratinocytes and modulate differentiation. Experimental cell research. 2001;271(2):315–328. doi: 10.1006/excr.2001.5390. [DOI] [PubMed] [Google Scholar]