Abstract
Background
Although the amygdala and insula are regarded as critical neural substrates perpetuating cigarette smoking, little is known about their circuit-level interactions with interconnected regions during nicotine withdrawal or following pharmacotherapy administration. To elucidate neurocircuitry associated with early smoking abstinence, we examined the impact of varenicline and nicotine, two modestly efficacious pharmacological cessation aids, on amygdala- and insula-centered circuits using resting-state functional connectivity (rsFC).
Methods
In a fMRI study employing a two-drug, placebo-controlled design, 24 overnight-abstinent smokers and 20 nonsmokers underwent ~17 days of varenicline and placebo pill administration and were scanned, on different days under each condition, wearing a transdermal nicotine or placebo patch. We examined the impact of varenicline and nicotine (both alone and in combination) on amygdala- and insula-centered rsFC using seed-based assessments.
Results
Beginning with a functionally-defined amygdala seed, we observed that rsFC strength in an amygdala-insula circuit was down-regulated by varenicline and nicotine in abstinent smokers. Using this identified insula region as a new seed, both drugs similarly decreased rsFC between the insula and constituents of the canonical default-mode network (DMN: posterior cingulate cortex, ventro/dorsomedial prefrontal cortex, parahippocampus). Drug-induced rsFC modulations were critically linked with nicotine withdrawal as similar effects were not detected in nonsmokers.
Conclusions
These results suggest that nicotine withdrawal is associated with elevated amygdala-insula and insula-DMN interactions. As these potentiated interactions were down-regulated by two pharmacotherapies, this effect may be a characteristic shared by pharmacological agents promoting smoking cessation. Decreased rsFC in these circuits may contribute to amelioration of subjective withdrawal symptoms. (http://www.clinicaltrials.gov, ID:NCT00830739).
Keywords: amygdala, insula, withdrawal, varenicline, nicotine, resting-state functional connectivity
INTRODUCTION
Abrupt cessation of cigarette smoking perturbs homeostasis maintained in the presence of chronic nicotine giving rise to the tobacco abstinence syndrome. Hallmark features of the syndrome include anxiety, irritability, and difficulty concentrating (1) making short-term cessation difficult for most smokers (2). The neurobiological mechanisms contributing to abstinence-induced aversive states are centered on the amygdala and its interconnected circuitry (3, 4). Another critical structure maintaining addiction is the insula, as circumscribed insular lesions are associated with sustained disruption of smoking behaviors (5). Since available pharmacological cessation aids are only modestly effective, elucidating the impact of nicotine withdrawal and pharmacotherapy administration on these regions may expedite development of improved cessation interventions.
The amygdala and insula are constituents of the neurocircuitry maintaining addiction (6, 7). These regions are anatomically (8) and functionally interconnected (9) and interactions between them are implicated at multiple stages of the addiction cycle (10). For example, during early abstinence, the insula is hypothesized to track homeostatically-relevant bodily sensations (11–13) and, in turn, alter circuit-level interactions with interconnected regions (e.g., amygdala, ventromedial prefrontal cortex), thereby modifying affective and motivational processes perpetuating drug use (6, 14, 15). Indirectly supporting such views, elevated activity within the amygdala and insula has been observed during smoking abstinence, following drug cue presentation, and to correlate with subjective use urges (16–18). However, little empirical data exist regarding circuit-level interactions between the amygdala, insula, and their interconnected brain regions during smoking abstinence or pharmacotherapy administration. As such, this study aimed to examine the impact of varenicline and nicotine, two pharmacological cessation aids, on amygdala and insula circuit dynamics during early smoking abstinence using resting-state functional connectivity (rsFC).
Circuit-level interactions between regions can be investigated using rsFC, the assessment of synchronized low-frequency fluctuations in the fMRI signal collected in the absence of explicit task demands (19, 20). Such rsFC assessments have been increasingly employed to interrogate functional circuits constrained to known neuroanatomical pathways (21), including those associated with the amygdala (9, 22) and insula (23, 24). Functional circuits identified ‘at rest’ closely correspond to those engaged during neuroimaging tasks (25), predict behavioral performance and personality traits (22, 26), are influenced by genetics (27), and can be pharmacologically manipulated (28, 29). Furthermore, rsFC may provide a systems-level perspective on the dysfunctional neurocircuitry underlying multifaceted neuropsychiatric disorders (30) including addiction (31). These systems-level perspectives highlight three intrinsic connectivity networks (ICNs): 1) the default mode network (DMN: 32), anchored by posterior cingulate and medial prefrontal cortices and thought to subserve endogenous information processing, 2) the executive control network (ECN: 24, 33), including lateral prefrontal and parietal regions and linked with exogenous information processing, and 3) the salience network (24), consisting of bilateral insula and anterior cingulate cortex and thought to ‘toggle’ dynamic activity between the DMN and ECN (34–37).
Varenicline, acting on nicotinic acetylcholine receptors (nAChRs), is believed to aid smoking cessation by ameliorating abstinence-induced withdrawal symptoms while also attenuating nicotine-induced pharmacological effects following re-exposure (38). In nicotine’s absence, varenicline acts primarily as a partial agonist at α4β2 nAChRs producing ~50–60% the relative action of nicotine; whereas in nicotine’s presence, the drug acts as an antagonist binding with higher affinity and preventing full activation by nicotine (39). In the clinic, varenicline reduces abstinence-induced affective and cognitive disturbances as well as the subjective rewarding aspects of a smoked cigarette (40). While this dual action profile may explain varenicline’s greater relative efficacy over other pharmacotherapies (41), supporting neurobiological evidence is based primarily on cellular and preclinical data (42). In this study, we interrogated the impact of varenicline and nicotine on amygdala/insula circuit dynamics to provide empirical support for varenicline’s dual action profile in the human brain.
To this end, we administered varenicline alone and in combination with nicotine to abstinent smokers and nonsmokers. All participants underwent, in a counterbalanced order, a regimen of varenicline and placebo pill administration (PILL factor) and completed fMRI scans near the end of each period wearing, on different days, a nicotine or placebo patch (PATCH factor). We hypothesized pharmacological manipulations would decrease rsFC in amygdala- and insula-centered circuits of abstinent smokers. More specifically, based on preclinical data regarding varenicline’s neuropharmacological properties (42), we anticipated a specific varenicline (PILL) x nicotine (PATCH) pharmacological interaction pattern across dependent variables (Fig. 1). If pharmacologically-induced rsFC modulations were critically associated with nicotine withdrawal, we further anticipated no drug effects in nonsmokers.
Figure 1.
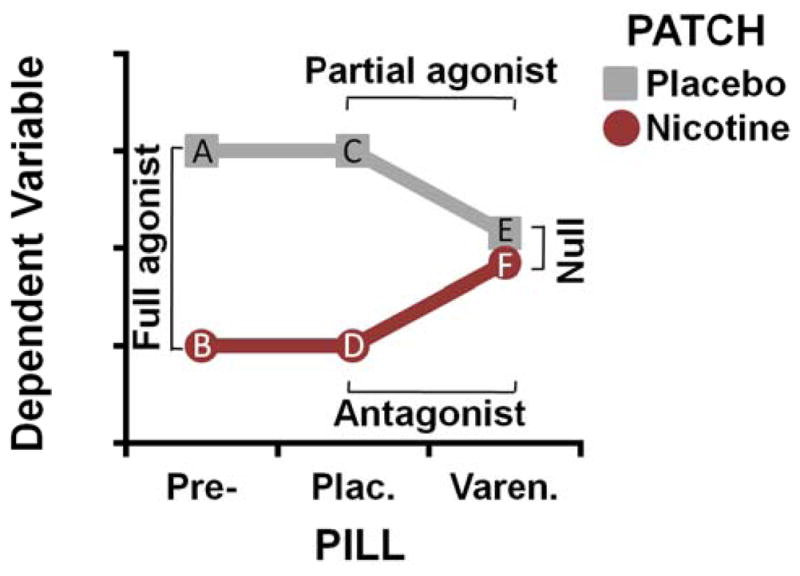
Schematic illustration of the idealized varenicline (PILL) x nicotine (PATCH) pharmacological interaction. Withdrawal-induced effects on the dependent variable (DV: e.g., amygdala- and insula-centric rsFC) were expected to be greatest following smoking deprivation and in the absence of drug administration (data points A and C). Administration of nicotine was then anticipated to reduce this withdrawal-induced elevation (data points B and D) yielding the full agonist effect (A vs. B, C vs. D). Similarly, administration of varenicline alone was expected to reduce the DV (data point E) thus yielding a partial agonist effect (C vs. E). Administration of varenicline in combination with nicotine (data point F) was expected to result in an attenuated nicotine-induced response, as varenicline binds to nAChRs with higher affinity than nicotine “blocking” the full agonist response and thus yielding an antagonist effect (D vs. F). These partial agonist and antagonist effects were anticipated to yield a null effect of nicotine versus placebo patch (E vs. F) in the presence of varenicline.
METHODS
Participants
Cigarette smokers (n=24; 12 females) and nonsmokers (n=20; 10 females) completed identical procedures. Participants were right-handed, 18–55 years of age, and reported no history of drug dependence (other than nicotine in smokers), neurological or psychiatric disorders or contra-indications for MRI scanning. Smokers were 36±10 years of age (mean±SD), reported daily cigarette use for 18±11 years, smoked 18±8 cigarettes/day, and were moderately nicotine dependent (Fagerström scores: 5±2). Nonsmokers reported no history of daily cigarette use and no smoking within two years preceding the study. Because nonsmokers were younger (30±7 years; p=0.05) than smokers, age was included as a covariate when comparing groups. Data from one male smoker and one male nonsmoker were excluded due to excessive head motion during scans. We obtained written informed consent in accordance with the NIDA-IRP Institutional Review Board.
Design and drugs
This was a within-subject, double-blind, placebo-controlled study involving 6 fMRI assessments (43). At three points during a varenicline administration regime (PILL: pre-pill, placebo, varenicline), participants were scanned twice, once each wearing a nicotine or placebo patch (PATCH). After two initial pre-pill sessions, participants were administered varenicline (17.0±4.2 days) and placebo pills (16.5±3.4 days) and again completed nicotine and placebo patch scans near the end of each pill interval (Fig. S1 in Supplement). As this was not a treatment study per se, other than a 12 h abstinence period before each neuroimaging day, our non-treatment seeking smokers were not explicitly instructed to alter their smoking behaviors.
Varenicline (Chantix®, Pfizer) and placebo pills were distributed in identical blister packs. Varenicline was administered according to standard guidelines (http://www.pfizer.com/products). Participants confirmed taking a study pill the morning of neuroimaging days. Given a ~24 h elimination half-life (44), we assumed varenicline carryover effects were negligible in those participants randomly assigned to first receive active medication as subsequent scanning under placebo pills occurred ~2 weeks after the last active dose1. Nicotine (NicoDerm CQ®, GlaxoSmithKline) or placebo patches were applied to the upper back at the beginning of neuroimaging days. We employed a multiple dosing strategy to match daily nicotine intake, nonsmokers: 7 mg patches; smokers: 21, 28, 35, or 42 mg patches (for 10–15, 16–20, 21–25, and >25 cigarettes/day, respectively). We instructed smokers to have their last cigarette 12 h before their scheduled arrivals. Upon arrival, participants were tested for recent drug/alcohol use and carbon monoxide levels (a guideline of ≤15 ppm verified overnight smoking abstinence).
rsFC analyses
We collected data with a Siemens 3T Magnetom Allegra scanner. An 8-min ‘resting’ scan was acquired ~2.5–3 h after patch application, during which participants were instructed to relax with eyes closed and remain still (see Supplemental Information for imaging parameters). We processed resting data as previously described (45) using AFNI, SPM5, and MATLAB. Functional data were slice-time and motion corrected, aligned with anatomical images, and normalized into Talairach space (3mm3 voxels). Images were spatially blurred (6mm) and time series underwent quadratic de-trending and band-pass filtering (0.01–0.1Hz). Nuisance signals from non-neuronal physiological processes were reduced by regressing each of the first three principal components from the time courses of white matter and cerebrospinal fluid voxels as covariates of no interest (46). Motion-correction parameters were also used to remove signals related to head movement. Furthermore, we employed two additional strategies to reduce potential motion-related confounds (Supplemental Information): 1) the censoring of volumes associated with large movements (47, 48), and 2) the use of a summary motion metric as a confounding variable in group-level analyses (49). For each subject and session, correlation coefficient (CC) images were calculated by correlating each voxel’s time course with a reference time course from a seed region of interest (ROI). Resulting subject-level CC images were Fischer’s Z-transformed (Z-images).
Amygdala-centric rsFC: Smoker drug effects
We performed two initial rsFC assessments using seeds placed at the center of mass in left (x=−22, y=−4, z=−16; 29 voxels) and right amygdala (x=24, y=−6, z=−16; 29 voxels) activation clusters identified at the group-level using a ‘functional localizer’ task (Supplemental Information) known to yield reliable amygdala reactivity (50, 51). To first identify ICNs associated with these amygdala seeds, we created rsFC maps by averaging each smoker’s Z-images across all sessions and submitting the session-averaged images to group-level, one-sample t-tests. Critically, to then identify regions whose rsFC with the amygdala was modulated by drugs, we analyzed these data in a whole-brain linear-mixed effects framework. Z-images from each smoker and session were entered into models including factors for PILL (pre-pill vs. placebo vs. varenicline) and PATCH (nicotine vs. placebo) along with mean motion and age as covariates. Given our a priori hypotheses, we focused on the whole-brain PILL x PATCH statistical maps (pcorrected<0.05: pvoxel-wise<0.005; cluster-extent: 46 voxels). Z-values (i.e., rsFC strength) from resulting ROIs were extracted for graphical examination by averaging across all voxels within the ROI. These procedures identified a left insula ROI whose rsFC with the left amygdala was modulated by drugs.
Insula-centric rsFC: Smoker drug effects
To examine drug-induced effects on insula-centric rsFC, we then performed an identical rsFC analysis now using the insula region (x=−42, y=12, z=4; 111 voxels) identified in the above amygdala-centric analysis as a seed. Given the exploratory nature of this assessment, we employed a more stringent threshold on the PILL x PATCH map (pcorrected<0.01: pvoxel-wise<0.001; cluster-extent: 33 voxels).
Specificity of drug effects
To test the specificity of drug-induced modulations in the amygdala/insula circuits of abstinent smokers, we employed both positive and negative control analyses. Using the above amygdala and insula seeds, we consistently observed drug-induced rsFC decreases. In contrast, nicotine administration to minimally-deprived smokers has been reported to increase rsFC between the posterior cingulate cortex (PCC) and medial PFC (mPFC) (29). As such, we predicted that nicotine would increase PCC-mPFC rsFC and thus provide a positive control for our amygdala and insula findings. We tested this prediction using a PCC seed (center coordinate obtained from the insula analysis above: x=2, y=−54, z=26, 29 voxels) and examined the whole-brain PATCH main effect (pcorrected<0.05).
If amygdala/insula circuit dynamics were critically associated with nicotine withdrawal, we predicted that drug-induced rsFC modulations would not be observed in nonsmokers. We tested this prediction using both a ROI and a whole-brain approach (Supplemental Information). First, we extracted nonsmokers’ rsFC values from those ROIs showing modulations within smokers and assessed drug effects ‘off-line’ in PILL x PATCH repeated-measures ANOVAs. Second, to directly examine differential pharmacological effects between groups across the whole-brain, we assessed the impact of the full agonist nicotine (vs. placebo patch) in the absence of varenicline in a GROUP (smoker vs. nonsmoker) x PATCH (nicotine vs. placebo) analysis (controlling for age and motion; pcorrected<0.05). Theoretically and empirically, the full agonist provides the most robust effects and thus the greatest power to detect group differences.
RESULTS
Amygdala-centric rsFC: Smoker drug effects
The left and right amygdala seeds identified ICNs that included bilateral amygdala and hippocampus, ventromedial PFC (vmPFC), and dorsomedial PFC (dmPFC; Fig. S2) consistent with previous reports (9, 22). We subsequently performed whole-brain PILL x PATCH analyses to identify regions whose rsFC with the amygdala was modulated by drugs in abstinent smokers. Focusing on the left seed, this analysis identified drug-induced rsFC modulations between the amygdala and left insular, right precentral, and right parietal regions (Fig. 2A, Table S1).
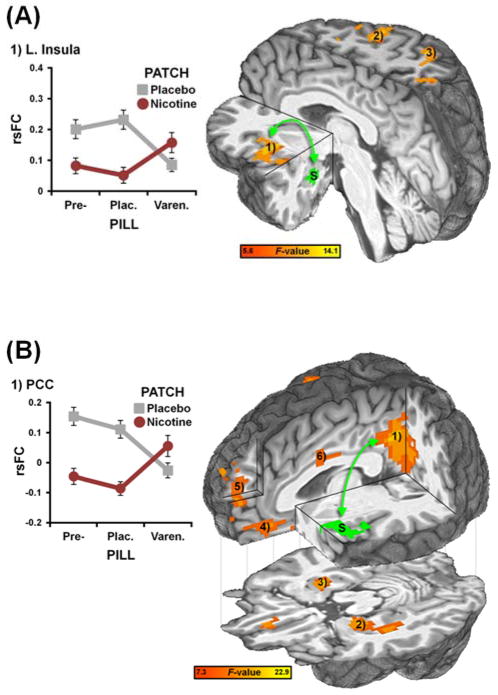
Figure 2.
rsFC strength in amygdala- and insula-centric circuits was decreased by nicotine and varenicline in a manner consistent with varenicline’s partial agonist profile. (A) A PILL x PATCH interaction analysis identified brain regions whose rsFC with a functionally-defined left amygdala seed (S, green) was modulated by drugs. Qualitatively, during pre-pill sessions, nicotine (vs. placebo patch) administration decreased rsFC strength between the amygdala and insula (1). This nicotine-induced decrease was internally replicated under placebo pill conditions. During active pill sessions, varenicline also decreased amygdala-insula rsFC strength when administered alone (graph, grey line) and attenuated nicotine’s impact when administered in combination (graph, red line). Similar drug-induced modulations were observed in the right precentral gyrus (2) and superior parietal lobule (3); see also Fig. S3 and Table S1. (B) A PILL x PATCH interaction analysis identified brain regions whose rsFC with a left insula seed (S, green: obtained from the amygdala-centric analysis) was modulated by drugs. Both varenicline and nicotine decreased rsFC strength between the insula and PCC (1). Similar drug-induced modulations were observed in the left parahippocampus extending into amygdala (2), right parahippocampus (3), vmPFC (4), dmPFC (5), and mid-cingulate cortex (6); see also Fig. S4 and Table S3. Data are reported as mean ± SEM.
Of particular interest, rsFC strength in an amygdala-insula circuit was modulated by both varenicline and nicotine in accord with our hypothesized PILL x PATCH pharmacological interaction (Fig. 2A). Specifically, nicotine decreased amygdala-insula rsFC under both pre-pill and placebo pill conditions but not under varenicline conditions2. This nicotine-induced decrease was absent under active pill sessions as varenicline: 1) also decreased amygdala-insula rsFC when administered alone, and 2) attenuated nicotine’s impact when administered in combination. Similar drug-induced modulations were observed for all identified ROIs (Fig. S3). The right amygdala seed also showed drug-induced rsFC modulations with the left insula (Table S2).
Insula-centric rsFC: Smoker drug effects
We then performed an additional rsFC assessment using the left insula region described above as a seed. This insula seed extracted an ICN anchored by bilateral insula and posterior medial prefrontal areas (Fig. S2) consistent with previous reports (23, 24). Critically, a whole-brain PILL x PATCH analysis identified regions whose rsFC with the insula was modulated by drugs including the PCC, left parahippocampus/amygdala, right parahippocampus, vmPFC, and dmPFC (Fig. 2B, Table S3).
As an exemplar, rsFC strength in an insula-PCC circuit was modulated by both varenicline and nicotine in accord with our hypothesized PILL x PATCH interaction (Fig. 2B). Qualitatively, insula-PCC connectivity strength was reduced by nicotine, but only in the absence of varenicline. No nicotine-induced decrease was observed under active pill sessions as varenicline: 1) also decreased insula-PCC rsFC, and 2) attenuated nicotine’s impact thereon. Similar drug-induced rsFC modulations were observed for all identified ROIs (Fig. S4). Critically, conjunction analyses indicated that elevated rsFC between the insula and PCC, among other regions, was both reduced by drugs and associated with greater subjective and objective measures of withdrawal severity (Fig. S5, Table S3).
Specificity of drug effects
To assess the directional and regional specificity of the drug-induced rsFC decreases reported above, we then conducted a PCC-centric rsFC analysis. We observed that nicotine administration increased PCC-mPFC rsFC (Fig. 3) thereby replicating previous findings (29) and providing a positive control for our amygdala and insula rsFC results.
Figure 3.
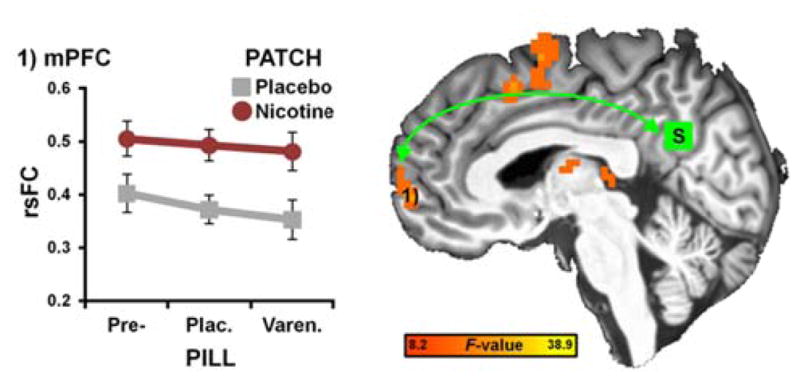
rsFC strength in a PCC-mPFC circuit was increased by nicotine in abstinent smokers. A whole-brain PATCH main effect identified brain regions whose rsFC with a PCC seed (S, green: center coordinate obtained from the insula-centric analysis in Fig. 2B; x = 2, y = −54, x = 26; 29 voxels) was modulated by nicotine. Across all PILL-levels, nicotine increased rsFC between the PCC and mPFC (1: x = 6, y = 60, x = 2; 56 voxels). No indications of a varenicline effect were observed.
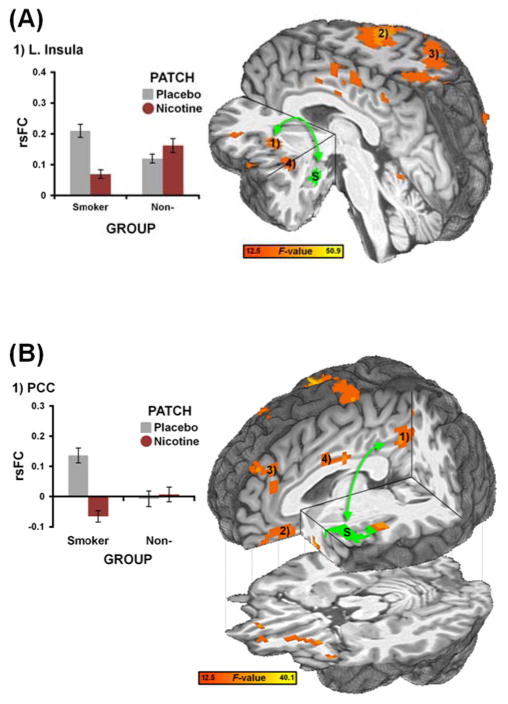
Finally, we examined the impact of these nicotinic drugs in nonsmokers. When considering the above ROIs that showed drug-induced changes in smokers, neither amygdala- (Fig. S3) nor insula-centric rsFC (Fig. S4) were modulated by drugs in nonsmokers. To more directly interrogate differential responses between smokers versus nonsmokers, we assessed the impact of nicotine in GROUP x PATCH analyses. Indicative of group specificity and thus providing a negative control, nicotine selectively decreased rsFC in the amygdala-insula (Fig. 4A, Fig. S6, Table S4) and insula-PCC circuits of smokers (Fig. 4B, Fig. S7, Table S5), yet produced no effect in nonsmokers. In contrast, heart rate was modulated by drugs in both smokers and nonsmokers (Fig. S8), confirming that nonsmokers did show an observable physiological response to the drugs.
Figure 4.
rsFC strength in amygdala- and insula-centric circuits was decreased by nicotine in abstinent smokers but not in nonsmokers. (A) A GROUP x PATCH interaction analysis identified brain regions whose rsFC with a left amygdala seed (S, green: same as in Fig. 2A) showed differential responses to nicotine challenge in smokers versus nonsmokers. Qualitatively, nicotine decreased rsFC between the amygdala and insula (1) in the smoker but not the nonsmoker group. Note: This whole-brain between-group strategy independently identified similar regions as those detected in the within-smoker analysis (c.f., Fig. 2A). Similar interaction patterns were observed in the precentral gyrus (2), parietal regions (3), and posterior insula (4); see also Fig. S6 and Table S4. (B) A GROUP x PATCH interaction analysis identified brain regions whose rsFC with a left insula seed (S, green: same as in Fig. 2B) showed differential responses to nicotine challenge in smokers versus nonsmokers. Nicotine decreased rsFC between the insula and PCC/precuneus (1) in the smoker but not the nonsmoker group. Similar interaction patterns were observed in the vmPFC (2), dmPFC (3), and mid-cingulate cortex (4); see also Fig. S7 and Table S5.
DISCUSSION
We examined the impact of varenicline and nicotine (administered both alone and in combination) on amygdala and insula circuit dynamics and provide the first empirical support for varenicline’s dual (partial agonist/antagonist) action profile in the human brain. While this dual mechanism of action may explain varenicline’s greater relative efficacy over other pharmacotherapies (41, 53), supporting neurobiological evidence has to date come only from cellular and preclinical data (42). Beginning with a functionally-defined amygdala seed, we observed that functional connectivity strength in an amygdala-insula circuit of abstinent smokers was down-regulated by varenicline and nicotine in a manner consistent with varenicline’s partial agonist properties. Similarly, nAChR stimulation decreased rsFC between the insula and, among other brain regions, components of the canonical DMN (e.g., PCC, vmPFC, dmPFC, and parahippocampus). These drug-induced modulations were contingent upon neuroadaptive changes associated with an extended smoking history that manifest during early abstinence, as similar effects were not observed in nonsmokers. These results provide a novel systems-level perspective on the neurobiological underpinnings of nicotine withdrawal that may contribute to aspects of the tobacco abstinence syndrome.
Linking the amygdala’s involvement with drug withdrawal (3, 4) and the insula’s critical role in nicotine addiction (5), we observed that amygdala-insula functional coupling was reduced by two smoking cessation aids. Whereas previous neuroimaging investigations have demonstrated that: 1) cigarette smoking or varenicline treatment dampens abstinence-induced elevated activity within the amygdala (4, 54, 55), and 2) drug cues and smoking urges increase activity within the insula (6), the functional interface between these regions has heretofore been unexplored during abstinence or nAChR stimulation. Circuit-level interactions between these regions have been theoretically implicated, although not empirically demonstrated, to play a role at multiple stages of the addiction cycle (10). For example, the insula is thought to monitor homeostatically-relevant bodily sensations during abstinence and, in turn, modify affective processes via interactions with the amygdala and vmPFC (10, 14). Our findings provide empirical evidence supporting such theoretical contentions. Increased amygdala-insula functional coupling has been linked with elevated subjective anxiety (56, 57) and irritability (10).
Towards a systems-level understanding of the insula’s role in nicotine addiction, we observed that the insula’s functional interaction with multiple components of the DMN was reduced by drug administration. The insula’s interactions with DMN regions appeared ‘maladaptive’ as they were associated with elevated subjective and objective measures of withdrawal severity (Fig. S5). These results suggest a novel neural mechanism that may contribute to the therapeutic actions of pharmacological cessation aids. Further interpretation of these insula-centric rsFC modulations is informed by a larger neuroimaging literature.
Complementing an interoceptive monitoring role, emerging evidence implicates the insula in modulating dynamic activity between two competitively interacting large-scale brain networks: one associated with internally-oriented cognitive operations (DMN) and the other with externally-oriented attentional processes (ECN: 30, 33, 34, 35). At ‘rest,’ activity between the DMN and ECN fluctuates in a temporally anti-correlated fashion (33), such that increased activity in one coincides with decreased activity in the other, suggesting these networks subserve opposing cognitive operations competing for limited processing resources. Perhaps one of the more heuristically useful perspectives to emerge from the nascent rsFC literature relates these competitive network dynamics to consequences during goal-directed behavior. Specifically, intermittent failures to adequately suppress DMN activity and/or maladaptive DMN-ECN interactions may represent sources of interference limiting goal-directed information processing (26, 58, 59). By ‘toggling’ activity between these functionally opposed networks (34–37), the insula has been suggested to facilitate processing of the currently most homeostatically-relevant stimuli arising from internal or external loci (24).
Accordingly, we suggest that during nicotine withdrawal, the insula may bias processing resources towards homeostatically-salient endogenous states through increased interaction with DMN regions at the expense of decreased exogenously-oriented attention mediated by the ECN. Such a shift in large-scale network dynamics could provide an unifying systems-level account of performance deficits across multiple cognitive domains during smoking abstinence (60). Stated simply, increased insula-DMN functional interactions may contribute to ‘difficulty concentrating’ during nicotine withdrawal. Supporting this proposal, nicotine decreases DMN activity and improves task performance in minimally-deprived smokers (61) and reduces DMN-ECN interactions following abstinence (28). The current results indicating that nAChR stimulation decreased insula-DMN circuit strength associate the insula’s role in nicotine addiction and involvement in large-scale “network switching” with nicotine’s performance-enhancing properties. Such a view is consistent with proposals implicating insula’s influence on network dynamics with nicotine addiction (31) and other neuropsychiatric conditions (30, 62).
Whereas the insula detects and/or represents interoceptive signals associated with a homeostatic disequilibrium (11, 16), we suggest that the insula’s interaction with DMN regions is involved in preparing the organism to respond to and alleviate such states. For example, increased insula-DMN interactions may be accompanied by ruminations about or planning for drug use. Insula’s elevated interaction with the PCC, amygdala, vmPFC, and parahippocampus, regions consistently activated during cue provocation (17, 18) may also enhance the salience of drug-associated stimuli. These insula-centric network dynamics are unlikely specifically associated with nicotine withdrawal but rather may reflect a general neurobiological mechanism evolved to maintain the organism’s survival that is usurped in addiction. As such, we speculate that similar circuit-level modulations would be observed in nonsmokers if homeostasis were perturbed (e.g., by thirst or hunger).
Based on the current results, it appears that a desirable characteristic of future pharmacological agents would be to decrease functional coupling in the amygdala-insula and/or insula-DMN circuits described. We suggest that decreased rsFC in these circuits during early smoking abstinence may contribute to the amelioration of subjective withdrawal symptoms such as anxiety, irritability, and difficulty concentrating.
To confirm the specificity of drug-induced rsFC decreases in the amygdala and insula circuits, we performed multiple control analyses. Providing a positive control, we demonstrated that nicotine increased PCC-mPFC rsFC in abstinent smokers as it does in minimally-deprived smokers (29). This analysis indicates that decreased connectivity in the amygdala and insula circuits was unlikely explained by non-selective pharmacological actions on the fMRI signal or neural transduction mechanisms. Additionally, this analysis provides evidence for the circuit and directional specificity of the drug effects observed in the amygdala and insula seed analyses. In the amygdala-insula and insula-PCC circuits, both varenicline and nicotine decreased rsFC. In contrast, within the PCC-mPFC, circuit only nicotine increased rsFC. These outcomes suggest that PCC-mPFC rsFC was modulated by nAChRs other than those upon which varenicline acts. Providing a negative control, we demonstrated that nicotine decreased amygdala and insula rsFC in smokers but not in nonsmokers. While it could be argued that the nicotine dose administered to nonsmokers was too small to induce rsFC modulations, nonsmoker’s lack of tolerance was a dose-limiting factor. However, the dose was sufficient to produce heart rate increases in nonsmokers comparable to those observed in smokers (Fig. S9). This analysis suggests that amygdala and insula circuit dynamics were reduced by drugs only during nicotine withdrawal.
Our findings should be considered in light of several remaining issues. First, head motion can have a systematic impact on rsFC measures (47, 49, 63) and our smoker group showed reduced in-scanner head movement following drug administration (Fig. S9). While we used a combination of three strategies to mitigate the confounding effects of motion (i.e., subject-level nuisance regressors, censoring, and group-level covariates) and therefore assume that drug-induced rsFC modulations were unlikely fully explained by motion-related artifacts, optimal approaches for dealing with motion remain an ongoing area of research (48, 64). Second, given insula’s role in monitoring autonomic arousal (12), our drug-induced rsFC modulations might reflect, at least in part, general shifts in autonomic tone as opposed to specific withdrawal-related CNS processes. We believe this is unlikely, as both smokers and nonsmokers showed drug-induced cardiovascular responses, yet only smokers showed rsFC modulations. Third, while we regressed from the BOLD time series nuisance signals derived using white-matter and cerebral spinal fluid voxels (46), heart rate and/or respiration data recorded during scanning could have further mitigated noise variation associated with non-neuronal physiological processes. Fourth, an additional “smoking as usual” condition may have provided further insight into the specificity of the observed circuit dynamics. Fifth, the potential laterality of insular function in relation to nicotine addiction remains an open issue. While the right insula has been implicated in large-scale network switching (34, 35) and right-lateralized insular lesions may produce more robust disruption of smoking behaviors (5), we predominately observed drug-induced changes associated with the left insula. Greater left insula gray matter density has been reported in smokers versus nonsmokers (65), consistent with the laterality herein. Finally, the degree to which amygdala and/or insula circuit alterations generalize to treatment-seeking smokers and/or predict treatment outcomes remains indeterminate.
This study highlights the utility of examining rsFC to elucidate the neural underpinnings of a multifaceted neuropsychiatric disorder. Previously, connectivity strength between anterior cingulate cortex and ventral striatum has been suggested to represent a “trait-like” circuit-level biomarker of nicotine addiction, as such functional connectivity: 1) inversely correlated with addiction severity, 2) was not impacted by nicotine administration, and 3) appeared modulated by nAChR genetics (27, 29). Here, we suggest that rsFC between insula and DMN regions may represent a “state-like” biomarker of nicotine withdrawal3, as such functional connectivity was: 1) modulated by two pharmacological cessation aids, 2) associated with withdrawal severity, and 3) unaltered by drugs in nonsmokers. Amygdala/insula circuitry may contribute to hallmark features of nicotine withdrawal such as anxiety, irritability and difficulty concentrating and provide a quantitative biometric usefully applied in the design and development of novel smoking cessation interventions.
Supplementary Material
Acknowledgments
This work was sponsored by the National Institute on Drug Abuse Intramural Research Program, National Institutes of Health, Department of Health and Human Services (NIDA-IRP/NIH/DHHS). We thank Eliscia Smith, Angela Neal, Kimberly Slater, Loretta Spurgeon, and the NIDA-IRP nurses, pharmacy, and recruitment staff for assistance with data collection.
FUNDING/SUPPORT: This work was sponsored by the National Institute on Drug Abuse Intramural Research Program, National Institutes of Health, Department of Health and Human Services (NIDA-IRP/NIH/DHHS).
Footnotes
No order effects (varenicline-first vs. placebo-first) were detected on any of the rsFC measures described below.
As ‘off-line’ follow-up tests on these data would constitute a circular analysis (52.), we provide a qualitative description of the PILL x PATCH interaction.
Neither amygdala-centric nor insula-centric rsFC correlated with FTND scores in the current study.
FINANCIAL DISCLOSURE: The authors report no biomedical financial interests or potential conflicts of interest.
PREVIOUS PRESENTATIONS: This study was presented in part at the annual meetings of the American College of Neuropsychopharmacology (December 4–8, 2011; Waikoloa, Hawaii) and the Society for Neuroscience (November 13–17, 2010; San Diego, California).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hughes JR. Effects of abstinence from tobacco: Valid symptoms and time course. Nic Tob Res. 2007;9:315–327. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- 2.Piasecki TM. Relapse to smoking. Clin Psychol Rev. 2006;26:196–215. doi: 10.1016/j.cpr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61–75. doi: 10.1016/j.brainres.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mihov M, Hurlemann R. Altered amygdala function in nicotine addiction: Insights from human neuroimaging studies. Neuropsychologia. 2012;50:1719–1729. doi: 10.1016/j.neuropsychologia.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 5.Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naqvi NH, Bechara A. The hidden island of addiction: The insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharm. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Rev. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- 9.Roy AK, Shehzad Z, Margulies DS, Kelly AMC, Uddin LQ, Gotimer K, et al. Functional connectivity of the human amygdala using resting state fMRI. Neuro Image. 2009;45:614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naqvi NH, Bechara A. The insula and drug addiction: An interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. 2010;214:435–450. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Craig AD. How do you feel - now? The anterior insula and human awareness. Nature Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 12.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 13.Craig AD. The sentient self. Brain Struct Funct. 2010;214:563–577. doi: 10.1007/s00429-010-0248-y. [DOI] [PubMed] [Google Scholar]
- 14.Paulus MP, Tapert SF, Schulteis G. The role of interoception and alliesthesia in addiction. Pharmacol Biochem Behav. 2009;94:1–7. doi: 10.1016/j.pbb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verdejo-Garcia A, Clark L, Dunn BD. The role of interoception in addiction: A critical review. Neuroscience and Biobehavioral Reviews. 2012;36:1857–1869. doi: 10.1016/j.neubiorev.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Garavan H. Insula and drug cravings. Brain Struct Funct. 2010;214:593–601. doi: 10.1007/s00429-010-0259-8. [DOI] [PubMed] [Google Scholar]
- 17.Chase HW, Eickhoff SB, Laird AR, Hogarth L. The Neural Basis of Drug Stimulus Processing and Craving: An Activation Likelihood Estimation Meta-Analysis. Biological Psychiatry. 2011;70:785–793. doi: 10.1016/j.biopsych.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, et al. Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. Neuroimage. 2012;60:252–262. doi: 10.1016/j.neuroimage.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional Connectivity in the Motor Cortex of Resting Human Brain Using Echo-Planar Mri. Mag Resonance Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 20.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 21.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cortex. 2010;21:1667–1673. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cauda F, D’Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. Functional connectivity of the insula in the resting brain. Neuroimage. 2011;55:8–23. doi: 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- 24.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. Correspondence of the brain’s functional architecture during activation and rest. PNAS. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly AMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuro Image. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Hong LE, Hodgkinson CA, Yang YH, Sampath H, Ross TJ, Buchholz B, et al. A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13509–13514. doi: 10.1073/pnas.1004745107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cole DM, Beckmann CF, Long CJ, Matthews PM, Durcan MJ, Beaver JD. Nicotine replacement in abstinent smokers improves cognitive withdrawal symptoms with modulation of resting brain network dynamics. Neuro Image. 2010;52:590–599. doi: 10.1016/j.neuroimage.2010.04.251. [DOI] [PubMed] [Google Scholar]
- 29.Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, et al. Association of Nicotine Addiction and Nicotine’s Actions With Separate Cingulate Cortex Functional Circuits. Arch Gen Psychiat. 2009;66:431–441. doi: 10.1001/archgenpsychiatry.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cog Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Sutherland MT, McHugh M, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: Lessons learned and a road ahead. Neuroimage. doi: 10.1016/j.neuroimage.2012.01.117. (in press) [DOI] [PMC free article] [PubMed]
- 32.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. PNAS. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. PNAS. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. PNAS. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-Mode and Task-Positive Network Activity in Major Depressive Disorder: Implications for Adaptive and Maladaptive Rumination. Biol Psychiatry. 2011;70:327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uddin LQ, Supekar KS, Ryali S, Menon V. Dynamic Reconfiguration of Structural and Functional Connectivity Across Core Neurocognitive Brain Networks with Development. J Neurosci. 2011;31:18578–18589. doi: 10.1523/JNEUROSCI.4465-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, et al. Pharmacological profile of the alpha 4 beta 2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 39.Rollema H, Coe JW, Chambers LK, Hurst RS, Stahl SM, Williams KE. Rationale, pharmacology and clinical efficacy of partial agonists of alpha(4)beta(2) nACh receptors for smoking cessation. Trends Pharmacol Sci. 2007;28:316–325. doi: 10.1016/j.tips.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC, et al. Varenicline improves mood and cognition during smoking abstinence. Biol Psych. 2009;65:144–149. doi: 10.1016/j.biopsych.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an alpha 4 beta 2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation - A randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 42.Rollema H, Hajos M, Seymour PA, Kozak R, Majchrzak MJ, Guanowsky V, et al. Preclinical pharmacology of the alpha 4 beta 2 nAChR partial agonist varenicline related to effects on reward, mood and cognition. Biochem Pharmacol. 2009;78:813–824. doi: 10.1016/j.bcp.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 43.Sutherland MT, Carroll AJ, Salmeron BJ, Ross TJ, Hong LE, Stein EA. Individual differences in amygdala reactivity following nicotinic receptor engagement in abstinent smokers. Neuroimage. doi: 10.1016/j.neuroimage.2012.10.043. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Faessel HM, Gibbs MA, Clark DJ, Rohrbacher K, Stolar M, Burstein AH. Multiple-dose pharmacokinetics of the selective nicotinic receptor partial agonist, varenicline, in healthy smokers. J Clin Pharmacol. 2006;46:1439–1448. doi: 10.1177/0091270006292624. [DOI] [PubMed] [Google Scholar]
- 45.Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, et al. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuro Image. 2010;53:593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuro Image. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Power J, Barnes K, Snyder A, Schlaggar B, Petersen S. Steps toward optimizing motion artifact removal in functional connectivity MRI; A reply to Carp. Neuroimage. doi: 10.1016/j.neuroimage.2012.03.017. (in press) http://dx.doi.org/10.1016/j.neuroimage.2012.1003.1017. [DOI] [PMC free article] [PubMed]
- 49.Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, et al. Impact of in-scanner head motion on multiple measures of functional connectivity: Relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60:623–632. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bigos KL, Pollock BG, Aizenstein HJ, Fisher PM, Bies RR, Hariri AR. Acute 5-HT reuptake blockade potentiates human amygdala reactivity. Neuropsychopharm. 2008;33:3221–3225. doi: 10.1038/npp.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sergerie K, Chochol C, Armony JL. The role of the amygdala in emotional processing: A quantitative meta-analysis of functional neuroimaging studies. Neurosci and Biobehav Rev. 2008;32:811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 52.Kriegeskorte N, Simmons WK, Bellgowan PSF, Baker CI. Circular analysis in systems neuroscience: The dangers of double dipping. Nat Neurosci. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline, an alpha 4 beta 2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation - A randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 54.Franklin T, Wang Z, Suh JJ, Hazan R, Cruz J, Li Y, et al. Effects of varenicline on smoking cue-triggered neural and craving responses. Arch Gen Psychiat. 2011;68:516–526. doi: 10.1001/archgenpsychiatry.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Loughead J, Ray R, Wileyto EP, Ruparel K, O’Donnell GP, Senecal N, et al. Brain activity and emotional processing in smokers treated with varenicline. Addict Biol. doi: 10.1111/j.1369-1600.2011.00324.x. (in press) [DOI] [PubMed] [Google Scholar]
- 56.Paulus MP, Stein MB. An insular view of anxiety. Biol Psych. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 57.Baur V, Hanggi J, Langer N, Jancke L. Resting-State Functional and Structural Connectivity Within an Insula-Amygdala Route Specifically Index State and Trait Anxiety. Biol Psychiatry. doi: 10.1016/j.biopsych.2012.06.003. (in press) [DOI] [PubMed] [Google Scholar]
- 58.Eichele T, Debener S, Calhoun VD, Specht K, Engel AK, Hugdahl K, et al. Prediction of human errors by maladaptive changes in event-related brain networks. PNAS. 2008;105:6173–6178. doi: 10.1073/pnas.0708965105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prado J, Weissman DH. Heightened interactions between a key default-mode region and a key task-positive region are linked to suboptimal current performance but to enhanced future performance. Neuroimage. 2011;56:2276–2282. doi: 10.1016/j.neuroimage.2011.03.048. [DOI] [PubMed] [Google Scholar]
- 60.Heishman SJ, Taylor RC, Henningfield JE. Nicotine and smoking: A review of effects on human performance. Exp Clin Psychopharm. 1994;2:345–395. [Google Scholar]
- 61.Hahn B, Ross TJ, Yang Y, Kim I, Huestis MA, Stein EA. Nicotine enhances visuospatial attention by deactivating areas of the resting brain default network. J Neurosci. 2007;27:3477–3489. doi: 10.1523/JNEUROSCI.5129-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sonuga-Barke EJ, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci Biobehav Rev. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 63.Van Dijk KRA, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carp J. Optimizing the order of operations for movement scrubbing: Comment on Power et al. Neuroimage. 2012 doi: 10.1016/j.neuroimage.2011.12.061. http://dx.doi.org/10.1016/j.neuroimage.2011.1012.1061. [DOI] [PubMed]
- 65.Zhang X, Salmeron BJ, Ross TJ, Geng X, Yang Y, Stein EA. Factors underlying prefrontal and insula structural alterations in smokers. Neuroimage. 2011;54:42–48. doi: 10.1016/j.neuroimage.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.