Abstract
p21-activated kinases (Paks) are a group of six serine/threonine kinases (Pak1-6) that are involved in a variety of biological processes. Recently, Paks, more specifically Pak1, -2, and -4, have been shown to play important roles in cardiovascular development and function in a range of model organisms including zebrafish and mice. These functions include proper morphogenesis and conductance of the heart, cardiac contractility, and development and integrity of the vasculature. The mechanisms underlying these effects are not fully known, but they likely differ among the various Pak isoforms and include both kinase-dependent and -independent functions. In this review, we discuss aspects of Pak function relevant to cardiovascular biology as well as potential therapeutic implications of small-molecule Pak inhibitors in cardiovascular disease.
Keywords: Protein kinases, Small GTPases, Signal transduction, Cardiovascular, Model organisms
Introduction
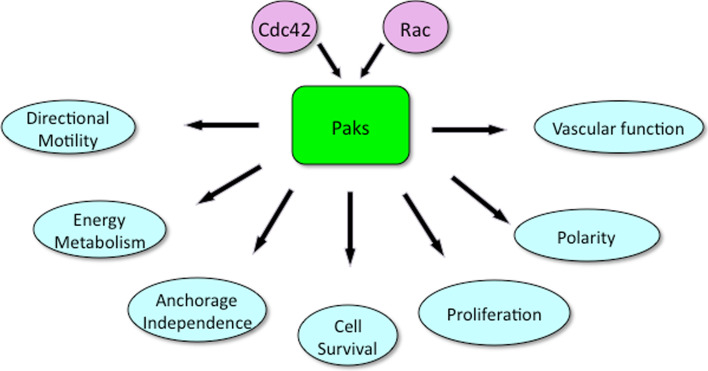
p21-activated kinases (Paks) were discovered in a biochemical screen as effector proteins for the small GTPases Cdc42 and Rac. Six distinct isoforms are encoded in mammalian cells, divided into two distinct groups: Pak1, -2, and -3, and Pak4, -5, and -6. Early in vitro work on Pak function emphasized the roles of these proteins in cell biological processes such as motility, polarity, survival, and proliferation (Fig. 1), but the recent development of Pak knockout animal models, particularly mouse models, has enabled a more comprehensive view of Pak function in development and in the function of particular organ systems [2, 4]. Furthermore, Pak knockout mice and zebrafish are beginning to reveal important roles for these kinases in cardiovascular development. In particular, Pak1, -2, and -4, have been shown to play important roles in heart and blood vessel development, as well as in the proper function of these systems in adult animals. While the exact mechanism of each isoform is not yet known, it is probable that these isoforms are distinct in function, operating through both kinase-dependent and -independent roles. In this review, we discuss aspects of Pak function relevant to cardiovascular biology as well as potential therapeutic implications of small-molecule Pak inhibitors in cardiovascular disease.
Fig. 1.
Cellular functions of Pak. Paks are activated by the small GTPases Cdc42 and Rac. Paks in turn activate signaling pathways that regulate a plethora of cellular events. In many but not all cases, activation is due to phosphorylation of protein substrates by Pak. The processes affected by Pak include directional motility and cell polarity (via phosphorylation of GEFs, GAPs, LIM kinase, Filamin A, p41Arc, etc.), energy metabolism (via phosphorylation of phosphoglucomutase 1 and perhaps other metabolic enzymes), cell survival (via activation of pro-survival proteins such as Akt and inactivation of BAD), cell proliferation (via phosphorylation of c-Raf and Mek1, as well as other growth-responsive signaling proteins), and vascular function (via phosphorylation of VE-cadherin)
Pak substrates of relevance to cardiovascular tissues
The Paks are known to phosphorylate a wide range of substrates, many of which might be relevant to the heart and vasculature (Fig. 2). While each Pak isoform likely has unique substrates dictated by tissue expression, subcellular localization, and intrinsic catalytic selectivity, there are nevertheless some common notable themes. For example, Pak1, -2, -3, and -4 have been shown to phosphorylate Raf-1 and Mek at sites that are required, in many cell types, for efficient activation of the Erk pathway [2]. Likewise, several of the Paks have been implicated in the activation of stress-activated kinases such as Jnk and p38 [4]. As these three mitogen-activated protein kinase (MAPK) pathways regulate key transcriptional events in cardiomyocytes, Pak-mediated effects on MAPKs is expected to be relevant to the function of these cells [14]. Paks also play a role in Akt signaling, though the relationship between these two kinases is complex, with some reports placing Akt downstream of Pak1 and others placing it upstream [24, 29]. Depending on the cellular context, both scenarios may well be true. As will be discussed below, Pak1-mediated activation of Akt, and also of the apoptotic regulator BAD, may promote cell survival in cardiomyocytes.
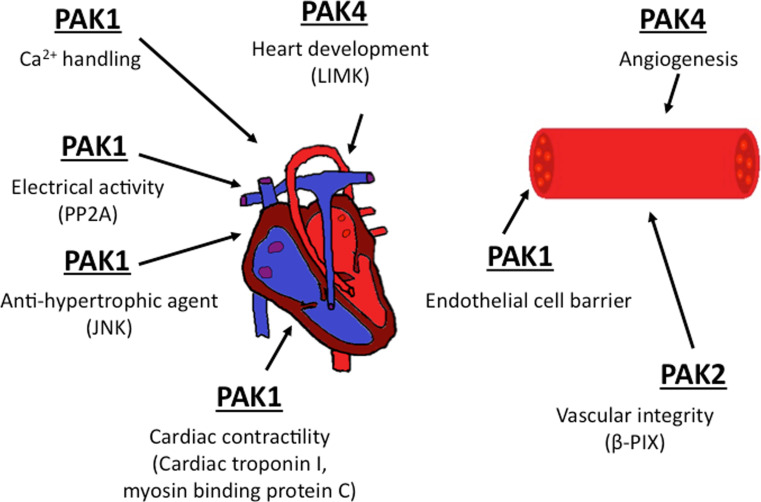
Fig. 2.
Pak roles in the cardiovascular system. Pak1, Pak2, and Pak4 are known to influence the development and/or function of the heart and blood vessels. In the heart, Pak1 has been linked to contractility, Ca2+ entry, and electrical activity through the indicated substrates and interactors. Pak4 has a major role in heart development during embryogenesis; this effect may be related to the Pak4 substrate LIMK. In blood vessels, loss of Pak2a or Pak2b in zebrafish is associated with hemorrhage, though the relevant substrates have yet to be identified. Pak1 also affects endothelial cell barrier function by regulating the stability of endothelial cell adhesions
Paks also phosphorylate a variety of proteins that affect the cytoskeleton and actin/myosin-based contractility. Such substrates include several guanine-nucleotide exchange factors (GEFs), guanine-nucleotide activating proteins (GAPs), and guanine-nucleotide dissociation inhibitors (GDIs), all of which directly modulate the activity of small GTPases of the Rho family [4]. Paks also phosphorylate LIM kinase, filamin A, stathmin, cortactin, and paxillin, which regulate cytoskeletal remodeling and focal adhesion formation. In addition, Pak1 has been shown to phosphorylate myosin light chain (MLC) and caldesmin, two proteins with important functions in contractility. In endothelial cells, phosphorylation of MLC by Pak2 is controversial, with one group reporting that Pak2 monophosphorylates MLC and induces cell retraction [31], and another that it phosphorylates and inactivates MLCK, leading to decreased MLC phosphorylation and limiting isometric tension [10].
In endothelial cells, Pak1/2 has been reported to phosphorylate VE-cadherin, thereby promoting beta-arrestin-dependent endocytosis of VE-cadherin in VEGF-treated cells. This event is associated with increased vascular permeability due to disassembly of intercellular junctions [9]. Interestingly, Pak2 and Pak4 may also be required downstream of Cdc42 for endothelial cell lumen formation during vascular morphogenesis, though the relevant substrates of these two kinases in lumen formation remain to be identified [19, 20].
Pak1
Pak1 is expressed at high levels in the heart and blood vessels of mammalian organisms and has been shown to have several distinct roles in cardiac function. At the organismal level, the Pak1 gene is not required for heart development, and standard Pak1 knock-out mice live a normal life-span without notable cardiac problems. However, cardiac function under stress conditions may be compromised. Using a conditional knock-out (cko) of Pak1 crossed to αMHC-Cre mice, Liu et al. [23] deleted Pak1 in cardiomyocytes of developing mice. While these heart-specific Pak1 cko mice, like constitutive Pak1 knockouts, did not show overt cardiac pathology, and had a normal lifespan under unstressed conditions, they developed greater hypertrophy and early heart failure when subjected to pressure overload induced by transverse aortic constriction or by chronic infusion of angiotensin II. In this setting, the Pak1 cko mice exhibited increased heart weight/tibia length ratios as well as increased cross-sectional area of cardiomyocytes, suggesting that Pak1 has anti-hypertrophic properties. Interestingly, wild-type mice treated with FTY720, a sphingosine-like analog that activates Pak1, were resistant to developing pressure overload-induced hypertrophy [23]. These effects were not seen in Pak1 cko mice, providing reasonable evidence for specificity of FTY720 in this setting—an important control, given that this compound is able to inhibit both sphingosine-1 phosphate and its receptor, sphingosine-1 phosphate receptor-1, modulators of vascular stability [8, 12, 27]. Interestingly, hypertrophy in Pak1 cko animals was found to be associated with impaired activation of the Jnk, but not the Erk pathway [23]. These data imply that Pak1 signals to Jnk in cardiomyocytes and that this signaling pathway can potentially be exploited to augment cardiac function in certain settings.
At the cellular level, Pak1 plays a pro-survival role in cardiomyocytes, perhaps by activating Akt (Fig. 3). Using cardiomyocytes from Pak1 knock-out mice, Mao et al. [24] showed that Pak1 is activated by hypertrophic stimuli and that Pak1 overexpression is accompanied by Akt activation, whereas Pak1 loss in accompanied by diminished Akt activation. As Pak1 was shown to directly phosphorylate Akt at Ser473 in vitro, these data suggest that Pak1 can act as a phosphoinositide-dependent kinase (Pdk)-2-like entity, responsible for Akt Ser473 phosphorylation in cardiomyocytes (Fig. 3a). Consistent with this view, activated Pak1 was shown to protect cardiomyocytes from cell death, and this effect was blocked by Akt inhibition. It should be noted, however, that Pak1 activation of Akt may be related to a scaffolding function as opposed to catalytic activity, as Higuchi et al. [11] reported that the Pak1 kinase domain serves as a scaffold to facilitate Akt stimulation by Pdk1 and to aid recruitment of Akt to the membrane. Resolving this issue may have therapeutic implications, as manipulation of Pak1 catalytic activity by small-molecule inhibitors or activators would not be expected to affect scaffolding functions. In this regard, it is interesting to note that in a rat model, FTY720 has been shown to activate Pak1 and Akt and to prevent arrhythmic events associated with ischemia/reperfusion injury [7]. These results suggest that, whatever the scaffold functions of Pak1, manipulating the catalytic function of Pak1 might be used for therapeutic benefit.
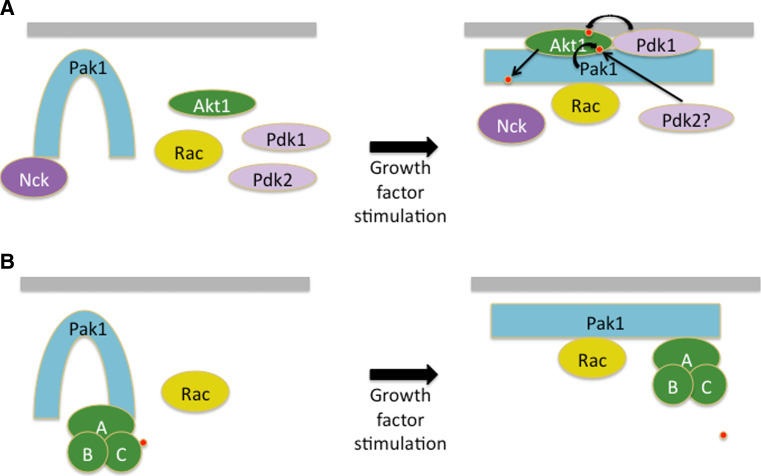
Fig. 3.
Scaffold activities of Pak1. While most models of Pak action posit that is functions via its kinase domain to phosphorylated substrates, some of its activities may be non-catalytic. a Currently, at least two models have been proposed to explain the effects of Pak1 on promoting Akt activity. In one model, Pak1 directly phosphorylates Akt1 on Ser473, acting in effect as a Pdk2. A second model posits that Pak1 is required for Akt activation because it serves as a scaffold for Pdk1 and to assist membrane recruitment for Akt. In this second model, Pak1 kinase activity is not required. The signaling is bidirectional, as Akt can also phosphorylate Pak1, disrupting the association of Pak1 with the adaptor protein Nck. Phosphorylation events are depicted as small red spheres. b Pak1 has also been proposed to act as a scaffold for PP2A. In this model, activation of Pak1 leads to a conformational change in PP2A resulting in autodephosphorylation of the C subunit (A conserved regulatory subunit, B variable regulatory subunit, C catalytic subunit)
Pak1 has also been shown to affect cardiac excitation and contractility. As with cell survival, the role of Pak1 in these processes may have catalytic as well as non-catalytic components. For example, many of the effects of active Pak1 on cardiac excitation and contractility appear to be related to dephosphorylation of key sarcomeric proteins through the serine/threonine protein phosphatase PP2A. Pak1 associates with, and activates, PP2A, and this association may explain the loss of phosphorylation noted for cardiac troponin I, and C-protein, with a concomitant increase in sensitivity to Ca2+, in cardiomyocytes expressing activated Pak1 [1, 15, 16]. In pacemaker cells of the sinoatrial node, a Pak1/PP2A signaling circuit acts to depress isoproterenol-induced upregulation of l-type Ca2+ current and delayed rectifier potassium current, and to suppress the chronotropic action of isoproterenol on pacemaker activity [13]. It is unclear whether the activation of PP2A by Pak1 requires the kinase activity of Pak1; rather, it may result from conformational changes that occur upon association of these two signaling proteins (Fig. 3b). If so, this would represent another example of an important non-catalytic function of Pak1.
In addition to its role in the heart, Pak1 function also affects blood vessel formation and integrity. As many experiments on angiogenesis have to date employed approaches that do not distinguish effects of Pak1 versus Pak2, we use the term Pak1/2 to denote such situations. For example, a dominant-negative peptide that inhibits Pak1/2 has been shown to impede tube formation in vitro and block angiogenesis in a chick chorioallantoic membrane assay [18]. Similarly, increased permeability of endothelial cells treated with serum, VEGF, bFGF, TNFalpha, histamine, or thrombin could be prevented by blocking Pak1/2 activity or by treating with inhibitors of myosin phosphorylation [28]. These data suggest that Pak1/2 regulates endothelial permeability induced by multiple growth factors and cytokines via an effect on cell contractility.
Birukova et al. [3] showed that the protective effect of atrial natriuretic peptide (ANP) on pulmonary vasculature following acute lung injury is weakened when Pak1 is knocked down by siRNA. These authors also showed that ANP activates Pak1 in pulmonary endothelial cells barrier, and that deletion of ANP in mice is associated with endothelial barrier dysfunction and loss of Pak1 activity in the lung. These results suggest a positive role for Pak1 in endothelial cell barrier protection in the setting of acute injury.
Pak2
The role of Pak2 in the heart and vessels has received less attention than that of Pak1, but this situation is rapidly changing due to increasing availability of genetic models. In zebrafish, loss-of-function mutation in the Pak2a gene is associated with cerebral hemorrhage, without any other obvious phenotype [5]. In this system, hemorrhage is likely related to defects in vascular integrity as opposed to vascular patterning [5]. Interestingly, the phenotype of Pak2 loss in zebrafish resembles that of βPix mutation, a gene encoding a Rac1-specific guanine-exchange factor that is known to interact with Pak2 [22]. In mice, loss of Pak2 is associated with fetal death at ~E8.0 due to multiple developmental defects, including prominent vascular defects [17]. Further studies of these mice are ongoing.
During vascular morphogenesis, Pak2 and -4 are known to be activated by Cdc42 during lumen formation; however, the direct targets of Pak2 and -4 remain elusive [19, 20]. As noted above, Pak1/2 has been reported to phosphorylate VE-cadherin in endothelial cells, promoting internalization of this cell adhesion molecule in VEGF-treated cells. In association, the disassembly of intercellular junctions causes an increase in vascular permeability [9]. This model, in which activation of Pak1/2 plays a role promoting vascular permeability, is seemingly at odds with the reported role of Pak1 in stabilizing the pulmonary endothelial cells barrier, as described above. If so, such opposing roles would be consistent with recent findings in mast cells, which indicate that Pak1 and Pak2 can play antagonistic roles in certain cell biological functions [21]. It is also possible that too much or too little signaling from Pak1/2 could yield similar phenotypes; i.e., increased permeability, due to improper VE-cadherin recycling to and from the cell surface.
Pak4
Pak4 deletion in mice is associated with embryonic lethality by ~E11.5, most likely due to heart defects. Up to E8.5, the hearts of Pak4-null mice appear to develop normally, with formation of the heart tube and proper looping. However, the common ventricle and the outflow tract are significantly smaller in the knockouts. By E9.5, the outflow tract is significantly smaller with thinning of the myocardium in the ventricles, and the heart rate is decreased and irregular. By E10.5, the embryos are much smaller than control littermates, show a lack of circulation, and no further development to the heart. Molecular analysis of this phenotype showed a significantly decreased level of phosphorylated LIM kinase (LIMK), a Pak4 substrate. Consistent with the known role of LIMK as a regulator of actin cytoskeleton dynamics through cofilin, the lack of active LIMK was associated with a decrease in polymerized F actin, impaired sarcomeric structure, and impaired contractility in hearts [26]. Coupled with the heart defects, Pak4-null mice displayed few embryonic blood vessels in the yolk sac and throughout the embryo [30]. Interestingly, Pak4 −/− mice displayed large vessels, but were lacking in the small branching vessels [30], suggesting a role for Pak4 in angiogenesis. Together, these results place Pak4 as an important regulator of cardiac contractility through LIMK and angiogenesis.
Conclusion
The Pak family of kinases has an integral place in the formation and function of the cardiovascular system. While many of the effects of Paks on these events can be attributed to phosphorylation, a growing body of literature suggests that scaffolding and perhaps other non-catalytic functions of Paks also play significant roles. While the pharmaceutical industry has begun to produce Pak inhibitors for the treatment of cancer [6, 25], such agents may need to be used with caution, given the role of Pak activity in heart failure and perhaps in vascular integrity. Indeed, Pak activators, such as FTY720 or more specific small molecules, might have therapeutic benefit in certain settings.
Abbreviations
- ANP
Atrial natriuretic peptide
- Cko
Conditional knock-out
- Erk
Extracellular-regulated kinase
- GAP
Guanine-nucleotide activating proteins
- GDI
Guanine-nucleotide dissociation inhibitor
- GEF
Guanine-nucleotide exchange factor
- LIMK
LIM kinase
- MAPK
Mitogen-activated protein kinase
- MLC
Myosin light chain
- Pak
p21-Activated kinase
- PDK
Phosphoinositide-dependent kinase
References
- 1.Ai X, Jiang A, Ke Y, Solaro RJ, Pogwizd SM. Enhanced activation of p21-activated kinase 1 in heart failure contributes to dephosphorylation of connexin 43. Cardiovasc Res. 2011;92:106–114. doi: 10.1093/cvr/cvr163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arias-Romero LE, Chernoff J. A tale of two Paks. Biol Cell. 2008;100:97–108. doi: 10.1042/BC20070109. [DOI] [PubMed] [Google Scholar]
- 3.Birukova AA, Xing J, Fu P, Yakubov B, Dubrovskyi O, Fortune JA, Klibanov AM, Birukov KG. Atrial natriuretic peptide attenuates LPS-induced lung vascular leak: role of PAK1. Am J Physiol Lung Cell Mol Physiol. 2010;299:L652–L663. doi: 10.1152/ajplung.00202.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- 5.Buchner DA, Su F, Yamaoka JS, Kamei M, Shavit JA, Barthel LK, McGee B, Amigo JD, Kim S, Hanosh AW, et al. pak2a mutations cause cerebral hemorrhage in redhead zebrafish. Proc Natl Acad Sci USA. 2007;104:13996–14001. doi: 10.1073/pnas.0700947104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chow HY, Jubb AM, Koch JN, Jaffer ZM, Stepanova D, Campbell DA, Duron SG, O’Farrell M, Cai KQ, Klein-Szanto AJ, et al. p21-Activated kinase 1 is required for efficient tumor formation and progression in a Ras-mediated skin cancer model. Cancer Res. 2012;72:5966–5975. doi: 10.1158/0008-5472.CAN-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egom EE, Ke Y, Musa H, Mohamed TM, Wang T, Cartwright E, Solaro RJ, Lei M. FTY720 prevents ischemia/reperfusion injury-associated arrhythmias in an ex vivo rat heart model via activation of Pak1/Akt signaling. J Mol Cell Cardiol. 2010;48:406–414. doi: 10.1016/j.yjmcc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaengel K, Niaudet C, Hagikura K, Lavina B, Muhl L, Hofmann JJ, Ebarasi L, Nystrom S, Rymo S, Chen LL, et al. The sphingosine-1-phosphate receptor S1PR1 restricts sprouting angiogenesis by regulating the interplay between VE-cadherin and VEGFR2. Dev Cell. 2012;23:587–599. doi: 10.1016/j.devcel.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8:1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 10.Goeckeler ZM, Masaracchia RA, Zeng Q, Chew TL, Gallagher P, Wysolmerski RB. Phosphorylation of myosin light chain kinase by p21-activated kinase PAK2. J Biol Chem. 2000;275:18366–18374. doi: 10.1074/jbc.M001339200. [DOI] [PubMed] [Google Scholar]
- 11.Higuchi M, Onishi K, Kikuchi C, Gotoh Y. Scaffolding function of PAK in the PDK1-Akt pathway. Nat Cell Biol. 2008;10:1356–1364. doi: 10.1038/ncb1795. [DOI] [PubMed] [Google Scholar]
- 12.Jung B, Obinata H, Galvani S, Mendelson K, Ding BS, Skoura A, Kinzel B, Brinkmann V, Rafii S, Evans T, Hla T. Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development. Dev Cell. 2012;23:600–610. doi: 10.1016/j.devcel.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ke Y, Lei M, Collins TP, Rakovic S, Mattick PA, Yamasaki M, Brodie MS, Terrar DA, Solaro RJ. Regulation of L-type calcium channel and delayed rectifier potassium channel activity by p21-activated kinase-1 in guinea pig sinoatrial node pacemaker cells. Circ Res. 2007;100:1317–1327. doi: 10.1161/01.RES.0000266742.51389.a4. [DOI] [PubMed] [Google Scholar]
- 14.Ke Y, Lei M, Wang X, Solaro RJ. Novel roles of PAK1 in the heart. Cell Logist. 2012;2:89–94. doi: 10.4161/cl.21497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ke Y, Sheehan KA, Egom EE, Lei M, Solaro RJ. Novel bradykinin signaling in adult rat cardiac myocytes through activation of p21-activated kinase. Am J Physiol Heart Circ Physiol. 2010;298:H1283–H1289. doi: 10.1152/ajpheart.01070.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ke Y, Wang L, Pyle WG, de Tombe PP, Solaro RJ. Intracellular localization and functional effects of P21-activated kinase-1 (Pak1) in cardiac myocytes. Circ Res. 2004;94:194–200. doi: 10.1161/01.RES.0000111522.02730.56. [DOI] [PubMed] [Google Scholar]
- 17.Kelly ML, Chernoff J. Mouse models of PAK function. Cell Logist. 2012;2:84–88. doi: 10.4161/cl.21381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiosses WB, Hood J, Yang S, Gerritsen ME, Cheresh DA, Alderson N, Schwartz MA. A dominant-negative p65 PAK peptide inhibits angiogenesis. Circ Res. 2002;90:697–702. doi: 10.1161/01.RES.0000014227.76102.5D. [DOI] [PubMed] [Google Scholar]
- 19.Koh W, Mahan RD, Davis GE. Cdc42- and Rac1-mediated endothelial lumen formation requires Pak2, Pak4 and Par3, and PKC-dependent signaling. J Cell Sci. 2008;121:989–1001. doi: 10.1242/jcs.020693. [DOI] [PubMed] [Google Scholar]
- 20.Koh W, Sachidanandam K, Stratman AN, Sacharidou A, Mayo AM, Murphy EA, Cheresh DA, Davis GE. Formation of endothelial lumens requires a coordinated PKCepsilon-, Src-, Pak- and Raf-kinase-dependent signaling cascade downstream of Cdc42 activation. J Cell Sci. 2009;122:1812–1822. doi: 10.1242/jcs.045799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kosoff R, Chow HY, Radu M, Chernoff J. Pak2 kinase restrains mast cell Fc{epsilon}RI receptor signaling through modulation of Rho protein guanine nucleotide exchange factor (GEF) activity. J Biol Chem. 2013;288:974–983. doi: 10.1074/jbc.M112.422295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Fraser SD, Faloon PW, Rollins EL, Vom Berg J, Starovic-Subota O, Laliberte AL, Chen JN, Serluca FC, Childs SJ. A betaPix Pak2a signaling pathway regulates cerebral vascular stability in zebrafish. Proc Natl Acad Sci USA. 2007;104:13990–13995. doi: 10.1073/pnas.0700825104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu W, Zi M, Naumann R, Ulm S, Jin J, Taglieri DM, Prehar S, Gui J, Tsui H, Xiao RP, et al. Pak1 as a novel therapeutic target for antihypertrophic treatment in the heart. Circulation. 2011;124:2702–2715. doi: 10.1161/CIRCULATIONAHA.111.048785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mao K, Kobayashi S, Jaffer ZM, Huang Y, Volden P, Chernoff J, Liang Q. Regulation of Akt/PKB activity by P21-activated kinase in cardiomyocytes. J Mol Cell Cardiol. 2008;44:429–434. doi: 10.1016/j.yjmcc.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murray BW, Guo C, Piraino J, Westwick JK, Zhang C, Lamerdin J, Dagostino E, Knighton D, Loi CM, Zager M, et al. Small-molecule p21-activated kinase inhibitor PF-3758309 is a potent inhibitor of oncogenic signaling and tumor growth. Proc Natl Acad Sci USA. 2010;107:9446–9451. doi: 10.1073/pnas.0911863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nekrasova T, Minden A. Role for p21-activated kinase PAK4 in development of the mammalian heart. Transgenic Res. 2012;21:797–811. doi: 10.1007/s11248-011-9578-7. [DOI] [PubMed] [Google Scholar]
- 27.Oo ML, Thangada S, Wu MT, Liu CH, Macdonald TL, Lynch KR, Lin CY, Hla T. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J Biol Chem. 2007;282:9082–9089. doi: 10.1074/jbc.M610318200. [DOI] [PubMed] [Google Scholar]
- 28.Stockton RA, Schaefer E, Schwartz MA. p21-activated kinase regulates endothelial permeability through modulation of contractility. J Biol Chem. 2004;279:46621–46630. doi: 10.1074/jbc.M408877200. [DOI] [PubMed] [Google Scholar]
- 29.Tang Y, Zhou H, Chen A, Pittman RN, Field J. The Akt proto-oncogene links Ras to Pak and cell survival signals. J Biol Chem. 2000;275:9106–9109. doi: 10.1074/jbc.275.13.9106. [DOI] [PubMed] [Google Scholar]
- 30.Tian Y, Lei L, Cammarano M, Nekrasova T, Minden A. Essential role for the Pak4 protein kinase in extraembryonic tissue development and vessel formation. Mech Dev. 2009;126:710–720. doi: 10.1016/j.mod.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Zeng Q, Lagunoff D, Masaracchia R, Goeckeler Z, Cote G, Wysolmerski R. Endothelial cell retraction is induced by PAK2 monophosphorylation of myosin II. J Cell Sci. 2000;113(Pt 3):471–482. doi: 10.1242/jcs.113.3.471. [DOI] [PubMed] [Google Scholar]