Abstract
Functional outcomes in long-term survivors of acute liver failure (ALF) are not well-characterized. The aim of this prospective study was to determine health related quality of life (HRQOL) in long-term adult ALF survivors. ALFSG registry participants completed the CDC HRQOL-14 and SF-36 questionnaires at a 1 and/or 2 year follow-up study visit. Responses were compared among ALF subgroups and to available U.S. general population controls. Among the 282 adult ALF patients, 125 had undergone liver transplantation (LT) while 157 were spontaneous survivors (SS), including 95 acetaminophen overdose (APAP) patients and 62 non-APAP SS. Acetaminophen SS patients reported significantly lower general health scores and more days of impaired mental and physical health, activity limitations due to poor health, pain, depression, and anxiety compared to the other groups (p < 0.001). There were no significant differences in coma grade, use of mechanical ventilation or ICP monitoring among the patient groups during their ALF hospitalization but the APAP SS had a significantly higher rate of psychiatric disease and substance abuse (p< 0.001). Compared to the U.S. general population, a greater proportion of the combined SS patients reported fair/poor health and >14 days of impaired physical/mental health and activity limitations due to poor health. In addition, a greater proportion of LT recipents reported >14 days of impaired physical/mental health. Similar results were observed using the SF-36 across the three ALF subgroups and compared to population controls. In conclusion, long-term adult ALF survivors report significantly lower quality of life scores compared to U.S. population controls. Furthermore, APAP SS patients report the lowest quality of life scores, possibly due to a higher rate of pre-morbid psychiatric and substance abuse disorders.
Keywords: Acute liver failure, quality of life, outcomes, liver transplantation, cerebral edema
Acute liver failure (ALF) is defined as the development of severe liver dysfunction with associated coagulopathy and encephalopathy in patients without known underlying liver disease. Although only 2000 to 3000 patients develop ALF in the U.S. each year, these patients are critically ill and require significant supportive care measures such as mechanical ventilation, vasopressors, and renal replacement therapy to achieve short-term survival.1 Despite the high initial mortality rate among patients with ALF, nearly 67% will ultimately survive, with 29% of these patients undergoing emergency liver transplantation.2 The pathogenesis of cerebral edema in ALF patients remains unclear but can lead to severe global impairment, seizures and even uncal herniation and death in some patients.3–5 Pilot studies have suggested that ALF patients with cerebral edema may have impaired cognition and survival during short-term follow-up.3–5 Whether ALF patients develop any long-term sequelae after such a catastrophic illness has not been well studied. In addition, there have been no large multicenter studies evaluating the long-term health-related quality of life (HRQOL) and functional outcomes in adult ALF survivors.
The Acute Liver Failure Study Group (ALFSG) is a consortium of 13 academic medical centers that has been prospectively studying the causes and outcomes of adult ALF patients in the U.S. since 1998. Much of the initial data from the ALFSG has focused on short-term (i.e. 3 weeks) outcomes such as survival and need for liver transplantation. However, a large proportion of these patients have now been followed up to 2 years from illness onset to determine their long-term clinical and functional outcomes. The aim of this study was to determine differences in HRQOL during long-term follow-up among spontaneous survivors (SS) and liver transplant (LT) recipients. In addition, we set out to determine if the severity of illness at ALF presentation was associated with long-term HRQOL outcomes. A secondary aim was to compare HRQOL outcomes in long-term survivors of ALF to age and gender matched US population controls.
Methods
US Acute Liver Failure Study Group
The ALFSG is a consortium of 13 U.S. academic referral centers funded by the National Institute of Diabetes and Digestive and Kidney Diseases to conduct an ongoing prospective observational study of the etiology, clinical features, and outcomes of adult patients with ALF. Enrollment criteria include the presence of coagulopathy defined as an international normalized ratio (INR) > 1.5 and any level of hepatic encephalopathy within 26 weeks of illness onset in a patient with no known underlying liver disease. The current study population consisted of consecutive adult ALF patients enrolled between January 1, 1998, and July 1, 2010 that completed a long-term follow-up visit.
Data collection
Local institutional review board approval was obtained at all participating sites and written informed consent was obtained from the patient’s durable power of attorney or next of kin at the time of enrollment. Detailed patient demographics, medical history, clinical features and laboratory values were collected at study enrollment. Serial laboratory and clinical parameters were prospectively recorded for up to 3 weeks after enrollment. At the end of three weeks, short-term outcomes were classified as spontaneous survival (SS), liver transplantation (LT), or death. Spontaneous survivors and LT recipients were to be followed at 12 and 24 months by the site investigator. At the long-term follow-up study visits subjects were queried regarding their current medication use, medical diagnoses, and interval health history since their ALF admission. In particular, subjects were queried regarding active substance abuse involving alcohol, prescription drugs, and illicit drugs. In addition, information regarding patients with active mood disorders or other psychiatric illnesses that required treatment, intervention, or monitoring were also recorded. Prior to Feb 2010, data forms were sent to the Data Coordinating Center (DCC) for review and entry into a central database. From Feb 2010 forward, data was entered into a web-based study database by the study sites. Periodic site visits were conducted by the ALFSG leadership to verify source documents. A Certificate of Confidentiality was obtained from the National Institutes of Mental Health for the entire study.
SF-36 questionnaire
The SF-36 (version 1.0) is a widely utilized quality of life instrument. The questionnaire is comprised of 8 scales: physical functioning, social functioning, role limitations due to physical health, role limitations due to emotional problems, mental health, vitality, bodily pain, and general health perceptions. Responses to questions in each scale are combined to create a score ranging between 0 (low) to 100 (high). In addition, these individual scale scores can be combined to create separate physical and mental health summary scores. The SF-36 questionnaire was to be completed at 12 and 24 months after ALF onset. Population norms were obtained from the SF-36 scoring manual which consists of 2,474 individuals with 47% between the ages of 18–44 and 57% female. Population norms were obtained only for the physical and health composite scores.
CDC HRQOL-14
The Center for Disease Control and Prevention (CDC) HRQOL-4 is a 4-item Healthy Days Core Module designed to assess the overall health of the U.S. general population. Since 1993, the CDC HRQOL-4 has been integrated into the state-based Behavioral Risk Factor Surveillance System (BRFSS). The CDC HRQOL-14 is a 14-item questionnaire that includes questions from the Health Days Core Module as well as the Activity Limitations Module and the Healthy Days Symptoms Module.6, 7 Each 1-day change in any of the Healthy Days Measures is considered clinically meaningful. As such, this instrument allows for direct comparisons of mean healthy days among differing patient groups, and the results can be compared to the general US population. The CDC HRQOL-14 questionnaire was completed by patients at the same study follow-up visit as the SF-36. Population norms were obtained from the BRFSS website data collected between 2006–2010.8 The normalized population consists of 1,934,859 individuals, with 28% between the ages of 18–44 years and 62% female. Population norms were aggregated over the years 2006–2010 by gender or age group (18–24,25–34,…,55–64,65–74,75+).
Statistical Analysis
SAS software version 9.2 (SAS Institute Inc., Cary, NC, U.S.A.) was utilized to perform statistical analysis. Baseline variables were described using counts and percentages for categorical data, or means and standard deviations (medians and interquartile ranges) for continuous normal (skewed) data. For variables identified as clinically relevant, statistical tests were performed using chi-square, ANOVA, or Kruskal-Wallis tests. Survey items are reported as counts for the CDC-QOL and standardized means and standard deviations for the SF-36 questionnaire. Comparison of measures between groups used a global ANOVA test or chi-square test followed by all Tukey multiple-comparison adjusted pairwise comparisons when significant. For comparisons against population norms, student’s t test or Wald test of proportions was used for pairwise comparisons.
Results
Demographic characteristics and clinical parameters
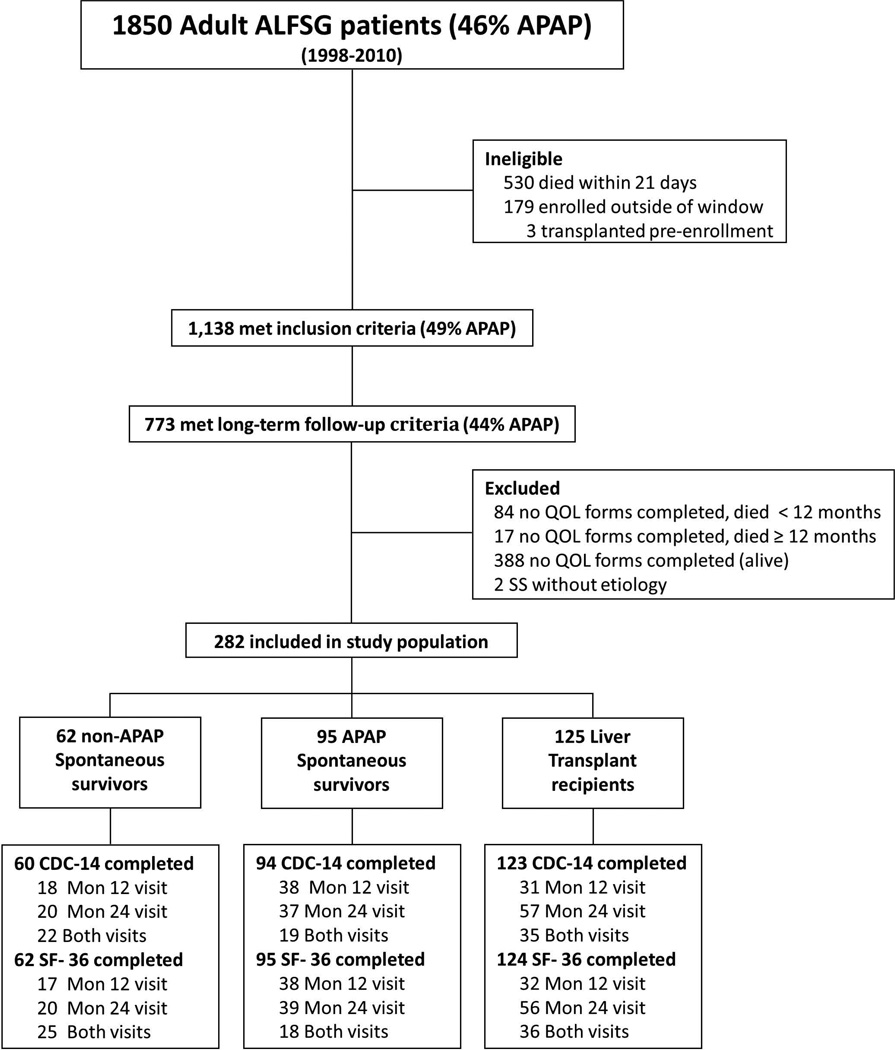
From 1/1/98 to 7/1/10, there were 1,850 adult ALF patients enrolled in the ALFSG. Among the 1,138 survivors at 3 weeks after enrollment, 773 patients had at least one long-term follow-up study visit completed via clinic visit, telephone interview, or chart review or had died beyond 21 days (Figure 1). There were 282 patients that completed at least one quality of life survey at months 12 or 24. Of these, 277 completed the CDC-14 (76 completed at both visits) and 281 completed the SF-36 (79 completed at both visits). The baseline characteristics of the 284 study patients were generally similar to the 405 excluded patients including the need for life support and initial illness severity. However, the included patients were significantly older, more likely to have undergone LT, have a higher BMI, and be non-Hispanic compared to the excluded patients (See Supplementary Table 1).
Figure 1.
Among the study population, 125 had undergone LT, while 157 were SS, including 95 due to acetaminophen overdose (APAP) and 62 due to non-acetaminophen etiologies (non-APAP). The APAP SS group included 58 subjects with non-intentional APAP overdose, 28 subjects with intentional APAP overdose, and 9 subjects with unknown intent. Two SS patients were missing etiology and were excluded from stratified analyses. The most common underlying etiologies for ALF in the LT recipients included drug-induced liver injury (DILI) (25%), indeterminate etiology (21%), and autoimmune hepatitis (18%); in the non-APAP spontaneous survivors, the most common etiologies of ALF included DILI (26%), hepatic ischemia (13%), and hepatitis A (13%). Thirteen (10.7%) of the LT recipients had developed ALF due to APAP overdose. No significant differences were noted in patient demographics including age, gender, race/ethnicity, employment status, years of education, and marital status (Table 1). The APAP SS demonstrated significantly higher rates of substance abuse and underlying psychiatric disease compared to the LT recipients and non-APAP SS (Table 1). However, the 58 non-intentional APAP SS were significantly less likely to have a psychiatric co-morbidity (48% vs 82%, p=0.003) compared to the intentional APAP patients but the proportion with a history of substance abuse was similar (47% vs 43%, p=0.75).
TABLE 1.
Baseline characteristics of adult ALFSG patient population
| N | Non-APAP SS (n=62) |
APAP SS (n=95) |
Liver transplant (n=125) |
p-value | |
|---|---|---|---|---|---|
| Age(yrs) | 282 | 42.5(14.2) | 38.2(13.2) | 40.4(13.0) | 0.52 |
| % Female | 282 | 71.0 | 74.7 | 64.8 | 0.27 |
| Race | 282 | 0.17** | |||
| White | 79.0 | 86.3 | 73.6 | ||
| Black | 14.5 | 7.4 | 18.4 | ||
| Other | 6.5 | 6.3 | 8.0 | ||
| Ethnicity (% non-Hispanic) | 282 | 96.8 | 97.9 | 92.0 | 0.13** |
| % Full/part time employed | 216 | 64.0 | 63.6 | 72.0 | 0.44 |
| Education (years) | 181 | 13.8 (2.0) | 12.4(2.2) | 13.3(2.7) | 0.56 |
| Marital Status (% married/significant other) | 250 | 56.1 | 39.1 | 52.8 | 0.07 |
| Etiology | 282 | ---^ | |||
| % Acetaminophen | 0 | 100 | 10.7 | ||
| % DILI | 25.8 | 0 | 24.6 | ||
| % Autoimmune hepatitis | 11.3 | 0 | 18.0 | ||
| % Hepatitis A | 12.9 | 0 | 1.6 | ||
| % Hepatitis B | 6.5 | 0 | 13.1 | ||
| % Ischemia | 12.9 | 0 | 0 | ||
| % Indeterminate | 11.3 | 0 | 21.3 | ||
| % Other† | 19.4 | 0 | 10.7 | ||
| Medical History | |||||
| Mean BMI (kg/m2) | 231 | 28.8(6.8) | 26.4(7.0) | 28.8(6.5) | 0.62 |
| % Psychiatric Disease | 282 | 37.1 | 57.9 | 18.4 | <0.001 |
| % Substance Abuse | 282 | 9.7 | 48.4 | 11.2 | <0.001 |
| % History IDU | 278 | 3.3 | 6.4 | 2.4 | 0.32** |
| % Hypertension | 282 | 24.2 | 13.7 | 13.6 | 0.13 |
| % Endocrine/Diabetes | 282 | 30.7 | 11.6 | 14.4 | 0.005 |
| Presenting Features and Clinical Complications | |||||
| Days from Jaundice to ALF* | 235 | 5.0(2.0) | 1.0(2.0) | 10.5(17.3) | <0.001 |
| INR* | 279 | 1.9(1.7) | 2.5(1.7) | 3.0(1.8) | <0.001 |
| ALT(IU/L)* | 279 | 1659 (3769) | 3195 (3769) | 711(1776) | <0.001 |
| AST(IU/L)* | 281 | 948 (6514) | 2600 (6514) | 622 (1511) | <0.001 |
| Bilirubin (mg/dL)* | 281 | 10.5(3.2) | 4.2(3.2) | 21.5(15.7) | <0.001 |
| Creatinine (mg/dL)* | 282 | 1.3(2.8) | 1.4(2.8) | 1.1(1.4) | 0.30 |
| % Admission grade ¾ encephalopathy | 280 | 36.1 | 40.0 | 34.7 | 0.71 |
| % Peak Grade ¾ encephalopathy | 282 | 43.6 | 47.4 | 55.2 | 0.27 |
| MELD | 278 | 13.6(4.7) | 11.4(6.8) | 15.9(5.1) | 0.001 |
| % Intubation | 281 | 30.7 | 41.1 | 30.7 | 0.22 |
| % CVVH or Dialysis | 112 | 31.8 | 51.4 | 36.4 | 0.25 |
| % ICP Monitor | 271 | 6.6 | 5.4 | 11.0 | 0.36** |
| % Any Infection | 282 | 38.7 | 40.0 | 37.6 | 0.94 |
Other includes pregnancy, Budd-Chiari, Hepatitis C, Hepatitis E, Mushroom, Wilsons, and miscellaneous
P-Value not provided because definition of strata imply different distribution
Listed as Median(IQR), non-parametric equivalent test (Kruskal-Wallis) used
Fisher’s Exact Test Data reported as Mean(std dev) or %
The LT recipients had significantly higher baseline serum total bilirubin and INR levels as compared to spontaneous survivors. APAP spontaneous survivors exhibited significantly higher aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels compared to the LT and non-APAP SS groups. However, there were no significant differences in baseline serum creatinine levels between the patient groups nor in rates of mechanical ventilation, use of renal replacement therapy, or intracranial pressure (ICP) monitoring during their ALF admission. The percentage of patients with stage 3 or 4 coma grade was also similar among LT recipients and the SS groups.
CDC HRQOL-14 results
ALFSG Adult Cohort
The CDC HRQOL-14 form was completed by 278 ALF patients at a median of 708 days after enrollment (range: 127 –3645 days). Within the Healthy Days Core Module, APAP spontaneous survivors were more likely to report fair or poor general health (Supplemental Table 2: 34% vs. 30% vs. 14%, p=0.001) compared to non-APAP spontaneous survivors and LT recipients. These patients also reported more days of impaired physical health (9.5 vs. 5.6 vs. 6.2, p=0.012), mental health (11.2 vs. 5.7 vs. 6.7, p=0.001), and activities limitations due to poor physical or mental health (9.1 vs. 4.9 vs. 4.5, p<0.001).
Within the Healthy Days Symptoms Module, APAP spontaneous survivors reported more days of pain (10.8 vs. 4.8 vs. 4.9, p<0.001), depression (11.2 vs. 5.3 vs. 5.7, p<0.001), and anxiety (12.7 vs. 4.8 vs. 6.7, p<0.001). No significant differences were noted between groups in regards to days of inadequate sleep or days of poor energy. Additionally, patients in these groups had no signficant differences in responses to questions within the Activity Limitations Module. Amongst the 73 ALF patients completing surveys at both months 12 and 24, there were no significant changes in the number of unhealthy days in the 39 SS patients during follow-up nor in the 34 LT recipients during follow-up (Data not shown).
ALFSG vs Population Controls
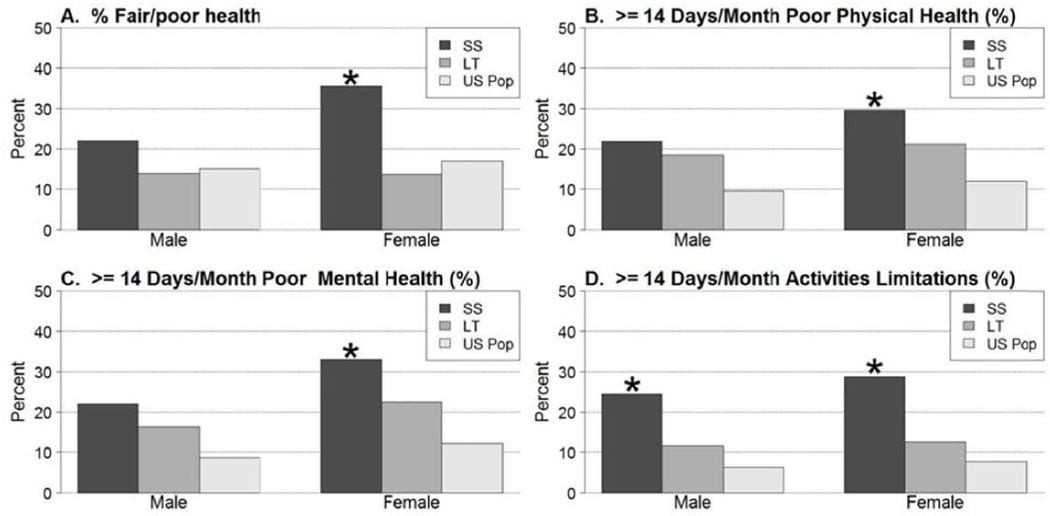
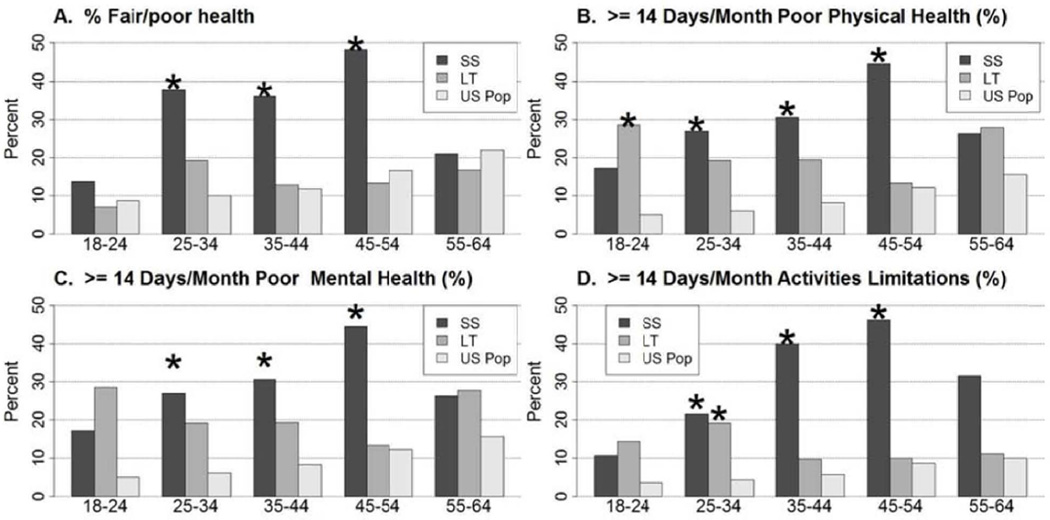
As compared to the U.S. general population, a higher proportion of spontaneous survivors (APAP and non-APAP combined) reported fair or poor general health (32% vs. 16%, p<0.001) (Figures 2 and 3, Supplemental Table 4). This finding was confined primarily to women and patients age 25 to 54. Transplant recipients reported similar general health status as compared to the U.S. general population. A higher percentage of both SS and LT recipients reported >14 days per month of poor physical health as compared to the U.S. general population (28% vs. 11%, p<0.001; 20% vs. 11%, p<0.001), with significant differences noted in patients of both genders for SS and females for LT, and in SS patients under age 55 and LT patients between the ages of 18–44. A greater proportion of both SS and LT recipients reported >14 days per month of poor mental health as compared to the U.S. general population (31% vs. 10%, p<0.001; 20% vs. 10%, p<0.001). Among spontaneous survivors, this finding was observed in both men and women between ages 25 to 54, but was limited to women and patients between ages 18 to 24 among transplant recipients. Both spontaneous survivors and transplant recipients were more likely to report >14 days per month of activities limitations due to poor physical or mental health (28% vs. 7%, p<0.001; 12% vs. 7%, p=0.02). Among spontaneous survivors, this finding was confined to patients between ages 25 to 64; there were no differences in the LT population.
Figure 2.
CDC HRQOL Healthy Days Core Module questions in 156 spontaneous survivors (SS) and 123 liver transplant (LT) recipients stratified by gender. Overall, female SS had significantly poorer HRQOL indices compared to the general population while the scores in male SS were only significantly different in Days of activity limitations. The male and female LT recipient scores were similar to the general US population. (SS; N=41 males and N=115 females) (LT; N=43 males and N=80 females). * p ≤0.001 when compared to the U.S. general population.
Figure 3.
CDC HRQOL Healthy Days Core Module questions in 156 spontaneous survivors (SS) and 123 liver transplant (LT) recipients stratified by age. The HRQOL scores were significantly lower in the SS patients in most age groupings while the LT recipients were generally similar to age matched population controls. (SS; Age 18–24 N=29, 25–34 N=37, 35–44 N=36, 45–54 N=27, 55–64 N=19) (LT; Age 18–24 N=14, 25–34 N=26, 35–44 N=31, 45–54 N=30, 55–64 N=18) * p ≤0.001 when compared to the U.S. general population.
SF-36 results
ALFSG Adult Cohort
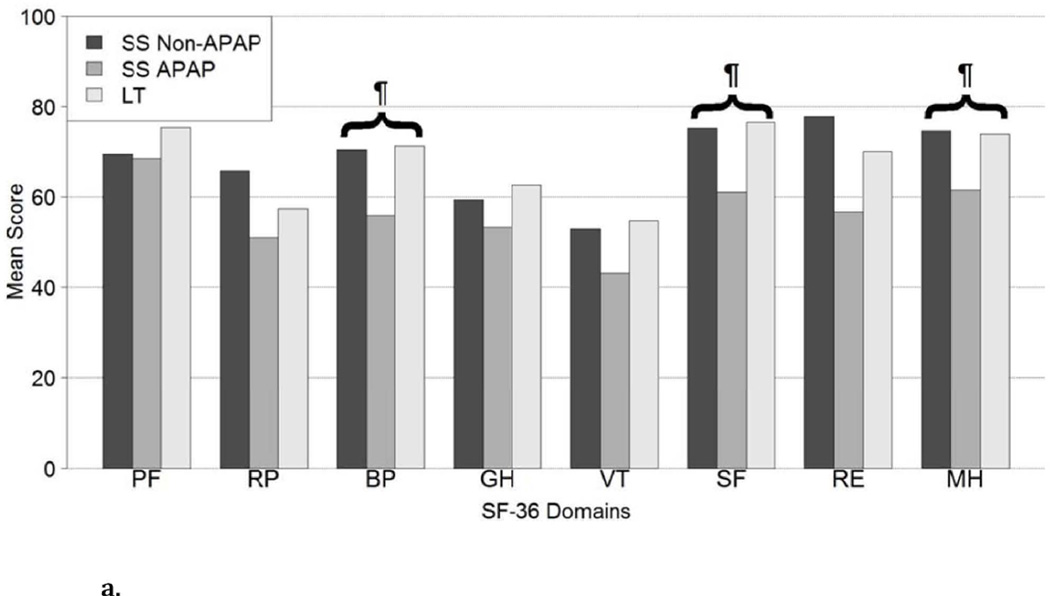
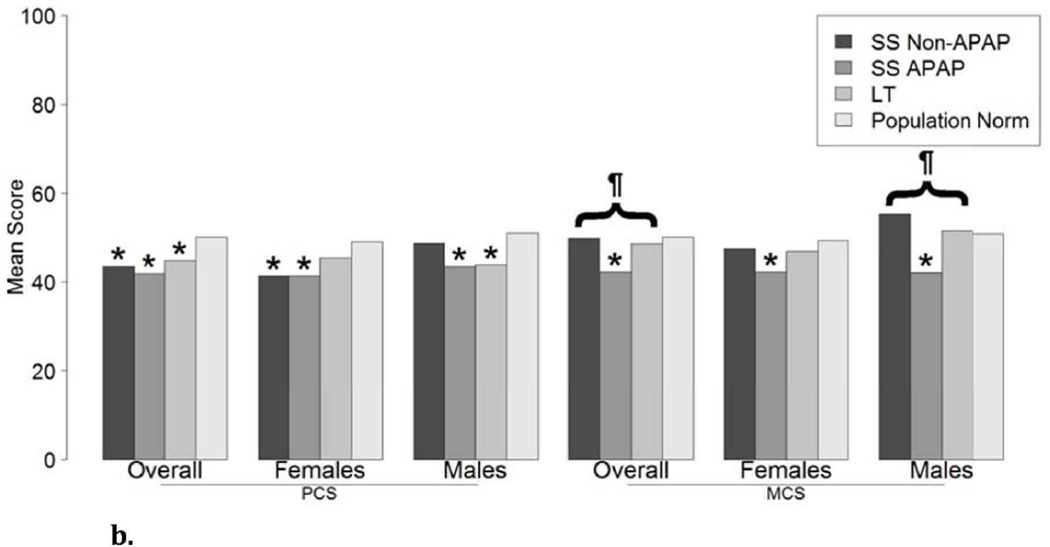
The SF-36 questionnaire was completed by 281 ALF patients at a median of 706 days after enrollment (Interquartile range 448, 859 days) which included 95 APAP SS, 62 non-APAP SS, and 124 LT recipients. Demographic data was similar to the patient population who completed the CDC HRQOL-14 questionnaire, with no significant difference between the 3 subgroups. As compared to non-APAP spontaneous survivors and LT recipients, APAP SS reported lower scale scores for pain (56 vs. 70 vs. 71, p<0.001), general health perceptions (53 vs. 59 vs. 63, p=0.024), energy/fatigue (43 vs. 53 vs. 55, p=0.003), social functioning (61 vs. 75 vs. 76, p<0.001), emotional health problems (57 vs. 78 vs. 70, p=0.006), and emotional well-being (62 vs. 75 vs. 74, p<0.001) (Figure 4A, Supplemental Table 3). There were no significant differences in scores between the 3 groups for the physical functioning, physical health problems, or physical health summary scales. However, the mental health summary scores were significantly lower in the APAP SS group compared to the other patient groups (Figure 4B). There were no significant differences in the mental or physical health summary scores over time in the 37 Spontaneous survivors nor in the 32 LT recipients with paired data (Data not shown).
Figure 4.
a. SF-36 scores in 95 APAP SS, 62 non-APAP SS, and 124 liver transplant recipients. SF-36 Domains: (PF) Physical functioning scale, (RP) Physical health problems scale, (BP) SF-36 Pain Scale, (GH) SF-36 General Health Perceptions Scale, (VT) Energy/fatigue scale, (SF) Social functioning scale, (RE) Emotional Health problems scale, (MH) Emotional well-being scale; ¶ represents overall statistical significance at alpha ≤0.001 comparing SS Non-APAP, SS APAP, LT.
b. SF-36 Component Scores: (PCS) Physical, (MCS) Mental; * represents statistical significance at alpha ≤0.001 when compared to the U.S. general population, ¶ represents overall statistical significance at alpha ≤0.001 comparing SS Non-APAP, SS APAP, LT.
ALFSG vs Population Controls
Gender adjusted physical and mental health summary scale scores were significantly lower in the APAP SS and some of the other subgroups compared to US population norms (Figure 4B).
Discussion
This is the first large, prospective multicenter study to assess QOL outcomes in long-term adult survivors of ALF. The results of this study demonstrate that ALF spontaneous survivors report significantly more days of poor physical and mental health during long-term follow-up as compared to the general U.S. population. Among the ALF patient subgroups, the APAP SS were significantly more likely to report impairment as compared to non-APAP spontaneous survivors and LT recipients. Similar findings were noted using both the CDC HRQOL-14 and SF-36 questionnaires (Figures 2 to 4).
Prior retrospective studies of HRQOL in ALF patients have focused primarily on patients who have undergone liver transplantation. During follow-up, ALF LT recipients report similar QOL scores as patients with cirrhosis undergoing LT.9 In addition, no significant differences in QOL have been noted between APAP and non-APAP ALF transplant recipients.10 However, ALF patients experience higher rates of depression, anxiety, and post-traumatic stress disorder after liver transplant compared to cirrhotic LT recipients, a finding that correlates with the higher rate of poor mental health among LT recipients observed in our study.11 This observation may be due to the fact that ALF patients are previously healthy, generally young individuals who suffered a sudden life-threatening illness whereas most cirrhotic LT recipients have been ill for several years and may have developed better coping skills. Furthermore, prior studies have shown that ALF LT recipients are less likely to resume employment compared to cirrhotic LT recipients, despite their younger age.9
The SF-36 questionnaire has been used to assess HRQOL and functional outcomes in patients with other chronic diseases, as well as in subjects who experience severe acute illness. In particular, the SF-36 has been administered to patients with various liver diseases including chronic hepatitis C and primary biliary cirrhosis.12–14 The SF-36 has also specifically been evaluated in LT recipients and individual domain scores from the current study are similar to prior data reported at 24 months after LT in cirrhotic patients.15 While cirrhotic LT recipients often report significantly improved QOL in the early post-transplant period compared to their pre-transplant status, post-transplant QOL usually remains lower than age matched population controls.16, 17 The durability of improved QOL after LT is also uncertain with several studies suggesting that patients’ psychological well-being may begin to erode as early as 1 year after LT.18, 19 In addition, episodes of rejection , osteoporosis, and recurrent disease are associated with further declines in QOL scores during long-term follow-up in cirrhotic LT recipients. 20–22 However, the QOL scores of LT recipients remain better than those reported in congestive heart failure patients.23 While the underlying etiology of cirrhosis affects HRQOL in the pre-transplant period, no significant differences in QOL based on etiology of cirrhosis have been noted 6 months after LT but more recent studies suggest that chronic HCV patients may have poorer HRQOL during long term follow-up.22, 24
Impaired QOL during long-term follow-up, as measured by the SF-36, has also been seen in previously healthy patients that have suffered from a severe acute illness such as acute renal failure, necrotizing pancreatitis, subarachnoid hemorrhage, and acute respiratory distress syndrome. 25–28 A stay in the intensive care unit with associated vital organ dysfunction may be associated with a reduced QOL, although this finding could also be attributable to pre-existing disease. 29 Of note, we did not find a significant relationship between markers of disease severity such as need for intubation, dialysis, or encephalopathy grade and SF-36 nor CDC-14 scores during long-term follow-up.
The CDC HRQOL-14 is a more recently developed instrument which was designed to assess the HRQOL of the general U.S. population. The 4-item Healthy Days Core Module has been utilized to assess long-term QOL in patients with several chronic diseases including osteoarthritis, rheumatoid arthitis, coronary artery disease, and diabetes mellitus.30–32 Studies using the CDC HRQOL-4 have shown that survivors of severe, life-threatening diseases, such as cerebrovascular accidents and breast cancer, report more unhealthy days per month when compared to the general U.S. population. In addition, one study of older adults indicated that patients with lower CDC-HRQOL-4 scores had a higher rate of hospitalization and death during follow-up.33 A key advantage of the CDC HRQOL-14 is the ability to quantify QOL impairment in terms of unhealthy days, and further make direct comparisons to the general population. In our study, APAP spontaneous survivors reported nearly twice as many days per month of impaired physical and mental health, activity limitation, pain, depression, and anxiety compared to the other groups (Figure 2) indicating that they have the poorest functional status during follow-up.
Our findings of increased days of pain, depression, and anxiety in APAP spontaneous survivors is consistent with prior data regarding the increased incidence of chronic pain and underlying psychiatric disease in this patient population. 34 As such, it is difficult to determine to what extent the long-term QOL impairment observed in this study is attributable to the ALF episode itself versus pre-existing medical or psychiatric factors since baseline QOL scores were not available in these patients. Our findings also demonstrate that non-APAP SS patients may experience substantial physical and mental health impairment during follow-up despite normalization of hepatic laboratory abnormalities. Among ALF liver transplant recipients, impaired mental and physical QOL domains are reported primarily in women and younger patients. As such, a significant subset of ALF survivors may benefit from targeted interventions including counseling, psychiatric care, and physical therapy.
Due to the low incidence of ALF and the limited study population size, we were unable to identify presenting laboratory or clinical parameters that correlated with long-term QOL scores. However, there were no significant differences in baseline coma grade or need for ICP monitoring among APAP spontaneous survivors, non-APAP spontaneous survivors, and LT recipients. Although prior studies have suggested that adherence to follow-up and immunosuppression compliance may be lower in LT recipients with APAP overdose, we were not able to compare QOL outcomes in APAP vs. non-APAP LT recipients due to the small number of patients.35
There were 405 ALFSG patients who did not complete the HRQOL forms and were therefore excluded from this analysis (Suppl Table 1). Overall, the included patients were older, more likely to be non- Hispanic and have undergone LT, and less likely to have had APAP overdose. Therefore, the QOL scores of the included ALFSG patients may intrinsically be better than those who did not complete the surveys since LT recipients had better QOL scores. The higher proportion of LT recipients captured in the current study group likely relates to the fact that LT recipients return more frequently for concurrent care than those who have recovered. In light of these limitations, confirmation of our findings in other large independent cohorts of ALF patients is needed. Nonetheless, the current study represents the largest study of QOL outcomes in ALF patients completed to date. In addition, the prospective enrollment of patients from multiple sites and administration of widely used and validated QOL instruments at predetermined study visits help improve the generalizability and importance of our findings.
In conclusion, our study demonstrates that long-term adult survivors of ALF exhibit diminished QOL as compared to age and gender matched population controls. Furthermore, the APAP SS report decreased QOL across several domains when compared to non-APAP spontaneous survivors and LT recipients. These results were somewhat surprising since LT recipients are known to have more frequent and severe cerebral edema and require long-term use of potentially neurotoxic immunosuppressive agents.36, 37 We speculate that the observed differences in long-term HRQOL outcomes in the ALF patient groups may be the result of other pre-existing medical and psychiatric co-morbidities rather than the patients initial ALF illness and hospital course. Ongoing studies of neuropsychological function by our group and others will help better define the type and severity of potential cognitive impairment that many long-term ALF survivors report to their physicians.
Supplementary Material
Acknowledgements
Dr. Rangnekar is supported by the T32 DK62708-01, NIDDK Training Grant in Gastrointestinal Epidemiology, and a Clinical and Translational Science Award from the Michigan Institute for Clinical and Health Research.
Grant support: We gratefully acknowledge the support provided by the members of The Acute Liver Failure Study Group. This study was funded by the National Institute of Diabetes, Digestive and Kidney Diseases (DK U-01-58369). Additional funding provided by the Tips Fund of Northwestern Medical Foundation and the Jeanne Roberts and Rollin and Mary Ella King Funds of the Southwestern Medical Foundation. Additionally, Dr. Rangnekar is supported by the T32 DK62708-01, NIDDK Training Grant in Gastrointestinal Epidemiology, and a Clinical and Translational Science Award from the Michigan Institute for Clinical and Health Research
Abbreviations
- ALF
Acute liver failure
- ALFSG
Acute liver failure study group
- ALT
Alanine aminotransferase
- APAP
Acetaminophen
- AST
Aspartate aminotransferase
- BRFSS
Behavioral Risk Factor Surveillance System
- CDC
Center for Disease Control and prevention
- CVVH
Continuous veno-venous hemofiltration
- DCC
Data coordinating center
- DILI
Drug induced liver injury
- HRQOL
Health-related quality of life
- ICP
Intracranial pressure
- INR
International normalized ratio
- LT
Liver transplant
- SS
Spontaneous survivor
Appendix
Members and institutions participating in the Acute Liver Failure Study Group 1998–2010 are as follows: W.M. Lee, M.D. (Principal Investigator), George A. Ostapowicz, M.D., Frank V. Schiødt, M.D., Julie Polson, M.D., University of Texas Southwestern, Dallas, TX; Anne M. Larson, M.D., Iris Liou, M.D., University of Washington, Seattle, WA; Timothy Davern, M.D., University of California, San Francisco, CA (current address: California Pacific Medical Center, San Francisco, CA), Oren Fix, M.D., University of California, San Francisco; Michael Schilsky, M.D., Mount Sinai School of Medicine, New York, NY (current address: Yale University, New Haven, CT); Timothy McCashland, M.D., University of Nebraska, Omaha, NE; J. Eileen Hay, M.B.B.S., Mayo Clinic, Rochester, MN; Natalie Murray, M.D., Baylor University Medical Center, Dallas, TX; A. Obaid S. Shaikh, M.D., University of Pittsburgh, Pittsburgh, PA; Andres Blei, M.D., Northwestern University, Chicago, IL (deceased), Daniel Ganger, M.D., Northwestern University, Chicago, IL; Atif Zaman, M.D., University of Oregon, Portland, OR; Steven H.B. Han, M.D., University of California, Los Angeles, CA; Robert Fontana, M.D., University of Michigan, Ann Arbor, MI; Brendan McGuire, M.D., University of Alabama, Birmingham, AL; Raymond T. Chung, M.D., Massachusetts General Hospital, Boston, MA; Alastair Smith, M.B., Ch.B., Duke University Medical Center, Durham, NC; Robert Brown, M.D., Cornell/Columbia University, New York, NY; Jeffrey Crippin, M.D., Washington University, St Louis, MO; Edwin Harrison, Mayo Clinic, Scottsdale, AZ; Adrian Reuben, M.B.B.S., Medical University of South Carolina, Charleston, SC; Santiago Munoz, M.D., Albert Einstein Medical Center, Philadelphia, PA; Rajender Reddy, M.D., University of Pennsylvania, Philadelphia, PA; R. Todd Stravitz, M.D., Virginia Commonwealth University, Richmond, VA; Lorenzo Rossaro, M.D., University of California Davis, Sacramento, CA; Raj Satyanarayana, M.D., Mayo Clinic, Jacksonville, FL; and Tarek Hassanein, M.D., University of California, San Diego, CA. The University of Texas Southwestern Administrative Group included Grace Samuel, Ezmina Lalani, Carla Pezzia, and Corron Sanders, Ph.D., Nahid Attar, the Statistics and Data Management Group included Joan S. Reisch, Ph.D., Linda S. Hynan, Ph.D., Janet P. Smith, Joe W. Webster and Mechelle Murray, and the Medical University of South Carolina Data Coordination Unit included Valerie Durkalski, Ph.D., Wenle Zhao, Ph.D., Catherine Dillon, and Tomoko Goddard
Footnotes
Disclosures: Dr. Rangnekar has no financial conflicts of interest. Dr. Fontana has served as a consultant to Bristol-Meyers Squibb, Vertex Pharmaceuticals, Tibotec, Merck, GlaxoSmithkline and Medtronic in the past year.
Author contributions:
Amol Rangnekar: study concept, analysis and interpretation, manuscript drafting and finalization.
Caitlyn Ellerbe: manuscript drafting and finalization, statistical analyses and interpretation.
Valerie Durkalski: study concept, manuscript drafting and finalization, statistical analyses and interpretation.
Robert J. Fontana: study concept, design, data acquisition, analysis and interpretation, manuscript drafting and finalization, overall supervision.
Brendan McGuire: Manuscript review and finalization
William M. Lee: manuscript drafting and finalization, overall supervision
References
- 1.Stravitz RT, Kramer AH, Davern T, Shaikh AO, Caldwell SH, Mehta RL, et al. Intensive care of patients with acute liver failure: recommendations of the U.S. Acute Liver Failure Study Group. Crit Care Med. 2007;35:2498–2508. doi: 10.1097/01.CCM.0000287592.94554.5F. [DOI] [PubMed] [Google Scholar]
- 2.Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SH, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 3.Jackson EW, Zacks S, Zinn S, Ryan J, Johnson MW, Gerber DA, et al. Delayed neuropsychologic dysfunction after liver transplantation for acute liver failure: a matched, case-controlled study. Liver Transpl. 2002;8:932–936. doi: 10.1053/jlts.2002.35550. [DOI] [PubMed] [Google Scholar]
- 4.Lewis MB, Howdle PD. Cognitive dysfunction and health-related quality of life in long-term liver transplant survivors. Liver Transpl. 2003;9:1145–1148. doi: 10.1053/jlts.2003.50239. [DOI] [PubMed] [Google Scholar]
- 5.Chan G, Taqi A, Marotta P, Levstik M, McAlister V, Wall W, et al. Long-term outcomes of emergency liver transplantation for acute liver failure. Liver Transpl. 2009;15:1696–1702. doi: 10.1002/lt.21931. [DOI] [PubMed] [Google Scholar]
- 6.Mielenz T, Jackson E, Currey S, DeVellis R, Callahan LF. Psychometric properties of the Centers for Disease Control and Prevention Health-Related Quality of Life (CDC HRQOL) items in adults with arthritis. Health Qual Life Outcomes. 2006;4:66. doi: 10.1186/1477-7525-4-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andresen EM, Catlin TK, Wyrwich KW, Jackson-Thompson J. Retest reliability of surveillance questions on health related quality of life. J Epidemiol Community Health. 2003;57:339–343. doi: 10.1136/jech.57.5.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. BRFSS home page. http://wwwcdcgov/brfss/questionnaires/indexhtm.
- 9.Aberg F, Hockerstedt K, Roine RP, Sintonen H, Isoniemi H. Influence of liver-disease etiology on long-term quality of life and employment after liver transplantation. Clin Transplant. 2012;26:729–735. doi: 10.1111/j.1399-0012.2012.01597.x. [DOI] [PubMed] [Google Scholar]
- 10.Sargent S, Wainwright SP. Quality of life following emergency liver transplantation for acute liver failure. Nurs Crit Care. 2006;11:168–176. doi: 10.1111/j.1362-1017.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 11.Guimaro MS, Lacerda SS, Aguilar MR, Karam CH, Kernkraut AM, Ferraz-Neto BH. Post-traumatic stress disorders, mood disorders, and quality of life in transplant recipients with acute liver failure. Transplant Proc. 2011;43:187–188. doi: 10.1016/j.transproceed.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Montali L, Tanaka A, Riva P, Takahashi H, Cocchi C, Ueno Y, et al. A short version of a HRQoL questionnaire for Italian and Japanese patients with Primary Biliary Cirrhosis. Dig Liver Dis. 2010;42:718–723. doi: 10.1016/j.dld.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Kamal SM, Ahmed A, Mahmoud S, Nabegh L, Gohary IE, Obadan I, et al. Enhanced efficacy of pegylated interferon alpha-2a over pegylated interferon and ribavirin in chronic hepatitis C genotype 4A randomized trial and quality of life analysis. Liver Int. 2011;31:401–411. doi: 10.1111/j.1478-3231.2010.02435.x. [DOI] [PubMed] [Google Scholar]
- 14.Snow KK, Bonkovsky HL, Fontana RJ, Kim HY, Sterling RK, Di Bisceglie AM, et al. Changes in quality of life and sexual health are associated with low-dose peginterferon therapy and disease progression in patients with chronic hepatitis C. Aliment Pharmacol Ther. 2010;31:719–734. doi: 10.1111/j.1365-2036.2010.04235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ratcliffe J, Longworth L, Young T, Bryan S, Burroughs A, Buxton M. Assessing health-related quality of life pre- and post-liver transplantation: a prospective multicenter study. Liver Transpl. 2002;8:263–270. doi: 10.1053/jlts.2002.31345. [DOI] [PubMed] [Google Scholar]
- 16.Younossi ZM, McCormick M, Price LL, Boparai N, Farquhar L, Henderson JM, et al. Impact of liver transplantation on health-related quality of life. Liver Transpl. 2000;6:779–783. doi: 10.1053/jlts.2000.18499. [DOI] [PubMed] [Google Scholar]
- 17.Tome S, Wells JT, Said A, Lucey MR. Quality of life after liver transplantation. A systematic review. J Hepatol. 2008;48:567–577. doi: 10.1016/j.jhep.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Goetzmann L, Klaghofer R, Wagner-Huber R, Halter J, Boehler A, Muellhaupt B, et al. Quality of life and psychosocial situation before and after a lung, liver or an allogeneic bone marrow transplant. Swiss Med Wkly. 2006;136:281–290. doi: 10.4414/smw.2006.11362. [DOI] [PubMed] [Google Scholar]
- 19.Moore D, Feurer I, Speroff T, Shaffer D, Nylander W, Kizilisik T, et al. Survival and quality of life after organ transplantation in veterans and nonveterans. Am J Surg. 2003;186:476–480. doi: 10.1016/j.amjsurg.2003.07.024. [DOI] [PubMed] [Google Scholar]
- 20.Bownik H, Saab S. Health-related quality of life after liver transplantation for adult recipients. Liver Transpl. 2009;15:S42–S49. doi: 10.1002/lt.21911. [DOI] [PubMed] [Google Scholar]
- 21.Desai R, Jamiesan NV, Gimson AE, Watson CJ, Gibbs P, Bradley JA, et al. Quality o f life up to 30 years following liver transplantation. Liver Transpl. 2008;14:1473–1479. doi: 10.1002/lt.21561. [DOI] [PubMed] [Google Scholar]
- 22.Saab S, Bownik H, Ayoub N, Younossi Z, Durazo F, Han S, et al. Differences in health-related quallity of life scores after orthotopic liver transplantation with respect to selected socioeconomic factors. Liver Transpl. 2011;17:580–590. doi: 10.1002/lt.22268. [DOI] [PubMed] [Google Scholar]
- 23.Duffy JP, Kao K, Ko CY, Farmer DG, McDiarmid SV, Hong JC, et al. Long-term patient outcome and quality of life after liver transplantation: analysis of 20-year survivors. Ann Surg. 2010;252:652–661. doi: 10.1097/SLA.0b013e3181f5f23a. [DOI] [PubMed] [Google Scholar]
- 24.Estraviz B, Quintana JM, Valdivieso A, Bilbao A, Padierna A, de Urbina JO, et al. Factors influencing change in health-related quality of life after liver transplantation. Clin Transplant. 2007;21:481–499. doi: 10.1111/j.1399-0012.2007.00672.x. [DOI] [PubMed] [Google Scholar]
- 25.Delannoy B, Floccard B, Thiolliere F, Kaaki M, Badet M, Rosselli S, et al. Six-month outcome in acute kidney injury requiring renal replacement therapy in the ICU: a multicentre prospective study. Intensive Care Med. 2009;35:1907–1915. doi: 10.1007/s00134-009-1588-z. [DOI] [PubMed] [Google Scholar]
- 26.Wong GK, Poon WS, Boet R, Chan MT, Gin T, Ng SC, et al. Health-related quality of life after aneurysmal subarachnoid hemorrhage: profile and clinical factors. Neurosurgery. 2011 doi: 10.1227/NEU.0b013e31820cd40d. [DOI] [PubMed] [Google Scholar]
- 27.Wright SE, Lochan R, Imrie K, Baker C, Nesbitt ID, Kilner AJ, et al. Quality of life and functional outcome at 3, 6 and 12 months after acute necrotising pancreatitis. Intensive Care Med. 2009;35:1974–1978. doi: 10.1007/s00134-009-1616-z. [DOI] [PubMed] [Google Scholar]
- 28.Mikkelsen ME, Shull WH, Biester RC, Taichman DB, Lynch S, Demissie E, et al. Cognitive, mood and quality of life impairments in a select population of ARDS survivors. Respirology. 2009;14:76–82. doi: 10.1111/j.1440-1843.2008.01419.x. [DOI] [PubMed] [Google Scholar]
- 29.Orwelius L, Nordlund A, Nordlund P, Simonsson E, Backman C, Samuelsson A, et al. Pre-existing disease: the most important factor for health related quality of life long-term after critical illness: a prospective, longitudinal, multicentre trial. Crit Care. 2010;14:R67. doi: 10.1186/cc8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Currey SS, Rao JK, Winfield JB, Callahan LF. Performance of a generic health-related quality of life measure in a clinic population with rheumatic disease. Arthritis Rheum. 2003;49:658–664. doi: 10.1002/art.11381. [DOI] [PubMed] [Google Scholar]
- 31.Brown DW, Balluz LS, Giles WH, Beckles GL, Moriarty DG, Ford ES, et al. Diabetes mellitus and health-related quality of life among older adults. Findings from the behavioral risk factor surveillance system (BRFSS) Diabetes Res Clin Pract. 2004;65:105–115. doi: 10.1016/j.diabres.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 32.Ford ES, Mokdad AH, Li C, McGuire LC, Strine TW, Okoro CA, et al. Gender differences in coronary heart disease and health-related quality of life: findings from 10 states from the 2004 behavioral risk factor surveillance system. J Womens Health (Larchmt) 2008;17:757–768. doi: 10.1089/jwh.2007.0468. [DOI] [PubMed] [Google Scholar]
- 33.Dominick KL, Ahern FM, Gold CH, Heller DA. Health-related quality of life among older adults with arthritis. Health Qual Life Outcomes. 2004;2:5. doi: 10.1186/1477-7525-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larson AM, Polson J, Fontana RJ, Davern TG, Lalani E, Hynan LS, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 35.Cooper SC, Aldridge RC, Shah T, Webb K, Nightingale P, Paris S, et al. Outcomes of liver transplantation for paracetamol (acetaminophen)-induced hepatic failure. Liver Transpl. 2009;15:1351–1357. doi: 10.1002/lt.21799. [DOI] [PubMed] [Google Scholar]
- 36.Sorensen LG, Neighbors K, Martz K, Zelko F, Bucuvalas JC, Alonso EM. Cognitive and academic outcomes after pediatric liver transplantation: Functional Outcomes Group (FOG) results. Am J Transplant. 2011;11:303–311. doi: 10.1111/j.1600-6143.2010.03363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karvellas CJ, Fix OK, Battenhouse H, Durkalski V, Sanders C, Lee WM. Outcomes and complications of intracranial pressure monitoring in acute liver failure: A retrospective cohort study (abstract) Hepatology. 2012;56(Suppl 1):A3. doi: 10.1097/CCM.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.